Highlights
-
•
Metastasis of endometrial cancer (EC) to bone is rare, occurring in <1.0% of cases.
-
•
The most common sites of bone metastasis in EC are the spine and hip.
-
•
Diagnosis of bone metastasis is associated with widely metastatic disease and poor prognosis.
-
•
The median overall survival following a diagnosis of bone metastasis was 11 months in our series.
-
•
87.5% of patients with bone metastasis were found to have microsatellite instability.
Keywords: Endometrial cancer, Microsatellite instability, Bone metastasis
Abstract
Metastasis to bone (BM) is an uncommon manifestation of advanced endometrial cancer (EC). The present study will review the clinicopathologic features of a cohort of patients with EC and BM. We conducted a multi-center retrospective review of patients with EC and BM. Demographic and clinical information was extracted from the medical records. Survival outcomes were determined using Kaplan-Meier Curves. Final analysis included 10 patients. The median age was 65 years (range 31–71). 80% had FIGO stage III/IV disease. The most common site of BM was the spine (66%). All patients presented with extraosseous dissemination at the time of diagnosis of BM and 70% were found to have multiple sites of BM. 80% of patients were diagnosed with BM in the recurrent setting. The median time to diagnosis of bone recurrence was 14 months (range: 0–44). Median survival after diagnosis of BM was 11 months (range: 1–22 months). Patients with endometrioid histology and single site of bone metastasis experienced improved survival (p = 0.04 and p = 0.05, respectively). Eight patients had immunohistochemistry or molecular tumor profiles available for review. Seven of these patients (87.5%) were found to have microsatellite instability (MSI). The most common mutation was hypermethylation of MLH-1 (43%). To our knowledge, this is the first report demonstrating a correlation between MSI and metastasis to bone. The identification of BM in EC is uncommon, but will alter treatment strategies and dramatically impact prognosis. Molecular tumor profiling should be performed to identify targeted therapy options and optimize adjuvant treatment strategies.
1. Introduction
Endometrial cancer (EC) represents the most common gynecologic malignancy in the United States, affecting 63,230 patients in 2018 (National Institutes of Health, 2018). The majority of EC is diagnosed at early stage with an overall good prognosis. However, approximately one-third of patients are diagnosed with advanced disease (Siegel et al., 2018, NCCN Guidelines, 2019). Despite excellent outcomes in early stage disease, patients presenting with advanced stage or with aggressive histologic subtypes have a higher incidence of recurrence and subsequently shorter survival. The reported 5-year overall survival (OS) of stage IV disease is a dismal 0–18% (Miller et al., 2012, Goff et al., 1994, Bristow et al., 2000). EC most commonly metastasizes by direct extension or lymphatic spread, and as a result, the majority of recurrences occur locally with in the pelvis and abdomen. Hematogenous spread occurs less frequently and most commonly manifests as lung and liver metastasis (Uccella et al., 2013, Mariani et al., 2001).
Although rare in EC, hematogenous spread to bone is a common feature of many non-gynecologic tumors. In fact, it is the most common site of distant metastasis in both breast and prostate cancer (Kennecke et al., 2010, Bubendorf et al., 2000). The true incidence of bony metastasis (BM) of EC is unknown, however several small series in the literature report an incidence of <1.0% (Uccella et al., 2013). At present, there is little known about the risk factors and pathologic mechanisms leading to development of BM in EC. The majority of affected patients initially present with advanced stage disease and high-grade histology, however most patients meeting these criteria do not go on to develop BM and instead will experience abdominopelvic failure. The present study will review the clinicopathologic features of a cohort of patients with EC metastatic to bone.
