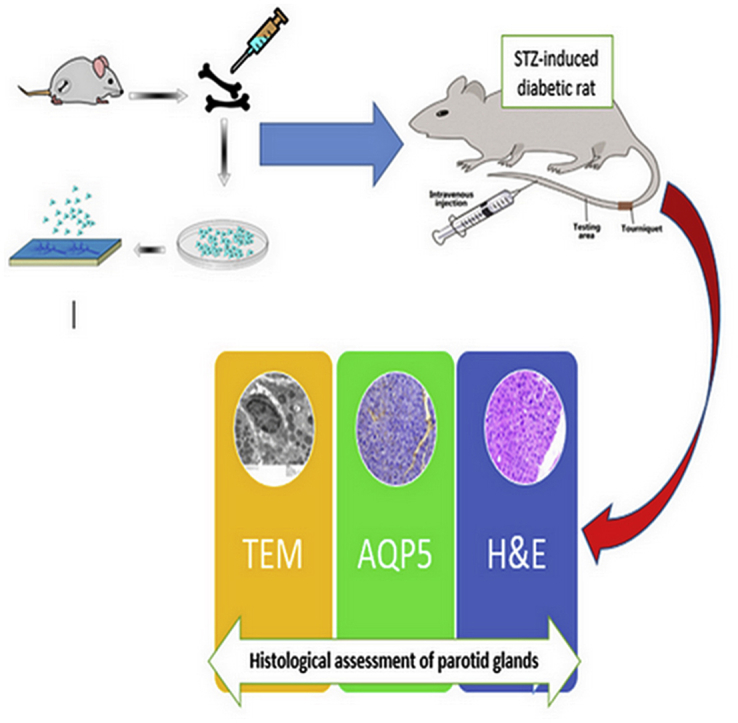
Background
The functional insufficiency of salivary glands constitute the common oral complaints in both type 1 and type 2 diabetes mellitus. The treatment with stem cell could decrease diabetic-induced hyposalivation and improve the quality of life for patients. Objective: The current study designed to assess the biological outcome of systemic injection of stem cells on the parotid salivary gland in streptozotocin induced diabetic. Methods: Twenty-four albino rats received intra-peritoneal injection of 50 mg/kg streptozotocin for induction of diabetes and were divided into two groups (n = 12): Group I (control) were kept without any manipulation, Group II received intravenous injection of 1×106 of mesenchymal stem cells of bone marrow derived for two days. All rats were sacrificed at 1, 3 weeks, then the parotid glands were isolated, fixed and processed for Heamatoxylin and Eosin examination, immunohistochemical staining for aquaporin-5. Results: group I groups showed intracellular cytoplasmic vacuoles and focal loss of salivary architecture, while group II showed maintenance of gland architecture. Immunohistochemical examination of aquaporin-5 showed significant difference between the two groups. Conclusion: bone marrow derived stem cell treatment considers as an improved methods in prevention and treatment of diabeticinduced hyposalivation.
Keywords: Stem cell therapy, Parotid salivary glands, Diabetes mellitus, Hyposalivation, Bone marrow derived stem cell, Aquaporin
Graphical abstract
Highlights
-
•
Salivary dysfunction have been described in diabetes mellitus.
-
•
Water secretion in salivary glands is regulated by called aquaporins on the secretory cells.
-
•
Stem cell-based therapy has great potential in treatment of diabetic-induced hyposalivation.
