Abstract
Psoriasis is a common non-contagious chronic inflammatory skin lesion, with frequent recurrence. It mainly occurs due to aberrant regulation of the immune system leading to abnormal proliferation of skin cells. However, the pathogenic mechanisms of psoriasis are not fully understood. Although most of the current therapies are mostly efficient, the side effects can result in therapy stop, which makes the effectiveness of treatment strategies limited. Therefore, it is urgent and necessary to develop novel therapeutics. Here, we investigated the efficacy of chrysin, a plant flavonoid, which we previously reported to possess strong antioxidant and anti-inflammatory effects, against psoriasis-like inflammation. Our results revealed that chrysin significantly attenuated imiquimod-induced psoriasis-like skin lesions in mice, and improved imiquimod-induced disruption of skin barrier. Moreover, the TNF-α, IL-17A, and IL-22-induced phosphorylation of MAPK and JAK-STAT pathways, and activation of the NF-κB pathway were also attenuated by chrysin pretreatment of epidermal keratinocytes. Most importantly, chrysin reduced TNF-α-, IL-17A-, and IL-22-induced CCL20 and antimicrobial peptide release from epidermal keratinocytes. Thus, our findings indicate that chrysin may have therapeutic potential against inflammatory skin diseases. Our study provides a basis for further investigating chrysin as a novel pharmacologic agent and contributes to the academic advancement in the field of Chinese herbal medicine.
Subject terms: Interleukins, Skin diseases
Introduction
Psoriasis is a frequently recurring non-contagious chronic skin disease, with obvious manifestations and easy clinical diagnosis1,2. The characteristics of this inflammatory disease involve aberrant keratinocyte proliferation, dermal angiogenesis, dendritic cell activation, release of pro-inflammatory cytokines, and recruitment of T lymphocytes, neutrophils, monocytes, and macrophages to skin3–6. Typical cutaneous manifestations of erythema and scaling represent vascular and epidermal violation, respectively, and some patients even exhibit psoriasis arthritis, resulting in swelling and inflammation of the joints, and nail lesions, caused by nail deformation7,8. The pathogenesis of psoriasis is not yet clear; however, increasing evidence shows that psoriasis is an immune-mediated disease9,10, which is related to immune dysfunction, and there is no universally effective therapeutic strategy. In addition, psoriasis can also be associated with cardiovascular disease and other comorbidities, such as hypertension, obesity, dyslipidaemia, metabolic syndrome, diabetes, smoking, and other cardiovascular risk factors11–13. According to the previous research and clinical experience, several cytokines are released from immune and inflammatory cells during psoriasis, such as TNF-α, IL-17A, and IL-226,14–17, which are speculated to act directly on human keratinocytes and cause a variety of pathological symptoms.
Polyphenols are natural compounds widely present in the Plantae kingdom18. Polyphenols act as protective agents in plants against a plethora of factors, such as ultraviolet radiation and bacterial and pest invasion. Recent studies have shown that polyphenols exhibit several beneficial biological activities, such as anti-angiogenic profile19,20, anti-inflammatory, antioxidant, antiviral, anti-allergic, and antitumor21–25. As a potent antioxidant reagent, polyphenols can scavenge free radicals in the body, inhibit a series of oxidation reactions to avoid DNA and cell oxidation, and further prevent cardiovascular diseases26,27. Flavonoids, also known as bioflavonoids, are the most abundant polyphenols found in the human diet. They are widely found in vegetables, cereals, fruits, rhizomes, flowers, bark, tea, and red wines28–30. So far, more than 5,000 different flavonoids have been identified31. Previous reports have demonstrated that flavonoids exhibit multiple biological activities. We have found in our previous research that many flavonoids have the effects of anti-photoaging on the skin32,33. In addition, in our previous related studies, it has been shown that the treatment of isoflavone extracts is beneficial to improve the inflammation response associated with psoriasis34. Isoflavone extracts have ameliorated imiquimod-induced psoriasis-like skin inflammation. It also inhibited the signal transduction induced by TNF-α, IL-17A, and IL-22 in human primary keratinocytes, both in the mRNA and the protein level34. Chrysin (C15H10O4) (Fig. 1) is a kind of flavonoid extracted from several plants, such as Passion-flower, honey, and propolis. It exhibits antioxidant35, antibacterial36, anti-anxiety37, hypoglycaemic38, antiviral39, anti-inflammatory40, antitumor41 and other activities. In addition to the effects of immunoregulation, our previous studies have also demonstrated that chrysin has photoprotective effects against both UVA- and UVB-induced damage and oxidative stress in HaCaT cells. Furthermore, in vivo experiments indicated that topical application of chrysin shows efficient percutaneous absorption and no skin irritation33. The advantage of chrysin is not only its antioxidant and anti-inflammatory effects, it is also cheap and can be easily extracted. Therefore, in this study, we will continue to use previous experimental models to evaluate the effects of chrysin on the skin injury and physiological parameters of mice in the imiquimod-induced psoriasis rodent model. In addition, we also use human primary keratinocytes to explore whether chrysin has the effects against the inflammatory response caused by pathogenic cytokines, including TNF-α, IL-17A, and IL-22.
Figure 1.
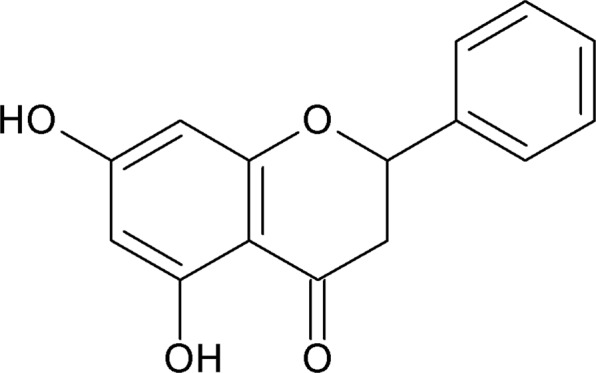
The structure of chrysin.
There are many strategies to treat psoriasis; however, the current management is usually unsatisfactory due to the possibility of poor efficacy, severe side effects, high expenses, or frequent recurrence. Therefore, it is urgent and important to develop novel drugs for the treatment of this inflammatory skin disease. In this study, we investigated whether chrysin has the potential to inhibit these cytokine-induced downstream signalling cascades in psoriasis and ameliorate physiological processes in human keratinocytes.
