Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal diseases, with an average 5-year survival rate of less than 10%. Unfortunately, the majority of patients have unresectable, locally advanced, or metastatic disease at the time of diagnosis. Moreover, traditional treatments such as chemotherapy, surgery, and radiation have not been shown to significantly improve survival. Recently, there has been a swift increase in cancer treatments that incorporate immunotherapy-based strategies to target all the stepwise events required for tumor initiation and progression. The results in melanoma, non-small-cell lung cancer and renal cell carcinoma are very encouraging. Unfortunately, the application of checkpoint inhibitors, including anti-CTLA4, anti-PD-1, and anti-PD-L1 antibodies, in pancreatic cancer has been disappointing. Many studies have revealed that the PDAC microenvironment supports tumor growth, promotes metastasis and consists of a physical barrier to drug delivery. Combination therapies hold great promise for enhancing immune responses to achieve a better therapeutic effect. In this review, we provide an outline of why pancreatic cancer is so lethal and of the treatment hurdles that exist. Particular emphasis is given to the role of the tumor microenvironment, and some of the latest and most promising studies on immunotherapy in PDAC are also presented.
Keywords: Pancreatic ductal adenocarcinoma, Tumor microenvironment, Immunotherapy, Gemcitabine, Treatment, Cancer stem cells
Core tip: Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and lethal malignancies. Treatments such as surgery, radiation, and chemotherapy have limited efficacy due to the extensive heterogeneity of genetic mutations and the dense stromal environment, among other causes. In recent years, immunotherapy has been successfully applied in the treatment of various types of cancers, and immunotherapy combined with the above treatments could create more favorable conditions for the fight against PDAC.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive lethal malignancy due to the lack of early diagnosis and limited response to treatments. It is the most prevalent type of pancreatic neoplasm, and it is developed in the exocrine compartment and accounts for more than 90% of pancreatic cancer cases. Despite scientific progress on the elucidation of PDAC tumor biology and the development of novel therapeutic regimes, it has an average 5-year survival rate of less than 10%[1] and is anticipated to become the second leading cause of cancer–related mortality by 2020. Almost 60%-70% of PDAC cases arise from the head of the pancreas, and these cases are usually diagnosed earlier than tumors arising from the body and tail, as the head of the pancreas contains the common bile duct[2]. Tumors of the body and tail are associated with a worse prognosis[3]. Weight loss, abdominal pain, and jaundice[4] are the most common symptoms observed in patients with PDAC, while less common symptoms include new-onset type 2 diabetes[5] and thromboembolic disease[6]. Classical treatments such as chemotherapy, surgery and radiation have been widely used, but they have not exhibited any significant improvements in clinical outcomes[7,8]. The overall survival for metastatic pancreatic cancer remains poor, and less than 20% of patients survive past the end of the first year[9]. Surgical resection and chemotherapy (gemcitabine and FOLFIRINOX, a combination of oxaliplatin, irinotecan, fluorouracil, and leucovorin) have managed to improve survival of patients with early-stage pancreatic cancer, but these treatments are not sufficient for patients with late stages of the disease[10]. Novel immunotherapies have provided promising results in various solid tumors, such as melanoma or renal cell carcinoma, in a number of cases surpassing chemotherapy as a first-line therapeutic selection[11]. Although immunotherapy began a new era in the field of cancer treatment, it is challenging in the context of PDAC as this type of cancer has a nonimmunogenic, immune-suppressive and therapy-resistant microenvironment.
TREATMENT HURDLES
PDAC development is associated with a poor prognosis due to its complicated and multifactorial nature. There is a lack of simple, early detection methods and is typically diagnosed at a late stage because symptoms do not appear until the disease has progressed and metastasized to distinct sites[12]. As mentioned above, surgical resection with chemotherapy provides the best treatment option for PDAC and is beneficial in patients whose cancer cells have not spread to critical abdominal vessels and adjacent organs[13].
