Abstract
There are growing concerns about the chronic and acute effects of synthetic additives such as antibacterial, fragrances, colourants and stabilizing agents used in the production of various household products. Many household products and materials including cosmetic products are reportedly suspected to be carcinogenic with some acting as endocrine disruptors among other effects. Thus, environmental-friendly alternatives such as products that are rich in bioactive phytochemicals are becoming consumers' preferred choice especially in the beauty and cosmetic sector. ‘Green’ preparation of medicinal soaps devoid of any synthetic additives was made from underutilized tropical seed of Citrus sinensis seed oil and some natural additives comprising of natural honey, Ocimum gratissimum leaves extract, Moringa oleifera seed oil and coconut oil. Precisely, the seed oil of the underexplored C. sinensis was obtained via soxhlet extraction and saponified with natural lye solution at different ratios to produce soaps of varying characteristics. The incorporation of honey and Ocimum gratissimum leaf extract provided additional antimicrobial, antioxidant and fragrance properties. Physico-chemical parameters of the oil and soaps were determined following standard procedures while the fatty acid profile of the trans-esterified oil was determined using GC–MS. The antimicrobial potential of the oil and soaps were assessed using agar diffusion method at concentrations 200 mg/mL and below. Linoleic acid (36%) and oleic acid (27%) were the most prominent in C. sinensis seed oil. The soap had antimicrobial potential comparable to commercial product. The soap samples recorded highest anti-bacteria activities (22.0 ± 1.0–23.0 ± 1.0) against Staphylococcus aureus and Bacillus subtilis and notable anti-fungi activities (18.0 ± 1.0) against Penicillium notatum and Candida albicans. Additionally, the oil showed moderate anti-parasite (anit-toxoplasma gondii) activity (EC50 ≤ 500 μg/mL) but with improved selectivity that precludes oxidative stress while the prepared medicinal soaps exhibited remarkable antioxidant property. The utilization of these locally sourced resources will prevent the daily introduction of synthetic antimicrobial and antioxidant chemicals into the environment. The initiative avail a sustainable production of environmentally-benign cosmetic products besides conversion of waste to wealth agrees which aligns with the Sustainable Development Goals (SDGs).
Keywords: Food science, Natural product chemistry, Organic chemistry, Antimicrobial, Medicinal chemistry, Toxoplasma gondii, Citrus sinensis, Green cosmetic, Green chemistry, Fatty acids
Food Science, Natural product chemistry; Organic chemistry; Antimicrobial; Medicinal Chemistry; Toxoplasma gondii; Citrus sinensis; Green Cosmetic; Green Chemistry; fatty acids.
1. Introduction
Many synthetic additives such as colorants, preservatives, fragrances and antibacterial have been found to be allergens and carcinogenic. For instance, various researches have implicated synthetic antioxidants butylated hydroxytoluene, butylated hydroxyanisole and parabens as potential carcinogens and endocrine disruptors (Kahl and Kappus, 1993; Gultekin et al., 2015; Nowak et al., 2018). These synthetic chemicals used as antioxidants and/or preservatives in foods and cosmetics are now known to promote irritation, carcinogenicity, mutagenicity and various other allergic reactions especially when used as additives in food and cosmetics (Joshi and Pawal, 2015; Suzuki, 2010). As such, there is need for extreme care in selecting the ingredients and additives for cosmetic formulation that afford consumers opportunity to achieve their targeted beauty desire without compromising human health and environmental safety.
The incorporation of raw natural honey (honeybees) into food and cosmetic products has been an age-long practice (Bergamo et al., 2019; Waheed et al., 2018; Chari, 2008). Honey in cosmetic is reported to possess both in vitro and in vivo potential against effect of Acne vulgaris on skin. It had efficacy in reducing lesion counts as well as skin microorganism concentration (Han et al., 2013). Several studies have indicated that honey has antioxidant, antimicrobial, skin smoothening and moisturizing effects (Boussaid et al., 2018; Alvarez-Suarez et al., 2010; Basualdo et al., 2007), wound healing (Molan and Betts, 2004), anti-inflammatory and antiviral activities (Bansal et al., 2005; Eteraf-Oskouei and Najafi, 2013) among others.
On the other hand, Ocimum gratissimum has been reported as natural cosmetic component to impact antimicrobial activity, anti-inflammatory activity and stimulate hair growth (Tareau et al., 2017; Fongnzossie et al., 2017). Interestingly, Cocos nucifera is an important seed oil widely applied in cosmetic products, such as soap, cream, shampoo, hair conditioner, toothpaste, deodorant, skin and hair care products as well as beauty make-ups (Tareau et al., 2017; Rodrigues et al., 2018).
In recent times, increased attention, particularly from the academia and industries has been given to the pharmacological potentials of fixed oils extracted from various herbs, plants and seeds in order to ascertain their multifunctional applicability including their classical roles as food and cosmetic substrate or additives. Various underutilized seed oils are now being investigated for possible mass production of cosmetic products (Atolani et al., 2016; Olabanji et al., 2016; Alander, 2004; Aliyu et al., 2012; Ameh et al., 2013; Getradeghana, 2000; Warra et al., 2011).
Citrus sinensis (Sweet orange) of the family Rutaceae is widely cultivated in Nigeria and many other tropical and subtropical regions (Atolani et al., 2012; Bovili, 1996; Jorge et al., 2016). More than 80 million tons of C. sinensis are produced annually (FAOSTAT, 2019) and citrus accounts for about 50% of the wastes produced by the juice industries in the form of peel and seed residues (Ozturk et al., 2018). Sweet orange is a major source of vitamins, especially vitamin C, folacin, thiamine, niacin, calcium, potassium and magnesium (Angew, 2007). The flavonoids and anthocyanin in the plant are reported to possess various pharmacological properties which include antioxidant and metal chelation properties (Atolani et al., 2012; Jorge et al., 2016; Ozturk et al., 2018; Tripoli et al., 2007). Some lipophilic components of plants have also shown anti-toxoplasma activity (Abugri at al., 2019; Adeyemi et al., 2019). Various value-added products such as ethanol, hesperidin and nanocellulose have been obtained from orange waste (Cypriano et al., 2018). Many seeds are reported to possess anti-microbial potentials (Atolani et al., 2019a, 2019b). Oranges are harvested in large quantity in African countries and the industries processing it into various food products, discards the seeds in large volume annually. Therefore, the aim of this research was to explore the underutilized seeds of C. sinensis for the production of natural antiseptic soaps with appreciable antioxidant and antimicrobial properties.
2. Materials and methods
2.1. Plant materials preparation
The fruit of C. sinensis (Sweet Orange) and Moringa oleifera leaves obtained within Ilorin metropolis were authenticated and assigned the specimen number UILH/001/996 and UILH/002/1008 respectively. The fruits of C. sinensis were squeezed manually to obtain the seeds from the flesh and dried at room temperature. After drying, the seeds were de-shelled manually, dried at ambient temperature and pulverized using an electric blender. Natural pure honey and undiluted coconut oil without additive was obtained directly from trusted sellers while Ocimum gratissimum leaves were collected fresh from source plant following authentication and documentation by the herbarium specialist.
2.2. Solvents and chemical reagents
Analytical grade solvents and reagents which include n-hexane, methanol, chloroform, potassium hydroxide, potassium iodide, glacial acetic acid and hydrochloric acid were used. Ashes used for lye preparation were obtained from a renowned baking centre in Kwara State, Nigeria.
2.3. Lipid extraction
The oil was obtained from the seed of C. sinensis by subjecting the pulverized seed material (110.10 g) to soxhlet extraction using n-hexane at 60 °C for 3 h (Atolani et al., 2016). The oil (38 g) was obtained after the removal of the extracting solvent in vacuo.
2.4. Physicochemical analysis
The physicochemical parameters which include saponification value, acid value, iodine value, free fatty acid were determined using standard procedures (AOAC, 1990; Atolani et al., 2016).
2.5. Lipid trans-esterification
The fatty acid profile of the seed oil was determined by converting the lipid to fatty acid methyl esters (FAMEs) following previous reported method (Atolani et al., 2016). Briefly, 2 g oil was refluxed for one hour with hydrochloric acid prepared in methanol (0.2 M) and the organic phase containing the FAMEs was concentrated in vacuo and dried over anhydrous magnesium sulphate. The extent of esterification was determined as a function of lipid used.
2.6. Determination of fatty acids composition
The FAMEs obtained was subjected to gas chromatograph (6890N, Agilent technologies) coupled to an Agilent technology inert electron ionisation Mass Detector, (5975B, Agilent technologies, CA). Fatty acids standards were first injected and the calibration kept for further analyses. Sample injection (with split ratio 5:1) was via analytics auto-sampler attached to the gas chromatograph equipped with a non-polar column: ZB 7 HG-G010-11 with size 30 m × 0.25 mm x 0.25 μm. Carrier gas (He) was set to 1 mL/min and the injection temperature kept at 250 °C while the temperature gradient was set to 100 °C (5 min) and thereafter increased to 180 °C (at 5 °C/min). The temperature was maintained isothermally and finally raised to terminate at 330 °C. The mass range of the mass spectrometer operated at 70 eV ionisation energy with source and quad temperatures set to 230 °C/150 °C in an electron impact mode was set to the range 35–600 m/z. FAMEs were identified by comparing their retention time with those of the authentic standards and further confirmed by comparison of mass fragmentation pattern with those of NIST library (Atolani et al., 2016).
