Abstract
Metformin is the safest and the most widely prescribed first-line therapy for managing hyperglycemia due to different underlying causes, primarily type 2 diabetes mellitus. In addition to its euglycemic properties, metformin has stimulated a wave of clinical trials to investigate benefits on aging-related diseases and longevity. Such an impact on the lifespan extension would undoubtedly expand the therapeutic utility of metformin regardless of glycemic status. However, there is a scarcity of studies evaluating whether metformin has differential cognitive effects across age, sex, glycemic status, metformin dose, and duration of metformin treatment and associated pathological conditions. By scrutinizing the available literature on animal and human studies for metformin and brain function, we expect to shed light on the potential impact of metformin on cognition across age, sex, and pathological conditions. This review aims to provide readers with a broader insight of (a) how metformin differentially affects cognition and (b) why there is a need for more translational and clinical studies examining multifactorial interactions. The outcomes of such comprehensive studies will streamline precision medicine practices, avoiding “fit for all” approach, and optimizing metformin use for longevity benefit irrespective of hyperglycemia.
Electronic supplementary material
The online version of this article (10.1007/s11357-019-00146-3) contains supplementary material, which is available to authorized users.
Keywords: Metformin, Cognition, Diabetes, Age, Sex, Gender, Brain function
Introduction
In recent years, the prescription rate for metformin is increased to 235/1000 population for the FDA-approved indications and up to 20.3/1000 person for off-label use (Le and Lee 2019). Apart from the role in maintaining glucose homeostasis, metformin has several potential anti-aging properties. The longevity benefit was observed in diabetic patients taking metformin when compared with diabetic subjects on non-metformin protocols, as well as non-diabetic subjects not taking metformin (Bannister et al. 2014). Recently, metformin has been purported to have a detrimental effect on cognition in male mice, supported by findings in recent clinical studies (Hervas et al. 2017; Kuan et al. 2017; Thangthaeng et al. 2017). Such surprising results that can affect the overall quality of life may outweigh metformin’s longevity benefits, especially if the target population for such benefit is non-diabetic.
At NIH RePORTER (https://projectreporter.nih.gov/reporter.cfm), there are currently 85 projects funded for “metformin and aging” and 17 of these or other projects involved targeting metformin and cognition. Further, there are currently eleven registered clinical trials (https://clinicaltrials.gov/ct2/home) focused on metformin, aging, and longevity. Of the trials identified, eight are directly addressing the benefit of metformin on age-related problems and their underlying molecular mechanisms (Table 1). Six clinical trials included both men and women. There is no information regarding the assessment of beneficial or harmful effects of metformin across sexes in any of these clinical trials. None of these longevity studies has focused on cognition or psychomotor elements of brain functions.
Table 1.
Clinical trials with a focus on metformin and aging
| Sr. | Clinical trials identifier | Name of the study | Sample size | Sex | Sex-based analysis | Age (years) | Condition or disease | Metformin dose (mg/day) | Metformin duration | Evaluation | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NCT 03309007 | A Double-Blind, Placebo-Controlled Trial of Anti-Aging, Pro-Autophagy Effects of Metformin in Adults With Prediabetes | 30 | Both | No details provided | 30–70 | Pre-diabetes | 1500 | 12 weeks | Autophagy | Change in leucocyte LC3 score, at 0, 4, and 12 weeks |
| 2 | NCT 02432287 | Metformin in Longevity Study (MILES) | 15 | Both | No details provided | 35–85 | Aging | 1700 | 12 weeks | Longevity gene expression changes | Increase in gene expression in muscle and adipose tissue using RNA sequencing |
| 3 | NCT 03451006 | Metformin and Aging Trial in the Elderly: A Pilot and Feasibility Study (MATE) | 12 | Both | No details provided | > 60 | Aging, inflammation, frailty | 2000 | 12 months | Effect of metformin in frailty | Change in frailty, balance score, gait speed, standing test from chair, change in senescent marker |
| 4 | NCT 02308228 | Metformin to Augment Strength Training Effective Response in Seniors (MASTERS) | 100 | Both | No details provided | > 65 | Aging | 1700 | 16 weeks | Interaction with resistance training adaptations | Muscle size, biopsy and CT vastus lateralis, muscle strength, muscle macrophage, muscle inflammatory gene expression, insulin sensitivity |
| 5 | NCT 03072485 | Phase 1 Study of the Effects of Combining Topical FDA-approved Drugs on Age-related Pathways on the Skin of Healthy Volunteers | 10 | Female | Not applicable | > 55 | Aging | Topical application | 4 weeks | Skin aging | Profile of gene transcript changes, Wrinkle score |
| 6 | NCT 01765946 | Metformin and Longevity Genes in Prediabetes | 38 | Both | No details provided | 40–75 | Pre-diabetic, aging | 1500 | 8 weeks (2 months) | Longevity gene expression changes | Longevity genes, Sirtuin-1, p66Shc, mTor, p53 in peripheral blood mononuclear cells, insulin sensitivity, monocyte polarization status |
| 7 | NCT 02745886 | Metformin Induces a Dietary Restriction-like State in Human | 60 | Male | Not applicable | 18–60 | Aging, overweight subjects | 1700 | 6 months | Calorie restriction like benefits | Gene expression profile, insulin sensitivity |
| 8 | NCT 03713801 | Impact of Metformin on Immunity | 50 | Both | No details provided | 63–90 | Aging, vaccine response impaired | 1500 | 12 weeks | Immune-response | Change in antibody response to PCV13 measurement of immune-phenotypes |
As a near obligate glucose consumer, the brain is one of the most metabolically active organs in the body. Therefore, the brain is more susceptible to manipulation of energy metabolism by glucose-lowering medicines. It is unclear whether the beneficial or deleterious effects on brain function accompanies the improved longevity associated with metformin. It is also ambiguous whether the effects of metformin on brain function are universal across broad demographics, such as sex, age, and existing pathological conditions. We attempt to address this critical concern by considering the effects of sex, age, and pathological conditions on metformin-dependent changes in brain function. Previous findings from in vitro studies, in vivo studies in rodents, cross-sectional and longitudinal data analysis, and clinical trials relevant to these questions were scrutinized. The goal of this update is to provide precision medicine-based insight for metformin treatment, optimizing longevity and cognitive benefits, and mitigating risks.
Metformin increases lifespan
Barzilai et al. 2016 and Novelle et al. 2016, in two separate papers, discussed the detail mechanisms for the anti-aging benefit of metformin (Barzilai et al. 2016; Novelle et al. 2016). Metformin collectively influences inflammation, cellular survival, stress, autophagy, and protein synthesis, which are significant players in aging/longevity (Algire et al. 2012; Batandier et al. 2006; Bridges et al. 2014; Cho et al. 2015; Duca et al. 2015; Foretz et al. 2010; Jadhav et al. 2013; Kickstein et al. 2010; Lien et al. 2014; Liu et al. 2011; Lu et al. 2015; Moiseeva et al. 2013; Nair et al. 2014; Perez-Revuelta et al. 2014; Saisho 2015; Song et al. 2015; Xie et al. 2011; Zheng et al. 2012). There is mounting evidence to suggest that metformin prolongs the lifespan of many species, ranging from nematodes-to-rodents (Anisimov et al. 2008; Anisimov et al. 2011; Cabreiro et al. 2013; De Haes et al. 2014). This longevity benefit is dependent on genotype, age, sex, dose, and duration of metformin therapy. Metformin substantially prolonged lifespan in female outbred mice by nearly 40% (Anisimov et al. 2008). The longevity benefit is higher by 14% when metformin treatment was initiated earlier rather than later in old age (Anisimov et al. 2011). In 129/sv and R6/2 mice, metformin extended the lifespan in male mice with only a subtle effect in female mice (Anisimov et al. 2010b; Ma et al. 2007). Although this effect occurred across several mouse breeds, not all demonstrated consistent prolongation of lifespan (Martin-Montalvo et al. 2013). This disparity could be due to metformin dose or genetic variation across studies. Drosophila, mice, and rats treated with very high doses did not extend lifespan, suggesting the need to fine-tune the dosage scheduling for longevity benefits (Martin-Montalvo et al. 2013; Slack et al. 2012; Smith Jr. et al. 2010). The strain used by Smith et al. 2010 to understand metformin-associated longevity also failed to replicate the CR benefit on longevity, further emphasizing underlying genetics for longevity benefit and further need for precision medicine-based approach. It is often discussed that metformin both directly (by altering genetics) and indirectly (by lowering disease burden) provides longevity benefit. The NIA’s intervention testing program (ITP) tested for longevity benefit in both male and female UM-HET3 mice using metformin (1000 ppm; 0.1%) alone or in combination with rapamycin (14 ppm) (Strong et al. 2016). Interestingly they observed metformin-associated 7% increase in longevity only in male mice (data pooled from all sites, statistically not significant, p = 0.35) and no such effect (0% change) in female mice irrespective of site. The major limitation of this study was site-specific variation in the benefit, which ranged from − 1 to 13%. While rapamycin alone treatment led to a uniform 10% increased longevity in both sexes. Further, the combination of metformin and rapamycin had synergistic longevity benefit up to 23% (statistically significant p = 0) in both male and female mice. These findings from ITP studies could suggest that when metformin may have failed to increase longevity in female in one particular strain, combination with other modality might help to achieve the same amount of benefit as that of male.
