Abstract
Increased inflammation associated with leaky gut is a major risk factor for morbidity and mortality in older adults; however, successful preventive and therapeutic strategies against these conditions are not available. In this study, we demonstrate that a human-origin Lactobacillus paracasei D3-5 strain (D3-5), even in the non-viable form, extends life span of Caenorhabditis elegans. In addition, feeding of heat-killed D3-5 to old mice (> 79 weeks) prevents high- fat diet-induced metabolic dysfunctions, decreases leaky gut and inflammation, and improves physical and cognitive functions. D3-5 feeding significantly increases mucin production, and proportionately, the abundance of mucin-degrading bacteria Akkermansia muciniphila also increases. Mechanistically, we show that the lipoteichoic acid (LTA), a cell wall component of D3-5, enhances mucin (Muc2) expression by modulating TLR-2/p38-MAPK/NF-kB pathway, which in turn reduces age-related leaky gut and inflammation. The findings indicate that the D3-5 and its LTA can prevent/treat age-related leaky gut and inflammation.
Electronic supplementary material
The online version of this article (10.1007/s11357-019-00137-4) contains supplementary material, which is available to authorized users.
Keywords: Aging, Cell wall, Cognition, Goblet cell, Inflammation, Leaky gut, Lipoteichoic acid, Metabolism, Mucin, Probiotics, Physical function
Introduction
The world’s population is rapidly aging with a concomitant increase in aging-related comorbidities like obesity, diabetes, cardiovascular diseases, cancer, infections, and cognitive decline (Hodes et al. 2016; Jaul and Barron 2017; Tabloski 2004). The mechanism(s) of how aging contributes to these health ailments are still unclear, but low-grade inflammation is often found to be higher in older adults and is a major risk factor for mortality and morbidity (Franceschi and Campisi 2014). The pathways leading to low-grade inflammation are not completely understood, but increased gut permeability (“leaky gut”) can allow non-selective diffusion of microbial and dietary antigens from the gut lumen into the lymphatic and blood circulation, which in turn promotes inflammatory responses locally (gut mucosa) and systemically (Konig et al. 2016; Ulluwishewa et al. 2011). Thus, effective strategies to reduce leaky gut and consequent inflammation are needed.
Emerging evidence suggests that detrimental perturbations in gut microbiome (dysbiosis) are associated with leaky gut, inflammation, and poor health outcomes in older adults. Gut permeability is tightly controlled by intestinal barriers including tight junction proteins and a mucus layer (Capaldo et al. 2017). The mucus layer functions as a physical barrier to separate gut microbes and host cells and protects from the abnormal leakage and invasion of pathogens and antigens into the circulation (Konig et al. 2016). The intestinal mucus layer covers the epithelial cells by making a viscoelastic gel layer, which is synthesized by goblet cells (Cornick et al. 2015). Intestinal goblet cells are interspersed between epithelial cells on the villi of intestinal lumen and constantly secrete mucins to form the mucus layer (Johansson 2012). Mucin 2 (Muc2) is the major mucin secreted from intestinal goblet cells and is known to protect leaky gut and invasion of microbes (Johansson 2012; Vemuri et al. 2018). Muc2 knockout (KO) mice develop colitis with a dramatically inflammatory gut mucosa (Van der Sluis et al. 2006) associated with a marked decrease in mucus layer’s thickness, thereby highlighting the importance of Muc2 in maintaining gut integrity and inflammation. A thin mucus layer may allow gut microbes to invade intestinal epithelial cells and to activate inflammatory responses in immune cells (Allaire et al. 2018). The mucus layer’s thickness is also dramatically reduced in the gut of older adults, which is also linked with increased leaky gut and inflammation (Elderman et al. 2017). Given the importance of the mucus layer in protection against microbial translocation, development of gut modulators to promote gut mucin production may be beneficial to ameliorate aging-related leaky gut and inflammation. We hypothesize that probiotics can be used to modulate gut microbiome and ameliorate aging-related leaky gut and inflammation.
Probiotics are live microorganisms that extend health benefits to the host/consumers upon administering in sufficient amounts (Hill et al. 2014). The most commonly used probiotics are non-spore-forming, gram-positive, lactic acid-producing bacteria such as specific Lactobacillus and Bifidobacterium strains that have been shown to have health-promoting effects against several human diseases (Azad et al. 2018; Landete et al. 2017; O'Toole et al. 2017). However, the precise mechanisms by which probiotics exert their benefits in aging remain largely unknown. Also, probiotics are thought to exert their beneficial effects as live cells; however, whether dead probiotics can also have beneficial effects is not well-known. Given the fact that commonly used probiotics like lactobacilli and bifidobacteria are Gram-positive, it has been hypothesized that bacterial molecules, such as bacterial cell wall constituents including peptidoglycan (PG) and lipoteichoic acid (LTA) and bacterial cytoplasm components including proteins and bacterial DNA, can have specific biological activities (Мokrozub et al. 2015).
Here, we demonstrate that LTA from the cell wall of a human-origin probiotic bacterium, L. paracasei strain D3-5 (called D3-5 hereafter), exhibits potent activity to stimulate mucin production and reduces aging-related leaky gut and inflammation, which in turn improves physical and cognitive functions in older mice. Mechanistically, LTA from D3-5 stimulated Muc2 expression in goblet cells via activation of toll-like receptor 2/p38-MAPK (TLR-2/p38-MAPK)/NF-κB pathway. Our results highlight the potential of a dead probiotic strain and its cell wall-derived LTA to prevent and/or treat aging-related leaky gut and inflammation and to improve overall cognitive and physical functions in older adults.
Results
Dead L. paracasei D3-5 feeding extends lifespan in Caenorhabditis (C.) elegans
The onset of aging is associated with decline in muscle mass, and in physical and cognitive functions. Wild-type C. elegans N2 is a widely used animal model in several anti-aging screening studies (Mack et al. 2019; Tissenbaum 2015b). In a previous study (Nagpal et al. 2018b), we isolated six probiotic Lactobacillus strains (viz. L. plantarum SK9, L. plantarum D6-2, L. paracasei D3-5, L. paracasei D10.4, L. rhamnosus D4-4, and L. rhamnosus D7-4) from healthy infants. Here, to investigate if these strains displayed anti-aging benefits, we screened them using the wild-type C. elegans N2 (Tissenbaum 2015a). Since longevity assay of C. elegans required addition of antibiotics and antimycotics to prevent contamination in long-term, all the bacteria used in C. elegans survival assay were considered killed by antibiotics. To control for the wide differences of bacterial genera and species as well as to prove the strain-dependent effects, we included two different strains but from the same genera and species.
Only two strains of dead probiotics, L. paracasei D3-5 and L. plantarum SK9, exhibited beneficial effects of extending lifespan and preserving better physical function when fed to wild-type C. elegans N2. Of these, D3-5 exhibited the strongest effects (Fig. 1a, b; Supplementary Figure S1a). Interestingly, D3-5 feeding declined muscle mass with significantly low rate compared to their control (E. coli OP50 strain)-fed and species mate L. paracasei 10-4-fed groups (Fig. 1c, d), suggesting that decline in physical function is associated with higher muscle mass in D3-5 fed C. elegans. Body length was shorter in the worms with lower survival including D10-4 fed worms (Fig. 1c; Supplementary Figure S1b). To determine whether D3-5 feeding increased muscle mass or persevered the decline of muscle in older worms, we calculated (i) the ratio of muscle mass vs body length (which gives similar measure of BMI in humans) and (ii) the longitudinal muscle mass decline by subtracting muscle mass on day 7 from day 11, within the same groups. First analysis showed no significant differences in the ratio of muscle mass/body length in worms fed with D3-5 and D10-4 compared to E. coli OP50 fed control worms at the adult age of day 7 (Fig. 1c, e), whereas second analysis demonstrated that muscle mass decline was significantly less in worms fed with D3-5 compared to E. coli OP-50 and D10-4 (Fig. 1d–f; Supplementary Figure S1c). These results suggest that D3-5 feeding preserved higher muscle mass at the older stage (day 11), without increasing it an adult age (day 7). No significant differences were observed in food intake (as measured by pharyngeal pumping rate) among all the groups (Supplementary Figure S1d). Other strains, regardless of genera and species, showed no significant changes in the lifespan of C. elegans compared to control OP50-fed worms. These results indicate that (i) certain dead probiotics are beneficial to aging-related ailments, in terms of extending life-span and preserving better physical function and muscle mass, and (ii) these beneficial effects are strain-specific, as not all probiotics from the same species/genera exhibit beneficial effects against aging.
