Abstract
The prevalence of obesity and an aging population are increasing worldwide. Both obesity and aging are independently known to be associated with cardiac dysfunction. However, in obese insulin-resistant subjects, the effects of aging on metabolic status and cardiac and mitochondrial functions are not completely understood. We hypothesized that in the obese insulin-resistant condition, aging induced by D-galactose increases cardiac senescence markers and aggravates the impairment of metabolic parameters, cardiac and mitochondrial function, and increases oxidative stress, inflammation, apoptosis, and autophagy. Sixty-four male Wistar rats were fed with either normal diet (ND) or high-fat diet (HFD) for 12 weeks. Then, rats were divided into vehicle groups (0.9% NSS, subcutaneous injection (SC)) or D-galactose groups (150 mg/kg/day, SC). After 0.9%NSS or D-galactose treatment for 4 weeks and 8 weeks, metabolic and cardiac functions were determined. The heart was then removed to determine mitochondrial functions and enable biochemical studies. After 4 weeks of D-galactose injection, ND rats treated with D-galactose (NDD4), HFD rats treated with vehicle (HFV4), and HFD rats treated with D-galactose (HFD4) had reduced cardiac function, impaired cardiac mitochondrial function and autophagy, and increased oxidative stress, inflammation, and apoptosis. Interestingly, after 8 weeks, HFD rats treated with D-galactose (HFD8) had the worst impairment of cardiac and mitochondrial function, autophagy, and apoptosis in comparison to the other groups. Aging induced by D-galactose aggravated cardiac dysfunction in obese insulin-resistant rats through the worsening of cardiac mitochondrial function, autophagy, and increased apoptosis in a time-dependent manner.
Keywords: D-galactose, Heart, Mitochondria, Obesity, Aging
Introduction
Cardiovascular diseases are the leading cause of death in the aging sector worldwide (Sacco et al. 2016). Aging is described by the progressive loss of physiological functions and is considered as being a major risk factor for the progression of cardiovascular diseases (Niccoli and Partridge 2012). Thus, understanding the underlying mechanisms involved in the aging process is essential and can lead to the development of new therapeutic strategies for age-associated diseases. Currently, an aging condition induced by chronic D-galactose exposure has been shown to have similar phenotypes to one described in naturally aging rodents (Aydin et al. 2012; Cebe et al. 2014; Yanar et al. 2011). There is an accumulation of evidence to demonstrate that administration of D-galactose in rodents can increase senescence markers in cardiac tissues (Cebe et al. 2014; Chang et al. 2017; Liu et al. 2012; Lu et al. 2010; Wu et al. 2017; Yu et al. 2015), increase oxidative stress, and decrease antioxidant proteins (Cebe et al. 2014; Chang et al. 2017; Wu et al. 2017). In addition, recent studies have shown that D-galactose increased cardiac inflammatory and apoptosis markers (Ali et al. 2015; Chang et al. 2016; Gao et al. 2015; Guo et al. 2017; Liang et al. 2017; Qian et al. 2008; Sun et al. 2014; Yu et al. 2015). At organ level, D-galactose induced aging-related conditions including a decrease in left ventricular ejection fraction (LVEF) and fractional shortening (FS) (Bei et al. 2018; Chang et al. 2016).
The prevalence of obesity also rises steadily among the older aged population (Boateng et al. 2017). Obesity is a metabolic health problem characterized by excessive fat accumulation, and its prevalence continues to increase worldwide even with significant efforts to reduce the rates of obesity (Popkin and Gordon-Larsen 2004). Obesity can cause the development of insulin resistance and cardiovascular diseases (CVDs) and consumption of a high-fat diet (HFD) is a well-known cause of obesity and insulin resistance (Riccardi et al. 2004). Our previous animal studies have shown that HFD consumption for 12 weeks could induce insulin resistance, and cardiac mitochondrial dysfunction, and lead to increased oxidative stress and sympathovagal imbalance (Apaijai et al. 2012; Pipatpiboon et al. 2012; Pratchayasakul et al. 2011b). Thus, these findings suggest that cardiac mitochondria play a major role during the progression of obese-insulin resistance.
Although either obesity or aging has been shown to lead to impaired cardiac function, the effects of aging induced by D-galactose on metabolic parameters, cardiac and mitochondrial functions, oxidative stress, inflammatory and apoptotic parameters, autophagy, and expression of cardiac senescence markers in obese insulin-resistant rats have never been investigated. In this study, we tested the hypothesis that aging induced by D-galactose aggravates cardiac dysfunction and metabolic impairment via increasing cardiac senescence markers, cardiac and mitochondrial dysfunction, oxidative stress, inflammatory and apoptotic markers, and autophagy in the presence of an obese-insulin resistant condition.
Methods
Animal model preparation
All rat experiments conformed to the NIH guidelines (Guide for the care and use of laboratory animals, NIH Publication No. 85-23, Revised 2011) and were carried out according to the protocol approved by the Institutional Animal Care and Use Committees of the Faculty of Medicine, Chiang Mai University, Thailand (approval no. 15/2561 on May 24, 2018). Sixty-four male Wistar rats weighing 200–220 g were obtained from the National Animal Center, Salaya Campus, Mahidol University, Thailand. All animals were maintained in environmentally controlled conditions (25 ± 0.5 °C, 12-h light/12-h dark cycle) and fed with normal rat chow and water ad libitum for 1 week to allow for acclimatization before the experiments.
Experimental design
Male Wistar rats were randomly assigned to be fed either a normal diet (ND, a diet containing 19.77% energy from fat), or a high-fat diet (HFD, a diet containing 52.98% energy from fat) (Pratchayasakul et al. 2011a). After 12 weeks of either ND or HFD consumption, rats were further divided into vehicle groups (0.9% NSS, subcutaneous injection (SC)) and D-galactose groups (150 mg/kg/day, SC). Rats were injected daily with either 0.9% NSS or 150 mg/kg/day of D-galactose for 4 or 8 weeks. Body weight and food intake of all rats were recorded throughout the experimental period. After D-galactose treatment for 4 weeks and 8 weeks, blood samples were collected from the tail vein for determination of metabolic parameters. An oral glucose tolerance test (OGTT), heart rate variability (HRV) for cardiac autonomic balance, and echocardiography were carried out. In addition, left ventricular (LV) function was determined using a pressure-volume (P-V) loop recording system. At the end of the study protocol, rats were anesthetized with Zoletil (50 mg/kg, Vibbac Laboratories, Carros, France) and Xylazine (0.15 mg/kg, Laboratorios Calier, S.A., Barcelona, Spain) by intramuscular injection. Then, the heart was rapidly removed for the determination of cardiac mitochondrial function and biochemical studies. All experimental protocols are illustrated in Fig. 1a. Cardiac tissues from eight rats per group were used for the determination of cardiac and mitochondrial functions, four rats for senescence-associated β-galactosidase (SA-β-gal) staining, and three rats per group for TUNEL staining. For the rest of the parameters, cardiac tissues from five rats per group were used.
