Abstract
Endometrial cancer arising from adenomyosis (EC-AIA) is extremely rare, and the typical magnetic resonance imaging (MRI) findings of EC-AIA have not been established. We report a case of EC-AIA that was detected preoperatively on MRI and conduct a literature review of the MRI findings of EC-AIA.
Keywords: Endometrial cancer, Uterine cancer, Adenomyosis, Magnetic resonance imaging
Introduction
Endometrial cancer is the second most prevalent gynecologic malignancy worldwide; an estimated 382,069 new cases and 89,929 deaths were projected for 2018 [1]. Among the types of benign gynecologic disease, adenomyosis is one of the most common, having a reported prevalence of 14% -66% [2]. It is not unusual for endometrial cancer to coexist with adenomyosis (EC-A); however, endometrial cancer arising from adenomyosis (EC-AIA) is extremely rare [3], and hence the characteristic magnetic resonance imaging (MRI) findings of EC-AIA have not been elucidated. We report a case of EC-AIA that was detected preoperatively by MRI.
Case
A 60-year-old woman (gravida 2, para 2) presented with the chief complaint of right lower abdominal pain. She had no significant medical or family history. Because the initial computed tomography (CT) displayed slight swelling of the uterine corpus, MRI of the pelvis without gadolinium-based contrast material was performed. The MRI revealed swelling of the posterior uterine wall with ill-defined signal intensity that was higher than myometrium on T2-weighted imaging (T2WI, Fig. 1A), and uniformly low signal intensity on T1-weighted imaging (T1WI, Fig. 1B). The posterior uterine wall had high signal intensity on diffusion weighted imaging (DWI) and low signal intensity on the apparent diffusion coefficient (ADC) map (Fig. 1C and D). Cytological analysis of endometrial tissue was negative. The serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 values were within normal limits (3.5 ng/mL and 4 U/mL, respectively). We made an initial diagnosis of adenomyosis showing atypical MRI findings, and the patient was treated conservatively and followed up regularly.
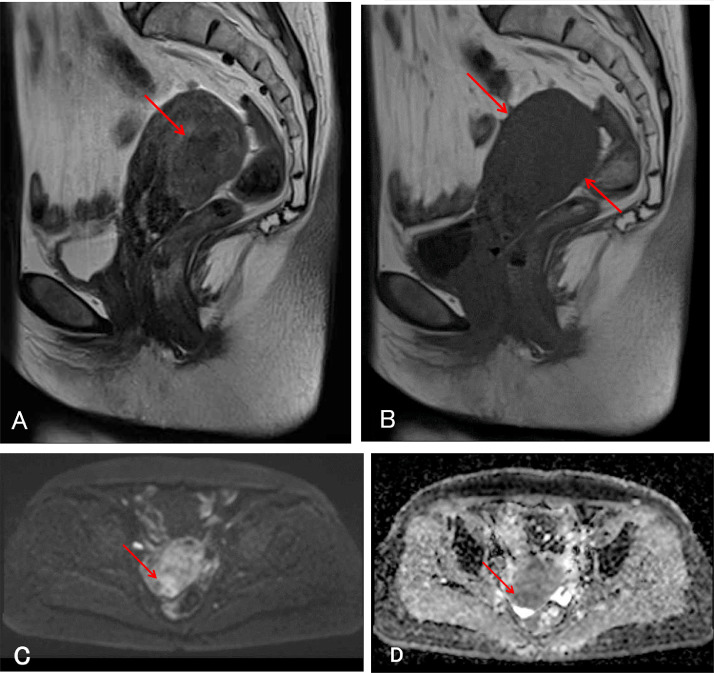
Fig. 1.
Initial magnetic resonance images (MRI) of the pelvis. (A) T2-weighted sagittal MRI demonstrates swelling of the posterior uterine wall and ill-defined signal intensity higher than that of myometrium (arrow). (B) T1-weighted sagittal MRI demonstrates uniform low signal intensity of the uterine corpus (arrows). (C) Diffusion weighted imaging (DWI) demonstrates high signal intensity in the posterior uterine wall (arrow). (D) Apparent diffusion coefficient (ADC) map demonstrates low signal intensity in the posterior uterine wall (arrow).