Fig. 1.
Feeding dead probiotics extends lifespan and improves physical function and muscle mass in C. elegans. a Selective strains of probiotics lactobacilli extended life-span of wild-type C. elegans N2 compared to E. coli OP50 strain-fed controls. b D3-5 feeding reduced physical function (movement) decline of C. elegans compared to their control OP50- and other lactobacilli strains-fed groups. c–e It also maintained higher body length (at day 7) (c), muscle mass (indicated by GFP-labelled MAH19 strain of C. elegans at × 4 magnification) at day 11 (d), and muscle mass/body length ratio at days 7 and 11 (e). f Muscle mass decline was prohibited with D3-5 feeding compared to their control OP50- and D10-4 fed groups. Values are mean of 150–195 in each group ± SEM (standard error of mean; error bars) done three times in five replicates (10–17 worms each replicate at each batch). Values with *p < 0.05, **p < 0.001 are statistically significant. ns: not significant
D3-5 feeding prevents HFD-induced metabolic dysfunctions in older mice
As D3-5 feeding prolonged the lifespan of C. elegans, we investigated its effects in older obese mice (> 79 weeks or > 18 months old that are equivalent to > 65–70 years old humans) that were fed with HFD to induce metabolic dysfunction. Ten weeks of D3-5 feeding prevented the development of glucose intolerance and insulin resistance (measured by oral glucose tolerance test [GTT] and insulin tolerance test [ITT], respectively) in HFD-fed older mice compared to their age, gender, and diet-matched counterparts (Fig. 2a, b). Body weight, fasting, and fed blood glucose levels and energy expenditure were not significantly changed after D3-5 treatment compared to control older mice (Supplementary Figure S2a-j). Further, feeding of D3-5 significantly reduced hepatic steatosis or non-alcoholic fatty liver disease (NAFLD) indicated by fat accumulation in the liver and decreased crown-like structures (indicator of inflammation) in the white adipose tissue (WAT) (Fig. 2c, d; Supplementary Figure S3a). WAT adipocyte size was also significantly reduced in D3-5 fed older mice compared to controls (Fig. 2e; supplementary Figure S3b). Overall, these results demonstrate that the D3-5 feeding prevented HFD-induced glucose intolerance, insulin resistance, hepatic steatosis/NAFLD, and inflammation in WAT with small adipocytes, indicating that D3-5 feeding ameliorates detrimental metabolic effects of HFD in older mice.
Fig. 2.

Feeding dead probiotics prevents HFD-induced metabolic dysfunctions in older mice. a, b Feeding of dead L. paracasei D3-5 prevented high fat diet (HFD)-induced glucose intolerance (a) and insulin resistance in older mice (b). c–e D3-5 feeding also prevented HFD-induced hepatic steatosis (fat accumulation in the liver) (upper panel—c) and reduced crown like structures (indicators of inflammation) (lower panel—c, d) and reduced adipocyte size of white adipose tissue (e) of older mice. Values are mean of n = 5–8 mice per group ± SEM (error bars). Values with *p < 0.05, **p < 0.001 are statistically significant. ns: not significant
D3-5 improves physical and cognitive functions, with reduced leaky gut and inflammation in older obese mice
Obese older adults commonly have poorer physical function, increased anxiety, and decreased cognition (learning and memory) (Dahl et al. 2013; DeJesus et al. 2016; Virta et al. 2013). Therefore, we tested the effects of D3-5 feeding on these ailments in older HFD-fed mice. We found that the older obese mice fed with D3-5 maintained better physical function, as evidenced by increased ambulatory and total motor activity (indicator of walking) compared to age- and gender-matched controls (Fig. 3a, b). In addition, other physical function indicator, i.e., hanging time on inclined screen (a measure of muscle strength), was significantly improved in D3-5-fed older mice (Fig. 3c). D3-5 feeding also significantly decreased anxiety (an another common ailment in older people) (Borta and Schwarting 2005) as indicated by (i) decreased rate of rearing and (ii) increased time spending in the center during open field test (Fig. 3d–g). We also found that the D3-5 feeding significantly increased cognitive function in terms of enhanced learning improvements during Morris Water Maze test in older mice compared to their non-treated controls (Fig. 3h). Because leaky gut and inflammation are often greater in older humans and mice and are major risk factors for poor health outcomes in older adults (Nagpal et al. 2018a), we next assessed the gut permeability and inflammation in these mice. We found that D3-5 feeding significantly decreased gut permeability (a marker of leaky gut) as assessed by reduced diffusion of FITC-dextran (3–5 kDa) from gut to the blood (Fig. 3i), and this effect was associated with lower expression of inflammatory genes including IL-6 and IL-1β in the colon of D3-5-fed older obese mice compared to controls (Fig. 3j, k). Together, these results indicate that D3-5 feeding led to better physical and cognitive functions and reduced anxiety/depression which was also associated with decreased gut permeability and inflammation in older HFD-fed obese mice.
Fig. 3.
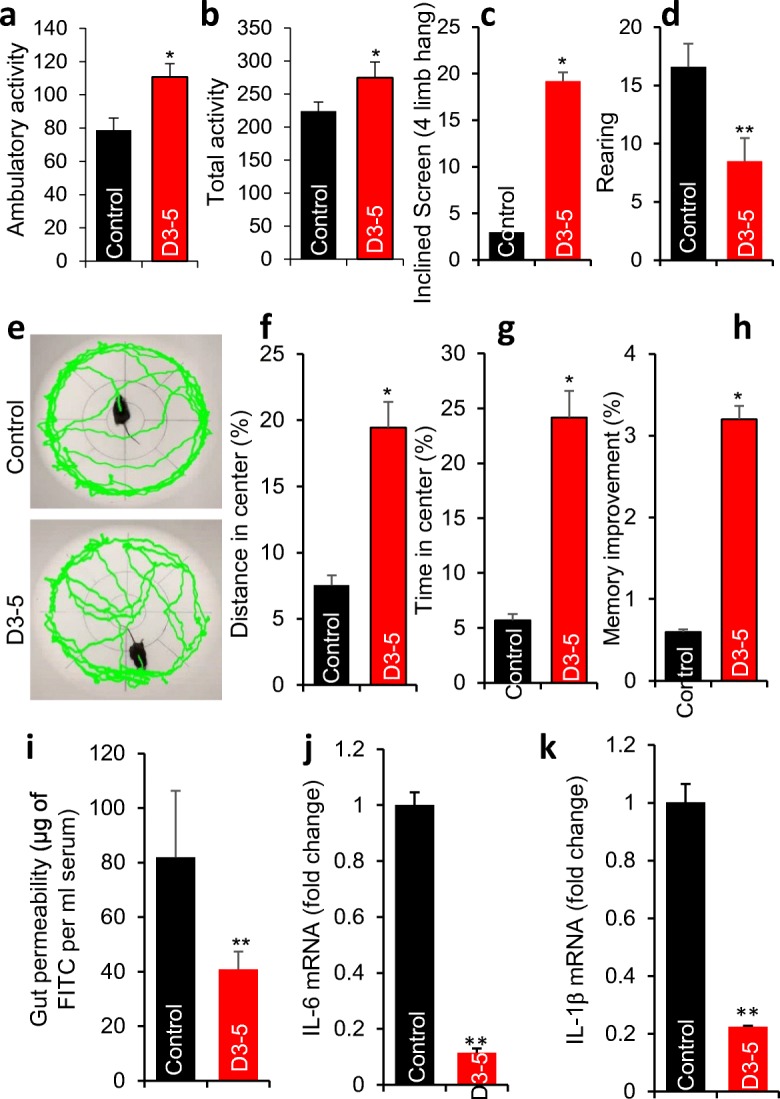
D3-5 feeding improves aging-related ailments like physical function, muscle strength, depression/anxiety, learning-memory, leaky gut, and inflammation in older obese mice. a, b Physical function [measured as ambulatory activity (a) and total activity (b)], muscle strength [measured as inclining on tilted screen (c)], anxiety/depression behavior [measured as rearing (d) and time spent in center during open field test (e–g)], and learning-memory behavior [measured during Morris Water Maze test (h)] were significantly enhanced in D3-5 fed older obese mice compared to their controls. Leaky gut [measured by appearance of FITC in blood leaked from gut (i)] and mRNA expression of inflammatory markers (like IL-6 and IL-1β) were significantly decreased in D3-5 fed older obese mice compared to their age-sex-matched controls (j, k). Values are mean of n = 5–8 mice per group ± SEM (error bars). Values with *p < 0.05, **p < 0.001 are statistically significant. ns: not significant
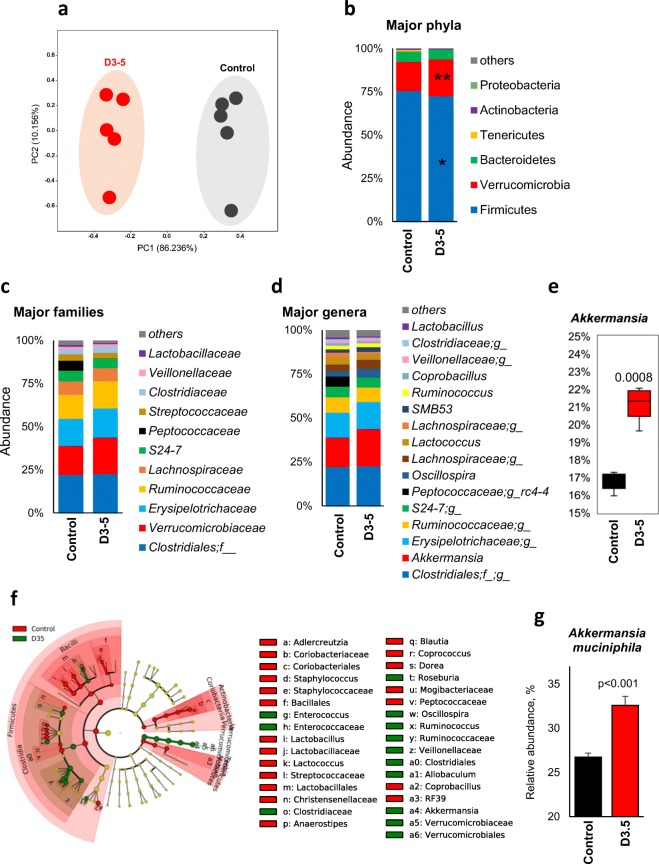
D3-5 beneficially modulates gut microbiome in older obese mice