In humans, there are indirect pieces of evidence for metformin-associated increased lifespan. Metformin early treatment can delay or prevent the onset of diabetes (Diabetes Prevention Program Research 2015). Metformin also improved health indicators for cardiovascular disease and atherosclerosis in males (Goldberg et al. 2013; Goldberg et al. 2017; Haffner et al. 2005). The cardiovascular risk reduction across the UK Prospective Diabetes Study (UKPDS) and the HOME Trial (NCT00375388) may justify the use of metformin as safe longevity-promoting therapy in patients with and without diabetes (Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group 1998; Kooy et al. 2009). To make this assertion, these findings will need to be reconciled with other studies conducted in non-diabetic subjects, such as the GIPS III study (NCT01217307), which demonstrated a little-to-no beneficial effect of metformin on cardiovascular health (Hartman et al. 2017; Lexis et al. 2014). In the CAMERA trial (NCT00723307), chronic metformin treatment for 18 months did not show any beneficial effect on cardiovascular health (Preiss et al. 2014). Interestingly, Han et al. 2019, meta-analyzed 40 studies comprising 1,066,408 patients and concluded that metformin reduced all-cause mortality and cardiovascular events in coronary artery disease patients. However, the authors also reported that overall, metformin did not effectively reduce the incidence of cardiovascular events in myocardial infarction and coronary artery disease patients in the absence of T2D (Han et al. 2019). There is a need to understand why metformin showed beneficial outcome in some studies and why showed no such effects in other studies. The answers to these questions could be deciding factors for precision medicine-based prescription of metformin for longevity benefits, especially in the absence of diabetes.
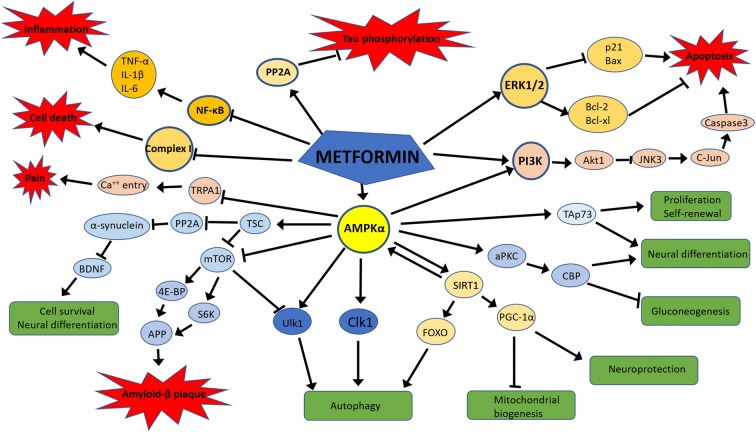
Metformin acts on neurons, astrocytes, and microglia (Fig. 1)
Fig. 1.
Mechanisms of action of metformin in the neuro-glial environment. Metformin via AMPKα-dependent pathways promote cell survival, neural differentiation, autophagy, neuroprotection, proliferation, and self-renewal. Metformin inhibits amyloid-β-plaque formation via AMPKα. Metformin inhibits inflammation via inhibiting NF-κB. Metformin activates apoptosis via PI3K pathway and inhibits apoptosis via the ERK1/2 pathway. Metformin promotes Tau dephosphorylation via PP2A pathway
As the major cells of the brain, neurons are among the most metabolically active cells in the body. Energy regulator-AMP-activated protein kinase (AMPK) signaling is highly expressed in the neurons. Within neurons, metformin primarily acts via AMPK to maintain energy homeostasis (Hardie 2014). Many recent studies explored AMPK-dependent, AMPK-independent, and energy independent effects of metformin in neuronal activity, differentiation, toxicity, autophagy, and survival. These differential effects on neurons were dose, duration, and disease-dependent (Aatsinki et al. 2014; Bayliss et al. 2016; Canto et al. 2009; Fatt et al. 2015; Ge et al. 2017; Hawley et al. 2010; Isakovic et al. 2007; Jang and Park 2018; Katila et al. 2017; Khedr et al. 2018; Kickstein et al. 2010; Matthes et al. 2018; Ou et al. 2018; Potts and Lim 2012; Price et al. 2012; Sesen et al. 2015; Song et al. 2015; St-Pierre et al. 2006; Wang et al. 2012; Wang et al. 2018b; Yan et al. 2017; Zhang et al. 2016; Zhu et al. 2015).
Apart from neurons, metformin also affects astrocytes and microglia. A higher concentration at 10 mM metformin increased glucose consumption, lactate production, and decreased oxygen consumption leading the primary astrocytes toward more glycolytic metabolism. (Hohnholt et al. 2017; Westhaus et al. 2017) Similarly, ketogenic activation in terms of increased acetoacetate and β-hydroxybutyrate production occurred at 1 mM metformin concentration. (Takahashi et al. 2014) Despite these metabolic changes, metformin was found to be protecting the astrocytes against apoptosis and cell death induced by oxygen and glucose deprivation. (Gabryel and Liber 2018).
It is well known that AMPK regulates the energy balance as well as the functional phenotype of microglia. (Lu et al. 2010; Sag et al. 2008) The microglia are highly plastic in response to micro-environment changes. Metformin-induced AMPK activation changes the microglia polarization toward M2 phenotype, which helps in tissue repair following an injury such as middle cerebral artery occlusion. (Jin et al. 2014). Further detailed refining of the underlying mechanism of metformin in microglia indicated AMPK-dependent release of TNF-α and AMPK-independent regulation IL-1β, IL-6, IL-10, TGF-β, nitric oxide, reactive oxygen species, NF-κB, p65, and PGC-1α. Interestingly, sex of the species and location of microglia determine the impact of metformin on microglia, as shown by selective microglial activation and reversal of neuropathic pain in male mice. (Inyang et al. 2019).
Metformin differently affects cognition (Table 2)
Table 2.
Studies with a focus on metformin and cognition
| Sr. | Species | Sex | Sex-based analysis | Age (years/weeks/months) | Pathological conditions | Metformin dose | Metformin duration | Evaluation | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| A. Human | ||||||||||
| 57–58/42–43 (M/F)% | No | 61–67 years | Diabetes and non-diabetic control not on metformin | No data | No data | Longevity | Longevity benefit over control | (Bannister et al. 2014) | ||
| Both | No | 40 and 65 years | T2DM | 0.5–2.0 g/day | 24 weeks | Cognition, anti-depressant effect | Better cognition, lessen depression | (Guo et al. 2014) | ||
| 60/40 (M/F)% | No | 51–99 years | Alzheimer disease/mild cognitive impairment or cognitively normal | No data | No data | Cognition | Impairment of cognition | (Moore et al. 2013) | ||
| Both | No | 55 years and more | Diabetes and non-diabetic control not on metformin | No data | 4–6 years | Cognition | Reduced risk of cognitive impairment | (Ng et al. 2014) | ||
| Not disclosed | No | 36–60 years | Diabetic vs healthy control | 1 g/day | 6 months | Cognition | Cognitive impairment | (Khattar et al. 2016) | ||
| Both | No | 67–80 years | Diabetes | No data | No data | Dementia | Lower association of dementia | (Cheng et al. 2014) | ||
| Both | No | 41–73 years | Diabetes | 1.7 g/day | 8–12 years | Cognition | No association with cognition | (Luchsinger et al. 2017) | ||
| Both | No | 54–75 years | Diabetes, Alzheimer disease, Parkinson disease | 130 g–385 g/year | Up to 400 days | Cognition and neurodegenerative disease | Cognitive impairment | (Kuan et al. 2017) | ||
| Both | No | 30–72 years | Diabetes, Huntington’s disease | No data, only intake association study | No data | Cognition and motor function | Cognitive impairment in control and cognitive benefit in HD | (Hervas et al. 2017) | ||
| Female | No | 56–70 years | Breast cancer survivor, obesity | 500–1500 mg/day | 6 months | Cognition | No effect on cognition | (Hartman et al. 2019) | ||
| Male | No | 25 and 30 years | Fragile X syndrome | 500–2000 mg/day | 1 year | Cognition and behavior | Improved cognition | (Protic et al. 2019) | ||
| Both | No | 55–80 years | Non-diabetic mild cognitive impairment and Alzheimer’s disease | 2000 mg/day | 8 weeks | Cognition and biomarkers | Improved cognition | (Koenig et al. 2017) | ||
| Both | No | > 60 years | Diabetic | NA | NA | Cognition | Higher risk for cognitive impairment | (Koo et al. 2019) | ||
| Both | No | 68.1 ± 7.8 years | Non-dementia vascular cognitive impairment | 500 mg t.i.d. | 1 year | Cognition | Improved cognition | (Lin et al. 2018) | ||
| Both | No | > 65 years | Dementia | NA | NA | Cognition | Better cognition compared with sulphonylureas | (Orkaby et al. 2017) | ||
| B. Mice | ||||||||||
| 1 | (129/Sv) | Male, female | Yes | 3 months | Non-diabetic | 100 mg/kg | 18 months | Longevity | Decreased lifespan in male and increased lifespan in female | (Anisimov et al. 2010a) |
| 2 | C57BL/6 | Male | No | 6 and 11 months | Non-diabetic | 0.1 to 1% metformin | Life-long | Longevity | Longevity benefit over control | (Martin-Montalvo et al. 2013) |
| 3 | C57BL/6 | Female | No | 8–10 weeks | cis-Diamineplatinum(II) dichloride (cisplatin) | 100 mg/kg | 7 days | Cognition | Prevention of cognitive impairment | (Zhou et al. 2016) |
| 4 | C57BL/6 | Male | No | 3, 12, and 24 months | Non-diabetic | 297 mg/kg | 3 months | Cognition | Cognitive impairment | (Thangthaeng et al. 2017) |
| 5 | C57BL/6J | Male | No | 12 months | High-fat diet-induced diabetes | 1% by weight | 6 months | Cognition and psychomotor | Prevention of cognitive impairment | (Allard et al. 2016) |
| 6. | C57BL/6 | Male | No | 3 months | Non-diabetic | 181.8 mg/kg | 3 months | Cognition and psychomotor | Impaired cognition | (Wenjun Li) |
| 6 | Outbred Swiss-derived female (SHR) | Female | No | 3 months | Non-diabetic | 100 mg/kg | No data | Longevity | Longevity benefit over control | (Anisimov et al. 2008) |
| 7 | Balb/c | Male | No | 3 months | Non-diabetic | 300 mg/kg | 14 days | Cognition and neurogenesis | Reversed AlCl3·6H2O-Induced memory and neurodegeneration deficit | (Ahmed et al. 2017) |
| 8 | APP/PS1 | Both | No | 12–13 months | Non-diabetic, Alzheimer disease mouse model | 5 g/l ad libitum in drinking water | 8 months | Cognition | Improved memory | (Matthes et al. 2018) |
| 9 | APP/PS1 | Female | No | 26 weeks | Non-diabetic Alzheimer disease mouse model | 200 mg/kg | 14 days | Cognition | Improved cognition | (Ou et al. 2018) |
| 10 | C57/129j | No detail | No | 2 months | Non-diabetic | 200 mg/kg | 38 days | Cognition and neurogenesis | Improved memory and increased neurogenesis | (Wang et al. 2012) |
