Abstract
Alzheimer disease (AD) is a chronic neurodegenerative disease with a multitude of contributing genetic factors, many of which are related to inflammation. The apolipoprotein E (APOE) ε4 allele is the most common genetic risk factor for AD and is related to a pro-inflammatory state. To test the hypothesis that microglia and AD-implicated cytokines were differentially associated with AD pathology based on the presence of APOE ε4, we examined the dorsolateral frontal cortex from deceased participants within a community-based aging cohort (n = 154). Cellular density of Iba1, a marker of microglia, was positively associated with tau pathology only in APOE ε4 positive participants (p = 0.001). The cytokines IL-10, IL-13, IL-4, and IL-1α were negatively associated with tau pathology, independent of Aβ1–42 levels, only in APOE ε4 negative participants. Overall, the association of mostly anti-inflammatory cytokines with less tau pathology suggests a protective effect in APOE ε4 negative participants. These associations are largely absent in the presence of APOE ε4 where tau pathology was significantly associated with increased microglial cell density. Taken together, these results suggest that APOE ε4 mediates an altered inflammatory response and increased tau pathology independent of Aβ1–42 pathology.
Subject terms: Alzheimer's disease, Neurodegeneration
Introduction
Alzheimer’s disease (AD), the leading cause of dementia, is a progressive neurodegenerative disorder with both genetic and environmental risk factors. Many of the genes associated with AD have small effects and are associated with immunological and inflammatory pathways in the brain1,2. In contrast, the apolipoprotein E (APOE) ε4 allele is associated with a dramatic increase in the risk of developing AD and a younger age of symptom onset3,4. APOE may contribute to the development of AD through a variety of mechanisms, including increased beta-amyloid (Aβ) levels as well as an altered neuroinflammatory state.
Studies examining human post-mortem brain tissue found an increased number of microglia within AD patients with APOE ε45,6. Minett et al.7 found that increased microglia levels were negatively associated with AD pathology in non-demented individuals while the opposite was observed in demented individuals. Furthermore, APOE ε4 was associated with increased microglial activation and worse tau pathology and clinical outcomes7. In addition, peripheral low-grade inflammation was associated with risk of AD in APOE ε4, but not ε2 or ε3 carriers8. These studies suggest the APOE ε4 allele may lead to AD pathology through an altered inflammatory state. However, the precise role of APOE ε4 in modulating the relationship between microglia and inflammatory cytokines with the development of AD pathologies in the human brain is largely unknown.
Multiple cytokines have been implicated in AD pathogenesis. These include both the generally pro-inflammatory cytokines IL-1α and β, TNF- α, and IFN-γ as well as the anti-inflammatory IL-4, IL-10, and IL-13. Pro-inflammatory cytokines may initially reduce pathology by clearing amyloid and tau but may become toxic over time. Anti-inflammatory cytokines have been associated with decreased levels of pro-inflammatory cytokines and increased neurogenesis in mouse models of AD9–12. A recent study by Taipa et al. found that a collection of both pro-inflammatory and anti-inflammatory cytokines were correlated with increased cognition and less cognitive decline in AD patients after one year, suggesting that a balance of cytokines is important for maintaining a less pathologic immune profile13. The effective loss of this balance and increased inflammation may contribute to the progression of AD pathology13,14. Overall, cytokines released from microglia may help determine the prevailing inflammatory state within the brain15. However, opposing results have been reported on the impact of cytokines on AD pathology and symptoms based primarily on mouse models and human blood and CSF15–20. Few studies have examined these relationships in human brain tissue and in relationship to APOE ε4 status. Thus, we aim to elucidate the effect of pro-inflammatory and anti-inflammatory cytokines on AD pathology within frontal cortical tissue in relationship to APOE genotype. Overall, the role of microglia in attenuating or facilitating the development of AD pathologies may be mediated by the specific factors they release and the balance between pro-inflammatory and anti-inflammatory cytokines.
Here we examine the dorsolateral frontal cortex, a region where Aβ develops early and tau pathology develops late, in a community-based aging cohort in order to study the age dependent relationships between known inflammatory cytokines and AD pathologies. We sought to test the hypothesis that the presence of the APOE ε4 allele alters the interaction between microglia and associated cytokines with AD pathology.
Methods
In the present study, we examined 154 autopsy participants from the Framingham Heart Study (FHS). The FHS is a community-based cohort that longitudinally tracks participants and their offspring using previously published selection criteria and protocols21. Participants who agreed to brain donation were enrolled and tissue was collected after death with informed consent from the next of kin. Methods were carried out in accordance with and approved by the institutional review boards from both the Boston University Medical Center and the Edith Nourse Rogers Memorial Veterans Hospital, Bedford, MA.
Pathological assessment
Neuropathological assessment was performed following procedures and criteria previously established by the Department of Veterans Affairs-Boston University brain bank22,23. Alzheimer disease was diagnosed based on the National Institute of Aging Reagan criteria. Alzheimer disease staging was performed according to the Braak and Braak staging for neurofibrillary tangles24 and the Consortium to Establish a Registry for Alzheimer Disease (CERAD) semi-quantitative criteria for neuritic plaques25. Neuritic plaques were defined as plaques with argyrophilic dystrophic neurites, with or without dense amyloid cores26.
Immunohistochemistry
Tissue was fixed in periodate-lysine-paraformaldehyde, tissue blocks were paraffin-embedded, and sections were cut at 10 µm for immunohistochemistry. Antigen retrieval for α-synuclein and β-amyloid was performed with formic acid treatment for two minutes. Sections were incubated overnight at 4 °C with antibodies to phosphorylated PHF-tau (AT8; Pierce Endogen, Rockford IL; 1:2000), ionized calcium binding adaptor molecule 1 (Iba1) (Wako, 1:500), and CD68 (Vector, 1:500). Sections were washed three times with phosphate-buffered saline (PBS; pH 7.4), and subsequently treated with biotinylated secondary antibody and labeled with a 3-amino-9-ethylcarbazol HRP substrate kit (Vector Laboratories, Burlingame, CA). The sections were then counterstained with Gill’s Hematoxylin (Vector Laboratories H-3401, Burlingame, CA) and subsequently cover slipped using Permount mounting medium.
