Abstract
Background
Subclinical hyperthyroidism/thyrotoxicosis originates from different causes and clinical conditions, sharing the laboratory constellation of a suppressed TSH in the presence of thyroid hormone concentrations within the reference range.
Aim
Presentation of hyperthyroidism can manifest itself in several ways. We questioned whether there is either a consistent biochemical equivalence of thyroid hormone response to these diagnostic categories, or a high degree of heterogeneity may exist both within and between the different clinical manifestations.
Methods
This secondary analysis of a former prospective cross-sectional trial involved 461 patients with untreated thyroid autonomy, Graves’ disease or on levothyroxine (LT4) after thyroidectomy for thyroid carcinoma. TSH response and biochemical equilibria between TSH and thyroid hormones were contrasted between endogenous hyperthyroidism and thyrotoxicosis (LT4 overdose).
Results
Concentrations of FT4, FT3, TSH, deiodinase activity and BMI differed by diagnostic category. Over various TSH strata, FT4 concentrations were significantly higher in LT4-treated thyroid carcinoma patients, compared to the untreated diseases, though FT3 levels remained comparable. They were concentrated in the upper FT4- but low deiodinase range, distinguishing them from patients with thyroid autonomy and Graves’ disease. In exogenous thyrotoxicosis, TSH and FT3 were less responsive to FT4 concentrations approaching its upper normal/hyperthyroid range.
Conclusions
The presence or lack of TSH feedforward activity determines the system response in the thyroid-active (hyperthyroidism) and no-thyroid response to treatment (thyrotoxicosis). This rules out a consistent thread of thyroid hormone response running through the different diagnostic categories. TSH measurements should therefore be interpreted conditionally and differently in subclinical hyperthyroidism and thyrotoxicosis.
Keywords: Subclinical hyperthyroidism, Thyrotoxicosis, Deiodinase
Introduction
Subclinical hyperthyroidism is a common thyroid disorder with a prevalence of up to 10% [1], [2], [3], [4], [5]. The current definition of subclinical hyperthyroidism is solely laboratory-based. Diagnosis is confirmed by measurement of a below-reference TSH value and thyroid hormone concentrations within their respective ranges [6], [7]. If either FT4 or FT3 concentrations exceed their respective upper reference limits, diagnosis is upgraded to overt hyperthyroidism. Although the laboratory constellation is the same in various conditions its causes differ. They include hyperactivity of the thyroid gland due to activating mutations in the path of the TSH receptor (toxic adenoma), hyper-stimulation of the thyroid gland by TSH receptor antibodies (Graves’ disease) or human chorionic gonadotropin (pregnancy), release of thyroid hormones from damaged thyroid tissue (thyroiditis) and exogenous intake of the drug levothyroxine (LT4) [5], [8]. The condition arising from glandular overproduction of thyroid hormones is conventionally termed hyperthyroidism, whereas the term thyrotoxicosis encompasses any other cause of the hormone excess [9]. Some authors prefer to focus on the clinical disorder instead of the biochemistry, as implied in the adjective “subclinical” [6]. Their argument is relevant because the treatment of subclinical hyperthyroidism is specifically guided by its cause [5], [10]. In contrast, many prognostic studies reporting on future outcomes and risks associated with single historic TSH measurements failed to account for this clinical distinction by disease [11], [12], [13], [14]. From a statistical perspective, this omission predictably leads to amalgamation bias whenever the averaged outcome of a heterogenous group is not shared among the members of that group [15], [16]. Clinical issues may arise if the relationship between TSH and thyroid hormones is altered in different conditions, so that TSH levels are inconsistent and deviate from the underlying thyroid hormone status [17].
Assuming the actual presentation of hyperthyroidism can manifest itself in several ways, it is of interest to examine such situations to discover if there is either a consistent thread of thyroid hormone response running through these groups, or if not, whether there exists a high degree of heterogeneity both within and between manifestations. This study examines the biochemically heterogeneous nature of subclinical hyperthyroidism/ thyrotoxicosis, comparing the interrelationships and equilibria between FT3, FT4 and TSH in three clinical entities (diagnostic categories), namely thyroid autonomy (toxic adenoma), Graves’ disease and thyrotoxicosis on LT4 therapy.
Materials and methods
Patients
The study group was part of a former prospective cross-sectional trial [18]. This secondary analysis involves 461 patients with untreated thyroid autonomy (toxic adenoma), untreated Graves’ disease and LT4-treated patients with thyroid carcinoma (Table 1). The trial was ethically approved and registered (www.ClinicalTrials.gov, NCT 01969552). Participants gave written informed consent. Thyroid autonomy was diagnosed by combined sonographic and scintigraphic imaging showing solitary or multiple hyperactive (“hot”) nodules. Diagnosis of Graves’ disease was initially established by clinical presentation (hyperthyroidism), thyroid imaging (typical hypoechogenic and hypervascularised ultrasound pattern and additional measurement of Technetium-99 m-Pertechnetate uptake in nine doubtful cases), and the presence of laboratory-based autoimmune markers at any time, not necessarily at presentation for the prospective study, namely elevated TSH receptor antibodies (TSH-R Ab), and facultatively measured thyroid peroxidase antibodies (TPO Ab). Thyroid carcinoma patients had all been thyroidectomised and 94% additionally received radioiodine treatment. Appropriate measurements were obtained when thyroid hormones were non-hypothyroid on stable LT4 medication. Patient history, thyroid medication and other drugs, age, BMI, laboratory tests (FT3, FT4, TSH), antibody status (TSH-R Ab, TPO Ab in suspected thyroid autoimmune disease only) and thyroid imaging were documented.
Table 1.