| 11 | ApoE TR | Male | No | 13 month | Alzheimer disease risk model | 300 mg/kg | 5 months | Cognition | Improved in ApoE3 TR mice only | (Zhang et al. 2019) |
| 12 | No | Female | No | No age (22–27-g body weight) | Ovariectomy | 7–15 mg/kg | 21 days | Cognition | Improved cognition | (Fatemi et al. 2019) |
| 13 | C57BL/6J | Male | No | 8–10 weeks | Streptozotocin-induced diabetes | 200 mg/kg | 6 weeks | Cognition | Improved cognition | (Wang et al. 2018a) |
| 14 | C57BL/6J | Male | No | 6 weeks | High-fat diet-induced diabetes | 0.2% w/w | 6 months | Cognition | Improved cognition | (Mamo et al. 2019) |
| C. Rats | ||||||||||
| 1 | Wistar | Male | No | Adult (180–200 g) | Scopolamine insult | 100–300 mg/kg | 14 days | Cognition | Prevention of cognitive impairment | (Mostafa et al. 2016) |
| 2 | Wistar | Male | No | 5 weeks (180–200 g) | High-fat diet-induced diabetes | 30 mg/kg | 21 days | Cognition and mitochondrial function | Prevents brain mitochondrial dysfunction and ultimately restores learning behavior | (Pintana et al. 2012) |
| 3 | Wistar | Male | No | 200–250 g | l-Methionine induced cognitive impairment | 30 mg/kg/ | 4 weeks | Short-term and long-term memory | Prevents short- and long-term memory | (Alzoubi et al. 2014) |
| 4 | Wistar | Male | No | 125–150 g | High-fat diet-induced diabetes | 1800 ppm (144 mg/kg diet) | 10 weeks | Cognition | No effect | (McNeilly et al. 2012) |
| 5 | Wistar | Male | No | 150–200 g | High-fat diet-induced stress and depression | 100 mg/kg/day | 4 weeks |
Cognition Lipid profile |
Reverted stress and high-fat diet-induced cognitive decline | (Khedr et al. 2018) |
| 6 | Wistar | Male | No | 250–300 g | Methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration | 50–150 mg/day | 21 days | Cognition, anxiety, depression, neurodegeneration | Dose dependent improved in cognition | (Keshavarzi et al. 2019) |
| 7 | Sprague-Dawley | Mae | No | 8 weeks | Hypobaric hypoxia induced cognitive impairment | 100 mg/kg | 21 days | Cognition | Prevented impairment in cognition | (Zhao et al. 2019) |
Human studies
Metformin has shown potential cognitive benefits in some disease conditions. There are very few pre-clinical or clinical studies, which have investigated the cognitive effect across age, sex, and blood glucose as individual factors or interfactorial interaction. Existing studies report changes across various cognitive evaluations, but with insufficient direct translational value to the broader population.
In a pilot cross-over study design using 2000 mg/day metformin for 8 weeks, Koenig et al. 2017 observed statistically significant favorable effect of metformin on executive function (Trail B test) and beneficial trends on measures of learning and memory (PAL total errors) and attention (DMS percent correct simultaneous) in non-diabetic MCI and AD patients. (Koenig et al. 2017) The study included 9 women and 11 men. The strength of the study was a cross-over design. The major limitation of the study was the pilot design with a small sample size. Similarly, in the Diabetes Prevention Program (DPP study), 2280 participants (776 on metformin, and 755 on placebo) were assessed for metformin and cognition relation. Overall this study observed no relation between metformin and cognition, which means that metformin was neither beneficial nor harmful in this study participants, and the authors concluded that metformin is cognitively safe. (Luchsinger et al. 2017) The cognitively safer outcome will support the use of metformin for longevity benefit. When looked at specific groups within the population, not all groups responded unequivocally. Especially the groups with higher hyperglycemia had worse cognition, and the aged population has consistently poor (non-significant) cognitive tests outcomes across all tests. (supplementary table 2 of the citation) Overall, men had significantly poor cognitive outcomes compared with women. (supplementary table 3 of the citation). Collectively, these factors indicate the role of hyperglycemia, age, and sex in cognitive outcomes in the presence of metformin.
On the contrary, metformin treatment was also associated with cognitive decline in diabetic subjects receiving at least 1 g metformin per day for over 6 months when compared with untreated, non-diabetic controls (Khattar et al. 2016). This study had several limitations. Firstly, the effects of age, sex, and disease severity were not controlled. Secondly, the controls used in this study were healthy, non-diabetic, age-matched subjects not receiving metformin medication, suggesting that cognitive effects observed in this study could be attributed to the severity of diabetes alone and may not be related to metformin. Future studies should consider using controls with pre-diabetic blood glucose levels with and without metformin treatment. Such an evaluation would provide a complete and more direct picture of the cognitive effects of metformin.
Similarly, the dementia screening program suggested an increased risk for cognitive impairment associated with diabetes and metformin, especially after adjustment for the age, sex, educational level, baseline test scores, hypertension, dyslipidemia, BMI, and baseline brain imaging abnormality (Koo et al. 2019). Kuan et al. (2017) used the Multivariate Cox proportional hazards regression model and supported the notion that metformin increased the risk for AD, vascular dementia, and Parkinson’s disease (PD) (Kuan et al. 2017). The strength of this study was a large sample size (4651 patients), adjustment for several confounding factors, and 12-year follow-up. The authors reported that higher doses (> 385 g per year) and longer duration of metformin treatment increased the risk for developing PD (3.54 times) and dementia (1.97 times). Although this was a large sample size study, which controls for both age (54–75 years) and sex, the authors did not elucidate age-sex interaction effects in their data analysis.
In another study, the authors reported a higher risk of cognitive impairment in diabetic patients taking metformin (Moore et al. 2013). However, the authors also acknowledged study limitations, such as the lack of data on diabetes duration, severity, metformin treatment duration, and use of concomitant anti-diabetic agents. Careful consideration and further assessment of the study by Hervas et al. 2017 provide more insight into metformin’s effect on cognition. The control group (without motor manifestation) showed cognitive impairment with metformin compared with the non-metformin group and beneficial effect of metformin on cognition in the Huntington’s disease (HD) group (Fig. 1 in the citation) (Hervas et al. 2017). In a clinical cohort of 67,731 elderly patients (> 65 years old), the results demonstrated an association between diabetic status and dementia, which was mitigated with prolonged metformin therapy (Cheng et al. 2014). However, there was no consideration of metformin dose or sex-dependent analysis. Another two observational studies involving T2DM patients suggested that metformin was associated with impaired cognitive performance due to vitamin B12 deficiency (Biemans et al. 2015; Moore et al. 2013). However, Khattar et al. ruled out such a notion (Khattar et al. 2016).
Overall, there were mixed and complex reporting of metformin-induced alterations in cognition in human studies. This further provides strength to the argument that metformin may not be “fit for all” cognitively safe medication when under consideration for longevity benefits. Individual risk and benefit assessment are necessary for such utilization.
Animal studies
Often animal experiments are performed for better-controlled study design. In the adult, male Wistar rats, 100 mg/kg/day metformin reversed scopolamine-induced cognitive impairment (Mostafa et al. 2016). Metformin attenuated impairments of spatial learning and memory, as well as short-term working memory. This effect was associated with reduced inflammation and oxidative stress-mediated through Akt activation. Similarly, metformin (100 mg/kg) reduced cisplatin-induced cognitive impairment in young (8–10 weeks) female C57BL/6 mice during novel object recognition and social discrimination testing (Zhou et al. 2016). The positive aspect of this study was the use of female mice, as behavioral studies tend to neglect females due to phenotype variability across the estrous cycle. However, the authors failed to mention a possible trend of a deleterious effect of metformin compared with saline control in behavior tests (Fig. 1 in the citation) (Zhou et al. 2016).
Another study compared middle-aged male (12 months) C57BL/6 mice receiving chronic metformin treatment and chronic high-fat diet (HFD). Results suggested an improved spatial learning, coordinated running performance, and reduced memory impairments (Allard et al. 2016). However, this study lacks the inclusion of a “control+metformin” treatment group. Metformin treatment lowered body weight, which may confound the outcome of cognitive tests (latency to reach platform parameter). The coordinated running performance is negatively influenced by higher body weights (Cook et al. 2002; Mao et al. 2015; McFadyen et al. 2003). The body weights of the mice in HFD with metformin treatment were significantly lower than HFD without metformin treatment (Allard et al. 2016). Similarly, in the Morris water maze test, the authors used latency to reach the platform, rather than path length as a learning measure. Higher body weight, reduced motor function, or slower swim speeds may influence escape latency, thereby skewing its utility as a learning measure in treatments that influence these variables.