Results
Chrysin pretreatment improves skin inflammation and epidermal hyperplasia in imiquimod (IMQ)-induced psoriasis-like model
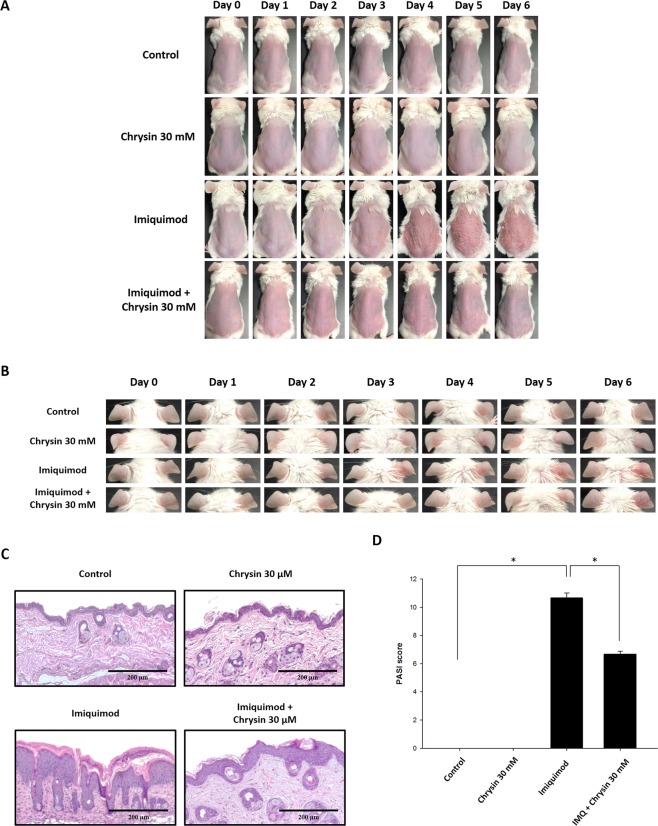
We first explored the anti-psoriatic activity of chrysin in the murine IMQ-induced psoriasis-like skin inflammation model. We compared the macroscopic and physiological characteristics of the control, IMQ-induced psoriasis-like skin, and IMQ-induced psoriasis-like skin with chrysin pretreatment. The results showed that the dorsal skin of the IMQ-treated mice exhibited redness and scaling from Day 4, and later, mouse skin conditions worsened. Similarly, we found that the mouse ears exhibited redness, thickness, and swelling, showing that IMQ induces psoriasis-like inflammation. However, in the group pretreated with chrysin, redness, scaling, swelling, and thickening of the skin and ears of mice were attenuated significantly. These results indicated an inhibitory effect of chrysin on IMQ-induced psoriasis-like skin inflammation (Figs. 2A,B).
Figure 2.
Chrysin improves imiquimod (IMQ)-induced skin inflammation. Chrysin was topically applied for 1 h on the skin and ears of mice before IMQ stimulation or vehicle cream administration for six consecutive days. (A) Phenotypic change in the appearance of skin of mice for six consecutive days. (B) Macroscopic changes in mouse ears. (C) Histological sections of mouse skin stained with haematoxylin and eosin. (D) Quantification of mouse skin histology examined using the PASI score. In each group, mice were stimulated with or without IMQ after pretreatment with chrysin. We performed in vivo experiments in at least six mice per treatment group, and data represent the mean ± SEM from at least six independent experiments. *p < 0.05 was considered to be statistically significant.
The histological effects of chrysin after IMQ-induced psoriasis-like skin inflammation were examined by haematoxylin and eosin (H&E) staining, as shown in Fig. 2C. The histology of IMQ-induced skin displayed significant inflammatory cell infiltrate and hyperkeratosis in the epidermal layer. The skin inflammation induced by IMQ treatment was improved by chrysin pretreatment. The histology of chrysin-pretreated skin exhibited significant improvement with respect to inflammatory symptoms. The total scoring of Psoriasis Area and Severity Index (PASI) showed that chrysin-pretreated groups showed significantly reduced PASI score compared with that of the IMQ group (Fig. 2D).
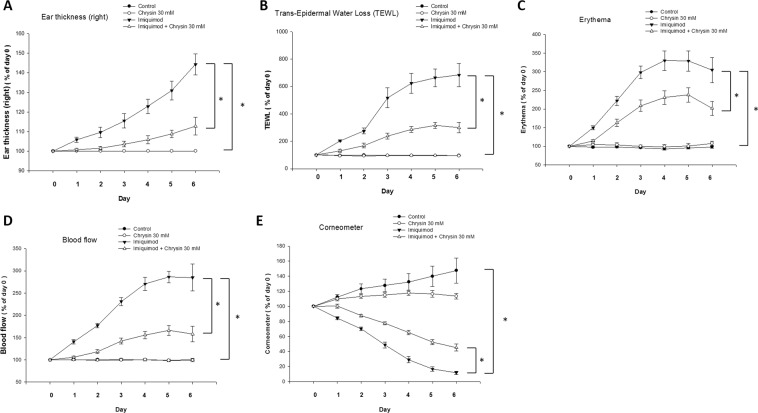
After IMQ stimulation, trans-epidermal water loss and values of physiological parameters, such as erythema, blood flow, and ear thickness, increased significantly. In addition, the surface skin hydration (corneometer) was greatly reduced. These results showed that IMQ induced an inflammatory state in the skin. The pretreatment by topical application of chrysin significantly reduced the trans-epidermal water loss, erythema, blood flow, and ear thickness, and increased the content of surface skin hydration. These findings indicated that chrysin plays an inhibitory role in IMQ-induced inflammation (Fig. 3).
Figure 3.
Chrysin improves physiology of mouse skin surface treated with IMQ. The effects of pre-treatment of chrysin on (A) ear thickness, and the physiological parameters such as (B) trans-epidermal water loss (TEWL), (C) erythema, (D) blood flow, and (E) skin hydration values of the skin surface of IMQ-treated mice were evaluated. The value of ear thickness and physiological parameters, including TEWL, erythema, and blood flow, were significantly lower compared to the IMQ only group (A–D). Skin hydration level was determined by Corneometer probe, and it increased after chrysin treatment following IMQ stimulation (E). Data represent the mean ± SEM from at least six independent experiments.
Chrysin inhibits signalling induced by TNF-α, IL-17A, or IL-22 in normal human epidermal keratinocytes (NHEKs)
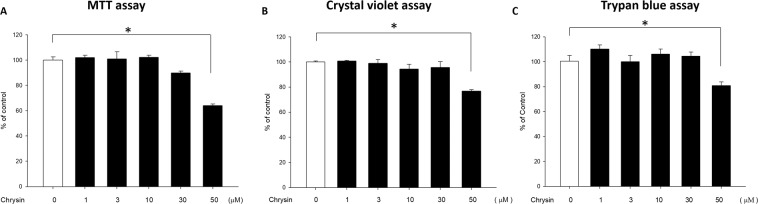
We first pretreated human primary keratinocytes with different concentrations of chrysin (0, 1, 3, 10, 30, and 50 μM), followed by MTT assay, crystal violet assay, and trypan blue assay. MTT assay revealed that pretreatment with different concentrations of chrysin did not cause cytotoxicity at concentrations between 0–30 μM; however, at 50 μM, chrysin began to cause toxicity in cells and affected cell viability (Fig. 4A). The results of crystal violet assay and trypan blue assay were consistent with that of MTT assay (Fig. 4B,C). As chrysin was not cytotoxic to NHEK at concentrations between 0–30 μM, we chose three concentrations of 3, 10, and 30 μM for further experiments.
Figure 4.
Chrysin exhibits no cytotoxicity in normal human epidermal keratinocytes (NHEKs). The (A) MTT assay, (B) crystal violet assay, and (C) trypan-blue exclusion method are the most common methods used to determine cell viability. We treated NHEKs with different concentrations of chrysin (1–50 μM), and the results showed that chrysin exhibited no cytotoxicity in the concentration range of 1–30 μM. However, at a concentration of 50 μM, the cell viability was decreased, it was shown that chrysin has cytotoxicity at this concentration. Therefore, in subsequent experiments, the concentrations of chrysin 3, 10, and 30 μM were selected. Results are expressed as a percentage of control value and are represented by mean ± SEM from at least three independent experiments. *p < 0.05 was considered to be statistically significant.