Immunoassay measurement
Frozen tissue from the dorsolateral frontal cortex was weighed and placed on dry ice. Freshly prepared, ice cold 5 M Guanidine Hydrochloride in Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl, pH 7.4) containing 1:100 Halt protease inhibitor cocktail (Thermo Fischer Scientific, Waltham, MA) and 1:100 Phosphatase inhibitor cocktail 2 & 3 (Sigma-Aldrich, St. Louis, MO) was added to the brain tissue at 5:1 (5 M Guanidine Hydrochloride volume (ml):brain wet weight (g)) and homogenized with Qiagen Tissue Lyser LT at 50 Hz for five min. The homogenate was then mixed (regular rocker) overnight at room temperature. The lysate was diluted with 1% Blocker A (Meso Scale Discovery (MSD), Rockville, Maryland, #R93BA-4) in wash buffer according to specific immunoassays: 1:300 for total tau and pTau231 (MSD #K15121D-2) and 1:4000 for beta-amyloids 1–40 and 1–42 (MSD #K15200E-2). Samples were subsequently centrifuged at 17,000 g and 4 °C for 15 minutes, after which the supernatant was applied to the immunoassays.
Levels of AD related cytokines including IFN-γ, IL-1β, IL-4, IL-10, IL-13, TNF-α, and IL-1α, were determined using the MSD proinflammatory panel 1 and cytokine panel 1. Ice cold RIPA buffer (Thermo Scientific, #89901) was added to the brain tissue at 5:1 and homogenized with Qiagen Tissue Lyser LT at 50 Hz for five min. The homogenate was centrifuged at 17,000 g and 4 °C for 15 minutes, then the supernatant was aliquoted for further use. Cytokine measurements were preformed according to MSD protocol using sandwich immunoassay techniques (MSD# K15210G). Brain homogenate was diluted with MSD diluent 21 and incubated for 2 hours with shaking at 500 rpm and subsequently washed three times with PBS + 0.05% Tween-20. Cytokine antibody was then added and the mixture was incubated for 2 hours with shaking at 500 rpm, and again subsequently washed three times with PBS + 0.05% Tween-20. 2X Read buffer T (MSD #R92TC-3) was added prior to analysis. Sulfo-tag conjugated anti-mouse secondary antibody was used for signal detection by the MSD platform, and an MSD SECTOR Imager 2400 was used to measure analyte levels.
Microscopic analysis of Tau and Microglia density
Tissue blocks from the dorsolateral frontal cortex were embedded in paraffin and cut at 10 μm (AT8) or 20 μm (Iba1 and CD68). Slides immunostained for tau (AT8), microglia overall (Iba1), and activated microglia (CD68) were scanned at 20× magnification with a Leica Aperio Scanscope (Leica Biosystems, Buffalo Grove, IL) as previously described27. Briefly, using ImageScope (Leica Biosystems, Buffalo Grove, IL), the gray matter was highlighted from the pia to the boundary between the white and gray matters. Leica’s image analysis and automated counting software (Aperio nuclear algorithm, Version 9) was calibrated for shape, size, and staining intensity to detect AT8-positive NFTs, Iba1-positive cells, and CD68-positive cells within the region of interest. Counts were normalized to the area measured and are presented as density within the analyzed region.
APOE genotyping
APOE genotype was determined from DNA extracted from participant brain tissue using single nucleotide polymorphism (National Center for Biotechnology Information SNPs rs429358 and rs7412) as previously described28. Participants were divided into APOE positive, which included genotypes ε2/ε4 (n = 2), ε3/ε4 (n = 30), and ε4/ε4 (n = 3), and APOE negative, including ε2/ε2 (n = 1), ε2/ε3 (n = 10), and ε3/ε3 (n = 98).
Statistical analysis
Statistical analysis was performed with SPSS version 25.0 (IBM inc., Armonk, NY) and Prism v8 (Graphpad Software, La Jolla, CA). IHC cellular density data – AT8, Iba1, CD68 – was analyzed as positive pixel count per mm2.
A total of 154 cases with complete data were included in the study. Levels of AT8, IL-10, IL-13, IL-4, IL-1β, IL-1α, and IFN-γ were normalized by logarithmic correction. Aβ measures were not normally distributed after log or square root corrections and therefore divided into quartiles and used as an ordinal variable. The raw data for Iba1, CD68, and TNF-α were normally distributed and needed no correction. Outliers were defined by values 3 times the interquartile range away from the upper and lower limits of the interquartile range for each measure and cases were removed from the dataset. In order to allow comparison, participants were divided into groups with and without an APOE ε4 allele.
To assess the variance between APOE ε4 carrier and non-carrier groups with respect to demographic and neuropathological characteristics we applied a chi-square test for proportions and independent samples T-tests. We used separate ordinal logistic regression models to estimate the relative odds of a one quartile increase in Aβ levels for a unit increase in microglial cell densities as well as cytokine levels adjusting for age and sex. Multiple linear regressions were used to test associations between microglial cell densities and tau pathology density and between cytokines and tau pathology adjusting for age, sex, and Aβ1–42. Analyses for each cytokine were run separately to avoid collinearity issues. Multiple comparisons were accounted for using Bonferroni correction. Binary logistic regressions were used to assess the relationship of drivers with dementia.
Results
Of the 154 participants from the FHS cohort analyzed there were 119 APOE ε4 negative and 35 were APOE ε4 positive. These two groups varied significantly by frequency of AD, cerebral amyloid angiopathy (CAA), and dementia such that the APOE ε4 carriers group had a higher prevalence. The mean level of tau pathology (AT8 density) was higher in APOE ε4 positive participants than APOE ε4 negative (Table 1). IL-13 and IL-4 had higher levels on average in APOE ε4 negative than APOE ε4 positive participants (Table 2). To test whether there was an effect of an interaction between APOE ε4 genotype and sex on tau pathology, a two-step linear regression adjusting for sex, APOE ε4, and sex-APOE ε4 interaction term was performed. The presence of APOE ε4 was significantly related to tau pathology (p = 0.007) independent of sex but the interaction term was not (p = 0.947). This suggests that the effect of APOE ε4 on tau pathology is independent of sex.