These escape latency results were replicated in adult male Wistar rats receiving HFD and metformin treatment (Pintana et al. 2012). Alzoubi et al. reported similar preventative effects of metformin after chronic l-methionine-induced cognitive impairment using radial arm maze performance, spatial learning, and memory metric (Alzoubi et al. 2014). Metformin treatment at 100 mg/kg/day reversed the cognitive decline associated with HFD and chronic restraint stress in male Wistar rats (Khedr et al. 2018). However, the cognitive assessment parameter used in the Morris water maze testing was a latency to reach the escape platform. Since HFD, chronic restraint stress, as well as metformin treatment altered the body weight in these rats the latency, may not be an accurate estimate for cognition. This confound of weight can be removed by re-analyzing the data using body weights as co-variate. Contrastingly, another study using Wistar rats reported no influence of metformin fortified diet on cognition, despite improving insulin sensitivity (McNeilly et al. 2012). The study used Matched-the-Position tasks for cognitive assessment.
Overall, Table 2 B and C indicates cognitively beneficial effects of metformin in the presence of major disease models and possible deleterious effects in the absence of disease model. This further suggests that metformin could be useful, cognitively safer, and even beneficial as longevity medicine in the presence of certain disease conditions. However, caution needs to be taken in the absence of such major diseases when it could be possible that longevity benefit is accompanied by cognitive deterioration and reduced quality of life.
The sex-dependent actions of metformin
Despite a higher prevalence of type 2 diabetes in men (CDC 2015), women with T2DM have poorer glycemic control and underachievement of desired hemoglobin A1c (HbA1c) levels (Chiu and Wray 2011; Nilsson et al. 2004). Women with diabetes have higher mortality, reduced lifespan, and more complications compared with men (Deshpande et al. 2008; Gregg et al. 2007). The influence of sex steroids may underlie the sex-specific differences in disease progression and treatment responses (Arnetz et al. 2014). Hormone-based sex differences are evident in the most oral anti-hyperglycemic agents on the market today, including metformin (Arnetz et al. 2014).
Previous work demonstrates that metformin’s bioavailability and glycemic control does not vary across sex or ethnicity in young adults (Karim et al. 2007). However, differences have been reported between sexes for metformin’s non-diabetic effects on physiology. In men, metformin increased plasma fatty acid levels, myocardial fatty acid utilization, and oxidation, and lower myocardial glucose utilization, indicating decreased fatty acid clearance (Lyons et al. 2013). Despite similar glucose control, only in men with lower testosterone levels, metformin decreased thyrotropin levels, Jostel’s thyrotropin index, and increased SPINA-GT when compared with normal testosterone level group (Krysiak et al. 2019). Another study found that men admitted to the intensive care unit for metformin-induced lactic acidosis had higher mortality compared with women (Biradar et al. 2010). Similarly, males with colorectal cancer had higher mortality when they were taking metformin compared with females colorectal patients on metformin (Park et al. 2017). On the other hand, in the Diabetes Prevention Program study, women receiving metformin to prevent T2DM were less adherent to treatment and reported a higher rate of adverse events (Walker et al. 2006). Metformin has also been associated with higher hospitalization and mortality rates in women (Pongwecharak et al. 2009).
Metformin exerted strong sex-dependent survival benefit in cases of colorectal cancer (CRC). This benefit was associated with longer duration of treatment with metformin (more than 22 months). After controlling for other clinically relevant factors, female diabetic patients with advanced stage CRC who had been treated with metformin had significantly lower CRC-related mortality, as compared with male counterparts (Park et al. 2017). In the Taiwanese population, Lee et al. (2011) reported similar findings with female CRC patients benefitting more than male CRC patients. However, the authors further reported the opposite sex effect with male hepatocellular cancer patients benefitting more than females (Lee et al. 2011). Interestingly, this differential effect on cancer-related mortality across sexes was associated with lower dose metformin (500 mg/day) (Lee et al. 2011).
There are also potential sex-specific differences in the effect of metformin on longevity dependent on the strain of the mice. In female mice, the highest to lowest longevity benefit with metformin was reported in SHR (+ 37.9%) (Anisimov et al. 2008), FVB/N (+ 8.0% and + 6.7%) (Anisimov et al. 2005; Anisimov et al. 2010a), 129/Sv (+ 4.4%) (Anisimov et al. 2010b), and HD (0%) (Ma et al. 2007) strains, while in male mice, the high to low longevity effect was noted in HD (+ 20.1%) (Ma et al. 2007) and 129/Sv (− 13.4%) (Anisimov et al. 2010b) strains.
Few pre-clinical or clinical studies have investigated the effect of metformin on cognitive function. The available data shows mixed results of metformin-induced cognitive alterations. (Table 2 A, B, and C) (Guo et al. 2014; Kuan et al. 2017; Moore et al. 2013; Mostafa et al. 2016; Ng et al. 2014; Ying et al. 2014; Zhou et al. 2016). In our lab, we found learning and memory impairments in non-diabetic, young adult and aged C57BL/6 male mice after receiving metformin at human equivalent doses (1.5–2.0 g/day). Data analysis has suggested that this cognitive impairment was nearly exclusive to male mice, suggesting sex as a critical determinant for future investigation.
The role of organic cation transporters in the sex difference of metformin
The transport of metformin across organs occurs through organic cation transporters (OCTs). There is increasing evidence that sex steroids affect the expression and function of OCTs. OCT2 expression is lower in female rat kidneys compared with males, resulting in a lower urinary excretion rate for metformin (Ma et al. 2016). The reduced elimination of metformin could lead to higher metformin accumulation in other organ systems of females, underscoring some of the aforementioned sex-dependent complications. Daily administration of testosterone (10 mg/0.1 ml of olive oil) for 7 days increased OCT2 mRNA levels, OCT2 protein expression, and OCT2 transport activity in both males (significant p < 0.05) and females (not significant p > 0.05) in Wistar rats. Conversely, daily administration of 17β-estradiol (1 mg/0.1 ml of olive oil) for 7 days decreased transport activity of the OCT2 protein in male rats only (Urakami et al. 1999). Given that sex hormone levels vary among men and women across the lifespan, it is possible that metformin effects could be age-dependent. However, metformin is also the substrate for MATE1 and MATE2. Estrogen therapy in ovariectomized mice decreased the expression on MATE2 transporters (Meetam et al. 2009b). In contrary to above discussion, a recent study found no sex differences in metformin accumulation in the kidney, liver, brain, intestine, heart, and lung within 2 h after a single dose of metformin (Ma et al. 2016). However, the time point of 2 h may not be a good indicator for chronically prescribed drug like metformin.
We determined the expression of OCT2 in the hippocampus of young (3 months), middle age (12 months), and old (22 months) male mice. We observed that OCT2 expression decreased over age in the hippocampus (Supplemental Fig. 2). Further, we assessed the OCT2 mRNA expression variation using qPCR in adult male and female mice (6 months). We noticed that the female cortex and hippocampus had higher OCT2 mRNA expression compared with male. However, when checked the translation of this OCT2 mRNA to proteins, we observed that at the age of 6 months, the OCT2a and b protein expression in hippocampus did not vary in male and female. This could be due to the inhibition of translation of mRNA to protein by higher estrogen in female mice at a younger age when compared with age-matched male mice. However, the translation inhibition may be reversed in an older female with lower levels of inhibitory estrogen.
In rats, metformin shows variable accumulation in the brain depending on the duration of metformin therapy (Labuzek et al. 2010—Table 2, columns 1 and 3) (Labuzek et al. 2010). At 2 h following a single dose of metformin, there was 2.5-fold increased concentration in the CSF, and 1.3-fold increased concentration in the cerebellum, as compared with plasma. Meanwhile, the hippocampus and frontal cortex had lower concentrations than plasma, with 0.3- and 0.5-fold differences respectively (Labuzek et al. 2010). At 3-week treatment, there was a 2.5- to 4-fold increased metformin concentration in the hippocampus, cerebellum, and frontal cortex, as well as a 14.5-fold increased concentration in the CSF, as compared with plasma (Labuzek et al. 2010). Regardless of the duration of metformin therapy, CSF always had the highest concentration of metformin, which is likely due to having the highest expression of OCT2 transporters. For diabetic and pre-diabetic patients, metformin is routinely administered chronically for months to years. It is possible that the long-term use could likely lead to rising accumulation of metformin in the brain, ultimately impacting the brain function in a time-dependent manner (Thangthaeng et al. 2017; Wenjun Li et al. 2019). We observed sex-dependent negative impact of metformin on short-term memory, cognitive flexibility, and delayed reversal in non-diabetic young adult C57BL6 male mice only and no such effect in females (Supplemental Fig. 3). In the male mice, although metformin enhanced locomotor, balanced performance, and induced anxiolytic effect, metformin impaired both short-term cognition, cognitive flexibility, and long-term spatial cognitive function (Wenjun Li et al. 2019).
