Abstract
Suzuki Indomobil Motor Plant (SIMP) Cakung, East Jakarta, Indonesia generates wastewater containing heavy metals such as nickel, zinc, chromium, copper, and COD derived from the metal coating process using the electroplating system. Electroplating wastewater produced by this company contains Nickel and COD above the quality standards set by the Government of DKI Jakarta (Governor Regulation No. 69/2013). This research aims to analyze and compare the efficiency and kinetics of Nickel complexes and COD removal using the Advanced Oxidation Process (AOP) and Electrocoagulation (EC) method. Electroplating wastewater generated by SIMP Cakung (ratio of plating wastewater to overflow plating wastewater is 1:30) in this study had characteristics of 379–568 ppm (effluent standard = 75 ppm) of COD, and 87.555–121 ppm (effluent standard = 1 ppm) of Nickel. Preliminary experiments with the factorial design method indicated that independent variables (pH, current density, ozone flow rate, and contact time) had a critical influence/significance on the removal efficiency of Nickel complexes, while the influence of the above variables in COD removal efficiency was not significant. Optimum operating conditions for Nickel complexes and COD removal using both AOP and EC reactor were found in this study as well as the reaction kinetics of the removal rate. Our study found that the optimum operating conditions for Nickel complexes and COD removal using the AOP reactor were at the pH of 10, the ozone flow rate of 2 L/min, the contact time of 60 min (99.75% and 51.25% for Nickel and COD removal, respectively). For the EC reactor, the optimum condition for Nickel and COD removal are pH of 6.5, the current density of 20 mA/cm2 and the contact time of 50 min (99.75% and 51.25% for Nickel and COD removal, respectively). In these conditions, the AOP reactor in its optimum condition could remove Nickel and COD more compared to the EC reactor. This finding suggests that AOP technology is not only reliable in removing Nickel from electroplating industrial wastewater, but also it could reduce the loading of COD for further treatment units by more than 50%. Further studies in the effect of the longer contact time and higher ozone flowrate on COD removal is suggested.
Keywords: Environmental chemical engineering, Waste treatment, Waste, Wastewater management, Electroplating wastewater, Wastewater treatment, Advanced oxidation process, Electrocoagulation
Environmental chemical engineering; Waste treatment; Waste; Wastewater management; Electroplating wastewater; Wastewater treatment; Advanced oxidation process; Electrocoagulation.
1. Introduction
SIMP Cakung is specialized in assembling two-wheeled machines (motorcycles) and four-wheeled engines and transmission (cars). Electroplating wastewater is generated in the metal plating (electroplating) process, which is one of the processes in the assembling of two-wheeled machines. Electroplating wastewater generated by SIMP Cakung highly contains heavy metals such as nickel, zinc, chromium, copper, and also less biodegradable compounds. These heavy metals are very harmful to the environment because they have toxic properties and inhibit the activity of microbes on subsequent biological treatment. In addition, they are also mutagenic and carcinogenic (Üstün, 2009).
In 2014, SIMP Cakung used Electrocoagulation (EC) method to treat wastewater combinations produced by domestic activities (canteen wastewater) and industrial wastewater. This method proved to be less effective because the concentrations of Nickel and COD still exceeded the effluent quality standard (Jakarta Governor Regulation No. 69/2013). Since 2015, the company has been modifying the treatment process by dividing wastewater into three types, the combination of canteen wastewater and industrial wastewater (other than electroplating wastewater) which have been treated by EC, overflow electroplating wastewater treated by conventional coagulation and flocculation, and electroplating wastewater which is not treated locally but immediately handed over to a third party with the license for hazardous waste treatment. This treatment of wastewater can meet quality standards, however, the conventional methods such as coagulation and flocculation used in the treatment are not environmentally friendly because of the hazardous and toxic sludge it produces in large quantities and also its high operational costs.
Electroplating industries generally use a chelating agent in their process, which can be either organic or inorganic compound (Wang et al., 2004). Chelating agent forms stable metal complexes so that the conventional precipitation process like EC is ineffective to remove metal compounds in electroplating wastewater. According to Hossain et al., 2013, the removal efficiency of an EC reactor increases as the pH of the reactor moves toward basic condition. Consequently, a high amount of chemicals is needed to neutralize alkalinity due to its high acidity of electroplating wastewater, and this action may decrease the effectiveness of biological treatment at the later stage (Durante et al., 2011; Juang et al., 2003). Furthermore, another study suggested that the larger the electric current density causes increases in the removal efficiency of metals (Nasrulah et al., 2014). That said, finding the optimum operating condition of an EC reactor in removing Nickel complexes and COD is necessary so that an economic and effective treatment could be obtained.