Table 1.
Demographic and neuropathological characteristics of cohort.
| APOE ε4 negative | APOE ε4 positive | p-value | |
|---|---|---|---|
| Demographics | |||
| Sample size (n) | 119 | 35 | — |
| Age at Deaths (yrs)b | 87.9 (0.843) | 85.9 (1.63) | 0.657 |
| Sex (% male)a | 43.7% | 45.7% | 0.788 |
| Dementiaa | 46.2% | 65.7% | 0.043 |
| Pathologya | |||
| AD | 28.6% | 60.0% | 0.001 |
| CTE | 0.84% | 2.86% | 0.354 |
| LBD | 27.7% | 37.1% | 0.285 |
| Neocortical LBD | 8.40% | 8.57% | 0.975 |
| FTLD | 11.8% | 11.4% | 0.957 |
| Remote Cortical Microinfarct# | 40.4% | 40.0% | 0.969 |
| Large Infarcts | 17.6% | 22.9% | 0.488 |
| Arteriolosclerosis | 86.6% | 91.4% | 0.441 |
| Atherosclerosis | 75.6% | 77.1% | 0.854 |
| CAA | 75.6% | 91.4% | 0.043 |
| IHC cellular density (cells/mm2)b | |||
| AT8 | 12466 (4614) | 29741 (9678) | 0.028 |
| Iba1 | 174.96 (4.04) | 181.98 (8.47) | 0.448 |
| CD68 | 166.85 (5.72) | 187.25 (12.69) | 0.162 |
| Immunoassay (pg/mL)b | |||
| Aβ1–42 | 864 (74) | 1330 (180) | 0.018 |
| Aβ1–40 | 177 (53) | 653 (156) | 0.007 |
LBD: Lewy body disease; AD: Alzheimer’s disease; CTE: Chronic Traumatic Encephalopathy; FTLD: Frontotemporal Lobar Degeneration; CAA: Cerebral Amyloid Angiopathy; Pathology prevalence represent the presence of pathology not the primary diagnosis; Control cases were defined as those lacking AD, CTE, LBD, or FTLD pathology. aχ2 test for proportions between ε4 allele absent and present groups; values presented as a percentage. bIndependent sample t-test for equality of means; values presented as mean (S.E.M). #Not all cases had remote cortical microinfarct data available (N = 144).
Table 2.
Levels of AD-related cytokines by APOE genotype.
| ε4 Negative | ε4 Positive | p-valuea | |
|---|---|---|---|
| IL-10 | 0.240 (0.007) | 0.244 (0.0153) | 0.119 |
| IL-13 | 4.02 (0.116) | 3.47 (0.135) | 0.003 |
| IL-4 | 0.302 (0.0135) | 0.261 (0.0204) | 0.004 |
| TNF-α | 0.517 (0.0161) | 0.517 (0.0236) | 0.211 |
| IL-1β | 4.38 (0.734) | 4.19 (0.716) | 0.406 |
| IL-1α | 1.46 (0.0990) | 1.34 (0.104) | 0.207 |
| IFN-γ | 1.54 (0.0452) | 1.65 (0.0941) | 0.242 |
Measures are presented as mean concentration (S.E.M) pg/mL; aindependent sample t-test for equality of means.
Associations between microglia and AD pathological markers
To test the hypothesis that the presence of the APOE ε4 allele altered the relationship between microglia and amyloid and tau pathology we examined these associations separately in participants with and without the APOE ε4 allele. Scatter plots of APOE ε4 positive and negative groups demonstrate significantly different associations between microglia cell density and AD pathology such that Iba1 was positively associated with the log of AT8 in APOE ε4 positive participants, but displayed no significant association in APOE ε4 negative participants (Fig. 1). In order to adjust for the effects of other potential drivers of pathology, we performed multiple regression analyses. A multiple linear regression showed that Iba1 cell density was significantly associated with greater AT8 positive tau pathology density (B = 0.013, p = 0.001) in APOE ε4 positive, but not APOE ε4 negative, participants adjusting for age, sex, and Aβ1–42. Iba1 cell density was not significantly associated with Aβ1–40 or Aβ1–42 via logistic ordinal regressions controlling for age and sex. Ordinal logistic and multiple linear regression analysis of amyloid and tau pathology with CD68 did not exhibit any significant relationships (Table 3). There is a clear difference in the relationship of Iba1, but not CD68, with tau pathology depending on the presence of the APOE ε4 allele. These results show that within APOE ε4 positive, but not APOE ε4 negative participants, microglial density is associated with increased tau pathology.
Figure 1.
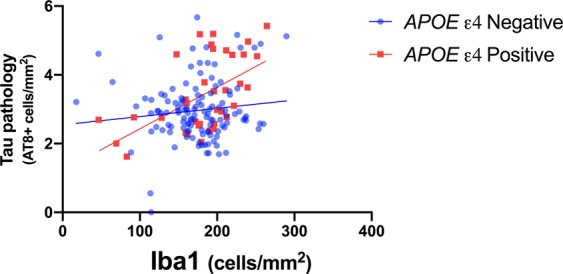
APOE ε4 allele alters the relationship between Iba1 and tau pathology. Scatter plots of Iba1 versus tau pathology (AT8 immunohistochemistry) are shown. The FHS cohort was divided into APOE ε4 positive and negative groups (red circles and blue squares respectively). Linear regressions demonstrated that Iba1 displayed significant positive relationship with tau pathology only in APOE ε4 carriers (B = 0.012, p = <0.001, 95% CI = 0.006–0.019).
Table 3.