1. Introduction
Diabetes mellitus (DM) considered as a collection of metabolic syndromes described by hyperglycemia and disorders in the metabolism of carbohydrates, proteins, and lipids.1 It is a glucose insufficiency syndrome characterized by deficiency in the secretion of insulin, which is the key anabolic hormone that create a major role in controlling numerous metabolic pathways, including carbohydrate metabolism, glycogen storage and fatty acid synthesis. There are two common types of DM, type 1 and type 2.2 Type 1 diabetes is described by the total insufficiency of insulin due to the damage of pancreatic beta cells, while type 2 is produced mainly by insulin resistance in the important organs, such as the liver, muscle, and adipose tissue.1
Saliva is a natural secretion which can mirror local and systemic deviations, since the saliva composition is affected by any nutritional, hormonal, immunologic, neurologic, and metabolic changes of the individual.3 The prevalence of caries, periodontal disease, and candida infections can be elevated in persons with raised blood glucose levels.4 DM is supposed to increase exposure to xerostomia, a quantitative and/or qualitative decline of saliva in the oral cavity. Diminished salivary secretion considered as a main cause of problems in the oral cavity by promoting aggregation of bacteria leading to many oral contaminations, severe thirst, change in the taste sensation, and rapid dental caries and bad breath.5
The progenitor stem cells are able to self-renew and differentiate into many cell types in vitro according to the micro-environment. The treatment of T2DM using numerous types of stem cells are still under research. These contain the bone marrow mononuclear stem cell (BM-MNSC), the hematopoietic stem cells, the mesenchymal stem cells (MSCs), the embryonic, the induced pluripotent stem cells, and others.6
The stem cells isolated from the bone marrow can be differentiated to many kinds of cells comprising the hematopoietic precursors, the MSCs and mature differentiated cells. As soon as the bone marrow derived mesenchymal cells are injected, various reactions may occur, the stem cells homing to the bone marrow and directed to the areas of damage then they are triggered to release many cytokines and growth factors.7 The BM-MNSCs can stimulate angiogenesis and vascularization in ischemic areas by paracrine effects,.8 Thus, they possibly activate the endogenous stem cells to proliferate and initiate the healing mechanism.9
Aquaporins (AQPs) form a groups of transmembranous protein channels that constitute the main role of transcellular water permeability. AQPs are permeable to water, and some of them have been penetrable to minutes molecules, containing cations and glycerol, and gases.10 According to AQPs construction and the permeability features, AQPs are divided into traditional AQPs, mainly permeable to water, ions and gases (AQP0, AQP1, AQP2, AQP4, AQP5, AQP6, AQP8).11
Water secretion is regulated by water channel proteins on the secretory cells, called aquaporin-5 (AQP5). It is supposed that any change in AQP5 may cause change in the salivary secretion.12
This study was designed mainly to find whether the diabetes mellitus disturb aquaporin-5 (AQP5) levels in rat parotid salivary gland and if the bone marrow derived stem cells has a protective outcome on diabetic induced morphological changes.
2. Materials and methods
2.1. The experimental animals
24 male white albino rats (200–250 gm body weight) were used in in this study, they were housed in Medical Experimental Research Center (MERC), Mansoura University, Egypt. The animal handling and experimental protocols was approved according to ethical committee for animal care and followed to the roles defined the controlling principle for the animals laboratory procedure in Faculty of Dentistry, Mansoura University (the code number A 16091019). The rats divided into two groups:
Group I (control): consisted of twelve12 rats, which received intra-peritoneal injection of 50 mg/kg streptozotocin for induction of diabetes
Group II (study): consisted of twelve12 rats, that received streptozotocin (STZ) for induction of diabetes as group I, then received an intravenous (I.V) injection of 1×106 of bone marrow cells for two days.13
2.2. Induction of diabetes
After fasting the rats for 18 h, the rats were injected by single intraperitoneal injection (IP) of 50 mg/kg STZ (Sigma Aldrich, Germany), freshly dissolved in dissolved in 0.1 ml citrate buffer (pH = 4.5). The diagnosis of diabetes was confirmed by measuring non fasting blood glucose levels using a glucometer (ACCU-Check, Roche Diagnostics GmbH, Mannheim, Germany). The blood glucose tests were taken 7 days after injection of STZ. The rats exhibiting blood glucose level over 300 mg/dl were used in this study.13
The level of rats’ blood glucose was examined every week and the results were listed in table. Statistical analysis for blood glucose level of all animals was made.
2.3. BM-MSCs manipulation and isolation
The tibiae and femurs of mice were flushed by Dulbecco's modified Eagle's medium (DMEM) added to 10% fetal bovine serum (FBS) - obtained from Lonza company, Swiss-, washed in PBS, suspended in the media enhanced with 1% penicillin–streptomycin, seeded in culture dishes, and incubated at 37 °C in 5% humidified CO2 for 2~3 days in order to develope many colonies. Once large colonies formed (80~90% confluence), cultures were double flushed with PBS and the cells were separated using 0.25% trypsin in 1 mm EDTA for 5 min at 37 °C. The suspension then centrifuged. The cell viability was determined by adding 10 μL trypan blue to 10 μL cell suspensions and mixing. Finally, 10 μL of the mixture was placed in a haemocytometer chamber (Cambridge Instruments, Buffalo, NY, United States), viable and nonviable cells were counted by hemocytometer, and then subculturing viable cells at 4×103 cells/cm2, the third passage were used for experiments.