One of the alternatives that can be implemented to treat electroplating wastewater is Advanced Oxidation Processes (AOP) (Malakootian et al., 2015). Electroplating wastewater is mainly non-biodegradable organic and inorganic compounds (low ratio of BOD5/COD); therefore, it needs a strong oxidizing agent to break down complex carbon chains and to oxidize inorganic compounds. One type of AOP that is easy to apply and environmentally friendly is ozone method (O3), which can be used to oxidize certain types of metals and transform them into insoluble oxidized compounds and then have them separated/recovered from wastewater by sedimentation/filtration process (Sato and Robbins, 2002). In addition, the ozone method is also a promising method to remove organic and inorganic compounds in wastewater (Khuntia et al., 2013, 2014). Ozone (in the form of gas) dissolved in wastewater should be kept as high as possible to maximize the oxidation process by expanding the contact surface between ozone gas and wastewater using microbubbles (Khuntia et al., 2014). Similar to EC, pH also plays an important role in determining the efficiency of wastewater treatment. According to Munter (2001), the decomposition rate of ozone in water increases as the pH rises. This finding suggested that the removal efficiency of metals and organic compound in wastewater will be higher at the basic condition. In addition, the ozone flow rate is also a sensitive operational parameter as it determines the number of oxidants in the reactor (Krishnan et al., 2016).
The general objective of this study is to select the best technology (AOP or EC) to remove Nickel and COD for wastewater derived from the electroplating system. This could be achieved by determining the optimum parameters by analyzing the Nickel and COD removal efficiency and their removal reaction kinetics.
2. Materials and methods
2.1. Experimental setup
The EC reactor is a batch system adapted from a study conducted by Vik et al. (1984). The capacity of the reactor is 13.392 L (with a height of 24 cm, a length of 31 cm and a width of 18 cm) and the shape of the reactor is rectangular made from glass and equipped with a circulation bath made from plastic (with a height of 24 cm, a length of 19 cm and a width of 19 cm). Inside the reactor, there were twelve aluminum plates of size 14 cm × 20 cm x 0.06 cm. The electrodes were connected in monopolar. The interelectrode distance was 2 cm. Twelve plates immersed to a 17.5 depth with an effective area total of 245 cm2. The electrodes were connected to direct current (DC) power supply providing electrical current in the range 0f 0–200A and voltage in the range 0–72 V. During the EC process, the 15 L sample was recirculated using a circulation pump to avoid the formation of an oxide layer on the cathode. The layout of the reactor shown in Figure 1a.
Figure 1.
Layouts of the system for EC (a) and AOP (b) reactor.
For AOP, a batch reactor of 100.4 L (diameter 30 cm, height 142 cm) receiving 40 L of electroplating wastewater was prepared (Figure 1b). Oxygen gas was supplied by a 2 m3 tube whereas Ozone was generated in the Ozone generator with a capacity of 1.2 g/h. The reactor is equipped with a gas Flowmeter (1–5 L/min), a circulation pump (18–30 L/min), and a Mazzei Injector (diameter 1.25 cm).
2.2. Design of the experiment
Several experimental conditions were chosen to find the most optimum condition in removing Nickel and COD. A 23 full factorial experimental design was constructed as seen in Figure 2. The experimental design consisted of two steps, the first step was aimed to optimize operational parameters by varying values of independent variables (current density, pH, and contact time for EC and Ozone flow rate, pH, and contact time for AOP reactor) (Berthouex and Brown, 2002) as shown in Figure 2. The optimum values of each independent variable were chosen based on the obtained highest efficiency of Nickel and COD removal. After the optimum value of the parameters had obtained from the first stage of the experiment, then it was continued to the second stage of the experiment to obtain more specified optimum values by using the same step as in the first stage but only for two parameters. The value of each independent variable in stage 2 experiment was chosen by adding and subtracting by one scale of the parameter unit to the optimum values obtained in the first stage of the experiment. For example, if the obtained optimum value of pH in the AOP reactor at the first stage is 11, then at the second stage we set two values of pH, 10 and 12. Then again, the most optimum condition in the second stage of the experiment was chosen based on the obtained highest efficiency of nickel and COD removal.
Figure 2.
Variables independents for full factorial experimental design.
The total number of experiments of this complete factorial design experiment was twelve experiments for each reactor. To ensure the variability of Nickel and COD removals were due to the variability of the independent variables, three ways ANOVA was performed to analyze the effect of each variable independent of the changes in removal efficiency of Nickel and COD for each reactor. In this study, we used Minitab to conduct the analyses at a confidence interval of 90% (α = 5%).
