Summary
The axon initial segment (AIS) cytoskeleton undergoes rapid and irreversible disruption prior to cell death after injury, and loss of AIS integrity can produce profound neurological effects on the nervous system. Here we described a previously unrecognized mechanism for ischemia-induced alterations in AIS integrity. We show that in hippocampal CA1 pyramidal neurons Nav1.6 mostly preserves at the AIS after disruption of the cytoskeleton in a mouse model of middle cerebral artery occlusion. Genetic removal of neurofascin-186 leads to rapid disruption of Nav1.6 following injury, indicating that neurofascin is required for Nav1.6 maintenance at the AIS after cytoskeleton collapse. Importantly, calcineurin inhibition with FK506 fully protects AIS integrity and sufficiently prevents impairments of spatial learning and memory from injury. This study provides evidence that calcineurin activation is primarily involved in initiating disassembly of the AIS cytoskeleton and that maintaining AIS integrity is crucial for therapeutic strategies to facilitate recovery from injury.
Subject Areas: Neuroscience, Molecular Neuroscience, Cellular Neuroscience
Graphical Abstract

Highlights
-
•
Ion channels are mostly retained at the AIS after ischemic injury
-
•
Neurofascin is required for clustering ion channels at the AIS after ischemia
-
•
Calcineurin inhibition protects AIS structural integrity and function from ischemia
-
•
Calcineurin inhibition protects cognitive function against impairment by ischemia
Neuroscience; Molecular Neuroscience; Cellular Neuroscience
Introduction
The axon initial segment (AIS) is a specialized ∼30-μm long proximal axon that contains ∼30- to 50-fold higher density of voltage-gated sodium channels (Nav) than the soma and dendrites (Kole et al., 2008). Owing to its unique property, the AIS is pivotal for generating an action potential in response to the integration of synaptic inputs under physiological conditions. Within the AIS subdomain, the cytoskeletal proteins including Ankyrin G (AnkG) and βIV-spectrin, together with neurofascin (Nfasc) and neuronal cell adhesion molecule (NrCAM), assemble Nav and voltage-gated potassium channels (Kv) to build an exquisite hierarchical structure. AnkG serves as an AIS master organizer to recruit Nav to the AIS during development and then βIV-spectrin tethers the AnkG-Nav complex to the axonal actin. In contrast, Nfasc is not required for AIS assembly and does not directly interact with channel-forming α subunits of Nav. Instead, Nfasc stabilizes the AIS by directly interacting with the extracellular matrix (Hedstrom et al., 2007, Ratcliffe et al., 2001, Zonta et al., 2011). It is interesting to know whether Nfasc is capable of stabilizing Nav at the AIS in the absence of AnkG under pathological conditions.
The AIS is very vulnerable to damage under pathophysiological conditions. For example, the AIS cytoskeleton is rapidly disrupted prior to cell death following medial cerebral artery occlusion (MCAO) in the peri-infarct and infarction core regions of the rodent brain (Hinman et al., 2013, Schafer et al., 2009). Inhibition of calpain only attenuates disruption of the AIS cytoskeleton in response to injury in vitro and in vivo, implicating that the AIS cytoskeletal proteins are proteolyzed partially through activation of the Ca2+-dependent calpain. However, inhibition of calcineurin with FK506 efficiently protects against cell deaths induced by ischemia in a mouse model of MCAO (Sharkey and Butcher, 1994). Moreover, blockade of calcineurin prevents activity-dependent AIS plasticity in cultured hippocampal neurons (Evans et al., 2013). These raise the question as to whether calcineurin is involved in regulating rapid AIS disruption under pathological conditions.
In the present study, we investigated the peri-infarct regions because axonal sprouting and synaptic reorganization in these regions are essential for recovery of human patients from neurological injury (Nudo, 2013, Wieloch and Nikolich, 2006). Given the critical role of the AIS in synaptic transmission, protection of the AIS in these regions after injury might be the first step to be considered for patient recovery. We thus began to compare the susceptibilities of the peri-infarct regions to ischemia using a commonly employed animal model of stroke MCAO that represents focal brain ischemia in human patients. We found that the AIS cytoskeleton was completely disrupted after 2 h of MCAO in hippocampal CA1 pyramidal neurons but not in the somatosensory cortex or striatum. In contrast, Nfasc and Nav1.6 were yet retained at the AIS after 4–6 h of MCAO. Genetic ablation of Nfasc promoted disruption of Nav1.6 at the AIS following injury, suggesting a critical role of Nfasc in the retention of Nav1.6 under pathophysiological conditions. Importantly, administration of a calcineurin inhibitor FK506 immediately after MCAO surgery completely prevented AnkG disruption, and impairments of AP generation, AP-dependent synaptic transmission, and spatial learning and memory resulting from ischemic injury.
Results
The Hippocampal AIS Cytoskeleton Is Preferentially Susceptible to Injury
To determine the vulnerability of the AIS to injury in different peri-infarct brain regions, mice were subjected to MCAO for various times by MCA thread insertion. The severity of AIS damage was examined by immunofluorescence (IF) staining of the scaffold protein AnkG in three peri-infarct regions: the somatosensory cortex, the hippocampus, and the thalamus (Figure 1A) (Popp et al., 2009). Compared with the uninjured (contralateral) side of the brain, the intensity and the length of AnkG staining were slightly reduced in the three regions of the injured side (ipsilateral) following 1 h of MCAO. As the occlusion time went longer, more than 50% of AnkG was preserved in the ipsilateral somatosensory cortex (SCC) after 6 h of MCAO, whereas AnkG was retained in the ipsilateral thalamus within 4 h of MCAO but absent at 6 h after the onset of occlusion (Figures 1B–1D). In contrast, AnkG was undetectable in the ipsilateral hippocampal CA1 region after 2 h of MCAO (Figures 1B–1D). Analogous results were observed in the hippocampal CA3 and DG regions (Figure S1). We next focused our further mechanical study on the hippocampus. Similar to AnkG, the AIS cytoskeletal protein βIV-spectrin was no longer observed at the AIS of ipsilateral hippocampal CA1 pyramidal cells after 2 h of MCAO (Figures S2A and S2B).
Figure 1.
