Abstract
Human foodborne infections with the zoonotic pathogen Campylobacter jejuni are on the rise and constitute a significant socioeconomic burden worldwide. The health-beneficial, particularly anti-inflammatory effects of vitamin C (ascorbate) are well known. In our preclinical intervention study, we assessed potential anti-pathogenic and immunomodulatory effects of ascorbate in C. jejuni-infected secondary abiotic IL-10−/− mice developing acute campylobacteriosis similar to humans. Starting 4 days prior peroral C. jejuni-infection, mice received synthetic ascorbate via the drinking water until the end of the experiment. At day 6 post-infection, ascorbate-treated mice harbored slightly lower colonic pathogen loads and suffered from less severe C. jejuni-induced enterocolitis as compared to placebo control animals. Ascorbate treatment did not only alleviate macroscopic sequelae of infection, but also dampened apoptotic and inflammatory immune cell responses in the intestines that were accompanied by less pronounced pro-inflammatory cytokine secretion. Remarkably, the anti-inflammatory effects of ascorbate pretreatment in C. jejuni-infected mice were not restricted to the intestinal tract but could also be observed in extra-intestinal compartments including liver, kidneys and lungs. In conclusion, due to the potent anti-inflammatory effects observed in the clinical murine C. jejuni-infection model, ascorbate constitutes a promising novel option for prophylaxis and treatment of acute campylobacteriosis.
Subject terms: Immunology, Antimicrobial responses, Bacterial infection, Acute inflammation, Pathogens
Introduction
Campylobacter jejuni are the most common cause of food-borne gastroenteritis with increasing prevalence worldwide1,2. In fact, human campylobacteriosis represents a socioeconomic burden given estimated disease-associated costs of approximately 2.4 billion Euro3. Most commonly, C. jejuni transfer via consumption of contaminated raw or undercooked meat and milk or the ingestion of contaminated surface water to humans4–8. The intestinal colonization of C. jejuni induces a strong inflammatory response of the innate immune system affecting both, absorptive and secretory functions of the gastrointestinal tract1. In fact, campylobacteriosis constitutes a classical sodium malabsorption syndrome9, which depending on the bacterial strain and the host immune status, results in illness of varying degree10. Whereas some patients remain asymptomatic or display mild symptoms, others develop fever, abdominal pain and watery diarrhea, or suffer from acute campylobacteriosis characterized by severe enterocolitis with inflammatory, bloody diarrhea1,11. In the majority of events, the disease is self-limited and treated symptomatically, whereas patients with immunosuppressive comorbidities require antibiotic treatment11,12. However, in few instances, post-infectious sequelae including Guillain-Barré syndrome, Miller Fisher syndrome, reactive arthritis and chronic inflammatory conditions of the intestinal tract might develop with a latent period of weeks or longer1,13.
Even though human campylobacteriosis is becoming increasingly important, the distinct cellular and molecular mechanisms of host-pathogen interactions are limited. Clinical investigations in human patients disclosed that severe courses of Campylobacter infection and post-infectious morbidities (e.g., Guillain-Barré syndrome) are induced by the Gram-negative bacterial cell wall constituent lipooligosaccharide (LOS), especially sialylated LOS, which leads to hyper-activating of immune response14. Further RNA sequencing studies in human volunteers confirmed the major role of LOS-induced toll-like receptor (TLR) -4 signaling pathways in the induction of acute campylobacteriosis9. These findings support the view that campylobacteriosis results from a LOS-induced, TLR-4 mediated hyperergic innate immune response, which is similar to the inflammatory events induced by other LOS producing pathogens like Neisseria meningitidis and N. gonorrhoeae. However, in vivo trials have been hampered by the limited availability of experimental models. Mice show a strong physiological colonization resistance against invading microorganisms due to the mouse specific gut microbiota composition and are therefore protected from infection with enteropathogenic bacteria such as C. jejuni15–17. Furthermore, when compared to human, mice have been shown to be about 10,000-fold more resistant against LOS and lipopolysaccharide (LPS) expressed by Gram-negative bacteria18–20. We could recently show that a depletion of the gastrointestinal microbiota upon broad-spectrum antibiotic application in IL-10−/− mice facilitates intestinal C. jejuni colonization resulting in the development of key symptoms of acute human campylobacteriosis including wasting and bloody diarrhea within several days post-infection21. The main reasons for these severe C. jejuni induced immunopathological responses mounting in acute ulcerative enterocolitis are (i.) the abrogation of colonization resistance following microbiota depletion and (ii.) the lack of IL-10 enhancing susceptibility of mice to C. jejuni LOS21. In consequence, secondary abiotic IL-10−/− mice challenged with C. jejuni show strong intestinal and extra-intestinal immune responses via LOS–induced TLR-4 signaling21–30. Most importantly, the major role of LOS-induced intestinal immunopathology during campylobacteriosis was independently confirmed in elegant infection experiments with microbiota depleted SIGGR−/− mice developing campylobacteriosis similar to secondary abiotic IL-10 deficient mice. In contrast to the latter, the SIGGR−/− mice rendered sensitive to LOS due to the lack of a central inhibitor of cellular LPS/LOS-induced signaling pathways31.
In the 1920s, vitamin C was first isolated by the Hungarian Nobel laureate Albert Szent Györgyi on track to unravel the options for treatment and prophylaxis of morbidities such as scurvy caused by deficiency of this (for humans essential) vitamin32,33. Patients suffering from scurvy exhibit poor wound healing due to weakening of collagenous structures and compromised immune cell functions34 and are therefore highly susceptible to infections32,35. Ascorbate, the biologically active form of vitamin C, exerts a strong reductive potential and acts as a potent antioxidant that can be reversibly oxidized to dehydroascorbic acid33,36. Due to these characteristics, ascorbate is involved in several pivotal host defenses including immune regulatory pathways35. This is further underlined by the fact that both, innate and adaptive immune cells such as neutrophils, monocytes and lymphocytes, respectively, can accumulate ascorbate against a concentration gradient and exhibit intracellular concentrations that are up to 100 times higher than in plasma36–38. The presence of ascorbate in cells and plasma protects from oxidative stress. It is known that during phagocytosis, human granulocytes release hydrogen peroxide (H2O2) into the extracellular medium subsequently causing oxidative stress39 and cell damage by lipid peroxidation and alteration of protein and nucleic acid structure40. Interestingly, due to this potent antioxidant property, ascorbate has been shown to neutralize H2O2 and to reduce the H2O2-induced apoptosis in periodontal tissues41. Moreover, endotoxin-induced oxidative stress due to reactive oxygen species (ROS) is associated with high cell mortality42. Increasing intracellular ascorbate concentrations, however, decrease ROS levels, thereby counteracting cell mortality43.
Ascorbate has been shown to exert antimicrobial effects against distinct bacterial species such as Mycobacterium tuberculosis44–47, Staphylococcus aureus, Escherichia coli48, Helicobacter pylori49,50 and Salmonella species51 in vitro. Beside direct effects of ascorbate due to its low pH, for instance, reducing cell viability, it is also known that ascorbate exerts indirect bactericidal effects in the presence of metal ions or oxygen52. However, bacterial biofilm protects the cells against external influences and causes increased tolerance to antibiotic compounds53. Interestingly, ascorbate disrupts bacterial biofilm formation by inhibiting production of extracellular polymeric substances in Bacillus subtilis54 and methicillin-resistant S. aureus (MRSA)55 and pyocyanin production in Pseudomonas aeruginosa56. Thus, vitamin C (alone or in combination with antibiotics) constitutes a promising treatment option to destabilize bacterial biofilms.
Studies in the early 1980s revealed that growth of enteropathogens such as C. jejuni could be inhibited by ascorbate at low concentrations52,57,58. The authors have elegantly shown that the antimicrobial activity of ascorbate against C. jejuni is not due to lowered pH, but rather depends on the oxidation of ascorbate to dehydroascorbate and other products. However, the antimicrobial effects of ascorbate against C. jejuni have not been examined further and the exact mechanisms underlying the ascorbate-mediated toxicity against C. jejuni are still not known in detail. Moreover, data regarding potential immunomodulatory effects of ascorbate in C. jejuni infection are completely missing. This prompted us to perform for the first time a preclinical ascorbate intervention study applying our well-established murine clinical model of acute campylobacteriosis. Therefore, microbiota depleted IL-10−/− mice were subjected to synthetic ascorbate application via the drinking water, perorally infected with C. jejuni and surveyed for gastrointestinal pathogen loads, clinical outcome and intestinal as well as extra-intestinal immune responses during campylobacteriosis.
