Abstract
Biodiversity loss and climate change simultaneously threaten marine ecosystems, yet their interactions remain largely unknown. Ocean acidification severely affects a wide variety of marine organisms and recent studies have predicted major impacts at the pH conditions expected for 2100. However, despite the renowned interdependence between biodiversity and ecosystem functioning, the hypothesis that the species’ response to ocean acidification could differ based on the biodiversity of the natural multispecies assemblages in which they live remains untested. Here, using experimentally controlled conditions, we investigated the impact of acidification on key habitat-forming organisms (including corals, sponges and macroalgae) and associated microbes in hard-bottom assemblages characterised by different biodiversity levels. Our results indicate that, at higher biodiversity, the impact of acidification on otherwise highly vulnerable key organisms can be reduced by 50 to >90%, depending on the species. Here we show that such a positive effect of a higher biodiversity can be associated with higher availability of food resources and healthy microbe-host associations, overall increasing host resistance to acidification, while contrasting harmful outbreaks of opportunistic microbes. Given the climate change scenarios predicted for the future, we conclude that biodiversity conservation of hard-bottom ecosystems is fundamental also for mitigating the impacts of ocean acidification.
Subject terms: Biodiversity, Biogeochemistry, Marine biology
Introduction
Ocean acidification (OA) caused by the ongoing increase in anthropogenic carbon dioxide (CO2) emissions represents one of the most important threats to marine biodiversity and ecosystem functioning1–5. Seawater acidification alters the carbonates system and affects the metabolism and physiology of calcifying marine species, leading to lower calcification rates and to malformation or dissolution of carbonate structures (e.g., organisms’ skeletons)2–11. These can in turn determine changes in the biotic potential, mobility, survival rates and can increase the risk of their extinction2–11. Changes in species abundance, composition and functions can alter ecosystem’s properties12–17, with impacts comparable or higher than other drivers of environmental change17–19. Moreover, whilst all species contribute to ecosystem functions, changes in the abundance of keystone species, ecosystem engineers and habitat forming species can have much larger cascading effects than the loss of other species20,21. For instance, the local extinction of habitat forming species have been shown to drive significant detrimental effects, in terms of habitat complexity, functional redundancy and ecosystem resilience22–25. Ocean acidification can thus alter ecosystem stability to a different extent, depending on the impact on specific taxa; the higher the functional role of the impacted taxa, the higher the possibility that OA will reduce or compromise key ecosystem functions26–29.
Increasing evidence indicates that the ecological interactions among the different components of multispecies assemblages can influence the response of each single species to environmental change30–36. This implies that the effects of OA observed in experiments conducted on a single species (i.e., one species isolated from its assemblages and habitat) might be different than those we can observe when the species is within complex networks of multispecific interactions, such as those occurring in their natural environment30,31.
So far, experimental studies tested only single species response to OA, while the impacts on multispecies assemblages remains untested. Moreover, whilst it is known that microbes play a key role in the functioning of marine ecosystems37–39 and on their host species40–42, only scarce information is available on the impact of OA on the microbe-host interactions in marine organisms.
In order to provide new insights on the impact of OA on marine life, we used as a model for our experiments, the coralligenous assemblages, which are formed by calcareous biogenic structures produced by calcifying algae and invertebrates, which are an hot spot of benthic biodiversity (including a large number of habitat forming species, such as the red coral C. rubrum and several calcifying algae and sponges) in several temperate seas43–46. These habitats are extremely vulnerable to fluctuations in environmental conditions, and the presence of calcareous structures makes these systems particularly threatened by warming and OA43–54. In the present study, we conducted long-term time-course acidification experiments (using the scenario of 1000 ppm of atmospheric CO2 predicted by 21004), to test for the responses of the red coral and of the entire coralligenous assemblage to OA. We tested the effects in terms of growth rates, and in terms of microbe-host associations. The analyses conducted on a Mediterranean coralligenous habitat indicate that increasing biodiversity values enable a stronger resistance to the impact of OA.
Methods
Experimental set-up
Corallium rubrum apical branches (n = 90, about 4 cm long with ca 200 polyps each, sampled from different coral colonies) and natural assemblages (n = 36 blocks of coralligenous assemblages, standardized to a volume of ca. 1 dm3 each) were collected at 30 ± 0.5 m depth in the Marine Protected Area of Portofino (Ligurian Sea, Italy). The sampling effort was selected based on previous experience to assure an optimal level of experimental replication, at the same time avoiding excess harvesting to minimize any potential impacts of sampling on the local population of the target species2. The experimental set-up followed the procedures described in ref. 2 with some modifications, as follows. After collection, all corals and natural coralligenous assemblages were placed in 50-L tanks containing air-bubbled seawater, filtered and maintained at the in situ temperature of 13.6 ± 0.5 °C and salinity of 37.5 ± 0.5. Constant temperature conditions were assured by continuous monitoring using a YSI TDS conductivity meter during the short transfer to the laboratory (6 hours). After 10 months of acclimation at the in situ temperature and salinity, the coral colonies and coralligenous assemblages were transferred to mesocosms filled with 10 L of 20-µm filtered seawater (collected in situ). To test the impact of OA according to the IPCC scenario of OA for the end of this century4, control mesocosms were exposed to current pCO2 levels (i.e., ca 400 ppm CO2) and an equal number of mesocosms were exposed to 1000 ppm CO2, reaching a final pH of approx. 7.7. To test the role of biodiversity in the response to OA, we included a first series of 6 mesocosms (3 controls and 3 acidified) containing C. rubrum alone, and a second series of 12 mesocosms (6 controls and 6 acidified) containing C. rubrum in association with coralligenous assemblages of 6 different values of biodiversity (i.e., replicated assemblages composed of organisms representing 6, 7, 8, 9, 10 or 11 different families). The biodiversity of the different systems analyzed was assessed considering the dominant organisms (i.e., the taxa covering more than 1% of the total area of the coralligenous blocks). In each mesocosm, five colonies of C. rubrum were grouped, either alone (in the first series of mesocosms) or in association with three blocks of coralligenous assemblages for each of the 6 different biodiversity treatments tested (in the second series). Despite 10 days have been already reported to be sufficient to detect negative impacts of acidification on C. rubrum2, we extended here the duration of the experiment to 86 days to increase the possibility to appreciate consistent effects on the red coral as well as on other potentially vulnerable coralligenous species.
A computerized Bronkhorst High-Tech BV series mass flow meter was used to acidify seawater by air-CO2 gas mixture bubbling at 1000 ppm CO22, while controls received bubbling at 400 ppm CO2. The flow rate was increased slowly up to final 150 ml min−1 to gradually reach the desired pH values in all mesocosms. All coral colonies and coralligenous assemblages survived during the whole experiment and after. Mesocosms were placed in a large tank with water supplied by three pumps, which re-circulated the water through the chiller (TECO SeaChill TR5) and within the water bath assuring homogeneous physical-chemical properties of the water mass inside each tank to avoid small-scale temperature or pH gradients. Seawater within each mesocosm was re-circulated continuously at the rate of 190 L h−1, with renewal of 20-µm filtered seawater (collected in situ) ensured for the entire duration of the experiment. Mesocosms were shaded to reproduce in situ light conditions from the selected depth using an opaque cloth, and partially covered with plastic wrap to facilitate equilibration between the gas mixtures and the experimental seawater and to minimize water evaporation. All mesocosms were fed three times a week with 2 mL of 104 Artemia salina nauplii and 2 mL of 105 microalgae (Nannochloropsis sp. and Tetraselmis sp.) in 20-µm filtered seawater.
Physical-chemical variables and carbonate parameters
During the experimental run, temperature and salinity were measured daily with an YSI TDS conductivity meter. pH was determined daily using a Crison pH electrode/meter calibrated with NBS buffers (accuracy ± 0.005). Alkalinity was determined at 8 different time points (at the start of the experiment and every approximately 1–2 weeks), by using an open-cell potentiometric titration procedure calibrated with certificated alkalinity standards55. Seawater samples (500 ml) for Total Alkalinity (TA) were preserved with 200 μl of 50% HgCl2 saturated solution to avoid any biological alteration and were stored in the dark at 4 °C until analysis. TA was determined on ~140 g subsamples using a titration system comprising of a 250 ml open titration cell thermo-regulated at 25 ± 0.1 °C, a Crison pH electrode/meter calibrated with certified DIN 19267 pH/mV standards (±0.5 mV) and a Crison Burette 1S (±0.001ml). The accuracy of the titrations was ±3 μmol kg−1. Parameters of the carbonate system, including pCO2, CO32−, HCO3− and DIC concentrations, saturation of aragonite (ΩAr) and calcite (ΩCa) were estimated from the measured values of pH, TA, temperature, salinity, phosphate and silicate concentrations using the program CO2Sys.xls 201156. The concentration of proteins, carbohydrates, and lipids was measured spectrophotometrically57. The sum of the carbohydrate, protein and lipid concentrations converted into carbon equivalents (by using the conversion factors of 0.40, 0.49 and 0.75 μg C μg−1, respectively) was defined as biopolymeric carbon (BPC)57,58.