Ischemic Injury Causes Rapid Disruption of the AIS Cytoskeleton AnkG in the Hippocampus
(A) Schematic of the regions (boxes) studied in (B).
(B) Representative confocal images of AnkG immunostaining from the box areas (A) of the somatosensory cortex (top), the CA1 region (middle), and the thalamus (bottom) of mice subjected to 1 h, 2 h, 4 h, or 6 h MCAO. Insets are enlarged from the yellow box areas.
(C and D) Quantification of the length (C) and the normalized fluorescence intensity (FI) (D) of AnkG immunostaining from the somatosensory cortex (black), the CA1 region (red), and the thalamus (blue) after various MCAO times. The ipsilateral FI was normalized to the contralateral from the same mouse.
(E) Representative image of AnkG immunoblot of hippocampal tissue homogenates from mice subjected to various times of MCAO.
(F) Quantification of the intensity of AnkG immunoblots after various MCAO times. p values are determined using one-way ANOVA with post hoc Bonferroni's multiple comparisons test. N represents mouse number. Data are presented as mean ± SEM. IS, ischemic injury; Contra, contralateral; Ipsi, ipsilateral.
To confirm these immunostaining results, we performed immunoblot analyses of hippocampal homogenates from sham or MCAO mice with antibodies against the C-terminal or spectrin-binding domains of AnkG. Immunoblots with both antibodies revealed a significant reduction in the amount of full-length AnkG in the contralateral compared with the ipsilateral after 1 h of MCAO. The full-length AnkG was rarely detected in the ipsilateral hippocampus with antibodies either against the C-terminal domain (Figures 1E and 1F) or against the spectrin-binding domain of AnkG after 2 h of MCAO (Figures S2C and S2D). Notably, no breakdown products of AnkG were detected in ipsilateral hippocampi after injury. Disruption of AnkG was not a consequence of cell death because we only observed a few TUNEL-positive cells in the hippocampus at this time point (Figure S1), consistent with the notion that cell death and AIS disruption are two independent events (Schafer et al., 2009). Collectively, these results suggest that the hippocampal AIS cytoskeleton is preferentially susceptible to injury.
Sodium Channels at the AIS Are Resistant to Injury-Induced Disruption
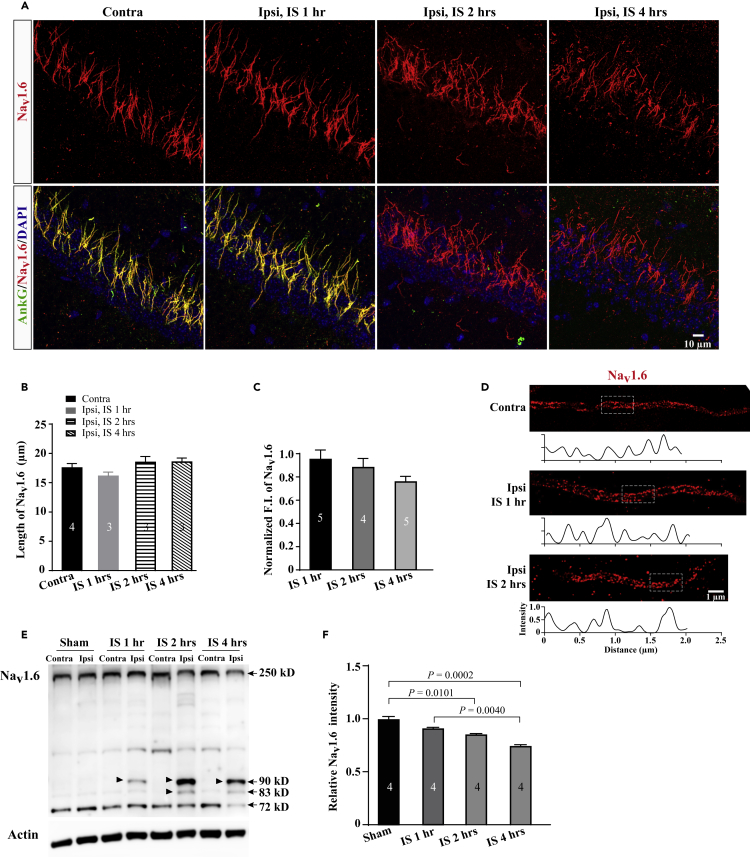
AnkG is required for assembly of Nav channel clustering at the AIS where action potentials are initiated (Hedstrom et al., 2007, Jenkins and Bennett, 2001, Kole et al., 2008, Zhou et al., 1998). To examine whether AnkG disruption led to disassembly of Nav, we performed IF staining of a Nav subtype Nav1.6 that is predominantly expressed in the hippocampal AIS. Unlike AnkG, the intensity and length of Nav1.6 at the AIS were not significantly reduced following 2 h of MCAO (Figures 2A–2C), but only the intensity was decreased by ∼26% at the 4-h time point (Figure 2C). Analogous results were observed for another AIS voltage-gated ion channel Kv7.2 (Figures S3A and S3B). To further examine whether nanoscale organizations of the AIS cytoskeleton and anchored ion channels were altered after injury, we performed super-resolution simulated emission depleted (STED) imaging to analyze the distribution of βIV-spectrin and Nav1.6 at the AIS. We found that Nav1.6 and βIV-spectrin showed periodic arrangements in the ipsilateral AIS comparable to the contralateral after 1 h of MCAO (Figures 2D and S3C). The periodic pattern of Nav1.6 was slightly altered after 2 h of MCAO when the AIS cytoskeleton was depleted (Figure 2D).
Figure 2.
Nav1.6 Largely Preserves at the AIS after Injury
(A) Representative confocal images of Nav1.6 immunostaining in the CA1 regions from mice subjected to 1 h, 2 h, or 4 h of MCAO.
(B and C) Quantification of the length (B) and the normalized FI (C) of Nav1.6 immunostaining from the CA1 pyramidal neurons after various times of MCAO.
(D) Super-resolution STED images of Nav1.6 immunostaining show periodic distribution at the AIS of hippocampal CA1 pyramidal neurons after 1 h or 2 h of MCAO. Boxes indicate analyzed areas showed below.
(E) Representative image of Nav1.6 immunoblot of hippocampal tissue homogenates from mice subjected to various times of MCAO. Arrow heads indicate fragments of small molecular size 110 kD and 95 kD that are proteolyzed from the full length of Nav1.6.