The major difficulties in treating pancreatic cancer lie at both the genetic and cellular levels. The extent of mutational changes in pancreatic tumors generates gene instability that appears to play an essential role in PDAC tumor growth and resistance to treatments. PDAC is characterized by considerable genetic heterogeneity not only among patients but also within a single primary tumor. Targeted treatments are effective in cancers that have a relatively high percentage of patients with the same cancer-causing mutation, such as EGFR in lung cancer[14] or BRAF in melanoma[15]. Pancreatic cancer, on the contrary, presents a variety of mutations that lead to cancer, and each mutation is present in a small percentage of patients[16].
The presence of multiple signaling pathway alterations could partially explain the presence of multiple resistance mechanisms. Although the underlying biology of PDAC has not been fully elucidated, key mutations of specific genes such as Kras, CDKN2A/p16, TP53 and SMAD4 and the concomitant activation of downstream signaling pathways appear to play an essential role in the resistance to treatments[17]. Additionally, the existence of cancer stem cells (CSCs) contributes to the acquisition of a more resistant tumor state. Pancreatic CSCs account for 0.5%-1.0% of all pancreatic cancer cells[18]; CSCs have an increased capacity for self-renewal and exhibit unique metabolic, autophagic and chemoresistance properties that allow them to escape any therapeutic interventions. CSCs are considered tumor-initiating cells that are able to promote tumor development and therapy resistance, leading to disease progression and relapse. One more reason why current treatment fails to exhibit considerable efficacy and beneficial clinical outcomes is that they do not adequately target CSCs[19].
Furthermore, the metastatic potential of PDAC is also responsible for the poor outcome and the lack of effective treatment modules. Recently, genomic and proteomic analyses in the primary PDAC tumor have revealed subclones with different metastatic potentials[20] and probably different responses to specific therapeutic regimes. Additionally, PDAC metastasizes microscopically early in the disease course, limiting the effectiveness of local therapies such as surgery and radiation[21].
Finally, multiple studies have demonstrated that components within the PDAC microenvironment are responsible for poor prognosis and the difficulty in establishing efficacious therapeutic strategies[22-24]. The tumor microenvironment (TME) is characterized by dense desmoplasia and extensive immunosuppression. Extensive desmoplasia results in decreased stromal vascularization, altered immune cell infiltration and hypoxia, inducing tumor growth and hindering drug activity[25].
TUMOR MICROENVIRONMENT
As mentioned above, the PDAC microenvironment is characterized by increased desmoplasia and the presence of several noncellular components, such as hyaluronic acid, and various cell types, such as cancer-associated fibroblasts (CAFs), pancreatic stellate cells (PSCs), muscle fibroblasts and immune cells. Cellular components account for 10%-30%, but the stroma generates most of the tumor mass[26]. The PSC and CAF components are the dominant cells of pancreatic cancers that produce the extracellular matrix in the TME[27]. These components are responsible for the generation of a rigid barrier that results in elevated tumor pressure, diminished vascularization and attenuated drug delivery. Conventional drugs, such as gemcitabine, cannot penetrate the rich and thick layer of the stoma in PDAC and result in drug resistance[28]. Targeting stroma has demonstrated contradictory results among preclinical studies. A study by Olive et al[29] in mouse models showed that inhibition of Sonic Hedgehog-dependent desmoplasia increased gemcitabine delivery and overall survival, while other studies exhibited results contradictory to those of conditional Shh ablation; however, Shh inhibition diminished stroma formation, induced a more aggressive phenotype and decreased survival[30,31]. Additionally, the limited availability of oxygen in the PDAC microenvironment and the minimal vascularization detected were identified as promising targets for therapy. However, clinical trials focused on VEGF-A inhibition combined with chemotherapy did not have the anticipated results. The dense ECM provoked elevated intratumoral pressure that negatively regulated vasculature and diffusion. This phenomenon was reversed with the use of hyaluronidase, but it had a limited beneficial effect because of the increased risk for thrombus[32]. In addition, the extensive immune suppression observed in PDAC comes as a result of the coordinated action of regulatory T cells (Treg), myeloid-derived suppressor cells (MDSCs) and macrophages, which block CD8+ T cell duties in tumor recognition and clearance.