2.7. Lye preparation
Wood ash was soaked in cold water for 24 h in order to obtain a concentrated lye solution (Zubair et al., 2018). The mixture was decanted, filtered and filtrate concentrated via evaporation to dryness to afford the lye, brownish crystals which were kept for further use.
2.8. Evaluation of lye properties
The concentrated lye solution obtained was characterized by measuring the conductivity and turbidity of the lye solutions on EC 214 Conductimeter and 2100N Turbidimeter respectively (Zubair et al., 2018).
2.9. Elemental characterization by X-Ray fluorescence (XRF) spectroscopy
Lye crystals obtained were further characterized by subjecting it to XRF analysis to determine the element composition (Atolani et al., 2013). XRF spectrometer ECLIPSE III (AMTEK INC. MA, US) was used for the XRF analysis. The lye crystal was further dried, pulverized and pelletized. The sample was placed in the sample chamber for irradiation. The sample chamber was placed at angle 45o to the source X-ray tube connected to the Si-PIN photodiode detector. The source X-ray tube was maintained at a voltage of 25 kV at a current of 50 μA. Samples was irradiated for 1000 s.
2.10. Saponification reaction with lye solution
Standard hot saponification procedure of Warra et al. (2011) and Atolani et al. (2016) was adopted. Briefly, warm lye solution (10 mL) was slowly and intermittently introduced into a boiling aliquot of C. sinensis seed oil (10 mL) and stirred with continuous heating until the creamy dark-brown soap formed. As applicable, additives were added at this stage to the thick creamy substance, followed by gentle stirring for additional 10 min before allowing it to cool and set for ten weeks.
2.11. Incorporation of natural additives
The medicinal soaps were prepared using the mixture thus: seed oil only; seed oil with honey and Ocimum gratissimum; seed oil with honey, Ocimum gratissimum, coconut oil and M. oleifera seed oil. After the initial saponification of the oil, the incorporation of the additives (a proportion of coconut oil, M. oleifera seed oil and honey) was performed as applicable. Fresh leaves of Ocimum gratissimum were briefly inserted into the semi-solid matter to impart fragrance and antibacterial properties to the soap.
2.12. Soap characterizations
The soap characterization followed standard procedure (Ameh et al., 2013; Zubair et al., 2018). The soap characteristics which include hardness, foamability, pH value and solubility were determined and compared with the commercial antiseptic soap, Septol.
2.13. Test for washing efficiency
The washing efficiency of the prepared soaps was determined using standard procedure (Ameh et al., 2013; Zubair et al., 2018). Precisely, one drop of palm oil was placed on four separate sheets of filter paper each. The filter papers containing the oil spots were then immersed in a separate test tubes containing soap solution (2 g soap per 100 mL water). The test tubes were then vigorously shaken for 2 min and the filter papers thereafter removed and rinsed with distilled water. The filter papers were observed visually and comparatively for their respective washing efficiency in removing the oil spots.
2.14. Total fatty matter evaluation
Standard procedure was adopted for the determination of the Total Fatty Matter, TFM (Zubair et al., 2018, Zubair et al., 2019). The TFM was determined by dissolving the soap (0.2 g) in 3 mL distilled water and 0.4 mL of 15% H2SO4 while heating until a clear solution was obtained. The mixture was allowed to settle for some time until fatty acids liberated from the soap cake. The formed cake was weighed, dried and used to determine the TFM.
2.15. Determination of free caustic alkali
The free caustic alkali was determined by titrating the solution of the soap (5 g) in 30 mL ethanol against H2SO4 solution (0.05 M) in the presence of 10 mL of 20% BaCl2 and few drops of phenolphthalein indicator. The free caustic alkali was thereafter calculated using the expression (Mak-Mensah and Firempong, 2011):
| Free caustic alkali = 0.31[Volume of acid (mL)/Weight of soap (g)] |
2.16. Determination of total alkali
The total alkali was calculated following the procedure reported by Olabanji et al. (2016). Soap sample (1 g) dissolved in 5 mL ethanol and warmed was titrated against NaOH (1.0 M) in the presence of 0.5 mL H2SO4 and phenolphthalein indicator.
2.17. Antioxidant assay
In order to establish the antioxidant potential of the seed oil and soap, the in vitro DPPH radical scavenging assay according to the procedure previously described (Adeosun et al., 2013; Atolani and Olatunji, 2016). The oil and soap solutions (2.4 mL each) were prepared at concentrations ranging from 10 to 500 μg/mg and 0.8 mL solution of freshly prepared DPPH solution (in methanol) at 0.1 mM. The resulting mixtures were thoroughly vortexed and incubated in the dark at room temperature for 10 min to attain a complete reaction. The absorbances of the solutions were read at 517 nm on a Unicam UV 500 Spectrophotometer (Thermo Spectronic, UK). The DPPH radical scavenging potential of the samples was expressed as percentage of DPPH radicals scavenged. Ascorbic acid was used as standard while the solution without sample served as control.
2.18. Assay for evaluation of anti-Toxoplasma activity
In this evaluation, we used the Toxoplasma gondii RH strain 2F (ATCC® 50839). To sustain the parasite stabilate, it was repeatedly passaged in in cultures of Human Fibroblast Foreskin (HFF ATCC®) monolayers. The cell medium consisted of DMEM (Nissui, Tokyo, Japan), GlutaMAX™-I and (fetal calf serum (10% v/v Gibco, Invitrogen, UK), and penicillin/streptomycin (100 U/mL; Biowhittaker, UK). Purified suspension of T. gondii tachyzoites was obtained by lysing infected HFF monolayers, followed by filtration and washing of cell lysates with fresh culture medium. The anti-parasite assay was performed as described elsewhere (Adeyemi et al., 2017a). In brief, various concentrations of oil extracts in culture medium (0, 125, 250, 500, 1000 μg/mL) were co-incubated with freshly purified tachyzoites in cultures of HFF monolayers in a 96-well optical bottom plate (Fisher Scientific, Pittsburgh, USA). This was followed by incubating plates in an atmosphere of 37 °C and 5% CO2 for 72 h. The negative drug control had only the culture medium, while sulfadiazine (5 μM) served as a positive drug control. Multiplicity of infection was 1:5 [parasite/host cell ratio] andparasite viability was determined by luminescence (Promega Beta-Glo Madison, USA). The assay was performed in triplicate and independently carried out three times.
2.19. Determination of cellular toxicity of the oil
The HFF cells maintained in normal cell culture medium as described above were allowed to grow to 70–80% confluence. This was followed by sub-culturing and seeding of 100000 cells per well in a 96-well plate. Then cells were incubated for 72 h in an atmosphere of 37 °C and 5% CO2. This was followed by treatment of cells with various concentrations of the oil (0–1000 μg/mL). Only the culture medium was added to the control well, while staurosporine served as positive drug control. After treatment, cells were incubated for another 72 h before colorimetric determination of the cell viability (Promega CellTitre-Aqueous One Solution Madison, USA). The assay was in triplicates and independently carried out three times.
2.20. Assay for determining reactive oxygen species (ROS) in cells
ROS within cells was measured as described elsewhere (Adeyemi et al., 2017a). This method is based on the oxidation of 2ʹ,7ʹ-dichlorodihydro- fluorescein diacetate (H2DCF-DA, Sigma, St Louis, MO, USA) to a fluorophore 2ʹ,7ʹ-dichlorofluorescein by intracellular peroxide. In brief, cultures of HFF monolayers treated with the oil in the absence/presence of T. gondii infection were incubated for 24 h in an atmosphere of 37 °C and 5% CO2. Thereafter, the cells were harvested, washed, and re-suspended in PBS containing the H2DCF-DA (final concentration100 μM). After 30–60 min incubation at 37 °C, fluorescence was recorded on a spectrofluorometer (Corona Electric, Japan) with excitation set at 485 nm and emission at 530 nm. To validate this assay, H2O2 (100 μM) was included as a positive control.
2.21. Assay for determining the mitochondrial membrane potential (MMP)
The MMP measurement was as described previously (Adeyemi et al., 2017a). In brief, cultures of HFF cells treated with C. sinensis oil in the absence/presence of T. gondii infection were incubated for 24 h at an atmosphere of 37 °C and 5% CO2. Thereafter, the cells were harvested, washed, and stained with 200 nM MitoRed (Dojindo Molecular Technologies Inc. Japan). Fluorescence was recorded on a spectrofluorometer with excitation set at 560 nm and emission at 580 nm.