2.3. Reaction kinetics of Nickel and COD removal
The second step of the study was to analyze the kinetic models of Nickel complexes and COD removal in optimum operating conditions for the only reactor that gave the highest removal efficiency. Initially, the pH of the wastewater is adjusted in accordance with the optimum pH obtained from step one and then settled for 30 min. After that, the supernatant was inserted in the reactor with optimum operational parameters obtained from step one, and then every 10 min the concentrations of Nickel and COD were measured by withdrawing samples from the reactor.
To determine the appropriate reaction order, then the Nickel and COD concentration was plotted versus time and modeled as zero, first and second-order reaction kinetic. The best-fitted model was chosen based on their R2 values. The kinetic model for Nickel complexes and COD removal is determined by equations described by the following equations.
| 1 |
| 2 |
| 3 |
where C is the Nickel or COD concentration and k is the reaction rate.
2.4. Sampling of electroplating wastewater
Electroplating wastewater was obtained from SIMP Cakung with plating wastewater-overflow plating wastewater ratio of 1:30 based on flow proportioning composite sample (USEPA, 2013). Plating wastewater was obtained from the metal plating (electroplating) process containing Nickel sulfate, boric acid, Nickel carbonate, phosphoric acid, saccharin, and saturated silicon carbide (SIMP, 2009). Overflow plating wastewater was obtained from the cleaning and washing process in the electroplating bath. After plating and overflow, the plating was mixed in the ratio of 1:30, its temperature, pH, Nickel, and COD was measured immediately to investigate the initial characteristics of electroplating wastewater.
2.5. Chemicals and measurements
All chemicals used in this research were analytical grade (Merck, Germany) and distilled water used as solvent throughout the experiment. The initial pH adjustment of electroplating wastewater used NaOH 1 N. EDTA powder pillows, Phthalate-Phosphate Reagent Powder Pillows, whereas PAN Indicator Solution 0.3% was used for Nickel analysis. COD Digestion Reagent vials were used for COD analysis. Pure oxygen gas was used as a source of the ozone generator.
Nickel and COD were measured in the supernatant after settling for 30 min and filtering it with a 2.5 μm cellulose filter (Whatman 42 Cat No 1442 125). The temperature was measured using SNI 06-6989.23-2005, pH using SNI 06-6989.11-2004, Nickel concentration was analyzed by DR 2800 Spectrophotometer (Method 8150, Nickel, 1- (2-Pyridylazo) -2-Naphthol (PAN) Method), and COD was analyzed by DR 2800 (Chemical Oxygen Demand Method 8000 and Reactor Digestion Method).
3. Results and discussions
3.1. Characteristics of electroplating wastewater
The characteristic of SIMP Cakung electroplating wastewater is shown in Table 1. With the ratio composition of the overflow electroplating: electroplating wastewater = 30: 1, Nickel concentrations were found in the range from 87.755 to 121 ppm, and COD concentrations were ranging from 379 to 568 ppm. The range of initial pH of wastewater is between 6.4 to 6.5. Concentrations of Nickel in electroplating wastewater were 1,160–1,200 ppm and pH was 2.3. Concentrations of Nickel in overflow electroplating wastewater was in the range of 55–65 ppm with pH values ranging from 6.4 to 6.5. The composite of overflow electroplating and electroplating wastewater diluted Nickel content in the electroplating wastewater that was quite high before the mixture and raise the pH of electroplating waste. It has to be noted that composting the two types of wastewater gave an advantage to the EC process since the rise of pH results in increases in the removal efficiency of the treatment unit. Hence, fewer chemicals are needed. In addition, the rise of pH could also assits in nickel removal with hydroxide precipitation mechanism.
Table 1.
Characteristics of the composite samples of Electroplating and Overflow Electroplating Wastewater.
| No. | Date of Sampling | Temperature (oC) | pH | Nickel (ppm) | COD (ppm) |
|---|---|---|---|---|---|
| 1. | 3 Februari 2016 | 32.4 | 6.4 | 121 | 379 |
| 2. | 23 March 2016 | 32.5 | 6.5 | 87.755 | 568 |
3.2. Determination of optimum condition with factorial design experiment for EC reactor
Results of the first and second stages of the experiment obtained from the EC reactor can be seen in Tables 2 and 3, respectively. The optimum condition obtained from the first stage experiment were: pH of 7.5, the current density of 20 A (7.42 mA/cm2) and contact time of 60 min (Condition 7). The optimum conditions were chosen based on the highest removal efficiency for Nickel and COD which were 98.46 % and 44.13 % respectively. With that removal efficiency, however, the Nickel concentration has not met the quality standard that is equal to 1 ppm KLH, 2014. Despite giving the highest removal for Nickel, Condition 8 was not chosen due to the COD removal was lower than that of Condition 7.