Characteristics of study group.
| Parameter | Untreated Toxic adenoma n = 258 | Untreated Graves’ disease n = 62 | LT4-treated carcinoma n = 141 | P value |
|---|---|---|---|---|
| Gender female/male | 71% / 29% | 73% / 27% | 67% / 33% | 0.85 |
| Age years | 65.0 [55, 74] | 53.0 [45, 62] | 54.0 [47, 62] | <0.001 |
| BMI kg/m2 | 26.3 [23.9, 29.5] | 26.2 [23.4, 27.9] | 28.4 [24.8, 32.2] | 0.001 |
| Thyroid volume ml | 26.0 [17.0, 43] | 20.0 [14.5, 29.8] | 0 [0, 0] | <0.001 |
| Weight-adjusted LT4 dose µg/kg | – | – | 1.69 [1.52, 2.02] | – |
| TSH within range/suppressed | 23% | 31% | 36% | 0.02 |
| 77% | 69% | 64% | ||
| FT4 within range/hyperthyroid | 96% | 86% | 74% | <0.001 |
| 4% | 14% | 26% | ||
| FT3 within range/hyperthyroid | 92% | 79% | 95% | <0.001 |
| 8% | 21% | 5% | ||
| FT3 pmol/l | 5.30 [4.96, 5.81] | 5.29 [5.06, 6.08] | 5.10 [4.70, 5.70] | 0.002 |
| FT4 pmol/l | 15.3 [13.6, 17.0] | 15.7 [14.3, 18.3] | 20.6 [18.8, 23.2] | <0.001 |
| TSH mIU/l | 0.25 [0.10, 0.38] | 0.29 [0.01, 0.44] | 0.15 [0.02, 0.75] | 0.81 |
| Deiodinase activity nmol/s | 32.6 [29.3, 36.6] | 32.7 [29.2, 38.3] | 23.2 [20.9, 25.5] | <0.001 |
| TPO Ab IU/ml | – | 68.1 [36.8, 558] | – | – |
| TSR-R Ab IU/l | – | 1 [0.20, 3.5] | – | – |
Data are median [IQR] or percentages. Significance was tested with Kruskal-Wallis or chi-square test, respectively. Range refers to the reference range.
Definition of hyperthyroidism/thyrotoxicosis
Subclinical hyperthyroidism/thyrotoxicosis was conventionally biochemically defined, and it was confirmed by measurement of a below-reference TSH value and thyroid hormone concentrations still within their respected reference range. Overt hyperthyroidism/ thyrotoxicosis was diagnosed if either FT4 or FT3 concentrations or both exceeded their respective upper reference limit while TSH was suppressed below its reference range. A distinction was made between hyperthyroidism and thyrotoxicosis. The former was caused by an overactive thyroid gland and endogenous thyroid hormone overproduction, the latter due to exogenous LT4 intake and drug overdosing.
Laboratory methods
TSH was measured with an automated direct chemiluminescence method (TSH3-Ultra ADVIA Centaur XP, Siemens Healthcare Diagnostics, Erlangen, Germany). The standard curve was calibrated with the 3rd WHO Standard for hTSH (IRP 81/565). Functional sensitivity was 0.008 mIU/l, intra-assay variation 1.4% to 2.4%, and inter-assay imprecision 0.9% to 2.9%. FT3 and FT4 were measured on the same platform, showing intra-assay CVs from 2.4% to 3.1% or 2.2% to 3.3% and inter-assay CVs from 2.3% to 3.9% or 2.5% to 4.0%, respectively. Assay performance characteristics have been reported [19]. Laboratory-evaluated reference intervals were as follows, 0.4 to 4 mIU/l for TSH, 3.1 to 6.8 pmol/l for FT3, 10 to 23 pmol/l for FT4.
TSH-R Abs were measured with an ELISA (EUROIMMUN AG, Lübeck, Germany), TPO Abs with a competitive chemiluminescence method (ADVIA Centaur XP, Siemens Healthcare Diagnostics, Erlangen, Germany). Reference ranges were for TSH-R Ab <2 IU/l and for TPO Ab <60 IU/ml.
Global deiodinase activity
SPINA-GD provides a measure of the maximum global activity of peripheral step-up deiodinases per unit of time (nmol/s) referred to as deiodinase activity and estimated from equilibrium levels of FT3, FT4 and constants for plasma protein binding, distribution and elimination with
Unlike the FT3-FT4 ratio, SPINA-GD accounts for non-linear enzyme kinetics, whilst not discriminating between the different types of deiodinases.
Thyroid ultrasound
Thyroid size, nodularity and echographic pattern were routinely documented in all subjects. Ultrasound relied on a 10 MHz transducer. The volume of each thyroid lobe was estimated according to a rounded ellipsoid formula (longitudinal diameter × width × depth × 0.5 cm3), and the summation of the lobe volumes yielded the thyroid volume. Reference values are <18 ml for female and <25 ml for male subjects. A thyroid volume <1 ml was regarded as athyreotic.
Thyroid scintigraphy
Technetium-99 m-Pertechnetate was used for thyroid scintigraphy. Imaging was performed using a parallel hole collimated gamma camera 20 min after application of 65–75 MBq of the tracer. In patients with larger thyroid nodule(s) (≥1 cm), it permitted to distinguish between hypofunctioning (“cold”) and hyperfunctioning nodules (“hot nodules”, toxic adenoma). Uptake measurements were also done in a few patients to differentiate Graves’ disease from autoimmune thyroiditis.
Statistical methods
Descriptive data are shown as median and interquartile range (IQR). TSH values were natural logarithmically transformed when indicated (lnTSH). Between-group comparisons for continuous variables were based on Wilcoxon’s rank-sum test for two groups or Kruskal-Wallis test for three groups. Chi-squared test was used to compare frequency variables. Correlations were based on Kendall’s tau rank correlation. To compare the curvilinear relationships between the diagnostic categories multivariable non-linear (third degree polynomial) models were fitted using maximum likelihood estimators and profiled 95% confidence intervals. Models were tested for interactions yielding complex inter-related responses and adjusted for covariates such as gender, age and BMI. For presentation, to visualise the complex interactions, the data were divided into clinically meaningful strata. All tests were two-sided with p < 0.05 denoting statistical significance. Variables were considered explanatory and no adjustments were made for multiple comparisons. The R statistical software package (version 3.6.1 for Mac) was used for all statistical tests [22].