2.3.1. Characterization
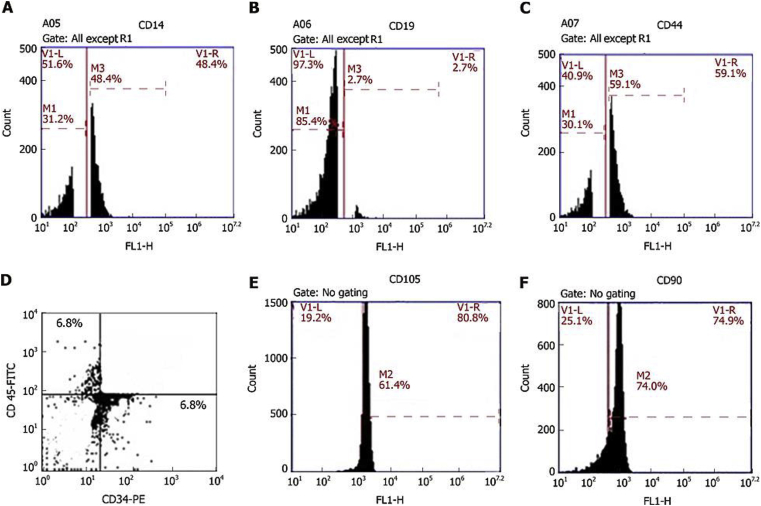
Four million BMSCs were trypsinized and harvested. They were washed and then resuspended in phosphate-buffered saline (PBS) enriched with 3% foetal bovine serum that contained a saturating concentration (1:100) of the six subsequent fluorescein isothiocyanate-conjugated monoclonal antibodies anti-CD14, anti-CD19, anti-CD44, anti-CD45, anti-CD90 and one phycoerythrin-conjugated monoclonal antibody, anti-CD34. The cells were incubated against isotype controls in the dark for 30 min at room temperature. Normal rat IgG peridinin chlorophyll protein complex was used as an isotype control to differentiate nonspecific background signals from specific antibody signals. Then, the cells were washed using 2 mL PBS and centrifuged for 5 min at 1500 rpm, and the resulting supernatant was discarded. The cells were suspended in 0.2 mL of 0.5% paraformaldehyde in PBS. Fluorescein activated cell sorting [(FACS) Canto, BD, United States)] was used for acquisition and analysis of CD34 and CD45, and the data were analysed with BD Cell Quest TM Pro version 6.0 software (dot plot). A BD Accuri C6 flow cytometer was used for the analysis of CD14, CD19, CD44, and CD90, and the data were analysed with BD Accuri C6 program software (histogram plot). All these steps were carried out at the Genetic Department, Children's Hospital, Mansoura University Fig. 1.
Fig. 1.
Plots of the flow cytometric analysis of bone marrow-derived stem cells. A: anti-CD14; B: anti CD19; C: anti-CD44; D: anti-CD34 (FL1-H) and anti-CD45 (FL2-H); E: anti-CD105; F: anti-CD90. A, B, C, E, and F are histograms of the relative fluorescence height (FL1-H) of surface markers against cell count, while D is a dot plot of the relative fluorescence height of the surface marker CD34-PE (FL1-H) against the relative fluorescence height of the surface marker CD45-FITC (FL2-H).
2.4. Bone marrow cells transplantation
After administration of anesthetizia by intraperitoneal injection of 30 mg/kg ketamine and 10 mg/kg xylaine. Rats of group II were treated once by intravenous injection (tail vein) with BM- MSCs at a dose of 1 × 106/10 μl HBSS/bovine serum albumin. While rats of group I was sham treated once by 10 μL HBSS/bovine serum albumin.14
2.5. Biopsy collection
The rats of each group were euthanized by sodium thiopental overdose 40 mg/kg by intrapritoneal injection after 1 and 3 weeks then the parotid glands were removed and fixed immediately in 10% formalin.
2.6. Sections of specimens were prepared for
-
1.
Haematoxylin & Eosin stain
-
2.
Immunohistochemical stains with rabbit anti-AQP5 polyclonal antibody by avidin-biotin complex method.