Aging affects metformin
Aging is associated with a physiological functional decline in various organ systems, including the hepatorenal metabolic-excretory system, psychomotor, and cognitive brain function (Costa et al. 2013; Shetty et al. 2014). With aging, sex hormonal decline occurs in both men and women (Bungum et al. 2011; Resnick et al. 2017). Sex hormones affect the expression and function of OCTs. Castrated rats showed OCT function loss, which is restored with the administration of testosterone (Meetam et al. 2009a). In male C57BL6 mice, OCTs are downregulated in the aging brain, as shown by lower mRNA and protein expression and activity (Wu et al. 2015). Contrastingly, the OCT function in ovariectomized mice was higher when compared with control and estrogen supplementation (Meetam et al. 2009b). In females, the sex hormone profile changes drastically across the lifespan. Near menopause initiation, there is a sudden drop in the estrogen:androgen ratio. This phase is dubbed as post-menopausal hyperandrogenism as characterized by higher levels of dehydroepiandrosterone and testosterone. This would necessarily mean that peri- and post-menopausal females may have a sudden increase in OCT expression and activity, further leading to significant differences in metformin bioaccumulation throughout the body and urinary excretion rates. Therefore, the age-associated altered sex steroids might affect the pharmacokinetics of metformin differently in men versus women requiring the specific calculation of the optimum dose to avoid any deleterious effects.
The direct action of metformin versus secondary glucose-lowering effect on cognition
Are the effects of metformin on longevity-related benefits universal? This critical question is thoroughly discussed by Konopka et al. 2019 and further supported by other studies (Konopka and Miller 2019). Metformin exerts antagonistic pleiotropy dependent on the presence or absence of pre-existing disease conditions such as T2DM, Alzheimer’s, aging, Huntington’s, Parkinson’s disease, or cancers or concomitant interventions such as exercise (Table 2A) (Konopka et al. 2019; Konopka and Miller 2019; Walton et al. 2019). The impact of metformin, whether it is due to control of sugar levels or more direct, is ambiguous.
Alteration in glucose levels has an impact on cognition in both males and females across the lifespan. Both acute and chronic blood sugar aberrations lead to cognitive impairment (Davis et al. 1996; Draelos et al. 1995; Frier 2001; Gonder-Frederick et al. 1994; Ryan and Geckle 2000; Sheen and Sheu 2016; Sommerfield et al. 2004). A homeostatic normoglycemic range between 4 and 15 mmol/l is necessary to achieve optimum cognitive and psychomotor function (Cox et al. 2005). This range could represent an analog similar to routine physiological parameters, such as blood pressure and body temperature. Thus, a metformin-induced normoglycemic range higher or lower than the endogenous, homeostatic limits may be a critical determinant of altered cognitive function observed in previous studies.
It is possible that the metformin-associated increased risk of dementia, cognitive loss, and AD progression is not related to glucose controlling effect. In a mechanistic study using cell line and primary neurons, Chen et al. 2009 found that metformin increased Aβ generation by upregulating beta-secretase 1 (BACE1) promoter activity in an AMPK-dependent manner (Chen et al. 2009). Similar studies performed on non-diabetic AD mouse models (APP/PS1) found the opposite effect by improving cognition and memory benefits of short-term and long-term metformin therapy. These cognitive benefits were unrelated to glycemic control and were due to the prevention of both amyloid plaque formation and tau phosphorylation via multiple AMPK-dependent pathways (Matthes et al. 2018; Ou et al. 2018), which suggest that the experimental design and model plays a critical role in outcome and conclusion.
Microvascular injury can lead to cerebrovascular inflammation and lead to vascular cognitive impairment (Fulop et al. 2018). Co-existing conditions such as hypertension might induce microvascular injury leading to an acceleration of AD pathophysiology as well as VCI (Csiszar et al. 2017). Metformin has been found to improve cognitive function in patients with non-dementia VCI, abnormal glucose metabolism by improving insulin resistance index (Lin et al. 2018). Similarly, cerebral ischemia leads to cognitive alterations by fluctuating brain energy metabolism via AMPK activation. It is currently unclear whether this activity is protective or deleterious for neural tissues, and if AMPK manipulation could be helpful (Pineda-Ramirez et al. 2017). Metformin has been examined as a means to affect the ischemia-related cognitive decline. One study found that sub-chronic metformin pre-treatment enhanced novel object recognition performance in a rat model of forebrain ischemia (Ashabi et al. 2014). Another study demonstrated that long-term pre-treatment with metformin in global cerebral ischemia-induced upregulation of the AMPK-BDNF-P70S6K pathway, subsequently enhancing learning and memory (Ghadernezhad et al. 2016). It was also shown that 7-day metformin pre-treatment (10 mg/kg) significantly reduced AMPK activation in ischemic brains. This effect was not observed with other dosages or duration of administration. These findings suggest an association between metformin, AMPK, and neuroprotection in cerebral ischemia, but also demonstrate a need to optimize intervention initiation time, duration, and the dose of metformin (Deng et al. 2016).
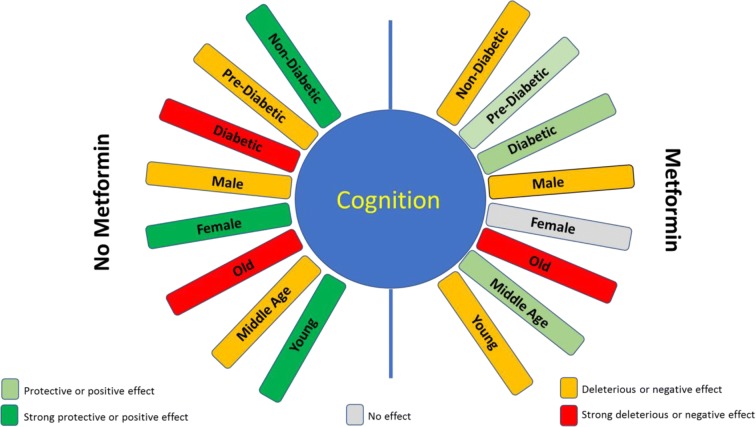
Conclusion: (Fig. 2)
Fig. 2.
Multifactorial interaction on the effect of metformin on cognition. The effect of metformin on cognition is mediated by a variety of factors, including age, sex, blood sugar levels, and associated disease condition. The color-coding in this predictive model is a summary of existing evidence on known mediators of metformin-induced cognitive changes. A more substantial clinical investigation should aim to extend these data to achieve a more accurate precision medicine approach to metformin pharmacotherapy
Based on this available information, it is clear that metformin affects longevity and neuro-cognition via AMPK-dependent and -independent mechanisms. Although various AMPK-independent mechanisms have been described over many years, these have not been well scrutinized in relation to cognition (Viollet et al. 2012). These effects of metformin were inconsistent and varied depending upon species (mice, rat, and human), sex, age, metformin dose, treatment duration, and associated pathological conditions (diabetes, stroke, AD, etc.). Figure 2 is not perfect and perhaps at the most primitive state just to put forth an idea that such a design and some form of the equation involving multiple factors may be useful in the future. There is a need for more translational studies that address these factors, to develop a comprehensive metformin dosing formula to achieve optimum anti-aging benefits and mitigate side effects. The differential and controversial impact of metformin on brain function described across the literature warrants a precision medicine-based approach to establish novel individualized therapy guidelines.
Electronic supplementary material
(DOCX 850 kb)
Acknowledgements
We have to express our appreciation to Ali Winters, Lab Assistant, UNTHSC for sharing her pearls of artistic wisdom with us while creating the figures of this manuscript.
Funding information
This work was financially supported by the National Institutes of Health grants 1R21NS087209-01A1 (SY), R01NS088596 (SY), and American Heart Association Grant 17POST33670981 (KC).