Associations of tau and Aβ pathologies with total (Iba1) and activated (CD68) microglia in APOE ε4 negative and positive participants.
| AT8 a | ε4 Negative | ε4 Positive | ||||
|---|---|---|---|---|---|---|
| B | 95% CI | p-value | B | 95% CI | p-value | |
| Iba1 | 0.002 | −0.002–0.005 | 0.398 | 0.013 | 0.006–0.020 | 0.001* |
| CD68 | 0.001 | −0.002–0.004 | 0.496 | 0.002 | −0.004–0.007 | 0.525 |
| Aβ1–42b | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Iba1 | 1.003 | 0.995–1.011 | 0.449 | 0.993 | 0.979–1.006 | 0.288 |
| CD68 | 1.003 | 0.998–1.009 | 0.271 | 0.995 | 0.987–1.004 | 0.257 |
| Aβ1–40b | OR | 95% CI | p-value | OR | 95% CI | p-value |
| Iba1 | 1.006 | 0.998–1.014 | 0.124 | 1.012 | 0.998–1.027 | 0.093 |
| CD68 | 1.003 | 0.998–1.009 | 0.229 | 1.005 | 0.996–1.014 | 0.251 |
aMultiple linear regressions were used to assess relationship of Iba1 and CD68 with AT8 cellular density adjusting for age, sex, and Aβ1–42. bOrdinal logistic regressions were used to assess relationship of Iba1 and CD68 with Aβ adjusting for age and sex OR, odds ratio; CI, confidence interval. *p < 0.05 after Bonferroni correction for multiple comparisons.
Associations between cytokines and AD pathological markers
We hypothesized that AD-associated cytokines would display no or weak associations with AD pathology in the absence of APOE ε4, and positive associations with pathology in APOE ε4 positive participants who are more likely to develop significant AD pathology. Within APOE ε4 negative participants, ordinal logistic regression analysis showed a negative association of Aβ1–42 with TNF-α (OR = 0.115, p = 0.030) controlling for age and sex (Table 4). However, within APOE ε4 positive participants, an ordinal logistic regression showed a positive association of Aβ1–42 with IFN-γ (p = 0.014), IL-10 (p = 0.012), and IL-4 (p = 0.005) adjusting for age and sex. The association of Aβ1–42 with IL-4 in the presence of APOE ε4 was still significant after a Bonferroni correction for multiple comparisons. Because high levels of TNF-α are associated with less Aβ1–42 in APOE ε4 negative participants, TNF-α may have a protective effect on Aβ accumulation that is lost with APOE ε4. In contrast, high levels of IL-10, IL-4, and IFN-γ were associated with increased Aβ1–42, suggesting an altered cytokine response in the presence of APOE ε4. No cytokines displayed a significant association with Aβ1–40.
Table 4.
Cytokine associations with Aβ1–42 pathology in APOE ε4 negative and positive participants.
| ε4 Negative | ε4 Positive | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| IL-10 | 0.880 | 0.00897–96.2 | 0.956 | 1.74 × 104 | 8.935–3.39 × 107 | 0.012 |
| IL-13 | 1.001 | 0.770–1.30 | 0.995 | 0.923 | 0.424–2.010 | 0.841 |
| IL-4 | 6.79 | 0.703–65.8 | 0.098 | 6.43 × 103 | 13.21–3.13 × 106 | 0.005* |
| TNF-α | 0.115 | 0.0162–0.815 | 0.030 | 0.0264 | 2.23 × 10-4–3.13 | 0.136 |
| IL-1β | 0.965 | 0.920–1.01 | 0.131 | 1.066 | 0.899–1.26 | 0.462 |
| IL-1α | 0.839 | 0.613–1.23 | 0.608 | 1.655 | 0.586–4.67 | 0.342 |
| IFN-γ | 1.59 | 0.803–1.60 | 0.182 | 5.45 | 0.385–6.75 | 0.014 |
Ordinal logistic regressions were run adjusting for age and sex; *p < 0.05 after Bonferroni correction for multiple comparisons; OR, odds ratio; CI, confidence interval.
To test the hypothesis that cytokine associations with tau pathology were altered by the presence of APOE ε4, we performed multiple linear regression analyses adjusting for age, sex, and Aβ1–42 (Table 5). In APOE ε4 negative participants, IL-10 (p = 0.015), IL-13 (p = 0.003), IL-4 (p = 0.021), and IL-1α (p = 0.005) were all significantly negatively associated with AT8 tau pathology (Table 5). The negative associations of AT8 tau pathology with increased IL-13 and IL-1α remained significant after a Bonferroni correction for multiple comparisons. In APOE ε4 positive participants, there were no significant associations of cytokines with AT8. The negative association of anti-inflammatory cytokines (i.e. IL-10, IL-13, IL-4) and IL-1α with tau pathology that is lost in APOE ε4 carriers suggests an altered interaction of the brain’s immune system with pathology based on APOE genotype.
Table 5.
Cytokine associations with tau pathology in APOE ε4 negative and positive participants.
| ε4 Negative | ε4 Positive | |||||
|---|---|---|---|---|---|---|
| B | 95% CI | p-value | B | 95% CI | p-value | |
| IL-10 | −1.463 | −2.634–−0.292 | 0.015 | −0.290 | −2.826–2.246 | 0.817 |
| IL-13 | −1.687 | −2.780–−0.594 | 0.003* | −2.530 | −6.324–1.264 | 0.183 |
| IL-4 | −0.901 | −1.662–−0.140 | 0.021 | −1.612 | −3.912–0.688 | 0.163 |
| TNF-α | −0.174 | −1.099–0.751 | 0.710 | −0.075 | −3.076–2.926 | 0.960 |
| IL-1β | −0.257 | −0.679–0.165 | 0.231 | 0.548 | −0.657–0.433 | 0.360 |
| IL-1α | −0.906 | −1.535–−0.276 | 0.005* | 0.578 | −1.491–2.643 | 0.573 |
| IFN-γ | −0.741 | −1.980–0.498 | 0.223 | −2.125 | −5.613–1.362 | 0.223 |
Multiple linear regressions were run adjusting for age, sex, and Aβ1–42; *p < 0.05 after Bonferroni correction for multiple comparisons. CI, confidence interval. Log transformed values were used when needed to ensure normality. Cytokine concentration in pg/mL.