We did not expect the feeding of dead D3-5 to have a major impact on gut microbiome, because of its dead form. Instead, we found that the gut microbiome signature was significantly different in older mice fed with D3-5 compared to their controls (Fig. 4). The β-diversity was dramatically different in D3-5 fed older mice compared to their controls (Fig. 4a), without significant changes in α-diversity indices (Supplementary Figure S4 a–d). However, microbial signatures were distinctly clustered in D3-5 compared to their controls (Supplementary Figure S4e). Further, D3-5 feeding significantly decreased the abundance of metabolically detrimental bacterial phyla including Firmicutes while increasing the abundance of metabolically beneficial Verrucomicrobia phyla and Verrucomibiaceae family (Fig. 4b, c). Feeding with D3-5 increased the abundance of Verrucomicrobiaceae, Verrucomicrobiales, Verrucomicrobiae, and Akkermansia species, while decreasing the ratios of Actinobacteria, Adlercreutzia, Coriobacteriales, and Coriobacteriia (Fig. 4d–f; Supplementary Figure S4f). The abundance of common mucin-degrading bacterial species Akkermansia muciniphila was significantly increased in D3-5-fed older mice compared to their controls (Fig. 4g).
Fig. 4.
D3-5 feeding results in favorable changes in gut microbiome. a Principal coordinate analysis (PCoA) showing the β-diversity clustering of gut microbiome from older obese mice fed with D3-5 and the control group. Major changes in bacterial phyla (b), families (c), and genera (d) in mice fed with D3-5 compared to their controls. e Abundance of Akkermansia was significantly increased in D3-5 fed mice compared to controls. f Cladograms of linear discrimination analysis (LDA) effect size (LEfSe) demonstrating the clustering of gut microbiome in D3-5-fed older mice compared to the controls. g Abundance of A. muciniphila was significantly increased in D3-5 treated older obese mice compared to their non-treated controls. Values are mean of n = 5 mice per group ± SEM (error bars). Values with *p < 0.05, **p < 0.001 are statistically significant. ns: not significant
D3-5 administration increases mucin production
To explain the dramatic increase of A. muciniphila by D3-5 feeding, we reasoned that mucin (food source for A. muciniphila) (Zhou 2017) levels may have increased in the gut of D3-5-fed older mice, leading to the increase in A. muciniphila as a feedback mechanism. Muc2 (a major isoform of mucin abundantly expressed in intestine) expression was significantly increased in duodenum, ileum, and colon of D3-5 fed older obese mice compared to their controls (Fig. 5a). D3-5 feeding also significantly increased the expression of mucin protein in colon along with increased mucin secretion in the feces compared to their controls (Fig. 5b, c). Mucin is secreted from goblet cells (Pelaseyed et al. 2014), and the goblet cell mass was significantly higher (34.6%) in D3-5 fed mice compared to their non-treated controls (Fig. 5d, e), suggesting that the observed increase in mucin production could be explained by a larger goblet cell mass.
Fig. 5.
D3-5 treatment enhances mucin production and goblet cell mass in the gut of older obese mice. a Real-time PCR results indicated that the expression of mucin 2 (Muc2) mRNA was significantly higher in the different regions of the intestine of D3-5 fed older mice compared to control mice. b, c Expression [measured by Western blotting in colon (b)] and production [measured using ELISA in feces (c)] of mucin was significantly higher in older obese mice fed with D3-5 compared to controls. d, e AB/PAS staining depicting more intestinal goblet cell mass in ileum (d) and their quantitative numbers (e) was significant in D3-5 fed older obese mouse gut compared to their controls. f–j Transplantation of fecal microbiome from older mice fed with D3-5 and their controls to gut-cleansed recipient mice also showed enhanced expression of Muc2 mRNA (f) and protein (g), marginal increase in fecal mucin (h), and goblet cell mass numbers (i, j). Real-time PCR reaction was performed in triplicates and repeated at least two to three times. Values are mean of n = 5–8 mice per group ± SEM (error bars). Values with *p < 0.05, **p < 0.001 are statistically significant. ns: not significant
To further explore whether D3-5-induced gut microbiome changes contribute in the modulation of goblet cell and mucin biology, we transplanted gut microbiome from D3-5 fed (FMT-D3-5) and their control mice (FMT-Control) to gut cleaned (GC; using antibiotics and polyethylene glycol [PEG] protocol) mice (Wrzosek et al. 2018). Muc2 mRNA and mucin protein as well as goblet cell mass were significantly increased in the gut of FMT-D3-5 recipient GC mice compared to FMT-control recipient GC mice (Fig. 5f–j). These results indicate that D3-5-induced gut microbiome modulation contributes in promoting goblet cell mass and mucin production.
Lipoteichoic acid (LTA) derived from the cell wall of D3-5 increases mucin production
In order to discover the specific cellular components of dead D3-5 which enhance the mucin production, we first fractionated cell wall and cytoplasmic components and subsequently treated CMT93 cells (a mouse goblet cell line) with these components. Muc2 mRNA expression and mucin content (labelled with PAS staining) were increased only in the cell wall fraction of D3-5-treated cells (Fig. 6a, b). To further determine which D3-5 cell wall constituents that enhanced mucin production, we fractionated the D3-5 cell wall into the peptidoglycan and LTA fractions, and found that the LTA fraction-treated CMT93 goblet cells exhibited significantly higher expression of Muc2 mRNA and mucin/PAS staining, suggesting that the LTA from the D3-5 cell wall activates mucin production from goblet cells (Fig. 6c, d). Interestingly, the LTA derived from L. paracasei D3-5 has only stimulated Muc2 mRNA expression in CMT-93 cells, while LTA derived from L. paracasei D10-4 demonstrated no effects (Fig. 7a), suggesting that LTA derived from D3-5 has unique biological activity to stimulate Muc2 mRNA expression in goblet cells.
Fig. 6.
Cell wall-derived lipoteichoic acid (LTA) of D3-5 enhances mucin production. a, b Cell wall fraction of D3-5 containing LTA increased mRNA expression of Muc2 (a) and production of mucin (PAS staining indicated in blue) in the mouse intestinal goblet cell line CMT93 cells (b). c, d D3-5 cell wall derived LTA (200 μg/ml) increased both Muc2 mRNA (c) and mucin production (d) compared to peptidoglycan (PGN)-enriched fraction. All the assays were performed in triplicate and were repeated at least two to three times. Values are mean of replicates and repeated batches ± SEM (error bars). Statistically significant (p values < 0.05) was obtained using Student’s t test and/or ANOVA. Values with *p < 0.05, are statistically significant. ns: not significant
Fig. 7.