2.22. Antimicrobial studies
The antimicrobial sensitivity of the oil and soap samples was examined using agar diffusion procedure (Ameh et al., 2013) against some economically important organisms (clinical isolate) of McFarland standard aseptically maintained on agar at 4 °C. The bacteria which were used include: Klebsiella pnamananae, Streptococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, Salmonella typhi and Escherichia coli, while fungi include Candida albicans, Penicillium notatum, Aspergillus niger and Rhizopus stolonifer. The prepared media was spread into sterilised petri-dishes and the organisms were inoculated following serial dilution of 1 × 106 CFU/mL. The sample solutions (1 mL) with highest concentration 100 mg/mL prepared in water were pipetted into each hole in the petri dish bored aseptically. Bacteria were incubated at 37 °C for 48 h while fungi were incubated at 37 °C for 96 h. The diameter zones of microbial inhibition were measured thereafter.
2.23. Data analysis
Data were analyzed by one-way ANOVA on GraphPad Prism 5 (San Diego, CA, USA) and presented as the mean of triplicates ± standard error of mean (SEM). IC50 values were obtained from a dose-response curve as the concentration causing a 50% inhibition or reduction in parasite and/or cell viability and the curve was fitted using a non-linear regression. Values at p < 0.05 are considered significant.
3. Results and discussion
3.1. Physicochemical characterization
The result of the physicochemical characterization of the oil is as depicted in Table 1.
Table 1.
Physicochemical characteristics of C. sinensis seed oil.
| Parameters | Results |
|---|---|
| Percentage yield (%) | 34.54 |
| Colour | Golden-yellow |
| State at ambient temperature | Liquid |
| Saponification value (mgKOH/g) | 193.55 |
| % Free fatty acid | 20.02 |
| Iodine value (I2g/100 g) | 83.45 |
| %Yield of the trans-esterified oil | 91.85 |
| Acid value (mgKOH/g) | 7.59 |
The C. sinensis seed oil had saponification value of 193.55 mg KOH/g. The relatively high saponification value of the oil indicates the presence of lower molecular weight fatty acids in the oils which therefore qualifies the oil to be classified as edible oil. The free fatty acid content was 20.02% showing that the oil would readily saponifies. However this high FFA content nullifies the edibility potential suggested by saponification potential. The iodine value of the oil was 83.45 gI2/100 g. This indicated that the oil is a potential source of unsaturated fatty acids which would be non-drying oil and thus can be recommended for saponification purposes (Guner et al., 2006). Taken together, the values obtained for the physicochemical properties were considerably in favour of the utilization of the oil from the indigenous seed of C. sinensis for soap production on commercial scale since the seed are obtained in large quantity especially in fruit juice industries.
3.2. Lye analysis
3.2.1. X-ray fluorescence (XRF) spectroscopy
In order to determine the major elemental composition of the lye, XRF spectroscopy analysis was carried out. The XRF results (Table 2) for the prepared lye sample showed the presence of potassium, calcium as major elements and trace amount of copper, iron, manganese, sirconium, barium and zinc. Toxic heavy metals such as elements cadmium, silver and mercury were not detected.
Table 2.
XRF spectroscopy result of lye.
| Elements | Conc. Value (wt%) ± SD |
|---|---|
| Ca | 0.2435 ± 0.0083 |
| K | 5.4347 ± 0.0723 |
| Ba | 0.0027 ± 0.0018 |
| Cu | 0.001 ± 0.0001 |
| Fe | 0.0093 ± 0.001 |
| Mn | 0.001 ± 0.0004 |
| Sr | 0.0034 ± 0.0005 |
| Zn | 0.0009 ± 0.0001 |
3.3. Conductivity and turbidity evaluations
The conductivity and turbidity of the prepared lye is as presented (Table 3). The lye has moderate conductivity and turbidity of 0.1 μS/cm and 199 NTU respectively.
Table 3.
Conductivity and Turbidity Result of Lye (wood ash).
| Parameter | Lye |
|---|---|
| Conductivity | 0.1 μS/cm |
| Turbidity | 199 NTU |
3.4. Fatty acids profile of the C. sinensis seed oil
FAMEs were prepared from the extracted C. sinensis seed oil and subjected to GC-MS analysis. The fatty acid profile of the oil as obtained from the GC-MS analysis is showed in Table 4.
Table 4.
Fatty acid compositions of C. sinensis.
| Peak No. | Fatty Acids | Short Name | Saturation | %Abundance |
|---|---|---|---|---|
| 1 | Palmitic acid | C16:0 | 16:0 | 21.10 |
| 2 | Palmitolinoleic acid | C16:1 | 16:1 | 0.39 |
| 3 | Heptadecenoic acid | C17:1 | 17:1 | 0.41 |
| 4 | Stearic acid | C18:0 | 18:0 | 4.80 |
| 5 | Elaidic acid | C18:1n9t | 18:1 | 1.11 |
| 6 | Oleic acid | C18:1n9c | 18:1 | 27.35 |
| 7 | Linoleic acid | C18:2n6c | 18:2 | 36.23 |
| 8 | α-Linolenic acid | C18:3n3 | 18:3 | 3.52 |
| 9 | Arachidic acid | C20:0 | 20:0 | 0.50 |
| 10 | Eicosadienoic acid | C20:2 | 20:2 | 0.35 |
| 11 | Heneicosylic acid | C21:0 | 21:0 | 0.36 |
| 12 | Arachidonic acid | C20:4n6 | 20:4 | 0.36 |
| 13 | Cis-11,14,17-eicosatrienoic acid | C20:3n3 | 20:3 | 0.36 |
| 14 | Behenic Acid | C22:0 | 22:0 | 0.34 |
| 15 | Cis-13,16-docosadienoic acid | C22:2n6 | 22:2 | 0.62 |
| 16 | Tricosylic acid | C23:0 | 23:0 | 1.05 |
| 17 | Lignoceric acid | C24:0 | 24:0 | 0.36 |
| 18 | Nervonic acid | C24:1 | 24:1 | 0.39 |
| 19 | Docosahexaenoic acid | C22:6n3 | 22:6 | 0.40 |
| Total Saturate | 28.49 | |||
| Total Unsaturate | 71.50 | |||
| Monounsaturate | 29.65 | |||
| Polyunsaturate | 41.84 |
The relatively high total lipid contents (34.5%) of the seeds (Table 1) makes the seed to be considered economically lucrative sources for industrial applications, specifically when compared with other oil seed crops such as corn and soybean which show a lipid content that is less than 20% (O'Brien, 2004). Linoleic acids (C18:2n6), oleic (C18:1n9) and palmitic (C16:0) were detected at high concentrations in the C. sinensis oil. Stearic acid (C18:0) and α-linolenic acid (C18:3n3) were also present in noticeable percentages in the oil at 4.80% and 3.52% respectively. The linolenic acid percentage of the C. sinensis seed oil was low compared to the common natural oils such as corn (10.0%), soybean (8%) and rapeseed (10%) oils but matches those of oils extracted from the seeds of sweet lemon (3.89%), orange (3.44%) and mandarin (3.57%) (Anwar et al., 2008).
The C. sinensis seed oil proved to be a good source of essential fatty acids (C18:2n6 + C18:3n3) containing approximately 40% of linoleic and linolenic fatty acids. This same feature was also observed with citrus seed oils by other authors (Ajewole and Adeyeye, 1993; Saïdani et al., 2004; Anwar et al., 2008). The palmitic and oleic acid which accounted for about 48% of the total fatty acid is known in the cosmetic industries as a major determinant of soap quality (Oghome et al., 2012). Thus, C. sinensis oil can therefore be adjudged to be suitable for soap production.
The high proportion of unsaturated fatty acids (71.50%) of the oil correlates with the trend observed in the iodine and saponification values. This characteristic was also observed in citrus seed oils by other authors (Atolani et al., 2012).
3.5. Physicochemical characteristics of prepared soaps
Different formulations were employed in the Green production of soaps from the extracted oil in order to explore the various potentials of the oil and afford optimization. The properties of the soaps produced based on the mixing ratio are as presented in Table 5.
Table 5.
Mixing ratio of the component used for the saponification.
| Soap Samples | Oil and Additives | Mixing Ratios | Colour | Washing Efficiency |
|---|---|---|---|---|
| CS1 | CSO | 1 | Cream | Excellent |
| CS2 | CSO + Additives | 0.8:0.2 | Brown | Very good |
| CS3 | CSO + CO + MO + Additives | 0.6:0.1:0.1:0.2 | Brown | Very good |
Where: CSO = C. sinensis Oil, CO = Coconut Oil, MO = M. oleifera Seed Oil, Additives = Ocimum gratissimum extract and Honey (1:1).
The C. sinensis soaps (CS1 to CS3) appeared creamy to brown in colour. Based on the physical observations, the mixing of the oil with coconut and M. oleifera seed oil reduced the washing efficiency as the highest washing efficiency was observed in the soap CS 1, that is, the soap without any additive incorporated. CS2 and CS3 with different proportions of additives have comparable washing efficiencies.
3.6. Physical characteristics of the soap samples
The physicochemical properties of the soaps produced was compared with a commercial soap (Septol) used as standard. The results of the physical characterization of the prepared soaps are as depicted in Table 6.
Table 6.