2. Methods
This is a multi-center retrospective review of patients with EC metastatic to bone. Institutional Review Board approval was obtained by each site. Tumor board registries from each institution were queried for cases of EC with BM. Inclusion criteria were patients with confirmed histologic diagnosis of primary EC and BM at initial presentation or recurrence. BM were diagnosed on the basis of radiographic or histologic confirmation. Demographic and clinical information extracted from the medical records of eligible patients included age, body mass index (BMI), race, stage, histology, grade, location of bony metastasis, time to bony recurrence, sites of metastatic disease, CA-125 level, treatments and survival status. Pathology reports were reviewed for microsatellite instability (MSI), based on absence of mismatch repair (MMR) genes by immunohistochemistry (IHC). Additionally, when available, molecular tumor profiles were reviewed. Descriptive statistics were employed to detail individual patient outcomes. Survival outcomes were determined using Kaplan-Meier Curves. Statistical significance was defined as P < 0.05. Analyses were performed using SPSS, Version 22.0. (IBM, USA).
3. Results
From 2012 to 2019, there were 1085 patients diagnosed with EC. Ten patients (0.09%) were diagnosed with BM in either the upfront or recurrent setting. There was a total of 32 osseous metastases (range 1–8 per patient). The median age was 65 years (range 31–71). Histology included 70% endometrioid and 30% serous carcinoma. All patients with endometrioid histology had moderately or poorly differentiated tumors. Eighty percent of the patients presented at advanced stage at initial diagnosis, defined as FIGO stage III/IV disease. The remaining two patients presented with FIGO stage I disease. In two patients, the diagnosis of BM was made at time of presentation of EC. In the remaining eight patients, BM were diagnosed at time of recurrence with a mean time of 14.4 months (range: 0–44 months) (Table 1). In five patients, diagnosis was confirmed by bone biopsy and histologic examination consistent with primary endometrial tumor. The remaining five patients were diagnosed on the basis of radiographic findings. 90% of patients presented with bone-related symptoms which prompted a further work up. There was only one incidental diagnosis of bone metastasis that were identified during simulation for adjuvant radiation therapy, after completion of 3 cycles of systemic chemotherapy (Table 2, Patient B). In this patient, bone lesions were not present on pre-operative CT scan, confirming that BM developed during chemotherapy.
Table 1.
Patient characteristics (N = 10).
| Characteristic | N (%)X |
|---|---|
| Age, median (range), years | 65 (31–71) |
| Body mass index, median (range) | 33 (24–48) |
| FIGO Stage at Initial Diagnosis | |
| I | 2 (20) |
| II | – |
| III | 5 (50) |
| IV | 3 (30) |
| Histology | |
| Endometrioid | 7 (70) |
| Serous | 3 (30) |
| FIGO grade* | |
| I | – |
| II | 2 (29) |
| III | 5 (71) |
| Bone metastasis at diagnosis | 2 (20) |
| Bone metastasis at recurrence | 8 (80) |
| Mean time from diagnosis to bone metastasis (months) | 14.4 (0–44) |
| Extent of bone metastasis | |
| Single | 3 (30) |
| Multiple | 7 (70) |
| Concurrent extraosseous metastases | 10 (100) |
| Treatment of bone metastasis | |
| Radiation | 3 (3) |
| Chemotherapy | 1 (10) |
| Chemotherapy + radiation | 2 (20) |
| Concurrent chemoradiation, followed by chemotherapy | 1 (10) |
| Chemotherapy + radiation, followed by immunotherapy | 1 (10) |
| Chemotherapy + anti-angiogenesis | 1 (10) |
| Immunotherapy | 1 (10) |
| Overall survival, median (range), months | 11 (1–22) |
| Presence of microsatellite instability** | 7 (87.5) |
Endometrioid histology only, all other histologies represent high-grade disease.
N = 8 patients with immunohistochemistry or molecular tumor profiles available for review.
Values are number (percentage) unless indicated otherwise.
Table 2.