Table 2.
Results from first stage experiment using EC reactor.
| Condition | Time (minutes) | pH | Current (Ampere) | Initial Nickel (ppm) | Final Nickel (ppm) | Initial COD (ppm) | Final COD (ppm) | Eff Nickel removal (%) | Eff COD removal (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 4.5 | 20 | 40.800 | 6.230 | 500 | 312 | 84.72 | 37.60 |
| 2 | 30 | 4.5 | 17 | 39.971 | 17.092 | 342 | 246 | 57.24 | 28.07 |
| 3 | 60 | 4.5 | 17 | 71.306 | 9.015 | 370 | 224 | 87.36 | 39.46 |
| 4 | 30 | 4.5 | 20 | 50.323 | 13.491 | 395 | 238 | 73.19 | 39.75 |
| 5 | 60 | 7.5 | 20 | 52.074 | 0.800 | 349 | 195 | 98.46 | 44.13 |
| 6 | 30 | 7.5 | 20 | 71.914 | 1.095 | 561 | 318 | 98.48 | 43.32 |
| 7 | 60 | 7.5 | 17 | 66.241 | 1.543 | 576 | 305 | 97.67 | 47.05 |
| 8 | 30 | 7.5 | 17 | 67.435 | 0.419 | 568 | 325 | 99.38 | 42.78 |
Values shown in bold indicate the highest value obtained in the respective experiment.
Table 3.
Results from the second stage experiment using EC reactor.
| Condition | Time (minutes) | pH | Current (Ampere) | Initial Nickel (ppm) | Final Nickel (ppm) | Initial COD (ppm) | Final COD (ppm) | Eff Nickel removal (%) | Eff COD removal (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 8.5 | 21 | 70.524 | 0.21 | 536 | 313 | 99.70 | 41.60 |
| 2 | 70 | 8.5 | 21 | 79.963 | 0.401 | 554 | 296 | 99.50 | 46.57 |
| 3 | 50 | 6.5 | 20 | 164.6 | 9.21 | 561 | 283 | 94.40 | 49.55 |
| 4 | 70 | 6.5 | 20 | 47.95 | 0.862 | 564 | 354 | 98.20 | 37.23 |
Values shown in bold indicate the highest value obtained in the respective experiment.
The optimum value obtained from the second stage was condition 1: pH of 8.5, the contact time of 50 min, and the current density of 21 A (7.79 mA/cm2). These optimum conditions were chosen because of maximum Nickel removal efficiency of 99.7% was obtained. The final Nickel concentration (0.21 ppm) obtained from this condition has met the effluent quality standard. That said, the removal efficiency for COD was 41.60 % and the final concentration was still relatively high (313 ppm).
The significance of the effect of variable independents on the removal efficiency of Nickel and COD was shown in Table 6. By using ANOVA three ways (α = 0.05), the effect of variable independents on the removals of the contaminants only significant if the p-value is less than 0.05. As seen in Table 6, for the EC reactor, only pH that could significantly explain the variability of Nickel and COD removals. It was expected as can be seen in Table 3, the only significant change of Nickel removal occurred when the pH of the system was raised to 7.5.
Table 6.
Results of ANOVA three ways on EC and AOP reactor.
|
EC reactor | ||||
|---|---|---|---|---|
| Independent Variable |
Nickel removal |
COD removal |
||
| F value | P-value | F value | P-value | |
| pH | 12.13 | 0.025 | 10.33 | 0.192 |
| Current | 0.25 | 0.642 | 0.54 | 0.595 |
| time |
2.31 |
0.203 |
2.02 |
0.39 |
| AOP Reactor | ||||
| Independent Variable | Nickel removal | COD removal | ||
| F value |
P-value |
F value |
P-value |
|
| pH | 2,507 | 0 | 0.89 | 0.519 |
| flowrate | 166.24 | 0.003 | 0.62 | 0.574 |
| time | 1,005.74 | 0.001 | 1.42 | 0.444 |
Values shown in bold indicate the highest value obtained in the respective experiment.