Fig. 1.
a Study protocol. b Effect of D-galactose on senescence marker expression. *p < 0.05 vs NDV at same week, †p < 0.05 vs NDD at same week, ‡p < 0.05 vs HFV at same week, #p < 0.05 vs 4 weeks within group. N = 4 per group. NDV normal diet vehicle, NDD normal diet D-galactose, HFV high-fat vehicle, HFD high-fat diet D-galactose, SA-β-gal senescence-associated β-galactosidase
Determination of metabolic parameters
Plasma was prepared from fasted blood samples and was kept frozen at − 80 °C until analysis of glucose, cholesterol, triglyceride, and insulin levels could be completed. Plasma insulin level was detected using a sandwich ELISA kit (Millipore, MI, USA). Plasma glucose and triglyceride levels were determined by colorimetric assay using a commercially available kit (Biotech, Bangkok, Thailand). Fasting plasma HDL and LDL levels were determined using commercially available kits (ERBA diagnostic, Mannheim, Germany) (Sivasinprasasn et al. 2015).
Tail-cuff blood pressure measurement and echocardiography
Rats were put in a restrainer to limit their movement. Volume-pressure recording sensors (VPRs) and occlusion cuffs (O-cuff) were attached to the tails. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded and analyzed using a CODA2 channel non-invasive blood pressure system (Kent Scientific Corporation, CT, USA) (Tunapong et al. 2017).
Echocardiography was used to evaluate LV function, as a non-invasive method. Rats were given light anesthesia using 2% isoflurane with oxygen (2 l/min). An echocardiography probe (S12, GE healthcare, CT, USA) was positioned on the chest at the parasternal short axis and linked to an echocardiography machine (GE vivid-i, GE healthcare, CT, USA). M-mode echocardiography was performed at the level of the papillary muscles. % Fractional shortening (%FS) was determined (Tunapong et al. 2017).
HRV measurement
Heart rate variability (HRV) measurement was performed by restraining the limbs of the rats in a prone position under 2.5% isoflurane inhalation anesthesia. A 27-gauge with 1-inch-long needle electrodes was inserted subcutaneously into the lead II electrocardiogram (ECG) position. Rats were allowed to gain full consciousness prior to ECG recording. During ECG recording, rats were kept restrained and prohibited from movement. The ECG signals were recorded for 20 min through a signal transducer (PowerLab 4/25T, ADInstruments, Sydney, Australia) and operated through a Chart 5.0 program (ADInstruments, Sydney, Australia). At least 300 consecutive RR intervals from the section of the tachogram were chosen for HRV analysis. The power spectra of RR intervals were obtained using a fast Fourier transform (FFT) algorithm. Three major oscillatory components were detected as a high-frequency band (HF; 0.6–3 Hz), a low-frequency band (LF; 0.2–0.6 Hz), and a very low-frequency band (VLF; below 0.2 Hz). Each spectral component was calculated as integrals under the respective part of the power spectral density function and was presented in absolute units (ms2). To minimize the effect of changes in total power on the LF and HF bands, LF and HF were expressed as normalized units by dividing them by the total power minus VLF. LF and HF bands of HRV were analyzed using an analytical program. An increased LF/HF ratio indicates cardiac sympathovagal imbalance (Tunapong et al. 2017).
P-V loop study
Rats were anesthetized by intramuscular injection with Zoletil (50 mg/kg, Vibbac Laboratories, Carros, France) and Xylazine (0.15 mg/ kg, Laboratories Carlier, SA, Barcelona, Spain), then placed in the supine position. Rats were ventilated with room air via a tracheostomy tube. The right carotid artery was identified and ligated, and then a P-V loop catheter (Scisence, Ontario, Canada) was inserted. The catheter tip was directed into the LV chamber to record LV pressure and volume. A 10-min period was allowed to get stable P-V loop signals. After stable signals were obtained from the P-V loop catheter, all loops recorded during a 20-min period were used for data analysis. The investigated parameters obtained from the P-V loop study included end-systolic pressure (ESP), end-diastolic pressure (EDP), maximum and minimum dP/dt (dP/dtmax and dP/dtmin), %ejection fraction (EF), and heart rate (HR). All P-V loop parameters were analyzed using Labscribe analytical software (Labscribe, Dover, NH, USA) (Palee et al. 2013).
SA-β-gal cytochemical staining
Senescence-associated β-galactosidase activity of the cardiac tissue was performed according to manufacturer’s protocol (Cell Signaling Technology). Briefly, after the rats were decapitated, cardiac tissue sections were fixed in 4% paraformaldehyde at room temperature for not more than 72 h and then washed with PBS and cryoprotected in 30% sucrose in PBS at 4 °C. Then, sections from the cardiac tissues were cut using a cryosectioner at 20 μm and were mounted on the glass slide. After 1 day, which allowed the samples to dry, the glass slides were washed once with PBS solution for 5 min at room temperature. Then, the samples were fixed with fixative solution for 30 min. After that, 1 ml of the β-galactosidase staining solution was added to each sample. The samples were then stored at 37 °C overnight. Blue precipitation in the cytoplasm was observed in the senescent cells at × 200 magnification (Chang et al. 2017). The number of SA-β-gal-positive cells was counted in four random fields with ImageJ software.
Cardiac mitochondrial function study
Mitochondrial ROS production, mitochondrial membrane potential changes, and mitochondrial swelling were determined to assess cardiac mitochondrial function by using the methods described in our previous study (Palee et al. 2013). The heart of each rat was removed at the end of the study and chopped into small pieces on an ice-cold plate. After that, cardiac tissues were homogenized and centrifuged to isolate cardiac mitochondria. Cardiac mitochondrial ROS production was measured by staining the cardiac mitochondria with dichlorohydrofluorescein diacetate (DCFHDA) dye for 25 min. Then, a fluorescent microplate reader (Gen5 Microplate Reader, BioTek Instruments, VT, USA) was used to detect the ROS level using the excitation wavelength of 485 nm and emission wavelength at 530 nm. To ascertain mitochondrial membrane potential change, 5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazolcarbocyanine iodide (JC-1) dye was used. The green fluorescence (JC-1 monomer) was excited at a wavelength of 485 nm and the emission was detected at 590 nm while the red fluorescence (JC-1 aggregates) was excited at a wavelength of 485 nm and the emission was detected at 530 nm. A decreased red/green fluorescence intensity ratio shows the depolarization of the mitochondrial membrane (Apaiajai et al. 2018). Mitochondrial swelling was determined by using spectrophotometry at 25 °C as described in a previous study (Apaiajai et al. 2018). Reduced light absorbance in a mitochondrial suspension at 540 nm indicates mitochondrial swelling. Cardiac mitochondrial morphology was also determined using a transmission electron microscope (TEM; JEM-1200 EX II, JEOL Ltd., Japan).
Determination of cardiac mitochondrial dynamics
With regard to cardiac mitochondrial dynamics, Western blot analysis was used to determine protein expression of the mitochondrial fission protein phosphorylation of dynamin-related protein 1 (Drp1) at serine 616 in the cytosolic fraction, Drp1, the mitochondrial fusion protein mitofusin 1/2 (MFN1/2), in isolation from the crude mitochondrial fraction from hearts. In brief, the isolated cardiac mitochondrial fraction was mixed with a loading buffer consisting of 5% mercaptoethanol, 0.05% bromophenol blue, 75 nM Tris, 2% SDS, and 10% glycerol at pH 6.8, and the mixture was boiled for 5 min and loaded into 10% gradient SDS-polyacrylamide gels. Proteins were then transferred to a nitrocellulose membrane with the presence of a glycine/methanol transfer buffer (containing 20 mM Tris, 0.15 M glycine, and 20% methanol) in a transfer system (Bio-Rad). The membranes were incubated in 5 % skim milk in 1× TBST buffer (containing 20 mM Tris (pH 7.6), 137 nM NaCl, and 0.05 % Tween-20) for 1 h at room temperature then exposed to anti-Drp1, phospho-Drp1 (Ser616), MFN1, MFN2, and VDAC (Cell Signaling Technology, Danvers, MA, USA) for 12 h. Bound antibody was detected by the conjugation of horseradish peroxidase with anti-rabbit IgG. Enhanced chemiluminescence (ECL) detection reagents were administered to visualize peroxidase reaction products.
