Summary
Despite many years of work on dopaminergic mechanisms of alcohol addiction, much of the evidence remains mostly correlative in nature. Fortunately, recent technological advances have provided the opportunity to explore the causal role of alterations in neurotransmission within circuits involved in addictive behaviors. Here, we address this critical gap in our knowledge by integrating an optogenetic approach and an operant alcohol self-administration paradigm to assess directly how accumbal dopamine (DA) release dynamics influences the appetitive (seeking) component of alcohol-drinking behavior. We show that appetitive reward-seeking behavior in rats trained to self-administer alcohol can be shaped causally by ventral tegmental area-nucleus accumbens (VTA-NAc) DA neurotransmission. Our findings reveal that phasic patterns of DA release within this circuit enhance a discrete measure of alcohol seeking, whereas tonic patterns of stimulation inhibit this behavior. Moreover, we provide mechanistic evidence that tonic-phasic interplay within the VTA-NAc DA circuit underlies these seemingly paradoxical effects.
Subject Areas: Animal Physiology, Neuroscience, Behavioral Neuroscience
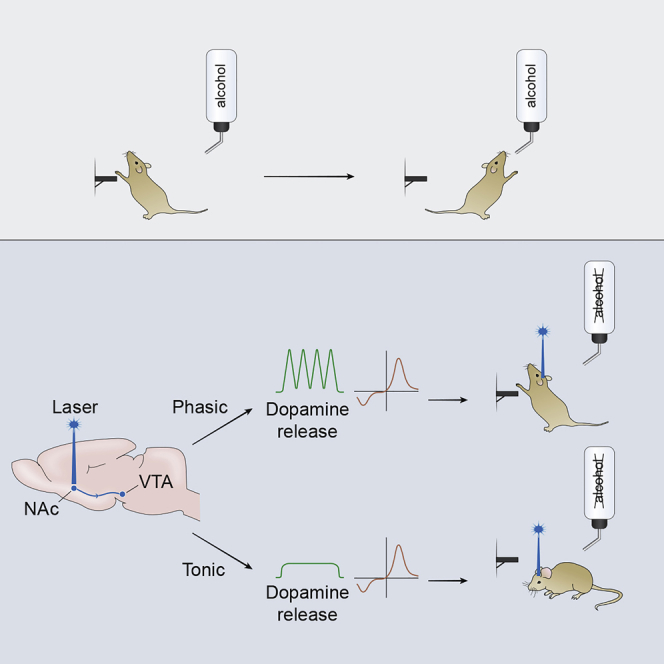
Graphical Abstract
Highlights
-
•
VTA-NAc DA transmission can bidirectionally modulate motivated behavior
-
•
Optogenetic increases in phasic DA release in the NAc enhance alcohol seeking
-
•
Optogenetic increases in tonic DA release in the NAc inhibit alcohol seeking
-
•
Phasic DA release can be decreased by the concurrent tonic activation
Animal Physiology; Neuroscience; Behavioral Neuroscience
Introduction
Alcohol use disorder (AUD) is a widespread neuropsychiatric disease, which can be defined as a pathological motivation to seek and consume alcohol. During the last several decades, researchers have sought to identify the neurobiological substrates responsible for these pathological drinking behaviors. The mesolimbic dopamine (DA) system has, perhaps, received the most attention as a key pathway that may become dysregulated in AUD and other addictive disorders. Indeed, many compelling studies have demonstrated that chronic exposure to all drugs of abuse promotes maladaptive alterations within the DAergic projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) (Volkow et al., 2011, Volkow and Morales, 2015, Koob and Volkow, 2016). Moreover, some data support the notion that VTA-NAc DA signaling can be involved in several elements associated with the development of addictive behavior, including reinforcement learning (Day et al., 2007, Flagel et al., 2011, Steinberg et al., 2013, Hart et al., 2014, Chang et al., 2016, Parker et al., 2011, Darvas et al., 2014), motivated drug and reward seeking (Phillips et al., 2003, Stuber et al., 2012, Wassum et al., 2013, Pascoli et al., 2015, Halbout et al., 2019), and drug and reward intake (Volkow et al., 2007, Mikhailova et al., 2016). However, despite more than 30 years of work on this topic, much of this evidence remains mostly correlative in nature with only a paucity of direct evidence that mesolimbic DA signaling regulates alcohol-drinking behaviors.
Fortunately, recent technological advances have provided the opportunity to explore the causal role of alterations in neurotransmission within circuits involved in addictive behaviors. To that end, recent studies have provided the first direct evidence that VTA-NAc DA neurotransmission directly modulates alcohol consumption (Bass et al., 2013, Juarez et al., 2017).
Importantly, alcohol drinking, as with many other addictive behaviors, can be deconstructed into distinct appetitive and consummatory components (Samson and Czachowski, 2003). Appetitive elements are those involved in seeking (motivated) behaviors directly related to the procurement of alcohol, whereas consummatory elements are behaviors associated with the actual act of drinking. Although dysregulation of both these processes likely contributes to the etiology of AUD, appetitive and consummatory behaviors can be differentially regulated and are likely mediated by distinct neural circuits. Notably, the first studies that sought to establish a causal role of mesolimbic DA signaling in alcohol drinking employed a procedure that did not allow for the discrete assessment of the seeking component of this behavior (Bass et al., 2013, Juarez et al., 2017). Here, we address this critical gap in our knowledge by integrating optogenetic stimulation of VTA-NAc DA release and an operant alcohol self-administration regimen that can dissociate appetitive and consummatory measures to directly assess how accumbal DA release dynamics influences the appetitive (motivational or seeking) component of alcohol drinking behavior. It should be pointed out that the chosen parameters of optostimulations were previously used to mimic tonic and phasic increases in DA transmission in behaving animals (Tsai et al., 2009, Adamantidis et al., 2011, Bass et al., 2013, Mikhailova et al., 2016). These stimulation protocols did not result in any behavioral indications of nonphysiological conditions such as hypo- or hyperdopaminergic states (Tsai et al., 2009, Bass et al., 2013, Mikhailova et al., 2016).