LTA induces mucin via TLR2 signaling. a Treatment of mouse intestinal goblet CMT-93 cells with 200 μg/ml LTA derived from D3.5 cell wall increased Muc2 mRNA expression, while LTA derived from D10.4 cell wall shows no effects. b mRNA expression of Muc2 was significantly increased in D3.5 LTA-treated CMT93 goblet cells, while this induction disappeared in TLR2 inhibitor (CU CPT22; 8 μM)-treated cells. c Mucin protein expression (measured by Western blot) in the colon was also increased in 5-day D3-5 and LTA-treated C57BL/6J (B6) young mice, while these effects of D3-5 and LTA treatment disappeared in age- and gender-matched TLR2 KO mice. d–g Similarly, goblet cell mass (AB/PAS staining) and numbers (d, e) were increased in D3-5 and LTA-treated B6 control mice, while these effects were not seen in TLR2 KO mice (f, g). Values are mean of n = 5–8 mice per group ± SEM (error bars). Values with *p < 0.05, **p < 0.001 are statistically significant. ns: not significant
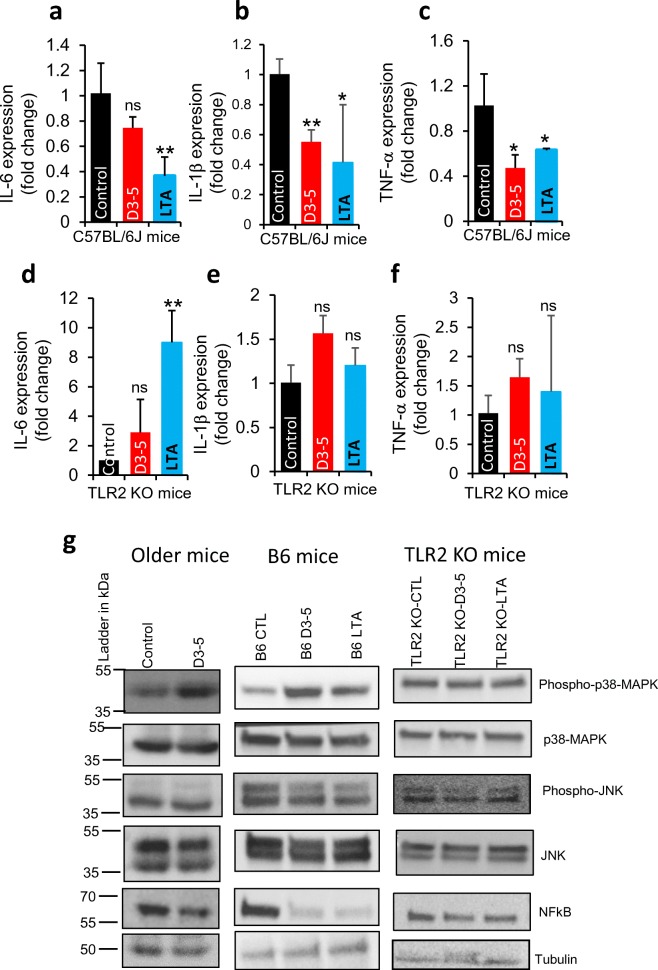
LTA activates TLR-2/p38-MAPK signaling to promote Muc2 expression and suppress NFκB to reduce inflammation
To further establish how D3-5-derived LTA increases Muc2 expression and mucin production, we hypothesized that LTA activates the toll-like receptor-2 (TLR-2) signaling and enhances Muc2 expression in intestinal goblet cells. LTA treatment-stimulated increase in Muc2 mRNA in CMT93 cells was significantly abolished in the presence of TLR2 inhibitor (CU CPT22) (Fig. 7b), suggesting that TLR2 signaling mediates the effects of LTA to enhance the expression of Muc2 in CMT93 goblet cells. In addition, oral administration of D3-5 and its cell wall-derived LTA for 1 week significantly increased Muc2 expression as well as enhanced goblet cell mass in the mouse gut. However, such effects of D3-5 and its cell wall LTA failed in TLR2 knockout (KO) mice (Fig. 7c–g), further indicating that LTA required TLR2 signaling activation to enhance goblet cell mass and mucin production. LTA also significantly reduced the inflammatory markers, i.e., IL-6, IL-1β, and TNF-α in the intestine of C57BL/6J mice; however, these effects were disappeared in the TLR2 KO mice (Fig. 8a–f), suggesting that LTA-reduced inflammation also depends on intact TLR2 signaling. These results suggest that LTA stimulated TLR2 signaling to enhance goblet cell mass and mucin production, which in turn reduced leaky gut and intestinal inflammation.
Fig. 8.
D3-5 cell wall-derived LTA activates TLR2-p38 MAPK signaling and suppresses NF-κB signaling. a–f D3-5 and its cell wall-derived LTA feeding significantly decreased mRNA expression of pro-inflammatory genes like IL-6, IL-1ββ and TNF-α in the colon of normal B6 (C57BL/6J) mice (a–c), while these effects are abolished in the TLR2 KO mouse colons (d–f). g Western blot analyses show that D3-5 and its cell wall-derived LTA increased abundance of phospho-p38-MAPK, and decreased NF-κB, with no changes in p38-MAPK, Phospho-JNK, and JNK in the colons of older mice and B6 young mice. Such effects disappeared in TLR2 KO mice. Values are mean of n = 5–8 mice per group ± SEM (error bars). Values with *p < 0.05, **p < 0.001 are statistically significant. ns not significant
To further discover which signaling mediators of the TLR2 pathway participate in response to LTA, we found that the levels of phosphorylated p38 MAPK proteins were significantly increased in the intestines of both old and young C57BL/6J mice, while this activation was abolished in TLR2 KO mice (Fig. 8g). However, total p38 MAPK as well as phospho- and total-JNK proteins did not significantly change upon LTA treatment (Fig. 8g), suggesting that p38-MAPK is activated downstream of TLR2, without impacting JNK signaling. The protein levels of NF-κB, a master regulator of inflammatory pathways, were significantly decreased in LTA-treated wild-type mice, but not in TLR2 KO mice (Fig. 8g). Together, these data show that LTA activation of TLR2-p38 MAPK signaling enhanced mucin production, suppressed NF-κB signaling, and reduced inflammatory cytokines.
Discussion
Increasing prevalence of age-related ailments including decline in physical and cognitive functions and increased predisposition to obesity, diabetes, cardiovascular diseases, and cancer is significantly associated with dysbiotic gut microbiome, leaky gut, and inflammation (Buford 2017; Nagpal et al. 2018a; Shimizu 2018). Herein, we demonstrated that a dead probiotic strain L. paracasei D3-5 isolated from infant gut enhanced life-span and maintained better physical function and muscle mass in C. elegans and prevented HFD-induced metabolic dysfunctions, leaky gut, and inflammation in older mice. Mechanistically, D3-5 cell wall-derived LTA enhanced goblet cells and mucin production by activating TLR2-p38 MAPK signaling and reducing inflammation by inhibiting NF-κB resulting in decreased expression of the pro-inflammatory cytokines IL-6, IL-1β, and TNF-α (Fig. 9).
Fig. 9.