Physical characteristics of the soap samples.
| Soaps | CS1 | CS2 | CS3 | Septol |
|---|---|---|---|---|
| pH (Day1) | 9.85 | 9.83 | 9.86 | - |
| pH (Week12) | 8.93 | 9.10 | 9.30 | 9.01 |
| Foam Height (cm) | 5.8 | 5.0 | 6.9 | 4.1 |
| Dissolution time (sec) |
300 |
380 |
310 |
540 |
| Texture | Hard | Soft | Soft | Hard |
| Total Fatty Matter | 0.55 | 0.95 | 0.75 | 0.65 |
| Free Caustic Alkali | 0.31 | 0.47 | 0.16 | 0.12 |
| Total Alkali | 0.50 | 0.34 | 0.31 | 0.11 |
Where; CS1 = C. sinensis Oil, CS2 = C. Sinensis + Ocimum gratissimum extract + Honey, CS3 = C. sinensis Oil + Coconut Oil + Moringa oleifera seed Oil + Ocimum gratissimum extract + Honey.
The pH of all the prepared soaps reduced slightly after week 12, implying a reduced alkalinity. The reduction in the pH is attributed to further neutralization of the unreacted alkali (lye) over time. The pH values of all the soaps were within acceptable limit 8.5–10 (Oyedele, 2002). The pH values obtained are also in agreement with literatures (Atolani et al., 2016; Ogunsuyi and Akinnawo, 2012; Vivian et al., 2014). Also, all the prepared soaps lather more than Septol soap, the commercial standard used. Sample CS3 had the highest foaming ability (Table 6). The inclusion of C. sinensis seed oil, coconut oil and Moringa oleifera seed oil together with Ocimum gratissimum leaf extract and honey improved the foam ability of the soap as observed in CS3 while the addition of only Ocimum gratissimum leaf extract and honey inhibit the foaming ability. It is evident that amongst all soaps produced, CS1 has the least solubility character as it took the longest time to dissolve. Free caustic alkali and total alkali of the produced soaps were higher than that of the Septol. Septol recorded a higher total fatty matter than only the CS1 soap.
3.7. Antioxidant activity of seed oils and soaps
The extent of DPPH radicals scavenged by the soap samples are as shown antioxidant activities of control (ascorbic acid), C. sinensis, CS1, CS3 and Septol are shown in Table 7.
Table 7.
DPPH scavenging activities of the oil and soaps.
| Percentage Reduction (%) | |||||
|---|---|---|---|---|---|
| Conc. (μg/mg) | C. sinensis Oil | CS1 | CS3 | Septol | Ascorbic Acid |
| 10 | 46.05 ± 8.35 | 56.82 ± 2.75 | 45.79 ± 4.81 | 44.42 ± 5.46 | 49.42 ± 1.74 |
| 50 | 43.97 ± 1.56 | 47.89 ± 3.36 | 49.54 ± 2.17 | 42.41 ± 1.14 | 56.12 ± 4.12 |
| 100 | 54.44 ± 0.69 | 55.30 ± 1.36 | 49.44 ± 1.52 | 46.05 ± 6.26 | 60.96 ± 10.08 |
| 200 | 54.07 ± 7.18 | 53.93 ± 4.58 | 52.53 ± 10.21 | 46.02 ± 1.77 | 65.01 ± 7.69 |
| 500 | 52.36 ± 0.80 | 54.59 ± 4.25 | 51.12 ± 0.74 | 45.96 ± 5.58 | 72.27 ± 0.53 |
| IC50 (μg/mg) | 12.3 ± 1.01 | 14.9 ± 0.65 | 11.4 ± 0.56 | 7.0 ± 0.34 | 35.9 ± 4.04 |
Mean of triplicate determinations ± SEM.
The reduction of DPPH radicals was determined by the decrease in the absorbance at 517 nm induced by the samples. DPPH is a stable free radical and accepts an electron (hydrogen radical) to become a stable diamagnetic molecule. The DPPH assay revealed that the C. sinensis seed oil and the soaps (CS1, CS3, and Septol) had appreciable scavenging capacity (Table 7) compared with the control (ascorbic acid). CS1 and CS3 recorded higher antioxidant capacity than Septol at all the tested concentrations. The antioxidant activity of citrus seed oil might be partly attributed to the presence of phenolic compounds (Lu and Foo, 2001).
Since cosmetic of natural sources is gaining more interest particularly among users who are now aware of the potential harmful effects of the synthetic additives (Soumanou and Adjou, 2016), the incorporation of natural additives has increased. The extract of the aromatic plant, O. gratissimum is one of the plants widely used in traditional medicine and natural cosmetic preparations for its flavouring and bioactivities (Fongnzossie et al., 2017). Owing to safety concerns about synthetics, the plant has found increased used and applications in food, perfumery, cosmetic and pharmaceutical industries (Aguiar et al., 2015; Pandey et al., 2014). In addition, the oil has been applied in various cosmetic formulations primarily for its antioxidant, moisturizing and smoothening effects on skin (Gupta et al., 2010; Krongrawa et al., 2018; Mahomoodally and Ramjuttun, 2016).
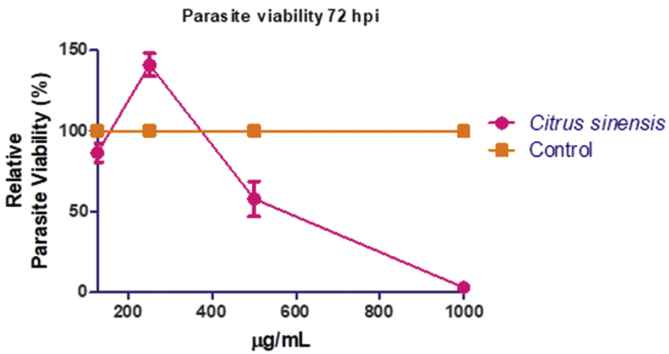
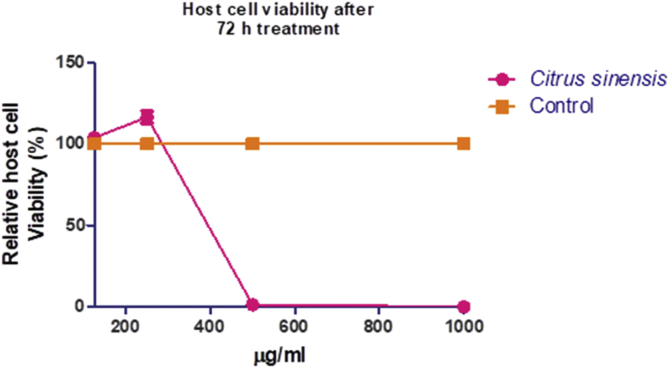
3.8. In vitro anti-parasite may be due to general toxicity
The anti-Toxoplasma assay revealed that C. senensis seed oil had moderate potency in restricting the in vitro growth of T. gondii (Figure 1) with EC50 of >500 μg/mL. Meanwhile, assay for cytotoxicity in mammalian host cells showed that C. senensis oil was dose-dependently cytotoxic to HFF cells with IC50 of <350 μg/mL (Figure 2). Furthermore, we estimated the selectivity index (SI) of the oil [ratio of the cytotoxicity (IC50) to the anti-parasite activity (EC50)] in order to determine anti-parasite efficacy. Data revealed that the C. senensis oil had SI of ≤1 relative to the reference drug, sulfadiazine, (SI ≤ 4), the drug currently used for treatment of toxoplasmosis (Table 8). This finding indicates that the C. senensis oil lacked selective anti-parasite action. Together, the findings implicate general cellular toxicity by C. senensis oil.
Table 8.
Fold activity against Toxoplasma gondii versus host cell (human foreskin fibroblast- HFF).
| Identifier | Anti-parasite activity EC50 (μg/mL) | Host cell cytotoxicity IC50 (μg/mL) | Selectivity index: IC50/EC50 |
|---|---|---|---|
| Citrus senensis oil | ≤500 | ≤350 | <1 |
| Sulfadiazine | ≤139 | ≤480 | <4 |
Values are expressed as Mean of replicates (n = 9).
Figure 1.
In vitro anti-parasite efficacy of Citrus senensis oil following after 72 h treatment post infection. Data presented as mean of nine replicates ± standard error of mean (SEM).
Figure 2.
Cytotoxicity of Citrus senensis oil in mammalian host cells (HFF) after 72 h treatment. Data presented as mean of nine replicates ± standard error of mean (SEM).
Table 9.