We reveal that phasic and tonic VTA-NAc DA release patterns bidirectionally modulate an appetitive alcohol drinking-related behavior. Specifically, we demonstrated that a high-frequency stimulation pattern (50 Hz) that evoked DA transients with temporal and concentration features similar to real-time DA fluctuations observed during drug-seeking behavior (Phillips et al., 2003, Owesson-White et al., 2009) resulted in an escalation of alcohol seeking. In contrast, applying low-frequency stimulation (5 Hz), and therefore shifting DA release into the tonic mode, where cells simultaneously fire at their basal frequency (Hyland et al., 2002, Floresco et al., 2003), suppressed this behavior. We also provide mechanistic evidence that tonic-phasic interplay within the VTA-NAc DA circuit underlies these seemingly puzzling effects on alcohol seeking.
Results
Viral Infusion and Operant Alcohol Self-Administration
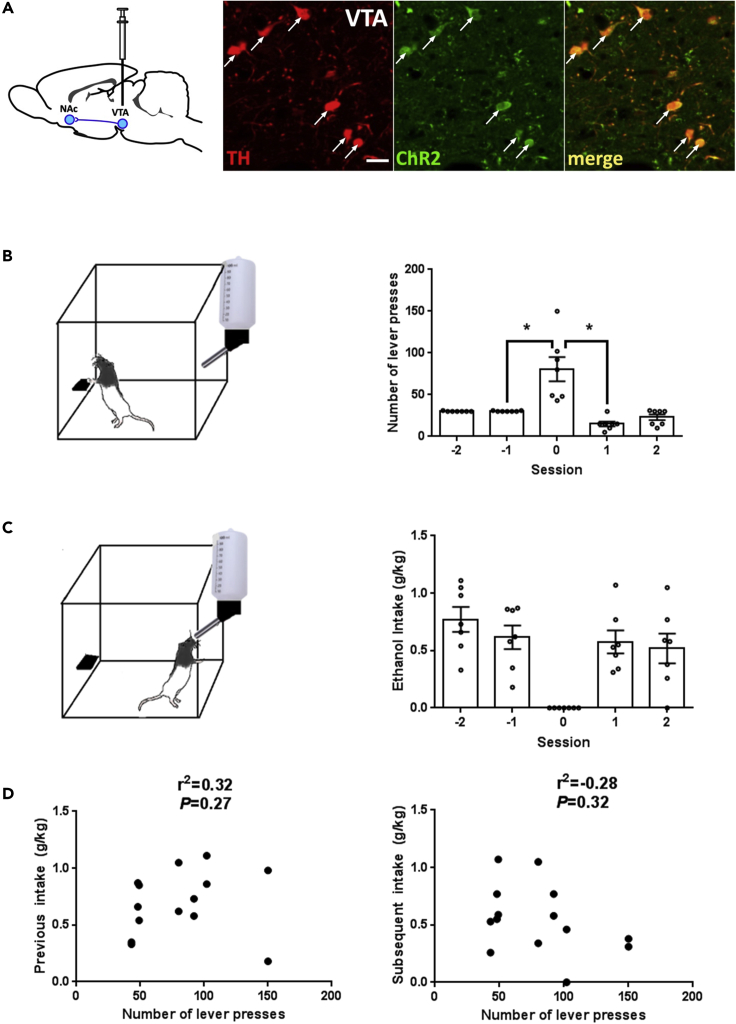
Adult male rats (n = 7) were trained to complete a daily 30 lever press response requirement to gain 20-min access to a sipper tube containing a 10% ethanol solution. After subjects displayed stable drinking behavior (Figure S1), they were anesthetized and received intra-VTA injections of an adeno-associated viral (AAV) construct, which restricted ChR2 expression to tyrosine hydroxylase (TH)-positive neurons (Fox et al., 2016, Mikhailova et al., 2016). Two days following the surgery, rats were returned to the operant drinking procedure, where they quickly resumed their original levels of alcohol intake (≈1.0 g/kg/20 min). These values were normally distributed (p = 0.204, Shapiro normality test). No significant difference was revealed between alcohol consumption measured as the weekly average per animal before and after the surgery (0.99 ± 0.18 g/kg versus 1.14 ± 0.14 g/kg, p = 0.527, paired t test). Four weeks after AAV infusion, robust colocalization of ChR2-EYFP and TH expression was observed in the VTA (Figure 1A, 97.2% ± 1.5% of ChR2-positive cells were also TH positive; n = 5) along with ChR2-positive terminals (Figure S2).
Figure 1.
Optogenetically Targeting DA Cells within the VTA of Rats Consuming Ethanol in an Operant Behavior Test
(A) Rats, previously trained to press a lever daily to gain access to a 10% ethanol solution for 20 min (see Figure S1), were anesthetized and injected in the VTA with a combination of DIO-ChR2-EYFP-AAV2/10 and TH-iCRE-AA2/10 via a Hamilton syringe (left panel). Immunohistochemical analysis confirmed that ChR2-EYFP was largely restricted to TH-positive cells in the targeted area (right panel). Scale bar = 25 μm. See Figure S2 for the nucleus accumbens.