Results
Ascorbate treatment and gastrointestinal pathogen loads in C. jejuni infected secondary abiotic IL-10−/− mice
We first assessed antimicrobial effects of synthetic ascorbate against C. jejuni in vitro. Studies on minimal inhibitory concentrations (MICs) of 20 different C. jejuni isolates revealed a MIC90 value of 2818 mg/L (16 mM) with MICs ranging between 352 and 2818 mg/L (Supplementary Fig. S1). A MIC of 1409 mg/L (8 mM) was determined for the C. jejuni infection strain 81–176.
Secondary abiotic IL-10−/− mice were subjected to synthetic ascorbate treatment via the drinking water starting four days prior C. jejuni infection and lasting until necropsy. The concentration of the applied ascorbate solution was 5 g/L and hence, 3.56 times the MIC of the C. jejuni infection strain 81–176. On days 0 and 1, mice were then perorally challenged with 109 viable C. jejuni bacteria by gavage and surveyed until day 6 post-infection (p.i.). Bacterial culture analysis of faecal samples over time revealed that ascorbate treatment did not affect intestinal colonization properties of the applied C. jejuni strain as indicated by comparable median loads of approximately 109 colony forming units per gram (CFU/g) derived from ascorbate or placebo (PLC) treated mice (n.s.; Supplementary Fig. S2). At day 6 p.i., colonic C. jejuni loads were slightly lower in ascorbate as compared to placebo treated mice (i.e., less than one log order of magnitude; p < 0.001; Fig. 1), whereas pathogen numbers were comparable in more proximal parts of the gastrointestinal tract including stomach, duodenum and ileum (n.s.; Fig. 1). Hence, ascorbate does only marginally affect intestinal C. jejuni colonization in vivo.
Figure 1.
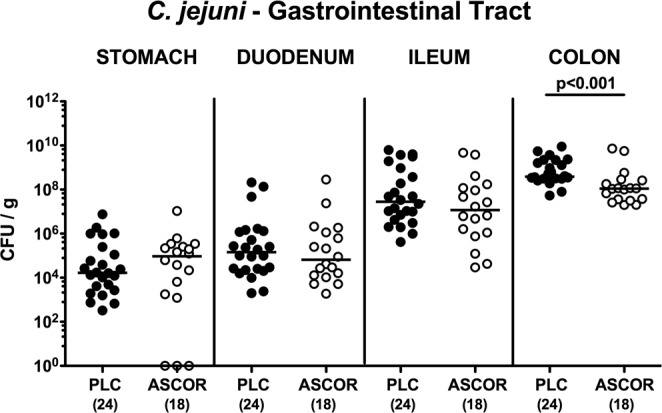
Gastrointestinal pathogen loads following ascorbate treatment of C. jejuni infected secondary abiotic IL-10−/− mice. Starting four days before peroral C. jejuni infection on days 0 and 1, secondary abiotic IL-10−/− mice were treated with synthetic ascorbate (ASCOR; open circles) or placebo (PLC; filled circles) via the drinking water. At day 6 post-infection, C. jejuni were isolated from distinct luminal parts of the gastrointestinal tract by culture and pathogenic loads expressed as colony forming units per gram (CFU/g). Medians, significance levels (p-values) assessed by the Mann-Whitney U test (for pairwise comparisons of PLC vs ASCOR in respective gastrointestinal compartment) and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments.
Ascorbate treatment ameliorates the clinical outcome of campylobacteriosis in C. jejuni infected secondary abiotic IL-10−/− mice
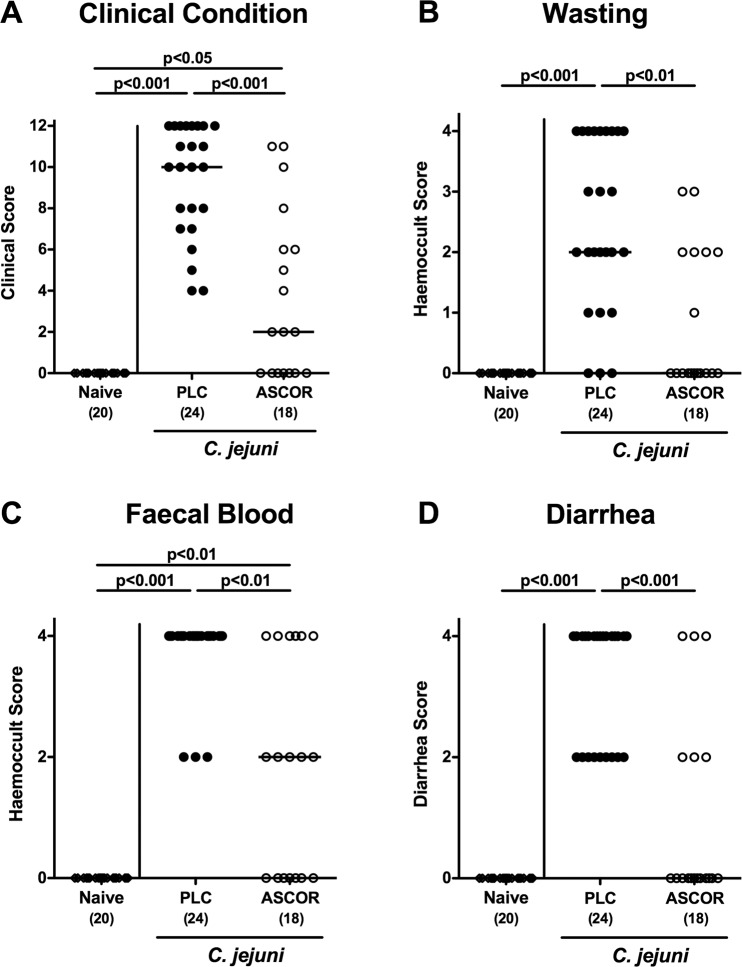
A kinetic survey of the clinical conditions of ascorbate versus PLC treated mice revealed that as early as day 5 p.i., the former suffered less distinctly from C. jejuni infection as compared to the latter (Supplementary Fig. S3). Upon necropsy (i.e., at day 6 p.i.), placebo treated mice were suffering from wasting and severe bloody diarrhea indicative for acute enterocolitis, whereas ascorbate treatment resulted in significantly reduced C. jejuni induced symptoms (Fig. 2A; Supplementary Fig. S3), particularly in less severe wasting, less pronounced diarrhea, less frequent abundance of blood in faecal samples and in better overall clinical appearance (p < 0.05–0.001 vs PLC; Fig. 2). Remarkably, almost 40% of infected mice from the ascorbate cohort were clinically uncompromised as indicated by cumulative clinical scores of 0 in 7 out of 18 cases (p < 0.001 vs PLC; Fig. 2A; Supplementary Fig. S3). Hence, ascorbate treatment alleviates clinical symptoms of campylobacteriosis.
Figure 2.
Macroscopic aspects of ascorbate treated mice following C. jejuni infection. Starting four days before peroral C. jejuni infection on days 0 and 1, secondary abiotic IL-10−/− mice were treated with synthetic ascorbate (ASCOR; open circles) or placebo (PLC; filled circles) via the drinking water. Applying a standardized clinical scoring system (see methods), (A) overall clinical outcome, (B) wasting, (C) abundance of faecal blood and (D) stool consistency were quantitatively surveyed at day 6 post-infection. Naive mice (open diamonds) served as uninfected controls. Medians, significance levels (p-values) assessed by the Kruskal Wallis test followed by Dunns correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments.