Analysis of the coralligenous assemblages
The biodiversity and the areal coverage of the benthic organisms of the coralligenous assemblages was inspected using a standardized photographic protocol and photoQuad software system for advanced image processing59. The photographic analysis was confirmed through parallel stereomicroscope identification of each component of the coralligenous assemblages60,61. The shift in the areal coverage of sponges and macroalgae in control and acidified mesocosms was calculated as the difference in the relative coverage (as % of the total surface area of the coralligenous) of each taxon, between the end and the start of the experiment. The net growth rates of the C. rubrum colonies, as well as the coral sclerites’ accretion, skeletal morphology and polyps’ activity, were determined using standard methods as previously described2. Briefly, the net growth rates of the C. rubrum colonies was measured using a buoyant weighting method62, a highly accurate technique for the determination of mass increase in living corals63. All coral colonies were suspended at aquaria filled with 20 µm filtered seawater (T = 13.6 °C, Sal = ~37.7) by a hook attached to an aluminum wire hanging from a Radwag bottom-loading scale to minimize the effect on the physiology and health of the coral colonies. The dry weights of the axial skeleton, of the sclerites and of the total skeleton (i.e., axial skeleton plus sclerites) were also determined. C. rubrum growth rates were calculated as the percentage weight difference (measured as buoyant weight) between the beginning and the end of the experiment, and normalized to the coral branch initial weight64. To calculate the mean basal diameter growth rate, first buoyant weight were converted into dry weights using the linear regression equation between buoyant weights and total (i.e., axial skeleton plus sclerites) dry skeleton weights65. Subsequently, dry weights were converted to basal diameters using the following relationship: W = 0.086 × G2.198. Where W = dry weight; G = basal diameter of the colony64. The annual basal diameter growth rates were finally calculated by normalizing to the duration of the interval (n = 86 days) and multiplying by 365. The accuracy and reliability of the adopted protocols for determining the growth rates was previously tested2, and confirmed also in the present experiments. The buoyant weight of the red corals in treated and control systems was checked to be significantly related with their axial skeleton dry weight and their total skeleton dry weight (i.e., scleraxis plus sclerites) (Supplementary Fig. S1).
To detect the effects of acidification on the sclerites accretion of C. rubrum, a calcein-labeling experiment was conducted2. At the end of the experiment, one coral colony from each replicated mesocosm was transferred in separated aquaria and kept under same conditions of temperature and pH as from where the colonies were withdrawn. An aliquot of calcein (Sigma C-0875, with a final concentration of 10 mg L−1) was added into each separated aquarium (treated and untreated coral branches) 120 h before the end of the bubbling experiment. Afterwards, their apical parts were soaked in 12% solution of sodium hypochlorite for 24 h, until all organic material was removed66. C. rubrum calcifies more rapidly in its apical regions where the sclerites are directly incorporated to form the medullar part of the axial skeleton67. The sclerites were then rinsed several times with reagent grade water (MilliQ), mounted on slides and analyzed under epifluorescence microscopy (excitation filter 450–490 nm) for total and fluorescent capstan and cross sclerites counting. The relative abundance of fluorescent (cross and capstan) sclerites was used as an estimate of newly-accreted calcium carbonate skeletal elements2. In addition, values of the fluorescent cross to capstan sclerites ratio were used to evaluate differences in the production of the two different skeleton elements among treatments.
To detect the effects of acidification on calcification, coral colonies were prepared for observation under Scanning Electron Microscope (SEM), analyzing the fine-scale skeletal morphology. At the end of the experiment, one colony was withdrawn from each mesocosm and the apical branches of the colonies were treated to remove all organic material using the same method adopted for sclerites analyses (described above). Further, skeletal samples were mounted on aluminum stubs using carbon adhesive tabs and subsequently coated with gold/palladium (Au/Pb) for five minutes using a Polaron Range sputter coater. SEM observations were conducted with a Philips® XL 20 microscope to assess the presence of skeletal abnormalities at low pH (e.g., malformation or dissolution of the skeleton). In addition, to quantify the possible effect of pH on the sclerites we used SEM in combination with a 0–2 visual rank-scale approach for determining the percentage of dissolution-damage68. Rank 0 corresponded to 0% damage, rank 1 corresponded to 0 < damage < 50%, and rank 2 corresponded to >50% damage, analyzing at least 100 sclerites per colony68.
The activity of each colony was assessed by determining the state of activity of its polyps (and expressed as the prevailing state of the polyps’ expansion)2. Each coral colony was assigned to three different expansion states of the polyp’s body as follows: prevailing number of totally expanded polyp and tentacles (value 2), tentacles or polyps emerging from the gastric cavity (value 1) and totally retracted polyps (value 0). The polyps’ activity was expressed as the percentage of polyps at the maximum expansion state relative to the total number of polyps. In addition, the percentage of open polyps per each colony was determined2. Only polyps of similar size were considered and the smallest polyps were excluded to avoid overestimating the number of retracted polyps. The air-CO2 gas mixtures were pumped carefully far from the coral colonies into the aquaria to avoid confounding effects on polyps’ activity.
Microbiological analyses
To check for the lack of pathogenic outbreaks potentially occurring in our mesocosms, virus and prokaryote counts from seawater samples of each mesocosm during the experiment were conducted by epifluorescence microscopy and SYBR Green I staining69.
Catalysed Reporter Deposition Fluorescence In Situ Hybridization (CARD-FISH) was conducted for the quantification of calcibacteria in the sponge H. columella. Briefly, calcibacteria were extracted from 1 g of fresh sponge tissue as previously described70. Aliquots of the calcibacteria extracts obtained and of the fresh sponge tissue71 were analysed by CARD-FISH72 using Horseradish Peroxidase (HRP)-labeled probes Eub-mix (Eub338, Eub338-II and Eub338-III) targeting Bacteria. The absence of nonspecific signals was routinely checked using the NON-338 probe72. Hybridization was performed at 35 °C for 2 hours, followed by washing of the hybridized samples into preheated (37 °C) washing buffer and treatment for Cy3-tyramide signal amplification (at 37 °C in the dark) and probe stabilization in PBS buffer (pH 7.6) amended with Triton X-100 (final concentration, 0.05%). Filters were then observed under epifluorescence microscopy under green light.
The presence of fungi was checked by scraping areas of the natural coralligenous substrates, followed by direct detection of fungi by calcofluor staining73. The frequency of fungal detection was calculated as the percentage of scraped spots (of standard surface area) in which fungi were detected, relative to the total number of spots analyzed.
Statistical analyses
To test for differences in the investigated variables between different experimental treatments, analysis of variance was carried out. In detail, a two-way analysis of variance was used to test for significant differences in the growth rates of the different coralligenous taxa between treatments (i.e., acidified vs control pH conditions) and to test for any potential tank effects (i.e., including “mesocosm” as a random factor, nested within the factor “treatment”)3.
The differences in the feeding behaviour, sclerite calcification and cross-to-capstan ratio were assessed using a two-way analysis of variance including factors treatment (acidified vs control conditions) and incubation type (red coral alone or in association with multispecies assemblages).
A distance-based permutational multivariate analysis of variance74 was used to test for significant differences in the integrity of the red coral sclerites (capstans and crosses), including factors treatment (acidified vs control), time (initial vs final time) and incubation type (red coral alone or with multispecies assemblages).
To assess the effect of acidification on the association between the sponge Hemimycale columella and calcibacteria, a three-way analysis of variance was used including factors treatment (acidified vs control), time (initial vs final) and biodiversity level (2 levels: low, i.e, multispecies assemblages including organisms of 6 families; and high, i.e., multispecies assemblages including organisms of 11 families).
A three-way analysis of variance was used to assess potential differences in the prokaryotic and viral abundance in the mesocosms during the experiment, including factors treatment (acidified vs control), time (initial, mid-term and final time) and incubation type (red coral alone or with multispecies assemblages).
To exclude potential differences in temperature and salinity among mesocosms during the experiment and to assess the significant changes in pH, total alkalinity and seawater carbonate system due to the acidification treatment, a mixed-design analysis of variance was used, including factors treatment (acidified vs control), time (86 subsequent days) and incubation type (red coral alone or with multispecies assemblages).