The high nickel removal was achieved in Condition 1 because of the formation of Al(OH)3 due to the reaction between the aluminum plates and water which has a large surface area to adsorb organic and inorganic materials and able to capture colloidal particles (Kobya et al., 2003). In addition, the high removal efficiency could be achieved due to the hydroxide precipitation reaction mechanism as a result of basic condition (pH 8.5) which increased the precipitation of Nickel ions. The selection of a pH ranges from acidic to alkaline (pH 4.5 to 8.5) was quite critical. According to Lekhlif et al. (2013) the hydrolysis of aluminum varies depending on the pH where at pH 4–9, the dominant species is Al(OH)3. This species is electrically neutral and presences as polymers which adsorb contaminants to form flocs and easily deposited.
3.3. Determination of optimum condition with factorial design experiment for AOP reactor
Results of the experiment using the AOP reactor in the first and second stages of a full factorial design 23 can be seen in Tables 4 and 5, respectively. The second running of factorial design only consists of five experiments, which was originally planned to run eight experiments, considering the final concentration of Nickel had met the quality standard (1 ppm), while the final COD concentration was still above the quality standard (75 ppm). Another reason was contact time if made two times longer, did not result in a significant decrease in the final concentration of COD as seen in the results shown in Condition 1 and 5 in Table 5. Electroplating wastewater required an additional NaOH 1 N as much as ± 1.261 ml/43 L of wastewater to get pH 11.6, while for pH 10 the wastewater only required ±224 ml/43 L waste (pH 11.6 required 5.6 times higher NaOH 1 N compared to pH 10).
Table 4.
Results from first stage experiment using AOP reactor.
| Condition | Ozone Flowrate (L/min) |
pH | Time (minutes) | Initial Nickel (ppm) | Final Nickel (ppm) | Initial COD (ppm) | Final COD (ppm) | Eff Nickel removal (%) | Eff COD removal (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2,5 | 8 | 90 | 63,764 | 35,996 | 571 | 360 | 43,55 | 36,95 |
| 2 | 4 | 8 | 90 | 74,943 | 53,066 | 567 | 407 | 29,19 | 28,22 |
| 3 | 2,5 | 11 | 90 | 54,935 | 0,955 | 593 | 350 | 98,26 | 40,98 |
| 4 | 4 | 11 | 90 | 36,816 | 2,535 | 562 | 344 | 93,11 | 38,79 |
| 5 | 2,5 | 8 | 180 | 90,378 | 9,258 | 552 | 386 | 89,76 | 30,07 |
| 6 | 4 | 8 | 180 | 88,186 | 22,871 | 550 | 364 | 74,07 | 33,82 |
| 7 | 2,5 | 11 | 180 | 27,239 | 0,151 | 548 | 362 | 99,45 | 33,94 |
| 8 | 4 | 11 | 180 | 24,488 | 0,987 | 535 | 378 | 95,97 | 29,35 |
Table 5.
Results from the second stage experiment using AOP reactor.
| Condition | Time (minutes) | pH | Ozone Flowrate (L/min) |
Initial Nickel (ppm) | Final Nickel (ppm) | Initial COD (ppm) | Final COD (ppm) | Eff Nickel removal (%) | Eff COD removal (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 10 | 2 | 33.023 | 0.082 | 560 | 273 | 99.75 | 51.25 |
| 2 | 60 | 10 | 3 | 28.294 | 0.663 | 627 | 283 | 97.66 | 54.86 |
| 3 | 60 | 11.6 | 2 | 17.176 | 0.026 | 512 | 256 | 99.85 | 50.00 |
| 4 | 60 | 11.6 | 3 | 19.760 | 0.028 | 537 | 225 | 99.86 | 58.10 |
Values shown in bold indicate the highest value obtained in the respective experiment.
The optimum condition obtained from the first stage experiment were: pH of 11, the ozone flow rate of 2.5 L/min and contact time of 90 min (Condition 3). This condition gave removal efficiency for Nickel and COD of 98.46 % and 44.13 %, respectively. The optimum values obtained from the second stage were: pH of 11.6, contact time of 60 min, and ozone flow rate of 3 L/min (Condition 4) which gave removal efficiency for Nickel and COD of 99.8 % and 58.1 %, respectively The optimum value of operational parameters were in accordance with the research conducted by Khuntia et al. (2015), produced optimum pH at 10 and a reaction time of 40 min to remove phenol in artificial wastewater using AOP of ozone microbubbles method (with ozone flow rate of 0.5 L/min up to 4.8 L/min).