In recent years, the impact of the TME on chemotherapy has become the target of many studies. Chemotherapy can induce immunogenic cell death in certain tumors, which could activate the immune system. Gemcitabine can affect the TME through the inhibition of the expansion of MDSCs and the induction of T2H cells, which leads to the polarization of M2 polarized TAMs[33]. Furthermore, other chemotherapeutic drugs, such as cisplatin or carboplatin, have been identified as inducers of IL-6 and prostaglandin E2 and IL-10-producing M2 polarized TAMs[34].
A highly heterogeneous subpopulation of cells is a characteristic of pancreatic cancer. This complex structure of cancer cells and stromal and immunosuppressive cells consequently alters the effect of immunotherapy. The predominant cell types in the PDAC TME are MDSCs, Tregs and macrophages[35]. Furthermore, several other cell types have also been identified in the PDAC TME, such as fibroblasts, ECM, and PSCs; there is also a high ratio of Treg/Teffs. The accumulated population of T cells in the TME leads T cells to exhaustion during an immune response.
Moreover, approximately 50% of PDAC tumors are characterized by the invasion of MDSCs and the upregulation of PD-L1 through IFN-γ[36]. Thus, PDAC tumors establish an immunosuppressive environment[37,38]. In more advanced tumors, several studies have identified that Tregs and Teffs inhibit the normal function of T cells and enhance the immunosuppressive environment of the TME[39].
Several studies also underline the lack of recognition by T cells of cancer antigens through the degradation of downregulation of major histocompatibility complex I in cancer cells. Furthermore, a mutation in the IFN-receptor 1 or 2 gene increases immune suppression in TME and helps cancer cells escape the T cell antitumor response[40]. Moreover, two phenotypes, often called “cold” and “hot” tumors, are categorized based on the degree of immune infiltration of T-lymphocytes[41]. Hot tumors are characterized by a variation in CD8+ and Tregs in response to immunotherapeutic drugs, and cold tumors, in the early stage of tumorigenesis, show a 20%-40% response to immune checkpoint inhibitors[37,42].
IMMUNOTHERAPY
PDAC is a disease with increased heterogeneity and exhibits unique immunologic hallmarks. The principal basis of cancer immunotherapy is to activate a patient’s T cells so that they can kill their tumors. The key steps are briefly described as follows: (1) Decrease of tumor-specific antigen presentation; (2) Activation of T cells; (3) Infiltration of T cells into tumors; (4) Recognition of cancer cells by T cells; and (5) Elimination of cancer cells[43]. There are several types of cancer immunotherapies, such as monoclonal antibodies, adoptive cell transfer[44-46], cancer vaccines[47,48], immune checkpoint inhibitors[49], and immune modulators, all currently tested in clinical trials for the determination of their efficacy. Promising results have been demonstrated after the administration of inhibitors against two major T cell response checkpoints, ipilimumab (anti-CTLA-4 IgG1 humanized antibody) and Nivolumab/ Pembrolizumab (anti-PD-1), in various immunogenic cancers, such as melanoma and non-small-cell lung cancer[50-52]. CTL-4 binds to its ligands on antigen-presenting cells (APCs) and exerts its immunosuppressive role by reducing T effector cell activation while increasing Treg activity[53].