(B) Individual data from seven rats are presented. Following virus injection, subjects acquired the 30 lever press response requirement. As expected, rats exhibited a significant increase in the number of lever presses when their action was not reinforced throughout the session (session 0). *p < 0.05 relative to reinforced session (Kruskal-Wallis test and Wilcoxon matched-pairs signed rank test), which was performed before (−1) or after (1) the non-reinforced session (session 0). Data are presented as mean ± SEM.
(C) Ethanol consumption was not significantly different between daily reinforced tests conducted prior to and following the extinction probe trials (p > 0.05). Data are mean ± SEM.
(D) No significant correlation was observed between lever responding during a 20-min non-reinforced session (extinction probe trial) and the amount of ethanol consumed on the two reinforced sessions preceding (left figure) or following (right figure) an extinction test (p > 0.05).
Operant Responding during Extinction Trial and Reinforced Sessions
To isolate a measure of alcohol seeking devoid of any consummatory behaviors, extinction probe trials were conducted once per week. During these trials, subjects were presented with all the alcohol-related cues, but completion of the lever press response requirement was not reinforced. Total lever presses during these 20-min extinction sessions represent a well-validated measure of appetitive or seeking behavior (Samson and Czachowski, 2003). As previously shown, lever press responding increased almost 3-fold during extinction trials (Figure 1B). As expected, the values obtained in the two reinforcing sessions that preceded the extinction trial were not normally distributed (p < 0.0003, Shapiro normality test) due to the ceiling effect of the 30 press requirement, whereas the data collected during the trial and the next session passed the normality test (p = 0.297 and p = 0.089). Therefore, statistical differences between the responses during the reinforced session and the extinction trial were confirmed using non-parametric analyses (Kruskal-Wallis and Wilcoxon matched-pairs signed rank test). The non-parametric one-way ANOVA on ranks revealed a significant difference between the sessions (p = 0.0001), whereas pairwise non-parametric tests confirmed significant differences between the extinction trial and reinforced sessions (p < 0.05).
Relationship between Alcohol Consummatory and Seeking Behaviors
Whether extinction trials can modify alcohol intake during subsequent reinforced sessions was also studied (Figure 1C). These values were normally distributed (p > 0.222, Shapiro normality test) and therefore analyzed with a repeated measures ANOVA test. No effect of the extinction trial on subsequent alcohol intake was found (F(2.445, 14.67) = 0.9918; p > 0.05).
Using Spearman correlation analysis, no relationship was observed between the number of lever presses completed during extinction trials and alcohol intake on the two reinforced sessions immediately preceding, or following, extinction probe trials (p > 0.05) (Figure 1D). Therefore, our data support the notion that consummatory and appetitive behaviors are not correlated with each other and are likely mediated by distinct neural circuits. It should also be noted that prior studies using this model have reported blood ethanol concentrations in excess of 60 mg% in cohorts with a similar range of intakes (ranged from 0.2 to 1.2 g/kg), blood alcohol levles that fall within the intoxicating range (Samson and Czachowski, 2003).
Optogenetic Manipulation of DA Release in the NAc
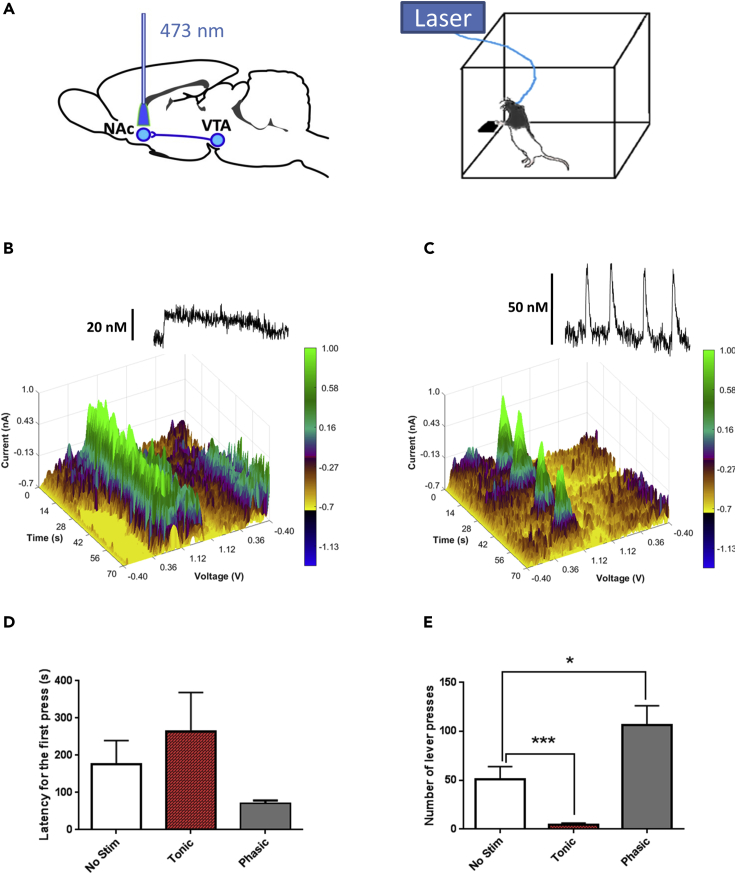
To determine the effect of VTA-NAc DA release on extinction trial responding, optical stimulations were delivered directly to the NAc during the first 10 min of these non-reinforced sessions (Figure 2A). To confirm that the optogenetic manipulations of DA release were effective, separate experiments, using in vivo fast scan cyclic voltammetry (FSCV), were conducted on another cohort of anesthetized rats (n = 7). Both phasic and tonic activation of the VTA-NAc circuitry resulted in substantial increases in accumbal DA concentrations (Figures 2B, 2C, and S3). These data passed the Shapiro-Wilk normality test (p > 0.868). The lower-frequency stimulation (5 Hz) applied for longer periods (60 s) mimicked tonic increase in DA dynamics (Figure 2B). However, brief (1 s) high-frequency (50 Hz) stimulations induced phasic patterns of NAc DA release (Figure 2C). As expected, the maximal amplitude of phasic DA efflux was significantly greater than the amplitude of tonic release (p = 0.002, unpaired t test) (Figure S3).