Patient characteristics and treatment.
| Age | Stage* | Histology/Grade** | Initial Treatment*** | Time from Diagnosis to BM (months) | Site of BM | Other Sites of Metastatic Disease | Molecular Tumor/IHC Profile | CA 125 Level at Diagnosis of BM (u/mL) | Survival Status after BM | |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 59 | IIIC1 | EM/G3 | RA TH BSO PPALND; CT 3C; EBRT + VBT; CT 1C | 9 | Spine: T10-L3 | BL pelvic LN | MSI-high (MLH1 absent); ER, PR positive | 8.4 | DOD, 13 months |
| B | 65 | IIIC2 | EM/G3 | ELAP TAH BSO PPALND; CT 3C | 4 | R sacrum, T3, T11, L3 | L pelvic LN & PA LN, lung | MSI-high (MSH6 absent) | 5.1 | Alive, with disease at 7 months |
| C | 31 | IA | EM/G3 | TAH BS ovarian transposition PPALND | 44 | L acetabulum | L pelvic sidewall mass | NA | 5.3 | Alive, NED at 22 months |
| D | 70 | IVB | EM/G2 | CT 5C, EBRT to L hip; RA TLH BSO PPALND | 0 | R iliac & hemisacrum, L femoral neck | R parametria, BL pelvic LN | MSI-high (MLH1 absent); ER, PR positive | 8.2 | Alive, with disease at 9 months |
| E | 67 | IIIC1 | EM/G2 | TAH BSO PPALND; CT 3C; EBRT; CT 3C | 13 | L iliac | L pelvic LN, vagina | MSI-high (MLH1 absent) | 10.3 | DOD, 17 months |
| F | 66 | IVB | S | CT 2C; palliative EBRT to T6; anastrozole | 0 | L iliac, T6 | Upper abdomen | MSI-high; BRCA positive | 151 | DOD, 6 months |
| G | 59 | IIIB | EM/G3 | ELAP TH BSO PPALND; EBRT; CT 6C | 10 | R iliac, L3-4 | R parametria, liver | MSI-high (MSH 6 absent) | 22.7 | DOD, 11 months |
| H | 71 | IIIC2 | S | ELAP TH BSO PPALND; CT 3C; EBRT; VBT; CT 3C | 12 | L iliac, T7-10, L1, L4, sternum | BL pelvic and PA LN | HER2/neu positive | 15.6 | DOD, 11 months |
| I | 63 | IB | S | ELAP RH BSO; refused adjuvant treatment | 12 | T11 | R pelvic LN, vaginal cuff | NA | NA | Alive, with disease at 3 months |
| J | 64 | IVB | EM/G2 | ELAP TH BSO PPALND omentectomy, CT 4C, PLD 6C | 40 | L1, L3, R 7th rib | Supraclavicular LN, PA & L pelvic LN, lung | MSI-high | 75.3 | Alive, with disease at 3 months |
BL: bilateral; BM: Bone metastasis BSO: bilateral salpingoophorectomy; C: cycles of chemotherapy; CT: carboplatin paclitaxel; DOD: died of disease; EBRT: external beam radiation therapy; ELAP: exploratory laparotomy; EM: Endometrioid; IHC: immunohistochemistry; L: left; NA: not available; NED: no evidence of disease; PA: paraaortic; PLD: Pegylated liposomal doxorubicin. PPALND: pelvic and paraaortic lymphadenectomy; R: right; RH: radical hysterectomy; TH: total hysterectomy; S: serous; VBT: vaginal brachytherapy.
Staging based on FIGO 2009 staging system.
Endometrioid only, other histologies all represent high grade disease.
Treatment modalities listed in sequence of treatment.