Similarly, binding of PD-1, which is predominantly expressed on T cells, with its ligands PDL-1/PDL-2, which are found on tumor cells and tumor-infiltrating lymphocytes, results in diminished T cell proliferation and antitumor cytokine release. Despite the encouraging evidence from the aforementioned cancer studies, these treatments exhibited poor efficacy to pancreatic cancer when administered alone. In a phase II study, ipilimumab was not able to induce tumor response in patients with advanced pancreatic cancer, and the anti-PD-L1 monoclonal antibody BMS-93655 had no efficacy in a phase I study[54-56]. The incompetence of these compounds to elicit pancreatic tumor growth inhibition was probably due to the immune quiescence, excessive desmoplasia and the lack of consensus expression of PD-L1 in this type of cancer[57]. Therefore, the incorporation of additional therapies for administration of combinatorial strategies appears to be the ideal approach to achieving the most efficient response. A broad spectrum of clinical trials in pancreatic cancer have been completed or are currently ongoing using immune checkpoint monotherapies, dual checkpoint combination therapies and checkpoint inhibitors combined with vaccines, cytotoxic chemotherapy and other inhibitory agents. Below, there are some examples of the therapeutic strategies followed in these clinical trials: (1) Monotherapies include the administration of various inhibitors against CTL-4 (ipilimumab, tremelimumab) and PD-1 (pembrolizumab, MPDL3280A, MEDI4736), and dual checkpoint inhibition including the combinations of these agents with each other or with other agents such as mogamulizumab (anti-CCR-5); (2) Immune checkpoint inhibitors in combination with chemotherapeutic agents consist of combinations of CTL4 and/or PD-1 inhibitors with conventional chemotherapeutic agents such as gemcitabine, Nab-paclitaxel, FOLFOX, and carboplatin[58-60]. A phase I clinical study investigating the efficacy of gemcitabine and tremelimumab in metastatic pancreatic cancer showed a partial response in some patients, and the disease remained stable for more than ten weeks[61]. In another study of unresectable pancreatic cancer, ipilimumab and gemcitabine combinatorial treatment had similar results[58]. Two clinical pilot studies based on the combination of chemotherapy and anti-PD-1 antibodies (pembrolizumab and FOLFOX for advanced GI cancer and pidilizumab and gemcitabine for resected pancreatic cancer) were initiated after increased tumor infiltration of CD8+ T cells and complete responses were observed in treated mice[59]; (3) Vaccine immunotherapy is based on the delivery of tumor antigens to APCs and the subsequent induction of an orchestrated immune response. Cancer-specific DNA alterations create neo-antigens, which results in a unique peptide sequence. Vaccine immunotherapies include whole-cell vaccines, DC vaccines, DNA and peptide vaccines, but despite the improved immune profiles, they have shown a poor clinical outcome[48]. The most widely studied vaccine in pancreatic cancer is GVAX, an allogenic irradiated whole-cell tumor vaccine genetically engineered to secrete granulocyte macrophage-colony stimulating factor (GM-CSF) and stimulate cytolytic activity against tumors[62]. In a phase I clinical study, GVAX administration in resectable pancreatic cancer before and after radiotherapy exhibited extended DFS[63], and in phase II clinical studies, GVAX in combination with cyclophosphamide or 5-FU-based chemoradiation demonstrated similar results regarding DFS and MS[64,65]. When combined with the aforementioned immune checkpoint inhibitor ipilimumab in a phase I trial in patients with advanced refractory pancreatic cancer, GVAX resulted in improved survival compared to ipilimumab alone, a fact that was associated with the extensive presence of T cells[66]. Other vaccines targeting KRAS, MUC1, VEGF-R, or survivin alone or in combination with GVAX are also under clinical investigation for the determination of their efficacy[60]; (4) Adoptive T cell immunotherapy is based on the modification of autologous T cells, engineered to express a chimeric antigen receptor (CAR) and stimulate the immune response against the tumor. Despite the impressive results gained by a clinical study utilizing CAR-T technology to target leukemia[67,68], the majority of patients receiving CAR-T cells targeting mesothelin, a membrane antigen overexpressed in pancreatic cancer, showed satisfying tolerance but failed to exhibit a good response[60]. In addition to mesothelin, other cancer-associated antigens are being tested in ongoing clinical trials as potential targets of CAR-T-based therapeutic regimes (anti-CEA, anti-CD-133, anti-ROR1, anti-WT1) alone or in combination with chemotherapy[60]; and (5) Immune modulating agents targeting the dense pancreatic microenvironment could also exert substantial antitumor activity. Promising data have been derived from the use of anti-CD40 agonistic antibodies along with gemcitabine in PDAC patients, where tumor regression was attributed to stromal alterations provoked by the effect of the anti-CD40 antibody[69,70]. Another molecule currently being tested in clinical trials against PDAC is CCR2, a chemokine receptor that mediates the chemotaxis of immune cells. In a phase 1 clinical trial, half of PDAC patients treated with PF-04136309, an inhibitor of CCR2, in combination with FOLFIRINOX, exhibited partial response and stable disease[71].