Figure 2.
Optogenetically Manipulating DA Release in the Rat NAc during Ethanol-Seeking Behavior and under Anesthetized Conditions
(A) Schematic configuration for optical activation of accumbal DA terminals that was used to trigger tonic and phasic patterns of DA transmission.
(B) Tonic DA release patterns were triggered by blue light applied for 1 min at 5 Hz with 1-s intervals between stimulations (10 min total). A three-dimensional color plot topographically represents electrochemical changes detected with voltammetry in the rat NAc over 70 s.
(C) Phasic DA release patterns were induced by blue light stimulation (1 s, 50 Hz) with 15-s intervals between each pulse train for the first 10 min of the operant session. A color plot depicts the increase in DA release (uninterrupted green rise) that is time locked to optical activation. Four divided green spikes denote DA transients, which are time-locked to optical stimulations. Therefore, two main patterns of DA transmission, phasic and tonic, could be optogenetically mimicked during the operant behavior test. See also Figures S3–S5.
(D and E) Changes in alcohol-seeking behavior of rats (n = 7) were evaluated by measures of the latency to the first lever press and total number of lever presses during non-reinforced sessions. (D) No significant changes in the latency to the first press were found between sessions with (tonic and phasic) and without stimulations (p > 0.05). (E) Shifting DA release in the NAc into a tonic mode resulted in a significant decrease in the number of lever presses. However, phasic activation of the NAc significantly increased this measure.
*p < 0.05; ***p < 0.001 (Mann-Whitney). Data are mean ± SEM.
Bidirectional Modulation of Alcohol-Seeking Behavior through Tonic or Phasic Stimulations
Neither pattern significantly altered the latency to the first lever press (p > 0.05, Mann-Whitney test) (Figure 2D). However, driving accumbal DA transmission into a tonic pattern dramatically decreased the number of lever presses (p = 0.0008, Mann-Whitney test), whereas phasic activation significantly increased this measure of seeking behavior (p = 0.034, Mann-Whitney test) (Figure 2E). Noticeably, both latency and lever press values were not normally distributed in the non-reinforced session without optogenetic stimulation (p = 0.008 and p = 0.036, respectively; Shapiro-Wilk test).
Our previous study demonstrated that phasic optoactivation of DA neurons with ChR2 expression facilitates reward-seeking behavior in operant tests (Adamantidis et al., 2011). Expectedly, no changes in this behavior following the same stimulation were observed in control animals, which were injected with AAV:eYFP (Adamantidis et al., 2011). This is in good agreement with multiple reports where light stimulation of mesolimbic DA neurons did not modify operant responses in the absence of opsin expression (Steinberg et al., 2013, Steinberg et al., 2014, Fischbach and Janak, 2019, Ilango et al., 2014). Therefore, it is unlikely that the bidirectional regulation of alcohol-seeking behavior observed following contrasting stimulation patterns resulted from artifactual activation of the brain tissue. Nevertheless, we explored the possibility that photostimulation itself (in the absence of ChR2 expression) could affect DA level (Figure S4). Repeated measures one-way ANOVAs found no significant effect of time on the signal during either high- (50 Hz; F(2.911, 11.64) = 0.7586, p = 0.536) or low- (5 Hz; F(3.498, 13.99) = 1.192, p = 0.354) frequency optical stimulation. However, profound DA release was triggered with an electrical stimulation under the same experimental conditions (F(1.807, 7.228) = 12.03, p < 0.01).