Based on the results of ANOVA three ways (Table 6), all independent variables have a significant impact on the removals of Nickel and COD as the p-values of those variables are below 0.05. Among the variables, pH was the most influential as the efficiency of Nickle removal raised from the range of 40–80 % at pH 8, became larger than 90% at pH 11. This shows that the variability of removals was significantly explained by the variability of pH. The difference with EC is that the other independent variables of AOP also significantly influencing the variability of Nickel removal. Table 6 also showed that there was not any independent variable that could significantly affect the variability of COD removals.
The COD content of electroplating wastewater of SIMP Cakung was very difficult to remove by ozone, thus requiring Ozonator capacity greater than 1.2 gr/hour for 40 L of wastewater treated. Research conducted by Zheng et al. (2015) on the original wastewater from acrylic fiber manufacturing industry, showed COD reduction of ±145 ppm (325 ppm to about 180 ppm) and an increase of ±55% in the ratio of biodegradability (BOD5/COD) (from 0.045 to around 0.07) in 45 min, with an Ozonator capacity of 5 gr/hour and 6 L of wastewater treated. The presence of certain ions in electroplating wastewater could reduce the ability of ozone and hydroxyl radicals to oxidize COD. One of the examples was carbonate ions that could act as inhibitors of ozone decomposition to produce hydroxyl radicals and also acted as a hydroxyl radical scavenger (Khuntia et al., 2015).
3.4. Reaction kinetics for Nickel and COD removal using AOP and EC reactor
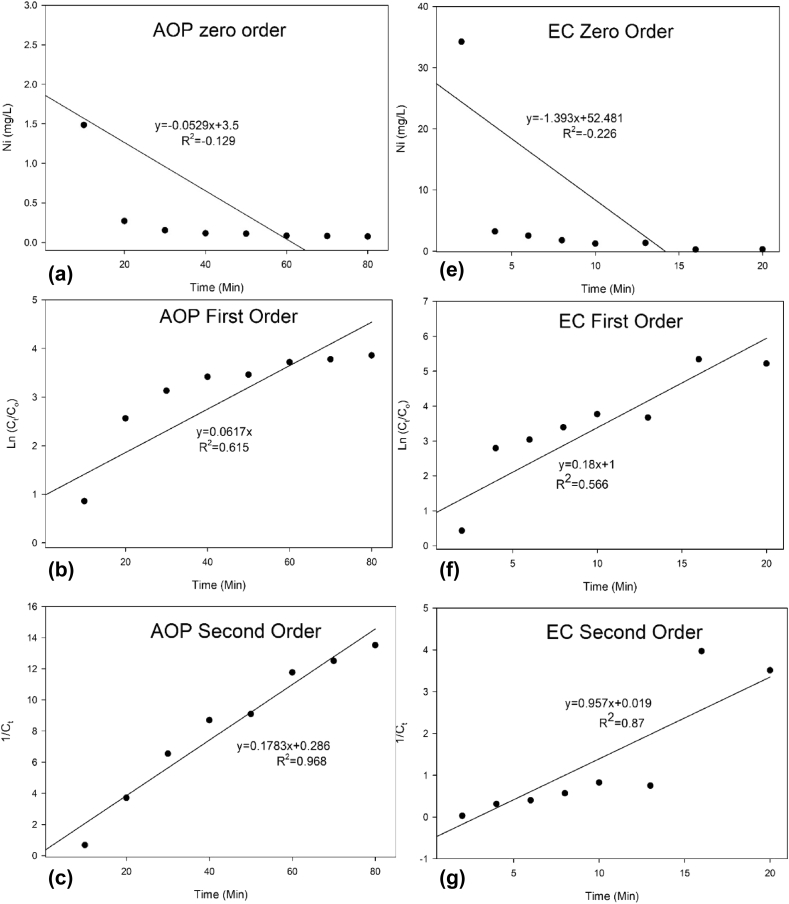
Based on the six charts in Figure 3, it can be seen that the reaction kinetics equation that gave the best fit to the experimental data was the second-order reaction for both AOP and EC removal. The reaction rate obtained from the second-order reaction for the EC reactor was 0.957 L/mg/min whereas, for the AOP reactor, the reaction rate was 0.1783 L/mg/min. The reaction rate obtained from the EC reactor was larger than that of the AOP reactor suggest that EC required a longer time to remove both Nickel and COD compared to AOP. From the results of a study conducted by Al-Shannag et al. (2014) the reaction kinetics for removing Nickel from electroplating wastewater using carbon steel electrodes using a current density of 2–4 mA/cm2 was a pseudo-first-order reaction. Differences in results are probably due to electrode type and the amount of current density that was used.