Determination of oxidative stress and inflammation in cardiac tissue
Cardiac malondialdehyde (MDA) concentrations were determined using a high-performance liquid chromatography (HPLC) system (Thermo Scientific, Bangkok, Thailand) as shown in our former study (Tanajak et al. 2016). Protein from cardiac tissues was mixed with 10% trichloroacetic acid (TCA) containing BHT then heated at 90 °C for 30 min and allowed to cool down at room temperature. The mixture was centrifuged, and the supernatant was mixed with 0.44 M H3PO4 and 0.6% thiobarbituric acid (TBA) solution to generate thiobarbituric acid reactive substances (TBARS). The solution was filtered through a syringe filter (polysulfone-type membrane, pore size 0.45 μm, Whatman International, Maidstone, UK) and analyzed using the HPLC system. Data were analyzed using BDS software (BarSpec Ltd., Rehovot, Israel), and plasma TBARS concentration was determined directly from a standard curve generated from a standard reagent for MDA at different concentrations and reported as MDA equivalent concentration. Cardiac tissue inflammation was detected using an Invitrogen rat TNF-α ELISA kit in accordance with the manufacturer’s protocol (Thermo Fisher Scientific).
Cardiac expression of autophagy and apoptotic proteins
For determination of autophagy and cardiac apoptotic protein, Western blot analysis was used for measurement of expression of proteins Beclin 1, p62, LC3II (Cell Signaling Technology, Danvers, MA, USA), caspase-3, and cleaved caspase-3. Anti-Beclin 1, p62, LC3II, caspase-3, and cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA), and anti-GAPDH (Sigma-Aldrich, St. Louis, MO, USA). Bound antibody was detected using horseradish peroxidase conjugated with anti-rabbit or anti-mouse IgG. Enhanced chemiluminescence (ECL) detection reagents were administered to visualize peroxidase reaction products (Palee et al. 2013).
TUNEL-positive cells for quantification of cardiomyocyte apoptosis
Terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) assay was used to detect cardiomyocyte apoptosis by using an in situ cell death detection kit (Roche, Basel, Switzerland). For in situ labelling, cardiac tissue slices from the ischemic area were placed in 1× PBS for 10 min after dehydration. The samples were covered with 50 μl of proteinase K solution (1:50) for 30 min followed by 50 μl of Cytonin™ for 120 min. For a positive control, the samples were covered with TACS nuclease 1:50 in TACS nuclease buffer. TUNEL-positive cells were detected with a fluorescence microscope (Nikon, Tokyo, Japan) at λex 494 nm and λem 512 nm. DAPI was detected at λex 358 nm and λem 461 nm. The apoptosis index was calculated as a percentage of the number of TUNEL-positive apoptotic cells over the total number of nucleated cells (DAPI staining) (Maneechote et al. 2018).
Statistical analysis
Data were expressed as mean ± SEM and were analyzed using GraphPad Prism 7.0 software. For baseline cardiac functions, unpaired t test was used. A two-way-ANOVA followed by TUKEY post hoc test was used to test the difference among the groups for cardiac and mitochondrial functions. For the rest of the parameters, two-way-ANOVA followed by LSD post hoc test was used to test the difference between the groups. A p value < 0.05 was considered as denoting a statistically significant difference.
Results
Both D-galactose-induced aging and HFD consumption caused the impairment of metabolic parameters
After 4 weeks and 8 weeks of D-galactose injection, the body weight, visceral fat, plasma insulin levels, glucose AUCg, HOMA index, TC, and LDL were determined in both normal diet and high-fat diet-fed rats. Rats in the high-fat diet treated with vehicle groups (HFV4 and HFV8) and in the high-fat diet with D-galactose injection groups (HFD4 and HFD8) showed significantly increased body weight compared to their respective NDV4, NDD4 and NDV8, NDD8 groups (Table 1). However, there was no significant difference in body weight between the NDV4 and NDD4 group as well as in between the NDV8 and NDD8 rats. In addition, there was no difference in food intake, plasma glucose, and TG levels between all groups. NDD4, HFV4, and HFD4 groups and NDD8, HFV8, and HFD8 groups showed significantly increased plasma insulin, LDL, glucose AUCg, and HOMA index when compared to NDV rats, suggesting that both D-galactose and long-term HFD caused an obese insulin-resistant condition (Table 1).
Table 1.
The effect of D-galactose on metabolic parameters in obese insulin-resistant rats
| Parameters | Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| NDV4 | NDD4 | HFV4 | HFD4 | NDV8 | NDD8 | HFV8 | HFD8 | |
| Body weight (g) | 467.8 ± 3.8 | 476.7 ± 13.4 | 620 ± 19.1*,† | 596.7 ± 15.6*,† | 482.7 ± 10.6 | 489.6 ± 8.7 | 648.3 ± 18.7*,† | 619.2 ± 16.9*,† |
| Food intake (g/day) | 24.3 ± 1.6 | 25.3 ± 0.9 | 25 ± 0.8 | 23.8 ± 1.8 | 24.7 ± 1.6 | 25.6 ± 1.2 | 26 ± 1.9 | 24.4 ± 0.5 |
| Visceral fat (g) | 20.5 ± 1.4 | 25.8 ± 2.3 | 52.3 ± 6.6*,† | 53.8 ± 6.7*,† | 21.9 ± 2 | 25.2 ± 1.3 | 56.2 ± 2.8 *,† | 55.3 ± 4.3*,† |
| Glucose (mg/dl) | 128.9 ± 6.5 | 129 ± 8.8 | 133.3 ± 3.4 | 129.9 ± 6.7 | 139.9 ± 16.1 | 145.1 ± 5.5 | 143.3 ± 16 | 145.5 ± 1.9 |
| Insulin (ng/ml) | 3.8 ± 0.6 | 9.9 ± 2.4* | 10.1 ± 1.1* | 10.4 ± 1.3* | 4.1 ± 1.1 | 11.6 ± 3.1* | 10.1 ± 2.4* | 12.3 ± 2.2* |
| HOMA index | 24.3 ± 3.5 | 49.7 ± 3.4* | 69.1 ± 5.7* | 60.2 ± 4* | 27.2 ± 3.3 | 78.5 ± 22.5* | 61.8 ± 11.8* | 52.5 ± 12.7* |
| Plasma glucose AUC (AUCg) (mg/dl × min × 104) | 2.4 ± 0.1 | 2.8 ± 0.3* | 2.7 ± 0.1* | 2.9 ± 0.4* | 2.1 ± 0.2 | 2.7 ± 0.2* | 2.8 ± 0.2* | 2.8 ± 0.1* |
| Cholesterol (mg/dl) | 79 ± 3.8 | 82.4 ± 3.9 | 99.9 ± 12.6*,† | 105.6 ± 9.2*,† | 76.4 ± 5 | 81.5 ± 2.5 | 110 ± 6.3*,† | 110.9 ± 6.9*,† |
| Triglyceride (mg/dl) | 89.8 ± 6.6 | 95.9 ± 4.8 | 112.2 ± 4 | 106.7 ± 2.3 | 83.2 ± 4.7 | 91.7 ± 6.5 | 112.9 ± 6.8 | 102.6 ± 14.5 |
| HDL (mg/dl) | 34.5 ± 1.4 | 31.6 ± 2.7 | 25.8 ± 1.3*,† | 24.6 ± 2.2*,† | 35.7 ± 2 | 30.4 ± 1.9 | 22.4 ± 2.2*,† | 23.2 ± 2.8*,† |
| LDL (mg/dl) | 23.69 ± 3.53 | 26.52 ± 4.77 | 38.97 ± 7.21*,† | 38.11 ± 3.98*,† | 27.03 ± 2.03 | 28.65 ± 3.08 | 42.93 ± 4.15*,† | 46.53 ± 1.67*,† |
Values are mean ± SEM (N = 8/group)
*p < 0.05 vs NDV at same week, †p < 0.05 vs NDD at same week, #p < 0.05 vs 4 weeks within group
NDV normal diet vehicle, NDD normal diet D-galactose, HFV high-fat vehicle, HFD high-fat diet D-galactose, HOMA homeostasis model assessment
Both D-galactose administration and obesity increased the expression of cardiac senescence markers
SA-β-gal staining of cardiac tissues was used as a cardiac senescence marker. Our results revealed that after 4 weeks and 8 weeks of D-galactose injections in both normal diet (NDD4 and NDD8) and high-fat diet-fed (HFD4 and HFD8) rats, a significant increase in the number of SA-β-gal-positive cells was demonstrated when compared to their NDV groups. Interestingly, HFD8 rats showed a significant increase in cardiac senescence markers when compared to other groups, suggesting that D-galactose-induced aging further increased cardiac senescence markers in a time-dependent manner in the presence of an obese insulin-resistant condition (Fig. 1b).