Exploring the Interplay between Optically Induced Tonic and Phasic DA Release in the NAc
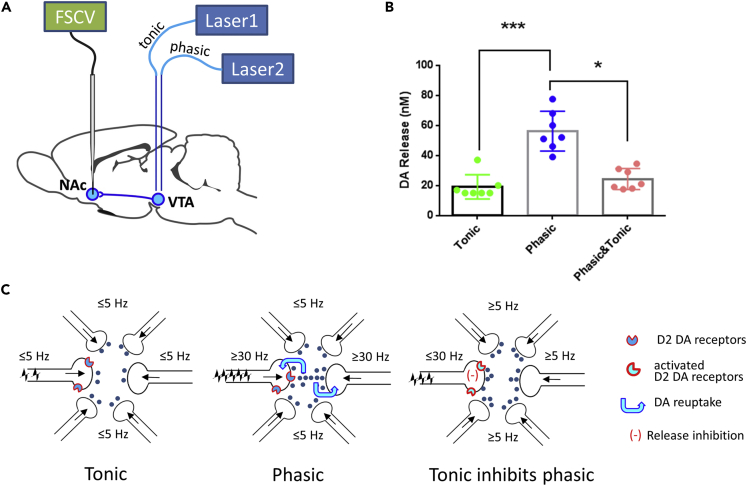
Why do tonic and phasic stimulation of VTA-NAC DA release have opposite effects on extinction probe trial responding? The finding that optically generating phasic patterns of accumbal DA activity promotes alcohol-seeking behavior is actually consistent with the findings of many prior studies that have revealed correlative relationships between subsecond phasic DA release and appetitive behavior for other reinforcers. If phasic DA release promotes alcohol seeking, one possible explanation for the decrease in extinction probe trial responding observed following tonic stimulation of accumbal DA terminals is that the sustained, low-frequency stimulation may prevent DA terminals from engaging in the phasic release that drives appetitive behavior. To explore this possibility, we combined in vivo optogenetic and FSCV methods to examine directly real-time interactions between tonic and phasic patterns of DA release in the NAc. Two optical fibers from separate lasers were inserted above the VTA of anesthetized rats (n = 7) that had been injected with the TH-ChR2 virus and a microelectrode for voltammetric detection was positioned in the NAc (Figure 3A). This configuration allowed for the measurement of real-time, subsecond NAc DA changes evoked by tonic or phasic patterns of VTA DA cell stimulation or by simultaneous delivery of both patterns.
Figure 3.
The Interaction between Phasic and Tonic DA Release Triggered by Optogenetic Activation of VTA-NAc DA Synapses
(A) Schematic representation of experiments, which combined real-time DA measurements performed with FSCV in the NAc and light activation of the VTA. Two optical fibers connected with separate lasers were inserted above the VTA of anesthetized rats (n = 7) to mimic coinciding tonic and phasic patterns of accumbal DA transmission with 5- and 50-Hz stimulations, respectively. See also Figures S3 and S4.
(B) The amplitude of phasic DA release was significantly higher than tonic DA elevation. However, combining tonic and phasic stimulations resulted in a reduced amplitude of DA release compared with that which was triggered by phasic activation alone. *p < 0.05; ***p < 0.001 (Kruskal-Wallis test and Dunn's multiple comparison test). Data are mean ± SEM.
(C) A simplified model of neurochemical events that take place at the level of presynaptic terminals when DA releases with tonic (left), phasic (middle), or overlapping (right) patterns. Tonic: when cells fire with low frequency (≤5 Hz), DA releases with a distinct pattern, which results in a relatively low but stable DA concentration in the extrasynaptic space. Phasic: when some cells begin to fire with higher frequency (≥30 Hz) and this firing is synchronized, higher DA concentrations are released. These DA transients exist briefly (from subseconds to 1–2 s), because the re-uptake mechanism through the DA transporter rapidly eliminates them (blue arrows), taking DA inside terminals. Phasic DA activity can be downregulated by a presynaptic feedback mechanism. This happens when DA transmission is shifting into the tonic mode (the majority of cells fire at a frequency of 5 Hz). The tonic DA concentrations activate D2 DA autoreceptors, and this action results in an inhibition of high frequency-induced phasic DA release.
As values of DA response to tonic stimulation were not normally distributed (p = 0.0004), a non-parametric test (Kruskal-Wallis) was used for the statistical analysis of these results (Figure 3B). As previously shown, phasic stimulation elicited higher elevations in DA concentrations relative to tonic optical activation (p < 0.001, Dunn's multiple comparisons test). Importantly, tonic stimulation delivered concurrently with phasic stimulation inhibited the large phasic NAc DA responses, yielding a response that was not different from tonic stimulation alone (p > 0.05, Dunn's multiple comparisons test).
A simplified model of the interaction between tonic and phasic modes of DA transmission is illustrated in Figure 3C. This model illustrates our interpretation of how optogenetically induced changes in alcohol-seeking behavior arise because of changes in DA dynamics at the level of accumbal terminals. Under basal conditions, VTA DA cells fire at a low frequency (4–6 Hz), resulting in a modest, but stable, DA concentration. Brief increases in the firing rate (≥30 Hz) lead to rapid DA transients, which are quickly terminated via re-uptake mechanisms. Importantly, this phasic pattern of DA activity has previously been observed in association with drug- and reward-seeking behaviors (Phillips et al., 2003, Owesson-White et al., 2009, Stuber et al., 2012, Wassum et al., 2013). Reproducing this pattern by high-frequency (50 Hz) optogenetic stimulation of accumbal DA terminals during an operant test leads to an increase in alcohol seeking. However, optogenetic delivery of 5-Hz stimulation during the period of lever presses for alcohol forces DA transmission into the tonic mode. Under this condition, more cells simultaneously fire with their maximal rate limited to the tonic firing rate of these cells, providing DA levels sufficient to activate presynaptic DA autoreceptors that regulate release of the neurotransmitter. The activation of this presynaptic feedback mechanism results in an inhibition of phasic DA release and, as a consequence, aclohol-seeking behavior is suppressed.
Discussion
Here, we show that appetitive reward-seeking behavior in rats trained to self-administer alcohol can be causally shaped by VTA-NAc DA neurotransmission. Our findings reveal that phasic patterns of subsecond accumbal DA release within this circuit enhance a discrete measure of alcohol seeking, whereas tonic patterns of stimulation within this pathway inhibit this behavior. We also demonstrate that tonic photoactivation of these VTA-NAc DA terminals occludes concurrent phasic DA release, which is likely needed to drive motivated behavioral responding for alcohol.