Results
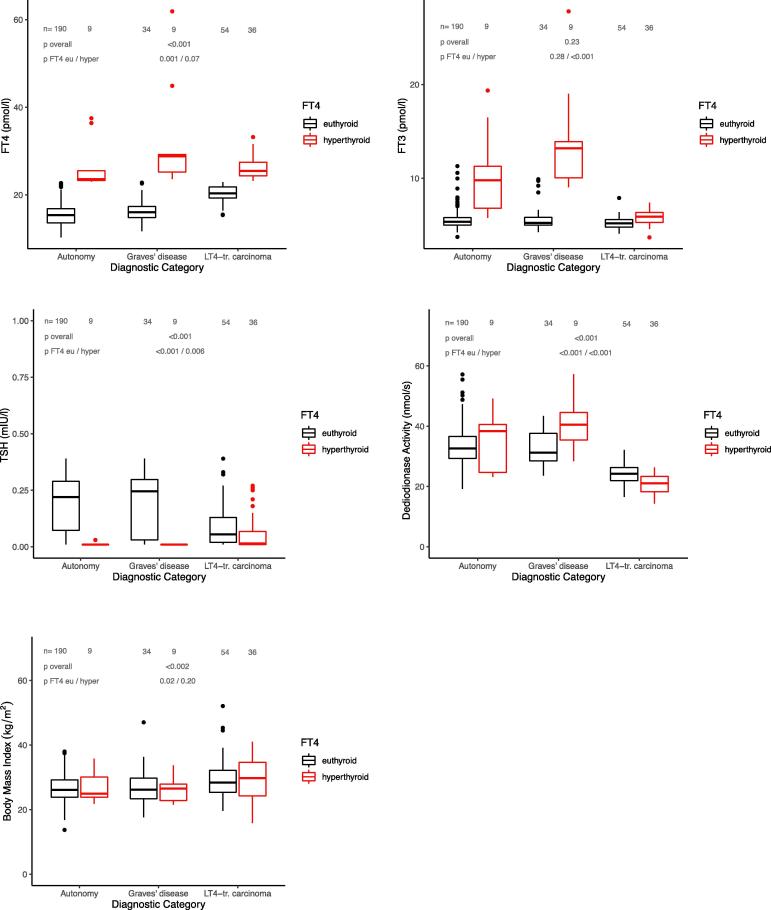
The characteristics of the prospectively sampled study group involving 461 patients with untreated thyroid autonomy, untreated Graves’ disease or on levothyroxine (LT4) after thyroidectomy for thyroid carcinoma are shown in Table 1. TSH levels were suppressed below the lower reference limit (<0.4 mIU/l) in 332 patients and in the euthyroid range in 129 patients, while FT4 concentrations were elevated in 56 patients, and FT3 concentrations in 41 patients. Thyroid parameters for each diagnostic category are shown in Table 1. Hyperthyroid FT4 concentrations unaccompanied by FT3 excess were more prevalent in the treatment category (Table 1, Fig. 1).
Fig. 1.
a–d. Thyroid parameters and body mass index in subclinically or overtly hyperthyroid patients (TSH < 0.4 mIU/l) with toxic adenoma, Graves’ disease or LT4-treated carcinoma, stratified by their FT4 concentration (euthyroid vs hyperthyroid). The hormonal patterns differed between the treatment categories. FT4 concentrations were higher in the treated patients, deiodinase activity was markedly lower, but FT3 concentrations similar. Body mass index was also different between diagnostic categories despite “normal” FT4. Statistical comparison between etiological groups overall and in FT4-subgroups is based on Kruskal-Wallis test.
Examining only patients with suppressed TSH (n = 332), hormonal parameters across the three diagnostic categories were as follows in untreated toxic adenoma (n = 199), untreated Graves’ disease (n = 43), and LT4-treated carcinoma (n = 90) in that order, FT4: 15.5 [13.6, 17.3], 16.7 [15.4, 21.9], 22.0 [20.1, 24.8] pmol/l, p < 0.001, FT3: 5.40 [5.02, 5.92], 5.50 [5.08, 9.13], 5.50 [5.00, 6.00] pmol/l, p = 0.23, TSH: 0.21 [0.05, 0.28], 0.18 [0.01, 0.29], 0.04 [0.01, 0.12] mIU/l, p < 0.001, deiodinase activity: 32.6 [29.3, 36.9], 32.0 [28.8, 38.8], 23.2 [20.9, 25.3] nmol/s, p < 0.001. BMI was also different among the diagnostic groups 26.0 [23.9, 29.2], 26.2 [23.1, 29.0], 28.9 [24.9, 33.5] kg/m2, p = 0.002. Fig. 1 shows the data further stratified by euthyroid vs hyperthyroid FT4 concentrations.