Figure 3.
Reaction Kinetics of Nickel Complexes Removal (a) AOP zeroth-order, (b) AOP first-order, (c) AOP second-order (d) EC zeroth-order, (e) EC first-order, and (f) EC second-order.
The rate of COD removal reaction obtained from both reactors was relatively slow (Figure 4). Among the three reaction kinetics, the second-order equation gave the best fit to the experimental data. The reaction rate obtained from the second-order reaction for the EC reactor was 4 × 10−5 L/mg/min whereas for the AOP reactor, the reaction rate was 7 × 10−7 L/mg/min. It is clearly seen that the rate of COD removal obtained from the EC reactor was larger than that obtained from the AOP reactor. This also indicated that AOP could remove COD faster than EC. That being stated, It could be seen COD concentration couldn't reach the quality standards. This might due to the small amount of ozone flow rate, current density and time that were set for the experiment.
Figure 4.
Reaction Kinetics of COD Removal (a) zeroth-order, (b) first-order, and (c) second-order.
It may be concluded that the AOP treatment unit alone is still not capable to remove COD. The secondary treatment using biological or chemical process is still needed to ensure that the quality of the wastewater effluent is in accordance with the quality standard. This study also revealed that the removal reaction rate of EC was larger than that of AOP for both Nickel and COD. The values of the reaction rate imply that both Nickel and COD was removed faster if the wastewater is treated by using AOP. This implies the less retention time required of treating the wastewater by using the AOP reactor which could be translated as less operational cost.
4. Conclusion
Based on this research, it can be concluded that: (1) the electroplating wastewater produced by SIMP Cakung with plating wastewater - overflow plating wastewater ratio of 1:30 were 87.755–121 ppm Nickel (>1 ppm) and COD 379–568 ppm (>75 ppm); (2) Optimum operating conditions for Nickel complexes and COD removal using AOP reactor were at the pH of 10, the ozone flow rate of 2 L/min, the contact time of 60 min (99.75% and 51.25% for Nickel and COD removal, respectively). For EC reactor, the optimum condition for Nickel and COD removal are pH of 6.5, the current density of 20 mA/cm2 and the contact time of 50 min (99.75% and 51.25% for Nickel and COD removal, respectively). Final Nickel concentration obtained has met the quality standard though the final concentration of COD could not meet the quality standard (288 ppm). (4) Reaction Kinetics of Nickel removal was a second-order kinetics with the value of reaction rate constant 0.1783 L/mg/min (R2 0.9687) whereas reaction kinetics of COD removal was also a second-order kinetics with the value of reaction rate constant 0.000007 L/mg/min (R2 0.6464) and COD removal efficiency 18.93% in 40 min (final COD concentration was 287 ppm). Comparing the performance of both reactors, it is suggested that the AOP reactor gave better removal efficiencies than those of EC reactors. Given the same contact time, the Nickel removal efficiency for both reactors is almost the same, however, for COD removal, the AOP reactor gave 58% removal efficiency whereas the removal efficiency of the EC reactor was only 41%. This indicates that by deploying the AOP treatment system, the organic contaminant loading for further biological or chemical treatment processes could be minimized.
Declarations
Author contribution statement
Setyo Sarwanto Moersidik: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Rudi Nugroho: Contributed reagents, materials, analysis tools or data.
Mira Handayani, Kamilawati Kamilawati: Performed the experiments; Analyzed and interpreted the data.
Mochamad Adhiraga Pratama: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This research was conducted in Pusat Teknologi Lingkungan, BPPT, Gedung Geostech Komplek Puspitek, Serpong, Tangerang, Indonesia. Special thanks are given to the Director of Pusat Teknologi Lingkungan, Mr. Rudi Nugroho and all of the staff.
References
- Al-Shannag M., Al-Qodah Z., Bani-Melhem K., Qtaishat M.R., Alkasrawi M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: kinetic study and process performance. Chem. Eng. J. 2014:749–756. [Google Scholar]
- Berthouex P., Brown L.C. second ed. Lewis Publishers; 2002. Statistics for Environmental Engineers. [Google Scholar]
- DPPL SIMP . 2009. Dokumen Pengelolaan Dan Pemantauan Lingkungan Hidup (DPPL) Jakarta. [Google Scholar]
- Durante C., Cuscov M., Isse A.A., Sandona G., Gennaro A. Water Research; 2011. Advanced Oxidation Processes Coupled with Electrocoagulation for the Exhaustive Abatement of Cr-EDTA; pp. 2122–2130. [DOI] [PubMed] [Google Scholar]
- Government of Special Capital Region of Jakarta, Governor Regulation No. 69/2013 About Wastewater Quality Standard for Activities and/or Business (23 July 2013).