(F) Quantification of the intensity of 250 kD Nav1.6 immunoblots after various MCAO times. The ipsilateral F.I. was normalized to the contralateral from the same mouse. p values in (C) and (F) are determined using one-way ANOVA with post hoc Bonferroni's multiple comparisons test. N represents mouse number. Data are presented as mean ± SEM.
Previous studies showed that Nav was subject to proteolysis after injury (Czogalla and Sikorski, 2005, Schafer et al., 2009, White et al., 2000). To examine the possibility that Nav1.6 might be broken down into fragments after injury, we performed immunoblot analyses of hippocampal homogenates with antibodies against Nav1.6. In the ipsilateral hippocampus, we identified low-molecular-weight fragments with ∼82 kD and ∼90 kD from Nav1.6. These bands showed up at the 1-h time point, and their density was increased as the MCAO time prolonged (Figure 2E). Compared with AnkG disruption, quantitative immunoblot analyses showed ∼80% retention of the full-length Nav1.6 at 2-h MCAO time point and then reduced to ∼70% at 4 h (Figure 2F). Together, these results suggest that Nav1.6 is resistant to injury-induced decline.
AP Generation Is Impaired after Injury
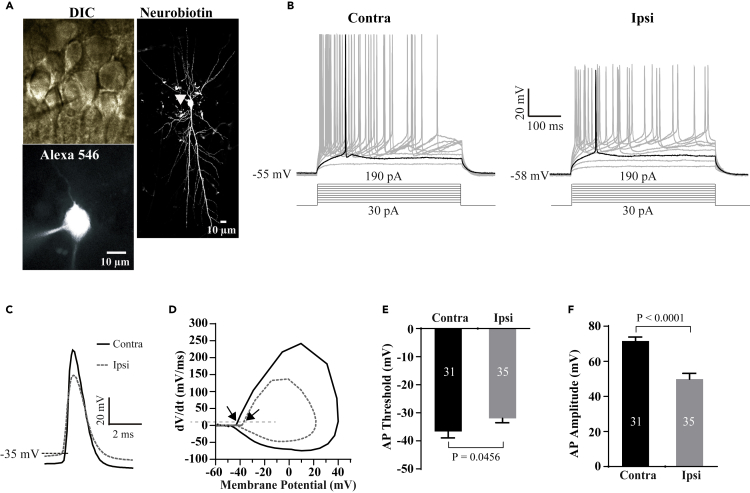
Could preserved Nav1.6 at the AIS generate APs in response to membrane depolarization? To address this question, we performed whole-cell electrophysiological recordings of APs on the CA1 pyramidal neurons of the contralateral and ipsilateral hippocampi following MCAO (Figure 3A). In contrast to the contralateral, we found a more positive AP threshold and a significant reduction in AP amplitude in the ipsilateral CA1 pyramidal cells after 1 h of MCAO (Figures 3B–3F), suggesting that functional alterations were prior to structural changes.
Figure 3.
Ischemic Injury Impairs Action Potential Generation
(A) Representative DIC and confocal images of a recorded hippocampal CA1 pyramidal neuron filled with Alexa 546 and neurobiotin. Arrow head indicates the AIS of the recorded neuron.
(B) Sample traces of changes in membrane voltages in response to a series of step currents in 20 pA increments from +30 pA to +190 pA in CA1 pyramidal neurons from the contralateral (left) and ipsilateral (right) hippocampi following 1 h of MCAO. Highlights show the traces containing the first AP initiated in the recorded neurons.
(C) Sample traces of the first APs were overlapped from the ipsilateral and contralateral CA1 pyramidal neurons.
(D) Phase-plane plots of membrane voltage vs its change rate in response to current injection in (C). The dash line indicates the change rate of 10 mV/ms. Arrows indicate AP thresholds.
(E and F) Summary of AP threshold and amplitude in the recorded neurons from the ipsilateral and contralateral hippocampal CA1 pyramidal neurons following 1 h of MCAO.p values in (E) and (F) are determined between ipsilateral and contralateral sides using unpaired t-test. N represents neuron number recorded. Data are presented as mean ± SEM.
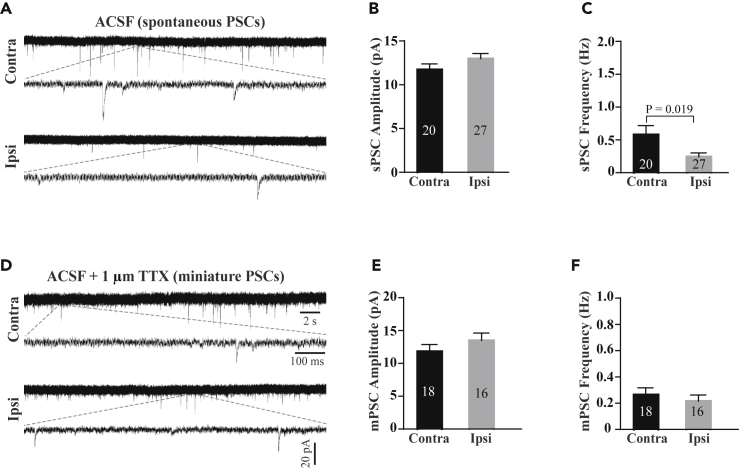
Under physiological conditions, AP propagation along the axon is required for spontaneous synaptic transmission among neurons. To examine whether impairment of AP generation could affect synaptic transmission, we performed whole-cell recordings of AP-dependent spontaneous postsynaptic currents (sPSCs) and AP-independent miniature PSCs (mPSCs) in hippocampal CA1 pyramidal neurons after injury. As we expected, sPSC frequency, but not amplitude, was significantly reduced (Figures 4A–4C), whereas neither the frequency nor the amplitude of mPSCs were altered after injury (Figures 4D–4F). These results together suggest that impairment of AP generation results in diminished synaptic transmission.
Figure 4.
Ischemic Injury Impairs Action Potential-Dependent Synaptic Transmission
(A) Sample traces of spontaneous postsynaptic currents (sPSCs) recorded in the CA1 pyramidal neurons with perfusion of physiological ACSF.
(B and C) Summary of sPSC amplitude (B) and frequency (C) in the recorded CA1 pyramidal neurons from the ipsilateral and contralateral hippocampi.