Four months later, the patient complained of continuous lower abdominal pain, and the following examinations were performed: a second pelvic MRI with gadolinium-based contrast material, contrast-enhanced CT (CECT), and fluorodeoxyglucose-positron emission tomography (FDG-PET). The second MRI showed increased thickening of the posterior uterine wall on T2WI and T1WI (Fig. 2A and B), and fat-saturated T1WI with gadolinium-based contrast material revealed strong and uniform enhancement of the posterior uterine wall (Fig. 2C). CECT revealed para-aorta lymphadenopathy and an ill-defined area of low density in the posterior uterine wall (Fig. 3). FDG-PET demonstrated hypermetabolism in the para-aortic lymph nodes and the posterior uterine wall (Fig. 4). Cytological examination of endometrium was again negative. CEA and CA125 values were highly elevated (28.4 ng/mL and 43 U/mL, respectively), but CA19-9 was within normal limits (15 U/mL). We then thought that the para-aorta lymphadenopathy could be metastasis of a uterine malignancy such as endometrial cancer or low-grade endometrial stromal sarcoma, and surgery was performed. At laparotomy, an intraoperative frozen section of the posterior uterine wall revealed adenocarcinoma, and therefore radical hysterectomy, bilateral adnexectomy, para-aortic lymphadenectomy, pelvic lymphadenectomy, and omentectomy were performed.
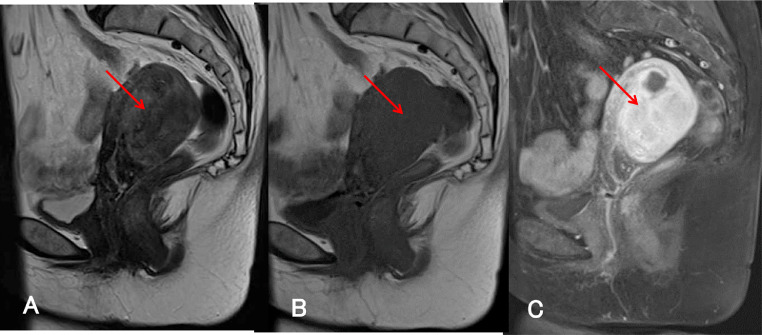
Fig. 2.
MRI acquired 4 months after the initial MRI. (A) T2-weighted sagittal and (B) T1-weighted sagittal MRI demonstrate increased thickening of the posterior uterine wall (arrow). (C) Fat-saturated T1-weighted sagittal MRI with gadolinium-based contrast material demonstrates strong and uniform enhancement of the posterior uterine wall (arrow).
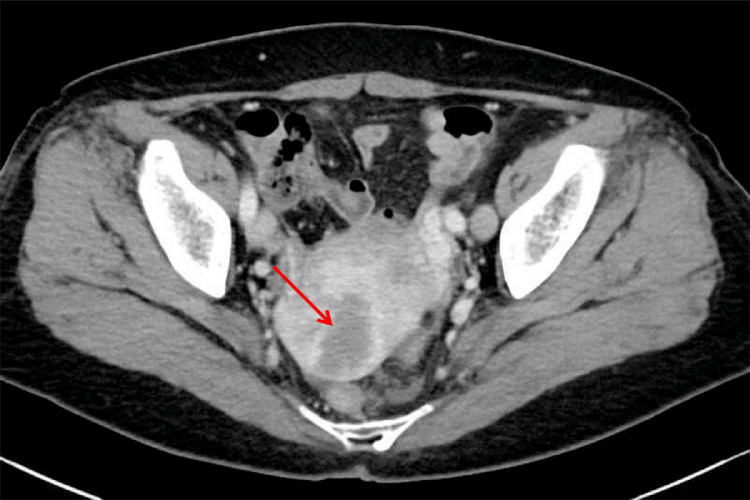
Fig. 3.
Contrast-enhanced computed tomography shows an ill-defined area of low density in the posterior uterine wall (arrow).