All analyses of variance were conducted on Euclidean distance similarity matrices, after checking the homogeneity of variance using the Cochran’s test. Post-hoc tests were carried out when significant differences were encountered. All statistical analyses were carried out using R75 and Primer 6+ software76.
Results and Discussion
All of the acidified systems reached the predicted seawater pH values, decreasing by approximately 0.4 units compared to the controls (Table 1 and Supplementary Fig. S2). This mimics with high accuracy the conditions assumed to occur in the future by the IPCC scenario of 1000 ppm of atmospheric CO24 and it is consistent with previous acidification experiments on the same population of C. rubrum2. The analysis of the available historical data77 highlights a significant trend of acidification of the water masses in the marine area from which the organisms were sampled (Supplementary Fig. S3). Indeed, we found in this site evidence of a decrease of −0.0025 pH units per year (Supplementary Fig. S3), which is consistent with current observations of the generalized trend of acidification for the whole Mediterranean basin78,79. This suggests that, in the future, this area might face longer periods at the low pH conditions tested in our study (i.e., pH < 7.8), highlighting the urgent need to understand the possible impacts of such environmental changes on these coralligenous assemblages and on the processes able to influence their response to OA.
Table 1.
Physical-chemical variables and main carbonate parameters measured in situ and in control or acidified treatments.
| System | S | T (°C) | pH | TA | DIC | pCO2 | HCO3− | CO32− | ΩAr | ΩCa | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (µmol kg−1) | (µmol kg−1) | (µatm) | (µmol kg−1) | (µmol kg−1) | |||||||
| in situ conditions | 13.6 ± 0.5 | 38.1 ± 0.2 | 8.06 ± 0.01 | 2586 ± 4 | 2332 ± 3 | 416 ± 1 | 2115 ± 3 | 210 ± 4 | 2.72 ± 0.01 | 4.17 ± 0.01 | |
| Control (86 days average) | Coral alone | 13.5 ± 0.1 | 37.6 ± 0.1 | 8.14 ± 0.01 | 2699 ± 61 | 2399 ± 53 | 362 ± 9 | 2169 ± 46 | 217 ± 8 | 4.80 ± 0.19 | 3.31 ± 0.13 |
| Coral + Coralligenous | 13.6 ± 0.1 | 37.5 ± 0.1 | 8.13 ± 0.01 | 2648 ± 60 | 2357 ± 53 | 363 ± 9 | 2135 ± 47 | 208 ± 7 | 4.62 ± 0.16 | 3.18 ± 0.11 | |
| Acidified Treatment (86 days average) | Coral alone | 13.4 ± 0.1 | 37.5 ± 0.1 | 7.84 ± 0.01 | 2843 ± 35 | 2669 ± 13 | 818 ± 97 | 2494 ± 21 | 144 ± 21 | 3.19 ± 0.46 | 2.20 ± 0.31 |
| Coral + Coralligenous | 13.6 ± 0.1 | 37.6 ± 0.1 | 7.84 ± 0.01 | 2790 ± 37 | 2621 ± 20 | 807 ± 87 | 2451 ± 24 | 139 ± 19 | 3.09 ± 0.42 | 2.13 ± 0.29 | |
| Acidified Treatment (on day 86) | Coral alone | 13.4 ± 0.2 | 37.8 ± 0.2 | 7.74 ± 0.01 | 2767 ± 50 | 2663 ± 39 | 1064 ± 33 | 2522 ± 33 | 100 ± 7 | 2.21 ± 0.16 | 1.53 ± 0.11 |
| Coral + Coralligenous | 13.4 ± 0.2 | 37.8 ± 0.1 | 7.74 ± 0.01 | 2692 ± 87 | 2588 ± 82 | 1020 ± 24 | 2451 ± 76 | 98 ± 5 | 2.18 ± 0.11 | 1.50 ± 0.08 | |
For the controls and acidified treatments, reported are salinity (S); temperature (T); total alkalinity (TA); dissolved inorganic carbon (DIC); partial pressure of carbon dioxide (pCO2); bicarbonates concentration (HCO3−); carbonates concentration (CO32−); aragonite saturation state (ΩAr); calcite saturation state (ΩCa).
In our acidified treatments, the carbonate parameters reflected the experimentally induced increase in pCO2, which reached final 1042 ± 36 µatm versus control values of 362 ± 9 µatm. The acidification treatment determined a significant decrease of average ΩAr compared with the controls (2.2 ± 0.1 versus 4.7 ± 0.2), ΩCa (1.5 ± 0.1 vs. 3.2 ± 0.1) and CO32− (99 ± 5 vs. 212 ± 8 µmol kg−1), and an increase in DIC (2626 ± 70 vs. 2378 ± 53 µmol kg−1) and HCO3− (2487 ± 66 vs. 2152 ± 47 µmol kg−1) compared to the controls (p < 0.01; Table 1 and Supplementary Fig. S2). All mesocosms displayed oversaturation of aragonite and calcite in the seawater and consistent values for the rest of the seawater physical-chemical variables during the entire duration of the experiments (Table 1 and Supplementary Fig. S2). These results confirm current evidences that, despite the high acidification levels, the Mediterranean waters are still largely oversaturated in both calcite and aragonite79.
Besides the red coral C. rubrum (Family Coralliidae), the dominant organisms of the coralligenous multispecies assemblages utilised in the present study belonged to the following families: Hildenbrandiaceae and Hapalidiaceae (algae), Hymedesmiidae, Ancorinidae, Clathrinidae, Leucosoleniidae and Sycettidae (sponges), Celleporidae, Smittinidae, Beanidae, Crisiidae and Schizoporellidae (bryozoans), Dendrophylliidae and Epizoanthidae (cnidarians), and Serpuloidae (polychaetes). The most abundant taxa in the coralligenous multispecies assemblages we tested were sponges and algae. Sponges were the dominant metazoa, accounting on average for 71 ± 23% of the surface covered by macrozoobenthos. Two main classes of Porifera were present: Calcarea (calcifying sponges including the species Sycon ciliatum, Leucosolenia sp., and Clathrina sp.), characterized by a calcareous skeleton, and Demospongiae, mainly characterized by a siliceous skeleton. Demosponges included two main groups: epilithic sponges (Haplosclerida n.c., Suberitidae, Aplysina sp., Phorbas sp., Hymedesmia sp., Dictyonella sp., Haliclona (Gellius) marismedi, Hemimycale columella Bowerbank, 1874 and endolithic sponges (Dercitus (Stoeba) plicatus and Jaspis incrustans). H. columella represented >94% of the total sponge coverage. The macroalgae included two main groups: turf algae (non-calcifying and mainly represented by Hildenbrandia cf. rubra -Sommerfelt- Meneghini, 1841) and crustose coralline algae (calcifying algae, almost entirely represented by Phymatolithon sp. Foslie, 1898).
As the red coral C. rubrum, also the dominant algae (Phymatolithon sp.) and sponge (H. columella) in the coralligenous assemblages we tested have been previously shown to be affected by low-pH seawater values2,3,7,80,81 and thus represented optimal models for our multispecies acidification experiment.
The acidification treatment significantly reduced the growth rates of different taxa compared to the controls (Fig. 1), determining a negative shift in the C. rubrum mass weight (by −0.18 ± 0.03% d−1, compared with control values of 0.10 ± 0.01% d−1) and a decrease in the areal coverage of other dominant organisms of the coralligenous assemblages (including calcifying algae, endolithic sponges and epilithic sponges; respectively by −0.31 ± 0.06, −0.16 ± 0.09 and −0.11 ± 0.03% d−1, compared with control values of 0.09 ± 0.06, 0.05 ± 0.03 and 0.10 ± 0.02% d−1; p < 0.01; Fig. 1).
Figure 1.

Impact of acidification on the dominant taxa. Reported are the changes in the growth rates of different coralligenous taxa due to the acidification treatment. The growth rates are expressed as shifts in mass weight for the red coral or in areal coverage for macroalgae and sponges. Reported are average values and SDs. Asterisks indicate significant differences (**p < 0.01) in the acidified treatment compared with the respective control.