Antimicrobial activities of C. Sinensis seed oil.
| Diameter Zone of Inhibition (mm) |
|||||||
|---|---|---|---|---|---|---|---|
| Tested Organisms | 200 | 100 | 50 | 25 | 12.5 | Gent. | MIC |
| Staphylococcus aureus | 17.0 ± 1.0 | 14.0 ± 0.0 | 10.0 ± 0.0 | - | - | 35.0 | 50.0 |
| Escherichia coli | 18.0 ± 0.0 | 15.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | - | 35.0 | 25.0 |
| Bacillus subtilis | 16.0 ± 0.0 | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | - | 36.0 | 25.0 |
| Pseudomonas aeruginosa | 18.0 ± 0.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | 35.0 | 12.5 |
| Salmonella typhi | 20.0 ± 0.0 | 18.0 ± 0.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 10.0 ± 0.0 | 36.0 | 12.5 |
|
Klebsiella pnamananae |
16.0 ± 0.0 |
14.0 ± 0.0 |
12.0 ± 0.0 |
10.0 ± 0.0 |
- |
35.0 |
25.0 |
| Fungi | Tioc. | ||||||
| Candida albicans | 16.0 ± 0.0 | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | - | 28.0 | 25.0 |
| Aspergillus niger | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | - | - | 27.0 | 50.0 |
| Penicillium notatum | 15.0 ± 1.0 | 13.0 ± 1.0 | 10.0 ± 0.0 | - | - | 26.0 | 50.0 |
| Rhizopus stolonifer | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | - | - | 26.0 | 50.0 |
Mean of triplicate determinations with SEM; Gent. means Gentamicin; Tioc means tioconazole. Sample was measure in mg/mL.
3.9. C. senensis oil anti-parasite action precludes oxidative stress
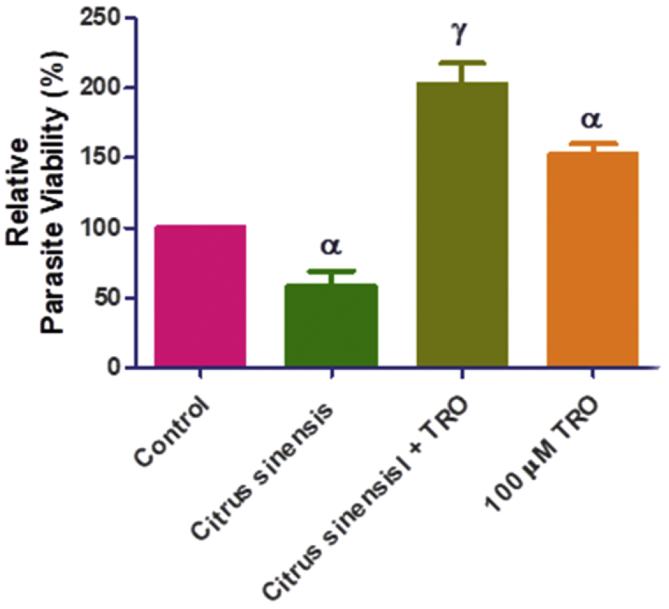
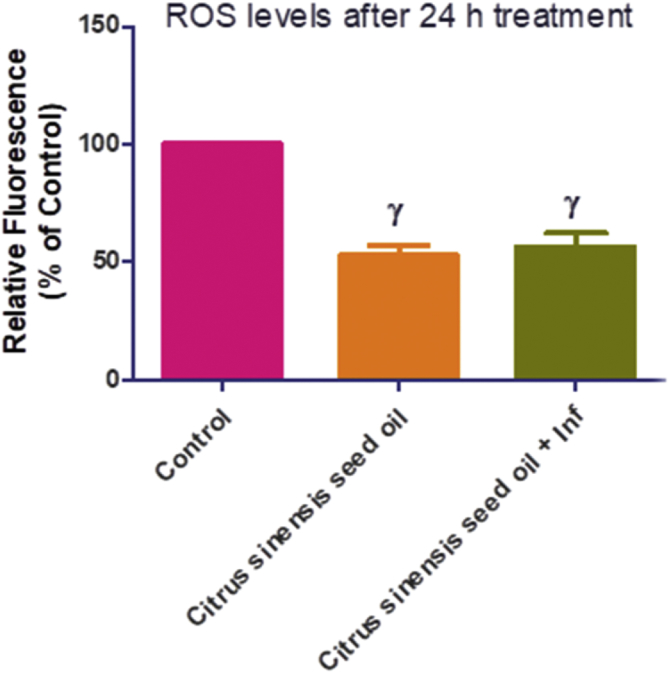
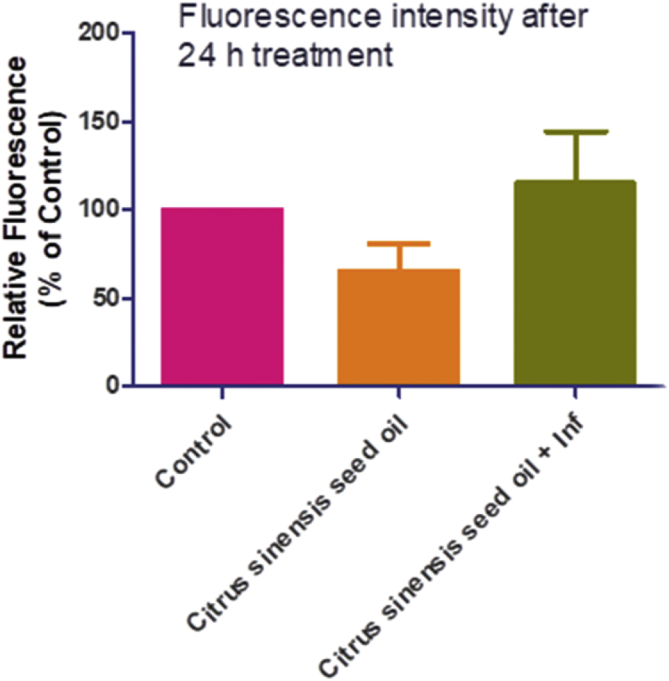
Additionally, we determined whether reactive oxygen species (ROS) was culpable in the anti-parasite action of C. senensis oil, by adding an antioxidant, Trolox (100 μM) to the screening assay. Data showed that the addition of trolox relieved the restriction of parasite growth by C. senensis oil (Figure 3), suggesting that oxidative stress might be contributing to the anti-parasite action of the oil extract. However, our assays to determine level of ROS production showed that C. senensis oil did not cause ROS production after 24 h treatment whether in the absence or presence of T. gondii infection (Figure 4). The oil extract actually reduced the ROS level by ≥ 50% when compared with the negative drug control. Probably the C. senensis oil did not predispose to the generation of ROS but utilizes alternative ways to restrict parasite growth. Moreover, reduction of ROS level by ≥ 50% compared to the negative control may indicate that the C. senensis oil extract might possess some level of antioxidant capacity. This may be related to the high DPPH scavenging activity observed in this study.
Figure 3.
In vitro anti-parasite efficacy of Citrus senensis oil following addition of antioxidant TROLOX. Data presented as mean of nine replicates ± standard error of mean (SEM). α at p < 0.05 versus control and γ at p < 0.0001 versus Citrus senensis.
Figure 4.
Cellular ROS level after 24 h treatment with Citrus senensis oil in the absence or presence of Toxoplasma gondii infection. Data presented as mean of nine replicates ± standard error of mean (SEM). γ at p < 0.0001 versus control.
Furthermore, we measured the mitochondria membrane potential (MMP) and data revealed that C. senensis oil mildly affected the cellular MMP (Figure 5) but not in the presence of T. gondii infection. The reason for this is unknown but might not be unconnected with alteration of physiological status of cells due to T. gondii infection (Adeyemi et al., 2017b, 2018). Taken together, findings support that the anti-parasite action of the C. senensis oil preclude oxidative stress.
Figure 5.
Cellular mitochondria membrane potential (MMP) after 24 h treatment with Citrus senensis oil in the absence or presence of Toxoplasma gondii infection. Data presented as mean of nine replicates ± standard error of mean (SEM).
3.10. Antimicrobial activity
Atolani et al. (2016), recommended that a wider range of anti-microbial tests would be necessary to ascertain the potential of natural antiseptic soaps. Therefore, in order to evaluate the antimicrobial potential of the prepared soaps in this study, a wide range of bacteria and fungi were employed as test organisms to determine the extent of antimicrobial activities of CS1 and CS3 soaps using the commercial antiseptic soap, Septol as a standard.
Results of antimicrobial evaluations (Tables 9, 10, 11, and 12) show that the seed oil possesses important antibacterial and antifungal activities. The result is in agreement with literature (Okunowo et al., 2013). Thus the antimicrobial activities obtained in this study have known scientific correlations. The antimicrobial activity was against a wide range of Gram positive and Gram negative bacteria as well as the screened fungi. These organisms have been implicated in skin and mucous membrane infections with reports of morbidity and mortality (Mahmoud, 2001).
Table 10.