Associations between cytokines and microglia cell density
In order to test the association between microglia and those cytokines related to tau pathology (unadjusted p < 0.05: IL-10, IL-13, IL-4, and IL-1α), linear regressions were performed adjusting for age and sex (Table 6). Iba1 microglia density was negatively associated with IL-4 in APOE ε4 positive participants (p = 0.040) and trended towards significance in APOE ε4 negative participants (p = 0.060). In APOE ε4 positive participants there was a trend towards a negative associate with Iba1 density and IL-10 (p = 0.055). Conversely, IL-lα exhibited a significant positive association with Iba1 microglia density in APOE ε4 positive participants (p = 0.023). None of these relationships survived Bonferroni correction for multiple comparisons. IL-13 did not display any significant association to Iba1 microglia density. Overall, the level of the anti-inflammatory cytokine IL-4 was negatively associated with microglia density and the pro-inflammatory cytokine IL-1α was associated with increased microglia density in APOE ε4 positive participants.
Table 6.
Cytokine associations with total microglial density (Iba1) in APOE ε4 negative and positive groups.
| ε4 Negative | ε4 Positive | |||||
|---|---|---|---|---|---|---|
| B | 95% CI | p-value | B | 95% CI | p-value | |
| IL-10 | −14.76 | −73.91–44.39 | 0.622 | −88.02 | −177.87–1.83 | 0.055 |
| IL-13 | 6.375 | −49.65–62.40 | 0.822 | −37.96 | −203.77–127.86 | 0.644 |
| IL-4 | −34.56 | −71.88–2.77 | 0.060 | −89.86 | −175.386–−4.384 | 0.040 |
| IL-1α | −18.62 | −50.32–13.28 | 0.251 | 94.42 | 14.032–174.807 | 0.023 |
Multiple linear regressions were run adjusting for age and sex. Log transformed values were used when needed to ensure normality. Cytokine concentration in pg/mL.
Relationship with dementia
To determine whether the two significant cytokines related to tau pathology (i.e. IL-13 and IL-1α) had an effect on dementia independent of tau pathology, we performed a logistic regression adjusting for tau pathology, Aβ1–42, Iba1, microglia density, age, and sex in APOE ε4 positive and negative groups. Only tau pathology was significantly associated with dementia (Table 7). However frontal cortex tau pathology displayed a greater effect on dementia in APOE ε4 positive participants (OR = 12.188, p = 0.038) than in APOE ε4 negative participants (OR = 2.542, p = 0.001).
Table 7.
Associations with dementia in APOE ε4 negative and positive participants.
| ε4 Negative | ε4 Positive | |||
|---|---|---|---|---|
| OR | p-value | OR | p-value | |
| AT8 | 2.518 | 0.001* | 12.188 | 0.038 |
| Aβ1–42 | 1.078 | 0.701 | 0.717 | 0.534 |
| Iba1 | 1.002 | 0.766 | 1.003 | 0.836 |
| IL-13 | 13.314 | 0.124 | 972.365 | 0.293 |
| IL-1α | 0.396 | 0.337 | 0.049 | 0.338 |
Binary logistic regression adjusting for age and sex; *p < 0.05 after Bonferroni correction for multiple comparisons. Cytokine concentration in pg/mL.
Sensitivity analyses
In order to control for possible confounds additional analyses were performed adjusting for comorbid pathologies, RNA integrity number (RIN, a measure of tissue quality), and CERAD score. Effect sizes were not changed with adjustments for comorbid pathologies, including arteriolosclerosis, atherosclerosis, and neocortical Lewy bodies or with adjustment for RIN. Adjusting for CERAD score did not significantly alter the association between Iba1 microglial density and AT8 tau pathology, suggesting the relationship is independent of Aβ plaque score.
Discussion
We examined the pathological relationships between microglia, associated cytokines, and AD pathologies within the dorsolateral frontal cortex in a community-based aging cohort of 154 participants from the Framingham Heart Study. Associations between inflammatory markers and AD pathologies were significantly altered by the presence of the APOE ε4 allele. In APOE ε4 positive participants, IL-4, IL-10, and IFN-γ were associated with increased levels of Aβ1–42. In contrast, higher levels of the cytokines IL-10, IL-13, IL-4, and IL-lα were associated with decreased tau pathology only in the absence of APOE ε4. Furthermore, higher Iba1 microglia density was associated with increased tau pathology in APOE ε4 positive participants. Compared to APOE ε4 negative participants, APOE ε4 carriers appear to adopt a potentially toxic association between inflammatory markers and Aβ1–42 and lose a potentially protective association between anti-inflammatory cytokines and the development of tau pathology.
While the exact role of APOE ε4 in the pathogenesis of AD remains unclear, our findings support its role in inflammation-mediated pathology. ApoE4 has been shown to both impair clearance of Aβ and worsen the inflammation caused by amyloid29,30. The presence of APOE ε4 has also been associated with increased pro-inflammatory cytokines and microglial activation. A recent study using a transgenic tau mouse model found that the presence of APOE ε4 increased tau, the reactivity of microglia, and the level of pro-inflammatory cytokines31. Pro-inflammatory cytokine release from microglia led to increased astrocyte activation further increasing inflammation. Furthermore, this study found that mice with APOE ε4 had increased expression of pro-inflammatory genes and down-regulation of homeostatic genes31.