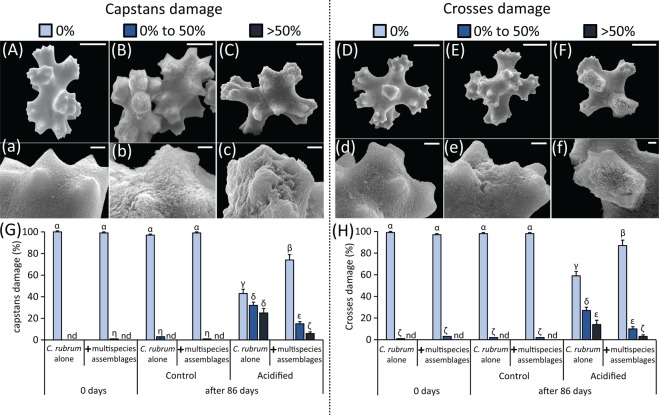
These results confirm the negative impact of acidification on the red corals when incubated alone2,82. Indeed, we found that the acidification treatment caused decalcification of C. rubrum calcium carbonate sclerites (57 ± 6% of damaged sclerites compared to no damages in the controls; p < 0.01; Fig. 2) and decrease of its feeding and calcification activities (ranging from −15 to −54% compared to the controls; p < 0.01; Supplementary Fig. S5). In detail, C. rubrum polyp’s activity decreased by 44 ± 10% and the percentage of open polyps by 32 ± 11%, while the newly accreted capstans decreased by 34 ± 8% and the crosses by 25 ± 10% (Supplementary Fig. S5). These results are consistent with evidences obtained in previous studies2. Indeed, while control levels of polyps’ expansion and sclerites accretion indicate healthy conditions of the colonies, their lower values under acidification suggest a decrease of C. rubrum feeding, respiration and calcification efficiency2,83. It is well known that calcification in C. rubrum is particularly sensitive to seawater acidification2,3, likely due to the composition of its skeleton and sclerites (Mg-rich calcite, which is more soluble than other CaCO3 forms)65 and its apparent inability to up-regulate pH at the site of calcification82. Such anomalies in calcification rates and malformation or dissolution of carbonate structures due to seawater acidification are consistent with evidences on tropical corals11,84,85.
Figure 2.
Impact of acidification on the red coral sclerites. Reported are the Scanning Electron Microscopy images of the red coral’s sclerites, including either capstans (A–C and respective enlarged details a-c) or crosses (D–F and respective enlarged details d-f), and indicative of different levels of damage ranked (A,a and D,d: <0% damage; B,b and E,e: between 0% and 50%; C,c and F,f: >50%). The bar plots show the frequency of the different levels of damage to red coral capstans (G) or crosses (H) for the different experimental systems [i.e., acidified and non-acidified control mesocosms, containing C. rubrum alone or in association with the coralligenous multispecies assemblages, at the start (0 days) and at the end (86 days) of the experiment]. Reported are average values and SDs (n.d., none detected). Greek letters are used to highlight the significant differences (p < 0.01) among the reported values, with α > β > γ > δ > ε > ζ > η in panel (G), and α > β > γ > δ > ε > ζ in panel (H).
Moreover, in the acidified systems containing C. rubrum alone, the red coral colonies showed a typical physiological adaptation to stressful conditions2,3,84, with a preferential production of smaller (crosses) over larger (capstans) coral sclerites (i.e., of +66.8 ± 13.2% compared to the controls; p < 0.01; Supplementary Fig. S5). Indeed, the increased proportion of small crosses has been suggested as an adaptation of the colonies to face the acidified conditions by decreasing the production of larger capstan and skeletal structures and thus limiting the energetic cost of calcification while preserving coenenchimal stiffness2,3,84.
Nevertheless, in the present study we show that the negative impacts of acidification were reduced if C. rubrum was associated to the natural coralligenous assemblages. In fact, the acidified red coral colonies in this case displayed higher integrity of their calcium carbonate sclerites (i.e., structural damages in only 20 ± 5% of the total sclerites; p < 0.01; Fig. 2). Consistently, C. rubrum associated with the natural coralligenous assemblages did not show a decrease of its feeding and calcification activity under acidified conditions (Supplementary Fig. S5). At the same time, C. rubrum associated with the natural coralligenous assemblages maintained its ability to keep the cross-to-capstan ratio at the same values of the non-acidified controls (Supplementary Fig. S6).
Previous studies hypothesized that benthic organisms would better contrasts seawater acidification in sediments rich in carbonates, as their dissolution may stabilize pH86. As such, we tested if the mitigation of the negative effects of acidification on C. rubrum was due to some buffering effect by the carbonatic coralligenous rocks. However, our study does not support this hypothesis, as in the acidified systems the carbonates parameters and pH values showed no significant differences in the mesocosms containing the red coral alone or in association with the coralligenous assemblages (Table 1 and Supplementary Fig. S2). At the same time, the continuous monitoring of the prokaryotic and viral abundances in the mesocosms confirmed the lack of significant differences among the experimental mesocosms, indicating the absence of bacterial or viral pathogenic outbreaks, and excluding that such events could play a role in the differential response to OA (Supplementary Fig. S4).
Therefore, different mechanisms should be invoked to explain the positive effects of the natural coralligenous assemblages on the resistance of C. rubrum to acidification.
In this regard, our study reports, for the first time, a significant and positive correlation between biodiversity and the species’ resistance to acidification (Fig. 3). In fact, we found that the negative effects of acidification on the red coral (in terms of mass loss due to acidification) decreased with increasing biodiversity of the associated coralligenous assemblages (y = 0.017 × −0.113; R2 = 0.976; p < 0.01; Fig. 3a). In detail, under low pH conditions, the red coral growth rates in the most biodiverse assemblages (0.08 ± 0.02% d−1) were on average 10 times higher than in the less biodiverse ones (−0.008 ± 0.004% d−1) (Fig. 3a).
Figure 3.
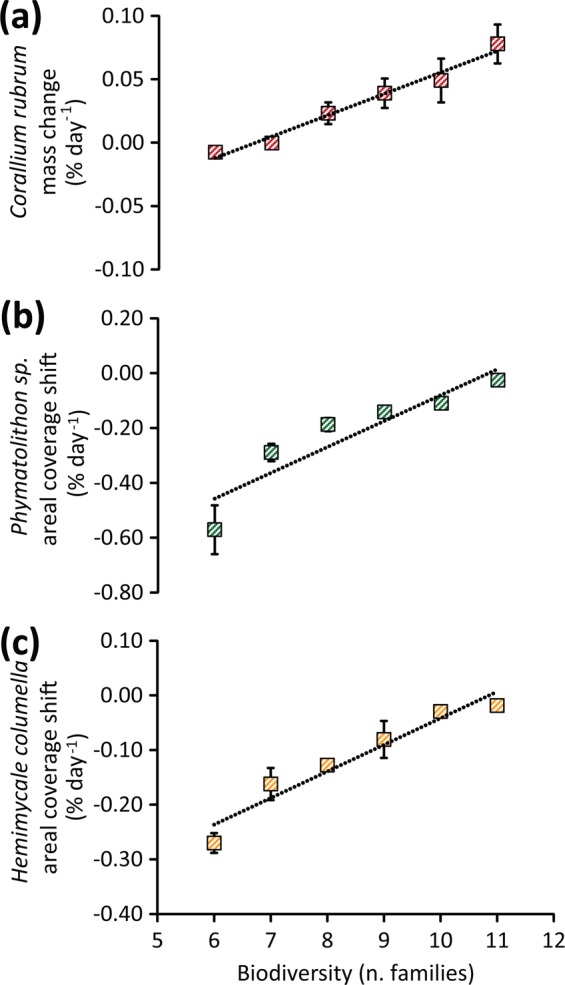
A higher biodiversity reduces the impacts of acidification. For all acidified mesocosms tested in the present study, reported are the positive relationships found between biodiversity (as number of different families contained in the natural coralligenous assemblages tested) and (a) the daily changes in mass weight of C. rubrum colonies, (b) the daily areal coverage shifts of Phymatolithon sp. (the dominant coralline alga) and (c) the daily areal coverage shifts of H. columella (the dominant epilithic sponge). Reported are average values and SDs.
Increasing biodiversity had positive effects also on the dominant coralline alga Phymatolithon sp. and the dominant epilithic sponge H. columella. These showed lower impacts of acidification (in terms of decrease in areal coverage) with higher biodiversity of the coralligenous assemblages (y = 0.094 × −1.025 and R2 = 0.846; y = 0.0486 × −0.5282 and R2 = 0.936 for the alga and the sponge, respectively; both p < 0.01; Fig. 3b,c). In detail, under acidified conditions, the decrease in areal coverage of Phymatolithon sp. in the most biodiverse assemblages (−0.03 ± 0.01% d−1) was on average 22 times lower than in the less biodiverse ones (−0.57 ± 0.09% d−1). While for H. columella, the decrease in areal coverage in the most biodiverse assemblages (−0.02 ± 0.01% d−1) was on average 14 times lower than in the less biodiverse ones (−0.27 ± 0.02% d−1).