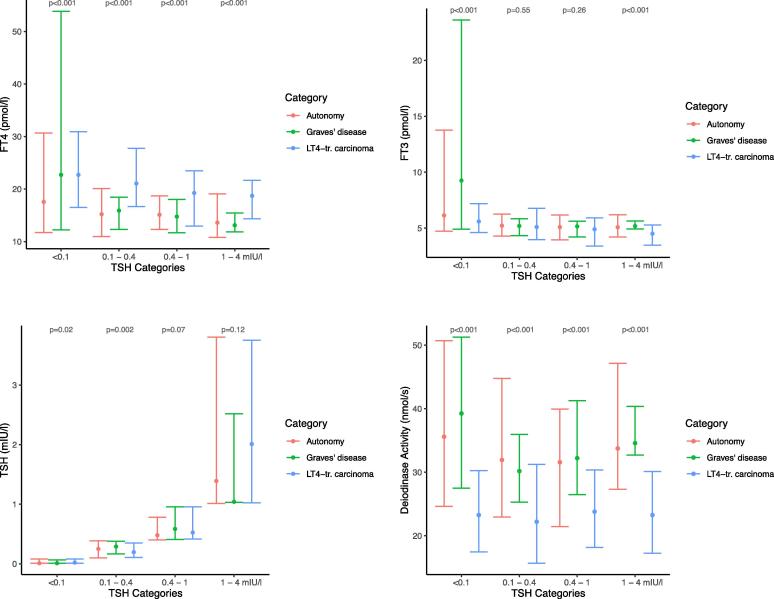
FT3 response was predicted from either TSH or FT4 concentrations using a non-linear regression model. Diagnostic category and the thyroid parameters interacted significantly (p < 0.001), suggesting that their combined influence is rather complex. This remained true when adjusting the models for gender, age and BMI (data not shown). To interpret and visualise the complex interactions data were stratified by a combination of clinically meaningful TSH bands and diagnostic categories (Fig. 2). FT4 concentrations were significantly higher in all TSH categories in LT4-treated thyroid carcinoma patients, compared to the untreated diseases, but deiodinase activity was substantially decreased (Fig. 2). FT3 levels remained comparable (Fig. 2).
Fig. 2.
a–d. FT4, FT3, TSH, and deiodinase activity stratified by diagnostic categories (thyroid autonomy, Graves’ disease, LT4-treated carcinoma) and TSH categories. The median and 95% confidence interval of the data is shown. Statistical difference between the disease categories in each TSH band was tested with Kruskal-Wallis test.
In patients with Graves’ disease, deiodinase activity was significantly positively correlated with TSH-R Ab titers (tau = 0.45, p < 0.001).
BMI was significantly lower over the full TSH range in the untreated diseases, compared to the LT4-treated condition (on average −2.1 kg/m2 [95%CI −1.1, −3.2], p < 0.001). After adjusting for lnTSH, age and gender the mean difference was −2.2 kg/m2 [95%CI −1.2, −3.3], p < 0.001.
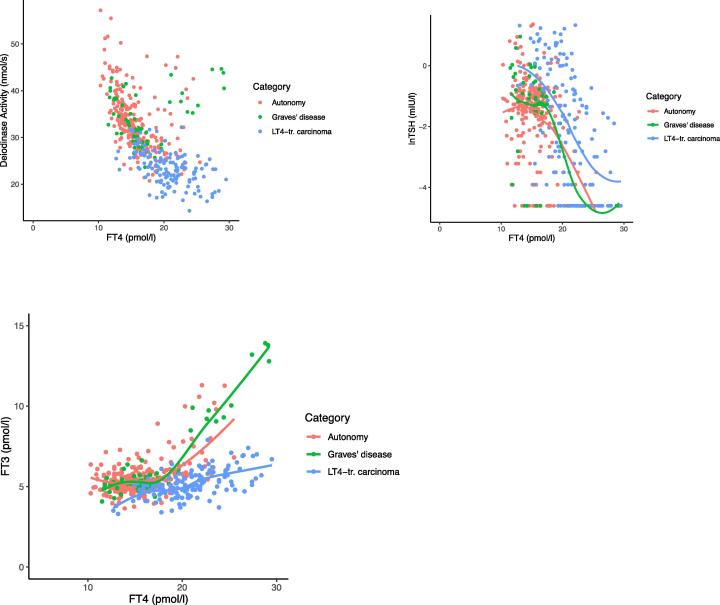
When plotting deiodinase activity against FT4 concentrations, LT4-treated patients were concentrated in the upper FT4 - low deiodinase range, visibly distinguishing them from patients with thyroid autonomy and Graves’ disease (Fig. 3a). Their TSH response to FT4 was significantly shifted to the right (Fig. 3b, lnTSH 1.1 mIU/l [95%CI 0.76, 1.43], p < 0.001). As a consequence of the decreased deiodinase activity, the non-linear response curve of FT3 to increasing FT4 concentrations was shifted to the right and markedly flatter (p < 0.001) toward the upper normal or hyperthyroid FT4 range in the LT4-treated patients (Fig. 3c).
Fig. 3.
a–c. Distribution of deiodinase activity (a) and relationship between TSH (b) or FT3 (c) and FT4, stratified by diagnostic category (thyroid autonomy, Graves’ disease, LT4-treated carcinoma). LT4-treated patients were concentrated in the upper FT4- but low deiodinase range, distinguishing them from the other two categories. Response curves for TSH and FT3 were derived by locally weighted scatterplot smoothing, and are significantly (p < 0.001) shifted between the diagnostic categories (see Results).
Discussion
Subclinical hyperthyroidism is not an etiological or clinical entity, rather a mix of different conditions sharing the same laboratory constellation, namely a suppressed TSH in the presence of thyroid hormone concentrations within the reference range. The present study shows that a high degree of biochemical heterogeneity exists both within and between the different manifestations of subclinical hyperthyroidism/thyrotoxicosis. This rules out the assumption of a consistent biochemical equivalence of the thyroid hormone response running through the different clinical entities. Rather our findings support a clear distinction between hyperthyroidism caused by an endogenously overactive thyroid gland and exogenous thyrotoxicosis (over-treatment with LT4). While the two conditions share a suppressed TSH the biochemical relationships between TSH and thyroid hormones differ widely between them.