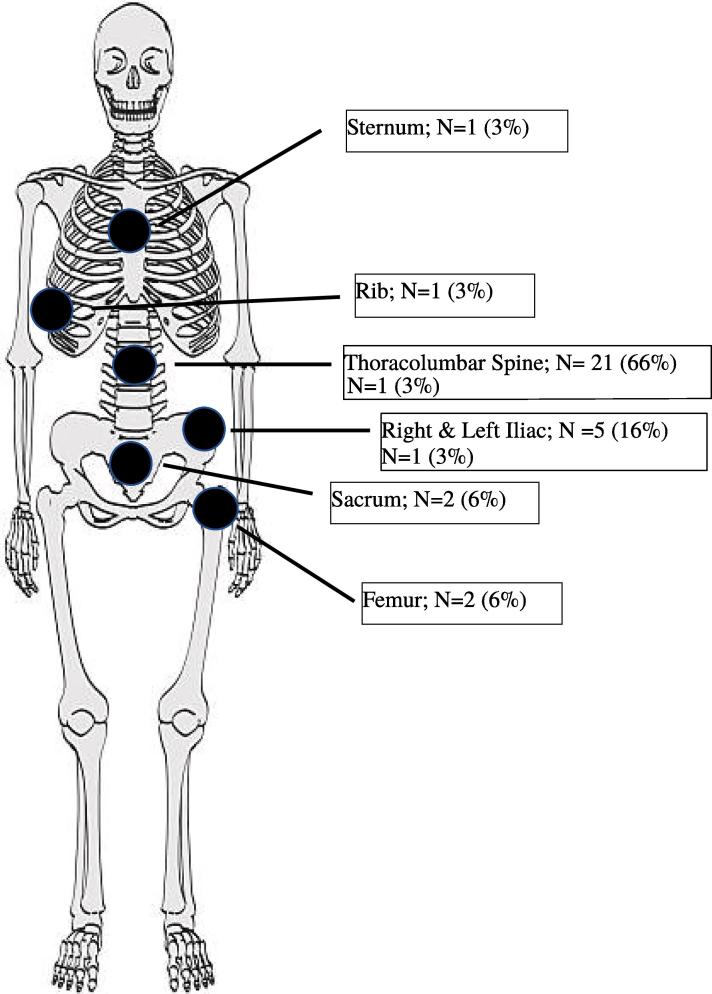
The most common sites of bony metastasis were the spine (66%) and the hip (22%). Six patients (60%) presented with distant bony failure, defined as bone metastasis outside of the pelvic bones or lumbar spine (Fig. 1). All patients presented with extraosseous dissemination at the time of diagnosis of bony metastasis. Multiple bony metastasis were identified in 7 (70%) patients. CA-125 level was elevated in only 2 (20%) patients. Of the eight patients presenting with BM in the recurrent setting, five were treated with platinum-based therapy in the upfront setting and all were resistant or refractory to platinum. Of these five patients, two were found to be platinum resistant with a mean time of 4.5 months from chemotherapy completion to the development of bone disease. The remaining three developed BM during platinum-based treatment.
Fig. 1.
Location of bone metastasis (N = 32).
Median overall survival (OS) after diagnosis of BM was 11 months (range: 1–22 months). Four patients are currently alive, all undergoing treatment, and one is alive with no evidence of disease. There was no difference in survival between those patients presenting with distant BM versus BM confined to the pelvis (p = 0.13). Patients with endometrioid histology experienced improved survival compared to serous histology (OS not yet achieved vs. 6 months; p = 0.04). We observed a trend toward improved OS in patients with single versus multiple lesions (OS not yet achieved vs. 9 months; p = 0.05).
Eight patients had MMR IHC or molecular tumor profiles available for review. Seven of these patients were found to have MSI (87.5%). The most common individual mutation was hypermethylation of MLH-1 which was identified in three patients (43%). Five MSI-high patients underwent germline testing, which were all negative. Two patients refused germline testing. Additionally, one patient was found to be positive for HER2/neu by IHC and confirmed by FISH (fluorescent in situ hybridization). A wide variety of treatment modalities were used in the management of BM. Three (30%) received radiation alone and three (30%) received a combination of chemotherapy and external beam radiation. Treatment was individualized for the remaining four patients including, single-agent pembrolizumab, EBRT chemotherapy followed by pembrolizumab, systemic chemotherapy alone and systemic chemotherapy with bevacizumab (Table 3).
Table 3.