Antimicrobial activities of soap, CS1.
| Diameter Zone of Inhibition (mm) |
|||||||
|---|---|---|---|---|---|---|---|
| Tested Organisms | 200 | 100 | 50 | 25 | 12.5 | Gent. | MIC |
| Staphylococcus aureus | 23.0 ± 1.0 | 19.0 ± 1.0 | 16.0 ± 0.0 | 13.0 ± 1.0 | 11.0 ± 1.0 | 35.0 | 12.5 |
| Escherichia coli | 21.0 ± 1.0 | 18.0 ± 0.0 | 15.0 ± 1.0 | 13.0 ± 1.0 | 11.0 ± 1.0 | 35.0 | 12.5 |
| Bacillus subtilis | 23.0 ± 1.0 | 19.0 ± 1.0 | 17.0 ± 1.0 | 14.0 ± 0.0 | 10.0 ± 0.0 | 36.0 | 12.5 |
| Pseudomonas aeruginosa | 18.0 ± 0.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | 35.0 | 12.5 |
| Salmonella typhi | 18.0 ± 0.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | 36.0 | 12.5 |
|
Klebsiella pnamananae |
20.0 ± 0.0 |
18.0 ± 0.0 |
16.0 ± 0.0 |
14.0 ± 0.0 |
10.0 ± 0.0 |
35.0 |
12.5 |
| Fungi | Tioc. | ||||||
| Candida albicans | 18.0 ± 0.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | 28.0 | 12.5 |
| Aspergillus niger | 15.0 ± 1.0 | 13.0 ± 1.0 | 10.0 ± 0.0 | - | - | 27.0 | 50.0 |
| Penicillium notatum | 15.0 ± 1.0 | 13.0 ± 1.0 | 10.0 ± 0.0 | - | - | 26.0 | 50.0 |
| Rhizopus stolonifer | 13.0 ± 1.0 | 10.0 ± 0.0 | - | - | - | 26.0 | 100.0 |
Mean of triplicate determinations with SEM; Gent. means Gentamicin; Tioc means tioconazole. Sample was measure in mg/mL.
Table 11.
Antimicrobial Activities of CS3 soap.
| Diameter Zone of Inhibition (mm) |
|||||||
|---|---|---|---|---|---|---|---|
| Tested Organisms | 200 | 100 | 50 | 25 | 12.5 | Gent. | MIC |
| Staphylococcus aureus | 23.0 ± 1.0 | 19.0 ± 1.0 | 17.0 ± 1.0 | 13.0 ± 1.0 | 11.0 ± 1.0 | 34.0 | 12.5 |
| Escherichia coli | 18.0 ± 0.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | 34.0 | 12.5 |
| Bacillus subtilis | 18.0 ± 0.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 10.0 ± 0.0 | - | 36.0 | 25.0 |
| Pseudomonas aeruginosa | 20.0 ± 0..0 | 18.0 ± 0.0 | 15.0 ± 1.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | 35.0 | 12.5 |
| Salmonella typhi | 21.0 ± 1.0 | 18.0 ± 0.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 10.0 ± 0.0 | 37.0 | 12.5 |
|
Klebsiella pnamananae |
19.0 ± 1.0 |
17.0 ± 1.0 |
14.0 ± 0.0 |
12.0 ± 0.0 |
10.0 ± 0.0 |
36. |
12.5 |
| Fungi | Tioc. | ||||||
| Candida albicans | 15.0 ± 1.0 | 13.0 ± 1.0 | 10.0 ± 0.0 | - | - | 26.0 | 50.0 |
| Aspergillus niger | 15.0 ± 1.0 | 13.0 ± 1.0 | 10.0 ± 0.0 | - | - | 27.0 | 50.0 |
| Penicillium notatum | 18.0 ± 0.0 | 15.0 ± 1.0 | 13.0 ± 1.0 | 10.0 ± 0.0 | - | 27.0 | 12.5 |
| Rhizopus stolonifer | 16.0 ± 0.0 | 14.0 ± 0.0 | 12.0 ± 0.0 | - | - | 27.0 | 50.0 |
Mean of triplicate determinations with SEM; Gent. means Gentamicin; Tioc means tioconazole.
Table 12.
Antimicrobial activities of septol (standard soap).
| Diameter Zone of Inhibition (mm) |
|||||||
|---|---|---|---|---|---|---|---|
| Tested Organisms | 200 | 100 | 50 | 25 | 12.5 | Gent. | MIC |
| Staphylococcus aureus | 25.0 ± 1.0 | 21.0 ± 1.0 | 17.0 ± 1.0 | 14.0 ± 0.0 | 11.0 ± 1.0 | 35.0 | 12.5 |
| Escherichia coli | 23.0 ± 1.0 | 18.0 ± 0.0 | 16.0 ± 0.0 | 11.0 ± 1 .0 | 10.0 ± 0.0 | 35.0 | 12.5 |
| Bacillus subtilis | 22.0 ± 0.0 | 18.0 ± 0.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 10.0 ± 0.0 | 36.0 | 12.5 |
| Pseudomonas aeruginosa | 22.0 ± 0.0 | 19.0 ± 1.0 | 17.0 ± 1.0 | 15.0 ± 1.0 | 11.0 ± 1.0 | 35.0 | 12.5 |
| Salmonella typhi | 21.0 ± 1.0 | 19.0 ± 1.0 | 15.0 ± 1.0 | 11.0 ± 1.0 | 10.0 ± 0.0 | 36.0 | 12.5 |
|
Klebsiella pnamananae |
21.0 ± 1.0 |
18.0 ± 0.0 |
16.0 ± 0.0 |
14.0 ± 0.0 |
10.0 ± 0.0 |
35.0 |
12.5 |
| Fungi | Tioc. | ||||||
| Candida albicans | 20.0 ± 0.0 | 18.0 ± 0.0 | 16.0 ± 0.0 | 13.0 ± 00 | 10.0 ± 0.0 | 28.0 | 12.5 |
| Aspergillus niger | 19.0 ± 1.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | 27.0 | 12.5 |
| Penicillium notatum | 19.0 ± 1.0 | 16.0 ± 0.0 | 14.0 ± 0.0 | 12.0 ± 0.0 | 10.0 ± 0.0 | 26.0 | 12.5 |
| Rhizopus stolonifer | 19.0 ± 1.0 | 17.0 ± 1.0 | 15 ± 1.0 | 12.0 ± 0. 0 | 10 ± 0.0 | 26.0 | 12.5 |
Mean of triplicate determinations with SEM; Gent. means Gentamicin; Tioc means tioconazole. Sample was measure in mg/mL.
It is evident (Table 9) that the C. sinensis seed oil showed appreciable level of anti-microbial activities against the tested organisms. At 50 mg/mL, the oil showed activity against all the test organisms with Salmonella typhi having the highest sensitivity. At 12.5 mg/mL, lower activities were recorded against Pseudomonas aeruginosa and Salmonella typhi. This oil had inhibitory activities against Salmonella typhi and Pseudomonas aeruginosa at all concentrations tested. Activities were however lower than the positive controls, gentamicin (bacteria) and tioconazole (fungi). Activities seem more pronounced against the fungi than the bacteria. The MIC for the antibacterial mostly was 25 mg/mL and 50 mg/mL for anti-fungi respectively.
CS1 soap exhibited appreciable level of antimicrobial activity against the tested organisms (Table 10). Broad activity was recorded against all test organisms at all concentrations except for the fungi Aspergillus niger, Penicillium notatum and Rhizopus stolonifer. Soap CS1 had highest sensitivity against Staphylococcus aureus, Escherichia coli and Bacillus subtilis and lowest inhibition against Rhizopus stolonifer fungi.
CS3 soap also exhibited appreciable level of antimicrobial activity against the tested organisms (Table 11). Broad activity was recorded against all the bacteria test organisms at all concentrations except for the Bacillus subtilis. However, at 12.5 mg/mL, the anti-fungi activity could not be sustained. This soap recorded its highest activity against Staphylococcus aureus, Salmonella typhi and lowest activity against Rhizopus stolonifer.
It was observed that CS1 soap (soap made from only C. sinensis oil) had higher antimicrobial activity than the CS3 soap which contains coconut and Moringa oleifera seed oils. The activity of CS1 was comparable with the standard soap, Septol (Table 12). It was noticed that mixing the C. sinensis seed oil with other oils (coconut oil and Moringa oleifera seed oil) reduces the soaps actions against these microbes.
The use of these natural soaps may help in the restricting growth of skin pathogen and consequently prevent skin diseases thereby avoiding the need to add synthetic antimicrobial agents. The use of antiseptic bath soaps is the earliest caution against bacteria and other pathogens that can cause colds, the flu, skin infections and other fatal communicable diseases (Atolani et al., 2016; Mwambete and Lyombe, 2011). Incorporation of synthetic additives like antimicrobial and fragrances, in soaps has many adverse effects such as irritation, environmental hazards among others, which should be avoided. Overuse of antibacterial agents in cosmetics products has been linked to bacterial resistance with capacity to induce more health havoc such as endocrine disruption and cancer initiation risks (Nowak et al., 2018). Triclosan, widely used in soaps, toothpastes and deodorants, has been detected in breast milk, and some recent studies have reported that it interferes with testosterone action in cells, impairs muscle functions and that it is allegedly carcinogenic (Chuanchen et al., 2001; Poole, 2002; Atolani et al., 2016). Alongside other cosmetic products, triclosan has been banned as additive in cosmetic products in some western countries. The catchall term "fragrance" has been reported as a potential mask for phthalates in soap, which act as endocrine disruptors linked to obesity, reproductive and developmental dysfunctions (Day, 2012). Also, many artificial fragrances present in most deodorants, shampoos, sunscreens, skin care, body care and baby products are reportedly carcinogenic or toxic to humans. Many scientific reports have linked rashes, skin discoloration and allergic skin irritation to many synthetic additives used in soaps and cosmetics (Atolani et al., 2016).