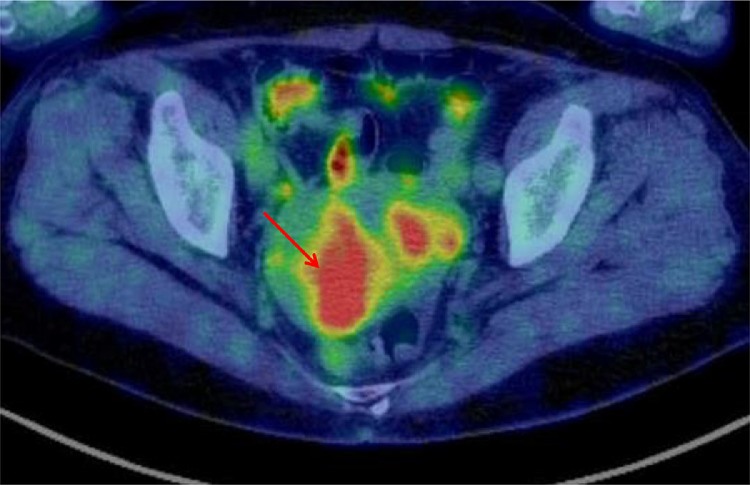
Fig. 4.
Fluorodeoxyglucose-positron emission tomography reveals a hypermetabolic area in the posterior uterine wall (arrow).
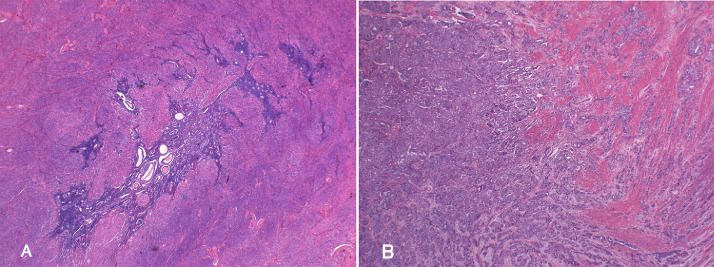
Macroscopic and microscopic examination of the surgical specimens revealed endometrial carcinoma (G2) of the uterine corpus arising from adenomyosis, pT3aN2M1. No masses were identifiable in the endometrial cavity. The specimen showed thickening of the posterior uterine wall. Diffuse endometrioid adenocarcinoma and adenomyotic glands were identified in the myometrium of the uterine corpus (Fig. 5A and B), and transitions from adenomyotic glandular epithelium to adenocarcinoma were observed. Also observed were serosal invasion, metastasis to the para-aortic and pelvic lymph nodes, and the presence of small disseminated nodules in the omentum and pelvic serosa. Postoperatively, the patient received 6 courses of monthly chemotherapy (carboplatin AUC 5 and paclitaxel 135 mg/m2). Follow-up examination at 8 months after surgery found no evidence of recurrence on CECT, and the tumor makers were within normal limits (CEA, CA125, and CA19-9).
Fig. 5.
Histopathological analysis of the uterine specimen. (A) Adenomyotic glands are diffusely distributed within the posterior uterine wall. Hematoxylin and eosin (H&E) staining: low power field. (B) Acinar and papillary proliferation of atypical duct resembling endometrial gland is seen in the posterior uterine wall. H&E staining: low power field.
Discussion
Colman and Rosenthal [4] proposed the following criteria for EC-AIA: (1) absence of carcinoma in normally situated endometrium and elsewhere in the pelvis; (2) carcinoma observed to arise from the epithelium of areas of adenomyosis rather than invading it from some other source; and (3) endometrial stromal cells must surround the aberrant glands to support the diagnosis of adenomyosis. In the present case of EC-AIA, although there was involvement of the omentum and the pelvic serosa, transitional figures were detected from adenomyotic glands to carcinomatous tissue. In addition, carcinomatous glands were scattered throughout the myometrium with thickening of the posterior uterine wall, resembling the pattern of adenomyosis. Based on these findings, we diagnosed this tumor as arising from adenomyosis. Machida et al. reported that EC-AIA has a worse prognosis than EC-A, and that EC-AIA is extremely rare [3].
MRI is now one of the most important modalities for diagnosing uterine disorders, but the typical MRI findings of EC-AIA have not been elucidated. A search for the MRI findings of EC-AIA in the English literature in PubMed and MEDLINE, using the keywords “endometrial cancer”, “uterine cancer”, and “adenomyosis”, returned 8 studies [5], [6], [7], [8], [9], [10], [11], [12]. We found no case series or reviews regarding the MRI findings of EC-AIA.