Notably, a higher biodiversity was associated with higher growth rates of the dominant coralligenous taxa also in the non-acidified controls (Supplementary Fig. S7), indicating that this feature was consistent across control and acidified treatments. In detail, under control conditions, the growth rates of C. rubrum, Phymatolithon sp. and H. columella in the most biodiverse assemblages were respectively 1.4, 7.2 and 9.2 times higher on average than in the less biodiverse ones (0.14 ± 0.01 vs −0.010 ± 0.001% d−1 for C. rubrum; 0.15 ± 0.04 vs −0.02 ± 0.01% d−1 for Phymatolithon sp.; 0.09 ± 0.03 vs −0.010 ± 0.005% d−1 for H. columella). Overall, these results indicate that, at the highest biodiversity tested in our study, the impact of acidification on otherwise highly vulnerable key organisms could be reduced by ca. 50 to >90%, depending on the species (Fig. 3a–c).
Several factors can contribute to explain such observed positive effects of increasing biodiversity in the fostering of the species’ resistance to acidification. On one hand, our results show that higher biodiversity was associated with higher availability of organic matter (R2 = 0.844; p < 0.01; Supplementary Fig. S8). Such positive link between biodiversity and trophic enrichment is consistent with previous evidences, which show the presence of highly biodiverse coralligenous structures to increase the benthic availability of trophic resources87,88, as well as with reports of higher biodiversity in hard-bottom ecosystems associated to higher organic matter contents89. As organic matter is a fundamental source of energy in the diet of C. rubrum (also supporting calcification)90,91 and sponges92,93, its higher availability in more biodiverse multispecies assemblages might contribute to explain the enhanced feeding activity and efficiency of the red coral and sponges and hence their higher resistance to acidification as observed in the present study.
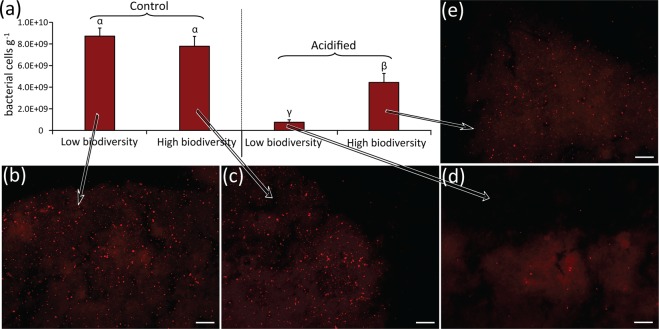
On the other hand, our study indicates that a higher biodiversity can favour and stabilise the cooperation between the large species of the coralligenous assemblages and their associated microbes and that this, in turn, can have positive effects on the species’ resistance to acidification. In fact, we report here that exposing the dominant demosponge species in our coralligenous assemblages (H. columella) to acidification caused a massive loss of the calcifying bacterial symbionts associated to its tissues (p < 0.01; Fig. 4). Nevertheless, in the more biodiverse assemblages, the loss of calcifying symbionts was much lower (p < 0.01; Fig. 4), suggesting a possible role of biodiversity in fostering a higher stability of host-microbe interactions that can increase host resistance to acidification. Our results thus add new evidence that sponges can thrive upon complex associations and symbioses with microbes93,94, and that preserving healthy microbiomes in highly biodiverse multispecies assemblages can contribute to increase the sponge resistance to acidification40,95,96.
Figure 4.
Impact of acidification on the association between the sponge Hemimycale columella and calcibacteria. The bar plot (a) reports the number of bacterial cells per gram of sponge dry weight, as determined by CARD-FISH on calcibacteria extracted from fresh H. columella sponge tissues. Average values and standard deviations are shown for samples collected at the end of the experiment (86 d) from acidified and non-acidified mesocosms containing low-biodiversity assemblages (i.e., only 6 families), or high-biodiversity assemblages (i.e., 11 families). The epifluorescence microscopy images show sections of the corresponding samples of H. columella sponge tissues from which calcibacteria were extracted, analyzed in parallel by CARD-FISH. (b), control (low-biodiversity, 6 families); (c), control (high-biodiversity, 11 families); (d), Acidified (low-biodiversity, 6 families); E, acidified (high-biodiversity, 11 families). Scale bar: 40 µm. Greek letters are used to highlight the significant differences (p < 0.01) among the reported values, with α > β > γ.
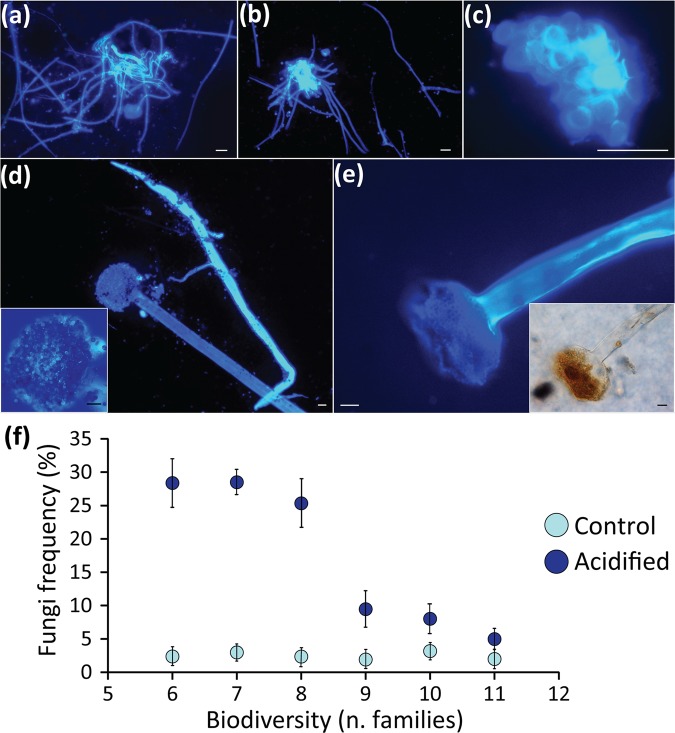
Recent evidences suggest that also unicellular fungi can be involved in complex networks of microbe-host interactions that can influence the responses of the sessile habitat-forming species to acidification97. In the present study, we only occasionally detected fungi on the surface of the coralligenous substrate at the start of the experiments (Fig. 5). However, under acidified conditions, we reported a clear fungal proliferation, with frequency of fungal detection on average 7 and up to >30 higher compared with the controls (p < 0.01; Fig. 5).
Figure 5.
Impact of acidification on unicellular fungi. Reported are microscopy images, illustrating examples of fungal structures colonizing the surface of the coralligenous assemblages tested in the present study. (a,b) fungal hyphae with septa and filamentous branching; (c) cluster of fungal spores; (d,e) sporangia with enlarged highlights showing details of the spores under epifluorescence or normal light microscopy (scale bars, 10 µm). The scatter plot (f) shows the relationships between biodiversity (as number of different families contained in the natural coralligenous assemblages tested) and the proliferation of fungi in acidified and non-acidified (control) mesocosms. Reported are average values and SDs.
This is consistent with current evidence showing that marine fungi can be favoured by acidification98,99. Notably, the proliferation of fungi decreased with increasing biodiversity (R2 = 0.847; p < 0.01; Fig. 5), suggesting that the more biodiverse coralligenous assemblages better contrasted the observed outbreak of opportunistic fungi (possibly including parasitic/pathogenic strains100,101. In Octocorallia, including the red coral C. rubrum, the sclerites can play a key role as physical and chemical barriers against fungal infection102. Therefore, our results suggest that biodiversity loss, coupled with reduced production of coral sclerites, can make the red coral more vulnerable to fungal infection, thus exacerbating the negative effects of OA on this species. Possible links between the proliferation of fungi under acidified conditions and the loss of calcifying bacteria reported in our study for H. columella, as well as with the observed decreased in the areal coverage of the dominant macroalgae might be hypothesized97 and deserve further investigation.
Another factor that can contribute to the increase of resistance to acidification at high biodiversity levels, is the fact that a higher biodiversity is associated to a higher functional redundancy26–29. Even if not specifically tested in the present study, we hypothesize that, the higher the biodiversity, the higher the probability of functional redundancy among species (considering either macro-, meio-and/or micro-organisms) which in turn can reduce the possibility to lose specific assemblage functions.
Finally, recent evidence suggested that, in coral reefs, the biogenic dissolution of carbonates by microbial borers (including endolithic bacteria, microalgae and fungi) may be a significant driver of carbonate dissolution in low pH conditions103, and this can be observed either in carbonatic substrates and on the skeleton/structures of living organisms104. We thus hypothesize that OA can not only alter microbe-host interactions but can also influence the activity of microborers, thus exacerbating the impact of acidification on the calcifying species.