Implications for amyloid pathology
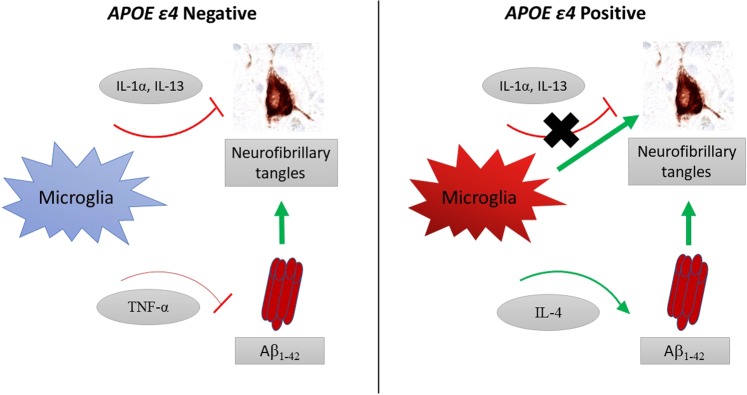
The role of microglia on the development of Aβ pathology is complex and varies depending on disease state and experimental paradigm. Our results support previous work in mouse models of Aβ pathology that demonstrated TNF-α decreased Aβ1–42 burden19 and further suggests that this potential protective effect of TNF-α is present only in APOE ε4 negative participants. In contrast, APOE ε4 positive participants appear to gain a positive association between increased levels of cytokines IFN-γ, IL-10, and IL-4 with Aβ1–42 (Table 3). It is important to note that the very large odds ratio for IL-10 and IL-4 are likely due to the low level of these cytokines in the brain. The cytokines IFN-γ, IL-10, and IL-4 have been proposed to increase amyloid deposition in mouse models of Aβ pathology, which is supported by our findings; however, they have also been associated with decreasing Aβ levels11,20,32,33. A study using APP transgenic mice found that increased IL-10 expression was associated with increased amyloid accumulation and increased ApoE expression, particularly within insoluble amyloid plaques20. It is proposed that in conjunction with increased IL-10, increased ApoE in these mice significantly impairs the phagocytic ability of microglia leading to the elevated amyloid accumulation. Our results agree with these findings suggesting a detrimental effect of IL-10, IL-4, and IFN-γ on amyloid, particularly in the presence of ApoE4. The difference between cytokine interactions with Aβ1–42 in APOE ε4 positive and negative participants may reflect an immune state that has an altered and detrimental function in the presence of APOE ε4 (Fig. 2).
Figure 2.
Schematic of significant immune-pathology interactions within APOE ε4 positive and negative participants. In APOE ε4 negative participants cytokines displayed negative associations with both tau and Aβ1–42, and microglia were not associated with neurofibrillary tangles. In contrast, in APOE ε4 carriers the negative association of cytokines with tau pathology was lost; microglia density was associated with more neurofibrillary tangles; and IL-4 was associated with increased Aβ1–42. Associations with p < 0.05 shown.
Implications for tau pathology
Microglia have been proposed to worsen tau pathology and to be toxic to neurons34–36. The reciprocal relationship, that tau induces microglial activation, has also been suggested based on findings in mice that expression of tau increases Iba1 microglia density and also alters the expression of genes involved in inflammation37,38. Simultaneous linear regression modeling supports the presence of a positive feedback loop between activated microglia cell density and tau pathology in human brain tissue27. We found a significant association between microglia density and tau pathology, suggesting a dysfunctional role of microglia in APOE ε4 positive, but not APOE ε4 negative, individuals.
A previous post-mortem human study examining the middle frontal cortex found that the association of Iba1 microglia density with tau pathology varied between individuals with and without dementia. In those with dementia Iba1 microglia density was positively associated with tau pathology but displayed a negative association in those without dementia7. Here we found that Iba1 microglia density was associated with increasing tau pathology in APOE ε4 carriers (Fig. 1), suggesting that an association with dementia may be due to APOE ε4 carrier status. Further studies with larger sample sizes are needed to help determine the impact of ApoE on the interaction of microglia with pathology.
Microglia may have a variety of phenotypes that represent different activation states and functions. CD68 is present within lysosomes which are increased in activated microglia with increased phagocytic activity7,39. Surprisingly, we found CD68 cell density was not significantly related with any markers of AD pathology. That total microglia density, but not CD68 positive microglial density, is associated with worse tau pathology suggests alternative phenotypes of activated microglia, or potentially astrocytes, may be involved in AD. This disconnect between Iba1 and CD68 positive microglia is supported by Minett et al. who found no association between CD68 and Iba1 labelled microglia, while finding weak relationships between other microglia markers – HLA-DR, MSR-A, and CD64 – that were also associated with AD pathology7. Future studies should further delineate microglia phenotypes and potential phagocytic or lysosomal impairments involved in human AD pathology.
The relationship between microglia and tau pathology has also been reported in transgenic mouse studies. A mouse line expressing tau and human APOE demonstrated that expression of APOE ε4 was associated with increased tau phosphorylation40. Furthermore, microglia mediated cell damage was shown to be greater in APOE ε4 expressing mice than those expressing APOE ε3 or APOE ε241,42. We found that the association of microglia and associated cytokines with tau pathology is altered by the presence of APOE ε4 in human frontal cortex as well.
The detrimental effect of microglia in the presence of APOE ε4 may be due in part to the loss of potentially protective cytokine interactions and their decreased levels in APOE ε4 positive participants (Table 2). Increasing levels of IL-10, IL-13, IL-4, and IL-1α were associated with decreased tau pathology in APOE ε4 negative participants while this effect is absent in APOE ε4 carriers (Table 5). The negative association of anti-inflammatory cytokines with tau pathology in APOE ε4 negative participants agrees with basic research studies in human cell lines that demonstrate a potentially protective effect43.
A variety of proinflammatory cytokines, including IL-1α, have been implicated in AD. IL-1α in its membrane bound form has been observed on activated microglia colocalized with Aβ plaques and neurons laden with tau pathology44,45. Additionally, in cultured human astrocytes IL-1α was found to induce tau phosphorylation46. Here we found that increased IL-1α was significantly associated with decreased tau pathology independent of Aβ1–42 levels within APOE ε4 negative participants and that increasing IL-1α levels were associated with increased Iba1 microglia density within APOE ε4 positive, but not APOE ε4 negative participants. Thus, the interaction between IL-1α and tau pathology appears to be altered by APOE ε4.
Tau pathology is one of the major pathological drivers of dementia in aging cohorts47–49. The cytokines IL-13 and IL-1α were significantly associated with less tau pathology in APOE ε4 negative participants and were not independently predictive of dementia. Instead, tau pathology density in the frontal cortex was significantly associated with dementia and had a much greater effect in APOE e4 positive than negative participants. Taken together this might suggest that IL-13 and IL-1α lessen the accumulation and detrimental effect of tau pathology in APOE ε4 negative participants and that this protective effect is lost in the presence of APOE ε4 (Fig. 2).