(D) Sample traces of miniature postsynaptic currents (mPSCs) recorded in CA1 pyramidal neurons after perfusion of physiological ACSF containing TTX (1 μm).
(E and F) Summary of mPSC amplitude (E) and frequency (F) in the recorded CA1 pyramidal neurons from the ipsilateral and contralateral hippocampi. p value in (C) is determined between ipsilateral and contralateral sides using unpaired t-test. N represents neuron number recorded. Data are presented as mean ± SEM.
Neurofascin Is Required for Clustering Sodium Channels at the AIS after Injury
Several lines of evidence showed that Nfasc plays an important role in maintenance of sodium channel clustering at the AIS by way of association with the extracellular matrix (Hedstrom et al., 2007, Zonta et al., 2011). To test whether Nav1.6 was anchored at the AIS by Nfasc after disruption of the AIS cytoskeleton, we carried out a series of experiments to demonstrate the expression of Nfasc at the AIS after injury. Nfasc remained unchanged and colocalization with Nav1.6 at the AIS after 2 h of MCAO (Figures 5A and 5B). Moreover, western blotting showed no difference between the ipsilateral and contralateral hippocampi after 4 h of MCAO (Figures 5C and 5D), suggesting that Nfasc is quite resistant to injury-induced decline. These results raise a question as to whether knockdown of Nfasc would cause rapid Nav1.6 disruption following MCAO.
Figure 5.
Nfasc Is Required for Nav1.6 Clustering at the AIS after Ischemic Injury
(A) Representative confocal images of Nfasc (green) and Nav1.6 (red) immunostaining in the hippocampal CA1 regions of mice subjected to 2 h of MCAO.
(B) Quantification of the normalized F.I. of Nfasc immunostaining in (A).
(C and D) Representative images (C) and quantification (D) of NF-186 immunoblot of hippocampal homogenates from mice subjected to sham or various times of MCAO.
(E–G) Representative confocal images (E and F) and quantification (G) of the normalized F.I. of Nav1.6 (magenta) immunostaining in the presence or absence of NF-186 in hippocampal pyramidal neurons after 2 h of MCAO. Scrambled or NF-186 sgRNA AAVs were delivered into the hippocampus of Emx1-Cre-dependent Cas9 expressing mice seven weeks before MCAO surgery. The scrambled sgRNA serves as a control. The ipsilateral F.I. was normalized to the contralateral from the same mouse. Note that Nav1.6 staining is not present at the AIS (Arrow heads) of a neuron expressing Cas9 (green) and NF-186 sgRNA (red). Boxes are enlarged in the right panels. p values in (F) are determined using one-way ANOVA with post hoc Bonferroni's multiple comparisons test. N indicates the number of mice. Data are presented as mean ± SEM.
To address this question, we took advantage of the Cre-dependent Cas9 knock-in mice to knockdown NF-186 that is predominantly expressed at the AIS. We first generated a mouse line with specific expression of Cas9 in the excitatory neurons by crossing the Cre-dependent Cas9 mouse to an Emx1-Cre driver. NF-186 sgRNA-3 that showed high efficacy of cleavage in cultured cells was selected to be constructed into the vector of adeno-associated virus (AAV)-U6-sgRNA-IRES-mCherry (Figure S4A). Next, the chimeric AAV1/2 viruses expressing sgRNA-3 were delivered via stereotactic injection into the hippocampal CA1 region of the Emx1-Cre/Cas9 mice. Seven weeks after injection, we found that the majority of NF-186 were ablated in infected neurons with sgRNA-3 AAVs (Figures S4B and S4C), which had no effect on AnkG expression at the AIS (Figure S4D). In contrast to scrambled AAVs and uninfected neurons in the same mice, Nav1.6 was little detectable in the infected neurons with sgRNA-3 AAVs after 2 h of MCAO (Figures 5E–5G), suggesting that Nfasc is required for clustering Nav1.6 at the AIS following ischemic injury.
Next we examined whether Nfasc anchored Nav1.6 by association with the EMC after injury. Chondroitinase ABC (ChABC) that is capable of digesting the essential EMC component, the chondroitin sulfate glycosaminoglycans (CS-GAGs), was applied into the hippocampal CA1 72 h before MCAO surgery (Figures S5A–S5C). We found that ChABC treatment had no effect on the clustering of Nav1.6 and Nfasc at the AIS in the absence of MCAO (Figures S5D–S5F) but caused a rapid disruption of Nav1.6 following 2 h of MCAO (Figure S5G), demonstrating that Nfasc together with the EMC clustered Nav1.6.
FK506 Fully Prevents Injury-Induced AIS Disruption
Calcineurin signaling was previously reported to play important roles in neuronal death induced by ischemic injury and in activity-dependent AIS plasticity (Evans et al., 2013, Sharkey and Butcher, 1994, Springer et al., 2000). To examine whether MCAO induced elevated calcineurin activation, which can cause NFAT to translocate into the nucleus, AAVs expressing fused NFAT-EGFP were injected into the hippocampus seven days prior to MCAO surgery. We found that the majority of NFAT-EGFP were located in the nucleus of the ipsilateral neurons compared with the cytosol of the contralateral neurons after 2 h of MCAO (Figures 6A and 6B). As disruption of the AIS cytoskeleton is an early response to injury independent of cell death, we asked whether inhibition of calcineurin with FK506 could prevent AIS disruption as well. To test this hypothesis, we subjected mice to MCAO surgery immediately followed by intravenous injection of FK506 at two different concentrations. After 2 h of MCAO, we found little AnkG preserved at the injured AIS following treatment with 0.5 mg/kg FK506. In contrast, administration of a higher concentration of FK506 (2.5 mg/kg) completely retained AnkG at the AIS (Figures 6C and 6D). We also compared FK506 with another calcineurin inhibitor (Cyclosporin A, CsA) and a calpain inhibitor (MDL 28170) for AIS protection. Although administration of CsA (20 mg/kg) retained the majority of AnkG (Figure S6), treatment with MDL 28170 (60 mg/kg) preserved only 20% of AnkG at the injured AIS (Figure S7), consistent with a previous study showing a partial protection of calpain inhibition against AIS disruption after injury (Schafer et al., 2009). Treatment with an Hsp90 inhibitor 17-AAG (25 mg/kg) had no effect on injury-induced disruption of AnkG (Figures S8A and S8B), ruling out the possibility that FK506 and CsA acted through inhibiting the Hsp90 stress response pathway (Owens-Grillo et al., 1995). Moreover, injury-induced NFAT-EGFP nuclear translocation was blocked by FK506 and CsA (Figures 6A and 6B). These results together suggest that inhibition of calcineurin with FK506 is sufficient to fully protect the AIS cytoskeleton from ischemic injury.