Ascorbate reduces apoptosis in the colon of C. jejuni infected secondary abiotic IL-10−/− mice
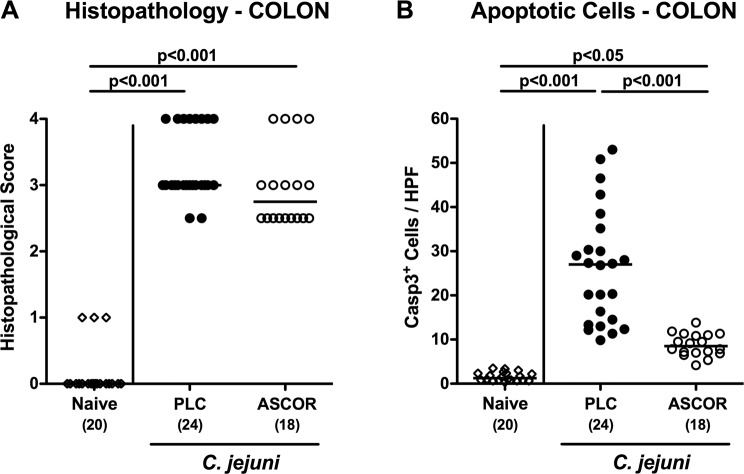
We next quantitatively assessed pathogen-induced histopathological changes in hematoxylin and eosin (H&E) stained colonic paraffin sections applying a standardized histopathological scoring system59. At day 6 p.i., ascorbate treated mice displayed a trend towards slightly lower histopathological scores as compared to PLC control animal (n.s.; Fig. 3A).
Figure 3.
Histopathological and apoptotic cell responses in colonic epithelia of ascorbate treated mice following C. jejuni infection. Starting four days before peroral C. jejuni infection on days 0 and 1, secondary abiotic IL-10−/− mice were treated with synthetic ascorbate (ASCOR; open circles) or placebo (PLC; filled circles) via the drinking water. (A) Histopathological changes were quantitated in hematoxylin and eosin stained colonic paraffin sections applying a standardized scoring system as described in methods. (B) The average numbers of apoptotic (positive for caspase3, Casp3+) colonic epithelial cells from six high power fields (HPF, 400x magnification) per mouse were assessed microscopically in immunohistochemically stained large intestinal paraffin sections at day 6 post-infection. Naive mice (open diamonds) served as uninfected and untreated controls. Medians, significance levels (p-values) assessed by the one-way ANOVA test followed by Tukey correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments.
Since apoptosis is regarded as a reliable parameter for the histopathological grading of intestinal inflammation15, we further quantitatively determined apoptotic colonic epithelial cell numbers following in situ immunohistochemical staining of colonic paraffin sections with a caspase3 antibody. In fact, C. jejuni infection was associated with multifold increases in colonic apoptotic epithelial cells at day 6 p.i. (p < 0.001 vs naive, whereas this increase was far less pronounced in ascorbate as compared to PLC treated mice (p < 0.001; Fig. 3B; Supplementary Fig. S4). Hence, ascorbate treatment decreases C. jejuni induced apoptotic responses in colonic epithelial cells.
Less distinct pro-inflammatory immune cell responses in the colon following ascorbate treatment of C. jejuni infected secondary abiotic IL-10−/− mice
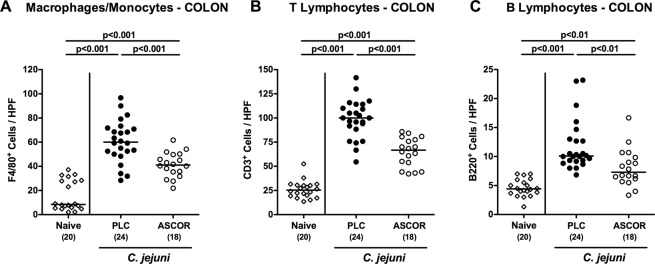
We next assessed large intestinal immune cell responses following ascorbate treatment of C. jejuni infected mice. C. jejuni infection was associated with marked increases of both, innate immune cell populations such as macrophages and monocytes (p < 0.001; Fig. 4A; Supplementary Fig. S5a) and adaptive immune cell subsets including T and B lymphocytes (p < 0.01–0.001; Fig. 4B,C; Supplementary Fig. S5b,c). At day 6 p.i., however, ascorbate treated mice displayed lower numbers of macrophages/monocytes as well as of T and B lymphocytes in their colonic mucosa and lamina propria as compared to infected PLC control animals (p < 0.01–0.001; Fig. 4; Supplementary Fig. S5). Hence, ascorbate treatment does not only alleviate macroscopic disease and colonic epithelial apoptosis following C. jejuni infection, but also dampens immune cell responses in the infected large intestines.
Figure 4.
Colonic immune cell responses in ascorbate treated mice following C. jejuni infection. Starting four days before peroral C. jejuni infection on days 0 and 1, secondary abiotic IL-10−/− mice were treated with synthetic ascorbate (ASCOR; open circles) or placebo (PLC; filled circles) via the drinking water. The average numbers of (A) macrophages and monocytes (positive for F4/80), (B) T lymphocytes (positive for CD3), and (C) B lymphocytes (positive for B220) in the colonic mucosa and lamina propria from six high power fields (HPF, 400x magnification) per mouse were assessed microscopically in immunohistochemically stained large intestinal paraffin sections at day 6 post-infection. Naive mice (open diamonds) served as uninfected and untreated controls. Medians, significance levels (p-values) assessed by the one-way ANOVA test followed by Tukey correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments.
Reduced pro-inflammatory mediator secretion in the intestinal tract following ascorbate treatment of C. jejuni infected secondary abiotic IL-10−/− mice
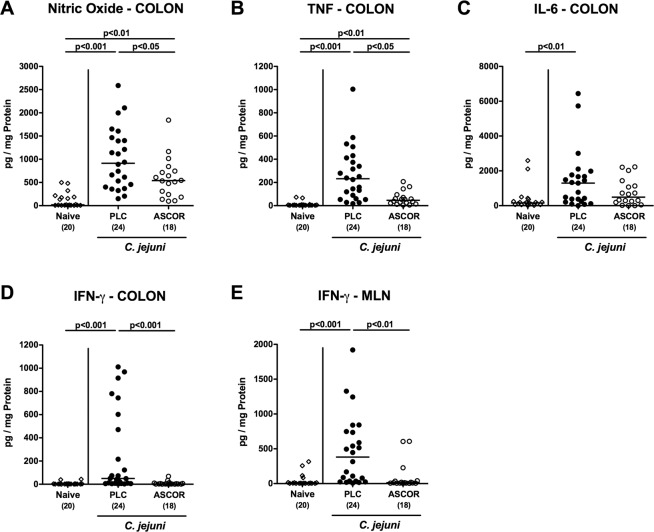
We further surveyed pro-inflammatory mediator responses in the intestines of ascorbate versus PLC treated C. jejuni infected mice. Six days following C. jejuni infection elevated nitric oxide (NO) and tumor necrosis factor (TNF) concentrations could be measured in colonic ex vivo biopsies (p < 0.01–0.001 vs naive; Fig. 5A,B), whereas ascorbate treated mice displayed lower mediator levels in their large intestines as compared to PLC control animals (p < 0.05; Fig. 5A,B). Remarkably, elevated IL-6 and interferon-γ (IFN-γ) concentrations were determined in colonic ex vivo biopsies at day 6 p.i. of PLC (p < 0.01 and p < 0.001 vs naive, respectively), but not ascorbate treated mice (Fig. 5C,D), which also held true for IFN-γ levels measured in mesenteric lymph nodes (MLN) at day 6 p.i. (p < 0.001; Fig. 5E). Hence, ascorbate treatment dampens pro-inflammatory mediator responses in the large intestines of C. jejuni infected mice.
Figure 5.
Intestinal inflammatory mediator responses in ascorbate treated mice following C. jejuni infection. Starting four days before peroral C. jejuni infection on days 0 and 1, secondary abiotic IL-10−/− mice were treated with synthetic ascorbate (ASCOR; open circles) or placebo (PLC; filled circles) via the drinking water. At day 6 post-infection. pro-inflammatory mediators including (A) nitric oxide, (B) TNF, (C) IL-6 and (D) IFN-γ were measured in supernatants of ex vivo biopsies derived from the colon as well as (E) IFN-γ concentrations assessed in mesenteric lymph nodes (MLN). Naive mice served as negative controls (open diamonds). Medians, significance levels (p-values) assessed by the Kruskall Wallis test followed by Dunns correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments.