D-galactose-induced aging aggravates LV dysfunction and sympathovagal imbalance in obese insulin-resistant rats
For the baseline cardiac functions, after 12 weeks of high-fat diet feeding, %FS was significantly decreased in high-fat diet-fed rats compared to their respective normal diet-fed rats (Fig. 2a). Furthermore, increased LF/HF ratio (Fig. 2c) and SBP, DBP, and MAP (Fig. 3) were found in 12 weeks of high-fat diet-fed rats compared to normal diet-fed rats for 12 weeks. The echocardiogram results displayed that there was significantly decreased %FS in NDD4, HFV4, and HFD4 rats compared to NDV4 rats. NDD8, HFV8, and HFD8 rats also had significantly decreased %FS compared to the NDV8 group. However, there was no significant difference in %FS among NDD4, HFV4, and HFD4 groups. Interestingly, HFD8 showed significantly decreased %FS in all groups (Fig. 2b). The LF/HF ratio of HRV was used as a marker of cardiac sympathovagal balance and our results revealed that the LF/HF ratio was higher in the NDD4, HFV4, and HFD4 and NDD8, HFV8, and HFD8 groups, when compared to their respective NDV group. Likewise, HFD8 had the worst impairment of HRV compared to other groups Fig. 2d.
Fig. 2.
Baseline cardiac functions and the effect of D-galactose-induced aging on echocardiographic parameters, heart rate variability, and P-V loop analysis. a Baseline fractional shortening. b Fractional shortening. c Baseline heart rate variability. d Heart rate variability. e P-V loop analysis. *p < 0.05 vs NDV at same week, †p < 0.05 vs NDD at same week, ‡p < 0.05 vs HFV at same week, #p < 0.05 vs 4 weeks within group. N = 8 per group. ND normal diet-fed rats, HF high-fat diet-fed rats, NDV normal diet vehicle, NDD normal diet D-galactose, HFV high-fat vehicle, HFD high-fat diet D-galactose, FS fractional shortening, LF/HF ratio low-frequency/high-frequency ratio
Fig. 3.
Baseline blood pressure and the effect of D-galactose-induced aging on blood pressure. *p < 0.05 vs NDV at same week, †p < 0.05 vs NDD at same week, ‡p < 0.05 vs HFV at same week, #p < 0.05 vs 4 weeks within group. N = 8 per group. ND normal diet-fed rats, HF high-fat diet-fed rats, SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial pressure
The P-V loop analysis was performed after 4 and 8 weeks of D-galactose injections. After 4 and 8 weeks of D-galactose injections, LV function parameters including LVESP, dP/dt max, and SV were significantly decreased in NDD4, HFV4, and HFD4 rats, compared to the NDV4 group and NDD8, HFV8, and HFD8 groups, when compared to NDV8 rats, whereas LVEDP and dp/dt min were significantly increased, compared to their respective NDV groups. At week 8, HFD8 showed significantly reduced LVESP, and SV and increased LVEDP, when compared to NDV8, NDD8, and HFV8 groups (Fig. 2e–j). As regards blood pressure, SBP, DBP, and MAP were significantly higher in the NDD4, HFV4, and HFD4 groups and NDD8, HFV8, and HFD8 groups when compared to their NDV counterpart groups (Fig. 3).
D-galactose-induced aging aggravated cardiac oxidative status in obese insulin-resistant rats
Cardiac tissue MDA was used as an indicator of oxidative stress. Our results demonstrated that at week 4, a significant increase in cardiac tissue MDA level in the HFD4 group was detected, when compared to NDV4 and NDD4 groups. At week 8, the HFD8 group showed significantly increased cardiac MDA level, when compared to NDV8, NDD8, and HFV8 groups (Fig. 4a). There was a significantly increased cardiac TNF-α level in the NDD4, HFV4, HFD4, NDD8, HFV8, and HFD8 groups compared to the NDV groups at week 4 (NDV4) and week 8 (NDV8) (Fig. 4b).
Fig. 4.
a The effect of D-galactose administration at different time points on cardiac tissue oxidative stress. b Inflammation. c Cleaved caspase-3/caspase-3 with representative images of Western blotting band at 4 weeks after D-galactose administration. d Cleaved caspase-3/caspase-3 with representative images of Western blotting band at 8 weeks after D-galactose administration in obese insulin-resistant rats. *p < 0.05 vs NDV at same week, †p < 0.05 vs NDD at same week, ‡p < 0.05 vs HFV at same week, #p < 0.05 vs 4 weeks within group. N = 5 per group. NDV normal diet vehicle, NDD normal diet D-galactose, HFV high-fat vehicle, HFD high-fat diet D-galactose, MDA malondialdehyde, TNF-α tumor necrosis factor-alpha
D-galactose-induced aging led to a worsening of cardiac cells apoptosis in obese insulin-resistant rats
Cleaved caspase-3/caspase-3 ratio and TUNEL assay were used as indicators of cardiac cell apoptosis. Our results showed that, at both 4 and 8 weeks, both D-galactose and high-fat diet-treated rats had a significantly increased ratio of cleaved caspase-3 and caspase-3 level, when compared to their NDV control groups (Fig. 4 c and d). In addition, the TUNEL assay was performed to allow the detection of apoptotic DNA fragmentation in cardiac cells. NDD4, HFV4, HFD4, NDD8, HFV8, and HFD8 rats showed the appearance of TUNEL-positive cells compared to the NDV groups (Fig. 5a–c). Interestingly, HFD8 had significantly increased TUNEL-positive cells among all groups, suggesting that D-galactose-induced aging further increased cardiac cell apoptosis in a time-dependent manner.