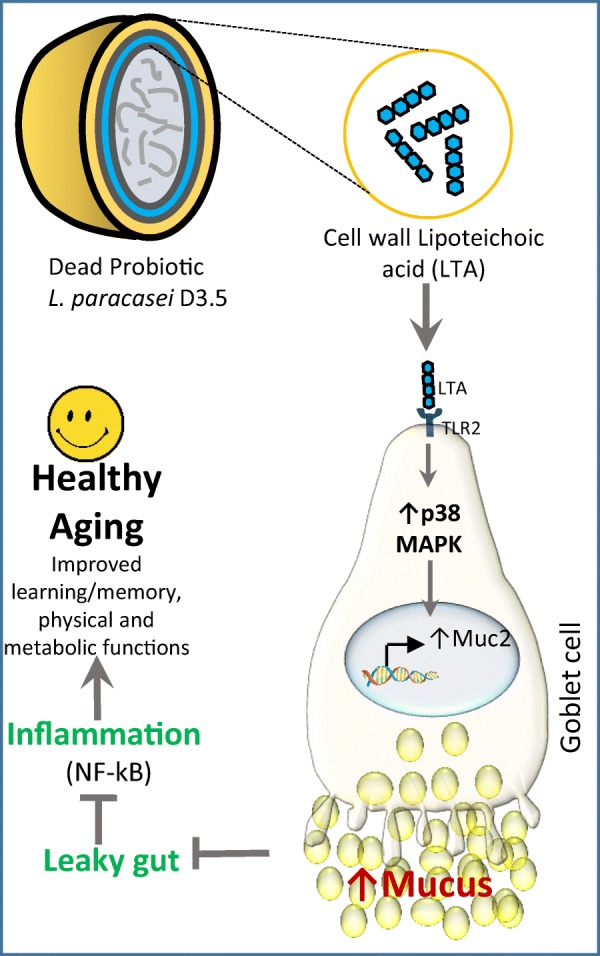
Proposed model of how dead L. paracasei D3-5 and its cell wall-derived LTA ameliorate leaky gut and inflammation, and promotes healthy aging. Mechanistically, D3-5-derived LTA activates TLR2/p38-MAPK signaling resulting in increased mucin production to suppress leaky gut and inhibit NF-κB to reduce inflammation
Although the precise mechanisms underlying increased inflammation in older adults remain elusive, increased gut epithelial permeability in patients presenting leaky gut is common in older adults and can serve as a major trigger for intestinal and systemic inflammation (Stehle Jr et al. 2012). Emerging evidence indicates that gut microbiome dysbiosis is associated with poor health outcomes in older adults and may contribute to the development of leaky gut and inflammation (Nagpal et al. 2018a). The abundance of certain detrimental Gram-negative bacteria are often increased in the older gut (Schiffrin et al. 2010), elevating the levels of pro-inflammatory molecule, i.e., LPS (Lipopolysaccharide), an endotoxin and major constituent of Gram-negative bacterial cell wall. LPS can diffuse in leaky gut conditions, causing endotoxemia and inflammation in local tissues as well as systemic inflammation (Mu et al. 2017). Therefore, gut microbiome modulators that are able to (i) reduce the abundance of Gram-negative bacteria and (ii) improve the gut barrier integrity by enhanced mucus layer thickness and expression of tight junctions could reduce leaky gut and inflammation (Kelly et al. 2015). Probiotics can be ideal candidates for this purpose because common probiotics are Gram-positive and therefore can balance the growth of Gram-negative bacteria in older gut, thereby reducing the LPS load and the associated inflammation.
In addition, specific strains of probiotics have been shown to induce mucin production. For example, bifidobacteria increase mucus production and protect from HFD-induced microbiota dysbiosis and obesity (Schroeder et al. 2018), and Lactobacillus GG upregulates Muc2 gene expression in intestinal epithelial cells (Wang et al. 2015). However, the impact of probiotics in age-related leaky gut, inflammation, and mucin biology remains elusive. Also, specific probiotics are known for their beneficial effects in several human diseases including diabetes, obesity, cardiovascular diseases, and cancer (Azad et al. 2018; Landete et al. 2017), but their impact on longevity is not well defined. In this study, we demonstrated that only a few selected human-origin probiotic strains exhibit beneficial effects in extending the lifespan of C. elegans. Among the six selected probiotic strains (Nagpal et al. 2018b), only two strains of lactobacilli like L. plantarum SK9 and L. paracasei D3-5 fed C. elegans show beneficial effects to extend life-span. D3-5 feeding showed the highest extension in life-span and hence was selected for further studies. These results indicate that not all probiotics have anti-aging effects and that these effects are highly strain-specific. Interestingly, D3-5 feeding also enhanced physical function and maintained higher muscle mass in older C. elegans, suggesting that the probiotic D3-5 prevented the aging-related muscle mass decline. The C. elegans longevity assay protocols require addition of antibiotics and antifungal to prevent contamination of the worm media. Thus, probiotics fed to C. elegans were considered and confirmed dead. That led us to discover that the dead D3-5 has anti-aging effects including increased life-span of C. elegans and preserved muscle mass and physical functions. These findings are unusually significant in several ways: (i) they establish a new paradigm that dead probiotics can also have beneficial effects, in contrary to the old paradigm suggesting that probiotics are needed to be alive to exhibit beneficial effects; (ii) dead probiotics eliminate the risk of leakage of live bacteria systemically in patients with severe leaky gut, thus increasing the risk of bacteremia/sepsis; and (iii) dead probiotics may offer an advantage for food industry applications as they can easily be supplemented in several food lines/products not amenable for live probiotics, such as beverages, baked products, and others.
Obesity and metabolic dysfunctions are common in older adults with poor health outcomes, and HFD-feeding induces these ailments much faster in older mice compared to younger ones (Dominguez and Barbagallo 2016; Nunes-Souza et al. 2016). We found that D3-5 feeding to older mice prevented the HFD-induced glucose intolerance, insulin resistance, hepatic steatosis (lipid accumulation in liver), and low-grade inflammation in adipose tissue. D3-5-fed older mice maintained better physical and cognitive functions with reduced leaky gut and inflammation, indicating that the anti-aging effects observed with D3-5 in C. elegans were translated to mammals, like older obese mice. Intriguingly, we found that the D3-5 also significantly modulated the gut microbiome composition in these older obese mice, as it considerably enhanced the abundance of a mucin-degrading bacterium A. muciniphila. Indeed, A. muciniphila is known to have beneficial effects against obesity, diabetes (Dao et al. 2016; Everard et al. 2013), and other human diseases along with anti-inflammatory effects (Naito et al. 2018; Ottman et al. 2017). We hypothesized that the increased abundance of A. muciniphila might be due to increased mucin production in the gut of D3-5 fed mice. Because mucin is a major food source for A. muciniphila, increased mucin production may enhance the growth of this bacteria. Interestingly enough, the intestine of D3-5 fed mice had significantly increased mucin production along with higher Muc2 expression and goblet cell mass. The fecal microbiome transplantation studies demonstrated that the D3-5 modulated gut microbiome enhances goblet cell mass and mucin production. However, these changes might be due to the presence of D3-5 and its cellular ingredients in the donor feces which might have been transferred to the recipient mice gut and showing these residual effects.
Using biochemical fractionation, we demonstrated that the D3-5’s cell wall-derived LTA is the key component responsible for enhancing Muc2 expression and mucin production from goblet cells. The LTA derived from L. paracasei D3-5 cell wall has this unique biological activity to stimulate Muc2 expression, while LTA of same species but different strain of probiotics like L. paracasei D10-4 has no effect on Muc2 mRNA expression. We further investigated the mechanism(s) by which LTA can stimulate goblet cells to produce mucin. We also found that LTA action depends on TLR2 signaling to induce Muc2 expression. Activation of TLR2 signaling further recruits and initiates downstream signaling cascade by phosphorylation of p38 MAPK (Kawai and Akira 2010; Ribeiro et al. 2010). Accordingly, D3-5 and LTA treatment increased phospho-p38-MAPK levels in the mouse intestine, indicating that LTA activates TLR2 and p38-MAPK signaling, which in turn increases Muc2 expression and goblet cell mass. Interestingly, our results also demonstrated that NF-κB protein levels were significantly decreased. Suppression of NF-κB signaling is known to diminish the expression of proinflammatory cytokines such as IL-6, IL-1β, and TNF-α (Tak and Firestein 2001). Thus, our results indicate that LTA downregulates NF-κB-mediated proinflammatory responses in the intestinal tissue milieu. Our future studies are planned to comprehend beneficial effects of LTA against aging-related leaky gut and inflammation, and determine the structural uniqueness and molecular mechanism(s), using cell culture, C. elegans, and mouse models. Altogether, our results demonstrated that a newly isolated human-origin probiotic strain D3-5 is beneficial to enhance longevity and ameliorate aging-related health ailments such as metabolic dysfunctions, leaky gut, and inflammation. D3-5 cell wall-derived LTA activated TLR2-p38 MAPK signaling that promoted Muc2 expression and mucin production from goblet cells thereby reducing the leaky gut and suppressing the NF-κB signaling eventually reducing the inflammation. These results demonstrated that the D3-5 and its cell wall-derived LTA could be used as a biotherapy to prevent and/or treat age-related gut microbiome dysbiosis, leaky gut, and inflammation in older adults.