Footnotes
Highlights
1. Metformin affects cognition.
2. Sex influences metformin pharmacokinetics and associated cognitive alterations.
3. Organic cation transporters and AMP-activated kinase might hold a key for metformin-associated cognitive alterations.
4. Age is a critical factor affecting metformin-associated cognitive alterations.
5. Metformin may inverse the pathological conditions induced cognitive variations.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aatsinki SM, Buler M, Salomaki H, Koulu M, Pavek P, Hakkola J. Metformin induces PGC-1alpha expression and selectively affects hepatic PGC-1alpha functions. Br J Pharmacol. 2014;171:2351–2363. doi: 10.1111/bph.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Mahmood Z, Javed A, Hashmi SN, Zerr I, Zafar S, Zahid S. Effect of metformin on adult hippocampal neurogenesis: comparison with donepezil and links to cognition. J Mol Neurosci. 2017;62:88–98. doi: 10.1007/s12031-017-0915-z. [DOI] [PubMed] [Google Scholar]
- Algire C, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila) 2012;5:536–543. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- Allard JS, Perez EJ, Fukui K, Carpenter P, Ingram DK, de Cabo R. Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behav Brain Res. 2016;301:1–9. doi: 10.1016/j.bbr.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzoubi KH, Khabour OF, Al-Azzam SI, Tashtoush MH, Mhaidat NM. Metformin eased cognitive impairment induced by chronic L-methionine administration: potential role of oxidative stress. Curr Neuropharmacol. 2014;12:186–192. doi: 10.2174/1570159X11666131120223201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, et al. Metformin slows down aging and extends life span of female SHR mice. Cell cycle (Georgetown, Tex) 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, et al. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle. 2010;9:188–197. doi: 10.4161/cc.9.1.10407. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging. 2010;2:945–958. doi: 10.18632/aging.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011;3:148–157. doi: 10.18632/aging.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnetz L, Ekberg NR, Alvarsson M. Sex differences in type 2 diabetes: focus on disease course and outcomes. Diabetes, Metab Syndrome Obes : Targets Ther. 2014;7:409–420. doi: 10.2147/DMSO.S51301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1alpha pathway. Metab Brain Dis. 2014;29:47–58. doi: 10.1007/s11011-013-9475-2. [DOI] [PubMed] [Google Scholar]
- Bannister CA, et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014;16:1165–1173. doi: 10.1111/dom.12354. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23:1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38:33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- Bayliss JA, Lemus MB, Santos VV, Deo M, Davies JS, Kemp BE, Elsworth JD, Andrews ZB. Metformin prevents nigrostriatal dopamine degeneration independent of AMPK activation in dopamine neurons. PLoS One. 2016;11:e0159381. doi: 10.1371/journal.pone.0159381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemans E, Hart HE, Rutten GE, Cuellar Renteria VG, Kooijman-Buiting AM, Beulens JW. Cobalamin status and its relation with depression, cognition and neuropathy in patients with type 2 diabetes mellitus using metformin. Acta Diabetol. 2015;52:383–393. doi: 10.1007/s00592-014-0661-4. [DOI] [PubMed] [Google Scholar]
- Biradar V, Moran JL, Peake SL, Peter JV. Metformin-associated lactic acidosis (MALA): clinical profile and outcomes in patients admitted to the intensive care unit. Crit Care Resusc. 2010;12:191–195. [PubMed] [Google Scholar]
- Bridges HR, Jones AJ, Pollak MN, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 2014;462:475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungum L, Jacobsson AK, Rosen F, Becker C, Yding Andersen C, Guner N, Giwercman A. Circadian variation in concentration of anti-Mullerian hormone in regularly menstruating females: relation to age, gonadotrophin and sex steroid levels. Hum Reprod. 2011;26:678–684. doi: 10.1093/humrep/deq380. [DOI] [PubMed] [Google Scholar]
- Cabreiro F, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2015) Age-adjusted rates of diagnosed diabetes per 100 civilian, non-institutionalized population, by sex, United States, 1980–2014
- Chen Y, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Lin CH, Tsai YW, Tsai CJ, Chou PH, Lan TH. Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol Ser, Biol Sci Med Sci. 2014;69:1299–1305. doi: 10.1093/gerona/glu073. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Wray LA. Gender differences in functional limitations in adults living with type 2 diabetes: biobehavioral and psychosocial mediators. Ann Behav Med: Publ Soc Behav Med. 2011;41:71–82. doi: 10.1007/s12160-010-9226-0. [DOI] [PubMed] [Google Scholar]
- Cho K, Chung JY, Cho SK, Shin HW, Jang IJ, Park JW, Yu KS, Cho JY. Antihyperglycemic mechanism of metformin occurs via the AMPK/LXRalpha/POMC pathway. Sci Rep. 2015;5:8145. doi: 10.1038/srep08145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MN, Bolivar VJ, McFadyen MP, Flaherty L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav Neurosci. 2002;116:600–611. doi: 10.1037/0735-7044.116.4.600. [DOI] [PubMed] [Google Scholar]
- Costa E et al. (2013) Aging is associated with impaired renal function, INF-gamma induced inflammation and with alterations in Iron regulatory proteins gene expression aging and disease 5:356-365 doi:10.14366/AD.2014.0500356 [doi] [DOI] [PMC free article] [PubMed]
- Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A, Grimm KJ, Clarke WL. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care. 2005;28:71–77. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- Csiszar A, et al. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 2017;39:359–372. doi: 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EA, Soong SA, Byrne GC, Jones TW. Acute hyperglycaemia impairs cognitive function in children with IDDM. J Pediatr Endocrinol Metab: JPEM. 1996;9:455–461. doi: 10.1515/JPEM.1996.9.4.455. [DOI] [PubMed] [Google Scholar]
- De Haes W, et al. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci U S A. 2014;111:E2501–E2509. doi: 10.1073/pnas.1321776111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T, et al. Pre-stroke metformin treatment is Neuroprotective involving AMPK reduction. Neurochem Res. 2016;41:2719–2727. doi: 10.1007/s11064-016-1988-8. [DOI] [PubMed] [Google Scholar]
- Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research G HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: a randomized clinical trial. Diabetes Care. 2015;38:51–58. doi: 10.2337/dc14-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draelos MT, Jacobson AM, Weinger K, Widom B, Ryan CM, Finkelstein DM, Simonson DC. Cognitive function in patients with insulin-dependent diabetes mellitus during hyperglycemia and hypoglycemia. Am J Med. 1995;98:135–144. doi: 10.1016/S0002-9343(99)80397-0. [DOI] [PubMed] [Google Scholar]
- Duca Frank A, Côté Clémence D, Rasmussen Brittany A, Zadeh-Tahmasebi Melika, Rutter Guy A, Filippi Beatrice M, Lam Tony K T. Metformin activates a duodenal Ampk–dependent pathway to lower hepatic glucose production in rats. Nature Medicine. 2015;21(5):506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi I, Delrobaee F, Bahmani M, Shamsizadeh A, Allahtavakoli M. The effect of the anti-diabetic drug metformin on behavioral manifestations associated with ovariectomy in mice. Neurosci Lett. 2019;690:95–98. doi: 10.1016/j.neulet.2018.10.024. [DOI] [PubMed] [Google Scholar]
- Fatt M, Hsu K, He L, Wondisford F, Miller FD, Kaplan DR, Wang J. Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Reports. 2015;5:988–995. doi: 10.1016/j.stemcr.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frier BM (2001) Hypoglycaemia and cognitive function in diabetes International journal of clinical practiceSupplement (123):30-37 [PubMed]
- Fulop GA, et al. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabryel B, Liber S. Metformin limits apoptosis in primary rat cortical astrocytes subjected to oxygen and glucose deprivation. Folia Neuropathol. 2018;56:328–336. doi: 10.5114/fn.2018.80866. [DOI] [PubMed] [Google Scholar]
- Ge XH, et al. Metformin protects the brain against ischemia/reperfusion injury through PI3K/Akt1/JNK3 signaling pathways in rats. Physiol Behav. 2017;170:115–123. doi: 10.1016/j.physbeh.2016.12.021. [DOI] [PubMed] [Google Scholar]
- Ghadernezhad N, Khalaj L, Pazoki-Toroudi H, Mirmasoumi M, Ashabi G. Metformin pretreatment enhanced learning and memory in cerebral forebrain ischaemia: the role of the AMPK/BDNF/P70SK signalling pathway. Pharm Biol. 2016;54:2211–2219. doi: 10.3109/13880209.2016.1150306. [DOI] [PubMed] [Google Scholar]
- Goldberg R, et al. Lifestyle and metformin treatment favorably influence lipoprotein subfraction distribution in the diabetes prevention program. J Clin Endocrinol Metab. 2013;98:3989–3998. doi: 10.1210/jc.2013-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Aroda VR, Bluemke DA, Barrett-Connor E, Budoff M, Crandall JP, Dabelea D, Horton ES, Mather KJ, Orchard TJ, Schade D, Watson K, Temprosa M, Diabetes Prevention Program Research Group Effect of long-term metformin and lifestyle in the diabetes prevention program and its outcome study on coronary artery calcium. Circulation. 2017;136:52–64. doi: 10.1161/CIRCULATIONAHA.116.025483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonder-Frederick LA, Cox DJ, Driesen NR, Ryan CM, Clarke WL. Individual differences in neurobehavioral disruption during mild and moderate hypoglycemia in adults with IDDM. Diabetes. 1994;43:1407–1412. doi: 10.2337/diab.43.12.1407. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC (2007) Mortality trends in men and women with diabetes, 1971 to 2000 Ann Intern Med 147:149–155 doi:0000605-200708070-00167 [pii] [DOI] [PubMed]
- Guo M, Mi J, Jiang QM, Xu JM, Tang YY, Tian G, Wang B. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2014;41:650–656. doi: 10.1111/1440-1681.12265. [DOI] [PubMed] [Google Scholar]