Figure 6.
FK506 Completely Protects the AIS Cytoskeleton and Function after Injury
(A and B) Representative confocal images (A) and quantification (B) show translocation of NFAT-GFP into the nucleus following injury; but this is blocked by 2.5 mg/kg FK506 or 20 mg/kg CsA. Insets are enlarged from the boxes. The ipsilateral F.I. ratio of the nuclear NFAT-EGFP to the cytosol is normalized to that of the contralateral from the same mouse.
(C) Representative confocal images of AnkG immunostaining of the CA1 regions from MCAO mice treated with vehicle (Veh), 0.5 mg/kg FK506 or 2.5 mg/kg FK506.
(D) Quantification of AnkG intensity normalized to the corresponding contralateral for different treatments.
(E–H) The overlap of sample traces (E), phase-plane plots (F), and summary of the threshold (G) and amplitude (H) of the first APs recorded from the ipsilateral and contralateral hippocampal CA1 pyramidal neurons of 2.5 mg/kg FK506 treatment.
(I–K) Sample traces (I) and summary of sPSC amplitude (J) and frequency (K) recorded from CA1 pyramidal neurons of 2.5 mg/kg FK506 treatment. p values in (B) are determined using one-way ANOVA with post hoc Bonferroni's multiple comparisons test. N in (B and D) denotes the number of mice and N in (F and G) indicates neuron number recorded. Data are presented as mean ± SEM.
Next we further examined the function of preserved AIS cytoskeleton by FK506 using whole-cell electrophysiological recordings of hippocampal CA1 pyramidal cells. We found no significant difference in AP amplitude and threshold between the ipsilateral and contralateral hippocampi of FK506-treated MCAO mice (Figures 6E–6H). Likewise, sPSC frequency was no longer changed following MCAO in FK506-treated mice (Figures 6I–6K).
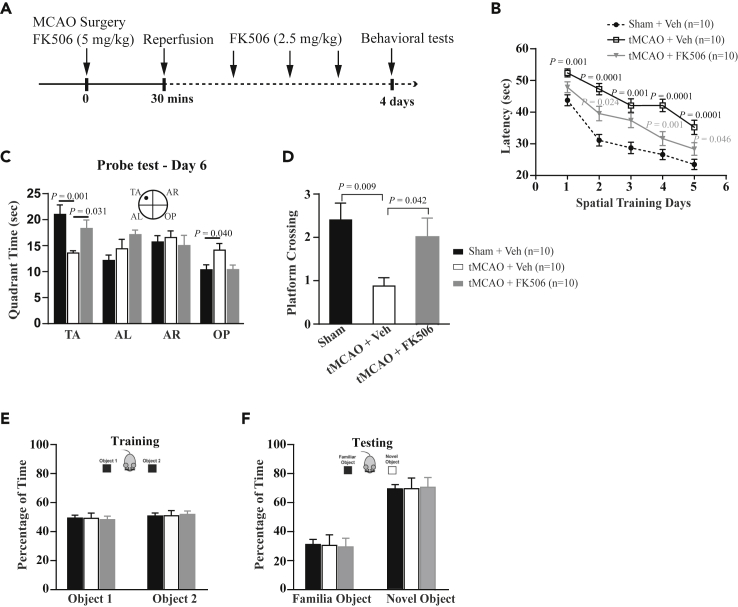
To address whether protection at the cellular level led to recovery of cognitive function, we subjected the MCAO-, sham-, and FK506-treatment mice to the hidden-platform version of Morris watermaze task with spatial cues (Morris et al., 1982) and new object recognition tasks for examining spatial and non-spatial memory, respectively. We chose the paradigm of 30-min transient MCAO (tMCAO) followed by re-perfusion because this mimicked 2 h of MCAO showing AnkG disruption but Nav1.6 and Nfasc preservation at the AIS (Figures 7A, S9A, and S9B). During the consecutive five-day training, the latencies for revealing the hidden platform were gradually decreased for both sham and MCAO mice, whereas the sham group achieved a significant shorter latency from the training day 2 compared with the MCAO mice (Figure 7B). For the probing task conducted on day 6, the sham group spent significantly more time in the target quadrant than the MCAO group who displayed no preference for the four quadrants (Figure 7C), and the sham group had more platform crossings than the MCAO group (Figures 7D and S9C), suggesting that the hippocampal-dependent spatial learning and memory is impaired after ischemic injury. These differences between the sham and MCAO groups were not due to changes in the swimming speed (Figures S9D and S9E). Mostly importantly, we found time spent in the target quadrant and platform crossings for FK506-treated MCAO mice similar to that of the sham group in the Morris watermaze task (Figures 7C, 7D, and S9C), indicating that spatial learning and memory of MCAO mice is fully protected by FK506.
Figure 7.
FK506 Fully Protects Hippocampal-Dependent Spatial Learning and Memory after Injury
(A) Schematic of the experimental paradigm of mice subjected to transient MCAO (tMCAO, 30 min of MCAO followed by reperfusion of four days) for behavioral tests.
(B–D) Behavioral test of standard hidden platform watermaze tasks for three groups of mice: sham + Veh, tMCAO + Veh, and tMCAO + FK506 (2.5 mg/kg). (B) Quantification of the latency to reach the platform for three groups of mice during the training days. The latency for the group of tMCAO + Veh is significant longer than those of the two groups of sham + Veh and tMCAO + FK506, but there are only significant differences in latency between sham + Veh and tMCAO + FK506 at day 2 (p = 0.007) and day 3 (p = 0.009). (C and D) Quantification of time spent in the quadrant areas of the maze (C) and the number of platform crossings (D) for three groups of mice in the probing test day (day 6). Time spent in TA and the number of platform crossings for the group of tMCAO + Veh are significantly reduced than those of the two groups of sham + Veh and tMCAO + FK506, but there is no difference between sham + Veh and tMCAO + FK506.