Methods
C. elegans culture and longevity assay
A wild-type strain of C. elegans N2 (Tissenbaum 2015a) and a muscle-specific GFP-labelled strain MAH19 (Kumsta and Hansen 2012) (procured from CGC, University of Minnesota) were cultured by standard procedures on NGM complete agar media and E. coli OP50 (Rea et al. 2005). The life span screening assay was carried out using liquid medium in 96-well plates according to a protocol described elsewhere (Solis and Petrascheck 2011) at room temperature (21–22 °C). In brief, 10–17 age-synchronized C. elegans (L1 larva) were cultured in each well with S-complete media containing Ampicillin, Carbenicillin, and Amphotericin B (for inhibiting bacterial and fungal contamination) and 100 μg/mL fluorodeoxyuridine (FUDR; for blocking fertilization) in 96-well plates. C. elegans were fed (0.3 × 108 cfu/mL) with six human-origin probiotic Lactobacillus strains (L. paracasei D3-5, L. paracasei D10-4, L. rhamnosus D4-4, L. rhamnosus D7-4, L. plantarum SK9, and L. plantarum D6-2) and were compared with E. coli OP50-fed controls. Fifty to 85 worms in five wells were used for each treatment, and the numbers of live worms were counted daily on the basis of body movement. Food intake was measured by counting the number of pharyngeal contractions per minute (pumping rate) using a microscope. The fraction of animals alive was expressed as the number of animals displaying body movement per total number of animals. Body length of the worms was measured using ImageJ tools. This assay was repeated three times using 10–17 worms in five replicates each time, accounting for 150–195 worms in each group for cumulative data presented in figures.
For the movement assay, C. elegans were picked into M9 buffer on a glass slide. The number of left- and right-ward body bends within 10 s was counted. One left-ward and one right-ward bend counted equal to one stroke. The number of strokes within 10 s was multiplied by six to calculate the movement rate per min (60 s). The counting of body strokes was repeated five times for each animal, and 10 animals were tested for each treatment, which accounted 50 measures per group. To determine the movement decline on 7, 11, and 14 days of age in worms, we subtracted data from 7th day movement activities. To determine food intake, rate of pharyngeal pumping within 1 min was counted, while worms kept on S-complete media containing bacteria (food) in 6-well plates at 20 °C under microscope without any disturbances. This assay was repeated five times for each animal, and 10 animals were tested for each group.
Mice
Older male C57BL/6J mice (78–80-week-old; equivalent to > 65 years of human age) were purchased from the Jackson Laboratory and were randomized in two groups: (1) control and (2) D3-5 (n = 5–8 animals in each group), after 2 weeks of acclimatization. Group 2 were fed with heat killed (70 °C incubation for 2 h and confirmed their death on MRS agar cultivation) D3-5 by supplementing the equivalent of 109 colony forming unit (cfu)/ml in drinking water, while control animals were fed with normal drinking water. Drinking water was freshly changed every day. Both groups of animals were fed with 60% kcal of fat high-fat diet (HFD; Research Diets). Body weight and food intake were measured weekly. Glucose and insulin tolerance tests, metabolic phenotyping, physical activity measures, behavior test, and gut permeability assays were performed as described below.
Fecal microbiome transplant and gut cleansed (GC) mice were prepared a modified method of Wrzosek et al. (2018). GC mice were fed with antibiotics cocktail (containing ampicillin 1 g/l, metronidazole 1 g/l, neomycin 1 g/l, and vancomycin 0.5 g/l and 3 g/l sweetener) in drinking water for 4 days, followed by orally gavage four doses of PEG (425 g/l, 200 μl/mouse) at 20 min interval on day 5 (this procedure depletes > 95% live microbial counts in the feces). Four hours after the last PEG dose, mice were administered with freshly prepared cecal microbiome slurry suspension (200 μl/mouse). Microbiome slurry was prepared using 500 mg of cecal contents dissolved in 5 ml of reduced PBS (phosphate buffer supplemented with 0.1% Resazurin (w/v) and 0.05% l-cysteine-HCl) under anaerobic condition (Kang et al. 2017). Control mice (called CCM-control) received microbiome from older-control mice, while CCM-D3-5 mice received microbiome from older-D3-5-treated mice, and continued for additional 3 days to enrich donor microbiome in recipient GC mice. After 1 week, GC animals were euthanized and tissues were collected and used for histology and gene and protein expression assays (using RT-PCR and western blots, respectively).
C57BL/6 wild-type and TLR2 KO mice (6–8 week-old) were randomized the mice into (1) control, (2) D3-5, and (3) LTA groups. Group 2 was fed with dead D3-5 bacteria (as described above), and group 3 was fed with LTA (6 mg/mL) based on the body weight (4 ml/kg) through oral gavage for 5 days. Control animals were fed with normal drinking water in the same way. Body weight, food and water intake, and feces were collected before, during, and after feeding period, and all the animals were then euthanized and tissues were collected for histological and gene expression analyses. All the animal experiments and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Wake Forest School of Medicine.
Glucose and insulin tolerance tests
Glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed in fasted (10–12 h for GTT and 4–6 h for ITT) mice by giving oral gavage of glucose (2.5 g/kg body weight) and intraperitoneal injection with insulin (0.75 U/kg body weight), respectively. Blood glucose was measured from tail puncture before (0 min) and 15, 30, 60, and 120 min after administration using Truetrack® glucometer (Ahmadi et al. 2019; Yadav et al. 2011)
Muscular endurance (incline screen)
Treadmill and muscular endurance tests were performed in blinded groups, as described before (Silverstein et al. 2015). For four-limb testing, mice were suspended by all four limbs onto a high wire mesh. Timer recording started from the mesh was inverted and stopped when the mouse fell onto the padding below.
Behavioral measures
Open field test to determine anxiety and depression was performed in a quiet and dim room. Mice were placed individually into the center of a clean and sanitized white cylindrical tank (30-cm height × 50-cm diameter), and mice movements were recorded using camera for 5 min. The measures of behavior parameters such as average moving speed, total distance travelled, percentage distance covered in the center, and percentage time spent in the center were assessed using a ToxTrac tool (Rodriguez et al. 2018).
Spatial learning and memory abilities were assessed using Morris Water Maze test (Nunez 2008). Tests were carried out in a clean and sanitized white cylindrical tank (30-cm height × 50-cm diameter) filled with tap water (25 °C) about 1 in below an island-like rescue platform placed at the center of the tank. During the pre-training, each mouse was trained three times in different directions. The water maze testing was performed at the same time next day with the same condition. Mice underwent 12 trials from four different directions. Time to reach the platform was recorded. All mice groups were blinded for the assessors.
Gut permeability assay
Intestinal/gut permeability was performed by giving oral gavage of FITC-dextran (3–5 kDa; 1 g/kg body weight) to mice pre-fasted for 4–6 h, and measuring the appearance of FITC fluorescence (excitation at 485 nm and emission at 520 nm) in serum with reference to the FITC standard curve, as earlier described by us (Ahmadi et al. 2019).
Histological analyses
Tissues from liver and intestine and white adipose tissue were collected, washed with PBS, and fixed in 10% formalin. Sections (0.5 μm thickness) were stained with hematoxylin and eosin (H&E) and imaged with AmScope microscope on × 20 magnification. Adipocyte size and distribution were assessed in ~ 480 random adipocytes from each mouse with ImageJ software. Crown-like structures (CLS)—indicative of inflammation in white adipose tissue (Apovian et al. 2008; Bagarolli et al. 2017; Ebke et al. 2014; Wijetunge et al. 2019)—were measured by counting the total number of CLS proportionate to the total number of adipocytes in each section. To analyze the goblet cell mass, sections were stained with Alcian Blue/PAS kit (Newcomer Supply, Middleton, WI) and goblet cells (blue dots under the pink background); numbers were counted by a person blinded for the groups.
Real-time PCR
Total RNA was extracted from cells or tissues using RNeasy and was reverse transcribed using High-Capacity cDNA reverse transcription kit. The cDNA was used to quantify the expression of Muc2, IL-6, IL-1β, and TNF-α using TaqMan Gene Expression Assays for real-time PCR. 18S rRNA was used as an internal control. Relative gene expression was calculated using ΔΔCT method and presented as relative fold change. All the assays were performed in triplicate and repeated minimum two to three times. Values presented as mean of replicates and combination of repeated batches.