- Haffner S, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol. 2019;18:96. doi: 10.1186/s12933-019-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu Rev Nutr. 2014;34:31–55. doi: 10.1146/annurev-nutr-071812-161148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman MHT, et al. Two-year follow-up of 4 months metformin treatment vs. placebo in ST-elevation myocardial infarction: data from the GIPS-III RCT. Clin Res Cardiol. 2017;106:939–946. doi: 10.1007/s00392-017-1140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman Sheri J., Nelson Sandahl H., Marinac Catherine R., Natarajan Loki, Parker Barbara A., Patterson Ruth E. The effects of weight loss and metformin on cognition among breast cancer survivors: Evidence from the Reach for Health study. Psycho-Oncology. 2019;28(8):1640–1646. doi: 10.1002/pon.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas D, Fornes-Ferrer V, Gomez-Escribano AP, Sequedo MD, Peiro C, Millan JM, Vazquez-Manrique RP. Metformin intake associates with better cognitive function in patients with Huntington’s disease. PLoS One. 2017;12:e0179283. doi: 10.1371/journal.pone.0179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnholt MC, Blumrich EM, Waagepetersen HS, Dringen R. The antidiabetic drug metformin decreases mitochondrial respiration and tricarboxylic acid cycle activity in cultured primary rat astrocytes. J Neurosci Res. 2017;95:2307–2320. doi: 10.1002/jnr.24050. [DOI] [PubMed] [Google Scholar]
- Inyang KE, Szabo-Pardi T, Wentworth E, McDougal TA, Dussor G, Burton MD, Price TJ. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol Res. 2019;139:1–16. doi: 10.1016/j.phrs.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakovic A, et al. Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci. 2007;64:1290–1302. doi: 10.1007/s00018-007-7080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav KS, Dungan CM, Williamson DL. Metformin limits ceramide-induced senescence in C2C12 myoblasts. Mech Ageing Dev. 2013;134:548–559. doi: 10.1016/j.mad.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Jang S, Park SH. Antidiabetic drug metformin protects neuronal cells against quinolinic acid-induced excitotoxicity by decreasing intracellular calcium. Chonnam Med J. 2018;54:24–30. doi: 10.4068/cmj.2018.54.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, et al. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun. 2014;40:131–142. doi: 10.1016/j.bbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Karim A, Slater M, Bradford D, Schwartz L, Zhao Z, Cao C, Laurent A. Oral antidiabetic drugs: bioavailability assessment of fixed-dose combination tablets of pioglitazone and metformin. Effect of body weight, gender, and race on systemic exposures of each drug. J Clin Pharmacol. 2007;47:37–47. doi: 10.1177/0091270006293755. [DOI] [PubMed] [Google Scholar]
- Katila N, et al. Metformin lowers alpha-synuclein phosphorylation and upregulates neurotrophic factor in the MPTP mouse model of Parkinson’s disease. Neuropharmacology. 2017;125:396–407. doi: 10.1016/j.neuropharm.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Keshavarzi S, Kermanshahi S, Karami L, Motaghinejad M, Motevalian M, Sadr S. Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: the role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicology. 2019;72:74–84. doi: 10.1016/j.neuro.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Khattar D, Khaliq F, Vaney N, Madhu SV. Is metformin-induced vitamin B12 deficiency responsible for cognitive decline in type 2 diabetes? Indian J Psychol Med. 2016;38:285–290. doi: 10.4103/0253-7176.185952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr SA, Elmelgy AA, El-Kharashi OA, Abd-Alkhalek HA, Louka ML, Sallam HA, Aboul-Fotouh S. Metformin potentiates cognitive and antidepressant effects of fluoxetine in rats exposed to chronic restraint stress and high fat diet: potential involvement of hippocampal c-Jun repression Naunyn Schmiedebergs. Arch Pharmacol. 2018;391:407–422. doi: 10.1007/s00210-018-1466-8. [DOI] [PubMed] [Google Scholar]
- Kickstein E, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A. 2010;107:21830–21835. doi: 10.1073/pnas.0912793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig AM, et al. Effects of the insulin sensitizer metformin in Alzheimer disease: pilot data from a randomized placebo-controlled crossover study Alzheimer dis. Assoc Disord. 2017;31:107–113. doi: 10.1097/WAD.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Miller BF. Taming expectations of metformin as a treatment to extend healthspan. Geroscience. 2019;41:101–108. doi: 10.1007/s11357-019-00057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, Musci RV, Safairad OD, Linden MA, Biela LM, Bailey SM, Hamilton KL, Miller BF. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019;18:e12880. doi: 10.1111/acel.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BK, Kim LK, Lee JY, Moon MK. Taking metformin and cognitive function change in older patients with diabetes. Geriatr Gerontol Int. 2019;19:755–761. doi: 10.1111/ggi.13692. [DOI] [PubMed] [Google Scholar]
- Kooy A, de Jager J, Lehert P, Bets D, Wulffele MG, Donker AJ, Stehouwer CD. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616–625. doi: 10.1001/archinternmed.2009.20. [DOI] [PubMed] [Google Scholar]
- Krysiak Robert, Szkróbka Witold, Okopień Bogusław. The Impact of Testosterone on Metformin Action on Hypothalamic‐Pituitary‐Thyroid Axis Activity in Men: A Pilot Study. The Journal of Clinical Pharmacology. 2019;60(2):164–171. doi: 10.1002/jcph.1507. [DOI] [PubMed] [Google Scholar]
- Kuan YC, Huang KW, Lin CL, Hu CJ, Kao CH. Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;79:77–83. doi: 10.1016/j.pnpbp.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Labuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopien B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacological Reports : PR. 2010;62:956–965. doi: 10.1016/S1734-1140(10)70357-1. [DOI] [PubMed] [Google Scholar]
- Le S, Lee GC. Emerging trends in metformin prescribing in the United States from 2000 to 2015. Clin Drug Investig. 2019;39:757–763. doi: 10.1007/s40261-019-00799-0. [DOI] [PubMed] [Google Scholar]
- Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexis CP, et al. Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: the GIPS-III randomized clinical trial. JAMA. 2014;311:1526–1535. doi: 10.1001/jama.2014.3315. [DOI] [PubMed] [Google Scholar]
- Lien F, et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J Clin Invest. 2014;124:1037–1051. doi: 10.1172/JCI68815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wang K, Ma C, Wang X, Gong Z, Zhang R, Zang D, Cheng Y. Evaluation of metformin on cognitive improvement in patients with non-dementia vascular cognitive impairment and abnormal glucose metabolism. Front Aging Neurosci. 2018;10:227. doi: 10.3389/fnagi.2018.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Fan Z, Edgerton SM, Yang X, Lind SE, Thor AD. Potent anti-proliferative effects of metformin on trastuzumab-resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactions. Cell Cycle. 2011;10:2959–2966. doi: 10.4161/cc.10.17.16359. [DOI] [PubMed] [Google Scholar]
- Lu DY, Tang CH, Chen YH, Wei IH. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem. 2010;110:697–705. doi: 10.1002/jcb.22580. [DOI] [PubMed] [Google Scholar]
- Lu J, et al. Activation of AMPK by metformin inhibits TGF-beta-induced collagen production in mouse renal fibroblasts. Life Sci. 2015;127:59–65. doi: 10.1016/j.lfs.2015.01.042. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Ma Y, Christophi CA, Florez H, Golden SH, Hazuda H, Crandall J, Venditti E, Watson K, Jeffries S, Manly JJ, Pi-Sunyer FX, Diabetes Prevention Program Research Group Metformin, lifestyle intervention, and cognition in the diabetes prevention program outcomes study. Diabetes Care. 2017;40:958–965. doi: 10.2337/dc16-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MR, et al. Impact of sex on the heart’s metabolic and functional responses to diabetic therapies am. J Physiol Heart Circ Physiol. 2013;305:H1584–H1591. doi: 10.1152/ajpheart.00420.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, Hoyt KR. Metformin therapy in a transgenic mouse model of Huntington’s disease. Neurosci Lett. 2007;411:98–103. doi: 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Ma YR, et al. Gender-related differences in the expression of organic Cation transporter 2 and its role in urinary excretion of metformin in rats. Eur J Drug Metab Pharmacokinet. 2016;41:559–565. doi: 10.1007/s13318-015-0278-1. [DOI] [PubMed] [Google Scholar]
- Mamo JC, et al. Probucol prevents blood-brain barrier dysfunction and cognitive decline in mice maintained on pro-diabetic diet. Diab Vasc Dis Res. 2019;16:87–97. doi: 10.1177/1479164118795274. [DOI] [PubMed] [Google Scholar]
- Mao JH, et al. Identification of genetic factors that modify motor performance and body weight using collaborative cross mice. Sci Rep. 2015;5:16247. doi: 10.1038/srep16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes F, et al. Inhibition of the MID1 protein complex: a novel approach targeting APP protein synthesis cell death. Discov. 2018;4:4. doi: 10.1038/s41420-017-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadyen MP, Kusek G, Bolivar VJ, Flaherty L. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–219. doi: 10.1034/j.1601-183X.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- McNeilly AD, Williamson R, Balfour DJ, Stewart CA, Sutherland C. A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia. 2012;55:3061–3070. doi: 10.1007/s00125-012-2686-y. [DOI] [PubMed] [Google Scholar]
- Meetam P, Srimaroeng C, Soodvilai S, Chatsudthipong V. Regulatory role of testosterone in organic cation transport: in vivo and in vitro studies. Biol Pharm Bull. 2009;32:982–987. doi: 10.1248/bpb.32.982. [DOI] [PubMed] [Google Scholar]
- Meetam P, Srimaroeng C, Soodvilai S, Chatsudthipong V. Role of estrogen in renal handling of organic cation, tetraethylammonium: in vivo and in vitro studies. Biol Pharm Bull. 2009;32:1968–1972. doi: 10.1248/bpb.32.1968. [DOI] [PubMed] [Google Scholar]
- Moiseeva O, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell. 2013;12:489–498. doi: 10.1111/acel.12075. [DOI] [PubMed] [Google Scholar]
- Moore EM, et al. Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36:2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa DK, Ismail CA, Ghareeb DA. Differential metformin dose-dependent effects on cognition in rats: role of Akt. Psychopharmacology. 2016;233:2513–2524. doi: 10.1007/s00213-016-4301-2. [DOI] [PubMed] [Google Scholar]