The fact that DA is released in the NAc through two distinct temporal patterns has been known for many years (Grace, 1991, Grace, 2000, Wightman and Robinson, 2002, Zweifel et al., 2009). However, for a variety of technical reasons, most studies have ignored these distinct temporal dynamics of DA neurotransmission and focused primarily on how the relative magnitude of DA release modifies behavioral output. Our findings reveal that, in addition to the importance of the magnitude of NAc dopamine release behaviors, the pattern through which this enhancement of DA occurs also plays a crucial role in shaping motivation to obtain abused drugs. To this point, we found that optogenetically increasing NAc DA release leads to opposite effects on alcohol seeking, depending on whether this increased release is generated by tonic or phasic patterns of synaptic activity. These findings have important implications for efforts related to developing new therapeutic strategies aimed at restoring normal synaptic DA activity, as the interventions may need to pay attention to the patterns through which DA signaling is modulated, not solely the magnitude of the DA release events.
As many prior studies have shown that phasic NAc DA release is associated with seeking behaviors for a wide range of reinforcers (Jones et al., 2010, Phillips et al., 2003, Wassum et al., 2013), it seems likely that this pattern of accumbal DA activity may promote alcohol seeking if the cause-effect relationship between these chemical changes and behavior exists. In fact, previous work has shown that electrically evoked phasic DA transients can promote a lever press for cocaine (Phillips et al., 2003). However, because electrical stimulation of the VTA can result in the release of multiple neurotransmitters in several brain areas, conclusions regarding a causal role of DA in the changes in drug seeking were limited. More direct evidence for a role of phasic DA in promoting motivated behavior comes from voltammetric studies showing that repeated cocaine exposure may enhance cue-evoked incentive motivation through augmented phasic DA signaling (Ostlund et al., 2014) and that these chemical alterations were positively correlated with lever press activity for reward, whereas slow DA changes (tonic) were not related to this activity (Wassum et al., 2013). Although these data strongly support a role for accumbal DA in reward-seeking behaviors, which originally was based mainly on pharmacological manipulations and lesion data (Berridge and Robinson, 1998, Berridge, 2007, Berridge et al., 2009), this evidence remains correlative in nature. In fact, based on compelling data, it was postulated that phasic VTA-NAc DA signaling serves a teaching function, encoding the association between cues that predicted rewarding events and reporting errors in reward prediction (Schultz, 2002, Bayer and Glimcher, 2005, Day et al., 2007, Zweifel et al., 2009, Steinberg et al., 2013, Hart et al., 2014). In this regard, our finding that, in rats trained to self-administer ethanol, lever press responding can be optogenetically manipulated through VTA-NAc DA transmission provides causal support for a distinct role of DA for seeking behavior in addition to DA's well established role for reinforcement learning. Moreover, the fact that tonic and phasic activation of this circuit has opposite effects on this measure of alcohol-seeking behavior emphasizes the importance of temporal DA release dynamics in modulating this measure of motivated behavior.
What might explain these divergent behavioral responses promoted by tonic and phasic increases in accumbal DA release? One likely hypothesis is that driving accumbal DA transmission in a tonic mode may occlude phasic DA release that normally encodes motivated behaviors. This hypothesis has existed for many years, based primarily on results obtained with pharmacological challenges (Grace, 2000, Phillips et al., 2003, Oleson et al., 2009). To test this idea directly, we integrated in vivo optogenetics and FSCV methods to examine the interaction between tonic and phasic patterns of accumbal DA neurotransmission. We found that both tonic and phasic stimulation of the VTA-NAc pathway increased DA release, with the magnitude of DA release being significantly greater with phasic stimulation. However, simultaneous delivery of tonic and phasic patterns of stimulation led to a marked inhibition of the large DA responses observed with phasic stimulation alone. Thus, sustained tonic stimulation of accumbal DA terminals may decrease extinction probe trial responding by preventing DA terminals from engaging in phasic signaling patterns that normally promote alcohol-seeking behaviors. In fact, a previous microdialysis study found that changes in tonic DA release are negatively correlated with the number of lever presses required to obtain reward (Ostlund et al., 2011). This is also consistent with a series of studies that have demonstrated that (−)-OSU6162, a monoamine stabilizer that increases tonic dopamine release in the NAc (Feltmann et al., 2016), significantly decreased alcohol-seeking behaviors in rats and suppressed craving measures in alcohol-dependent individuals (Steensland et al., 2012, Khemiri et al., 2015).
At first glance, the results of this study may seem inconsistent with our prior findings that tonic photoactivation of VTA DA neurons decreased alcohol intake in an intermittent two-bottle choice procedure, whereas phasic stimulation of these cells did not significantly modulate alcohol drinking (Bass et al., 2013). However, there is strong evidence that seeking and consummatory elements of alcohol drinking behavior are distinct processes. For example, a meta-analysis of the drinking behavior of 234 rats using the same operant procedure employed in this study found no correlation between prior day alcohol intake and extinction probe trial responding (Samson and Czachowski, 2003). Moreover, intra-NAc infusion of raclopride, a D2 receptor antagonist, significantly reduced extinction probe trial responding while having no effect on alcohol intake, if the operant response requirement was removed (Samson and Chappell, 2004). Here, we provide additional support for the dissociation between appetitive and consummatory alcohol-drinking behaviors, as we found no relationship between extinction responding and alcohol drinking on the session preceding or following the extinction sessions.