Western blot
Total proteins from cells and tissues were extracted using homogenization lysis buffer as earlier described by us (Yadav et al. 2011). Proteins were resolved by SDS-PAGE electrophoresis and transferred to PVDF membrane for Western blotting. Membranes were developed with primary antibodies (anti-Mucin 2, phospho-p38 MAPK, p38-MAPK, phospho-SAPK/JNK, SAPK/JNK, and NF-kB p65 from Cell Signaling) followed by secondary antibody and developing with chemiluminiscent ECL kit and imaged on PXi with the GeneSys software. Tubulin was used as the loading control.
Fecal mucin
Fecal mucin content was determined using an ELISA kit (Cosmo BioCo. Ltd) to quantify mucin within the feces in triplicate from each mice (Crowther and Wetmore 1987).
Gut microbiome analyses
The gut microbiota composition was analyzed using 16S rRNA sequencing with MiSeq Illumina platform and analyzed using bioinformatics pipelines, as per our previously described protocols (Ahmadi et al. 2019; Nagpal et al. 2018b). In brief, genomic DNA was extracted from ~ 200 mg of mice feces (n = 5 in each group) using the Qiagen DNA Stool Mini Kit (Qiagen, CA, USA). Primers 515 F (barcoded) and 806 R were used to amplify the V4 region of bacterial 16S rDNA. After being purified and quantified with AMPure® magnetic purification beads and Qubit-3 fluorimeter, respectively, equal amounts (8 pM) of the amplicons were applied for sequencing on an Illumina MiSeq sequencer (using Miseq reagent kit v3). The sequences were de-multiplexed, quality filtered, clustered, and analyzed with the Quantitative Insights into Microbial Ecology (QIIME) and R-based analytical tools (Navas-Molina et al. 2013). Linear discriminatory analysis (LDA) effect size (LEfSe) was used to identify unique bacterial taxa that drive differences between different groups (Ahmadi et al. 2019; Nagpal et al. 2018b). Microbiome analyses were performed from n = 5 animals from each group.
Cell wall, cytoplasm, peptidoglycan, and lipoteichoic acid preparation
We prepared bacterial cell wall and cytoplasmic fractions as described by Kim et al. (2002) with slight modifications. In brief, L. paracasei D3-5 was cultivated in MRS at 37 °C up to log phase, and cell pellets were disrupted with a high pressure homogenizer (EmulsiFlex®-C3, AVESTIN, Inc., Canada) in citrate buffer. After removing cell debris, the supernatants were ultra-centrifuged at 70,000g for 30 min (Beckman). The resulting supernatant was designated as cytoplasmic fraction, while the pellet as cell wall fraction. Further, the peptidoglycan-wall teichoic acid (PGN-WTA) was further fractionated using SDS according to the protocol developed by Heß et al. (2017). In brief, 4% SDS was added to the supernatant and incubated at 100 °C for 30 min, followed by stirring overnight at room temperature. Then, this solution was centrifuged (30,000g, 15 min), and the pellets were washed four times with citrate buffer and five times with ethanol, and lyophilized to form PGN-WTA powder. Lipoteichoic acid (LTA) from D3-5 cell wall fraction was also extracted according to Heß et al. (2017). L. paracasei D3-5 cells were disrupted as described above, and cell homogenates were stirred with an equal volume of butanol for 30 min. Aqueous and organic phases were separated through centrifugation at 21,000g for 15 min. The aqueous phase containing LTA was collected and dialyzed (3.5 kDa cutoff membrane) against water which was changed every 24 h for 5 days, and the resultant LTA solution inside the dialysis membrane was lyophilized to get the LTA powder.
Cell culture and treatment
The mouse mucin-secreting goblet cells CMT-93 were grown in 12-well plate (for RNA extraction) and 6-well plate (for protein extraction) for 24 h; cells were treated with 1% (v/v) of cell wall, cytoplasm, PGN, or LTA fractions derived from D3-5 bacterial cells. Cells were collected after 14-h treatment, and RNA and proteins were used for real-time PCR and Western blotting, respectively. TLR2 inhibitor (CU CPT22, 8 μM) was used to determine whether LTA action is involving TLR2 signaling in mucin production. Mucin production in cells was visualized using PAS Kit (Sigma), following the manufacturer’s protocols.
Statistical analyses
All the assays were performed at least two to three times with three to five replicates at each time and/or n = 5–8 animals in each group, and the values presented in graphs/tables are mean ± standard error of means. Statistical differences among groups/treatments were analyzed using two-tailed unpaired Student’s t test and/or ANOVA. Bacterial diversity and OTU abundance between groups were compared using Kruskal-Wallis test followed by Dunn’s post hoc analysis, and heatmaps were created in R statistical software package (version 3.4.3; https://www.rproject.org/). P values with < 0.05 were considered statistically significant.
Electronic supplementary material
(PDF 780 kb)
Acknowledgments
We would like to thank Sandy Sink for help in metabolic phenotyping studies, and Cynthia Zimmerman from Wake Forest Regenerative Medicine Histology Core for tissue processing and slide preparations. We are also thankful to Dr. John Parks and Dr. Martha Alexander Miller for providing valuable advices, suggestions, and departmental resources. C. elegans strains were generously provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We are also thankful to all the participating researchers, technicians, and the staff members of the animal facilities and laboratory members for their consistent help during the study.
Author contributions
SW, SA, RN, SJ, SPM, KK, XZ, and ZW performed and/or helped in various experiments conducted in this study as well as data compilations and interpretations, and writing first draft of manuscript. DAM, SBK, and DWK significantly contributed as intellects for experimental designs and data interpretations. HY conceived the project idea, supervised the study, compiled and interpreted data, and wrote the manuscript.
Funding information
This work was supported by National Institutes of Health grant R01DK081842 (DAM), R01HL142930 (KK), R01HL132035 (XZ), R01AG059416, U13AG040938, R01AG052419, U24AG058556, KL2TR001421 (SBK), R01AG018915, R01AG045551, and U24AG059624 (DWK); the Pepper Older Americans for Independence Center (P30AG21332); and the Department of Defense funding W81XWH-18-1-0118, GRANT12726940/AZ180098, R01AG018915 (HY), as well as funds and services provided from the Center for Diabetes, Obesity and Metabolism, Wake Forest Baptist Medical Center, and the National Center for Advancing Translational Sciences (NCATS), the National Institutes of Health-funded Wake Forest Clinical, and Translational Science Institute (WF CTSI) through Grant Award Number UL1TR001420.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmadi S, Nagpal R, Wang S, Gagliano J, Kitzman DW, Soleimanian-Zad S, Sheikh-Zeinoddin M, Read R, Yadav H. Prebiotics from acorn and sago prevent high-fat-diet-induced insulin resistance via microbiome-gut-brain axis modulation. J Nutr Biochem. 2019;67:1–13. doi: 10.1016/j.jnutbio.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Apovian CM, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad MAK, Sarker M, Li T, Yin J. Probiotic species in the modulation of gut microbiota: an overview. Biomed Res Int. 2018;2018:9478630. doi: 10.1155/2018/9478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagarolli RA, et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J Nutr Biochem. 2017;50:16–25. doi: 10.1016/j.jnutbio.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Borta A, Schwarting RKW. Inhibitory avoidance, pain reactivity, and plus-maze behavior in Wistar rats with high versus low rearing activity. Physiol Behav. 2005;84:387–396. doi: 10.1016/j.physbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Buford TW (2017) (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome 5:80. 10.1186/s40168-017-0296-0 [DOI] [PMC free article] [PubMed]
- Capaldo CT, Powell DN, Kalman D. Layered defense: how mucus and tight junctions seal the intestinal barrier. J Mol Med (Berl) 2017;95:927–934. doi: 10.1007/s00109-017-1557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3:e982426. doi: 10.4161/21688370.2014.982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RS, Wetmore RF. Fluorometric assay of O-linked glycoproteins by reaction with 2-cyanoacetamide. Anal Biochem. 1987;163:170–174. doi: 10.1016/0003-2697(87)90108-4. [DOI] [PubMed] [Google Scholar]
- Dahl AK, Hassing LB, Fransson EI, Gatz M, Reynolds CA, Pedersen NL. Body mass index across midlife and cognitive change in late life. Int J Obes. 2013;37:296–302. doi: 10.1038/ijo.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao MC, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- DeJesus RS, et al. Associations between anxiety disorder diagnoses and body mass index differ by age, sex and race: a population based study. Clin Pract Epidemiol Ment Health. 2016;12:67–74. doi: 10.2174/1745017901612010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez LJ, Barbagallo M. The biology of the metabolic syndrome and aging. Curr Opin Clin Nutr Metab Care. 2016;19:5–11. doi: 10.1097/MCO.0000000000000243. [DOI] [PubMed] [Google Scholar]