Deiodinase activity was markedly reduced in thyrotoxicosis, compared to hyperthyroidism, owing to a lack of TSH stimulation, inhibition by LT4 and the absence of a functioning thyroid gland [23]. This deficiency of athyreotic patients, affecting their ability to convert the supplied T4 into the biologically more active T3, has been previously recognised [24], [25], [26]. It results in a disjoint between TSH, FT4 and FT3, and equivalent FT3 levels equilibrate with much lower TSH values and higher FT4 concentrations post thyroidectomy on LT4 replacement, compared to the preoperative natural state [25]. This, in turn, compromises the utility of TSH as a marker of true euthyroidism in these patients [26]. Pathophysiologically, the shifted equilibria may be explained by the loss of feedforward control, i.e. the lacking TSH-dependent up-regulation of both glandular T3 secretion and deiodinase activities in the absence of a functioning thyroid gland [27], [28], [29]. Although direct TSH control is also relinquished in hyperthyroidism, a strong feedforward signal on glandular T3 production and up-regulation of deiodinase activity is maintained in both Graves’ disease by TSH receptor antibodies substituting for the pituitary hormone TSH and toxic adenomas through TSH- or G protein- mutations activating the TSH receptor [30]. This is however not the case in thyrotoxicosis, where the lack of thyroidal feedforward activity results in relational dysbalance between the controlling element TSH and the peripheral thyroid hormones FT4 and FT3 [23]. In patients with Graves’ disease, we showed in this study that deiodinase activity correlated positively with antibody titers. In addition, genetic factors have also been reported to influence deiodinase activity and FT3 concentrations, and CW7-carriers in particular showed the highest FT3 levels in stress-triggered Graves’ disease [31].
The present findings show that the homeostatic equilibria are expressed differently in exogenous thyrotoxicosis, compared to hyperthyroidism. The FT3 response to increasing FT4 concentrations was both shifted and also considerably less responsive in LT4-treated patients. Unlike patients with endogenous hyperthyroidism, LT4-treated patients showed no acceleration of their T3 generation toward the upper normal or hyperthyroid FT4 range and lacked the surge in serum FT3 levels observed in the former conditions (Fig. 3). It is important to note that the enzymatic deiodination of T4 that generates T3 follows a nonlinear function based on Michaelis-Menten-Hill kinetics, thereby producing an exponential rise in FT3 concentrations when the availability of substrate increases. This became apparent as FT4 progressed into the upper part of its reference interval, but well before it exceeded that range. The clinical relevance of the FT3 surge may be inferred from large populations studies, particularly the Rotterdam Study [32], [33], [34]. Although FT3 concentrations were not directly measured, this study documented an elevated risk of atrial fibrillation associated with increasing FT4 concentrations in the upper reference range [34].
Athyreotic patients lack the apparatus, mechanisms and control of T3 feedforward activity [23]. As a result, FT3 generation from a similar amount of T4 falls short in those patients, compared to patients with an overactive thyroid. Intracellular T3 levels, which could only be measured in experimental animals, were equally found to be inadequate in various tissues despite TSH normalisation on LT4 replacement [35], [36]. This may, at least in part, explain observed differences in the expression of the clinically hyperthyroid phenotype including weight and body mass index between the disease categories in this study. Though not examined in the present study relief of hypothyroid symptoms and complaints of hyperthyroid symptoms have been linked to conversion efficiency and achieved concentrations of circulating FT3 on LT4 medication [37].
Shifted responses and wide individual variations may require - unless the lacking amount of T3 is directly substituted - acceptance of FT4 concentrations above their upper reference limit and/or suppressed TSH levels in a substantial portion of patients without a functioning thyroid remnant, in order to raise their FT3 concentration sufficiently [38]. Another prospective study measuring serum biochemical markers in patients on LT4 following total thyroidectomy extends the clinical relevance of our findings by demonstrating that markers were closest to euthyroid in patients with mildly suppressed TSH levels, as opposed to those with normal or strongly suppressed TSH [39]. Conversely, application of risk profiles of such patients should give results vastly different from endogenous hyperthyroidism because the T4 excess is dissociated from the T3 response, unaccompanied by the FT3 surge present in endogenous hyperthyroidism.
While numerous studies reported on adverse cardiovascular outcomes and bone loss in association with subclinical hyperthyroidism, partly with conflicting outcomes, the underlying risk-relationship has not been well defined [14], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]. However, a consistent thyroid hormone response running through the different clinical entities sharing a suppressed TSH can be ruled out (Fig. 3). Risk profiles associated with the laboratory constellation of subclinical hyperthyroidism/ thyrotoxicosis can therefore be expected to vary considerably, depending on the cause of the condition and the particular relation of TSH with the underlying thyroid hormones [54]. Predictably, reported prognostic outcomes were inconsistent in large population studies when using either TSH or thyroid hormones in the risk assessment [33], [34].
Long-term risks of elevated FT4 concentrations in LT4-treated patients in the absence of TSH suppression and without T3 excess are largely unknown. While documenting an increased cardiovascular and mortality risk in association with high normal FT4 concentrations in the presence of normal TSH, the Rotterdam study included an insufficient sample of treated patients to inform about the risks of thyrotoxicosis [34]. In a large retrospective cohort study, patients with differentiated thyroid carcinoma had a 2.5-fold increased risk for atrial fibrillation, compared to the control group, which was independent from established risk factors, and this was remarkably not associated with TSH levels [44]. A retrospective case-control study in US veterans found that osteoporosis, but not fractures, was more common in patients with thyroid cancer than controls [55]. The effect of low TSH was small, compared to other major influences, such as female gender and older age, and lower TSH was only associated with osteoporosis, but not with increased fracture risk [55]. A small study in a high-risk group of postmenopausal women with thyroid carcinoma showed that trabecular bone scores were lower in cases, compared to controls, but did not significantly change on suppressive LT4-treatment during a 4-year (median) follow-up, nor did bone mineral density measured by DEXA [56]. These studies suggest that the long-term risk of TSH suppressive therapy for the deterioration of bone status is far less than that of untreated hyperthyroidism, presumably due to different T3 effects. Apart from TSH-associated risk, T4 increase has been directly linked to growth promotion of malignant tumours, as it enhances non-classical hormone signalling through the integrin receptor [57], [58]. A low ratio of FT3 to FT4 was independently correlated with various adverse outcomes, most strongly dyslipidaemia [59].