- Nair V, Sreevalsan S, Basha R, Abdelrahim M, Abudayyeh A, Rodrigues Hoffman A, Safe S. Mechanism of metformin-dependent inhibition of mammalian target of rapamycin (mTOR) and Ras activity in pancreatic cancer: role of specificity protein (Sp) transcription factors. J Biol Chem. 2014;289:27692–27701. doi: 10.1074/jbc.M114.592576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimer’s Dis: JAD. 2014;41:61–68. doi: 10.3233/JAD-131901. [DOI] [PubMed] [Google Scholar]
- Nilsson PM, Theobald H, Journath G, Fritz T. Gender differences in risk factor control and treatment profile in diabetes: a study in 229 swedish primary health care centres. Scand J Prim Health Care. 2004;22:27–31. doi: 10.1080/02813430310003264. [DOI] [PubMed] [Google Scholar]
- Novelle MG, Ali A, Dieguez C, Bernier M, de Cabo R. Metformin: a hopeful promise in aging research. Cold Spring Harb Perspect Med. 2016;6:a025932. doi: 10.1101/cshperspect.a025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkaby AR, Cho K, Cormack J, Gagnon DR, Driver JA. Metformin vs sulfonylurea use and risk of dementia in US veterans aged >/=65 years with diabetes. Neurology. 2017;89:1877–1885. doi: 10.1212/WNL.0000000000004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Z, et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun. 2018;69:351–363. doi: 10.1016/j.bbi.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Park JW, Lee JH, Park YH, Park SJ, Cheon JH, Kim WH, Kim TI. Sex-dependent difference in the effect of metformin on colorectal cancer-specific mortality of diabetic colorectal cancer patients. World J Gastroenterol. 2017;23:5196–5205. doi: 10.3748/wjg.v23.i28.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Revuelta BI, Hettich MM, Ciociaro A, Rotermund C, Kahle PJ, Krauss S, Di Monte DA. Metformin lowers Ser-129 phosphorylated alpha-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death Dis. 2014;5:e1209. doi: 10.1038/cddis.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Ramírez Narayana, Gutiérrez Aguilar Germán Fernando, Espinoza-Rojo Mónica, Aguilera Penélope. Current evidence for AMPK activation involvement on resveratrol-induced neuroprotection in cerebral ischemia. Nutritional Neuroscience. 2017;21(4):229–247. doi: 10.1080/1028415X.2017.1284361. [DOI] [PubMed] [Google Scholar]
- Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012;91:409–414. doi: 10.1016/j.lfs.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Pongwecharak J, Tengmeesri N, Malanusorn N, Panthong M, Pawangkapin N. Prescribing metformin in type 2 diabetes with a contraindication: prevalence and outcome. Pharm World Sci. 2009;31:481–486. doi: 10.1007/s11096-009-9303-2. [DOI] [PubMed] [Google Scholar]
- Potts MB, Lim DA. An old drug for new ideas: metformin promotes adult neurogenesis and spatial memory formation. Cell Stem Cell. 2012;11:5–6. doi: 10.1016/j.stem.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss D, et al. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:116–124. doi: 10.1016/S2213-8587(13)70152-9. [DOI] [PubMed] [Google Scholar]
- Price NL, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protic D, Aydin EY, Tassone F, Tan MM, Hagerman RJ, Schneider A (2019) Cognitive and behavioral improvement in adults with fragile X syndrome treated with metformin-two cases. Mol Genet Genomic Med:e745. 10.1002/mgg3.745 [DOI] [PMC free article] [PubMed]
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, Ellenberg SS, Gill TM, Shumaker SA, Pleasants DD, Barrett-Connor E, Bhasin S, Cauley JA, Cella D, Crandall JP, Cunningham GR, Ensrud KE, Farrar JT, Lewis CE, Molitch ME, Pahor M, Swerdloff RS, Cifelli D, Anton S, Basaria S, Diem SJ, Wang C, Hou X, Snyder PJ. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA. 2017;317:717–727. doi: 10.1001/jama.2016.21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CM, Geckle MO. Circumscribed cognitive dysfunction in middle-aged adults with type 2 diabetes. Diabetes Care. 2000;23:1486–1493. doi: 10.2337/diacare.23.10.1486. [DOI] [PubMed] [Google Scholar]
- Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisho Y. Metformin and inflammation: its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets. 2015;15:196–205. doi: 10.2174/1871530315666150316124019. [DOI] [PubMed] [Google Scholar]
- Sesen J, Dahan P, Scotland SJ, Saland E, Dang VT, Lemarié A, Tyler BM, Brem H, Toulas C, Cohen-Jonathan Moyal E, Sarry JE, Skuli N. Metformin inhibits growth of human glioblastoma cells and enhances therapeutic response. PLoS One. 2015;10:e0123721. doi: 10.1371/journal.pone.0123721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen YJ, Sheu WH. Association between hypoglycemia and dementia in patients with type 2 diabetes. Diabetes Res Clin Pract. 2016;116:279–287. doi: 10.1016/j.diabres.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Shetty RA, Ikonne US, Forster MJ, Sumien N. Coenzyme Q10 and alpha-tocopherol reversed age-associated functional impairments in mice. Exp Gerontol. 2014;58:208–218. doi: 10.1016/j.exger.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C, Foley A, Partridge L. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS One. 2012;7:e47699. doi: 10.1371/journal.pone.0047699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L., Elam C. F., Mattison J. A., Lane M. A., Roth G. S., Ingram D. K., Allison D. B. Metformin Supplementation and Life Span in Fischer-344 Rats. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65A(5):468–474. doi: 10.1093/gerona/glq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfield AJ, Deary IJ, Frier BM. Acute hyperglycemia alters mood state and impairs cognitive performance in people with type 2 diabetes. Diabetes Care. 2004;27:2335–2340. doi: 10.2337/diacare.27.10.2335. [DOI] [PubMed] [Google Scholar]
- Song YM, et al. Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway. Autophagy. 2015;11:46–59. doi: 10.4161/15548627.2014.984271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Strong R, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Shinichi, Iizumi Takuya, Mashima Kyoko, Abe Takato, Suzuki Norihiro. Roles and Regulation of Ketogenesis in Cultured Astroglia and Neurons Under Hypoxia and Hypoglycemia. ASN Neuro. 2014;6(5):175909141455099. doi: 10.1177/1759091414550997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangthaeng N, Rutledge M, Wong JM, Vann PH, Forster MJ, Sumien N. Metformin impairs spatial memory and visual acuity in old male mice. Aging Dis. 2017;8:17–30. doi: 10.14336/AD.2016.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352:854–865 [PubMed]
- Urakami Y, Nakamura N, Takahashi K, Okuda M, Saito H, Hashimoto Y, Inui K. Gender differences in expression of organic cation transporter OCT2 in rat kidney. FEBS Lett. 1999;461:339–342. doi: 10.1016/S0014-5793(99)01491-X. [DOI] [PubMed] [Google Scholar]
- Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, et al. Adherence to preventive medications: predictors and outcomes in the diabetes prevention program. Diabetes Care. 2006;29:1997–2002. doi: 10.2337/dc06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton RG, et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: a randomized, double-blind, placebo-controlled, multicenter trial: the MASTERS trial. Aging Cell. 2019;18:e13039. doi: 10.1111/acel.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang L, Zhou J, Qin A, Chen Z. The protective effect of formononetin on cognitive impairment in streptozotocin (STZ)-induced diabetic mice biomed. Pharmacother. 2018;106:1250–1257. doi: 10.1016/j.biopha.2018.07.063. [DOI] [PubMed] [Google Scholar]
- Wang S, et al. Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic. Neuropathy Diabetes. 2018;67:98–109. doi: 10.2337/db17-0503. [DOI] [PubMed] [Google Scholar]
- Li Wenjun, Chaudhari Kiran, Shetty Ritu, Winters Ali, Gao Xiaofei, Hu Zeping, Ge Woo-Ping, Sumien Nathalie, Forster Michael, Liu Ran, Yang Shao-Hua. Metformin Alters Locomotor and Cognitive Function and Brain Metabolism in Normoglycemic Mice. Aging and disease. 2019;10(5):949. doi: 10.14336/AD.2019.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus A, Blumrich EM, Dringen R. The antidiabetic drug metformin stimulates glycolytic lactate production in cultured primary rat astrocytes. Neurochem Res. 2017;42:294–305. doi: 10.1007/s11064-015-1733-8. [DOI] [PubMed] [Google Scholar]
- Wu KC, Lu YH, Peng YH, Tsai TF, Kao YH, Yang HT, Lin CJ. Decreased expression of organic cation transporters, Oct1 and Oct2, in brain microvessels and its implication to MPTP-induced dopaminergic toxicity in aged mice. J Cereb Blood Flow Metab. 2015;35:37–47. doi: 10.1038/jcbfm.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Han C, Wang G, Waddington JL, Zheng L, Zhen X. Activation of AMPK/mTORC1-mediated autophagy by metformin reverses Clk1 deficiency-sensitized dopaminergic neuronal death. Mol Pharmacol. 2017;92:640–652. doi: 10.1124/mol.117.109512. [DOI] [PubMed] [Google Scholar]
- Ying MA, Maruschak N, Mansur R, Carvalho AF, Cha DS, McIntyre RS. Metformin: repurposing opportunities for cognitive and mood dysfunction. CNS Neurol Disord Drug Targets. 2014;13:1836–1845. doi: 10.2174/1871527313666141130205514. [DOI] [PubMed] [Google Scholar]
- Zhang CS, et al. Metformin activates AMPK through the Lysosomal pathway cell. Metab. 2016;24:521–522. doi: 10.1016/j.cmet.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lin Y, Dai X, Fang W, Wu X, Chen X. Metformin treatment improves the spatial memory of aged mice in an APOE genotype-dependent manner. FASEB J. 2019;33:7748–7757. doi: 10.1096/fj.201802718R. [DOI] [PubMed] [Google Scholar]
- Zhao M, et al. Metformin administration prevents memory impairment induced by hypobaric hypoxia in rats. Behav Brain Res. 2019;363:30–37. doi: 10.1016/j.bbr.2019.01.048. [DOI] [PubMed] [Google Scholar]
- Zheng Z, et al. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61:217–228. doi: 10.2337/db11-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kavelaars A, Heijnen CJ. Metformin prevents Cisplatin-induced cognitive impairment and brain damage in mice. PLoS One. 2016;11:e0151890. doi: 10.1371/journal.pone.0151890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XC, et al. Chronic metformin preconditioning provides Neuroprotection via suppression of NF-kappaB-mediated inflammatory pathway in rats with permanent cerebral ischemia. Mol Neurobiol. 2015;52:375–385. doi: 10.1007/s12035-014-8866-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 850 kb)