(E and F) Quantification of time spent on two identical objects during training (E) and on the familiar object and novel object during test (F) for three groups. Time spent on the novel object is significantly longer than that of the familiar object, but there is no difference in time spent on the novel object between three groups. TA, target quadrant; AL, adjacent left quadrant; AR, adjacent right quadrant, and OP, opposite quadrant. p values in (B and C) and in (D) are determined using two-way and one-way ANOVA with post hoc Bonferroni's multiple comparisons test, respectively. p values (gray) indicate significance between tMCAO + FK506 and tMCAO + Veh. p values (black) indicate significance between tMCAO + Veh and sham + Veh. N denotes the number of mice studied. Data are presented as means ± SEM.
Next, we subjected mice to new object recognition task for testing non-spatial memory. Generally, the new object recognition task mostly represents functions of the perirhinal and prefrontal cortex (Albasser et al., 2015, Barker and Warburton, 2011, Winters et al., 2004), although it is yet under debate (Cohen et al., 2013). After a short-term recognition for getting familiar with two objects, the sham-, MCAO-, and FK506-treatment groups showed no difference for the time spent on the new or familiar objects (Figure 7E). Three groups preferred to explore the novel object compared with the familiar one (Figure 7F), suggesting that non-spatial memory is not impaired after ischemic injury.
Taken together, these results suggest that inhibition of calcineurin with FK506 fully protect not only the cytoskeletal structure but also AIS function and spatial learning and memory following ischemic injury.
Discussion
The AIS cytoskeleton rapidly and irreversibly disassembles in response to injury. Given that the AIS plays a pivotal role in synaptic transmission by generating APs, disruption of the AIS cytoskeleton could lead to many neurological disorders associated with damaged brain regions. By comparing the peri-infarct regions, we found that the AIS cytoskeleton was more rapidly disrupted in the hippocampus than the striatum and somatosensory cortex, consistent with previous findings demonstrating that neuronal degeneration selectively occurred in the hippocampus and that hippocampal-dependent spatial memory tended to be impaired after injury in animal models and human patients (Bartsch et al., 2015, Pulsinelli et al., 1982, Schmidt-Kastner and Freund, 1991).
NavChannels Are Resistant to Injury-Induced Decline at the AIS
AnkG is crucial for AIS assembly during development, and either genetic ablation or silence of AnkG expression by shRNA failed to recruit Nav channels, NF-186, NrCAM, and βIV-spectrin into the AIS (Hedstrom et al., 2007, Huang and Rasband, 2018, Jenkins and Bennett, 2001, Zhou et al., 1998). A previous study reported that Nav1.6 and AnkG were concurrently disrupted in the infarct core area of the cortex in a focal ischemia/reperfusion (I/R, 90 min of MCAO followed by reperfusion) (Schafer et al., 2009). To examine the stability of Nav, we prolonged MCAO up to 6 h and examined an alteration in Nav1.6 density in the peri-infarct hippocampus. In contrast to complete AnkG disruption after 2 h of MCAO, we found that the majority of Nav1.6 preserved at the AIS till 6 h of MCAO.
Nav1.6 is mainly distributed in the distal AIS and responsible for AP generation due to its activation at a low threshold (Hu et al., 2009). AP generation is impaired in the CA1 pyramidal neurons, whereas Nav1.6 density is not altered at the time point of 1 h of MCAO. Because breakdown fragments of Nav1.6 were observed at this time point, a small amount of Nav1.6 proteolysis might be enough to impair AIS function but did not influence the intensity. But we cannot rule out other possibilities that (1) the density of other Nav channels such as Nav1.2 that is predominantly located in the proximal AIS was reduced after injury; and (2) Nav phosphorylation was increased after injury. In support of this, p38-mitogen-activated protein kinases (MAPKs) has been reported to be activated in the brain following ischemic injury (Alessandrini et al., 1999, Barone et al., 2001, Sugino et al., 2000), and p38 activation then phosphorylates Nav1.6 serine 553, which can result in a reduction of Nav1.6 current density (Wittmack et al., 2005).
Given that the majority of Nav1.6 preserved in the hippocampal AIS after disruption of the cytoskeleton in response to injury, we examined the hippocampal-dependent spatial learning and memory and the perirhinal cortex-involved non-spatial memory in a mouse model of I/R injury (30 min of MCAO followed by reperfusion), which have mostly mimicked the condition of 2 h of MCAO in terms of AnkG disruption and retentions of Nav1.6 and Nfasc. We found that ischemic injury caused severe impairment of spatial memory rather than non-spatial memory, implicating that the hippocampus is more likely damaged than the perirhinal cortex.
Nfasc Is Required for NavChannel Maintenance at the AIS after Injury
Unlike AnkG, Nfasc is not required for AIS assembly but might stabilize the AIS ion channels by interacting with both AnkG and brevican-based ECM (Hedstrom et al., 2007). Consistent with this idea, our results demonstrate that Nfasc and the vast majority of Nav1.6 preserve at the AIS in the absence of AnkG following a prolonged ischemic injury. Knockout of Nfasc in Cre-dependent cas9 knock-in mice and destruction of the chondroitin sulfate proteoglycans (CSPGs) with ChABC abolish Nav1.6 clustering at the AIS after injury, suggesting that Nfasc contributes to maintenance of Nav1.6 after injury. Because the current model of the AIS architecture shows that Nfasc does not directly interact with the pore-forming α subunits of Nav1.6 at the AIS domain, how Nfasc stabilizes Nav1.6 in the absence of AnkG after injury needs further investigations.
Previous studies have reported that a regulatory subunit Navβ1 directly interacts with NF-186 and NrCAM (McEwen and Isom, 2004), and Navβ4 associates with Navα via a covalent disulfide at the AIS (Buffington and Rasband, 2013). Therefore, we speculate that the β subunit may serve as a linker between Nfasc and Nav1.6 α subunits although other possibilities cannot be ruled out. For instance, some unidentified anchoring molecules that directly interact with both Nfasc and Navα can cluster Nav1.6 at the AIS. Or under pathophysiological conditions Nfasc directly binds to the Nav1.6 α subunits in the absence of AnkG. At least, a previous study showed that an Nfasc-binding protein brevican was upregulated in the optic nerve following ischemia (Reinhard et al., 2017). Further experiments are needed to address these questions.