2.7. Computer digital assisted image analysis
5 slides were taken from every group, 5 random fields from each slides have been examined. Slides were photographed by Olympus® digital camera that was connected on Olympus® microscope with 1/2 X photo adaptor, using 400X objective lens. Photos examined on Intel® Core I3® based computer using Video Test Morphology® software (Russia) with a definite fixed routine for measuring the quantity and the area of staining.
2.8. Statistical analysis
The collected data was evaluated by Statistical Package for Social Science software computer program version 17 (SPSS, Inc., Chicago, IL, USA). The mean and standard deviation express the quantitative data. The study groups were compared using student's t-test. P value less than 0.05 was considered statistically significant.
3. Results
3.1. Haematoxylin & Eosin stain results
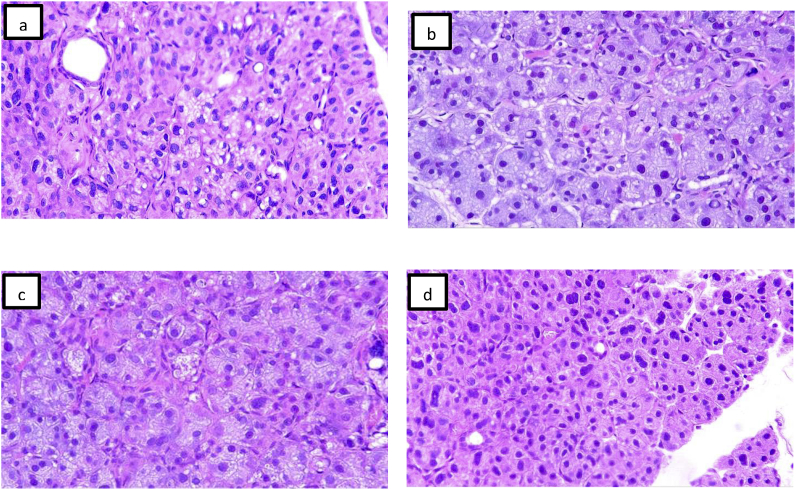
Group I: After 1 week, there were cytoplasmic vacuoles and pyknotic nucleus, focal loss of salivary architecture. After 3 weeks, there was more cytoplasmic vacuoles and pyknotic nucleus with focal areas of degeneration Fig. 2(a and b).
Fig. 2.
Group I (a,b) after one and three weeks after diabetes induction, the parotid glands showed large numbers of cytoplasmic vacuoles with shrunken nuclei and loss of acinar outlines. (c,d) group II after one and three weeks, there were less numbers of cytoplasmic vacuoles and maintained acinar outlines (H&E, X100).
Group II: After 1week: the sections showed less amount of cytoplasmic vacuoles and preserved normal salivary architecture. After 3 weeks: slides showed proper parenchymal architecture consisted of acini and intralobular ducts Fig. 2(c and d).
3.2. Immunohistochemical stains (Anti-aquporin-5) results
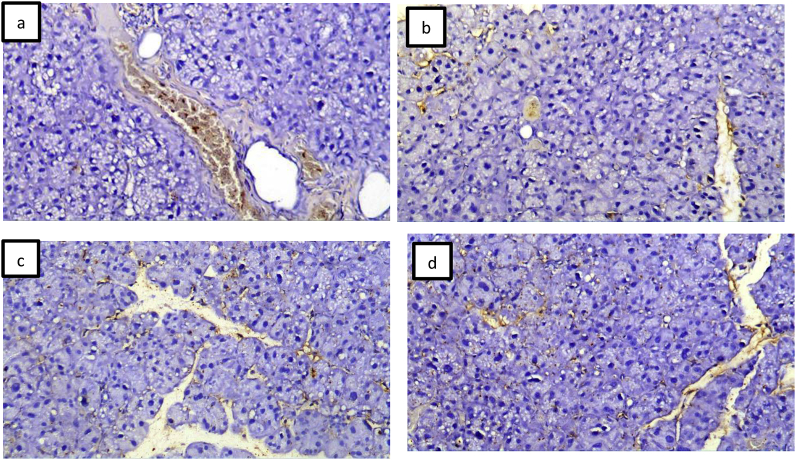
AQP5 was presented on the cellular membrane of the gland acini and cytoplasm of the duct system. After one and three week's group I showed reduction in AQP5 expression compared to groups II Fig. 3.