4. Conclusion
C. sinensis seed, a neglected bio-resource has been investigated for the chemical composition of the oil, potential application in cosmeceuticals and its biological activities. Though, C. sinensis seed is a typical waste product in the environment and natural fruit juice industries, soap made from C. sinensis seed oil, is herein reported to possess cosmeceutical application especially, antiseptic with excellent properties such as good solubility, foaming ability, texture, colour, low free caustic alkali, antimicrobial activity, antioxidant potential, anti-parasite and low cytotoxicity. Based on these results, it could be concluded that the bioactivities recorded for C. sinensis seed oil (and soaps) is based on the chemical compositions herein reported. Since most cosmetic consumers now prefer “green”, natural and more benign products which is also more environmental-friendly, the application of the seed oil is highly recommended for further exploration. The result obtained in this research further add credence to the concept of conversion of waste to wealth initiative which could ameliorate poverty whilst making the terrestrial and aquatic environmental safer. Obviously, these results would be useful resource for the soap and cosmetic industries.
Declarations
Author contribution statement
Atolani O.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Olatunji G.A.: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Adamu N., Adeyemi O.S.: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Areh E.T.: Performed the experiments; Analyzed and interpreted the data.
Oguntoye O.S.: Performed the experiments; Wrote the paper.
Zubair M.F., Fabiyi O.A., Oyegoke R.A., Tarigha D.E.: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Kambizi L.: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by The World Academy of Sciences (grant number: 15-244 RG/CHE/AF/AC_G – FR3240287031).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
O. Atolani, Email: tolanvent@yahoo.com.
L. Kambizi, Email: kambizil@cput.ac.za.
References
- Abugri D.A., Jaynes J.M., Witola W.H. Anti-Toxoplasma activity of Sorghum bicolor-derived lipophilic fractions. BMC Res. Notes. 2019;12:688. doi: 10.1186/s13104-019-4732-z. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeyemi O.S., Atolani O., Awakan O.J., Olaolu T.B., Nwonuma C.O., Alejolowo O., Otohinoyi D.A., Rotimi D., Owolabi A., Batiha G.E. In vitro screening to identify anti- toxoplasma compounds and in silico modeling for bioactivities and toxicity. Yale J. Biol. Med. 2019;92:369–383. [PMC free article] [PubMed] [Google Scholar]
- Adeosun C.B., Sinmisola S., Opeifa A.O., Atolani O. Essential oil from the stem bark of Cordia sebestena scavenged free radicals. J. Acute Med. 2013;3:138–141. [Google Scholar]
- Adeyemi O.S., Murata Y., Sugi T., Kato K. Inorganic nanoparticles caused death of Toxoplasma gondii through alteration of redox status and mitochondrial membrane potential. Int. J. Nanomed. 2017;12:1647–1661. doi: 10.2147/IJN.S122178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeyemi O.S., Murata Y., Sugi T., Han Y., Kato K. Modulation of host HIF-1α activity and the tryptophan pathway contributes to the anti-Toxoplasma gondii potential of nanoparticles. Biochem. Biophys. Rep. 2017;11:84–92. doi: 10.1016/j.bbrep.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeyemi O.S., Murata Y., Sugi T., Han Y., Kato K. Exploring amino acid-capped nanoparticles for selective anti-parasitic action and improved host biocompatibility. J. Biomed. Nanotechnol. 2018;14(5):847–867. doi: 10.1166/jbn.2018.2544. [DOI] [PubMed] [Google Scholar]
- Aguiar J.J.S., Sousa C.P.B., Araruna M.K.A., Silva M.K.N., Portelo A.C., Lopes J.C., Carvalho V.R.A., Figueredo F.G., Bitu V.C.N., Coutinho H.D.M., Miranda T.A.S., Matias F.F. Antibacterial and modifying-antibiotic activities of the essential oils of Ocimum gratissimum L. and Plectranthus amboinicus L. Eur. J. Integr. Med. 2015;7(2):151–156. [Google Scholar]
- Ajewole K., Adeyeye A. Characterisation of Nigerian citrus seed oils. Food Chem. 1993;47:77–78. [Google Scholar]
- Alander I. Sheabutter – a multi-functional ingredient for food and cosmetics. Lipid Technol. 2004;16(9):202–205. [Google Scholar]
- Aliyu M.S., Tijjani M.B., Doko M.H.I., Garba I., Ibrahim M.M., Abdulkadir S.M., Abba D., Zango U.U. Antimicrobial activity of Sabulun Salo a local traditional medicated soap. Nigerian J. Basic Appl. Sci. 2012;20(1):35–38. [Google Scholar]
- Alvarez-Suarez J.M., Tulipani S., Díaz D., Estevez Y., Romandini S., Giampieri F., Damiani E., Astolfi P., Bompadre S., Battino M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010;48:2490–2499. doi: 10.1016/j.fct.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Ameh A.O., Muhammad J.A., Audu H.G. Synthesis and characterization of antiseptic soap from neem oil and Shea butter oil. Afr. J. Biotechnol. 2013;12(29):4656–4662. [Google Scholar]
- Angew O.N. Functional foods. Trends Food Sci. Technol. 2007;30:19–21. [Google Scholar]
- Anwar F., Naseer R., Bhanger M.I., Ashraf S., Talpur F.N., Aladedunye F.A. Physico-chemical characteristics of citrus seeds and seed oils from Pakistan. J. Am. Oil Chem. Soc. 2008;85:321–330. [Google Scholar]
- AOAC . Association of Official Analytical Chemists Washington, D.C.; USA: 1990. Official Method of Analysis 15th Edition. [Google Scholar]
- Atolani O., Adeniji A., Omonike O.O., Olatunji G.A. Energy dispersive - XRF metal analysis and cancer cell line cytotoxicity of Kigelia pinnata root. J. Biol. Active Prod. Nat. 2013;3(3):194–199. [Google Scholar]
- Atolani O., Areh E.T., Oguntoye O.S., Zubair M.F., Fabiyi O.A., Oyegoke R.A., Tarigha D.E., Adamu N., Adeyemi O.S., Kambizi L., Olatunji G.A. Chemical composition, antioxidant, anti-lipooxygenase, antimicrobial, anti-parasite and cytotoxicity of Polyalthia longifolia seed oil. Med. Chem. Res. 2019;22(12) [Google Scholar]
- Atolani O., Oguntoye H., Areh E.T., Adeyemi O.S., Kambizi L. Chemical composition, anti-toxoplasma, cytotoxicity, antioxidant, and anti-inflammatory potential of Cola gigantea seed oil. Pharmaceut. Biol. 2019;57(1):154–160. doi: 10.1080/13880209.2019.1577468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atolani O., Olabiyi E.T., Issa A.A., Azeez H.T., Onoja E.G., Ibrahim S.O., Olatunji G.A. Green synthesis and characterisation of natural antiseptic soaps from the oils of underutilised tropical seed. Sustain. Chem. Pharm. 2016;4:32–39. [Google Scholar]
- Atolani O., Olatunji G.A. Chemical composition, antioxidant and cytotoxicity potential of Daniellia oliveri (Rolfe) Hutch. & Dalz. Turkish. J. Pharmacol. Sci. 2016;13(1):84–94. [Google Scholar]
- Atolani O., Omere J., Otuechere C.A., Adewuyi A. Antioxidant and cytotoxicity effects of seed oils from edible fruits. J. Acute Dis. 2012;1(2):130–134. [Google Scholar]
- Bansal V., Medhi B., Pandhi P. Honey–a remedy rediscovered and its therapeutic utility. Kathmandu Univ. Med. J. 2005;3(3):305–309. [PubMed] [Google Scholar]
- Basualdo C., Sgroy V., Finola M.S., Marioli J.M. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet. Microbiol. 2007;124:375–381. doi: 10.1016/j.vetmic.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Bergamo G., Seraglio S.K.T., Gonzaga L.V., Fett R., Costa A.C.O. Physicochemical characteristics of bracatinga honeydew honey and blossom honey produced in the state of Santa Catarina: an approach to honey differentiation. Food Res. Int. 2019;116:745–754. doi: 10.1016/j.foodres.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Boussaid A., Chouaibi M., Rezig L., Hellal R., Donsì F., Ferrari G., Hamdi S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem. 2018;11(2):265–274. [Google Scholar]
- Bovili H. Orange: source of natural compounds. Aromes Ingred. Addit. 1996;7:41–42. [Google Scholar]
- Chari R.V. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc. Chem. Res. 2008;41:98e107. doi: 10.1021/ar700108g. [DOI] [PubMed] [Google Scholar]