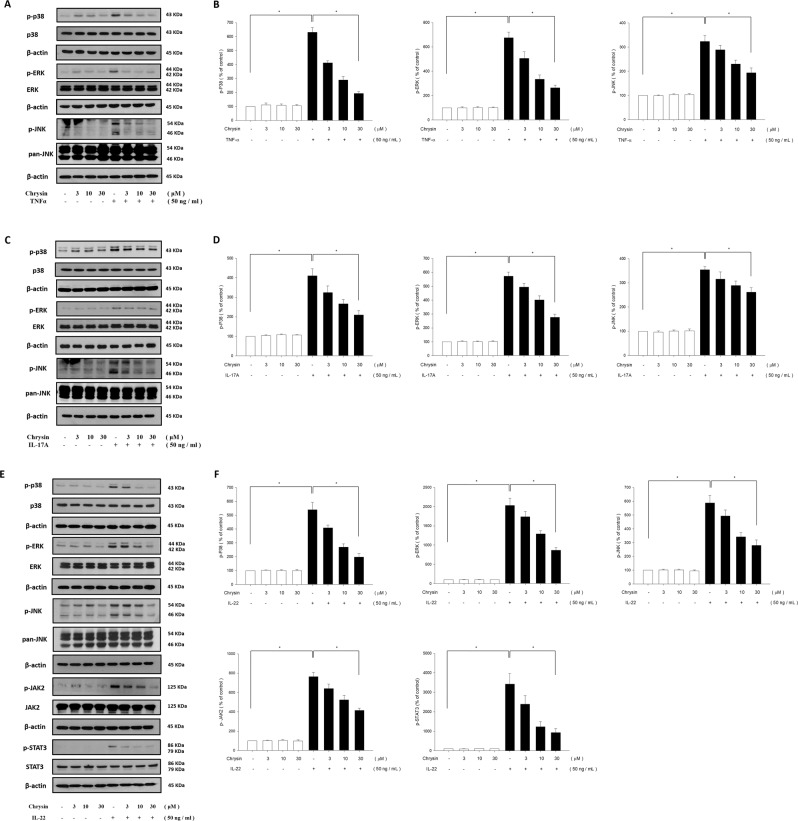
TNF-α-stimulated NHEK cells showed a significant increase in levels of the mitogen-activated protein kinase (MAPK) pathway components, including p38 kinase, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), when compared with those in the controls. Pretreatment with chrysin at various concentrations (3, 10, and 30 μM) significantly attenuated the TNF-α-stimulated increase in phosphorylation of p38 kinase, ERK, and JNK proteins (Fig. 5A,B). We also investigated the effect of chrysin on IL-17A-induced MAPK pathway activation using western blot analysis. The results showed that the phosphorylation of p38 kinase, ERK, and JNK activated only after IL-17A treatment. Pretreatment with chrysin at the working concentrations (3, 10, and 30 μM) inhibited the IL-17A-induced MAPK phosphorylation in a dose-dependent manner in NHEK (Fig. 5C,D). In addition, we examined the effects of chrysin on IL-22-induced phosphorylation of MAPK protein in NHEK using western blot analysis. The results showed that IL-22 treatment upregulated the phosphorylation of p38 kinase, ERK, and JNK, and chrysin pretreatment downregulated the activation of MAPK proteins following IL-22 stimulation (Fig. 5E,F).
Figure 5.
Chrysin inhibits phosphorylation of MAPK and JAK/STAT pathway components induced by TNF-α, IL-17A, and IL-22 in normal human epidermal keratinocytes (NHEK). NHEK were pretreated with different doses of chrysin, and stimulated with either (A) TNF-α, (C) IL-17A, or (E) IL-22, and the quantification data are shown in the right panel (B,D,F). The total protein was extracted from the cells and associated protein expression was determined via western blotting. The results are presented as a percentage of the control. *p < 0.05 was considered to be statistically significant.
Previous studies showed that the JAK-STAT pathway is a classical signal transduction pathway and is triggered by IL-22 stimulation. Therefore, we investigated the effects of chrysin on the activation of these related signalling molecules. After chrysin treatment, western blot was performed to identify the protein expression of phosphorylated JAK2 and STAT3. As shown in Fig. 5E,F, p-JAK2 and p-STAT3 levels were decreased by chrysin pretreatment compared with the IL-22 only group. These results suggested that TNF-α, IL-17A, and IL-22 indeed elicit psoriatic inflammation, and chrysin can significantly inhibit the production of these proinflammatory mediators.
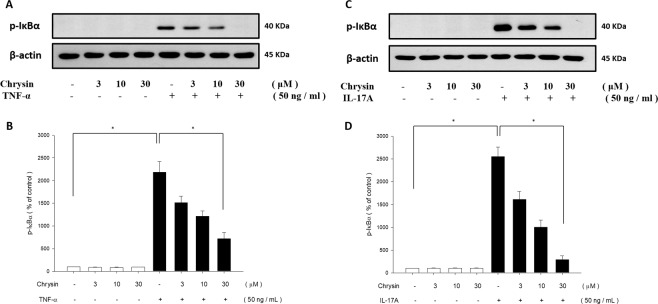
Chrysin suppressed TNF-α- or IL-17A-induced NF-κB pathway activation in NHEKs
NF-κB pathway is the key regulatory pathway during inflammation, and is considered to be a crucial mediator in the pathogenesis of psoriasis42. Therefore, we investigated the effects of chrysin on TNF-α- or IL-17A-mediated induction of α isoform of IκB (IκBα) in the NF-κB signalling pathway using western blot analysis. TNF-α- or IL-17A-stimulated NHEK cells showed a significant increase in IκBα protein levels when compared to the controls. In addition, pretreatment with chrysin downregulated the phosphorylation of IκBα protein. These results indicated that TNF-α and IL-17A treatments promote the NF-κB pathway, and this effect was reduced by chrysin pretreatment (Fig. 6A–D).
Figure 6.
Chrysin inhibits TNF-α and IL-17A-induced phosphorylation of IKK/NF-κB pathway in NHEK. The phosphorylation of IκBα protein in both TNF-α (A) and IL-17A (C) only groups was significantly upregulated under pre-treatment with chrysin, and the expression of IκBα protein was inhibited by chrysin pre-treatment. The quantification data are shown in the below panel (B,D). The total protein was extracted from the cells and IκBα expression was determined via western blotting. The results are presented as a percentage of the control. *p < 0.05 was considered to be statistically significant.