In summary, our findings provide causal evidence that tonic-phasic interplay within the VTA-NAc DA circuit can significantly modulate an alcohol-seeking behavior. Our data demonstrate that shifting accumbal DA transmission into a tonic mode, while rats are engaged in alcohol-seeking behavior, diminishes further motivation to obtain alcohol. We further show that this effect is likely mediated by an inhibition of phasic DA release that normally promotes this behavior. These findings may have important clinical implications for studies seeking to manipulate mesolimbic DA release as a treatment for AUD. Given recent evidence that NAc stimulation shows some promise in the treatment of AUD (Azevedo and Mammis, 2018, Salib et al., 2018), these results may guide optimal therapeutic parameters of stimulation that could be particularly effective in eliminating maladaptive drinking behaviors.
Limitations of the Study
Our neurochemical experiments with dual stimulation of the VTA were designed to test empirically a long-existing general hypothesis regarding the interaction between tonic and phasic DA release patterns in the NAc. Although this experiment was conducted on alcohol-naive subjects, it should be noted that in our initial observations, we did not find any significant alcohol intake-related changes in optogenetically evoked DA signals. Nevertheless, future studies, using models of alcohol use disorder (e.g., chronic intermittent alcohol vapor), should be conducted to determine whether prolonged alcohol exposure affects tonic-phasic interplay. Furthermore, this study was focused on the appetitive component of alcohol-drinking behavior. Previously, we and others provided evidence that VTA-NAc DA transmission modulates alcohol consumption in an intermittent two-bottle choice procedure. Whether this is also the case in operant models of alcohol self-administration should be clarified in future experiments.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We are grateful to the WFU Biology Microscopic Core Imaging Facility under direction of Dr. Glen Marrs for use of the Zeiss LSM 710 confocal microscope. We thank A. Chappell for excellent guidance with behavioral experiments and Dr. Benjamin Rowland for his assistance with statistical analysis. This study was funded by NIH grants AA022449 (E.A.B.), AA17531 (J.L.W.), P50 AA026117 (E.A.B. and J.L.W.), DA024763 (C.E.B.), and T32AA007565 (A.L.D.) and the Tab Williams Family Endowment Fund (E.A.B.).
Author Contributions
E.A.B., C.E.B., and K.D.B. designed electrochemical experiments and analyzed the data. V.P.G. and A.L.D. performed electrochemical and ethanol self-administration studies. E.A.B., K.D.B., and J.L.W. designed optogenetic experiments and drafted the manuscript.
Declaration of Interests
The authors declare no competing financial interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100877.
Supplemental Information
References
- Adamantidis A.R., Tsai H.C., Boutrel B., Zhang F., Stuber G.D., Budygin E.A., Tourino C., Bonci A., Deisseroth K., de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo C.A., Mammis A. Neuromodulation therapies for alcohol addiction: a literature review. Neuromodulation. 2018;21:144–148. doi: 10.1111/ner.12548. [DOI] [PubMed] [Google Scholar]
- Bass C.E., Grinevich V.P., Gioia D., Day-Brown J.D., Bonin K.D., Stuber G.D., Weiner J.L., Budygin E.A. Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front. Behav. Neurosci. 2013;7:173. doi: 10.3389/fnbeh.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer H.M., Glimcher P.W. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. Dissecting components of reward: 'liking', 'wanting', and learning. Curr. Opin. Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.Y., Esber G.R., Marrero-Garcia Y., Yau H.-J., Bonci A., Schoenbaum G. Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nat. Neurosci. 2016;19:111–116. doi: 10.1038/nn.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M., Wunsch A.M., Gibbs J.T., Palmiter R.D. Dopamine dependency for acquisition and performance of Pavlovian conditioned response. Proc. Natl. Acad. Sci. U S A. 2014;111:2764–2769. doi: 10.1073/pnas.1400332111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J.J., Roitman M.F., Wightman R.M., Carelli R.M. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat. Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Feltmann K., Fredriksson I., Wirf M., Schilstrom B., Steensland P. The monoamine stabilizer (-)-OSU6162 counteracts downregulated dopamine output in the nucleus accumbens of long-term drinking Wistar rats. Addict. Biol. 2016;21:438–449. doi: 10.1111/adb.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach S., Janak P.H. Decreases in cued reward seeking after reward-paired inhibition of mesolimbic dopamine. Neuroscience. 2019;412:259–269. doi: 10.1016/j.neuroscience.2019.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel S.B., Clark J.J., Robinson T.E., Mayo L., Czuj A., Willuhn I., Akers C.A., Clinton S.M., Phillips P.E.M., Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco S.B., West A.R., Ash B., Moore H., Grace A.A. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Fox M.E., Mikhailova M.A., Bass C.E., Takmakov P., Gainetdinov R.R., Budygin E.A., Wightman R.M. Cross-hemispheric dopamine projections have functional significance. Proc. Natl. Acad. Sci. U S A. 2016;113:6985–6990. doi: 10.1073/pnas.1603629113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A.A. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace A.A. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95:S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Halbout B., Marshall A.T., Azimi A., Liljeholm M., Mahler S.V., Wassum K.M., Ostlund S.B. Mesolimbic dopamine projections mediate cue-motivated reward seeking but not reward retrieval in rats. Elife. 2019;8:e43551. doi: 10.7554/eLife.43551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A.S., Rutledge R.B., Glimcher P.W., Phillips P.E.M. Phasic dopamine release in the rat rucleus accumbens symmetrically encodes a reward prediction error term. J. Neurosci. 