- Chuanchen R., Beinlich K., Hoang T.T., Becher A., Karkhoff-Schweizer R.R., Schweizer H.P. Cross-resistance between triclosan and antibiotics is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxb mutants over-expressing MexCD-OprJ. Antimicrob. Agents Chemother. 2001;45:428–432. doi: 10.1128/AAC.45.2.428-432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypriano D.Z., da Silva L.L., Tasic L. High value-added products from the orange juice industry waste. Waste Manag. 2018;79:71–78. doi: 10.1016/j.wasman.2018.07.028. [DOI] [PubMed] [Google Scholar]
- Day R. Fall into Organics. 2012. https://patch.com/illinois/deerfield/bp--fall-into-organics
- Eteraf-Oskouei T., Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran. J. Basic Med. Sci. 2013;16(6):731–742. [PMC free article] [PubMed] [Google Scholar]
- Fongnzossie E.F., Tize Z., Fogang Nde P.J., Nyangono Biyegue C.F., Bouelet Ntsama I.S., Dibong S.D., Nkongmeneck B.A. Ethnobotany and pharmacognostic perspective of plant species used as traditional cosmetics and cosmeceuticals among the Gbaya ethnic group in Eastern Cameroon. South Afr. J. Bot. 2017;112:29–39. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAOSTAT, 2018) 2018. http://www.fao.org/faostat/en/#home
- Getradeghana B.T. Evaluation of African traditional soap. Global J. Pure Appl. Sci. 2000;6:174–179. [Google Scholar]
- Gultekin F., Yasar S., Gurbuz N., Ceyhan B.M. Food additives of public concern for their carcinogenicty. J. Nutr. Health Food Sci. 2015;3(4):1–6. [Google Scholar]
- Güner F.S., Yağı Y.Y., Erciyes A.T. Polymers from triglyceride oils. Prog. Polym. Sci. 2006;31:633–670. [Google Scholar]
- Gupta A., Malviya R., Singh T.P., Sharma P.K. Indian medicinal plants used in hair care cosmetics: a short review. Phcog. J. 2010;2(10):361–364. [Google Scholar]
- Han S.M., Lee K.G., Pak S.C. Effects of cosmetics containing purified honeybee (Apis mellifera L.) venom on acne vulgaris. J. Integr. Med. 2013;11(5):320–326. doi: 10.3736/jintegrmed2013043. [DOI] [PubMed] [Google Scholar]
- Jorge N., Da Silva A.C., Aranha C.P.M. Antioxidant activity of oils extracted from orange (Citrus sinensis) seeds. Ann. Br. Acad. Sci. 2016;88(2):951–958. doi: 10.1590/0001-3765201620140562. [DOI] [PubMed] [Google Scholar]
- Joshi L.S., Pawal H.A. Herbal cosmetics and cosmeceuticals: an overview. Nat. Prod. Chem. Res. 2015;3(2):1–8. [Google Scholar]
- Kahl R., Kappus H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebensm. Unters. Forsch. 1993;196(4):329–338. doi: 10.1007/BF01197931. [DOI] [PubMed] [Google Scholar]
- Krongrawa W., Limmatvapirat S., Pongnimitprasert N., Meetam P., Limmatvapirat C. Formulation and evaluation of gels containing coconut kernel extract for topical application. Asian J. Pharm. Sci. 2018;13(5):415–424. doi: 10.1016/j.ajps.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Foo L.Y. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2001;68:81–85. [Google Scholar]
- Mahmoud A.G. Emerging infections and the skin. J. Invest. Dermatol. Symp. Proc. 2001;6:188–196. [Google Scholar]
- Mahomoodally M.F., Ramjuttun P. A quantitative ethnobotanical survey of phytocosmetics used in the tropical island of Mauritius. J. Ethnopharmacol. 2016;193:45–59. doi: 10.1016/j.jep.2016.07.039. [DOI] [PubMed] [Google Scholar]
- Mak-Mensah E.E., Firempong C.K. Chemical characteristics of toilet soap prepared from neem (Azadirachta indica A. Juss) seed oil. Asian J. Plant Sci. Res. 2011;1(4):1–7. [Google Scholar]
- Molan P.C., Betts J.A. Clinical usage of honey as a wound dressing: an update. J. Wound Care. 2004;13:353–356. doi: 10.12968/jowc.2004.13.9.26708. [DOI] [PubMed] [Google Scholar]
- Mwambete K.D., Lyombe F. Antimicrobial activity of medicated soaps commonly used by Dares Salaam residents in Tanzania. Indian J. Pharmaceut. Sci. 2011;73:92–98. doi: 10.4103/0250-474X.89765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak K., Ratajczak–Wrona W., Górska M., Jabłońska E. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol. 2018;474:238–251. doi: 10.1016/j.mce.2018.03.014. [DOI] [PubMed] [Google Scholar]
- O’Brien R.D. CRC Press; Boca Raton, USA: 2004. Fats and Oils: Formulating and Processing for Applications; pp. 12–43. [Google Scholar]
- Oghome P., Eke M.U., Kamalu C.I.O. 2012. Characterization of fatty acid used in soap manufacturing in Nigeria: laundry, toilet, Medicated and Antiseptic Soap, 2(4) pp. 2930–2934. [Google Scholar]
- Ogunsuyi O., Akinnawo C. Quality assessment of soaps produced from palm bunch ash-derived Alkali and Coconut Oil. J. Appl. Sci. Environ. Manag. 2012;16:363–366. [Google Scholar]
- Okunowo W.O., Oyedeji O., Afolabi L.O., Matanmi E. Essential oil of grape fruit (Citrus paradisi) peels and its antimicrobial activities. Am. J. Plant Sci. 2013;4:1–9. [Google Scholar]
- Olabanji I.O., Ajayi S.O., Akinkunmi E.O., Kilanko O., Adefemi G.O. Physicochemical and in vitro antimicrobial activity of the oils and soap of the seed and peel of Citrus sinensis. Afr. J. Microbiol. Res. 2016;10(8):245–253. [Google Scholar]
- Oyedele A.O. The skin tolerance of shea fat employed as excipient in topical preparations. Nigerian J. Nat. Prod. Med. 2002;66:26–29. [Google Scholar]
- Ozturk B., Parkinson C., Gonzalez-Miquel M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Separ. Purif. Technol. 2018;206:1–13. [Google Scholar]
- Pandey A.K., Singh P., Tripathi N.N. Chemistry and bioactivities of essential oils of some Ocimum species: an overview. Asian Pac. J. Trop. Biomed. 2014;4(9):682–694. [Google Scholar]
- Poole K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 2002;92:S55–S64. [PubMed] [Google Scholar]
- Rodrigues F., de la Cádiz-Gurrea M.L., Nunes M.A., Pinto D., Vinha A.F., Linares I.B., Oliveira M.B.P.P., Carretero A.S. 2018. Cosmetics. Polyphenols: Properties, Recovery, and Applications; pp. 393–427. [Google Scholar]
- Saïdani M., Dhifi W., Marzouk B. Lipid evaluation of some Tunisian citrus seeds. J. Food Lipids. 2004;11:242–250. [Google Scholar]
- Soumanou M.M., Adjou E.S. Sweet fennel (Ocimum gratissimum) oils. Essential oils in food preservation. Flavor. Saf. 2016:765–773. [Google Scholar]
- Suzuki D. The “Dirty Dozen” Ingredients Investigated in the David Suzuki Foundation Survey of Chemicals in Cosmetics. Backgrounder. 2010:1–15. [Google Scholar]
- Tareau M.A., Palisse M., Odonne G. As vivid as a weed… Medicinal and cosmetic plant uses amongst the urban youth in French Guiana. J. Ethnopharmacol. 2017;203:200–213. doi: 10.1016/j.jep.2017.03.031. [DOI] [PubMed] [Google Scholar]
- Tripoli E., La Guardia M., Giammanco S., Di Majo D., Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007;104:466–479. [Google Scholar]
- Vivian O.P., Nathan O., Osano A., Mesopirr L., Omwoyo W.N. Assessment of the physicochemical properties of selected commercial soaps manufactured and sold in Kenya. Open J. Appl. Sci. 2014;4:433–440. [Google Scholar]
- Waheed M., Hussain M.B., Javed A., Mushtaq Z., Hassan S., Shariati M.A., Khan M.U., Majeed M., Nigam M., Mishra A.P., Heydari M. Honey and cancer: a mechanistic review. Clin. Nutr. 2018 doi: 10.1016/j.clnu.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Warra A.A., Wawata I.G., Gunu S.Y., Atiku F.A. Soap preparation from Soxhlet extracted Nigerian Cotton seed oil. Adv. Appl. Sci. Res. 2011;2(5):617–623. [Google Scholar]
- Zubair M.F., Atolani O., Ibrahim S.O., Oguntoye O.S., Abdulrahim H.A., Oyegoke R.A., Olatunji G.A. Chemical and biological evaluations of potent antiseptic cosmetic products obtained from Momordica charantia seed oil. Sustain. Chem. Pharm. 2018;9:35–41. [Google Scholar]
- Zubair M.F., Atolani O., Ibrahim S.O., Oguntoye O.S., Oyegoke R.A., Olatunji G.A. Fatty acids composition, antimicrobial potential and cosmeceutical utilization of Prosopis africana seed oil. J. Mex. Chem. Soc. 2019;2018(3):62. Published by Mexican Chemical Society. [Google Scholar]