Tau pathology is strongly associated with brain atrophy and worsening AD50–52. Additionally, tau pathology has previously been shown to correlate with decreased brain decreased brain volumes specifically within the FHS cohort53. While our study focuses on the dorsolateral frontal cortex, AD pathology involves several brain regions and appears to spare others54,55. In aging and AD, NFTs likely begin within the entorhinal cortex and then extend to limbic areas and into the neocortex, while the opposite has been observed for Aβ pathology, which may begin in the neocortex and then extend to subcortical regions24,55. Here we modeled the dorsolateral frontal cortex which is a region where Aβ and tau pathologies converge in intermediate to severe AD. The associations here likely reflect other cortical regions, including visual association areas that have been previously implicated in early AD in this cohort56. In addition, cortical layers are differentially affected by pathology such that NFTs in AD tend to involve the deep cortical layers while the plaque pathology may begin more superficially. The patterns of gliosis and regional production of cytokines within different cortical layers requires further study. Further work is needed to determine the progression of pathology in different brain regions over the course of AD.
Limitations
There were several limitations to this study. Although the FHS brain donation cohort was recruited from the larger FHS community-based study, there is likely an autopsy-based selection bias. Cytokines may be rapidly metabolized and influenced by post-mortem interval. However, adjustment for RIN, a marker of tissue degradation, showed similar results. The associations between numerous cytokines and cell types and pathologies were studied based on a priori hypotheses. Nevertheless, future studies will need to confirm these results as well as determine the role of additional cytokines and cell types.
Conclusions
The associations between microglia and related cytokines with AD pathologies in human frontal cortex were altered between APOE ε4 positive and negative participants in a community aging cohort. Increased levels of IL-10, IL-13, IL-4, and IL-1α were associated with lower levels of tau pathology in APOE ε4 negative participants, suggesting a potential protective role of the immune system in absence of APOE ε4. The negative association of both anti-inflammatory and pro-inflammatory cytokines with tau pathology is absent in APOE ε4 positive individuals, who accumulate increased levels of microglia and tau pathology. Overall, APOE ε4 appears to alter the associations of microglia and inflammatory cytokines with AD pathologies. This altered immune response may partially explain the more severe Aβ and tau pathology and increased risk for AD within APOE ε4 carriers. These findings provide support for a modulatory role of APOE in immune and glial cell function in the human brain and highlight the need to stratify by APOE genotype when studying immune interactions in AD.
Acknowledgements
This study received support from the National Institute on Aging (P30AG13846 and supplement 0572063345, RF1AG054156, R56AG057768, R01AG057768, R01AG016495, R01AG033040), US Department of Veterans Affairs (I01CX001038), Alzheimer’s Association (NIRG-305779, AARF-17-529888). The views, opinions and/or findings contained in this article are those of the authors and should not be construed as an official Veterans Affairs position, policy or decision, unless so designated by other official documentation.
Author contributions
Study design and conception: J.F., N.A. and T.S. Acquisition, analysis, and/or interpretation of data: J.F., N.A., J.C., O.S., W.X., V.A., R.N., S.S., G.M., G.J., H.R., R.A. and T.S. Drafting and revising of the manuscript: All authors have contributed to drafting and/or critically revising the manuscript. All authors have given final approval of the version to be published and agree to be accountable for the work.
Data availability
All materials, data, and associated protocols will be made available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Giri M, Zhang M, Lu Y. Genes associated with Alzheimer’s disease: an overview and current status. Clin. Interv. Aging. 2016;11:665–681. doi: 10.2147/CIA.S105769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Sci. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 4.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/s1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overmyer M, et al. Reactive microglia in aging and dementia: an immunohistochemical study of postmortem human brain tissue. Acta Neuropathol. 1999;97:383–392. doi: 10.1007/s004010051002. [DOI] [PubMed] [Google Scholar]
- 6.Egensperger R, Kösel S, Eitzen U, Graeber MB. Microglial Activation in Alzheimer Disease: Association with APOE Genotype. Brain Pathol. 2006;8:439–447. doi: 10.1111/j.1750-3639.1998.tb00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minett T, et al. Microglial immunophenotype in dementia with Alzheimer’s pathology. J. Neuroinflammation. 2016;13:135. doi: 10.1186/s12974-016-0601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao Q, et al. Association of Chronic Low-grade Inflammation With Risk of Alzheimer Disease in ApoE4 Carriers. JAMA Netw. Open. 2018;1:e183597. doi: 10.1001/jamanetworkopen.2018.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szczepanik AIL-4. IL-10 and IL-13 modulate Aβ(1–42)-induced cytokine and chemokine production in primary murine microglia and a human monocyte cell line. J. Neuroimmunology. 2001;113:49–62. doi: 10.1016/s0165-5728(00)00404-5. [DOI] [PubMed] [Google Scholar]
- 11.Kiyota T, et al. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010;24:3093–3102. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiyota T, et al. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012;19:724–733. doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taipa R, et al. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging. 2019;76:125–132. doi: 10.1016/j.neurobiolaging.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Sochocka M, Diniz BS, Leszek J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017;54:8071–8089. doi: 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog. Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Zheng C, Zhou XW, Wang JZ. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-alpha, TGF-beta and IFN-gamma. Transl. Neurodegener. 2016;5:7. doi: 10.1186/s40035-016-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sackmann V, et al. Anti-inflammatory (M2) macrophage media reduce transmission of oligomeric amyloid beta in differentiated SH-SY5Y cells. Neurobiol. Aging. 2017;60:173–182. doi: 10.1016/j.neurobiolaging.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Bagyinszky E, et al. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J. Neurol. Sci. 2017;376:242–254. doi: 10.1016/j.jns.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Chakrabarty P, Herring A, Ceballos-Diaz C, Das P, Golde TE. Hippocampal expression of murine TNFalpha results in attenuation of amyloid deposition in vivo. Mol. Neurodegener. 