Amelioration of inflammatory responses in extra-intestinal compartments following ascorbate treatment of C. jejuni infected secondary abiotic IL-10−/− mice
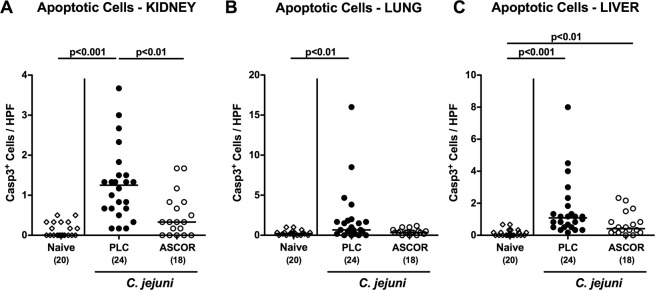
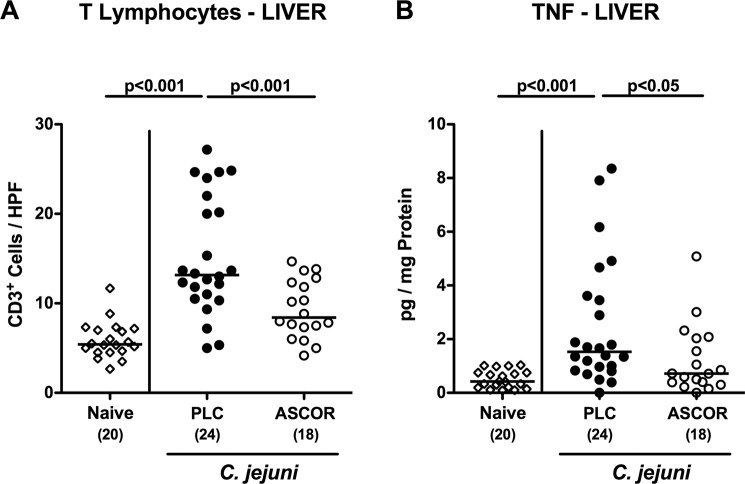
We further addressed whether the ascorbate associated disease-alleviating effects could also be observed in extra-intestinal compartments. We therefore quantitatively assessed apoptotic cell responses in kidneys, lungs and liver applying in situ immunohistochemistry. Six days following C. jejuni infection, increased numbers of caspase3+ cells could be determined in the kidneys and lungs of PLC (p < 0.01–0.001 vs naive), but not of ascorbate treated mice (Fig. 6A,B; Supplementary Fig. S6a,b). C. jejuni infected mice from either cohort displayed elevated hepatic apoptotic cell numbers (p < 0.01–0.001 vs naive), but with a trend towards lower numbers following ascorbate as compared to PLC application (n.s.; Fig. 6C; Supplementary Fig. S6c). Furthermore, PLC (p < 0.001 vs naive), but not ascorbate treated mice exhibited increased numbers of CD3+ T lymphocytes in their livers (Fig. 7A; Supplementary Fig. S6d), that was accompanied by elevated C. jejuni induced hepatic TNF secretion in the former, but not the latter cohort (p < 0.05 vs PLC; Fig. 7B).
Figure 6.
Extra-intestinal apoptosis in ascorbate treated mice following C. jejuni infection. Starting four days before peroral C. jejuni infection on days 0 and 1, secondary abiotic IL-10−/− mice were treated with synthetic ascorbate (ASCOR; open circles) or placebo (PLC; filled circles) via the drinking water. At day 6 post-infection, the average numbers of apoptotic cells (positive for caspase-3, Casp3+) from six high power fields (HPF, 400x magnification) per mouse were assessed microscopically in immunohistochemically stained paraffin sections derived from (A) liver, (B) kidneys and (C) lungs. Naive mice served as uninfected and untreated controls (open diamonds). Medians, significance levels (p-values) assessed by the Kruskal Wallis test followed by Dunns correction and numbers of analyzed animals (in parentheses) are indicated. Data were pooled from four independent experiments.
Figure 7.
Hepatic inflammatory immune responses in ascorbate treated mice following C. jejuni infection. Starting four days before peroral C. jejuni infection on days 0 and 1, secondary abiotic IL-10−/− mice were treated with synthetic ascorbate (ASCOR; open circles) or placebo (PLC; filled circles) via the drinking water. At day 6 post-infection, the average numbers of (A) T lymphocytes (positive for CD3) from six high power fields (HPF, 400x magnification) per mouse were assessed microscopically in immunohistochemically stained paraffin sections derived from liver ex vivo biopsies. Furthermore, (B) TNF concentrations were determined in supernatants of hepatic ex vivo biopsies taken at day 6 p.i. Naive mice served as negative controls (open circles). Numbers of mice (in parentheses), medians (black bars) and significance levels (p-values) determined by the one-way ANOVA test followed by Tukey correction are indicated. Data shown were pooled from three independent experiments.
We finally surveyed whether viable pathogens had translocated from the intestinal tract to extra-intestinal including systemic tissue sites following ascorbate treatment. C. jejuni translocation frequencies to MLN, spleen and liver were rather comparable following either treatment regimen at day 6 p.i. (Supplementary Fig. S7), whereas viable bacteria were isolated from the kidneys and lungs of PLC treated mice in single cases only, but in none of the animals from the ascorbate cohort (Supplementary Fig. S7). Of note, all blood samples taken at day 6 p.i. were C. jejuni-culture negative. Hence, the disease-alleviating effects of ascorbate treatment in C. jejuni infected mice were not restricted to the intestinal tract but could also be observed in extra-intestinal compartments.
Discussion
In this preclinical intervention study, we addressed for the first time potential anti-pathogenic and immunomodulatory properties of vitamin C during experimental acute campylobacteriosis in a clinical murine infection model. Following adding ascorbate to the drinking water starting four days prior C. jejuni infection of microbiota depleted IL-10−/− mice the excessively high gastrointestinal pathogen loads (of up to 109 viable bacterial cells per g faeces) within the stably infected animals could only marginally be lowered by less than one order of magnitude until day 6 p.i. This result was not surprising given that the ascorbate concentration within the drinking water exceeded approximately 3.5 times the MIC against the applied C. jejuni strain but is reduced by mixing with the secretory body fluids in the intestinal tract of the animals. The potent antibacterial properties of ascorbate directed against food-borne pathogens such as C. jejuni52,57,58 and Salmonella species51,60,61 have been further supported by several previous in vitro studies. Initially, it was hypothesized that the antimicrobial effects of vitamin C were particularly due to its pH lowering properties62. But this is not the case for the antimicrobial activity of ascorbate against C. jejuni, which depends on oxidation of ascorbate to dehydroascorbic acid and other products, as confirmed in independent studies in vitro52,58.
Despite the high gastrointestinal pathogen burdens, ascorbate treatment resulted in much better overall clinical conditions of C. jejuni infected mice including less severe diarrhea and less frequent abundance of faecal blood. It is noteworthy that almost 40% of mice from the ascorbate cohort were clinically uncompromised whereas mice from the placebo group suffered from full-blown campylobacteriosis as indicated by wasting and bloody diarrhea. The better macroscopic outcome upon ascorbate pretreatment of C. jejuni infected mice was accompanied by less apoptotic colonic epithelial cell responses. In support, Helicobacter pylori-induced apoptosis in human gastric epithelial cells was shown to be dampened after ascorbate treatment63, whereas another study revealed that intracellular accumulation of ascorbate could suppress apoptotic pathways in human monocytes in vitro64. Moreover, co-incubation with ascorbate promoted proliferative properties of human peripheral T lymphocytes65,66.
In addition to exerting antimicrobial effects, vitamin C has been shown to exhibit potent immunomodulatory, particularly anti-inflammatory properties both, in vitro and in vivo67–69. In our present study, ascorbate treatment resulted in less pronounced C. jejuni-induced pro-inflammatory innate as well as adaptive immune responses as indicated by lower numbers of macrophages and monocytes as well as of T and B lymphocytes, respectively, within the inflamed colonic mucosa and lamina propria that were accompanied by less intestinal secretion of pro-inflammatory mediators including nitric oxide, TNF, and IFN-γ and IL-6 at day 6 p.i. In support, ascorbate co-incubation resulted in down-regulated TNF levels in human whole blood70 and in splenic mouse T cell cultures71, whereas decreased nitric oxide, TNF, IFN-γ, and IL-6 concentrations could be determined in Staphylococcus aureus infected murine peritoneal macrophages in vitro40. Furthermore, ascorbate application to healthy subjects enhanced natural killer cell activities, lymphocyte proliferation and chemotaxis72,73, further underlining the potent immunomodulatory, anti-inflammatory effects of vitamin C69.
Notably, the anti-inflammatory effects of ascorbate treatment in C. jejuni infected mice were not restricted to the intestinal tract, but could also be observed in extra-intestinal organs as indicated by less distinct apoptosis in liver, kidneys and lungs and lower T cell numbers and lower TNF concentrations in livers of ascorbate versus placebo treated mice. Interestingly, translocation frequencies of viable C. jejuni from the intestinal tract to the liver were rather comparable following either treatment regimen, whereas no viable bacteria at all could be isolated from the kidneys and lungs following ascorbate pretreatment and in single cases only in placebo control mice at day 6 p.i.