CONCLUSION
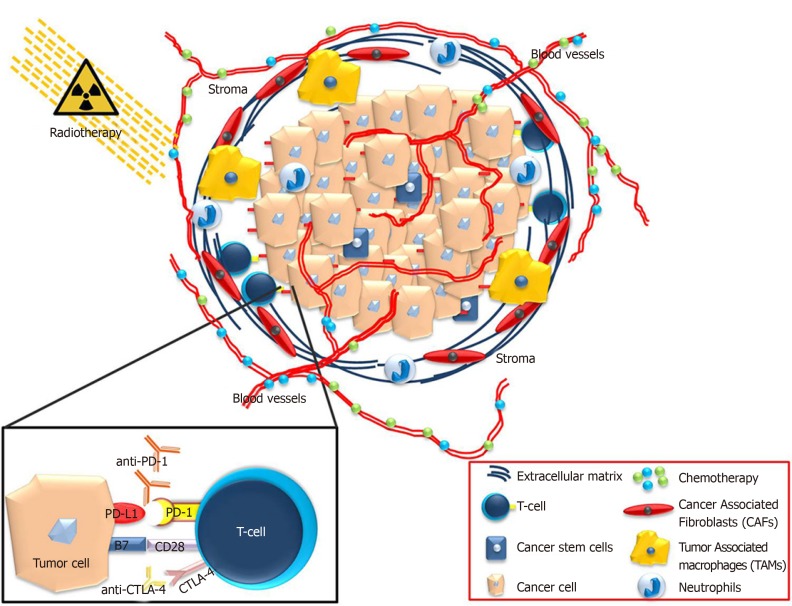
Pancreatic cancer remains a devastating disease with poor prognosis. This is due to factors such as the lack of early diagnostic markers, delayed detection, diverse genetics and rapid metastasis. The extensive TME that grows around the tumor plays crucial roles in this disease. Due to the dense and immunosuppressive TME, the penetrance of therapeutic regimes for the elimination of cancer cells is hindered. The interaction between the microenvironment and cancer cells remains to be further elucidated. However, in recent years, immunotherapy has been successfully applied in the treatment of various types of cancers. Combination therapies have been developed to optimize the clinical outcome and prolong the survival of patients with pancreatic cancer (Figure 1).
Figure 1.
Τhe pancreatic ductal adenocarcinoma microenvironment consists of a significant hurdle for the efficient application of chemotherapy drugs or immunotherapeutic compounds. Combination treatments of chemotherapy, immunotherapy and radiation might render pancreatic ductal adenocarcinoma microenvironment more vulnerable to inhibition and promote effective treatment strategies.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: April 3, 2019
First decision: November 11, 2019
Article in press: December 13, 2019
Specialty type: Oncology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin JM, Mohamed SY S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
Contributor Information
Panagiotis Sarantis, Molecular Oncology Unit, Department of Biological Chemistry, Medical School, National and Kapodistrian University of Athens, Athens 11527, Greece.
Evangelos Koustas, Molecular Oncology Unit, Department of Biological Chemistry, Medical School, National and Kapodistrian University of Athens, Athens 11527, Greece.