Our findings contrast the thyroid-active (hyperthyroidism) and no-thyroid response to treatment (thyrotoxicosis), determined either by activation or lack of the physiological feedforward path on T3. Outcome measures cannot therefore rely on the assumption of response equivalence, which would require a consistent translation of the signalling chain from TSH to TSH receptor, FT4 and finally onto FT3. This questions the utility of TSH measurement alone as a clinical predictor in subclinical hyperthyroidism/ thyrotoxicosis, rather rendering it strongly dependent on the underlying cause and clinical entity. This is similar to the hypothyroid situation where the decision whether to treat or not to treat has been separated from the definition and diagnosis of subclinical hypothyroidism [60].
Conclusion
The present study demonstrates a biochemically heterogenous expression of hormone activities in subclinical/overt hyperthyroidism and exogenous thyrotoxicosis. From a clinical, biochemical and statistical perspective, the two conditions can and should be clearly distinguished, particularly when assessing risks in association with TSH measurement. Our findings support calls from other authors to abandon the term subclinical hyperthyroidism which refers solely to a laboratory constellation, putting stronger emphasis on etiological and clinical entities.
Data availability
Clinical data are available from the original prospective study (www. ClinicalTrials.gov, NCT 01969552) [18]. Deidentified data will be shared with investigator support for similar projects.
Contributions
RL collected clinical data, RH drafted the manuscript, JEM, RL and JWD contributed additional ideas and text to the final jointly approved article.
Disclosure Statement
JWD is co-owner of the intellectual property rights for the patent “System and Method for Deriving Parameters for Homeostatic Feedback Control of an Individual” (Singapore Institute for Clinical Sciences, Biomedical Sciences Institutes, Application Number 201208940-5, WIPO number WO/2014/088516). RH, JEM and RL declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
CRediT authorship contribution statement
Rudolf Hoermann: Conceptualization, Methodology, Data curation, Formal analysis, Writing - original draft, Visualization. John E.M. Midgley: Conceptualization, Writing - review & editing. Rolf Larisch: Investigation, Data curation, Writing - review & editing. Johannes W. Dietrich: Conceptualization, Writing - review & editing.
Acknowledgement
None.
References
- 1.Vanderpump M.P., Tunbridge W.M., French J.M., Appleton D., Bates D.M., Clark F. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf). 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 2.Hollowell J.G., Staehling N.W., Flanders W.D., Hannon W.H., Gunter E.W., Spencer C.A. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National health and nutrition examination survey (NHANES 3) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 3.Rosario P.W. Natural history of subclinical hyperthyroidism in elderly patients with TSH between 0.1 and 0.4 miu/l: A prospective study. Clin Endocrinol (Oxf) 2010;72:685–688. doi: 10.1111/j.1365-2265.2009.03696.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooper D.S., Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142–1154. doi: 10.1016/S0140-6736(11)60276-6. [DOI] [PubMed] [Google Scholar]
- 5.Carlé A., Andersen S.L., Boelaert K., Laurberg P. Management of endocrine disease: Subclinical thyrotoxicosis: prevalence, causes and choice of therapy. Eur J Endocrinol. 2017;176:R325–R337. doi: 10.1530/EJE-16-0276. [DOI] [PubMed] [Google Scholar]
- 6.Wiersinga W.M. Guidance in subclinical hyperthyroidism and subclinical hypothyroidism: are we making progress. Eur Thyroid J. 2015;4:143–148. doi: 10.1159/000438909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross D.S., Burch H.B., Cooper D.S., Greenlee M.C., Laurberg P., Maia A.L. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 8.Charkes N.D. The many causes of subclinical hyperthyroidism. Thyroid. 1996;6:391–396. doi: 10.1089/thy.1996.6.391. [DOI] [PubMed] [Google Scholar]
- 9.Braverman LE, Utiger RD. Introduction to thyrotoxicosis. In: Braverman LE, Utiger RD, eds. J.B. Lippincott Company; 1995;645–647.
- 10.Biondi B., Kahaly G.J. Cardiovascular involvement in patients with different causes of hyperthyroidism. Nat Rev Endocrinol. 2010;6:431. doi: 10.1038/nrendo.2010.105. [DOI] [PubMed] [Google Scholar]
- 11.Völzke H., Schwahn C., Wallaschofski H., Dörr M. Review: The association of thyroid dysfunction with all-cause and circulatory mortality: is there a causal relationship? J Clin Endocrinol Metab. 2007;92:2421–2429. doi: 10.1210/jc.2007-0179. [DOI] [PubMed] [Google Scholar]
- 12.Ochs N., Auer R., Bauer D.C., Nanchen D., Gussekloo J., Cornuz J. Meta-analysis: Subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med. 2008;148:832–845. doi: 10.7326/0003-4819-148-11-200806030-00225. [DOI] [PubMed] [Google Scholar]
- 13.Rieben C., Segna D., Da Costa B.R., Collet T.H., Chaker L., Aubert C.E. Subclinical thyroid dysfunction and the risk of cognitive decline: a meta-analysis of prospective cohort studies. J Clin Endocrinol Metab. 2016;101:4945–4954. doi: 10.1210/jc.2016-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumgartner C., Da Costa B.R., Collet T.H., Feller M., Floriani C., Bauer D.C. Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation. 2017;136:2100–2116. doi: 10.1161/CIRCULATIONAHA.117.028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran P.J., Bauer D.J. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol. 2011;62:583–619. doi: 10.1146/annurev.psych.093008.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher A.J., Medaglia J.D., Jeronimus B.F. Lack of group-to-individual generalizability is a threat to human subjects research. Proc Natl Acad Sci U S A. 2018;115:E6106–E6115. doi: 10.1073/pnas.1711978115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoermann R., Midgley J.E.M., Larisch R., Dietrich J.W. Recent advances in thyroid hormone regulation: toward a new paradigm for optimal diagnosis and treatment. Front Endocrinol (Lausanne) 2017;8:364. doi: 10.3389/fendo.2017.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoermann R., Midgley J.E.M., Giacobino A., Eckl W.A., Wahl H.G., Dietrich J.W. Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment. Clin Endocrinol (Oxf) 2014;81:907–915. doi: 10.1111/cen.12527. [DOI] [PubMed] [Google Scholar]
- 19.Larisch R., Giacobino A., Eckl W.A., Wahl H.G., Midgley J.E.M., Hoermann R. Reference range for thyrotropin. Post hoc assessment. Nuklearmedizin. 2015;54:112–117. doi: 10.3413/Nukmed-0671-14-06. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich J.W., Landgrafe G., Fotiadou E.H. TSH and thyrotropic agonists: Key actors in thyroid homeostasis. J Thyroid Res. 2012;2012 doi: 10.1155/2012/351864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietrich J.W., Landgrafe-Mende G., Wiora E., Chatzitomaris A., Klein H.H., Midgley J.E.M. Calculated parameters of thyroid homeostasis: emerging tools for differential diagnosis and clinical research. Front Endocrinol (Lausanne) 2016;7:57. doi: 10.3389/fendo.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2019; ISBN 3-900051-07-0. Available from https://www.R-project.org/.