2011;6:16. doi: 10.1186/1750-1326-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakrabarty P, et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron. 2015;85:519–533. doi: 10.1016/j.neuron.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsao CW, Vasan RS. Cohort Profile: The Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int. J. Epidemiol. 2015;44:1800–1813. doi: 10.1093/ije/dyv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vonsattel JP, Del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol. 2008;115:509–532. doi: 10.1007/s00401-007-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mez J, et al. Assessing clinicopathological correlation in chronic traumatic encephalopathy: rationale and methods for the UNITE study. Alzheimers Res. Ther. 2015;7:62. doi: 10.1186/s13195-015-0148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 25.Mirra SS, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurol. 1991;41:479–479. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 26.Montine TJ, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cherry JD, et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol. Commun. 2016;4:112. doi: 10.1186/s40478-016-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein TD, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015;130:21–34. doi: 10.1007/s00401-015-1435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tai LM, et al. APOE-modulated Abeta-induced neuroinflammation in Alzheimer’s disease: current landscape, novel data, and future perspective. J. Neurochem. 2015;133:465–488. doi: 10.1111/jnc.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez GA, Tai LM, LaDu MJ. & Rebeck, G. W. Human APOE4 increases microglia reactivity at Abeta plaques in a mouse model of Abeta deposition. J. Neuroinflammation. 2014;11:111. doi: 10.1186/1742-2094-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Y, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nat. 2017;549:523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mastrangelo MA, Sudol KL, Narrow WC, Bowers WJ. Interferon-{gamma} differentially affects Alzheimer’s disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am. J. Pathol. 2009;175:2076–2088. doi: 10.2353/ajpath.2009.090059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillot-Sestier MV, et al. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85:534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchihara T, et al. ApoE immunoreactivity and microglial cells in Alzheimer’s disease brain. Neurosci. Lett. 1995;195:5–8. doi: 10.1016/0304-3940(95)11763-M. [DOI] [PubMed] [Google Scholar]
- 35.Maphis N, et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. 2015;138:1738–1755. doi: 10.1093/brain/awv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki A, et al. Microglial activation in brain lesions with tau deposits: comparison of human tauopathies and tau transgenic mice TgTauP301L. Brain Res. 2008;1214:159–168. doi: 10.1016/j.brainres.2008.02.084. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, et al. Expression of Tau40 induces activation of cultured rat microglial cells. PLoS One. 2013;8:e76057. doi: 10.1371/journal.pone.0076057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wes PD, et al. Tau overexpression impacts a neuroinflammation gene expression network perturbed in Alzheimer’s disease. PLoS One. 2014;9:e106050. doi: 10.1371/journal.pone.0106050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabinowitz, S. S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli [published erratum appears in J. Exp. Med. 1992 Jan 1;175(1):309]. J. Exp. Med.174, 827–836, 10.1084/jem.174.4.827 (1991). [DOI] [PMC free article] [PubMed]
- 40.Tesseur I, et al. Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am. J. Pathol. 2000;156:951–964. doi: 10.1016/S0002-9440(10)64963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maezawa I, Nivison M, Montine KS, Maeda N, Montine TJ. Neurotoxicity from innate immune response is greatest with targeted replacement of E4 allele of apolipoprotein E gene and is mediated by microglial p38MAPK. FASEB J. 2006;20:797–799. doi: 10.1096/fj.05-5423fje. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH. Phosphorylation Sites on Tau Identified by Nanoelectrospray Mass Spectrometry. J. Neurochemistry. 2002;74:1587–1595. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee M, McGeer E, McGeer PL. Activated human microglia stimulate neuroblastoma cells to upregulate production of beta amyloid protein and tau: implications for Alzheimer’s disease pathogenesis. Neurobiol. Aging. 2015;36:42–52. doi: 10.1016/j.neurobiolaging.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Sheng JG, Mrak RE, Griffin WST. Enlarged and phagocytic, but not primed, interleukin-1α-immunoreactive microglia increase with age in normal human brain. Acta Neuropathologica. 1998;95:229–234. doi: 10.1007/s004010050792. [DOI] [PubMed] [Google Scholar]
- 45.Griffin WS, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl Acad. Sci. USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanji K, et al. Interleukin-1 induces tau phosphorylation and morphological changes in cultured human astrocytes. Neuroreport. 2003;14:413–417. doi: 10.1097/01.wnr.0000059783.23521.7c. [DOI] [PubMed] [Google Scholar]
- 47.Armstrong RA. A critical analysis of the ‘amyloid cascade hypothesis’. Folia Neuropathologica. 2014;3:211–225. doi: 10.5114/fn.2014.45562. [DOI] [PubMed] [Google Scholar]
- 48.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurol. 1992;42:631–631. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 49.Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurol. 1992;42:1681–1681. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- 50.Double KL, et al. Topography of brain atrophy during normal aging and alzheimer’s disease. Neurobiol. Aging. 1996;17:513–521. doi: 10.1016/0197-4580(96)00005-x. [DOI] [PubMed] [Google Scholar]
- 51.Risacher SL, et al. Alzheimer disease brain atrophy subtypes are associated with cognition and rate of decline. Neurol. 2017;89:2176–2186. doi: 10.1212/WNL.0000000000004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavedo E, et al. Plasma tau correlates with basal forebrain atrophy rates in people at risk for Alzheimer disease. Neurol. 2020;94:e30–e41. doi: 10.1212/WNL.0000000000008696. [DOI] [PubMed] [Google Scholar]
- 53.Kaur B, et al. Association between neuropathology and brain volume in the Framingham Heart Study. Alzheimer Dis. Assoc. Disord. 2014;28:219–225. doi: 10.1097/WAD.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braak H, Braak E, Bohl J, Lang W. Alzheimer’s disease: amyloid plaques in the cerebellum. J. Neurological Sci. 1989;93:277–287. doi: 10.1016/0022-510x(89)90197-4. [DOI] [PubMed] [Google Scholar]
- 55.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 56.McKee AC, et al. Visual association pathology in preclinical Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006;65:621–630. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All materials, data, and associated protocols will be made available.