- Ebke LA, et al. Tight association between macrophages and adipocytes in obesity: implications for adipocyte preparation. Obesity (Silver Spring) 2014;22:1246–1255. doi: 10.1002/oby.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderman M, et al. The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PLoS One. 2017;12:e0184274. doi: 10.1371/journal.pone.0184274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Heß N, Waldow F, Kohler TP, Rohde M, Kreikemeyer B, Gómez-Mejia A, Hain T, Schwudke D, Vollmer W, Hammerschmidt S, Gisch N (2017) Lipoteichoic acid deficiency permits normal growth but impairs virulence of Streptococcus pneumoniae. Nat Commun 8. 10.1038/s41467-017-01720-z [DOI] [PMC free article] [PubMed]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Hodes RJ, et al. Disease drivers of aging. Ann N Y Acad Sci. 2016;1386:45–68. doi: 10.1111/nyas.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaul E, Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health. 2017;5:335. doi: 10.3389/fpubh.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS One. 2012;7:e41009. doi: 10.1371/journal.pone.0041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C et al (2017) Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat Diet. MBio:8. 10.1128/mBio.00470-17 [DOI] [PMC free article] [PubMed]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Woo HJ, Kim KH, Kim E-R, Jung H-K, Juhn H-N, Lee HJ. Antitumor activity of Lactobacillus plantarum cytoplasm on teratocarcinoma-bearing mice. J Microbiol Biotechnol. 2002;12:998–1001. [Google Scholar]
- Konig J, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7:e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta C, Hansen M. C. elegans rrf-1 mutations maintain RNAi efficiency in the soma in addition to the germline. PLoS One. 2012;7:e35428. doi: 10.1371/journal.pone.0035428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landete JM, Gaya P, Rodriguez E, Langa S, Peiroten A, Medina M, Arques JL. Probiotic bacteria for healthier aging: immunomodulation and metabolism of phytoestrogens. Biomed Res Int. 2017;2017:5939818. doi: 10.1155/2017/5939818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack HI, Heimbucher T, Murphy CT (2019) The nematode Caenorhabditis elegans as a model for aging research Drug Discovery Today: Disease Models
- Mu Q, Kirby J, Reilly CM, Luo XM. Leaky gut as a danger signal for autoimmune diseases. Front Immunol. 2017;8:598. doi: 10.3389/fimmu.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R, et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4:267–285. doi: 10.3233/NHA-170030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R, Wang S, Ahmadi S, Hayes J, Gagliano J, Subashchandrabose S, Kitzman DW, Becton T, Read R, Yadav H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci Rep. 2018;8:12649. doi: 10.1038/s41598-018-30114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Uchiyama K, Takagi T. A next-generation beneficial microbe: Akkermansia muciniphila. J Clin Biochem Nutr. 2018;63:33–35. doi: 10.3164/jcbn.18-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Molina JA, et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013;531:371–444. doi: 10.1016/B978-0-12-407863-5.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Souza V, Cesar-Gomes CJ, Da Fonseca LJ, Guedes Gda S, Smaniotto S, Rabelo LA. Aging increases susceptibility to high fat diet-induced metabolic syndrome in C57BL/6 mice: improvement in glycemic and lipid profile after antioxidant therapy. Oxidative Med Cell Longev. 2016;2016:1987960. doi: 10.1155/2016/1987960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez J (2008) Morris Water Maze Experiment. J Vis Exp. 10.3791/897 [DOI] [PMC free article] [PubMed]
- O'Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- Ottman N, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One. 2017;12:e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaseyed T, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro CM, Hermsen T, Taverne-Thiele AJ, Savelkoul HF, Wiegertjes GF. Evolution of recognition of ligands from Gram-positive bacteria: similarities and differences in the TLR2-mediated response between mammalian vertebrates and teleost fish. J Immunol. 2010;184:2355–2368. doi: 10.4049/jimmunol.0900990. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Zhang H, Klaminder J, Brodin T, Andersson PL, Andersson M. ToxTrac: a fast and robust software for tracking organisms. Methods Ecol Evol. 2018;9:460–464. doi: 10.1111/2041-210X.12874. [DOI] [Google Scholar]
- Schiffrin EJ, Morley JE, Donnet-Hughes A, Guigoz Y. The inflammatory status of the elderly: the intestinal contribution. Mutat Res. 2010;690:50–56. doi: 10.1016/j.mrfmmm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Schroeder BO, Birchenough GMH, Stahlman M, Arike L, Johansson MEV, Hansson GC, Backhed F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe. 2018;23(27-40):e27. doi: 10.1016/j.chom.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y. Gut microbiota in common elderly diseases affecting activities of daily living. World J Gastroenterol. 2018;24:4750–4758. doi: 10.3748/wjg.v24.i42.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein MG, Ordanes D, Wylie AT, Files DC, Milligan C, Presley TD, Kavanagh K. Inducing muscle heat shock protein 70 improves insulin sensitivity and muscular performance in aged mice. J Gerontol A Biol Sci Med Sci. 2015;70:800–808. doi: 10.1093/gerona/glu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis GM, Petrascheck M (2011) Measuring Caenorhabditis elegans life span in 96 well microtiter plates. J Vis Exp. 10.3791/2496 [DOI] [PMC free article] [PubMed]
- Stehle JR, Jr, Leng X, Kitzman DW, Nicklas BJ, Kritchevsky SB, High KP. Lipopolysaccharide-binding protein, a surrogate marker of microbial translocation, is associated with physical function in healthy older adults. J Gerontol Series A: Biomed Sci Med Sci. 2012;67:1212–1218. doi: 10.1093/gerona/gls178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabloski PA. Global aging: implications for women and women’s health. J Obstet Gynecol Neonatal Nurs. 2004;33:627–638. doi: 10.1177/0884217504268655. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA. Using C. elegans for aging research. Invertebr Reprod Dev. 2015;59:59–63. doi: 10.1080/07924259.2014.940470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA. Using C. elegans for aging research. Invertebr Reprod Dev. 2015;59:59–63. doi: 10.1080/07924259.2014.940470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Vemuri R, et al. Gut microbial changes, interactions, and their implications on human lifecycle: an ageing perspective. Biomed Res Int. 2018;2018:4178607. doi: 10.1155/2018/4178607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virta JJ, Heikkila K, Perola M, Koskenvuo M, Raiha I, Rinne JO, Kaprio J. Midlife cardiovascular risk factors and late cognitive impairment. Eur J Epidemiol. 2013;28:405–416. doi: 10.1007/s10654-013-9794-y. [DOI] [PubMed] [Google Scholar]
- Wang YM, Ge XZ, Wang WQ, Wang T, Cao HL, Wang BL, Wang BM. Lactobacillus rhamnosus GG supernatant upregulates serotonin transporter expression in intestinal epithelial cells and mice intestinal tissues. Neurogastroenterol Motil. 2015;27:1239–1248. doi: 10.1111/nmo.12615. [DOI] [PubMed] [Google Scholar]
- Wijetunge S, Ratnayake R.M.C.J., Kotakadeniya H.M.S.R.B., Rosairo S., Albracht-Schulte K., Ramalingam L., Moustaid-Moussa N., Kalupahana N.S. (2019) Association between serum and adipose tissue resistin with dysglycemia in South Asian women. Nutr Diabetes 9:5 doi:10.1038/s41387-019-0071-3, [DOI] [PMC free article] [PubMed]
- Wrzosek L, Ciocan D., Borentain P., Spatz M., Puchois V., Hugot C., Ferrere G., Mayeur C., Perlemuter G., Cassard A.M. (2018) Transplantation of human microbiota into conventional mice durably reshapes the gut microbiota. Sci Rep 8:6854 doi:10.1038/s41598-018-25300-3 [DOI] [PMC free article] [PubMed]
- Yadav H, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J Funct Foods. 2017;33:194–201. doi: 10.1016/j.jff.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Мokrozub VV, Lazarenko LM, Sichel LM, Babenko LP, Lytvyn PM, Demchenko OM, Melnichenko YO, Boyko NV, Biavati B, DiGioia D, Bubnov RV, Spivak MY (2015) The role of beneficial bacteria wall elasticity in regulating innate immune response. EPMA Journal 6. 10.1186/s13167-015-0035-1 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 780 kb)