Adriana Papadimitropoulou, Center of Basic Research, Biomedical Research Foundation of the Academy of Athens, Athens 11527, Greece.
Athanasios G Papavassiliou, Molecular Oncology Unit, Department of Biological Chemistry, Medical School, National and Kapodistrian University of Athens, Athens 11527, Greece.
Michalis V Karamouzis, Molecular Oncology Unit, Department of Biological Chemistry, Medical School, National and Kapodistrian University of Athens, Athens 11527, Greece; First Department of Internal Medicine, “Laiko” General Hospital, Medical School, National and Kapodistrian University of Athens, Athens 11527, Greece. mkaramouz@med.uoa.gr.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Corbo V, Tortora G, Scarpa A. Molecular pathology of pancreatic cancer: from bench-to-bedside translation. Curr Drug Targets. 2012;13:744–752. doi: 10.2174/138945012800564103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Postgrad Med J. 2008;84:478–497. doi: 10.1136/gut.2006.103333. [DOI] [PubMed] [Google Scholar]
- 4.Porta M, Fabregat X, Malats N, Guarner L, Carrato A, de Miguel A, Ruiz L, Jariod M, Costafreda S, Coll S, Alguacil J, Corominas JM, Solà R, Salas A, Real FX. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005;7:189–197. doi: 10.1007/BF02712816. [DOI] [PubMed] [Google Scholar]
- 5.De Souza A, Khawaja KI, Masud F, Saif MW. Metformin and pancreatic cancer: Is there a role? Cancer Chemother Pharmacol. 2016;77:235–242. doi: 10.1007/s00280-015-2948-8. [DOI] [PubMed] [Google Scholar]
- 6.Khorana AA. Cancer and coagulation. Am J Hematol. 2012;87 Suppl 1:S82–S87. doi: 10.1002/ajh.23143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol. 2015;21:3157–3165. doi: 10.3748/wjg.v21.i11.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamska A, Domenichini A, Falasca M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo SC, Nathan H, Cameron JL, Olino K, Edil BH, Herman JM, Hirose K, Schulick RD, Choti MA, Wolfgang CL, Pawlik TM. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;118:2674–2681. doi: 10.1002/cncr.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaccaro V, Sperduti I, Milella M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;365:768–9; author reply 769. doi: 10.1056/NEJMc1107627. [DOI] [PubMed] [Google Scholar]
- 11.Santoni M, Massari F, Di Nunno V, Conti A, Cimadamore A, Scarpelli M, Montironi R, Cheng L, Battelli N, Lopez-Beltran A. Immunotherapy in renal cell carcinoma: latest evidence and clinical implications. Drugs Context. 2018;7:212528. doi: 10.7573/dic.212528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 2013;6:321–337. doi: 10.1177/1756283X13478680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty S, Singh S. Surgical resection improves survival in pancreatic cancer patients without vascular invasion- a population based study. Ann Gastroenterol. 2013;26:346–352. [PMC free article] [PubMed] [Google Scholar]
- 14.Bethune G, Bethune D, Ridgway N, Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010;2:48–51. [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng L, Lopez-Beltran A, Massari F, MacLennan GT, Montironi R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine. Mod Pathol. 2018;31:24–38. doi: 10.1038/modpathol.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant TJ, Hua K, Singh A. Molecular Pathogenesis of Pancreatic Cancer. Prog Mol Biol Transl Sci. 2016;144:241–275. doi: 10.1016/bs.pmbts.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuel N, Hudson TJ. The molecular and cellular heterogeneity of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;9:77–87. doi: 10.1038/nrgastro.2011.215. [DOI] [PubMed] [Google Scholar]