Fig. 3.
Group I (a, b) showed reduced immunoreactivity with the acini and ducts. Group II (c, d) showed increased immunoreactivity on the cell membranes and cytoplasm of ducts (immunohistochemical stain with AQP5,X100).
3.3. Statistical results
-
a.
Table (1) showing mean ± SD for blood glucose level after diabetic induction and its statistical analysis results for the different groups:
Table 1.
showing mean ± SD for blood glucose level after diabetic induction and its statistical analysis results for the different groups.
| Group I | Group II | ANOVA | |||
|---|---|---|---|---|---|
| F | P value | ||||
| One week | Mean | 384.25 | 191.67 | 20.08 | 0.000 |
| SD | 32.74 | 74.70 | |||
| Two weeks | Mean | 436.38 | 196.17 | 14.9 | 0.000 |
| SD | 42.27 | 150.16 | |||
| Three weeks | Mean | 514.25 | 148.33 | 221.8 | 0.000 |
| SD | 22.11 | 59.54 | |||
Non significant: at P > 0.05.Significant: at P < 0.05.
Non significant: at P > 0.05.Significant: at P < 0.05.
-
b.
Table (2) showing comparison in AQP5 expression between the two studied groups.
Table 2.
Comparison between the two studied groups.
| Control | Study group | t(p) | |
|---|---|---|---|
| 1 week | |||
| Mean ± SD. | 0.52 ± 0.14 | 1.0 ± 0.15 | 5.246a(0.001a) |
| Median (Min. –Max.) | 0.56(0.36–0.68) | 1.04(0.82–1.18) | |
| 3 weeks | |||
| Mean ± SD. | 0.58 ± 0.04 | 1.91 ± 0.17 | 17.093a(<0.001a) |
| Median (Min. –Max.) | 0.59(0.53–0.63) | 1.96(1.71–2.11) | |
t: Student t-test.
p: p value for comparing between the studied groups.
Statistically significant at p ≤ 0.05.
Using Paired student's t-Test for comparing between groups, the test showed a significant difference among two groups at 1 week examination period (t = 5.246, P = 0.001) and at 3 weeks examination period (t = 17.093, P < 0.001).
4. Discussion
Diabetes mellitus (DM) considered one of the most widespread chronic disease. The proper treatment should include carefully controlling to maintain the system and organ vitality, when not properly controlled, it lead to neuropathy, renal diseases, blindness, arteriosclerosis, and glandular dysfunction, comprising the salivary glands.15
The present study, after diabetic induction, parotid salivary glands showed large numbers of cytoplasmic vacuoles with shrunken nuclei and loss of acinar outlines, that comes in accordance with Anderson et al. that early histological studies showed that there were reduction in the size of serous acini of diabetic rats, in addition the diabetic rats showed morphological alterations in gland architecture and dentistry of secretory protein. These comprise the development of autophagic vacuoles and increase numbers of lipid globules.16 Ivanovski et al. reported that patients with diabetes mellitus suffer from damage of salivary gland which lead to decreased amount of saliva than in healthy individuals. Oother authors explain that the lower salivary flow associated with elevation in diuresis or polyuria, a reduction in extracellular fluid and therefore decreased in the saliva formation.17
In healthy persons, the formation of ROS and its neutralization were balanced by the production of antioxidant defense, mostly owing to the defense mechanisms of the cells via formation of antioxidants and antioxidant enzymes, that provide defense of cells and tissues from oxidative damage. In diabetes patients, the formation of the controlling mechanisms could be of significance role in tissues liability to oxidative stress. Also, other studies reported that diabetes stimulate deviations in the antioxidant controlling mechanism in several body tissues.18
In our current study, results revealed more regular glandular architecture of the parotid gland after intravenous injection of BMD stem cells, which comes in acceptance with Khalili et al. in their study on treatment of NOD mice (non-obese diabetic) after induction of Sjogren's-like disease using multipotent mesenchymal stromal cells (MSCs) as a treatment protocol for autoimmune diseases because of the anti-inflammatory and immunostimulator abilities of stem cells.19