- 23.Hoermann R., Midgley J.E.M., Larisch R., Dietrich J.W. Relational stability in the expression of normality, variation, and control of thyroid function. Front Endocrinol (Lausanne) 2016;7:142. doi: 10.3389/fendo.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gullo D., Latina A., Frasca F., Le Moli R., Pellegriti G., Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0022552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito M., Miyauchi A., Morita S., Kudo T., Nishihara E., Kihara M. TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy. Eur J Endocrinol. 2012;167:373–378. doi: 10.1530/EJE-11-1029. [DOI] [PubMed] [Google Scholar]
- 26.Hoermann R., Midgley J.E.M., Larisch R., Dietrich J.W. Is pituitary TSH an adequate measure of thyroid hormone-controlled homoeostasis during thyroxine treatment. Eur J Endocrinol. 2013;168:271–280. doi: 10.1530/EJE-12-0819. [DOI] [PubMed] [Google Scholar]
- 27.Ishii H., Inada M., Tanaka K., Mashio Y., Naito K., Nishikawa M. Induction of outer and inner ring monodeiodinases in human thyroid gland by thyrotropin. J Clin Endocrinol Metab. 1983;57:500–505. doi: 10.1210/jcem-57-3-500. [DOI] [PubMed] [Google Scholar]
- 28.Citterio C.E., Veluswamy B., Morgan S.J., Galton V.A., Banga J.P., Atkins S. De novo triiodothyronine formation from thyrocytes activated by thyroid-stimulating hormone. J Biol Chem. 2017;292:15434–15444. doi: 10.1074/jbc.M117.784447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berberich J., Dietrich J.W., Hoermann R., Müller M.A. Mathematical modeling of the pituitary-thyroid feedback loop: role of a TSH-T3-shunt and sensitivity analysis. Front Endocrinol (Lausanne) 2018;9:91. doi: 10.3389/fendo.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vassart G., Dumont J.E. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- 31.Vita R., Lapa D., Trimarchi F., Vita G., Fallahi P., Antonelli A. Certain HLA alleles are associated with stress-triggered Graves’ disease and influence its course. Endocrine. 2017;55:93–100. doi: 10.1007/s12020-016-0909-6. [DOI] [PubMed] [Google Scholar]
- 32.Yeap B.B., Alfonso H., Hankey G.J., Flicker L., Golledge J., Norman P.E. Higher free thyroxine levels are associated with all-cause mortality in euthyroid older men: The health in men study. Eur J Endocrinol. 2013;169:401–408. doi: 10.1530/EJE-13-0306. [DOI] [PubMed] [Google Scholar]
- 33.Heeringa J., Hoogendoorn E.H., Van Der Deure W.M., Hofman A., Peeters R.P., Hop W.C.J. High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch Int Med. 2008;168:2219–2224. doi: 10.1001/archinte.168.20.2219. [DOI] [PubMed] [Google Scholar]
- 34.Chaker L., Heeringa J., Dehghan A., Medici M., Visser W.E., Baumgartner C. Normal thyroid function and the risk of atrial fibrillation: the Rotterdam study. J Clin Endocrinol Metab. 2015;100:3718–3724. doi: 10.1210/jc.2015-2480. [DOI] [PubMed] [Google Scholar]
- 35.Escobar-Morreale H.F., Obregón M.J., Escobar Del Rey F. Morereale De Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. 1995;96:2828–2838. doi: 10.1172/JCI118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werneck De Castro J.P., Fonseca T.L., Ueta C.B., Mcaninch E.A., Abdalla S.M., Wittmann G. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest. 2015;125:769–781. doi: 10.1172/JCI77588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larisch R., Midgley J.E.M., Dietrich J.W., Hoermann R. Symptomatic relief is related to serum free triiodothyronine concentrations during follow-up in levothyroxine-treated patients with differentiated thyroid cancer. Exp Clin Endocrinol Diabetes. 2018;126:546–552. doi: 10.1055/s-0043-125064. [DOI] [PubMed] [Google Scholar]
- 38.Hoermann R., Midgley J.E.M., Larisch R., Dietrich J.W. Individualised requirements for optimum treatment of hypothyroidism: Complex needs, limited options. Drugs Cont. 2019;8:1–18. doi: 10.7573/dic.212597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito M., Miyauchi A., Hisakado M., Yoshioka W., Ide A., Kudo T. Biochemical markers reflecting thyroid function in athyreotic patients on levothyroxine monotherapy. Thyroid. 2017;27:484–490. doi: 10.1089/thy.2016.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collet T.-H., Gussekloo J., Bauer D.C., Den Elzen W.P.J., Cappola A.R., Balmer P. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Int Med. 2012;172:799–809. doi: 10.1001/archinternmed.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gencer B., Collet T.-H., Virgini V., Bauer D.C., Gussekloo J., Cappola A.R. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040–1049. doi: 10.1161/CIRCULATIONAHA.112.096024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biondi B., Wartofsky L. Treatment with thyroid hormone. Endocr Rev. 2014;35:433–512. doi: 10.1210/er.2013-1083. [DOI] [PubMed] [Google Scholar]
- 43.Abrahamsen B., Jørgensen H.L., Laulund A.S., Nybo M., Brix T.H., Hegedüs L. Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures - the OPENTHYRO register cohort. J Bone Miner Res. 2014;29:2040–2050. doi: 10.1002/jbmr.2244. [DOI] [PubMed] [Google Scholar]