Location and treatment of bone metastasis.
| Patient | Location of BM | Treatment of BM | Response to Treatment |
|---|---|---|---|
| A | Spine: T10-L3 | EBRT | PR, followed by progression and abdominopelvic metastasis at 9 months |
| B | R sacrum, T3, T11, L3 | Gemcitabine + bevacizumab | PR, currently receiving gemcitabine + bevacizumab |
| C | L acetabulum | Concurrent chemoradiation: EBRT + cisplatin, followed by carboplatin + paclitaxel | CR, currently under observation off therapy |
| D | R iliac & hemisacrum, L femoral neck | EBRT, followed by carboplatin + paclitaxel, followed by pembrolizumab | PR, currently receiving pembrolizumab |
| E | L iliac | EBRT | PR, followed by progression at 4 months |
| F | L iliac, T6 | EBRT | PD, with progression in the upper abdomen at 3 months |
| G | R iliac, L3-4 | PLD | SD, followed by progression in the bone and upper abdomen at 4 months |
| H | L iliac, T7-10, L1, L4, sternum | EBRT, followed by PLD | PR, followed by progression in the retroperitoneum at 5 months |
| I | T11 | EBRT, followed by carboplatin + paclitaxel | PR, currently receiving carboplatin + paclitaxel |
| J | L1, L3, R 7th rib | Pembrolizumab | SD, currently receiving pembrolizumab |
BM: Bone metastasis; CR: complete response; EBRT: External beam radiation therapy; PLD: pegylated liposomal doxorubicin; PD: progressive disease, PR: partial response; SD: stable disease.
4. Discussion
BM is an uncommon finding in EC. Several small retrospective series have reported an incidence of only 0.8% (Uccella et al., 2013, Keheo et al., 2010, Ghosh and Rao, 2015). Interestingly, the reported incidence of subclinical BM is up to 25% in an anatomopathological studies of patients with EC undergoing autopsy. It should be noted however that in these studies, the vast majority of patients harbored metastasis at numerous sites, including the liver and lungs (Abdul-Karim et al., 1990). These findings suggest that hematogenous dissemination to bone represents a late metastatic pattern and signifies advanced, aggressive disease.
The most commonly reported locations of BM are the spine and pelvic bones (Kennecke et al., 2010). Consistent with these reports, all patients in the current study presented with metastasis at one or both of these locations. Seven patients presented with metastasis to the pelvic bones, including the sacrum and iliac bones, and six patients presented with metastasis to the spine. Additionally, four patients presented with a combination of both pelvic bone and spinal metastasis. When comparing the location of initial disease to the location of bone metastasis, all patients with pelvic bone involvement were found to have positive pelvic lymph nodes on the ipsilateral side. This suggests that despite the fact that BM is considered a hematologic phenomenon, the close proximity of other metastatic disease may imply local factors influence disease spread.
Although there is no “tumor marker” specific to EC, CA-125 has been utilized to help predict the extent of disease at presentation and in some cases to monitor for recurrence (NCCN Guidelines, 2019). Retrospective data demonstrates correlation between elevated CA-125 and the presence of lymph node metastasis as well as advanced stage disease in patient with EC (Hoon Chung et al., 2006, Yildiz et al., 2012). Interestingly, only two patients in our cohort displayed an elevation in CA 125. One (patient J) had an elevated, but down-trending CA 125 at the time of development of BM. This is likely due to the theory that CA-125 increases as a result of intra-peritoneal processes (Rump et al., 2004). Therefore, metastasis outside the abdomen, including the bone, would not necessarily produce an abnormality in this serum analyte. Nevertheless, it is still interesting based on the fact that the majority of patients in the present report had concurrent disease at both bone and intra-abdominal locations. Normal CA-125 does not preclude the possibility of metastatic disease.
The prognosis of patients with EC found to have metastatic disease to the bone is extremely poor with dismal median OS of only 10–12 months (Uccella et al., 2013, Keheo et al., 2010). Consistent with the present study, the presence of multiple BM has been associated with worse prognosis (Uccella et al., 2013). An additional factor utilized to stratify prognosis is the degree of “platinum-sensitivity” or lack thereof (Pfisterer and Ledermann, 2006). Although this designation system originates from ovarian cancer literature, it has also been applied to EC and which, like ovarian cancer, demonstrates a direct correlation with platinum-free interval and survival (Nagao et al., 2013). In the present study, six patients were either resistant or refractory to platinum-based regimens, defined as recurrence within 6-months of platinum-based therapy or progression on platinum therapy, respectively. This demonstrates the aggressive nature of tumors metastasizing and suggests they are less likely to respond to traditional regimens.
