Abstract
Objectives
Ureaplasma urealyticum (UU), Chlamydia trachomatis (CT), and Neisseria gonorrhoeae (NG) are highly prevalent worldwide and may lead to some genital diseases. The objective of this large‐scale study was to estimate the prevalence characteristics of UU, CT, and NG among women in Taizhou, Zhejiang Province, China.
Methods
A total of 13 303 women who visited the gynecologic outpatient service of Taizhou First People's Hospital in Taizhou from 2013 to 2018 were analyzed. The testing of UU, CT, and NG was performed on the collected vaginal swabs using real‐time fluorescence quantitative polymerase chain reaction (RT‐PCR) method.
Results
The overall infection rates of UU, CT, and NG were 62.04%, 10.20%, and 4.09% in the Taizhou‐based population, respectively. The age‐specific prevalence showed that younger women (age <25 years) were the preferred period for the positive detection of UU or CT, while elder women (age ≥40 years) had the highest prevalence of NG. In addition, the UU‐CT co‐infection pattern (7.32%) predominated in the study population, and CT was significantly associated with UU and NG.
Conclusions
Our novel data demonstrated that UU, CT, and NG infection are prevalent among women in Taizhou, and comprehensive UU, CT, and NG screening guidelines and treatment policies for this population are warranted.
Keywords: Chlamydia trachomatis, Neisseria gonorrhoeae, prevalence, Ureaplasma urealyticum
1. BACKGROUND
Ureaplasma urealyticum (UU), Chlamydia trachomatis (CT), and Neisseria gonorrhoeae (NG) are the sexually transmitted pathogen and highly prevalent worldwide. UU is belonging to the Ureaplasma group, which are the smallest free‐living microorganisms. It is considered responsible for some genital diseases, such as nongonococcal urethritis, cervicitis, testicular inflammation, infertility, and prostatitis.1 CT is a non‐motile and Gram‐negative bacterium with a diameter of 0.2‐1.4 µm that tend to live inside the epithelial cells of human.2, 3 It is also the most common curable sexually transmitted organism that causes pelvic inflammatory disease and infects 4‐5 million new cases every year worldwide.4, 5 NG is a Gram‐negative diplococcus and the causal agent of more than 100 million cases of gonorrhea around the world each year.6 Not all colonized patients show obvious signs and symptoms of UU, CT, or NG infections. However, these sexually transmitted infections (STIs) may lead to adverse pregnancy outcome, such as membranes, and low birthweight in newborn and preterm delivery.7, 8
Compared with men, women, especially women in low‐income countries, are more susceptible to STIs than men. We conducted this study on 13 303 women from gynecological outpatients to evaluate the prevalence characteristics of UU, CT, and NG in the Taizhou area of Zhejiang Province, China, from 2013 to 2018; therefore, policy makers could identify and address their specific needs for the prevention, testing, and treatment of STIs.
2. MATERIALS AND METHODS
2.1. Study population
This is a large‐scale and cross‐sectional study. We recruited 13 303 women who visited the gynecologic outpatient service of Taizhou First People's Hospital (Taizhou, China) for various reasons, between January 1, 2013, and August 31, 2018, and had a testing of UU, CT, or NG. The mean age of the patients is 32.30 years (range: 13‐89 years). The inclusion criteria for the study were as follows: (a) women who were living in the Taizhou area; (b) women who were attending first time for UU, CT, or NG screening in the past 12 months; and (c) pregnancy and lactation women were also included. The exclusion criteria for the study were as follows: (a) children under the age of 12; (b) women who were in their second round of screening or more; (c) women who did use vaginal medication in the past 7 days; and (d) women who were receiving antibiotics in the past 2 weeks. The study was approved by the Institutional Medical Ethics Review Board of Taizhou First People's Hospital in Zhejiang Province. All participants signed the informed consent form. Anonymous was ensured during the data analyzed.
2.2. Clinical specimens, laboratory testing, and molecular biological methods
Vaginal swabs were obtained by a gynecologist and transported to molecular biology laboratory within 1 hour, and all specimens were completely detected within 24 hours. The presence of UU, CT, and NG was detected using real‐time fluorescence quantitative polymerase chain reaction (RT‐PCR) method (Hangzhou ACON Biotechnology Co., Ltd) in the StepOnePlus Real‐Time PCR System (Applied Biosystems). For each assay, amplification reaction mixtures consisted of: 0.4 µL Taq DNA polymerase, 35.6 µL of PCR buffer [10 mmol/L Tris‐HCl (pH8.9), 50 mmol/L KCl, 4 mmol/L MgCl2, 100 µmol/L of deoxynucleoside triphosphate (dTTP, dATP, dGTP, dCTP), 100 nmol/L of primers], and 4 µL of sample DNA. PCR conditions were as follows: pre‐denaturation at 95°C for 3 minutes, 40 cycles of denaturation at 94°C for 15 seconds, and annealing at 60°C for 30 seconds. Negative control, positive control, and weak positive control were performed in each assay (Hangzhou ACON Biotechnology Co., Ltd). Positive UU (CT or NG) diagnostic criteria were as follows: Cycle threshold value ≤37 was diagnosed as positive, and cycle threshold value >37 was diagnosed as negative. Clinical and Laboratory Standards Institute guidelines were used to ensure the accuracy of the testing results. At the same time, the testing is included in the external quality assessment (EQA), which is usually organized by National Center for Clinical Laboratory (NCCL) of China (twice every year). The results of EQA indicate that the quality of products from this company should be reliable. The duplex measurements might perform if the cycle threshold value was 37‐40. The limit of detection of this assay is 500 copies/mL.