Overall, our results agree with ecological theories predicting that a higher biodiversity promotes higher stability and resistance to environmental changes16,26–29,105, as well as with experimental evidences that, in biodiversity-rich assemblages, microbe-host interactions can be more stable than in low-biodiversity assemblages, due to the increased potential functional redundancy40,42,106. Our study supports the perspective that the complex networks of biotic interactions occurring between microbes and large sessile species in multispecies assemblages can significantly influence the species’ response to environmental alterations brought on by global change17,28–31,40,42,106,107. We show here that a higher biodiversity and healthy microbe-host interactions, can largely contribute to mitigate the negative impacts of acidification on otherwise highly vulnerable species. These results support the current perspective that marine conservation actions help the oceans to mitigate and adapt to climate change by promoting intact and complex ecosystems with high diversity and abundance of species108–113. Despite the understanding of the specific mechanisms underlying the observed positive effects of a high biodiversity deserve further investigation, our timely results allow highlighting that the biodiversity conservation of hard-bottom ecosystems will be crucial in the future also to increase their stability and resistance to the threats of OA.
Supplementary information
Acknowledgements
This work was supported by the project MERCES (Marine Ecosystem Restoration in Changing European Seas; European Union’s Horizon 2020 research and innovation program, grant agreement no.689518). Thanks to Barbara Calcinai and Chiara Pennesi for their support in taxonomic classification of coralligenous multispecies assemblages.
Author contributions
R.D. conceived the study, R.D., C.Co. and A.D. designed the experimental approach. B.P. and C.Ce. conducted sampling activity and species identification. B.P., E.R., M.L.M., C.Co., C.Ce. and A.D. contributed to the experimental work and laboratory analyses. E.R., B.P. and R.D. contributed to data elaboration and interpretation and all co-Authors contributed to write and to finalize the manuscript.
Data availability
All data of this study are included in the present manuscript and its Supplementary Information file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Eugenio Rastelli and Bruna Petani.
Contributor Information
Eugenio Rastelli, Email: eugenio.rastelli@szn.it.
Roberto Danovaro, Email: r.danovaro@univpm.it.
Supplementary information
is available for this paper at 10.1038/s41598-020-59886-4.
References
- 1.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean Acidification: The Other CO2 Problem. Annu. Rev. Mar. Sci. 2009;1:169–92. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- 2.Cerrano C, et al. Red coral extinction risk enhanced by ocean acidification. Sci. Rep. 2013;3:1457. doi: 10.1038/srep01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramanti L, et al. Detrimental effects of ocean acidification on the economically important Mediterranean red coral (Corallium rubrum) Glob. Change Biol. 2013;19:1897–1908. doi: 10.1111/gcb.12171. [DOI] [PubMed] [Google Scholar]
- 4.Pörtner, H. O. et al. Ocean systems. In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds: Field, C. B. et al.). Cambridge University Press, Cambridge, United Kingdom and New York, USA, pp. 411–484. (2014).
- 5.Gattuso JP, et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science. 2015;349:aac4722. doi: 10.1126/science.aac4722. [DOI] [PubMed] [Google Scholar]
- 6.Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010;13:1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin C, Rodolfo-Metalpa R, Picton B, Hall-Spencer JM. Effects of ocean acidification on sponge communities. Mar. Ecol.-Evol. Persp. 2014;35:41–49. doi: 10.1111/maec.12093. [DOI] [Google Scholar]
- 8.Martin, S., & Hall-Spencer, J. M. Effects of Ocean Warming and Acidification on Rhodolith/Maërl Beds. In Rhodolith/Maërl Beds: A Global Perspective Springer. International Publishing, pp. 55–85 (2017).
- 9.Wernberg T, Smale DA, Thomsen MS. A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob. Change Biol. 2012;18:1491–1498. doi: 10.1111/j.1365-2486.2012.02656.x. [DOI] [Google Scholar]
- 10.Kroeker KJ, Gambi MC, Micheli F. Community dynamics and ecosystem simplification in a high-CO2 ocean. Proc. Natl. Acad. Sci. USA. 2013;110:12721–12726. doi: 10.1073/pnas.1216464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster T, Falter JL, McCulloch MT, Clode PL. Ocean acidification causes structural deformities in juvenile coral skeletons. Sci. Adv. 2016;2:e1501130. doi: 10.1126/sciadv.1501130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loreau M, et al. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 13.Naeem, S., Bunker, D. E., Hector, A., Loreau, M. & Perrings, C. Biodiversity, Ecosystem-Functioning, and Human Wellbeing. Oxford University Press, New York. (2009).
- 14.Duffy JE. Why biodiversity is important to the functioning of real‐world ecosystems. Front. Ecol. Environ. 2009;7:437–444. doi: 10.1890/070195. [DOI] [Google Scholar]
- 15.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 16.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 17.Tilman D, Isbell F, Cowles JM. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 2014;45:471–493. doi: 10.1146/annurev-ecolsys-120213-091917. [DOI] [Google Scholar]
- 18.Tilman D, Reich PB, Isbell F. Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proc. Natl. Acad. Sci. USA. 2012;109:10394–10397. doi: 10.1073/pnas.1208240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper DU, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486:105–108. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 20.Paine RT. A note on trophic complexity and community stability. Am. Nat. 1969;103:91–93. doi: 10.1086/282586. [DOI] [Google Scholar]
- 21.Smith, C., et al. Report on identification of keystone species and processes across regional seas. Deliverable 6.1, DEVOTES Project. 105 pp. https://hal.archives-ouvertes.fr/hal-01790558 (2015).
- 22.Coleman FC, Williams SL. Overexploiting marine ecosystem engineers: potential consequences for biodiversity. Trends Ecol. Evol. 2002;17:40–44. doi: 10.1016/S0169-5347(01)02330-8. [DOI] [Google Scholar]
- 23.Heithaus MR, Frid A, Wirsing AJ, Worm B. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 2008;23:202–210. doi: 10.1016/j.tree.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Linares C, et al. Immediate and delayed effects of a mass mortality event on gorgonian population dynamics and benthic community structure in the NW Mediterranean Sea. Mar. Ecol. Progr. Ser. 2005;305:127–137. doi: 10.3354/meps305127. [DOI] [Google Scholar]
- 25.Ponti M, et al. Ecological shifts in Mediterranean coralligenous assemblages related to gorgonian forest loss. PLoS One. 2014;9:e102782. doi: 10.1371/journal.pone.0102782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yachi S, Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl Acad. Sci. USA. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hector A, Bagchi R. Biodiversity and ecosystem multifunctionality. Nature. 2007;448:188–90. doi: 10.1038/nature05947. [DOI] [PubMed] [Google Scholar]
- 28.Loreau M, de Mazancourt C. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol. Lett. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- 29.Mori AS, Furukawa T, Sasaki T. Response diversity determines the resilience of ecosystems to environmental change. Biol. Rev. 2013;88:349–364. doi: 10.1111/brv.12004. [DOI] [PubMed] [Google Scholar]
- 30.Nagelkerken I, Connell SD. Global alteration of ocean ecosystem functioning due to increasing human CO2 emissions. Proc. Natl. Acad. Sci. USA. 2015;112:13272–13277. doi: 10.1073/pnas.1510856112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. A framework for community interactions under climate change. Trends Ecol. Evol. 2010;25:325–331. doi: 10.1016/j.tree.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008;11:1351–1363. doi: 10.1111/j.1461-0248.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 33.Davis AJ, Jenkinson LS, Lawton JH, Shorrocks B, Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998;391:783–786. doi: 10.1038/35842. [DOI] [PubMed] [Google Scholar]
- 34.González-Megías A, Menéndez R. Climate change effects on above- and below-ground interactions in a dryland ecosystem. Philos. T. R. Soc. B. 2012;367:3115–3124. doi: 10.1098/rstb.2011.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liancourt P, et al. Plant response to climate change varies with topography, interactions with neighbors, and ecotype. Ecology. 2013;94:444–453. doi: 10.1890/12-0780.1. [DOI] [PubMed] [Google Scholar]
- 36.Suttle KB, Thomsen MA, Power ME. Species interactions reverse grassland responses to changing climate. Science. 2007;315:640–642. doi: 10.1126/science.1136401. [DOI] [PubMed] [Google Scholar]
- 37.Falkowski PG, Fenchel T, Delong EF. The microbial engines that drive earth’s biogeochemical cycles. Science. 2008;320:1034–1039. doi: 10.1126/science.1153213. [DOI] [PubMed] [Google Scholar]
- 38.Gasol, J. M., & Kirchman, D. L. Microbial ecology of the oceans. John Wiley & Sons. 528 pp. (2018).