In these 8 cases and the present case, the MRI findings of EC-AIA were classified into 3 pattern types: (1) uterine wall thickening with ill-defined areas of high signal intensity on T2WI [5,6], (2) well-defined mass of high signal intensity on the uterine wall on T2WI [10,11], and (3) solid papillary projections within a cystic mass attached to the uterine subserosa (ie, cystic adenomyosis origin) [7], [8], [9],12]. The cystic component was hyper-intense on T1WI and T2WI. The solid papillary projections showed enhancement on T1WI with gadolinium-based contrast material. Our case of EC-AIA exhibited the uterine wall-thickening pattern.
ADC maps were available for only 3 cases: 2 of cystic adenomyosis origin [7,12] and the present case (ie, wall-thickening pattern). In those of cystic adenomyosis origin, solid papillary projections within the cystic mass showed low signal intensity. In the present case, the posterior uterine wall showed low signal intensity.
In conclusion, we experienced a case of endometrial cancer arising from adenomyosis. Because the MRI findings of EC-AIA vary and there are few examples of each pattern, it will be necessary to accumulate a larger number of cases before the characteristic MRI findings can be fully evaluated.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Vercellini P, Vigan´ø P, Somigliana E, Daguati R, Abbiati A, Fedele L. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol. 2006;20:465–477. doi: 10.1016/j.bpobgyn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Machida H, Maeda M, Cahoon SS, Scannell CA, Garcia-Sayre J, Roman LD. Endometrial cancer arising in adenomyosis versus endometrial cancer coexisting with adenomyosis: are these two different entities? Arch Gynecol Obstet. 2017;295:1459–1468. doi: 10.1007/s00404-017-4375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colman HI, Rosenthal AH. Carcinoma developing in areas of adenomyosis. Obstet Gynecol. 1959;14:342–348. [PubMed] [Google Scholar]
- 5.Motohara K., Tashiro H., Ohtake H., Saito F., Ohba T., Katabuchi H. Endometrioid adenocarcinoma arising in adenomyosis: elucidation by periodic magnetic resonance imaging evaluations. Int J Clin Oncol. 2008;13:266–270. doi: 10.1007/s10147-007-0725-3. [DOI] [PubMed] [Google Scholar]
- 6.Taga S, Sawada M, Nagai A, Yamamoto D, Hayase R. A case of endometrioid adenocarcinoma arising from adenomyosis. Case Rep Obstet gynecol. 2014;2014 doi: 10.1155/2014/569295. 3 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heo SH, Lee KH, Kim JW, Jeong YY. Unusual manifestation of endometrioid adenocarcinoma arising from subserosal cystic adenomyosis of the uterus: emphasis on MRI and positron emission tomography CT findings. Br J Radiol. 2011;84:e212–e214. doi: 10.1259/bjr/24318075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori M, Furusawa A, Kino N, Uno M, Ozaki Y, Yasugi T. Rare case of endometrioid adenocarcinoma arising from cystic adenomyosis. J Obstet Gynaecol Res. 2015;41:324–328. doi: 10.1111/jog.12513. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa Y, Takano K, Higa S, Tanabe M, Wada A, Sugita M. Endometrial carcinoma coexisting with pregnancy, presumed to derive from adenomyosis: A case report. Int J Gynecol Cancer. 2001;11:488–490. doi: 10.1046/j.1525-1438.2001.01066.x. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki T, Sugiyama T, Nanjo H, Hoshi N, Murakami M, Sugita A. Endometrioid adenocarcinoma arising from adenomyosis: report and immunohistochemical analysis of an unusual case. Pathol Int. 2001;51:308–313. doi: 10.1046/j.1440-1827.2001.01200.x. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi K, Yamanaka Y, Hamana S, Ohara N, Maruo T. Invasive adenocarcinoma arising from uterine adenomyosis involving the rectosigmoid colon. Int J Gynecol Cancer. 2004;14:1004–1006. doi: 10.1111/j.1048-891X.2004.14541.x. [DOI] [PubMed] [Google Scholar]
- 12.Baba A, Yamazoe S, Dogru M, Ogawa M, Takamatsu K, Miyauchi J. Clear cell adenocarcinoma arising from adenomyotic cyst: A case report and literature review. J Obstet Gynaecol Res. 2016;42:217–223. doi: 10.1111/jog.12866. [DOI] [PubMed] [Google Scholar]