The here presented immunomodulatory properties of an externally applied vitamin during acute C. jejuni induced enterocolitis are further supported by our very recent study surveying the health beneficial (i.e, anti-inflammatory) effects of vitamin D in the same clinical murine campylobacteriosis model. Following pre-treatment with synthetic 25-OH cholecalciferol, secondary abiotic IL-10−/− mice (i.) harbored comparably high C. jejuni loads in their gastrointestinal tract alike placebo controls upon peroral infection, but (ii.) suffered less frequently from diarrhea in the midst of infection, displayed (iii.) less pathogen-induced apoptotic, but (iv.) more pronounced counter-regulatory regenerative colonic cell responses that were accompanied by (v.) less distinct recruitment of both, innate und adaptive immune cells to the infected intestines, and by (vi.) less secretion of pro-inflammatory mediators in the intestinal tract (i.e, colon, ileum und MLN) as well as in the liver. Furthermore, as opposed to placebo controls (vii.) vitamin D treated mice displayed an uncompromised colonic epithelial barrier function which was accompanied by (viii.) less distinct bacterial translocation from the inflamed gut to extra-intestinal compartments in the latter as compared to the former74.
The pharmacokinetic properties of ascorbate in the vertebrate host are well studied75–79. Murine investigations on absorption, tissue distribution and retention of ascorbate76–78 revealed that maximum ascorbate concentrations could be measured in liver and kidneys (alike in the urine of humans75) approximately three hours following peroral single dose application76. Whereas in the lungs, adrenal glands, skin, white fat and pancreas peak levels were detectable as early as 6 hours, in the spleen increasing ascorbate concentrations could still be assessed until 24 hours following application76. The risk of adverse effects and intoxication upon high-dose ingestion may be considered negligible in subjects with intact renal function, given that vitamin C is water-soluble and concentrations exceeding the daily demands will be excreted via the kidneys80.Together, ascorbate application can be considered as safe and has been pharmaceutically approved for the treatment of gastrointestinal morbidities in humans33,60,81. Interestingly, several inflammatory morbidities within the gastrointestinal tract have been shown to be associated with reduced ascorbate plasma concentrations82,83.
In conclusion, our pre-clinical intervention study provides strong evidence for the first time that ascorbate constitutes a promising compound exerting potent anti-inflammatory and hence, disease-alleviating effects in non-self-limiting acute campylobacteriosis. Furthermore, food supplementation with ascorbate might be a useful tool to enhance host defense mechanisms in livestock animals directed against enteropathogens including C. jejuni thereby lowering pathogenic loads or even preventing from pathogen acquisition. Further studies are needed, however, to unravel the underlying mechanism in more detail.
Material and Methods
Ethical statement
In vivo experiments were carried out according to the European Guidelines for animal welfare (2010/63/EU) following agreement by the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, registration number G0172/16 and G0247/16). Animal welfare was monitored twice a day.
Determination of minimal inhibitory concentrations of ascorbate
To determine the antimicrobial effect of synthetic ascorbate, 20 C. jejuni isolates including the reference strain 81–176 (used for infection of mice, see below) and the DSM 4688 strain (for quality control) were tested in three independent experiments for their minimal inhibitory concentration (MIC) by the broth microdilution and macrodilution method. Settings of inoculum density, growth medium and conditions as well as incubation time were applied following the recommendations of the Clinical and Laboratory Standards Institute (CLSI) given in the document VET01-Ed584. Twofold serial dilutions ranging from 0.03–32.0 mmol/L (6–5636 µg/mL) for ascorbate were tested. Stock solutions were prepared in Mueller-Hinton broth (Oxoid, Germany) and adjusted to pH 7.3.
Generation of secondary abiotic/gnotobiotic mice
In the identical unit of the Forschungseinrichtungen für Experimentelle Medizin (FEM, Charité - University Medicine Berlin), IL10−/− mice (C57BL/6j background) were bred, raised and housed under specific pathogen free (SPF) conditions. Mice were kept under standard conditions (22–24 °C room temperature, 55 ± 15% humidity, 12 h light/12 h dark cycle) in cages including filter tops within an experimental semi-barrier (accessible only with lab coat, overshoes, caps and sterile gloves) and had free access to autoclaved standard chow (food pellets: ssniff R/M-H, V1534-300, Sniff, Soest, Germany) as well as to autoclaved drinking water (ad libitum).
The depletion of the murine commensal intestinal microbiota in order to abrogate the physiological colonization resistance and hence, to assure stable intestinal C. jejuni colonization was achieved by application of five different antibiotics to the mice15,85. Briefly, 3-week old mice were treated with an antibiotic cocktail containing vancomycin (500 mg/L; Cell Pharm, Germany), ciprofloxacin (200 mg/L; Bayer Vital, Germany), imipenem (250 mg/L; MSD, Germany), metronidazole (1 g/L; Fresenius, Germany), and ampicillin plus sulbactam (1 g/L; Ratiopharm, Germany) within autoclaved drinking water (ad libitum) over a period time of 8 weeks. Three days prior infection the antibiotic treatment was withdrawn to assure antibiotic washout.
Treatment with synthetic ascorbate
Starting four days before C. jejuni infection and lasting until the end of the experiment, three-month old, sex-matched secondary abiotic IL-10−/− mice (maximum of three animals per cage) were treated with ascorbate (Sigma Aldrich, München, Germany) that had been sterile-filtered and added to the autoclaved tap water (ad libitum) and changed every other day. For ascorbate treatment, a daily dosage of 1 g per kg body weight was calculated86. Considering a body weight of approximately 25 g per mouse and a daily drinking volume of approximately 5 mL, the final concentration of the ascorbate solution was 5 g/L (pH 7.0). Mice from the placebo (PLC) cohort received autoclaved tap water only. Of note, the daily inter-individual drinking volumes between cages within and between respective cohorts were comparable. In four individual experiments, n = 5/5/4/4 ascorbate treated mice and n = 6/6/6/6 PLC controls were analyzed.
C. jejuni infection
As previously described, mice were challenged with 109 CFU of the C. jejuni strain 81–176 (that had initially been isolated from a diseased patient suffering from bloody diarrhea) in a volume of 0.3 mL phosphate buffered saline (PBS; Gibco, life technologies, UK) on two successive days (days 0 and 1) by oral gavage15. In order to avoid contaminations, mice were kept and handled under strict aseptic conditions.
Evaluation of clinical conditions
The clinical conditions of mice were evaluated daily (starting four days before and lasting until day 6 after C. jejuni infection) and quantitated via standardized cumulative clinical scores (maximum 12 points), addressing the abundance of blood in faeces (0: no blood; 2: microscopic detection of blood by the Guajac method using Haemoccult, Beckman Coulter/PCD, Germany; 4: macroscopic blood visible), stool consistency (0: formed faeces; 2: pasty faeces; 4: liquid faeces), and the clinical aspect (0: normal; 2: ruffled fur, less locomotion; 4: isolation, severely compromised locomotion, pre-final aspect) as described earlier27.
Sampling methods
Six days after the infection, mice were sacrificed by inhalation of isoflurane (Abbott, Germany). Luminal gastrointestinal samples (i.e., from colon, ileum, duodenum, and stomach) and ex vivo biopsies from intestinal (colon, ileum, MLN) and extra-intestinal (liver, kidneys and lungs) compartments were taken under aseptic conditions. To generate individual serum probes, cardiac blood was collected (approximately 1.0 mL). Intestinal samples for microbiological, immunological and immunohistopathological assays were taken in parallel from each mouse.
Histopathology
For histopathological analyses, sections (thickness 5 µm) of formalin-fixed (5%) and paraffin-embedded colonic ex vivo biopsies were used, stained with hematoxylin and eosin (H&E), and examined by light microscopy (100x magnification). The histopathological changes in the large intestines were quantitatively evaluated following an established histopathological scoring system ranging from 0 to 4 as described earlier59. Score 1: intact epithelium with minimal inflammatory cell infiltrates in the mucosa. Score 2: mild hyperplasia and mild goblet cell loss with mild inflammatory cell infiltrates in the mucosa and submucosa. Score 3: moderate goblet cell loss with moderate inflammatory cell infiltrates in the mucosa. Score 4: marked goblet cell loss, multiple crypt abscesses and crypt loss with marked inflammatory cell infiltration into in the mucosa and submucosa.