- 44.Klein Hesselink E.N., Lefrandt J.D., Schuurmans E.P., Burgerhof J.G.M., Groen B., Gansevoort R.T. Increased risk of atrial fibrillation after treatment for differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2015;100:4563–4569. doi: 10.1210/jc.2015-2782. [DOI] [PubMed] [Google Scholar]
- 45.Inoue K., Tsujimoto T., Saito J., Sugiyama T. Association between serum thyrotropin levels and mortality among euthyroid adults in the United States. Thyroid. 2016;26:1457–1465. doi: 10.1089/thy.2016.0156. [DOI] [PubMed] [Google Scholar]
- 46.Chaker L., Baumgartner C., Den Elzen W.P.J., Collet T.-H., Ikram M.A., Blum M.R. Thyroid function within the reference range and the risk of stroke: An individual participant data analysis. J Clin Endocrinol Metab. 2016;101:4270–4282. doi: 10.1210/jc.2016-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaker L., Korevaar T.I.M., Rizopoulos D., Collet T.H., Völzke H., Hofman A. Defining optimal health range for thyroid function based on the risk of cardiovascular disease. J Clin Endocrinol Metab. 2017;102:2853–2861. doi: 10.1210/jc.2017-00410. [DOI] [PubMed] [Google Scholar]
- 48.Aubert C.E., Floriani C., Bauer D.C., Da Costa B.R., Segna D., Blum M.R. Thyroid function tests in the reference range and fracture: individual participant analysis of prospective cohorts. J Clin Endocrinol Metab. 2017;102:2719–2728. doi: 10.1210/jc.2017-00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hung C.L., Yeh C.C., Sung P.S., Hung C.J., Muo C.H., Sung F.C. Is partial or total thyroidectomy associated with risk of long-term osteoporosis: a nationwide population-based study. World J Surg. 2018;42:2864–2871. doi: 10.1007/s00268-018-4573-2. [DOI] [PubMed] [Google Scholar]
- 50.Siru R., Alfonso H., Chubb S.A.P., Golledge J., Flicker L., Yeap B.B. Subclinical thyroid dysfunction and circulating thyroid hormones are not associated with bone turnover markers or incident hip fracture in older men. Clin Endocrinol (Oxf) 2018;89:93–99. doi: 10.1111/cen.13615. [DOI] [PubMed] [Google Scholar]
- 51.Lillevang-Johansen M., Abrahamsen B., Jørgensen H.L., Brix T.H., Hegedüs L. Over- and under-treatment of hypothyroidism is associated with excess mortality: A register-based cohort study. Thyroid. 2018;28:566–574. doi: 10.1089/thy.2017.0517. [DOI] [PubMed] [Google Scholar]
- 52.Thayakaran R., Adderley N.J., Sainsbury C., Torlinska B., Boelaert K., Šumilo D. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: Longitudinal study. BMJ. 2019;366 doi: 10.1136/bmj.l4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neves J.S., Leitão L., Baeta Baptista R., Bigotte Vieira M., Magriço R., Viegas Dias C. Lower free triiodothyronine levels within the reference range are associated with higher cardiovascular mortality: An analysis of the NHANES. Int J Cardiol. 2019;285:115–120. doi: 10.1016/j.ijcard.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Bassett J.H., O’shea P.J., Sriskantharajah S., Rabier B., Boyde A., Howell P.G., Weiss R.E., Roux J.P., Malaval L., Clement-Lacroix P., Samarut J., Chassande O., Williams G.R. Thyroid hormone excess rather than thyrotropin deficiency induces osteoporosis in hyperthyroidism. Mol Endocrinol. 2007;21:1095–1107. doi: 10.1210/me.2007-0033. [DOI] [PubMed] [Google Scholar]
- 55.Papaleontiou M., Banerjee M., Reyes-Gastelum D., Hawley S.T. Haymart MR. Risk of osteoporosis and fractures in patients with thyroid cancer: A case-control study in U.S. veterans. Oncologist. 2019;24:1166–1173. doi: 10.1634/theoncologist.2019-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim E.H., Jeon Y.K., Pak K., Kim I.-J., Kim S.-J., Shin S. Effects of thyrotropin suppression on bone health in menopausal women with total thyroidectomy. J Bone Metab. 2019;26:31. doi: 10.11005/jbm.2019.26.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis P.J., Tang H.Y., Hercbergs A., Lin H.Y., Keating K.A., Mousa S.A. Bioactivity of thyroid hormone analogs at cancer cells. Front Endocrinol (Lausanne) 2018;9:739. doi: 10.3389/fendo.2018.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hercbergs A. Clinical implications and impact of discovery of the thyroid hormone receptor on integrin αvβ3 – a review. Front Endocrinol. 2019;10 doi: 10.3389/fendo.2019.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterson S.J., McAninch E.A., Bianco A.C. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy. J Clin Endocrinol Metab. 2016;101:4964–4973. doi: 10.1210/jc.2016-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jonklaas J., Bianco A.C., Bauer A.J., Burman K.D., Cappola A.R., Celi F.S. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Clinical data are available from the original prospective study (www. ClinicalTrials.gov, NCT 01969552) [18]. Deidentified data will be shared with investigator support for similar projects.