Although there is limited data on the management of EC metastatic to bone, a large body of literature exists regarding management of BM in breast cancer (BC). Treatment strategies include systemic therapy, bone modifying agents (BMA), radiation, surgery and analgesia. Utilization of these modalities vary based on overall disease burden, time of presentation (primary versus recurrent disease) and symptoms. The primary goal of systemic therapy is control and mitigation of disease, conversely the goal of radiation, surgery and analgesia is largely palliative (Van Poznak et al., 2017). BMA have been studied extensively in BC, specifically the bisphosphonates zoledronic acid and pamidronate, and the RANK-L inhibitor denosumab. The primary use of these medications is to prevent skeletal related adverse events (SRE), including fracture, hypercalcemia, surgery to bone and spinal cord compression, which will affect up to 65% of untreated patients and contribute to decreased quality of life (Van Poznak et al., 2017, Stopeck et al., 2010). Although there is no prospective literature on the use of BMA in gynecologic cancer, the use of these agents maybe warranted in patients at high risk for SRE. This is demonstrated by our patient who developed a femur neck fracture at the location of BM (patient D).
To our knowledge, no study has correlated molecular tumor profiles of EC with BM. In the present study, eight patients had IHC or molecular tumor profile data available. Of these patients, seven (87.5%) were found to have microsatellite instability (MSI). MSI is a result of loss of function of mismatch repair (MMR) genes, which leads to accumulation of single base-pair mismatches, as well as small insertions and deletion in tandem repeats. MSI is identified in 20–40% of endometrial carcinomas, the majority of which are due to sporadic loss of function, rather than germline mutations observed in hereditary nonpolyposis colorectal cancer (HNPCC) syndrome (Black et al., 2006, Kwon et al., 2011). Generally, these tumors are associated with endometrioid histology, however, the relationship between MSI and clinical outcomes in EC is not clearly defined. Multiple studies have reported improvement in OS in patients with MMR deficient tumor compared to their MMR proficient counterpart receiving the same adjuvant therapy. However, others have observed no difference or worse survival in MMR deficient tumors (Black et al., 2006, Kwon et al., 2011, Shikama et al., 2016, McMeekin et al., 2016). Additionally, there are conflicting reports in the literature regarding the correlation between MSI status and stage of disease at diagnosis. Several studies report no correlation, while other investigators report a more advanced disease stage at diagnosis associated with MSI high tumors (Black et al., 2006, Kwon et al., 2011, Shikama et al., 2016, McMeekin et al., 2016). The later corresponds to the present study, in which all MSI – high patients presented with stage III and IV disease. A recent large data base analysis of patients with colorectal cancer (CRC) from Australia addressed the risk of metastasis to specific distant sites, including lung and bone, in relation to the presence of MSI. However, they found no difference in patterns of metastatic disease between MSS and MSI CRC (Prasanna et al., 2018). Although these findings in CRC cannot be generalized to EC, these findings are worth noting due to the lack of literature available regarding patterns of metastasis of MSI-high EC.
At present, there is no standardized therapy for patients with EC metastatic to bone. Limited data is available and suggests that multimodality therapy is associated with improved outcomes, however these findings did not achieve statistical significance (Uccella et al., 2013). As demonstrated in the current study, a variety of modalities have been utilized based on physician preference and experience, as well as individualization based on the patient’s specific disease. Current data demonstrates that MSI – high EC exhibit good response to programmed cell death ligand 1 (PD-L1) inhibition. A recent large prospective study examined the effects of pembrolizumab on MMR deficient tumors previously treated with at least one line of standard therapy. They observed an overall response rate of 54% with an additional 23% of patients experiencing stable disease (Le, 2017). Our cohort also included one patient who tested positive for HER2/neu. Literature in BC demonstrates HER2/neu positive patients carries a higher propensity for bony spread compared to HER2/neu negative cancer (Kennecke et al., 2010). Additionally, a recent phase II evaluation of the addition of trastuzumab to carboplatin-paclitaxel demonstrates superior outcomes in HER2/neu positive serous EC compared to chemotherapy alone (Fader et al., 2018). These findings illustrate potential therapeutic pathways for patients with BM.