- 39.Danovaro R, et al. Marine viruses and global climate change. FEMS Microbiol. Rev. 2011;35:993–1034. doi: 10.1111/j.1574-6976.2010.00258.x. [DOI] [PubMed] [Google Scholar]
- 40.Ribes M, et al. Restructuring of the sponge microbiome favors tolerance to ocean acidification. Environ. Microbiol. Rep. 2016;8:536–544. doi: 10.1111/1758-2229.12430. [DOI] [PubMed] [Google Scholar]
- 41.Torda G, et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Change. 2017;7:627–636. doi: 10.1038/nclimate3374. [DOI] [Google Scholar]
- 42.Bourne DG, Morrow KM, Webster NS. Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annu. Rev. Microbiol. 2016;70:317–340. doi: 10.1146/annurev-micro-102215-095440. [DOI] [PubMed] [Google Scholar]
- 43.Casas-Güell E, et al. Structure and biodiversity of coralligenous assemblages dominated by the precious red coral Corallium rubrum over broad spatial scales. Sci. Rep. 2016;6:36535. doi: 10.1038/srep36535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ballesteros E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanography and Marine Biology - An Annual Review. 2006;44:123–195. [Google Scholar]
- 45.Kipson, S. et al. Preliminary list of typical/indicator species within Croatian Coralligenous Monitoring Protocol. In Second Mediterranean Symposium on the conservation of Coralligenous and other Calcareous Bio-Concretions (pp. 219-220) RAC/SPA publ. (2014).
- 46.Garrabou J, Ballesteros E, Zabala M. Structure and dynamics of north-western Mediterranean rocky benthic communities along a depth gradient. Estuar. Coast. Shelf S. 2002;55:493–508. doi: 10.1006/ecss.2001.0920. [DOI] [Google Scholar]
- 47.Cornwall CE, et al. Diurnal fluctuations in seawater pH influence the response of a calcifying macroalga to ocean acidification. P. Roy. Soc. B-Biol. Sci. 2013;280:20132201. doi: 10.1098/rspb.2013.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivest EB, Comeau S, Cornwall CE. The role of natural variability in shaping the response of coral reef organisms to climate change. Curr. Clim. Change Rep. 2017;3:271–281. doi: 10.1007/s40641-017-0082-x. [DOI] [Google Scholar]
- 49.Vargas CA, et al. Species-specific responses to ocean acidification should account for local adaptation and adaptive plasticity. Nat. Ecol. Evol. 2017;1:0084. doi: 10.1038/s41559-017-0084. [DOI] [PubMed] [Google Scholar]
- 50.Anthony KRN, Kleypas JA, Gattuso JP. Coral reefs modify their seawater carbon chemistry-implications for impacts of ocean acidification. Glob. Change Biol. 2011;17:3655–3666. doi: 10.1111/j.1365-2486.2011.02510.x. [DOI] [Google Scholar]
- 51.Cornwall CE, et al. Resistance of corals and coralline algae to ocean acidification: physiological control of calcification under natural pH variability. P. Roy. Soc. B-Biol. Sci. 2018;285:20181168. doi: 10.1098/rspb.2018.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroeker, K. J. et al. Ecological change in dynamic environments: Accounting for temporal environmental variability in studies of ocean change biology. Glob. Change Biol. (2019). [DOI] [PubMed]
- 53.Bevilacqua S, Guarnieri G, Farella G, Terlizzi A, Fraschetti S. A regional assessment of cumulative impact mapping on Mediterranean coralligenous outcrops. Sci. Rep. 2018;8:1757. doi: 10.1038/s41598-018-20297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zunino S, Canu DM, Zupo V, Solidoro C. Direct and indirect impacts of marine acidification on the ecosystem services provided by coralligenous reefs and seagrass systems. Glob. Ecol. Conserv. 2019;18:e00625. doi: 10.1016/j.gecco.2019.e00625. [DOI] [Google Scholar]
- 55.Dickson, A. G., Sabine, C. L., & Christian, J. R. Guide to Best Practices for Ocean CO2 Measurements. PICES Special Publication. 3, 191 pp. (2007).
- 56.Pelletier, G. J., Lewis, E., Wallace, D. W. R. CO2SYS.XLS: a Calculator for the CO2 System in Seawater for Microsoft Excel/VBA. Version 16. Washington State Department of Ecology, http://www.ecy.wa.gov/programs/eap/models.html (2011).
- 57.Danovaro, R. Methods for the Study of Deep-sea Sediments, Their Functioning and Biodiversity. CRC Press, BocaRaton, FL, pp. 414 (2010).
- 58.Pusceddu A, Dell’Anno A, Fabiano M, Danovaro R. Quantity and bioavailability of sediment organic matter as signatures of benthic trophic status. Mar. Ecol. Progr. Ser. 2009;375:41–52. doi: 10.3354/meps07735. [DOI] [Google Scholar]
- 59.Trygonis V, Sini M. photoQuad: A dedicated seabed image processing software, and a comparative error analysis of four photoquadrat methods. J. Exp. Mar. Biol. Ecol. 2012;424:99–108. doi: 10.1016/j.jembe.2012.04.018. [DOI] [Google Scholar]
- 60.Cabioc’h, J. et al. Guide des algues des mers d’Europe. Manche et Atlantique. Méditerranée Ed.: Delachaux et Niestlé, Paris. 272 pp. (2006).
- 61.Calcinai B, et al. Comparison between the sponge fauna living outside and inside the coralligenous bioconstruction. A quantitative approach. Mediterr. Mar. Sci. 2015;16:413–418. doi: 10.12681/mms.900. [DOI] [Google Scholar]
- 62.Form AU, Riebesell U. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Change Biol. 2012;18:843–853. doi: 10.1111/j.1365-2486.2011.02583.x. [DOI] [Google Scholar]
- 63.Jokiel, P. L., Maragos, J. E., & Franzisket, L. Coral growth: buoyant weight technique. Coral reefs: research methods. UNESCO, Paris, 529–541 (1978).
- 64.Tsounis G, Rossi S, Gili JM, Arntz WE. Red coral fishery at the Costa Brava (NW Mediterranean): case study of an overharvested precious coral. Ecosystems. 2007;10:975–986. doi: 10.1007/s10021-007-9072-5. [DOI] [Google Scholar]
- 65.Ries JB, Cohen AL, McCorkle DC. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology. 2009;37:1131–1134. doi: 10.1130/G30210A.1. [DOI] [Google Scholar]
- 66.Manzello DP. Coral growth with thermal stress and ocean acidification: lessons from the eastern tropical Pacific. Coral Reefs. 2010;29:749–758. doi: 10.1007/s00338-010-0623-4. [DOI] [Google Scholar]
- 67.Debreuil J, et al. Comparative analysis of the soluble organic matrix of axial skeleton and sclerites of Corallium rubrum: Insights for biomineralization. Comp. Biochem. Phys. B. 2011;159:40–48. doi: 10.1016/j.cbpb.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Gabay Y, Fine M, Barkay Z, Benayahu Y. Octocoral tissue provides protection from declining oceanic pH. PloS One. 2014;9:e91553. doi: 10.1371/journal.pone.0091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel A, et al. Virus and prokaryote enumeration from planktonic aquatic environments by epifluorescence microscopy with SYBR Green I. Nat. Protoc. 2007;2:269–276. doi: 10.1038/nprot.2007.6. [DOI] [PubMed] [Google Scholar]
- 70.Garate L, Blanquer A, Uriz MJ. Calcareous spherules produced by intracellular symbiotic bacteria protect the sponge Hemimycale columella from predation better than secondary metabolites. Mar. Ecol. Progr. Ser. 2015;523:81–92. doi: 10.3354/meps11196. [DOI] [Google Scholar]
- 71.Uriz MJ, Agell G, Blanquer A, Turon X, Casamayor EO. Endosymbiotic calcifying bacteria: a new cue to the origin of calcification in metazoa? Evolution. 2012;66:2993–299. doi: 10.1111/j.1558-5646.2012.01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pernthaler A, Pernthaler J, Amann R. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 2002;68:3094–3101. doi: 10.1128/AEM.68.6.3094-3101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barone G, et al. Benthic deep-sea fungi in submarine canyons of the Mediterranean Sea. Progr. Oceanogr. 2018;168:57–64. doi: 10.1016/j.pocean.2018.09.011. [DOI] [Google Scholar]
- 74.Anderson, M. J. Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef: Statistics Reference Online, 1–15 (2014).
- 75.R Core Team R. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ (2014).
- 76.Anderson, M. J., Gorley, R. N. & Clarke, K. R. Permanova+ for Primer: Guide to Software and Statistical Methods. Primer-E, Plymouth, UK. (2008).
- 77.Regione Liguria online portal. http://rgetrasweb.regione.liguria.it/qpg/Tree.do?codNodo=3282.