Immunohistochemical assays
In situ immunohistochemical analyses were conducted as previously reported87,88. Briefly, paraffin sections (5 μm) derived from ex vivo biopsies of interest (i.e., colon, liver, kidneys, lungs) were stained with primary antibodies directed against cleaved caspase 3 (Asp175, Cell Signaling, Beverly, MA, USA, 1:200) to detect apoptotic epithelial cells; with F4/80 (# 14–4801, clone BM8, eBioscience, San Diego, CA, USA, 1:50) to detect macrophages/monocytes; with CD3 (#N1580, Dako, 1:10) to detect T lymphocytes; and furthermore, with B220 (No. 14-0452-81, eBioscience; 1:200) to detect B lymphocytes. The sections were incubated with the primary antibody for 30 min followed by another 30 min of incubation with the respective secondary antibody (for anti-caspase-3 and anti-CD3 staining: biotinylated donkey anti-rabbit antibody; for anti-F4/80 and anti-B220: biotinylated rabbit anti-rat antibody; all from Dianova, Hamburg, Germany). As detection system, the Streptavidin-Alkaline Phosphatase Kit (Dako) using Fast Red as chromogen was applied. To generate negative controls, the primary antibodies had been excluded. The examination of positively stained cells was undertaken by light microscopy (magnification 100x and 400x). The average number of respective positively stained cells for each mouse was calculated within at least six high power fields (HPF, 0.287 mm2, 400x magnification) by a blinded independent investigator. An Axiolmager Z1 microscope was used for the generation of images, which were subsequently processed with the Axiovision software (Carl Zeiss MicroImaging, Jena, Germany).
The colonization and translocation of C. jejuni
The C. jejuni loads were quantitatively surveyed in faeces samples taken every days after infection, and further, upon necropsy in gastrointestinal luminal samples (taken from stomach, duodenum, ileum and colon) and in homogenized ex vivo biopsies derived from MLN, spleen, liver, kidneys and lungs as well as in cardiac blood by culture as stated earlier15,89. The detection limit of viable pathogens was ≈100 CFU per g. For the determination of cumulative translocation rates of viable C. jejuni into respective extra-intestinal compartments, the ratio of the sum of culture-positive mice and the total numbers of analyzed animals (in %) out of four experiments were calculated.
Measurements of intestinal and extra-intestinal pro-inflammatory mediators
Longitudinally sliced and in PBS washed colonic ex vivo biopsies (strips of approximately 1 cm2) as well as ex vivo biopsies derived from liver (approximately 1 cm3) and MLN (3–4 lymph nodes) were cultured for 18 h at 37 °C in 24-flat-bottom well culture plates (Nunc, Germany) containing 500 μL serum-free RPMI 1640 medium (Gibco, life technologies, UK) that was supplemented with penicillin (100 U/mL) and streptomycin (100 µg/mL; PAA Laboratories, Germany). Using the Mouse Inflammation Cytometric Bead Assay (CBA; BD Biosciences, Germany) the culture supernatants and serum samples were analyzed for TNF, IFN-γ and IL-6 on a BD FACS Canto II flow cytometer (BD Biosciences). The nitric oxide concentrations were determined by the Griess reaction85,90. The pro-inflammatory mediator levels were normalized to the protein concentrations measured in the supernatant of the respective organ homogenate88.
Data analysis
Data from four independently performed experiments were pooled and analyzed. The Mann-Whitney test (GraphPad Prism v7, USA) was used for determination of medians and levels of significance for pairwise comparisons of not normally distributed data, whereas for multiple comparisons, the one-sided ANOVA with Tukey post-correction or the Kruskal-Wallis test with Dunn’s post-correction were used. Two-sided probability (p) values ≤ 0.05 were considered significant.
Supplementary information
Acknowledgements
We thank Alexandra Bittroff-Leben, Ines Puschendorf, Ulrike Fiebiger, Anna-Maria Schmidt, Gernot Reifenberger, and the staff of the animal research facility at Charité - University Medicine Berlin for excellent technical assistance and animal breeding. We further thank Dr. Anja A. Kühl (Department of Medicine I for Gastroenterology, Infectious Diseases and Rheumatology/Research Center ImmunoSciences (RCIS), Charité – Universitätsmedizin Berlin) for histopathological scoring and taking representative photomicropgraphs of immunhistochmically stained paraffin sections. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin. This work was supported from the German Federal Ministries of Education and Research (BMBF) in frame of the zoonoses research consortium PAC-Campylobacter to SM, SB, MMH (IP7/01KI1725D) and to ET, SK, CK (IP5/01Kl1725C) and from the Federal Ministry for Economic Affairs and Energy following a resolution of the German National Parliament, Deutscher Bundestag (ZIM, ZF4117908 AJ8) to SB and MMH. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author contributions
S.M.: Performed experiments, co-wrote paper. U.E.: Performed experiments, co-edited paper. E.T.: Performed experiments, analyzed data, co-edited paper. S.K.: Performed experiments, analyzed data, co-edited paper. C.K.: Performed experiments, analyzed data, co-edited paper. S.B.: Provided advice in experimental design, critically discussed results, co-edited paper. M.M.H.: Designed and performed experiments, analyzed data, wrote paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Stefan Bereswill and Markus M. Heimesaat.
Supplementary information
is available for this paper at 10.1038/s41598-020-59890-8.
References
- 1.Backert, S., Tegtmeyer, N., Cróinín, T. Ó., Boehm, M. & Heimesaat, M. M. In Campylobacter (ed. Günter Klein) 1–25 (Academic Press, 2017).
- 2.Fitzgerald C. Campylobacter. Clin. laboratory Med. 2015;35:289–298. doi: 10.1016/j.cll.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Authority EFS, Prevention ECFD. & Control. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA. J. 2018;16:e05500. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronowski C, James CE, Winstanley C. Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol. Lett. 2014;356:8–19. doi: 10.1111/1574-6968.12488. [DOI] [PubMed] [Google Scholar]
- 5.Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane JA, Mehra RK, Carrington SD, Hickey RM. The food glycome: a source of protection against pathogen colonization in the gastrointestinal tract. Int. J. Food Microbiol. 2010;142:1–13. doi: 10.1016/j.ijfoodmicro.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Hermans D, et al. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 2012;12:89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- 8.Newell, D., Mughini-Gras, L., Kalupahana, R. A. & Wagenaar, J. 85–110 (2016).