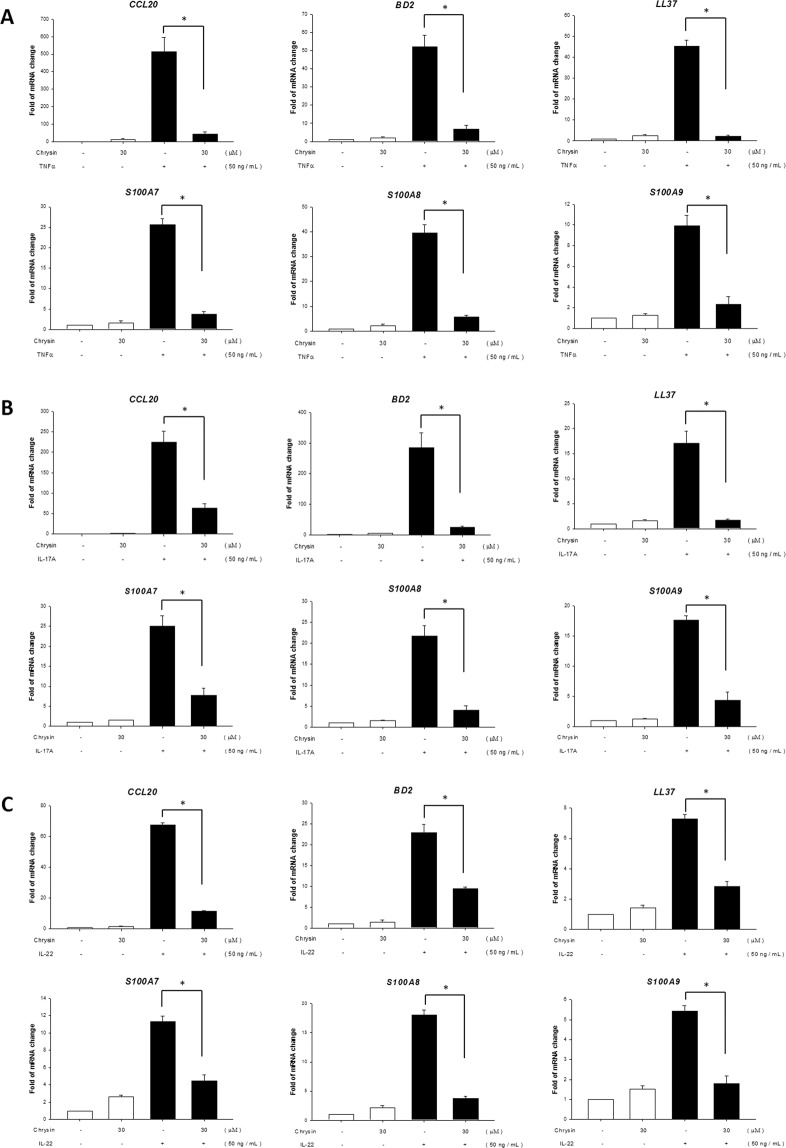
Chrysin downregulated the mRNA expression of CCL20 and AMPs in NHEKs induced by TNF-α, IL-17A, or IL-22 recombination proteins
In addition to providing a basic physical barrier, the skin is also an outpost of the immune response. These immune reactions are crucial responses to injury or infection, and can also regulate the skin symbiotic microbiota43. While the skin is infected with harmful pathogens (mostly microorganisms), keratinocytes are activated to secrete antimicrobial peptides and proteins (AMPs), cytokines, and chemokines to trigger an immune response. Chemokines, like CCL20, and AMPs, including cathelicidin, β-defensins, and S100 proteins, are speculated to intensify psoriasis lesions44, and excessive production of AMPs is an important characteristic of psoriasis lesions43–45. Previous studies have reported that TNFα, IL-17A, and IL-22 upregulate the expression of CCL20 and AMPs46–48. Therefore, in this study, we investigated whether chrysin can downregulate the expression of CCL20 and AMPs via TNFα, IL-17A, and IL-22 stimulation. Our results showed that the expression of CCL20, S100A7, S100A8, S100A9, hBD2, and LL-37 were significantly increased by TNFα, IL-17A, and IL-22 stimulation. In the group pretreated with chrysin, the expression of CCL20 and AMPs were significantly suppressed (Fig. 7A–C).
Figure 7.
Chrysin downregulates the expression of TNF-α, IL-17A, and IL-22-induced genes at the mRNA level. After pre-treatment with chrysin, NHEK were stimulated with TNF-α (A), IL-17A (B), and IL-22 (C), and RT-qPCR was used to detect the effects of chrysin on the mRNA expression of CCL20, S100A7, S100A8, S100A9, hBD2, and LL-37. Data are expressed as fold induction of relevant mRNA sequences compared to untreated controls. Data represent mean ± SEM from at least three independent experiments performed in triplicates; *p < 0.05 was considered to be statistically significant.
Discussion
This study showed that chrysin can ameliorate the inflammation in psoriasis, and the underlying mechanism involves the regulation of three signalling pathways, namely MAPK, JAK-STAT, and IKK/NF-κB pathways. This is consistent with the results of our previous study, wherein chrysin was found to protect skin cells against the photodamage caused by UVA and UVB via the regulation of MAPK pathway and inhibition of ROS production33. In this study, we explored several factors that contributed to psoriasis, such as TNF-α, IL-17A, and IL-22 and found that chrysin could ameliorate the inflammatory reactions induced by these factors.
The most important pathological characteristics of psoriasis include hyperinflammation, epidermal keratinocyte proliferation and abnormal differentiation, and angiogenesis. The pathogenesis of psoriasis is a highly complex molecular mechanism, involving a variety of immune and inflammatory mediators. Several innate immune cells, adaptive immune cells, and epidermal keratinocytes are stimulated by various cytokines, which is the main cause for the sustained development of psoriatic lesions. However, based on previous research and clinical experiences, the immune and inflammatory cells release a variety of cytokines, especially TNF-α, IL-17A, and IL-22, which are believed to affect epidermal keratinocytes directly, and are the most important cytokines responsible for a variety of pathological processes.
Conventional treatments of psoriasis include topical and systemic medication49,50. Systemic steroids are widely used as anti-inflammatory drugs in the treatment of several skin diseases; however, they exhibit potential side effects, such as skin atrophy, striae, and telangiectasias51, or systemic response, including inhibition of the hypothalamic-pituitary-adrenal axis and other endocrinological complications, such as diabetes and osteoporosis52. In addition, the side effects of steroids in the cardiovascular, gastrointestinal, and neurological systems are well known. Besides these common side effects, some reports have indicated that psoriasis leads to a deterioration of the rebound phenomenon after systemic steroid withdrawal53. Moreover, morphological changes from non-pustular to pustular psoriasis can become difficult to control after discontinuation of steroid therapy, making it difficult to perform a risk-benefit analysis54,55. The use of steroids may also cause erythroderma in patients with psoriasis56. Although steroids are very effective as first-line treatments in many cases, the efficacy of the drug weakens over time in most cases, and higher doses are then required to maintain the treatment response. Therefore, steroids are not conducive to long-term use57. In contrast biological agents, which are proteins produced by organisms, have advantages, such as lower side effects, less hepatotoxicity and nephrotoxicity, and selective towards immune system. However, their disadvantages are that they are expensive and less popular. At present, the main biological agents are antibodies and agents against TNF, IL-12/23, IL-17, JAK, and IκB kinase58. TNF therapy has long been used to treat various inflammatory diseases, including psoriasis59, and mainly neutralizes TNF secretion by inflammatory cells. IL-12/23 Inhibitors interfere with IL-12 and IL-23-mediated cell signal transduction60, while IL-17 inhibitor neutralizes the effects of IL-17A61 and the downstream signalling pathways. In psoriasis, JAK inhibitors show good therapeutic effects. In a mouse model of contact dermatitis, topical application of JAK inhibitors could effectively inhibit the infiltration of lymphocyte, the phosphorylation of STAT3, and the proliferation of keratinocyte62. Recently, many studies have speculated that NF-κB pathway may be a new target for the treatment of psoriasis, and IκB kinase inhibitor can improve the severity of psoriasis63. However, compared with steroids and biological agents, Chinese herbal medicine ingredients are more advantageous, as they can be isolated from natural plants, can be conveniently obtained, have no usage restrictions, and show only few or no side effects. In modern medicine, there are more and more Chinese medicine treatments for psoriasis are extensively investigated64. Indigo naturalis has been used as a traditional Chinese medicine for the treatment of skin diseases. In recent years, many reports indicated that topical application of indigo naturalis and indirubin were effective and safe for the treatment of psoriasis, especially plaque psoriasis and nail psoriasis, and it also has the inhibitory effect on the inflammatory response of human neutrophils. It has also been found that indigo naturalis up-regulated the expression of claudin-1 and the function of tight junction in human epidermal cells65–67. However, although Chinese herbal medicine can be an alternative, safety, adverse effects and efficacy of long-term medication are still important issues that need to be addressed in the future. It is important to identify novel herbal ingredients that can be efficiently and safety used in abovementioned therapeutic approaches/strategies against psoriasis and other skin inflammatory diseases.