- 78.Flecha S, et al. Trends of pH decrease in the Mediterranean Sea through high frequency observational data: indication of ocean acidification in the basin. Sci. Rep. 2015;5:16770. doi: 10.1038/srep16770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hassoun AER, et al. Acidification of the Mediterranean Sea from anthropogenic carbon penetration. Deep-Sea Res. Pt. I. 2015;102:1–15. doi: 10.1016/j.dsr.2015.04.005. [DOI] [Google Scholar]
- 80.Bradassi F, Cumani F, Bressan G, Dupont S. Early reproductive stages in the crustose coralline alga Phymatolithon lenormandii are strongly affected by mild ocean acidification. Mar. Biol. 2013;160:2261–2269. doi: 10.1007/s00227-013-2260-2. [DOI] [Google Scholar]
- 81.Noisette F, Egilsdottir H, Davoult D, Martin S. Physiological responses of three temperate coralline algae from contrasting habitats to near-future ocean acidification. J. Exp. Mar. Biol. Ecol. 2013;448:179–187. doi: 10.1016/j.jembe.2013.07.006. [DOI] [Google Scholar]
- 82.Le Goff C, et al. In vivo pH measurement at the site of calcification in an octocoral. Sci. Rep. 2017;7:11210. doi: 10.1038/s41598-017-10348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Previati M, Scinto A, Cerrano C, Osinga R. Oxygen consumption in Mediterranean octocorals under different temperatures. J. Exp. Mar. Biol. Ecol. 2010;390:39–48. doi: 10.1016/j.jembe.2010.04.025. [DOI] [Google Scholar]
- 84.Cohen AL, Holcomb M. Why corals care about ocean acidification: uncovering the mechanism. Oceanography. 2009;22:118–127. doi: 10.5670/oceanog.2009.102. [DOI] [Google Scholar]
- 85.Andersson AJ, Gledhill D. Ocean acidification and coral reefs: effects on breakdown, dissolution, and net ecosystem calcification. Annu. Rev. Mar Sci. 2013;5:321–348. doi: 10.1146/annurev-marine-121211-172241. [DOI] [PubMed] [Google Scholar]
- 86.Blackford J, et al. Detection and impacts of leakage from sub-seafloor deep geological carbon dioxide storage. Nat. Clim. Change. 2014;4:1011–1016. doi: 10.1038/nclimate2381. [DOI] [Google Scholar]
- 87.Cerrano C, et al. Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodiv. Conserv. 2010;19:153–167. doi: 10.1007/s10531-009-9712-5. [DOI] [Google Scholar]
- 88.Bianchelli S, Pusceddu A, Canese S, Greco S, Danovaro R. High meiofaunal and nematodes diversity around mesophotic coral oases in the Mediterranean Sea. PloS One. 2013;8:e66553. doi: 10.1371/journal.pone.0066553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bianchelli S, Buschi E, Danovaro R, Pusceddu A. Biodiversity loss and turnover in alternative states in the Mediterranean Sea: a case study on meiofauna. Sci. Rep. 2016;6:34544. doi: 10.1038/srep34544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsounis G, et al. Diet and seasonal prey capture rates in the Mediterranean red coral (Corallium rubrum L.) Mar. Biol. 2006;149:313–325. [Google Scholar]
- 91.Anthony KR, Fabricius KE. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Biol. Ecol. 2000;252:221–253. doi: 10.1016/S0022-0981(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 92.De Goeij JM, et al. Surviving in a marine desert: the sponge loop retains resources within coral reefs. Science. 2013;342:108–110. doi: 10.1126/science.1241981. [DOI] [PubMed] [Google Scholar]
- 93.Rix L, et al. Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop. Mar. Ecol. Progr. Ser. 2018;589:85–96. doi: 10.3354/meps12443. [DOI] [Google Scholar]
- 94.Bayer K, Kamke J, Hentschel U. Quantification of bacterial and archaeal symbionts in high and low microbial abundance sponges using real-time PCR. FEMS Microbiol. Ecol. 2014;89:679–690. doi: 10.1111/1574-6941.12369. [DOI] [PubMed] [Google Scholar]
- 95.Bordenstein SR, Theis KR. Host biology in light of the microbiome: ten principles of holobionts and hologenomes. PLoS Biol. 2015;13:e1002226. doi: 10.1371/journal.pbio.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hester ER, Barott KL, Nulton J, Vermeij MJ, Rohwer FL. Stable and sporadic symbiotic communities of coral and algal holobionts. ISME J. 2016;10:1157–1169. doi: 10.1038/ismej.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Williams GJ, et al. Ocean warming and acidification have complex interactive effects on the dynamics of a marine fungal disease. Proc. R. Soc. B-Biol. Sci. 2014;281:20133069. doi: 10.1098/rspb.2013.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krause E, Wichels A, Giménez L, Gerdts G. Marine fungi may benefit from ocean acidification. Aquat. Microb. Ecol. 2013;69:59–67. doi: 10.3354/ame01622. [DOI] [Google Scholar]
- 99.Krause E, Wichels A, Erler R, Gerdts G. Study on the effects of near-future ocean acidification on marine yeasts: a microcosm approach. Helgoland Mar. Res. 2013;67:607. doi: 10.1007/s10152-013-0348-1. [DOI] [Google Scholar]
- 100.Peters Esther C. Coral Reefs in the Anthropocene. Dordrecht: Springer Netherlands; 2015. Diseases of Coral Reef Organisms; pp. 147–178. [Google Scholar]
- 101.Jephcott TG, et al. Ecological impacts of parasitic chytrids, syndiniales and perkinsids on populations of marine photosynthetic dinoflagellates. Fungal Ecol. 2016;19:47–58. doi: 10.1016/j.funeco.2015.03.007. [DOI] [Google Scholar]
- 102.Toledo-Hernández C, et al. The role of sclerites in the defense against pathogens of the sea fan Gorgonia ventalina (Octocorallia) J. Exp. Mar. Biol. Ecol. 2016;483:20–24. doi: 10.1016/j.jembe.2016.06.002. [DOI] [Google Scholar]
- 103.Tribollet A, Godinot C, Atkinson M, Langdon C. Effects of elevated pCO2 on dissolution of coral carbonates by microbial euendoliths. Global Biogeochem. Cy. 2009;23:GB3008. doi: 10.1029/2008GB003286. [DOI] [Google Scholar]
- 104.Tribollet A. Dissolution of dead corals by euendolithic microorganisms across the northern Great Barrier Reef (Australia) Microb. Ecol. 2008;55:569–580. doi: 10.1007/s00248-007-9302-6. [DOI] [PubMed] [Google Scholar]
- 105.Cadotte MW, Dinnage R, Tilman D. Phylogenetic diversity promotes ecosystem stability. Ecology. 2012;93:S223–S233. doi: 10.1890/11-0426.1. [DOI] [Google Scholar]
- 106.Louca S, et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018;2:936–943. doi: 10.1038/s41559-018-0519-1. [DOI] [PubMed] [Google Scholar]
- 107.Konopka A, Lindemann S, Fredrickson J. Dynamics in microbial communities: unraveling mechanisms to identify principles. ISME J. 2015;9:1488–1495. doi: 10.1038/ismej.2014.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Micheli F, et al. Evidence that marine reserves enhance resilience to climatic impacts. PloS one. 2012;7:e40832. doi: 10.1371/journal.pone.0040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roberts CM, et al. Marine reserves can mitigate and promote adaptation to climate change. Proc. Natl. Acad. Sci. USA. 2017;114:6167–6175. doi: 10.1073/pnas.1701262114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hisano M, Searle EB, Chen HY. Biodiversity as a solution to mitigate climate change impacts on the functioning of forest ecosystems. Biol. Rev. 2018;93:439–456. doi: 10.1111/brv.12351. [DOI] [PubMed] [Google Scholar]
- 111.Hisano M, Chen HY, Searle EB, Reich PB. Species-rich boreal forests grew more and suffered less mortality than species-poor forests under the environmental change of the past half-century. Ecol. Lett. 2019;22:999–1008. doi: 10.1111/ele.13259. [DOI] [PubMed] [Google Scholar]
- 112.Duffy JE, Lefcheck JS, Stuart-Smith RD, Navarrete SA, Edgar GJ. Biodiversity enhances reef fish biomass and resistance to climate change. Proc. Natl. Acad. Sci. USA. 2016;113:6230–6235. doi: 10.1073/pnas.1524465113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mellin C, Aaron MacNeil M, Cheal AJ, Emslie MJ, Julian Caley M. Marine protected areas increase resilience among coral reef communities. Ecol. Lett. 2016;19:629–637. doi: 10.1111/ele.12598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data of this study are included in the present manuscript and its Supplementary Information file.