Potential Mechanisms Underlying AIS Protection by FK506 after Injury
Given that the majority of Nav1.6 preserved in the hippocampal AIS after disruption of the cytoskeleton in response to injury, we examined the hippocampal-dependent spatial learning and memory and the perirhinal cortex-involved non-spatial memory in a mouse model of I/R injury (30 min of MCAO followed by reperfusion), which have mostly mimicked the condition of 2 h of MCAO in terms of AnkG disruption and retentions of Nav1.6 and Nfasc. We found that ischemic injury caused severe impairment of spatial memory rather than non-spatial memory, implicating that the hippocampus is more likely damaged than the perirhinal cortex. Treatment with a calcineurin inhibitor FK506 completely prevented injury-induced disruption of the AIS cytoskeleton, AIS function, and spatial memory impairments. Calpain has been previously reported to partially proteolyze the AIS cytoskeletal proteins following ischemic injury, raising the possibility that in addition to calpain other proteases are involved in the breakdown of the cytoskeleton protein. How calcineurin works in concert with calpain or other proteases remains an open question.
How does injury cause a cytosolic Ca2+ surge to activate calcineurin? Blockade of NMDA receptors is previously reported to have no effect on disruption of the AIS cytoskeleton after injury. In this study, we found that blockade of L-type voltage-gated calcium channels (VGCCs) prevents disruption of the AIS cytoskeleton after injury (Figure S8C), suggesting that Ca2+ influx through VGCCs activated calcineurin to trigger injury-dependent AIS disruption. This is consistent with previous studies demonstrating that blockade of the L-type VGCC prevents calcineurin-mediated activity-dependent AIS plasticity and activation of the caspase apoptotic cascade (Evans et al., 2013, Springer et al., 2000). However, inhibition of purinergic P2X7 receptors had no effect on AIS disruption induced by 2 h of MCAO (Figure S8C), which is inconsistent with a previous report showing that inhibition of P2X7 receptors could prevent AIS disruption in a rat model of ischemia/reperfusion (I/R, 90 min of MCAO followed by reperfusion) (Del Puerto et al., 2015). This discrepancy may be due to different animal species, ischemic time, and/or injury models as well as different treatment types. In contrast to our study that drugs were applied immediately following surgery, P2X7 receptor inhibitors were administered 3 h after injury in the previous study.
In conclusion, we have shown that calcineurin signaling regulates disruption of the AIS cytoskeletal protein after injury. Owing to protection of cell survival and AIS integrity, calcineurin inhibitors might be potential therapeutic drugs for treating ischemic patients.
Limitations of the Study
One caveat of this study is that sPSCs recorded from CA1 pyramidal cells demonstrate AIS function of the presynaptic neurons rather than postsynaptic CA1 pyramidal cells. Moreover, given that genetic knockout of calcineurin in the forebrain has been shown to impair synaptic plasticity and working memory associated with the hippocampus (Zeng et al., 2001), maintaining certain level of calcineurin activity is crucial for normal brain function. Thus future work focuses on whether partial and transient inhibition of calcineurin would be better suited to be a therapeutic treatment and for determining the optimal window for calcineurin inhibitors applied into ischemia patients.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Matthew N. Rasband (Baylor College of Medicine, Houston, Texas, USA) for helpful discussions. We thank Dr. Tian Chi (ShanghaiTech University, Shanghai, China) and Dr. Ke Tang (Nanchang University, Nanchang, China) for kindly providing the Cas9 and Emx1-Cre knock-in mice. We thank Dr. Antos Christopher (ShanghaiTech University) for kindly providing NFAT-EGFP vectors. We also thank the facilities of Imaging Core of Life School of Science and Technology at ShanghaiTech University for technical supports. This work was supported by the National Key Research and Development Program of China (2017YFC1001300) and the National Natural Science Foundation of China (31671062 (S.H.), 31771130 (G.Z.)), the Shanghai Municipal Government and ShanghaiTech University.
Author Contributions
Y. Z., X. W., Z. G., and S. H. conceived the project and designed the experiments. Y. Z. generated data for immunostaining, immunoblot, and animal behavioral tests. X. W. did electrophysiological recordings, behavioral tests, and immunostaining experiments. X. C. made AAV viruses. J. L. designed sgRNAs. C. T. performed super-resolution imaging. J. C. collected a part of confocal images. C. X. performed behavioral experiments. Y. Z., X. W., G. X., and S. H. wrote the paper. All of the authors discussed the results and contributed to the preparation of the manuscript.
Declaration of Interests
The authors declare no competing financial interests.
Published: February 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100880.
Contributor Information
Guisheng Zhong, Email: zhongsh@shanghaitech.edu.cn.
Shuijin He, Email: heshj@shanghaitech.edu.cn.