In this study, the topical application of chrysin suppressed the IMQ-induced psoriasis-like skin inflammation in the murine model, ameliorated the skin-related physiology, including trans-epidermal water loss (TEWL), erythema, blood flow speed, and ear thickness, and increased surface skin hydration (corneometer). Furthermore, pretreatment with chrysin reduced the IMQ-induced inflammation and infiltration of inflammatory cells, as observed in the histopathological analysis. We also found that chrysin effectively suppressed the downstream responses of these cytokines in epidermal keratinocytes, including phosphorylation of the MAPK pathway, activation of IκBα in the NF-κB pathway, and triggering of the JAK-STAT pathway at protein levels. Furthermore, chrysin treatment downregulated the expression of CCL20 and antimicrobial peptides (AMPs), consisting of S100A7, S100A8, S100A9, hBD2, and LL-37, at mRNA levels.
Many skin diseases, such as atopic dermatitis, contact dermatitis, psoriasis, and even ultraviolet radiation are related to skin inflammation. Inflammation of the skin is often accompanied by an increase in vascular permeability, together with the release of NO and prostaglandins, which trigger accumulation of tissue fluid in the lesion and occurrence of oedema. At the same time, leukocytes (especially neutrophils) also migrate to the skin lesions. The production of matrix metalloproteinase, in particular MMP-9, degrades the main structural component of connective tissue, collagen, in the epidermis and dermis68, which contributes to such migration of leukocytes69. Other cytokines released from epidermal cells or antigen-specific cells play a crucial role in inflammation of the skin, such as TNF-α, IL-1, and IL-6. Therefore, the above mentioned mediators can be used as indicators for exploring skin inflammation4. TNF-α plays a key role in inflammatory and barrier-impaired skin diseases, and also affects the regulation of many downstream molecules, such as cytokines, chemokines, enzymes, and proteins. Therefore, by elucidating the effects of Chinese herbal medicines on the cellular signalling pathways induced by TNF-α, we could reduce the effects of these inflammatory molecules, and identify the potential and feasibility of Chinese herbal medicines for the treatment of inflammatory skin diseases.
Cytokines involved in both Th1 and Th17 pathways are found in the skin of psoriasis patients, including IL-17A, IL-17F, IL-19, IL-20, IL-22, IL-23, IL-24, IL-26, and TNF-α, which can be detected in serum and lesions. The synergistic effect of IL-17 and IL-22 promotes the expression of antimicrobial peptides in keratinocytes, such as β-defensin-2 (BD-2), S100A7 (psoriasin), cathelicidin (LL37), and S100A8/9 (calprotectin), all of which may lead to the development of psoriasis in individuals with a higher resistance to skin infections70,71. In this study, the results showed that three cytokines, TNF-α, IL-17A, and IL-22, induced mRNA expression of CCL20 and antimicrobial peptides, and chrysin pretreatment downregulated their mRNA levels significantly. In recent years, an increasing number of studies have reported that interleukin-36 (IL-36), which belongs to the IL-1 superfamily, affects the balance between pro-inflammatory and anti-inflammatory branches, and easily leads to tissue inflammation. IL-36 cytokines are mainly expressed at the barrier sites in the body, such as the skin epithelium, and the spectrum of psoriasis is one of the famous examples72. Therefore, IL-36 is likely to be an important diagnostic tool for dermatitis, and additional keratinocyte-linked cytokines like the inflammasome-related IL-1β, IL-36α, and IL-36γ should be investigated in the future. Previous studies have indicated that IL-17 exhibits the ability to activate transcription factors, such as NF-κB, in many cell types, including fibroblasts, macrophages, chondrocytes, intestinal epithelial cells, and myofibroblasts of the colon and pancreas73. When cells are stimulated by IL-17, the IL-17 receptor (IL-17R) is triggered to activate ERK1 and ERK2 and the stress-induced JNK-1 and JNK-2, as well as the p38 MAPK pathway; these signal transduction pathways contribute to the upregulation of IL-6, IL-1, and NF-κB74. Our results showed that the addition of TNF-α, IL-17A, and IL-22 induced the phosphorylation of p38, ERK, and JNK, and TNF-α and IL-17A activated the expression of IκBα in the NF-κB pathway. Previous studies have reported that IL-22 induces the phosphorylation of IKK and IκB in HaCaT cells75. However, we have not explored the effects of IL-22 on the NF-κB pathway in this study. If we can further explore the effects of this pathway, we can better understand the mechanism of chrysin on the pathogenesis of psoriasis. The phosphorylation of these related proteins was suppressed by the pretreatment of chrysin in a dose-dependent manner. These results demonstrated that chrysin effectively improved psoriasis-associated inflammation and exhibited anti-psoriatic potential.
With the advent of cellular molecular biotechnology, it has become increasingly clear that many molecules related to the skin barrier, as well as molecules that maintain skin hydration, such as filaggrin and junction protein, play a decisive role in maintaining normal physiological functions of the skin. Therefore, exploring and reducing the external factors that affect the above skin properties is an important research area in pathology and future drug development. Cornification (or keratinization) is the process of terminal differentiation of epidermal keratinocytes and plays an important role in the formation of the skin barrier76,77. In psoriatic lesions, abnormal differentiation of keratinocytes is observed. In particular, the expression of some crucial differentiation markers, such as keratin 10, loricrin, and filaggrin, is suppressed, leading to hypogranulosis in the psoriatic lesion epithelium. The three cytokines, TNF-α, IL-17A, and IL-22, affect the differentiation of keratinocytes and result in downregulation of the abovementioned differentiated proteins. In addition, proliferation and thickening of the skin in psoriatic lesions can also be observed, which is related to the hyperplasia of keratinocytes. Previous evidence showed that among the several cytokines that are pathogenic to psoriasis, IL-22 most likely promotes the proliferation of keratinocytes78,79. In the inflammatory pathway, we found that chrysin can effectively inhibit the expression of inflammation-related proteins and mRNAs induced by TNF-α, IL-17A, and IL-22. In future, we hope to address whether chrysin can reverse the inhibition of TNF-α, IL-17A, or IL-22-induced differentiation in keratinocytes in psoriasis.
In conclusion, the current study demonstrates the potential of chrysin in the treatment of inflammatory skin diseases, such as psoriasis, via its ability to relieve their symptoms. The findings also suggest that it may be useful as a daily health care supplement for prevention of inflammation.
Materials and Methods
Ethics statement
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of Fu Jen Catholic University (approval #A10367). The principles of the 3Rs (Replacement, Reduction, and Refinement) were followed to optimize the experimental design. The human primary epidermal keratinocytes were cells from human foreskins. The foreskins were provided by the Mackay Memorial Hospital, after obtaining the consent for use (#13MMHIS022) from the Institutional Review Board. There was no interaction between the researcher and the foreskin donors, and the foreskins do not have any relevant information to identify the donors. All experiments were performed in accordance with relevant guidelines and regulations.