2.3. Statistical analyses
All statistical analyses were conducted using SPSS 17.0 software (SPSS, Inc). Chi‐square test or Fisher's exact test was used to assess the statistical significance of any differences in prevalence or distributions, and the difference in age between women screened for UU, CT, or NG was tested with t‐test. Furthermore, Spearman linear correlation test was used to verify a trend analysis. P value was two‐sided, and statistical tests were considered significant if P value was .05 or less.
3. RESULTS
3.1. Characteristics of the study population
Among 13 303 women screened, we obtained 6190 specimens for UU testing, 11 242 specimens for CT testing, and 8289 specimens for NG testing. In this population, UU, CT, and NG were found in 3840 (62.04%), 1147 (10.20%), and 339 (4.09%) women, respectively. The average age of women screened for UU, CT, and NG was 31.93 years (SD: 8.57, range: 14‐89 years), 31.82 years (SD: 9.02, range: 14‐89 years), and 32.31 years (SD: 9.84, range: 13‐89 years), respectively. The average age of the UU‐positive, CT‐positive, and NG‐positive groups was 31.49 years (SD: 8.38), 29.24 years (SD: 8.83), and 37.37 years (SD: 14.33), respectively, whereas the average age of the UU‐negative, CT‐negative, and NG‐negative groups was 32.66 years (SD: 8.82), 32.11 years (SD: 8.99), and 32.09 years (SD: 9.54), respectively. The differences between positive and negative groups of UU, CT, and NG regarding average age were statistically significant (t = 5.252, 10.264, −6.721, all P < .001).
3.2. Prevalence of UU, CT, and NG among the study population
The infection rates of UU, CT, and NG were 62.04% (3840/6190, 95% CI 60.83%‐63.24%), 10.20% (1147/11242, 95% CI 9.64%‐10.76%), and 4.09% (339/8289, 95% CI 3.66%‐4.52%) in the Taizhou‐based population, respectively. The prevalence of UU was significantly higher than that of CT (χ 2 = 5251.161, P < .001) or NG (χ 2 = 5251.161, P < .001), whereas the prevalence of CT was significantly higher than that of NG (χ 2 = 253.636, P < .001).
3.3. Prevalence of UU, CT, and NG in different age groups
The distribution of UU, CT, and NG according to age groups is shown in Figure 1. The highest prevalence of UU or CT infection was observed in younger women (age <25 years), while the highest prevalence of NG infection was observed in elder women (age ≥40 years). Comparing age groups showed that the prevalence rates of UU and CT decreased with increasing age (r = −.057, −.103, all P < .001); at the same time, the prevalence rate of NG peaked the first time below the age of 25 years and decreased thereafter until the age between 35 and 39 years, where it reached the lowest level, after that, it increased and exhibited its second peak in the older age group (≥40 years).
Figure 1.
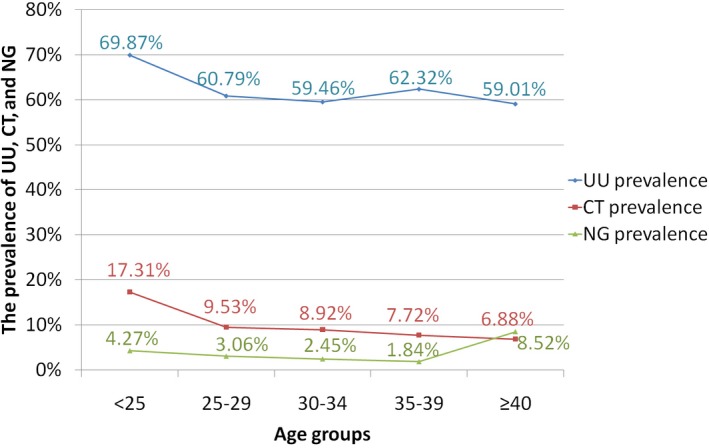
The prevalence of UU, CT, and NG in different age groups
3.4. Prevalence of UU, CT, and NG during 2013‐2018
The rates of UU and NG infection were significantly different during 2013‐2018 (χ 2 = 23.471, 30.773, all P < .001), while no difference was found in the rate of CT infection during 2013‐2018 (P = .211; Figure 2). Spearman linear correlation analysis indicated that UU and NG did not increase from 2013 to 2018 (P = .735, .790).