Fig. 5.
The effect of D-galactose-induced aging on cardiac apoptosis using the TUNEL-positive cells in obese insulin-resistant rats. a Representative figure of TUNEL-positive cells at 4-week D-galactose. b Representative figure of TUNEL-positive cells at 8-week D-galactose. c Percentage of TUNEL-positive cells at both 4- and 8-week D-galactose. *p < 0.05 vs NDV at same week, †p < 0.05 vs NDD at same week, ‡p < 0.05 vs HFV at same week, #p < 0.05 vs 4 weeks within group. N = 3 per group. NDV normal diet vehicle, NDD normal diet D-galactose, HFV high-fat vehicle, HFD high-fat diet D-galactose
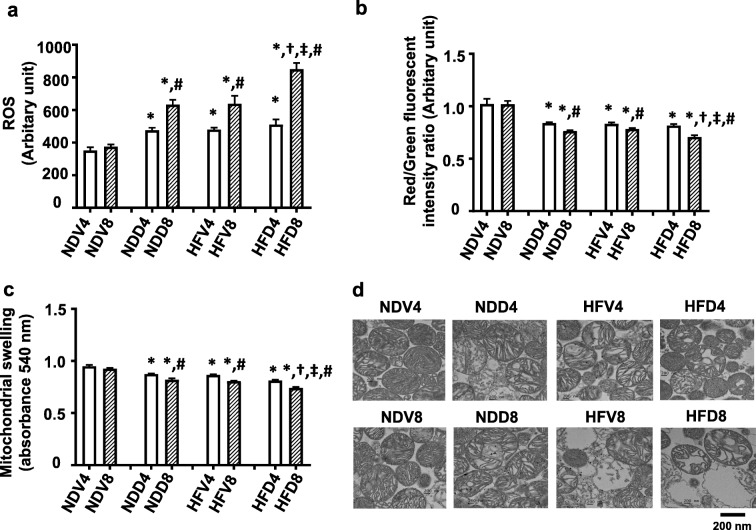
D-galactose-induced aging exacerbates cardiac mitochondrial dysfunction in obese insulin-resistant rats
Cardiac mitochondrial function was determined by measuring mitochondrial ROS production, membrane potential depolarization, and swelling. At both week 4 and week 8, NDD, HFV, and HFD showed significantly increased mitochondrial ROS level, depolarization of mitochondrial membranes, and swelling, when compared with their respective NDV groups (Fig. 6a–c). The representative transmission electron micrographs of cardiac mitochondria demonstrated that mitochondria in NDD4, HFV4, HFD4, NDD8, HFV8, and HFD8 groups showed unfolding of cristae, indicating mitochondrial swelling compared to NDV4 and NDV8 groups (Fig. 6d). Interestingly, HFD8 rats had the greatest level of mitochondrial swelling of all groups suggesting that D-galactose-induced aging further exacerbates cardiac mitochondrial dysfunction in a time-dependent manner.
Fig. 6.
The effect of D-galactose-induced aging on cardiac mitochondrial function in obese insulin-resistant rats. a Cardiac mitochondrial ROS production. b Cardiac mitochondrial membrane potential. c Cardiac mitochondrial swelling. d TEM representative images of cardiac mitochondria. *p < 0.05 vs NDV at same week, †p < 0.05 vs NDD at same week, ‡p < 0.05 vs HFV at same week, #p < 0.05 vs 4 weeks within group. N = 8 per group. NDV normal diet vehicle, NDD normal diet D-galactose, HFV high-fat vehicle, HFD high-fat diet D-galactose, ROS reactive oxygen species, TEM transmission electron microscopy
Both D-galactose-induced aging and obese insulin resistance led to the impairment of the cardiac mitochondrial fusion process
The expression of mitochondrial fusion proteins MFN1 and MFN2 in the heart were determined. At both weeks 4 and 8, NDD, HFV, and HFD groups showed a significant decrease in MFN1 and MFN2 expression, compared to their respective NDV groups (Fig. 7 a and b). When it came to the mitochondrial fission process, the expression levels of Drp1 in the mitochondrial fraction and the phosphorylated form of Drp1 at serine 616 in cytosol were determined. Our results demonstrated that at week 4, only HFD4 rats had significantly increased phosphorylated Drp1 in the cytosol, compared to NDV4, NDD4, and HFV4 groups (Fig. 7c). After 8 weeks of D-galactose injections HFD8 group had significantly increased Drp1 expression in the mitochondria and phosphorylated Drp1 in the cytosol, when compared to the NDV8, NDD8, and HFV8 groups. However, there was no significant difference in Drp1 level in mitochondria and phosphorylated Drp1 in cytosol in the NDV4, NDD4, and HFV4 and NDV8, NDD8, and HFV8 groups (Fig. 7 c and d).
Fig. 7.
The effect of D-galactose-induced aging on cardiac mitochondrial fusion and fission proteins in obese insulin-resistant rats. a Mitochondrial MFN1 level. b Mitochondrial MFN2 level. c Phosphorylated Drp1 at serine 616 in cytosol. d Mitochondrial Drp1 level. *p < 0.05 vs NDV at same week, †p < 0.05 vs NDD at same week, ‡p < 0.05 vs HFV at same week, #p < 0.05 vs 4 weeks within group. N = 5 per group. NDV normal diet vehicle, NDD normal diet D-galactose, HFV high-fat vehicle, HFD high-fat diet D-galactose, MFN1 mitofusin 1, MFN2 mitofusin 2, Drp1 dynamin-related protein 1, VDAC voltage-dependent anion channels
D-galactose-induced aging exacerbated the impairment of autophagic processes in obese insulin-resistant rats
To facilitate the assessment of cardiac autophagy, Beclin-1, p62, and LC3II were determined. Our results showed that at week 4, Beclin-1 was significantly decreased in the HFD4 group, when compared to NDV4 group. Additionally, NDD8, HFV8, and HFD8 rats showed significantly reduced Beclin-1, when compared with the NDV8 group. Interestingly, the HFD8 group had a significantly higher p62 level than other groups, indicating that D-galactose-induced aging aggravates autophagy impairment in the obese condition in a time-dependent manner (Fig. 8a–c). However, LC3II level was not different among the groups (Fig. 8d).
Fig. 8.
The effect of D-galactose-induced aging on autophagy in obese insulin-resistant rats. a Beclin-1 expression at 4-week D-galactose. b Beclin-1 expression at 8-week D-galactose. c p62 and d LC3II with representative Western blotting bands. *p < 0.05 vs NDV at same week, †p < 0.05 vs NDD at same week, ‡p < 0.05 vs HFV at same week, #p < 0.05 vs 4 weeks within group. N = 5 per group. NDV normal diet vehicle, NDD normal diet D-galactose, HFV high-fat vehicle, HFD high-fat diet D-galactose
Discussion
The major findings from this study are firstly the administration of 150 mg/kg/day D-galactose significantly increased cardiac senescence marker expression and secondly, aging induced by D-galactose in rats caused the development of metabolic impairment, high blood pressure, cardiac sympathovagal imbalance, and cardiac dysfunction in a time-dependent pattern. These adverse cardiac effects were due to the impairment of cardiac mitochondrial function, increased oxidative stress, inflammation and impaired regulation of mitochondrial dynamics, resulting in cardiac cell apoptosis and leading finally to cardiac dysfunction. These findings indicate the development of an established D-gal-induced aging rat. The third finding was that in the presence of an obese insulin-resistant condition, aging induced by D-galactose worsens cardiac dysfunction via aggravating cardiac mitochondrial dysfunction and impaired autophagy.