Materials
The chrysin used in this study was purchased from Sigma-Aldrich (St Louis, MO; CAS: 480-40-0) with a purity of 97% and was dissolved in dimethyl sulfoxide (DMSO). Antibodies (Ab) against p-ERK and ERK were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Abs against p-IκBα, p-STAT3, P38, STAT3, p-JAK2, JAK2, and β-actin were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Abs against p-P38, p-JNK and JNK were purchased from R&D Systems (Minneapolis, MN). TNF-α, IL-17A, and IL-22 were purchased from PeproTech (Rocky Hill, NJ).
Animals
Male mice BALB/c (8–11 weeks) were used for all experiments. Mice were purchased from the National Laboratory Animal Center, Taipei, Taiwan. Animals were individually housed in polypropylene cages with controlled temperature (21–25 °C), light (12/12 h light/dark cycle), and humidity (60 ± 5%). All animals had ad libitum access to standard food and water.
IMQ-induced psoriasis-like skin inflammation in mice
Chrysin (30 mM) or vehicle was applied to the dorsal shaved back and the right ears of mice. After 1 h, mice received a 62.5 mg topical dose of commercially available imiquimod cream (Aldara 5%; Meda AB, Solna, Sweden) or vehicle cream (Vaselina Pura, Laboratorios Rida, Valencia, Spain) on the same positions for six consecutive days. Skin physiology-related values, including trans-epidermal water loss (TEWL), erythema, skin hydration by MPA-580 (Courage & Khazaka, Cologne, Germany), and blood flow were measured with FLO-N1 (Omegawave, Tokyo, Japan) daily before chrysin treatment. The Mexameter® MX 18 is available as a probe that connects to the MPA systems, which is a tool to measure the two components, mainly responsible for the colour of the skin: melanin and haemoglobin (erythema) by reflectance. For use, the probe was pressed on the measurement site for ~1 s to measure the melanin and erythema indices. FLO-N1, a non-contact type of instrument, was used to measure tissue blood flow, blood volume, and flow velocity. In addition, the thickness of both ears in mice were measured and photographed for indicating the changes in the appearance of the skin and ears. At the end of the experiment, mice were sacrificed and tissues were collected and stored at −80 °C for subsequent homogenization or fixation in formalin.
Histopathological analysis
Mouse tissues were fixed with 4% paraformaldehyde at 4 °C overnight. Routine methods were used to prepare formalin-fixed paraffin-embedded blocks of the mice’s skin tissues. These tissues were then cut into 5-μm sections, and stained with haematoxylin and eosin (H&E). ZEISS Axioskop 40 Inverted System microscope (NY, United States) and SPOT Cam software (Sterling Heights, MI) were used to visualize the Images from H&E staining. Psoriasis Area and Severity Index (PASI) is an objective scoring system. The erythema, scaling, and thickness were scored independently from 0 to 4 (0 no infection,1 mild, 2 intermediate, 3 severe, 4 very severe), and the total score was used as an index of psoriasis severity (scores 0–12).
Cell culture
The primary keratinocytes were isolated from human foreskin tissue, and were grown in Keratinocyte-SFM (Gibco BRL/Invitrogen, Carlsbad, CA). The primary keratinocytes were used between passages 2 to 4 in this study. Normal human keratinocytes were plated in 35-mm culture dishes, and 24 h prior the stimulus, keratinocyte medium was switched and chrysin (3, 10, or 30 μM) was added. The control medium contained an equal volume of DMSO. Finally, cells were stimulated with either TNF-α (50 ng ml−1), IL-17A (50 ng ml−1), or IL-22 (50 ng ml−1) from PeproTech (Rocky Hill, NJ).
Cell viability assays (MTT, trypan blue assay, and crystal violet assay)
Cell viability was determined as previously described32,34 by MTT, trypan blue, and crystal violet assays. In MTT assay, cells were pretreated with DMSO or chrysin for 24 h. After a brief wash, MTT (0.5 mg/mL in Keratinocyte-SFM) was used for the quantification of metabolically-active live cells and were analysed photometrically at 550 nm. In crystal violet assay, cells were treated as described, fixed with methanol, and then stained with 0.1% crystal violet solution for 1 h staining. Then, the cells were washed thrice with double-distilled water, followed by acetic acid to dissolve the cells. The optical density (OD) was then read using a Tecan Sunrise spectrophotometer (Tecan, Crailsheim, Germany) at 550 nm. The Trypan Blue exclusion method was performed according to the manufacturer’s protocols. The normal human keratinocytes were pretreated with chrysin, and then suspended and stained with equal volume of trypan blue dye. The cells were counted using a dual-chamber haemocytometer and a light microscope.
Western blot analysis
After 5 and 15 min of stimulation using TNF-α, IL-17A, or IL-22, the total proteins were extracted by applying RIPA lysis buffer. Protein concentration was measured with the Pierce protein assay kit (Pierce, Rockford, IL). The proteins were separated by electrophoresis on 10% SDS–polyacrylamide gels, and then transferred onto a PVDF membrane (Millipore, Darmstadt, Hesse, Germany). After blocking with 5% non-fat dry milk for 1 h at room temperature, the membranes were probed using the indicated specific antibodies and visualized with ECL solution.
Real-time quantitative RT-PCR
After 6 and 8 h of stimulation with TNF-α, IL-17A, or IL-22, total RNA was isolated using the total RNA isolation kit (GeneDireX®, Vegas, NV) according to manufacturer’s instructions and reverse-transcribed into cDNA using SuperScript™ III First-Strand Synthesis System kit (Invitrogen, Carlsbad, CA). The qPCR was performed using the CFX96™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA), following the previously described conditions34 with SYBR green (Kapa Biosystems, Wilmington, MA). Primer sequences used in the PCR reactions are listed in Table 1. Data were normalized relative to β-actin expression and evaluated using the equation: fold change = 2−ΔΔCT.
Table 1.
Primer sequences for RT-qPCR.
| Gene | Primer sequence (5′-3′) |
|---|---|
| Human CCL2080 | F: TACTCCACCTCTGCGGCGAATCAGAA |
| R: GTGAAACCTCCAACCCCAGCAAGGTT | |
| Human S100A781 | F: GCATGATCGACATGTTTCACAAATACAC |
| R: TGGTAGTCTGTGGCTATGTCTCCC | |
| Human S100A882 | F: TGAAGAAATTGCTAGAGAC |
| R: CTTTATCACCAGAATGAGGA | |
| Human S100A978 | F: GCTCCTCGGCTTTGACAGAGTGCAAG |
| R: GCATTTGTGTCCAGGTCCTCCATGATGTGT | |
| Human BD283 | F: CCAGCCATCAGCCATGAGGGT |
| R: GGAGCCCTTTCTGAATCCGCA | |
| Human LL-3784 | F: GCAGTCACCAGAGGATTGTGAC |
| R: CACCGCTTCACCAGCCC | |
| Human β-Actxin85,86 | F: CGGGGACCTGACTGACTACC |
| R: AGGAAGGCTGGAAGAGTGC |
Statistical analysis
Data were expressed as the mean ± SEM of in vivo experiments at least six mice per treat group, and three cell culture replicates in the in vitro experiments by using GraphPad Prism Program 6 software (GraphPad Software San Diego, CA). Comparison of the mean difference with and without chrysin treatment was made using one-way ANOVA followed by Dunnett’s t-test for multiple comparisons. We considered p < 0.05 to be statistically significant.