2014;34:698–704. doi: 10.1523/JNEUROSCI.2489-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland B.I., Reynolds J.N., Hay J., Perk C.G., Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ilango A., Kesner A.J., Broker C.J., Wang D.V., Ikemoto S. Phasic excitation of ventral tegmental dopamine neurons potentiates the initiation of conditioned approach behavior: parametric and reinforcement-schedule analyses. Front. Behav. Neurosci. 2014;8:155. doi: 10.3389/fnbeh.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.L., Day J.J., Aragona B.J., Wheeler R.A., Wightman R.M., Carelli R.M. Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biol. Psychiatry. 2010;67:737–744. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B., Morel C., Ku S.M., Liu Y., Zhang H., Montgomery S., Gregoire H., Ribeiro E., Crumiller M., Roman-Ortiz C. Midbrain circuit regulation of individual alcohol drinking behaviors in mice. Nat. Commun. 2017;8:2220. doi: 10.1038/s41467-017-02365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemiri L., Steensland P., Guterstam J., Beck O., Carlsson A., Franck J., Jayaram-Lindstrom N. The effects of the monoamine stabilizer (-)-OSU6162 on craving in alcohol dependent individuals: a human laboratory study. Eur. Neuropsychopharmacol. 2015;25:2240–2251. doi: 10.1016/j.euroneuro.2015.09.018. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailova M.A., Bass C.E., Grinevich V.P., Chappell A.M., Deal A.L., Bonin K.D., Weiner J.L., Gainetdinov R.R., Budygin E.A. Optogenetically-induced tonic dopamine release from VTA-nucleus accumbens projections inhibits reward consummatory behaviors. Neuroscience. 2016;333:54–64. doi: 10.1016/j.neuroscience.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson E.B., Talluri S., Childers S.R., Smith J.E., Roberts D.C., Bonin K.D., Budygin E.A. Dopamine uptake changes associated with cocaine self-administration. Neuropsychopharmacol. 2009;34:1174–1184. doi: 10.1038/npp.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund S.B., LeBlanc K.H., Kosheleff A.R., Wassum K.M., Maidment N.T. Phasic mesolimbic dopamine signaling encodes the facilitation of incentive motivation produced by repeated cocaine exposure. Neuropsychopharmacol. 2014;39:2441–2449. doi: 10.1038/npp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund S.B., Wassum K.M., Murphy N.P., Balleine B.W., Maidment N.T. Extracellular dopamine levels in striatal subregions track shifts in motivation and response cost during instrumental conditioning. J. Neurosci. 2011;31:200–207. doi: 10.1523/JNEUROSCI.4759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White C.A., Ariansen J., Stuber G.D., Cleaveland N.A., Cheer J.F., Wightman R.M., Carelli R.M. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur. J. Neurosci. 2009;30:1117–1127. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J.G., Wanat M.J., Soden M.E., Ahmad K., Zweifel L.S., Bamford N.S., Palmiter R.D. Attenuating GABA(A) receptor signaling in dopamine neurons selectively enhances reward learning and alters risk preference in mice. J. Neurosci. 2011;31:17103–17112. doi: 10.1523/JNEUROSCI.1715-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V., Terrier J., Hiver A., Lüscher C. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron. 2015;88:1054–1066. doi: 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Phillips P.E., Stuber G.D., Heien M.L., Wightman R.M., Carelli R.M. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Salib A.N., Ho A.L., Sussman E.S., Pendharkar A.V., Halpern C.H. Neuromodulatory treatments for alcohol use disorder: a review. Brain Sci. 2018;8:95. doi: 10.3390/brainsci8060095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson H.H., Chappell A.M. Effects of raclopride in the core of the nucleus accumbens on ethanol seeking and consumption: the use of extinction trials to measure seeking. Alcohol. Clin. Exp. Res. 2004;28:544–549. doi: 10.1097/01.alc.0000121649.81642.3f. [DOI] [PubMed] [Google Scholar]
- Samson H.H., Czachowski C.L. Behavioral measures of alcohol self-administration and intake control: rodent models. Int. Rev. Neurobiol. 2003;54:107–143. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Steensland P., Fredriksson I., Holst S., Feltmann K., Franck J., Schilstrom B., Carlsson A. The monoamine stabilizer (-)-OSU6162 attenuates voluntary ethanol intake and ethanol-induced dopamine output in nucleus accumbens. Biol. Psychiatry. 2012;72:823–831. doi: 10.1016/j.biopsych.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Steinberg E.E., Boivin J.R., Saunders B.T., Witten I.B., Deisseroth K., Janak P.H. Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg E.E., Keiflin R., Boivin J.R., Witten I.B., Deisseroth K., Janak P.H. A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 2013;16:966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber G.D., Britt Jp Fau - Bonci A., Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol. Psychiatry. 2012;71:1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.C., Zhang F., Adamantidis A., Stuber G.D., Bonci A., de Lecea L., Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S., Wang G.J., Swanson J.M., Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch. Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Fowler J.S., Tomasi D., Telang F. Addiction: beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum K.M., Ostlund S.B., Loewinger G.C., Maidment N.T. Phasic mesolimbic dopamine release tracks reward seeking during expression of Pavlovian-to-instrumental transfer. Biol. Psychiatry. 2013;73:747–755. doi: 10.1016/j.biopsych.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R.M., Robinson D.L. Transient changes in mesolimbic dopamine and their association with 'reward'. J. Neurochem. 2002;82:721–735. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Zweifel L.S., Parker J.G., Lobb C.J., Rainwater A., Wall V.Z., Fadok J.P., Darvas M., Kim M.J., Mizumori S.J.Y., Paladini C.A. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Natl. Acad. Sci. U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.