- 9.Bucker R, et al. Campylobacter jejuni impairs sodium transport and epithelial barrier function via cytokine release in human colon. Mucosal Immunol. 2018;11:575–577. doi: 10.1038/mi.2017.78. [DOI] [PubMed] [Google Scholar]
- 10.Poly F, Guerry P. Pathogenesis of Campylobacter. Curr. Opin. Gastroenterol. 2008;24:27–31. doi: 10.1097/MOG.0b013e3282f1dcb1. [DOI] [PubMed] [Google Scholar]
- 11.Kist M, Bereswill S. Campylobacter jejuni. Contrib. Microbiol. 2001;8:150–165. doi: 10.1159/000060405. [DOI] [PubMed] [Google Scholar]
- 12.Walker RI, et al. Pathophysiology of Campylobacter enteritis. Microbiol. Rev. 1986;50:81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keithlin J, Sargeant J, Thomas MK, Fazil A. Systematic review and meta-analysis of the proportion of Campylobacter cases that develop chronic sequelae. BMC Public. Health. 2014;14:1203. doi: 10.1186/1471-2458-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortensen NP, et al. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 2009;11:988–994. doi: 10.1016/j.micinf.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Bereswill S, et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. Plos One. 2011;6:e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masanta WO, et al. Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin. Dev. Immunol. 2013;2013:526860. doi: 10.1155/2013/526860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiebiger U, Bereswill S, Heimesaat MM. Dissecting the Interplay Between Intestinal Microbiota and Host Immunity in Health and Disease: Lessons Learned from Germfree and Gnotobiotic Animal Models. Eur. J. Microbiol. Immunol. 2016;6:253–271. doi: 10.1556/1886.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren HS, et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. J. Infect. Dis. 2010;201:223–232. doi: 10.1086/649557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson SA, Care AS, Skinner RJ. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol. Reprod. 2007;76:738–748. doi: 10.1095/biolreprod.106.056143. [DOI] [PubMed] [Google Scholar]
- 20.da Silva AMT, et al. Shock and multiple-organ dysfunction after self-administration of Salmonella endotoxin. N. Engl. J. Med. 1993;328:1457–1460. doi: 10.1056/NEJM199305203282005. [DOI] [PubMed] [Google Scholar]
- 21.Haag LM, et al. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10−/− mice via Toll-like-receptor-2 and -4 signaling. PLoS One. 2012;7:e40761. doi: 10.1371/journal.pone.0040761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haag LM, et al. Campylobacter jejuni infection of infant mice: acute enterocolitis is followed by asymptomatic intestinal and extra-intestinal immune responses. Eur. J. Microbiol. Immunol. 2012;2:2–11. doi: 10.1556/EuJMI.2.2012.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alutis ME, et al. Selective gelatinase inhibition reduces apoptosis and pro-inflammatory immune cell responses in Campylobacter jejuni-infected gnotobiotic IL-10 deficient mice. Eur. J. Microbiol. Immunol. 2014;4:213–222. doi: 10.1556/EUJMI-D-14-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bereswill S, et al. Lactobacillus johnsonii ameliorates intestinal, extra-intestinal and systemic pro-inflammatory immune responses following murine Campylobacter jejuni infection. Sci. Rep. 2017;7:2138. doi: 10.1038/s41598-017-02436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bereswill S, Grundmann U, Alutis ME, Fischer A, Heimesaat MM. Campylobacter jejuni infection of conventionally colonized mice lacking nucleotide-oligomerization-domain-2. Gut Pathog. 2017;9:5. doi: 10.1186/s13099-017-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekmekciu I, Fiebiger U, Stingl K, Bereswill S, Heimesaat MM. Amelioration of intestinal and systemic sequelae of murine Campylobacter jejuni infection by probiotic VSL#3 treatment. Gut Pathog. 2017;9:17. doi: 10.1186/s13099-017-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heimesaat MM, et al. The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front. Cell Infect. Microbiol. 2014;4:77. doi: 10.3389/fcimb.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimesaat MM, Grundmann U, Alutis ME, Fischer A, Bereswill S. Microbiota Composition and Immune Responses During Campylobacter Jejuni Infection in Conventionally Colonized IL-10(−/−) Mice Lacking Nucleotide Oligomerization Domain 2. Eur. J. Microbiol. Immunol. 2017;7:1–14. doi: 10.1556/1886.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heimesaat MM, Grundmann U, Alutis ME, Fischer A, Bereswill S. Absence of Nucleotide-Oligomerization-Domain-2 Is Associated with Less Distinct Disease in Campylobacter jejuni Infected Secondary Abiotic IL-10 Deficient Mice. Front. Cell Infect. Microbiol. 2017;7:322. doi: 10.3389/fcimb.2017.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt AM, et al. Protease Activity of Campylobacter jejuni HtrA Modulates Distinct Intestinal and Systemic Immune Responses in Infected Secondary Abiotic IL-10 Deficient Mice. Front. Cell Infect. Microbiol. 2019;9:79. doi: 10.3389/fcimb.2019.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl M, et al. A novel mouse model of Campylobacter jejuni gastroenteritis reveals key pro-inflammatory and tissue protective roles for Toll-like receptor signaling during infection. Plos Pathog. 2014;10:e1004264. doi: 10.1371/journal.ppat.1004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemilä H. Vitamin C and Infections. Nutrients. 2017;9:339. doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorice A, et al. Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini Rev. Med. Chem. 2014;14:444–452. doi: 10.2174/1389557514666140428112602. [DOI] [PubMed] [Google Scholar]
- 34.Ang A, Pullar JM, Currie MJ, Vissers MC. Vitamin C and immune cell function in inflammation and cancer. Biochemical Soc. Trans. 2018;46:1147–1159. doi: 10.1042/BST20180169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr, A. C. & Maggini, S. Vitamin C and Immune Function. Nutrients9, 10.3390/nu9111211 (2017). [DOI] [PMC free article] [PubMed]
- 36.Strohle A, Wolters M, Hahn A. Micronutrients at the interface between inflammation and infection–ascorbic acid and calciferol: part 1, general overview with a focus on ascorbic acid. Inflamm. Allergy Drug. Targets. 2011;10:54–63. doi: 10.2174/187152811794352105. [DOI] [PubMed] [Google Scholar]
- 37.Washko P, Rotrosen D, Levine M. Ascorbic acid in human neutrophils. Am. J. Clin. Nutr. 1991;54:1221S–1227S. doi: 10.1093/ajcn/54.6.1221s. [DOI] [PubMed] [Google Scholar]
- 38.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl Acad. Sci. USA. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Root RK, Metcalf JA. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J. Clin. investigation. 1977;60:1266–1279. doi: 10.1172/jci108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dey S, Bishayi B. Killing of S. aureus in murine peritoneal macrophages by Ascorbic acid along with antibiotics Chloramphenicol or Ofloxacin: Correlation with inflammation. Microb. Pathog. 2018;115:239–250. doi: 10.1016/j.micpath.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 41.Wu W, et al. Effect of vitamin C administration on hydrogen peroxide-induced cytotoxicity in periodontal ligament cells. Mol. Med. Rep. 2015;11:242–248. doi: 10.3892/mmr.2014.2712. [DOI] [PubMed] [Google Scholar]
- 42.Baxter F. Septic Shock. Can. J. Anaesth. 1997;44:59–72. doi: 10.1007/bf03014326. [DOI] [PubMed] [Google Scholar]
- 43.Qiao H, May JM. Macrophage differentiation increases expression of the ascorbate transporter (SVCT2) Free. Radic. Biol. Med. 2009;46:1221–1232. doi: 10.1016/j.freeradbiomed.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boissevain CH, Spillane JH., Jr. A note on the effect of synthetic ascorbic acid (vitamin C) on the growth of the tubercle bacillus. Am. Rev. Tuberculosis. 1937;35:661–662. [Google Scholar]
- 45.St JL. Der Einfluss von Vitamin C und Vitamin B 1 auf das Wachstum der Tuberkelbacillen. Klinische Wochenschr. 1937;16:1423–1425. doi: 10.1007/BF01777258. [DOI] [Google Scholar]
- 46.Sirsi M. Antimicrobial action of vitamin C on M. tuberculosis and some other pathogenic organisms. Indian. J. Med. Sci. 1952;6:5. [Google Scholar]
- 47.Myrvik QN, Volk WA. Comparative study of the antibacterial properties of ascorbic acid and reductogenic compounds. J. Bacteriol. 1954;68:622. doi: 10.1128/JB.68.5.622-626.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kallio J, Jaakkola M, Maki M, Kilpelainen P, Virtanen V. Vitamin C inhibits staphylococcus aureus growth and enhances the inhibitory effect of quercetin on growth of Escherichia coli in vitro. Planta Med. 2012;78:1824–1830. doi: 10.1055/s-0032-1315388. [DOI] [PubMed] [Google Scholar]