Although bone metastasis in endometrial carcinoma are uncommon occurrences, they should always be considered in the differential diagnosis of patients presenting with known EC and bone pain. The identification of bone metastasis will alter treatment strategies as well has dramatically impact prognosis. Consideration should be given to the incorporation of bone modifying agents to decrease skeletal related adverse events and improve quality of life. Additionally, molecular tumor profiling should be performed to identify targeted therapy options and optimize adjuvant treatment strategies.
CRediT authorship contribution statement
Jennifer McEachron: Conceptualization, Writing - original draft, Formal analysis, Investigation. Carolyn Chatterton: Formal analysis, Writing - original draft. Victoria Hastings: Investigation. Constantine Gorelick: Conceptualization, Resources. Katherine Economos: Conceptualization, Resources. Yi-Chun Lee: Conceptualization, Writing - review & editing, Resources. Marguax J. Kanis: Conceptualization, Writing - review & editing, Resources.
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
References
- Abdul-Karim F.W., Kida M., Wentz W.B., Carter J.R., Sorensen K., Macfee M. Bone metastasis from gynecologic carcinomas: a clinicopathologic study. Gynecol. Oncol. 1990;39(2):108–114. doi: 10.1016/0090-8258(90)90414-g. [DOI] [PubMed] [Google Scholar]
- Black D., Soslow R.A., Levine D.A., Tornos C., Chen S.C., Hummer A.J. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J. Clin. Oncol. 2006;24(11):1745–1752. doi: 10.1200/JCO.2005.04.1574. [DOI] [PubMed] [Google Scholar]
- Bristow R.E., Zerbe M.J., Rosenshein N.B., Grumbine F.C., Montz F.J. Stage IVB endometrial carcinoma: the role of cytoreductive surgery and determinants of survival. Gynecol. Oncol. 2000;78:85–91. doi: 10.1006/gyno.2000.5843. [DOI] [PubMed] [Google Scholar]
- Bubendorf L., Schopfer A., Wagner U., Sauter G., Moch H., Willi N., Gasser T.C., Mihatsch M.J. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Human Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- Fader A.N., Roque D.M., Siegel E., Buza N., Hui P., Abdelghany O. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous caricnomas that overexpress human epidermal growth factor receptor 2/neu. J. Clin. Oncol. 2018;36(30):2044–2051. doi: 10.1200/JCO.2017.76.5966. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Rao P.B. Osseous metastases in gynecological epithelial malignancies: a retrospective institutional study and review of the literature. J. Clin. Diagnostic Res. 2015;9(12):XC10–XC13. doi: 10.7860/JCDR/2015/15063.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B.A., Goodman A., Muntz H.G., Fuller A.F., Jr, Nikrui N., Rice L.W. Surgical stage IV endometrial carcinoma: a series of 47 cases. Gynecol. Oncol. 1994;52:237–240. doi: 10.1006/gyno.1994.1038. [DOI] [PubMed] [Google Scholar]
- Hoon Chung H., Weon Kim J., Park N., Song Y., Sang S., Lee H. Use of preoperative serum CA-125 levels for prediction of lymph node metastasis and prognosis in endometrial cancer. Acta Obstetricia et Gynecologic Scandinavica. 2006;85:1501–1505. doi: 10.1080/00016340601022777. [DOI] [PubMed] [Google Scholar]
- Keheo S.M., Zivanovic O., Ferguson S.E., Barakat R.R., Soslow R. Clinicopathologic features of bone metastases and outcomes in patients with primary endometrial cancer. Gynecol. Oncol. 2010;117:229–233. doi: 10.1016/j.ygyno.2010.01.047. [DOI] [PubMed] [Google Scholar]