Previous studies have demonstrated that the administration of 60–150 mg/kg/day D-galactose in mice and rats at the age of 2–5 months old for 6–8 weeks can increase senescence markers in cardiac tissue (Bo-Htay et al. 2018; Cebe et al. 2014; Chang et al. 2017; Wu et al. 2017). In this study, we demonstrated that the administration of 150 mg/kg/day D-galactose for either 4 weeks or 8 weeks significantly increased cardiac senescence marker expression, indicating that we successfully established a mimetic aging model induced by D-galactose. It is well known that when excessive senescent cells accumulate in the heart, it can decrease the regenerative capacity and induce low-grade inflammation, promoting aging and age-related disorders (Childs et al. 2017; Olivieri et al. 2018; Sikora et al. 2011). In this study, there was no aggravation of metabolic impairments in the normal diet-fed rats treated with D-galactose for 4 or 8 weeks as shown by similar levels of increased plasma insulin and HOMA index (Table 1). A growing body of evidence indicates that D-galactose administration significantly increases the expression of oxidative stress, inflammation, apoptosis, and impaired autophagy, leading to cardiac dysfunction (Bei et al. 2018; Bo-Htay et al. 2018; Cebe et al. 2014; Chang et al. 2017; Chen et al. 2016; Wu et al. 2017). Our results also demonstrated that giving of a D-galactose injection for 4 weeks significantly increased oxidative stress, inflammation, apoptosis and impaired autophagy and cardiac dysfunction in normal diet-fed rats. In addition, after 8 weeks of D-galactose administration, cardiac oxidative stress and apoptosis were increased to a greater degree than that observed in normal diet-fed rats treated with D-galactose for 4 weeks. This was shown by increased cardiac MDA level and a further increase in TUNEL-positive cells, indicating that excess oxidative stress and apoptosis occur in a time-dependent manner following D-galactose administration (Table 2). However, similar level of the increased inflammation was found in both 4 and 8 weeks of D-galactose-treated rats fed with a normal diet, suggesting that the inflammation reached the maximal level at 4 weeks (Table 2). Additionally, cardiac sympathetic overactivity was aggravated after 8 weeks of D-galactose administration in normal diet-fed rats compared to D-galactose 4 weeks rats which might be due to excessive increased oxidative stress after 8 weeks of D-galactose administration (Table 2).
Table 2.
Table summarizing the effect of D-galactose on cardiometabolic impairment in high-fat diet-induced obese insulin-resistant rats
| Groups | Cardiometabolic impairment | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolic disturbance | LV dysfunction | Cardiac autonomic imbalance | Oxidative stress | Inflammation | Apoptosis | Autophagy impairment | Mitochondrial impairment | Aging marker | ||||||||||
| Week | Week | Week | Week | Week | Week | Week | Week | Week | ||||||||||
| 4 | 8 | 4 | 8 | 4 | 8 | 4 | 8 | 4 | 8 | 4 | 8 | 4 | 8 | 4 | 8 | 4 | 8 | |
| NDV | ||||||||||||||||||
| NDD | ↑ | ↑ | ↑ | ↑↑ | ↑ | ↑↑ | ↑ | ↑ | ↑ | ↑ | ↑↑ | ↑ | ↑↑ | ↑ | ↑↑ | ↑ | ↑ | |
| HFV | ↑ | ↑ | ↑ | ↑↑ | ↑ | ↑↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑↑ | ↑ | ↑↑ | ↑ | ↑ |
| HFD | ↑ | ↑ | ↑ | ↑↑↑ | ↑ | ↑↑↑ | ↑ | ↑↑ | ↑ | ↑ | ↑ | ↑↑↑ | ↑ | ↑↑↑ | ↑ | ↑↑↑ | ↑ | ↑↑↑ |
NDV normal diet vehicle, NDD normal diet D-galactose, HFV high-fat vehicle, HFD high-fat diet D-galactose
Although it is well known that the administration of D-galactose provides a model for aging, the effect of D-galactose-induced aging on cardiac mitochondrial functions and morphology are not well established. Our results demonstrated that the cardiac mitochondrial function in rats fed with D-galactose for 8 weeks was impaired to a greater degree, compared with rats treated with D-galactose for 4 weeks. This was indicated by an increase in the detrimental cardiac mitochondrial ROS production, mitochondrial depolarization, and mitochondrial swelling. Furthermore, both 4-week and 8-week D-galactose-treated rats exhibited an imbalance of cardiac mitochondrial dynamics as indicated by a significant decrease in mitochondrial fusion (MFN1 and MFN2 levels). However, the cardiac mitochondrial fission marker (Drp1) did not change in both 4-week and 8-week D-galactose-treated rats. In addition, previous studies have shown that impaired autophagy plays an important role in the progression of various forms of cardiovascular disease (Chen et al. 2016; Lin et al. 2019; Wang et al. 2019). Consistent with a previous in vitro study (Chen et al. 2016), our study also demonstrated that impaired autophagy occurred in normal diet rats treated with D-galactose for 4 weeks and further impairment of autophagy was detected in 8-week D-galactose-administered rats fed with normal diet. This autophagy was represented by decreased Beclin 1 and increased p62 expression compared with D-galactose 4-week rats (Table 2). Thus, our findings suggested that D-galactose aggravated LV dysfunction in normal diet-fed rats by increasing oxidative stress, apoptosis, autonomic imbalance, mitochondrial dysfunction, and impaired autophagy in a time-dependent manner (Table 2).
In addition to aging, it is well known that obesity is one of the major risk factors for cardiac dysfunction. Obesity can induce insulin resistance and cardiac dysfunction by an increase in visceral fat mass, and free fatty acid metabolites, and altered adipokine release, leading to an increased oxidative stress level (Shinlapawittayatorn et al. 2018). Our previous study demonstrated that after 16 weeks of a high-fat diet consumption, obese rats had increased oxidative stress, apoptosis and mitochondrial dysfunction when compared to normal diet fed rats, leading to LV dysfunction (Tanajak et al. 2016). Consistent with that study, our current study also showed metabolic impairment to be associated with consumption of a high-fat diet as indicated by insulin resistance, increased body weight, visceral fat mass, LDL level, and LV dysfunction. These were caused by increased oxidative stress, apoptosis, and mitochondrial dysfunction in rats fed with a high-fat diet for 16 weeks the comparisons being drawn to normal diet-fed rats. Interestingly, additional impairment of LV dysfunction was found in rats fed with a high-fat diet for 20 weeks as indicated by further impairment in autophagy and mitochondrial dysfunction than rats fed with a high-fat diet for 16 weeks as shown in Table 2.