- 18.Lee CJ, Li C, Simeone DM. Human pancreatic cancer stem cells: implications for how we treat pancreatic cancer. Transl Oncol. 2008;1:14–18. doi: 10.1593/tlo.08013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida GJ, Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016;107:5–11. doi: 10.1111/cas.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Large TYS, Bijlsma MF, Kazemier G, van Laarhoven HWM, Giovannetti E, Jimenez CR. Key biological processes driving metastatic spread of pancreatic cancer as identified by multi-omics studies. Semin Cancer Biol. 2017;44:153–169. doi: 10.1016/j.semcancer.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parente P, Parcesepe P, Covelli C, Olivieri N, Remo A, Pancione M, Latiano TP, Graziano P, Maiello E, Giordano G. Crosstalk between the Tumor Microenvironment and Immune System in Pancreatic Ductal Adenocarcinoma: Potential Targets for New Therapeutic Approaches. Gastroenterol Res Pract. 2018;2018:7530619. doi: 10.1155/2018/7530619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foucher ED, Ghigo C, Chouaib S, Galon J, Iovanna J, Olive D. Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment. Front Immunol. 2018;9:1044. doi: 10.3389/fimmu.2018.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren B, Cui M, Yang G, Wang H, Feng M, You L, Zhao Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17:108. doi: 10.1186/s12943-018-0858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uzunparmak B, Sahin IH. Pancreatic cancer microenvironment: a current dilemma. Clin Transl Med. 2019;8:2. doi: 10.1186/s40169-019-0221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 27.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10:1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binenbaum Y, Na'ara S, Gil Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist Updat. 2015;23:55–68. doi: 10.1016/j.drup.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng X, Kim JY, Ghafoory S, Duvaci T, Rafiee R, Theobald J, Alborzinia H, Holenya P, Fredebohm J, Merz KH, Mehrabi A, Hafezi M, Saffari A, Eisenbrand G, Hoheisel JD, Wölfl S. Methylisoindigo preferentially kills cancer stem cells by interfering cell metabolism via inhibition of LKB1 and activation of AMPK in PDACs. Mol Oncol. 2016;10:806–824. doi: 10.1016/j.molonc.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Q, Li Y, Niu Z, Zong Y, Wang M, Yao L, Lu Z, Liao Q, Zhao Y. Atorvastatin (Lipitor) attenuates the effects of aspirin on pancreatic cancerogenesis and the chemotherapeutic efficacy of gemcitabine on pancreatic cancer by promoting M2 polarized tumor associated macrophages. J Exp Clin Cancer Res. 2016;35:33. doi: 10.1186/s13046-016-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, van der Burg SH. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013;73:2480–2492. doi: 10.1158/0008-5472.CAN-12-3542. [DOI] [PubMed] [Google Scholar]
- 35.Aliru ML, Schoenhals JE, Venkatesulu BP, Anderson CC, Barsoumian HB, Younes AI, K Mahadevan LS, Soeung M, Aziz KE, Welsh JW, Krishnan S. Radiation therapy and immunotherapy: what is the optimal timing or sequencing? Immunotherapy. 2018;10:299–316. doi: 10.2217/imt-2017-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karakhanova S, Link J, Heinrich M, Shevchenko I, Yang Y, Hassenpflug M, Bunge H, von Ahn K, Brecht R, Mathes A, Maier C, Umansky V, Werner J, Bazhin AV. Characterization of myeloid leukocytes and soluble mediators in pancreatic cancer: importance of myeloid-derived suppressor cells. Oncoimmunology. 2015;4:e998519. doi: 10.1080/2162402X.2014.998519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmood J, Shukla HD, Soman S, Samanta S, Singh P, Kamlapurkar S, Saeed A, Amin NP, Vujaskovic Z. Immunotherapy, Radiotherapy, and Hyperthermia: A Combined Therapeutic Approach in Pancreatic Cancer Treatment. Cancers (Basel) 2018;10 doi: 10.3390/cancers10120469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young K, Hughes DJ, Cunningham D, Starling N. Immunotherapy and pancreatic cancer: unique challenges and potential opportunities. Ther Adv Med Oncol. 2018;10:1758835918816281. doi: 10.1177/1758835918816281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, Mitchem JB, Plambeck-Suess SM, Worley LA, Goetz BD, Wang-Gillam A, Eberlein TJ, Denardo DG, Goedegebuure SP, Linehan DC. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amedei A, Niccolai E, Benagiano M, Della Bella C, Cianchi F, Bechi P, Taddei A, Bencini L, Farsi M, Cappello P, Prisco D, Novelli F, D'Elios MM. Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol Immunother. 2013;62:1249–1260. doi: 10.1007/s00262-013-1429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen PL, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167:397–404.e9. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilmi M, Bartholin L, Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J Gastroenterol. 2018;24:2137–2151. doi: 10.3748/wjg.v24.i20.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Wolfgang CL, Zheng L. Precision Immuno-Oncology: Prospects of Individualized Immunotherapy for Pancreatic Cancer. Cancers (Basel) 2018;10 doi: 10.3390/cancers10020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le DT, Jaffee EM. Next-generation cancer vaccine approaches: integrating lessons learned from current successes with promising biotechnologic advances. J Natl Compr Canc Netw. 2013;11:766–772. doi: 10.6004/jnccn.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell. 2002;2:25–28. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 49.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 51.Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L, Pluzanski A, Reckamp KL, Burgio MA, Kohlhäeufl M, Waterhouse D, Barlesi F, Antonia S, Arrieta O, Fayette J, Crinò L, Rizvi N, Reck M, Hellmann MD, Geese WJ, Li A, Blackwood-Chirchir A, Healey D, Brahmer J, Eberhardt WEE. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057) J Clin Oncol. 2017;35:3924–3933. doi: 10.1200/JCO.2017.74.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blank CU, Enk A. Therapeutic use of anti-CTLA-4 antibodies. Int Immunol. 2015;27:3–10. doi: 10.1093/intimm/dxu076. [DOI] [PubMed] [Google Scholar]
- 54.Foley K, Kim V, Jaffee E, Zheng L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016;381:244–251. doi: 10.1016/j.canlet.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahin IH, Askan G, Hu ZI, O'Reilly EM. Immunotherapy in pancreatic ductal adenocarcinoma: an emerging entity? Ann Oncol. 2017;28:2950–2961. doi: 10.1093/annonc/mdx503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng L. PD-L1 Expression in Pancreatic Cancer. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A, 3rd, Mulcahy M. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. Oncologist. 2019 doi: 10.1634/theoncologist.2019-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 60.Thind K, Padrnos LJ, Ramanathan RK, Borad MJ. Immunotherapy in pancreatic cancer treatment: a new frontier. Therap Adv Gastroenterol. 2017;10:168–194. doi: 10.1177/1756283X16667909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH, Jr, Bagalà C, Colombi F, Cagnazzo C, Gioeni L, Wang E, Huang B, Fly KD, Leone F. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25:1750–1755. doi: 10.1093/annonc/mdu205. [DOI] [PubMed] [Google Scholar]
- 62.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, Laheru DA, Goggins M, Hruban RH, Jaffee EM. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, Lillemoe KD, O'Reilly S, Abrams RA, Pardoll DM, Cameron JL, Yeo CJ. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 64.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, Onners B, Tartakovsky I, Choi M, Sharma R, Illei PB, Hruban RH, Abrams RA, Le D, Jaffee E, Laheru D. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Jr, Donehower RC, Jaffee EM, Laheru DA. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beatty GL. Engineered chimeric antigen receptor-expressing T cells for the treatment of pancreatic ductal adenocarcinoma. Oncoimmunology. 2014;3:e28327. doi: 10.4161/onci.28327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O'Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O'Dwyer PJ. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA, Yano M, Fowler KJ, Lockhart AC, Suresh R, Tan BR, Lim KH, Fields RC, Strasberg SM, Hawkins WG, DeNardo DG, Goedegebuure SP, Linehan DC. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–662. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]