Supplemental Information
References
- Albasser M.M., Olarte-Sanchez C.M., Amin E., Brown M.W., Kinnavane L., Aggleton J.P. Perirhinal cortex lesions in rats: novelty detection and sensitivity to interference. Behav.Neurosci. 2015;129:227–243. doi: 10.1037/bne0000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandrini A., Namura S., Moskowitz M.A., Bonventre J.V. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc. Natl. Acad. Sci. U S A. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker G.R., Warburton E.C. When is the hippocampus involved in recognition memory? J. Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone F.C., Irving E.A., Ray A.M., Lee J.C., Kassis S., Kumar S., Badger A.M., Legos J.J., Erhardt J.A., Ohlstein E.H. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med. Res. Rev. 2001;21:129–145. doi: 10.1002/1098-1128(200103)21:2<129::aid-med1003>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Bartsch T., Dohring J., Reuter S., Finke C., Rohr A., Brauer H., Deuschl G., Jansen O. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J. Cereb. Blood Flow Metab. 2015;35:1836–1845. doi: 10.1038/jcbfm.2015.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington S.A., Rasband M.N. Na+ channel-dependent recruitment of Navbeta4 to axon initial segments and nodes of Ranvier. J. Neurosci. 2013;33:6191–6202. doi: 10.1523/JNEUROSCI.4051-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S.J., Munchow A.H., Rios L.M., Zhang G., Asgeirsdottir H.N., Stackman R.W., Jr. The rodent hippocampus is essential for nonspatial object memory. Curr. Biol. 2013;23:1685–1690. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czogalla A., Sikorski A.F. Spectrin and calpain: a 'target' and a 'sniper' in the pathology of neuronal cells. Cell. Mol. Life Sci. 2005;62:1913–1924. doi: 10.1007/s00018-005-5097-0. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Puerto A., Fronzaroli-Molinieres L., Perez-Alvarez M.J., Giraud P., Carlier E., Wandosell F., Debanne D., Garrido J.J. ATP-P2X7 receptor modulates axon initial segment composition and function in physiological conditions and brain injury. Cereb.Cortex. 2015;25:2282–2294. doi: 10.1093/cercor/bhu035. [DOI] [PubMed] [Google Scholar]
- Evans M.D., Sammons R.P., Lebron S., Dumitrescu A.S., Watkins T.B., Uebele V.N., Renger J.J., Grubb M.S. Calcineurin signaling mediates activity-dependent relocation of the axon initial segment. J. Neurosci. 2013;33:6950–6963. doi: 10.1523/JNEUROSCI.0277-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom K.L., Xu X., Ogawa Y., Frischknecht R., Seidenbecher C.I., Shrager P., Rasband M.N. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J.Cell Biol. 2007;178:875–886. doi: 10.1083/jcb.200705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman J.D., Rasband M.N., Carmichael S.T. Remodeling of the axon initial segment after focal cortical and white matter stroke. Stroke. 2013;44:182–189. doi: 10.1161/STROKEAHA.112.668749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Tian C., Li T., Yang M., Hou H., Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Huang C.Y., Rasband M.N. Axon initial segments: structure, function, and disease. Ann. N. Y. Acad. Sci. 2018;1420:46–61. doi: 10.1111/nyas.13718. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins S.M., Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J.Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole M.H., Ilschner S.U., Kampa B.M., Williams S.R., Ruben P.C., Stuart G.J. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- McEwen D.P., Isom L.L. Heterophilic interactions of sodium channel beta1 subunits with axonal and glial cell adhesion molecules. J. Biol. Chem. 2004;279:52744–52752. doi: 10.1074/jbc.M405990200. [DOI] [PubMed] [Google Scholar]
- Morris R.G., Garrud P., Rawlins J.N., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nudo R.J. Recovery after brain injury: mechanisms and principles. Front. Hum.Neurosci. 2013;7:887. doi: 10.3389/fnhum.2013.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens-Grillo J.K., Hoffmann K., Hutchison K.A., Yem A.W., Deibel M.R., Jr., Handschumacher R.E., Pratt W.B. The cyclosporin A-binding immunophilin CyP-40 and the FK506-binding immunophilin hsp56 bind to a common site on hsp90 and exist in independent cytosolic heterocomplexes with the untransformed glucocorticoid receptor. J. Biol. Chem. 1995;270:20479–20484. doi: 10.1074/jbc.270.35.20479. [DOI] [PubMed] [Google Scholar]
- Popp A., Jaenisch N., Witte O.W., Frahm C. Identification of ischemic regions in a rat model of stroke. PLoS One. 2009;4:e4764. doi: 10.1371/journal.pone.0004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli W.A., Brierley J.B., Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann. Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Ratcliffe N.R., Kennedy S.M., Morganelli P.M. Immunocytochemical detection of Fcgamma receptors in human atherosclerotic lesions. Immunol.Lett. 2001;77:169–174. doi: 10.1016/s0165-2478(01)00217-6. [DOI] [PubMed] [Google Scholar]
- Reinhard J., Renner M., Wiemann S., Shakoor D.A., Stute G., Dick H.B., Faissner A., Joachim S.C. Ischemic injury leads to extracellular matrix alterations in retina and optic nerve. Sci. Rep. 2017;7:43470. doi: 10.1038/srep43470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D.P., Jha S., Liu F., Akella T., McCullough L.D., Rasband M.N. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 2009;29:13242–13254. doi: 10.1523/JNEUROSCI.3376-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R., Freund T.F. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Sharkey J., Butcher S.P. Immunophilins mediate the neuroprotective effects of FK506 in focal cerebral ischaemia. Nature. 1994;371:336–339. doi: 10.1038/371336a0. [DOI] [PubMed] [Google Scholar]
- Springer J.E., Azbill R.D., Nottingham S.A., Kennedy S.E. Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury. J. Neurosci. 2000;20:7246–7251. doi: 10.1523/JNEUROSCI.20-19-07246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino T., Nozaki K., Takagi Y., Hattori I., Hashimoto N., Moriguchi T., Nishida E. Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J. Neurosci. 2000;20:4506–4514. doi: 10.1523/JNEUROSCI.20-12-04506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B.C., Sullivan J.M., DeGracia D.J., O'Neil B.J., Neumar R.W., Grossman L.I., Rafols J.A., Krause G.S. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J. Neurol. Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [Review] [DOI] [PubMed] [Google Scholar]
- Wieloch T., Nikolich K. Mechanisms of neural plasticity following brain injury. Curr.Opin.Neurobiol. 2006;16:258–264. doi: 10.1016/j.conb.2006.05.011. [Review] [DOI] [PubMed] [Google Scholar]
- Winters B.D., Forwood S.E., Cowell R.A., Saksida L.M., Bussey T.J. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J. Neurosci. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmack E.K., Rush A.M., Hudmon A., Waxman S.G., Dib-Hajj S.D. Voltage-gated sodium channel Nav1.6 is modulated by p38 mitogen-activated protein kinase. J. Neurosci. 2005;25:6621–6630. doi: 10.1523/JNEUROSCI.0541-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Chattarji S., Barbarosie M., Rondi-Reig L., Philpot B.D., Miyakawa T., Bear M.F., Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- Zhou D., Lambert S., Malen P.L., Carpenter S., Boland L.M., Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J.Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta B., Desmazieres A., Rinaldi A., Tait S., Sherman D.L., Nolan M.F., Brophy P.J. A critical role for Neurofascin in regulating action potential initiation through maintenance of the axon initial segment. Neuron. 2011;69:945–956. doi: 10.1016/j.neuron.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.