Acknowledgements
This work is supported by the research grants from Fu-Jen Catholic University, Ministry of Science and Technology (MOST104-2320-B-030-008-MY3 and MOST 105-2314-B-195-006-MY3), MacKay Memorial Hospital (MMH-107-98), and the Cathay General Hospital (104-CGH-FJU-03) in Taiwan. The fifth author is partially supported by an United States National Science Foundation grant DMS-1408839 and a McDevitt Endowment Fund at Georgetown University.
Author contributions
Funding acquisition, Chi-Ming Pu; Investigation, Chi-Feng Hung and Hsin-Ju Li; Methodology, Nan-Lin Wu and Chien-Yu Hsiao; Resources, Chi-Feng Hung, Nan-Lin Wu, Chi-Ming Pu, and Chien-Yu Hsiao; Writing – original draft, Chi-Feng Hung and Hsin-Ju Li; Writing – review & editing, Chi-Feng Hung, Hsin-Ju Li, Nan-Lin Wu, and Der- Chen Chang.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can. Fam. Physician. 2017;63:278–285. [PMC free article] [PubMed] [Google Scholar]
- 2.Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun. Rev. 2014;13:490–495. doi: 10.1016/j.autrev.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Peters BP, Weissman FG, Gill MA. Pathophysiology and treatment of psoriasis. Am. J. Health Syst. Pharm. 2000;57:645–659. doi: 10.1093/ajhp/57.7.645. [DOI] [PubMed] [Google Scholar]
- 4.Nestle FO, Kaplan DH, Barker J. Psoriasis. N. Engl. J. Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 5.Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin. Rev. Allergy Immunol. 2013;44:183–193. doi: 10.1007/s12016-012-8307-1. [DOI] [PubMed] [Google Scholar]
- 6.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Invest. Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 7.Sobolewski P, Walecka I, Dopytalska K. Nail involvement in psoriatic arthritis. Reumatologia. 2017;55:131–135. doi: 10.5114/reum.2017.68912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raposo I, Torres T. Nail psoriasis as a predictor of the development of psoriatic arthritis. Actas Dermosifiliogr. 2015;106:452–457. doi: 10.1016/j.ad.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun. Rev. 2003;2:119–125. doi: 10.1016/S1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 10.David T, Ling SF, Barton A. Genetics of immune-mediated inflammatory diseases. Clin. Exp. Immunol. 2018;193:3–12. doi: 10.1111/cei.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prey S, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J. Eur. Acad. Dermatol. Venereol. 2010;24(Suppl 2):23–30. doi: 10.1111/j.1468-3083.2009.03564.x. [DOI] [PubMed] [Google Scholar]
- 12.Gkalpakiotis S. Psoriasis comorbidities. Cas. Lek. Cesk. 2017;156:119–122. [PubMed] [Google Scholar]
- 13.Puig, L. Cardiometabolic Comorbidities in Psoriasis and Psoriatic Arthritis. Int J Mol Sci19 (2017). [DOI] [PMC free article] [PubMed]
- 14.Nickoloff BJ. Cracking the cytokine code in psoriasis. Nat. Med. 2007;13:242–244. doi: 10.1038/nm0307-242. [DOI] [PubMed] [Google Scholar]
- 15.Zaba LC, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 2007;204:3183–3194. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb AB, et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J. Immunol. 2005;175:2721–2729. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
- 17.Cheuk S, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol. 2014;192:3111–3120. doi: 10.4049/jimmunol.1302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Q, Heilmann J, Konig B. Natural phenolic metabolites with anti-angiogenic properties - a review from the chemical point of view. Beilstein J. Org. Chem. 2015;11:249–264. doi: 10.3762/bjoc.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 1. Curr. Oncol. 2006;13:14–26. [PMC free article] [PubMed] [Google Scholar]
- 20.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 2. Curr. Oncol. 2006;13:99–107. [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad S, Phromnoi K, Yadav VR, Chaturvedi MM, Aggarwal BB. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010;76:1044–1063. doi: 10.1055/s-0030-1250111. [DOI] [PubMed] [Google Scholar]
- 22.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Pietta PG. Flavonoids as antioxidants. J. Nat. Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 24.Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant. Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Prochazkova D, Bousova I, Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Raj P, et al. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014;95:63–71. doi: 10.1016/j.lfs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Niu L, et al. Polyphenols in Regulation of Redox Signaling and Inflammation During Cardiovascular Diseases. Cell Biochem. Biophys. 2015;72:485–494. doi: 10.1007/s12013-014-0492-5. [DOI] [PubMed] [Google Scholar]
- 28.Ayoub M, de Camargo AC, Shahidi F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016;197:221–232. doi: 10.1016/j.foodchem.2015.10.107. [DOI] [PubMed] [Google Scholar]
- 29.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amawi H, Ashby CR, Jr., Tiwari AK. Cancer chemoprevention through dietary flavonoids: what’s limiting? Chin. J. Cancer. 2017;36:50. doi: 10.1186/s40880-017-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arct J, Pytkowska K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008;26:347–357. doi: 10.1016/j.clindermatol.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Huang CH, et al. Photoprotective Effects of Cycloheterophyllin against UVA-Induced Damage and Oxidative Stress in Human Dermal Fibroblasts. PLoS One. 2016;11:e0161767. doi: 10.1371/journal.pone.0161767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu NL, et al. Chrysin protects epidermal keratinocytes from UVA- and UVB-induced damage. J. Agric. Food Chem. 2011;59:8391–8400. doi: 10.1021/jf200931t. [DOI] [PubMed] [Google Scholar]
- 34.Li HJ, Wu NL, Lee GA, Hung CF. The Therapeutic Potential and Molecular Mechanism of Isoflavone Extract against Psoriasis. Sci. Rep. 2018;8:6335. doi: 10.1038/s41598-018-24726-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rashid S, et al. Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol. Mech. Methods. 2013;23:337–345. doi: 10.3109/15376516.2012.759306. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, Park JH, Cho MH, Lee J. Flavone reduces the production of virulence factors, staphyloxanthin and alpha-hemolysin, in Staphylococcus aureus. Curr. Microbiol. 2012;65:726–732. doi: 10.1007/s00284-012-0229-x. [DOI] [PubMed] [Google Scholar]
- 37.Kasala ER, et al. Chemopreventive and therapeutic potential of chrysin in cancer: mechanistic perspectives. Toxicol. Lett. 2015;233:214–225. doi: 10.1016/j.toxlet.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y, Gong FL, Zhao GB, Li J. Chrysin suppressed inflammatory responses and the inducible nitric oxide synthase pathway after spinal cord injury in rats. Int. J. Mol. Sci. 2014;15:12270–12279. doi: 10.3390/ijms150712270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fale PL, et al. Acetylcholinesterase inhibition, antioxidant activity and toxicity of Peumus boldus water extracts on HeLa and Caco-2 cell lines. Food Chem. Toxicol. 2012;50:2656–2662. doi: 10.1016/j.fct.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 40.Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012;33:83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Pick A, et al. Structure-activity relationships of flavonoids as inhibitors of breast cancer resistance protein (BCRP) Bioorg Med. Chem. 2011;19:2090–2102. doi: 10.1016/j.bmc.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 42.Goldminz AM, Au SC, Kim N, Gottlieb AB, Lizzul PF. NF-kappaB: an essential transcription factor in psoriasis. J. Dermatol. Sci. 2013;69:89–94. doi: 10.1016/j.jdermsci.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Bangert C, Brunner PM, Stingl G. Immune functions of the skin. Clin. Dermatol. 2011;29:360–376. doi: 10.1016/j.clindermatol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J. Dermatol. 2012;39:225–230. doi: 10.1111/j.1346-8138.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckert RL, et al. S100 proteins in the epidermis. J. Invest. Dermatol. 2004;123:23–33. doi: 10.1111/j.0022-202X.2004.22719.x. [DOI] [PubMed] [Google Scholar]