Bone marrow derived stem cells can be simply isolated by using needle aspirates, and can be divided in great numbers of multipotent mesenchymal stromal cells (MSCs). Many studies were designed to evaluate the impact of the consumption of MSCs as a new strategy for treatment of autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, and diabetes mellitus.20
In the current work, statistical analysis of blood glucose level indicate significant difference between groups and lowering blood glucose level after BMDSc injection, This could be supported by Fotino et al. who stated that tissue repair and regeneration may be achieved by the use of bone marrow-derived stem cell (BMSC) transplantation, and in stimulating immune reactions. As well as, current clinical investigations are assessing the role of BMSC in improvement the function of beta-cell mass with type 1 and type 2 diabetes.21
Regarding immunohistochemical expression of AQP5 in the parotid glands in cell membrane of acini and cytoplasm of duct system. These results supported by Akamatsu et al. AQP5 positive staining was restricted to the apical membranes of acini.22 It is well recognized that aquaporin-5 (AQP5) is represented in the salivary glands, mostly restricted at the apical membrane of the acinar cells. The physiological significance of AQP5 in transcellular water transfer were explained by decreased salivary flow under pilocarpine injections in AQP5-null mice compared with normal mice. In addition, it has been reported the significance of AQP5 in this transcellular water transfer process, and the stimulation of saliva discharge.23
Statistical analysis of AQP5 showed significance difference among groups at 1 and 3 weeks. These results recommend the potential pathophysiological significance of AQP5 in the salivary glands and accepted by Shikawa et al. that induced diabetic rats were incapable of activation the AQP5 releasing from cell membrane in interlobular duct of parotid glands, the deficiency in AQP5 transferring can be responsible for the formation of xerostomia.23
Other studies described achievements consuming BMDCs for preserving functional activity of salivary gland comes in agreement with Tran and his colleagues who isolate entire BMDCs cells and injected the soluble intracellular components into mice with irradiation-injured SGs. BMDCs were considered as an effective treatment therapy. Also, it comprises only the cell by-products and not whole live BMDCs which transmit the risk of differentiating into unwanted/tumorigenic cell types in SGs.24
The present explanations recommend that there was proper homeostatic non-inflammatory transmission of BMSCs into the parotid glands, which reduce the morphological changes of parotid glands. This experimental transportation was somewhat rapid and might previously be noticed on the first week. In the salivary glands, MSCs mobilized from the bone marrow appeared to donate mainly to the enhancements in tissue performance by local cytokine-mediated interactions with the tissue stem cells.25
Furthermore, the proper mechanism of this enhancement may not explaine absolutely. Tran and coworkers suggested that enhanced salivary gland function resulted from a grouping of several mechanisms, such as cell fusion, vasculogenesis, transdifferentiation, and paracrine signaling. Basically, no chemotactic or mobilizing agents were used in the present study, in order to avoid their various side effects and toxicities.
5. Conclusion
BMDSC stem cells were a valuable means for studying in vivo fate of MSCs as cellular stem cell therapy for salivary glands complications during DM. Profound homeostatic and non-inflammatory movement of MSCs from the bone marrow into the salivary glands increase the salivary secretion and reserve the glandular architecture.
Declaration of competing interest
The authors have no conflict of interest to declare.
Contributor Information
Mona Denewar, Email: monadenewar@gmail.com.
Laila E. Amin, Email: lailaamin@mans.edu.eg, lailalsyd@yahoo.com.