- Hossain M., Mahmud M.I., Parvez M.S., Cho H.M. Impact of current density, operating time and ph of textile wastewater treatment by electrocoagulation process. Environ. Eng. Res. 2013;18(3):157–161. [Google Scholar]
- Juang R.S., Lin S.H., Wang T.Y. Removal of metal ions from complexed solutions in fixed bed using A strong-acid ion exchange resin. Chemosphere. 2003:1221–1228. doi: 10.1016/S0045-6535(03)00578-2. [DOI] [PubMed] [Google Scholar]
- Khuntia S., Majumder S.K., Ghosh P. Removal of ammonia from water by ozone microbubbles. Ind. Eng. Chem. Res. 2013:318–326. [Google Scholar]
- Khuntia S., Majumder S.K., Ghosh P. Oxidation of as (III) to as(V) using ozone microbubbles. Chemosphere. 2014:120–124. doi: 10.1016/j.chemosphere.2013.10.046. [DOI] [PubMed] [Google Scholar]
- Khuntia S., Majumder S.K., Ghosh P. Quantitative prediction of generation of hydroxyl radicals from ozone microbubbles. Chem. Eng. Res. Des. 2015:231–239. [Google Scholar]
- Kobya M., Can O.T., Bayramoglu M. Treatment of textile wastewater by electrocoagulation using iron and aluminum electrodes. J. Hazard Mater. 2003:163–178. doi: 10.1016/s0304-3894(03)00102-x. [DOI] [PubMed] [Google Scholar]
- Krishnan S., Rawindran H., Sinnathambi C.M., Lim J.W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. IOP Conf. Series: Mater. Sci. Eng. 2017;206 [Google Scholar]
- Lekhlif B., Ourdrhiri L., Zidane F., Drogui P., Blais J.F. Study of the electrocoagulation of electroplating industry wastewaters charged by Nickel (II) and Chromium (VI) J. Master Environ. 2013:111–120. [Google Scholar]
- Malakootian M., Yousefi N., Fatehizadeh A., Van Ginkel S.W., Ghorbani M., Rahimi S., Ahmadian M. Nickel (II) removal from industrial plating effluent by fenton process. Environ Eng Manag. 2015:837–842. [Google Scholar]
- Munter R. Advanced oxidation processes – current status and prospects. Proc. Estonian Acad. Sci. 2001;50(2):59–80. [Google Scholar]
- Nasrullah M., Siddique M.N.I., Wahid Z. Effect of high current density in electrocoagulation process for sewage treatment. Asian J. Chem. 2014;26:4281–4285. [Google Scholar]
- National Standardization Agency of Indonesia (SNI) National Standardization Agency of Indonesia (SNI). Water Quality Measurement: Measurement of Temperature by Using Thermometer (Standard No. 06-6989.23-2005).
- National Standardization Agency of Indonesia (SNI). Water Quality Measurement: Measurement of Acidity by Using pH meter (Standard No. 06-6989.11:2004).
- Sato M., Robbins E.I. 2002 November. United States of America patent No. U. S. Jpn. Outlook 6,485,696 B1. [Google Scholar]
- The Ministry of Environment of Indonesia (KLH). The Minister of Environment Decree No. 5/2014 about Various Industrial Wastewater Standard (25 November 2014).
- USEPA . 2013, February 28. Wastewater Sampling. Athens, Georgia. [Google Scholar]
- Üstün G.E. Occurrence and removal of metals in urban wastewater treatment plants. J. Hazard Mater. 2009:833–838. doi: 10.1016/j.jhazmat.2009.07.073. [DOI] [PubMed] [Google Scholar]
- Vik E.A., Carlson D.A., Eikum A.S., Gjessing E.T. Electrocoagulation of potable water. Water Res. 1984;18(11):1355–1360. [Google Scholar]
- Wang L.K., Hung Y.T., Lo H.H., Yapijakis C. Marcel Dekker Inc; New York: 2004. Handbook of Industrial and Hazardous Wastes Treatment. [Google Scholar]
- Zheng T., Wang Q., Zhang T., Shi Z., Tian Y., Shi S., Wang J. Microbubble enhanced ozonation process for advanced treatment of wastewater produced in acrylic fiber manufacturing industry. J. Hazard Mater. 2015:412–420. doi: 10.1016/j.jhazmat.2015.01.069. [DOI] [PubMed] [Google Scholar]