Currently, the prevalence of obesity and insulin resistance are increasing dramatically among young people and adults worldwide; however, the effects of aging on cardiometabolic impairments under these conditions are not clear. Therefore, we aimed to determine the effects of aging induced by D-galactose in rats on obese insulin resistance at different time points of D-galactose administration. Our results demonstrated that aging induced by D-galactose aggravated cardiac dysfunction in a time-dependent manner in the presence of an obese insulin-resistant condition. The deterioration in the cardiac function was due to the impairment of mitochondrial functions as indicated by increased mitochondrial ROS production, mitochondrial depolarization, and mitochondrial swelling in aging obese rats in a time-dependent manner when compared to high-fat diet rats treated with vehicle and normal diet D-galactose rats. In addition to the impaired mitochondrial fusion, increased mitochondrial fission was also detected in both high-fat diet-fed rats treated with D-galactose for 4 and 8 weeks when compared to high-fat diet rats treated with vehicle, indicating that D-galactose-induced aging also aggravated the imbalance of the cardiac mitochondrial dynamics in obese insulin-resistant rats.
It has been shown that aging is associated with reduced cellular autophagy and progressive accumulation of damaged proteins and undigested materials, leading to heart failure (De Meyer et al. 2010), and obesity can also foster autophagy impairment (Sun et al. 2019). Interestingly, our results indicated that autophagy impairment was more severe in high-fat diet-fed rats treated with D-galactose for 4 and 8 weeks when compared to their respective vehicle-treated rats and normal diet-fed rats treated with D-galactose. In addition, the TUNEL-positive cells were significantly further increased in aged-obese rats in a time-dependent manner compared to normal diet-fed rats receiving D-galactose and high-fat diet vehicle rats. Therefore, the deterioration of the impaired autophagy and increased apoptosis as found in the aged-obese rats in this study could be responsible for the aggravation of LV dysfunction found in the aged-obese rats (Table 2).
In conclusion, our findings demonstrated for the first time that aging induced by D-galactose exacerbated the deterioration of cardiac dysfunction in obese insulin-resistant rats through the worsening of cardiac mitochondrial function, autophagy, and increased apoptosis in a time-dependent manner. These findings also raise the awareness of the potential adverse effects of aging on left ventricular function, especially when associated with obesity.
Limitations
The present study has limitations as we only performed SA-β-gal staining to detect the aging of the cardiac senescent cells. In order to better support senescence of cells, additional cardiac senescent marker such as cardiac p16INK4a expression need to be carried out in future studies. Regarding mitochondrial functions, we determined mitochondrial ROS production, membrane depolarization, and swelling. In order to have a better evaluation of the alterations of mitochondrial function, citrate synthase activity, complex I activity, ATP content, and NAD+ levels need to be measured in the future research. Furthermore, hallmarks of alterations of cardiac structures are missing in the present study. However, a previous study has demonstrated that H and E staining showed no alteration in cardiac architecture. Moreover, cardiac fibrosis detected by Masson’s trichrome staining was not found in D-galactose-treated mice compared with control mice (Bei et al. 2018). Future research will need to target on the effect of aging induced by D-galactose and high-fat diet-induced obesity on cardiac morphology.
Funding information
This work was supported by a NSTDA Research Chair Grant from the National Science and Technology Development Agency Thailand (NC), Thailand Research Fund Grants RTA6080003 (SCC), RSA5880015 (KS), RSA6180056 (SP), and the Chiang Mai University Center of Excellence Award (NC).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali T, Badshah H, Kim TH, Kim MO. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model. J Pineal Res. 2015;58:71–85. doi: 10.1111/jpi.12194. [DOI] [PubMed] [Google Scholar]
- Apaiajai N, Chunchai T, Jaiwongkam T, Kerdphoo S, Chattipakorn SC, Chattipakorn N. Testosterone deprivation aggravates left-ventricular dysfunction in male obese insulin-resistant rats via impairing cardiac mitochondrial function and dynamics proteins. Gerontology. 2018;64:333–343. doi: 10.1159/000487188. [DOI] [PubMed] [Google Scholar]
- Apaijai N, Pintana H, Chattipakorn SC, Chattipakorn N. Cardioprotective effects of metformin and vildagliptin in adult rats with insulin resistance induced by a high-fat diet. Endocrinology. 2012;153:3878–3885. doi: 10.1210/en.2012-1262. [DOI] [PubMed] [Google Scholar]
- Aydin S, et al. Comparison of oxidative stress biomarkers in renal tissues of D-galactose induced, naturally aged and young rats. Biogerontology. 2012;13:251–260. doi: 10.1007/s10522-011-9370-3. [DOI] [PubMed] [Google Scholar]
- Bei Y, Wu X, Cretoiu D, Shi J, Zhou Q, Lin S, Wang H, Cheng Y, Zhang H, Xiao J, Li X. miR-21 suppression prevents cardiac alterations induced by d-galactose and doxorubicin. J Mol Cell Cardiol. 2018;115:130–141. doi: 10.1016/j.yjmcc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Bo-Htay C, Palee S, Apaijai N, Chattipakorn SC, Chattipakorn N. Effects of d-galactose-induced ageing on the heart and its potential interventions. J Cell Mol Med. 2018;22:1392–1410. doi: 10.1111/jcmm.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boateng GO, Adams EA, Odei Boateng M, Luginaah IN, Taabazuing MM. Obesity and the burden of health risks among the elderly in Ghana: a population study. PLoS One. 2017;12:e0186947. doi: 10.1371/journal.pone.0186947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebe T, Yanar K, Atukeren P, Ozan T, Kuruç AI, Kunbaz A, Sitar ME, Mengi M, Aydın MS, Eşrefoğlu M, Aydın S, Cakatay U. A comprehensive study of myocardial redox homeostasis in naturally and mimetically aged rats. Age. 2014;36:9728. doi: 10.1007/s11357-014-9728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YM, Chang HH, Kuo WW, Lin HJ, Yeh YL, Padma Viswanadha V, Tsai CC, Chen RJ, Chang HN, Huang CY. Anti-apoptotic and pro-survival effect of alpinate oxyphyllae fructus (AOF) in a d-galactose-induced aging heart. Int J Mol Sci. 2016;17:466. doi: 10.3390/ijms17040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YM, Chang HH, Lin HJ, Tsai CC, Tsai CT, Chang HN, Lin SL, PadmaViswanadha V, Chen RJ, Huang CY. Inhibition of cardiac hypertrophy effects in D-galactose-induced senescent hearts by alpinate oxyphyllae fructus treatment evid based complement. Alternat Med. 2017;2017:2624384. doi: 10.1155/2017/2624384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gao J, Sun W, Li L, Wang Y, Bai S, Li X, Wang R, Wu L, Li H, Xu C. Involvement of exogenous H2S in recovery of cardioprotection from ischemic post-conditioning via increase of autophagy in the aged hearts. Int J Cardiol. 2016;220:681–692. doi: 10.1016/j.ijcard.2016.06.200. [DOI] [PubMed] [Google Scholar]
- Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, van Deursen JM. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer GR, De Keulenaer GW, Martinet W. Role of autophagy in heart failure associated with aging. Heart Fail Rev. 2010;15:423–430. doi: 10.1007/s10741-010-9166-6. [DOI] [PubMed] [Google Scholar]
- Gao J, He H, Jiang W, Chang X, Zhu L, Luo F, Zhou R, Ma C, Yan T. Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer’s disease. Behav Brain Res. 2015;293:27–33. doi: 10.1016/j.bbr.2015.06.045. [DOI] [PubMed] [Google Scholar]
- Guo XH, Li YH, Zhao YS, Zhai YZ, Zhang LC. Antiaging effects of melatonin on the myocardial mitochondria of rats and associated mechanisms. Mol Med Rep. 2017;15:403–410. doi: 10.3892/mmr.2016.6002. [DOI] [PubMed] [Google Scholar]
- Liang CY, Liang YM, Liu HZ, Zhu DM, Hou SZ, Wu YY, Huang S, Lai XP (2017) Effect of Dendrobium officinale on D-galactose-induced aging mice. Chin J Integr Med. 10.1007/s11655-016-2631-x [DOI] [PubMed]
- Lin B, Feng D, Xu J. Cardioprotective effects of microRNA-18a on acute myocardial infarction by promoting cardiomyocyte autophagy and suppressing cellular senescence via brain derived neurotrophic factor. Cell Biosci. 2019;9:38. doi: 10.1186/s13578-019-0297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang J, Chen X, Guo C, Guo Y, Wang H. Ginkgo biloba extract EGB761 protects against aging-associated diastolic dysfunction in cardiomyocytes of D-galactose-induced aging rat. Oxidative Med Cell Longev. 2012;2012:418748. doi: 10.1155/2012/418748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Ye Q, Liu CM, Shan Q, Wang YJ. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-kappaB pathway activation. Cereb Cortex. 2010;20:2540–2548. doi: 10.1093/cercor/bhq002. [DOI] [PubMed] [Google Scholar]
- Maneechote C, Palee S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Differential temporal inhibition of mitochondrial fission by Mdivi-1 exerts effective cardioprotection in cardiac ischemia/reperfusion injury. Clin Sci (Lond) 2018;132:1669–1683. doi: 10.1042/CS20180510. [DOI] [PubMed] [Google Scholar]
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22:R741–R752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Olivieri F, Prattichizzo F, Grillari J, Balistreri CR. Cellular Senescence and inflammaging in age-related diseases mediators. Inflamm. 2018;2018:9076485. doi: 10.1155/2018/9076485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palee S, Weerateerangkul P, Chinda K, Chattipakorn SC, Chattipakorn N. Mechanisms responsible for beneficial and adverse effects of rosiglitazone in a rat model of acute cardiac ischaemia-reperfusion. Exp Physiol. 2013;98:1028–1037. doi: 10.1113/expphysiol.2012.070433. [DOI] [PubMed] [Google Scholar]
- Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. PPARgamma agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology. 2012;153:329–338. doi: 10.1210/en.2011-1502. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Effects of estrogen in preventing neuronal insulin resistance in hippocampus of obese rats are different between genders. Life Sci. 2011;89:702–707. doi: 10.1016/j.lfs.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011;88:619–627. doi: 10.1016/j.lfs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Qian YF, Wang H, Yao WB, Gao XD. Aqueous extract of the Chinese medicine, Danggui-Shaoyao-San, inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Cell Biol Int. 2008;32:304–311. doi: 10.1016/j.cellbi.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–456. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Roth GA, Reddy KS, Arnett DK, Bonita R, Gaziano TA, Heidenreich PA, Huffman MD, Mayosi BM, Mendis S, Murray CJ, Perel P, Piñeiro DJ, Smith SC Jr, Taubert KA, Wood DA, Zhao D, Zoghbi WA. The heart of 25 by 25: achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke: a modeling study from the American Heart Association and World Heart Federation. Circulation. 2016;133:e674–e690. doi: 10.1161/CIR.0000000000000395. [DOI] [PubMed] [Google Scholar]
- Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N. The influence of obese insulin-resistance on the outcome of the ischemia/reperfusion insult to the heart. Curr Med Chem. 2018;25:1501–1509. doi: 10.2174/0929867324666170616105639. [DOI] [PubMed] [Google Scholar]
- Sikora E, Arendt T, Bennett M, Narita M. Impact of cellular senescence signature on ageing research. Ageing Res Rev. 2011;10:146–152. doi: 10.1016/j.arr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Sivasinprasasn S, Sa-Nguanmoo P, Pratchayasakul W, Kumfu S, Chattipakorn SC, Chattipakorn N. Obese-insulin resistance accelerates and aggravates cardiometabolic disorders and cardiac mitochondrial dysfunction in estrogen-deprived female rats. Age. 2015;37:28. doi: 10.1007/s11357-015-9766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Tan Y, Rexiati M, Dong M, Guo W. Obesity is a common soil for premature cardiac aging and heart diseases - role of autophagy. Biochim Biophys Acta Mol basis Dis. 2019;1865:1898–1904. doi: 10.1016/j.bbadis.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Sun SL, Guo L, Ren YC, Wang B, Li RH, Qi YS, Yu H, Chang ND, Li MH, Peng HS. Anti-apoptosis effect of polysaccharide isolated from the seeds of Cuscuta chinensis Lam on cardiomyocytes in aging rats. Mol Biol Rep. 2014;41:6117–6124. doi: 10.1007/s11033-014-3490-1. [DOI] [PubMed] [Google Scholar]
- Tanajak P, et al. Fibroblast growth factor 21 (FGF21) therapy attenuates left ventricular dysfunction and metabolic disturbance by improving FGF21 sensitivity, cardiac mitochondrial redox homoeostasis and structural changes in pre-diabetic rats. Acta Physiol (Oxford) 2016;217:287–299. doi: 10.1111/apha.12698. [DOI] [PubMed] [Google Scholar]
- Tunapong W, Apaijai N, Yasom S, Tanajak P, Wanchai K, Chunchai T, Kerdphoo S, Eaimworawuthikul S, Thiennimitr P, Pongchaidecha A, Lungkaphin A, Pratchayasakul W, Chattipakorn SC, Chattipakorn N. Chronic treatment with prebiotics, probiotics and synbiotics attenuated cardiac dysfunction by improving cardiac mitochondrial dysfunction in male obese insulin-resistant rats. Eur J Nutr. 2017;57:2091–2104. doi: 10.1007/s00394-017-1482-3. [DOI] [PubMed] [Google Scholar]
- Wang S, Kandadi MR, Ren J. Double knockout of Akt2 and AMPK predisposes cardiac aging without affecting lifespan: Role of autophagy and mitophagy. Biochim Biophys Acta Mol basis Dis. 2019;1865:1865–1875. doi: 10.1016/j.bbadis.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Hou CL, Mu XP, Sun C, Zhu YC, Wang MJ, Lv QZ. H2S donor NaHS changes the production of endogenous H2S and NO in D-galactose-induced accelerated ageing. Oxidative Med Cell Longev. 2017;2017:5707830. doi: 10.1155/2017/5707830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanar K, Aydın S, Cakatay U, Mengi M, Buyukpınarbaşılı N, Atukeren P, Sitar ME, Sönmez A, Uslu E. Protein and DNA oxidation in different anatomic regions of rat brain in a mimetic ageing model. Basic Clin Pharmacol Toxicol. 2011;109:423–433. doi: 10.1111/j.1742-7843.2011.00756.x. [DOI] [PubMed] [Google Scholar]
- Yu Y, et al. Fibroblast growth factor 21 protects mouse brain against D-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol Biochem Behav. 2015;133:122–131. doi: 10.1016/j.pbb.2015.03.020. [DOI] [PubMed] [Google Scholar]