- Kennecke Hagen, Yerushalmi Rinat, Woods Ryan, Cheang Maggie Chon U., Voduc David, Speers Caroline H., Nielsen Torsten O., Gelmon Karen. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- Kwon J.S., Scott J.L., Gilks C.B., Daniels M.S., Sun C.C., Lu K.H. Testing women with endometrial cancer to detect Lynch syndrome. J. Clin. Oncol. 2011;29(16):2247–2252. doi: 10.1200/JCO.2010.32.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le MMR deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017 doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani A., Wbb M.J., Keeney G.L., Calori G., Podratz K.C. Hematogenous dissemination in corpus cancer. Gynecol. Oncol. 2001;80(2):233–238. doi: 10.1006/gyno.2000.6058. [DOI] [PubMed] [Google Scholar]
- McMeekin D.S., Tritchler D.L., Cohn D.E., Mutch D.G., Lankes H.A., Geller M.A. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2016;34(25):3026–3068. doi: 10.1200/JCO.2016.67.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D., Filiaci V., Gleming G., Mannel R., Cohn D., Matsumoto T., Tewari K. Randomized phase II noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol. Oncol. 2012;125(3):771. [Google Scholar]
- Nagao S., Nishio S., Michimae J., Tanabe H., Okada S., Otsuki T. Applicability of the concept of “platinum sensitivity” to recurrent endometrial cancer: The SGSG-012/GOTIC-004/Intergroup study. Gynecol. Oncol. 2013;131(3):567–573. doi: 10.1016/j.ygyno.2013.09.021. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, 2018. Cancer Facts: Uterine cancer. Surveillance, Epidemiology, and End Results Program.
- NCCN Guidelines, 2019. Uterine Neoplasms. Version 2.2019.
- Pfisterer J., Ledermann J.A. Management ofplatinum-sensitive recurrent ovarian cancer. Semin. Oncol. 2006;33:S12–S610. doi: 10.1053/j.seminoncol.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Prasanna T., Karapetis C.S., Roder D., Tie J., Padbury R., Price T. The survival outcome of patients with metastatic colorectal cancer based on site of metastases and impact of molecular markers and site of primary cancer on metastatic patter. Acta Oncol. 2018;15(11):1438–1444. doi: 10.1080/0284186X.2018.1487581. [DOI] [PubMed] [Google Scholar]
- Rump A., Morikawa Y., Tanaka M., Minami S., Umesaki N., Takeuchi M., Miyajima A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004;279(10):91980–91988. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- Shikama A., Minaguchi T., Matsumoto K., Akiyama-Abe A., Nakamura Y., Michikami H. Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas. Gynecol. Oncol. 2016;140(2):226–233. doi: 10.1016/j.ygyno.2015.11.032. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H. Denosumab compared with zolendronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol. 2010;28(35):5132–5138. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- Uccella S., Morris J.M., Bakkum-Gamez J.N., Kenney G.L., Podratz K.C., Mariani A. Bone metastases in endometrial cancer: report on 19 patients and review of the medical literature. Gynecol. Oncol. 2013;130:474–482. doi: 10.1016/j.ygyno.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Poznak, C., Somerfield, M.R., Barlow, W.E., Sybil Beirmann, B.J., Bosserman, L.D., Clemons, M.J., et al. 2017. Role of Bone-Modifying Agents in Metastatic Breast Cancer: an American Society of Clinical Oncology – Cancer Care Ontario Focused Guideline Update. 35 (35), 3978–3986. [DOI] [PubMed]
- Yildiz A., Yetimalar H., Kasap B., Aydin C., Tatar S. Euro. J. Obstet. Gynecol. Reprod. Biol. 2012;164(2):191–195. doi: 10.1016/j.ejogrb.2012.05.038. [DOI] [PubMed] [Google Scholar]