- 48.Nomura I, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J. Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 49.Lebwohl M, Ting PT, Koo JY. Psoriasis treatment: traditional therapy. Ann. Rheum. Dis. 2005;64(Suppl 2):ii83–86. doi: 10.1136/ard.2004.030791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rebora A. Conventional therapies for psoriasis. Reumatismo. 2007;59(Suppl 1):77–80. doi: 10.4081/reumatismo.2007.1s.77. [DOI] [PubMed] [Google Scholar]
- 51.Abraham A, Roga G. Topical steroid-damaged skin. Indian. J. Dermatol. 2014;59:456–459. doi: 10.4103/0019-5154.139872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mrowietz U, Domm S. Systemic steroids in the treatment of psoriasis: what is fact, what is fiction? J. Eur. Acad. Dermatol. Venereol. 2013;27:1022–1025. doi: 10.1111/j.1468-3083.2012.04656.x. [DOI] [PubMed] [Google Scholar]
- 53.Brodell RT, Williams L. A corticosteroid-induced flare of psoriasis. How to control or, better yet, avoid. Postgrad. Med. 1999;106:31–32. doi: 10.3810/pgm.1999.12.813. [DOI] [PubMed] [Google Scholar]
- 54.Brenner M, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br. J. Dermatol. 2009;161:964–966. doi: 10.1111/j.1365-2133.2009.09348.x. [DOI] [PubMed] [Google Scholar]
- 55.Chowaniec O, Blichowska T. [Case of generalized pustular psoriasis caused by corticosteroid treatment of simple psoriasis and pemphigus] Przegl Dermatol. 1979;66:541–544. [PubMed] [Google Scholar]
- 56.Boyd AS, Menter A. Erythrodermic psoriasis. Precipitating factors, course, and prognosis in 50 patients. J. Am. Acad. Dermatol. 1989;21:985–991. doi: 10.1016/S0190-9622(89)70287-5. [DOI] [PubMed] [Google Scholar]
- 57.Adcock IM, Barnes PJ. Molecular mechanisms of corticosteroid resistance. Chest. 2008;134:394–401. doi: 10.1378/chest.08-0440. [DOI] [PubMed] [Google Scholar]
- 58.Woo, Y. R., Cho, D. H. & Park, H. J. Molecular Mechanisms and Management of a Cutaneous Inflammatory Disorder: Psoriasis. Int J Mol Sci18 (2017). [DOI] [PMC free article] [PubMed]
- 59.Taylor PC. Anti-tumor necrosis factor therapies. Curr. Opin. Rheumatol. 2001;13:164–169. doi: 10.1097/00002281-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Papp KA, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br. J. Dermatol. 2013;168:844–854. doi: 10.1111/bjd.12214. [DOI] [PubMed] [Google Scholar]
- 61.Gordon KB, et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 2016;375:345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 62.Fridman JS, et al. Preclinical evaluation of local JAK1 and JAK2 inhibition in cutaneous inflammation. J. Invest. Dermatol. 2011;131:1838–1844. doi: 10.1038/jid.2011.140. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, et al. Targeting NF-kappa B with a natural triterpenoid alleviates skin inflammation in a mouse model of psoriasis. J. Immunol. 2009;183:4755–4763. doi: 10.4049/jimmunol.0900521. [DOI] [PubMed] [Google Scholar]
- 64.Meng S, et al. Psoriasis therapy by Chinese medicine and modern agents. Chin. Med. 2018;13:16. doi: 10.1186/s13020-018-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin YK, et al. Anti-inflammatory effects of the extract of indigo naturalis in human neutrophils. J. Ethnopharmacol. 2009;125:51–58. doi: 10.1016/j.jep.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 66.Lin YK, et al. Indigo naturalis upregulates claudin-1 expression in human keratinocytes and psoriatic lesions. J. Ethnopharmacol. 2013;145:614–620. doi: 10.1016/j.jep.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 67.Lin YK, et al. Anti-psoriatic effects of indigo naturalis on the proliferation and differentiation of keratinocytes with indirubin as the active component. J. Dermatol. Sci. 2009;54:168–174. doi: 10.1016/j.jdermsci.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 68.Jablonska-Trypuc A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 69.Nagaoka I, Hirota S. Increased expression of matrix metalloproteinase-9 in neutrophils in glycogen-induced peritoneal inflammation of guinea pigs. Inflamm. Res. 2000;49:55–62. doi: 10.1007/s000110050559. [DOI] [PubMed] [Google Scholar]
- 70.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273–279. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mohamed MF, Abdelkhalek A, Seleem MN. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 2016;6:29707. doi: 10.1038/srep29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buhl AL, Wenzel J. Interleukin-36 in Infectious and Inflammatory Skin Diseases. Front. Immunol. 2019;10:1162. doi: 10.3389/fimmu.2019.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin. Immunol. 2007;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23:1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cho KA, et al. Interleukin-17 and Interleukin-22 Induced Proinflammatory Cytokine Production in Keratinocytes via Inhibitor of Nuclear Factor kappaB Kinase-alpha Expression. Ann. Dermatol. 2012;24:398–405. doi: 10.5021/ad.2012.24.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elias PM. Stratum corneum defensive functions: an integrated view. J. Invest. Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 77.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 78.Boniface K, et al. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 79.Ma HL, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harper EG, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J. Invest. Dermatol. 2009;129:2175–2183. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aries MF, et al. Anti-inflammatory and immunomodulatory effects of Aquaphilus dolomiae extract on in vitro models. Clin. Cosmet. Investig. Dermatol. 2016;9:421–434. doi: 10.2147/CCID.S113180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Altmae S, et al. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol. Hum. Reprod. 2010;16:178–187. doi: 10.1093/molehr/gap102. [DOI] [PubMed] [Google Scholar]
- 83.Li D, et al. A novel lipopeptide from skin commensal activates TLR2/CD36-p38 MAPK signaling to increase antibacterial defense against bacterial infection. PLoS One. 2013;8:e58288. doi: 10.1371/journal.pone.0058288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu CA, Huang DY, Lin WW. Beclin-1-independent autophagy positively regulates internal ribosomal entry site-dependent translation of hypoxia-inducible factor 1alpha under nutrient deprivation. Oncotarget. 2014;5:7525–7539. doi: 10.18632/oncotarget.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian H, et al. Ethanol extract of propolis protects macrophages from oxidized low density lipoprotein-induced apoptosis by inhibiting CD36 expression and endoplasmic reticulum stress-C/EBP homologous protein pathway. BMC Complement. Altern. Med. 2015;15:230. doi: 10.1186/s12906-015-0759-4. [DOI] [PMC free article] [PubMed] [Google Scholar]