References
- 1.Bakianian Vaziri P., Vahedi M., Mortazavi H., Abdollahzadeh Sh, Hajilooi M. Evaluation of salivary glucose, IgA and flow rate in diabetic patients: a case-control study. J Dent. 2010;7:13–18. [PMC free article] [PubMed] [Google Scholar]
- 2.Motta M., Bennati E., Ferlito L., Passamonte M., Malaguarnera M. Value and significance of new diagnostic criteria of diabetes mellitus in older people. Arch Gerontol Geriatr. 2007;45:103–108. doi: 10.1016/j.archger.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Wolff A., Zuk-Paz L., Kaplan I. Major salivary gland output differs between users and non-users of specific medication categories. Gerodontology. 2008;25:210–216. doi: 10.1111/j.1741-2358.2008.00223.x. [DOI] [PubMed] [Google Scholar]
- 4.Ravindran R., Gopinathan D.M., Sukumaran S. Estimation of salivary glucose and glycogen content in exfoliated buccal mucosal cells of patients with type II diabetes mellitus. J Clin Diagn Res. 2015;9:89–93. doi: 10.7860/JCDR/2015/11633.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro A.M. Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev Diabet Stud. 2012;9:385–406. doi: 10.1900/RDS.2012.9.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezania A., Bruin J.E., Riedel M.J. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H., Park J.S., Choi Y.J. Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem Cell. 2009;27:1686–1696. doi: 10.1002/stem.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duong Van Huyen J.P., Smadja D.M., Bruneval P. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod Pathol. 2008;21:837–846. doi: 10.1038/modpathol.2008.48. [DOI] [PubMed] [Google Scholar]
- 9.Nakano-Doi A., Nakagomi T., Fujikawa M. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cell. 2010;28:1292–1302. doi: 10.1002/stem.454. [DOI] [PubMed] [Google Scholar]
- 10.Musa-Aziz R., Chen L.M., Pelletier M.F., Boron W.F. Relative Co2/NH3 selectivities of AQP1, AQP4, AQP5, amtB, and RhAG. Proc Natl Acad Sci USA. 2009;106:5406–5411. doi: 10.1073/pnas.0813231106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yool A.J. Functional domains of aquaporin-1: keys to physiology, and targets for drug discovery. Curr Pharmaceut Des. 2007;13:3212–3221. doi: 10.2174/138161207782341349. [DOI] [PubMed] [Google Scholar]
- 12.Yeghiazarians Y., Zhang Y., Prasad M., Shih H., Saini S.A., Takagawa J. Injection of bone marrow extract into infarcted hearts result in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250–1256. doi: 10.1038/mt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 14.Dominici M., Le Blanc K., Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Aitken-Saavedra J., Rojas-Alcayaga G., Maturana-Ramírez A. Salivary gland dysfunction markers in type 2 diabetes mellitus patients. J Clin Exp Dent. 2015;7(4):501–505. doi: 10.4317/jced.52329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mednieks M.I., Szczepanski A., Clark B., Hand A.R. Protein expression in salivary glands of rats with streptozotocin diabetes. Int J Exp Pathol. 2009;90:412–422. doi: 10.1111/j.1365-2613.2009.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivanovski K., Naumovski V., Kostadinova M., Pesevska S., Drijanska K., Filipce V. Xerostomia and salivary levels of glucose and urea in patients with diabetes. Prilozi. 2012;33:219–229. [PubMed] [Google Scholar]
- 18.Townsend D.M., Tew K.D., Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalili S., Liu Y., Kornete M. Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjö gren’s-like disease. PloS One. 2012;7(6):e38615. doi: 10.1371/journal.pone.0038615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fotino C., Ricordi C., Lauriola V., Alejandro R., Pileggi A. Bone marrow-derived stem cell transplantation for the treatment of insulin-dependent diabetes. Rev Diabetic Stud. 2010;7(2):144–159. doi: 10.1900/RDS.2010.7.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akamatsu T., Parvin M.N., Murdiastuti K. Expression and localization of aquaporins, members of the water channel family, during development of the rat submandibular gland. Pflügers Archiv. 2003;446:641–651. doi: 10.1007/s00424-003-1109-9. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa Y., Inoue N., Zhenfang Y., Nakae Y. Molecular mechanisms and drug development in aquaporin water channel diseases: the translocation of aquaporin-5 from lipid rafts to the apical plasma membranes of parotid glands of normal rats and the impairment of it in diabetic or aged rats. J Pharmacol Sci. 2004:271–275. doi: 10.1254/jphs.fmj04004x6. [DOI] [PubMed] [Google Scholar]
- 23.Tran S.D., Liu Y., Xia D. Paracrine effects of bone marrow soup restore organ function, regeneration, and repair in salivary glands damaged by irradiation. PloS One. 2013;8 doi: 10.1371/journal.pone.0061632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lombaert I.M., Wierenga P.K., Kok T., Kampinga H.H., deHaan G., Coppes R.P. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Canc Res. 2006;12(6):1804–1812. doi: 10.1158/1078-0432.CCR-05-2381. [DOI] [PubMed] [Google Scholar]
- 25.Lombaert I.M., Wierenga P.K., Kok T., Kampinga H.H., deHaan G., Coppes R.P. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res. 2006;12(6):1804–1812. doi: 10.1158/1078-0432.CCR-05-2381. [DOI] [PubMed] [Google Scholar]