- 49.Zhang ZW, Farthing MJ. The roles of vitamin C in Helicobacter pylori associated gastric carcinogenesis. Chin. J. Dig. Dis. 2005;6:53–58. doi: 10.1111/j.1443-9573.2005.00194.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang HM, Wakisaka N, Maeda O, Yamamoto T. Vitamin C inhibits the growth of a bacterial risk factor for gastric carcinoma: Helicobacter pylori. Cancer. 1997;80:1897–1903. doi: 10.1002/(SICI)1097-0142(19971115)80:10<1897::AID-CNCR4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh, T. et al. A Combination of Linalool, Vitamin C, and Copper Synergistically Triggers Reactive Oxygen Species and DNA Damage and Inhibits Salmonella enterica subsp. enterica Serovar Typhi and Vibrio fluvialis. Appl Environ Microbiol85, 10.1128/AEM.02487-18 (2019). [DOI] [PMC free article] [PubMed]
- 52.Juven BJ, Kanner J. Effect of ascorbic, isoascorbic and dehydroascorbic acids on the growth and survival of Campylobacter jejuni. J. Appl. Bacteriol. 1986;61:339–345. doi: 10.1111/j.1365-2672.1986.tb04295.x. [DOI] [PubMed] [Google Scholar]
- 53.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. antimicrobial agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Pandit, S. et al. Low Concentrations of Vitamin C Reduce the Synthesis of Extracellular Polymers and Destabilize Bacterial Biofilms. Frontiers in Microbiology8, 10.3389/fmicb.2017.02599 (2017). [DOI] [PMC free article] [PubMed]
- 55.Ali Mirani Z, et al. Ascorbic acid augments colony spreading by reducing biofilm formation of methicillin-resistant Staphylococcus aureus. Iran. J. basic. Med. Sci. 2018;21:175–180. doi: 10.22038/ijbms.2018.20714.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Mowafy SA, Shaaban MI. & Abd El Galil, K. H. Sodium ascorbate as a quorum sensing inhibitor of Pseudomonas aeruginosa. J. Appl. Microbiology. 2014;117:1388–1399. doi: 10.1111/jam.12631. [DOI] [PubMed] [Google Scholar]
- 57.Juven BJ, Kanner J, Weisslowicz H, Harel S. Effect of Ascorbic and Isoascorbic acids on Survival of Campylobacter jejuni in Poultry Meat (1) J. Food Prot. 1988;51:436–437. doi: 10.4315/0362-028X-51.6.436. [DOI] [PubMed] [Google Scholar]
- 58.Fletcher RD, Albers AC, Chen AK, Albertson JN., Jr. Ascorbic acid inhibition of Campylobacter jejuni growth. Appl. Env. Microbiol. 1983;45:792–795. doi: 10.1128/AEM.45.3.792-795.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erben U, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014;7:4557–4576. [PMC free article] [PubMed] [Google Scholar]
- 60.Hernandez-Patlan, D. et al. Evaluation of the Dietary Supplementation of a Formulation Containing Ascorbic Acid and a Solid Dispersion of Curcumin with Boric Acid against Salmonella Enteritidis and Necrotic Enteritis in Broiler Chickens. Animals (Basel)9, 10.3390/ani9040184 (2019). [DOI] [PMC free article] [PubMed]
- 61.Hartzen SH, Frimodt-Moller N, Frolund Thomsen V. The antibacterial activity of a siderophore. 1. In vitro activity of deferoxamine alone and in combination with ascorbic acid on Staphylococcus aureus. APMIS: acta pathologica, microbiologica, et. immunologica Scandinavica. 1989;97:419–424. doi: 10.1111/j.1699-0463.1989.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 62.v. Gagyi J. Ueber die bactericide und antitoxische Wirkung des Vitamin C. J. Mol. Med. 1936;15:190–195. [Google Scholar]
- 63.Chen X, Liu R, Liu X, Xu C, Wang X. L-ascorbic Acid-2-Glucoside inhibits Helicobacter pylori-induced apoptosis through mitochondrial pathway in Gastric Epithelial cells. Biomed. Pharmacother. 2018;97:75–81. doi: 10.1016/j.biopha.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 64.Perez-Cruz I, Carcamo JM, Golde DW. Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood. 2003;102:336–343. doi: 10.1182/blood-2002-11-3559. [DOI] [PubMed] [Google Scholar]
- 65.Huijskens MJ, et al. Technical advance: ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J. Leukoc. Biol. 2014;96:1165–1175. doi: 10.1189/jlb.1TA0214-121RR. [DOI] [PubMed] [Google Scholar]
- 66.Molina N, Morandi AC, Bolin AP, Otton R. Comparative effect of fucoxanthin and vitamin C on oxidative and functional parameters of human lymphocytes. Int. Immunopharmacol. 2014;22:41–50. doi: 10.1016/j.intimp.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 67.Carcamo JM, Borquez-Ojeda O, Golde DW. Vitamin C inhibits granulocyte macrophage-colony-stimulating factor-induced signaling pathways. Blood. 2002;99:3205–3212. doi: 10.1182/blood.v99.9.3205. [DOI] [PubMed] [Google Scholar]
- 68.Bowie AG, O’Neill LA. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J. Immunol. 2000;165:7180–7188. doi: 10.4049/jimmunol.165.12.7180. [DOI] [PubMed] [Google Scholar]
- 69.Mousavi S, Bereswill S, Heimesaat MM. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur. J. Microbiology Immunol. 2019 doi: 10.1556/1886.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartel C, Strunk T, Bucsky P, Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27:101–106. doi: 10.1016/j.cyto.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 71.Maeng HG, et al. Vitamin C enters mouse T cells as dehydroascorbic acid in vitro and does not recapitulate in vivo vitamin C effects. Immunobiology. 2009;214:311–320. doi: 10.1016/j.imbio.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Levy R, Shriker O, Porath A, Riesenberg K, Schlaeffer F. Vitamin C for the treatment of recurrent furunculosis in patients with imparied neutrophil functions. J. Infect. Dis. 1996;173:1502–1505. doi: 10.1093/infdis/173.6.1502. [DOI] [PubMed] [Google Scholar]
- 73.Heuser G, Vojdani A. Enhancement of natural killer cell activity and T and B cell function by buffered vitamin C in patients exposed to toxic chemicals: the role of protein kinase-C. Immunopharmacol. Immunotoxicol. 1997;19:291–312. doi: 10.3109/08923979709046977. [DOI] [PubMed] [Google Scholar]
- 74.Mousavi S, et al. Vitamin D in Acute Campylobacteriosis-Results From an Intervention Study Applying a Clinical Campylobacter jejuni Induced Enterocolitis Model. Front. immunology. 2019;10:2094. doi: 10.3389/fimmu.2019.02094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lykkesfeldt J, Tveden-Nyborg P. The Pharmacokinetics of Vitamin C. Nutrients. 2019;11:2412. doi: 10.3390/nu11102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iwama M, Shimokado K, Maruyama N, Ishigami A. Time course of vitamin C distribution and absorption after oral administration in SMP30/GNL knockout mice. Nutr. 2011;27:471–478. doi: 10.1016/j.nut.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 77.Aumailley L, et al. Vitamin C modulates the metabolic and cytokine profiles, alleviates hepatic endoplasmic reticulum stress, and increases the life span of Gulo−/− mice. Aging. 2016;8:458–483. doi: 10.18632/aging.100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vissers MC, Bozonet SM, Pearson JF, Braithwaite LJ. Dietary ascorbate intake affects steady state tissue concentrations in vitamin C-deficient mice: tissue deficiency after suboptimal intake and superior bioavailability from a food source (kiwifruit) Am. J. Clin. Nutr. 2011;93:292–301. doi: 10.3945/ajcn.110.004853. [DOI] [PubMed] [Google Scholar]
- 79.Tsukaguchi H, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nat. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 80.Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian. J. Clin. Biochem. 2013;28:314–328. doi: 10.1007/s12291-013-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aditi A, Graham DY. Vitamin C, gastritis, and gastric disease: a historical review and update. Digestive Dis. Sci. 2012;57:2504–2515. doi: 10.1007/s10620-012-2203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ichim TE, et al. Intravenous ascorbic acid to prevent and treat cancer-associated sepsis? J. Transl. Med. 2011;9:25. doi: 10.1186/1479-5876-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.El Halabi, I. et al. Ascorbic Acid in Colon Cancer: From the Basic to the Clinical Applications. International journal of molecular sciences19, 10.3390/ijms19092752 (2018). [DOI] [PMC free article] [PubMed]
- 84.CLSI. VET01-A5: Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard. 5th Edition. Clinical and Laboratory Standards Institute, Wayne, PA. Volume 34 (2018).
- 85.Heimesaat MM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 86.Kockar C, Ozturk M, Bavbek N. Helicobacter pylori eradication with beta carotene, ascorbic acid and allicin. Acta Medica. 2001;44:97–100. [PubMed] [Google Scholar]
- 87.Heimesaat MM, et al. Impact of Campylobacter jejuni cj0268c knockout mutation on intestinal colonization, translocation, and induction of immunopathology in gnotobiotic IL-10 deficient mice. Plos One. 2014;9:e90148. doi: 10.1371/journal.pone.0090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heimesaat MM, Giladi E, Kuhl AA, Bereswill S, Gozes I. The octapetide NAP alleviates intestinal and extra-intestinal anti-inflammatory sequelae of acute experimental colitis. Peptides. 2018;101:1–9. doi: 10.1016/j.peptides.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 89.Heimesaat MM, et al. Survey of extra-intestinal immune responses in asymptomatic long-term Campylobacter jejuni-infected mice. Eur. J. Microbiol. Immunol. 2013;3:174–182. doi: 10.1556/EuJMI.3.2013.3.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free. Radic. Biol. Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.