Figure 2.
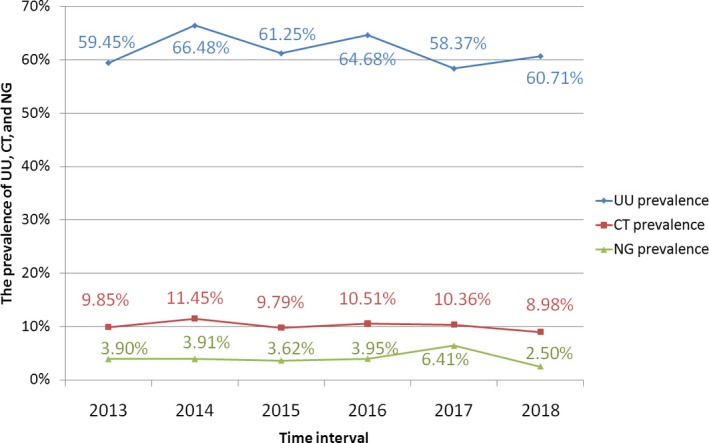
Distribution of UU, CT, and NG in Taizhou, 2013‐2018
3.5. The co‐infection of UU, CT, and NG
The most frequently present co‐infection was UU and CT in 7.32% (349/4765), followed by UU and NG in 2.64% (70/2647), CT and NG in 0.74% (56/7596), and the least frequently present co‐infection was UU, CT, and NG in 0.69% (18/2590). The distribution of UU, CT, and NG co‐infection was determined on the basis of age (Figure 3). The prevalence of UU‐CT co‐infection exhibited its peak below the age of 25 years (13.64%, 127/931) and decreased thereafter. Spearman linear correlation analysis indicated that the prevalence of UU‐CT co‐infection decreased with increasing age (r = −.095, P < .001). No strong evidence of a difference in the distribution of UU‐NG co‐infection, CT‐NG co‐infection, and UU‐CT‐NG co‐infection regarding age was observed (χ 2 = 7.824, 4.666, 1.757, P = .948, .902, .697).
Figure 3.
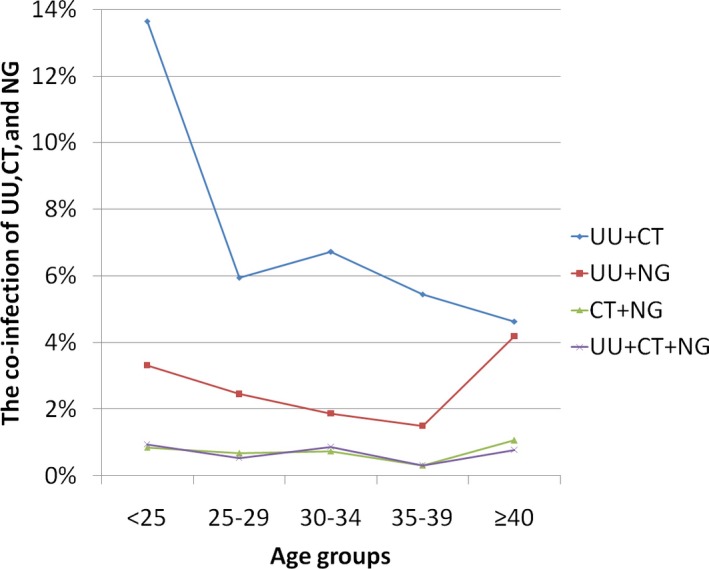
The positive rates of UU, CT, and NG co‐infection in different age groups
3.6. The relationship between the infection of UU, CT, and NG
Table 1 shows the relationship between the infection of UU, CT, and NG. The infection rate of CT was higher in the UU‐positive group (349/3134, 11.14%) than that in the UU‐negative group (81/1631, 4.97%; χ 2 = 49.737, P < .001). There was no significant difference for the infection rate of NG between the UU‐positive group (4.02%) and the UU‐negative group (2.65%; χ 2 = 3.220, P = .073). The infection rate of CT was higher in the NG‐positive group (22.49%) than that in the NG‐negative group (10.19%; χ 2 = 38.428, P < .001). Therefore, CT was significantly associated with UU and NG, whereas no association between NG and UU was found.
Table 1.
The relationship between the infection of UU, CT, and NG
| Positive number | Total number | % | Statistical significance, P | |
|---|---|---|---|---|
| CT infection | ||||
| UU‐positive group | 349 | 3134 | 11.14 | χ 2 = 49.737, P < .001 |
| UU‐negative group | 81 | 1631 | 4.97 | |
| NG infection | ||||
| UU‐positive group | 70 | 1743 | 4.02 | χ 2 = 3.220, P = .073 |
| UU‐negative group | 24 | 904 | 2.65 | |
| CT infection | ||||
| NG‐positive group | 56 | 249 | 22.49 | χ 2 = 38.428, P < .001 |
| NG‐negative group | 749 | 7347 | 10.19 | |
4. DISCUSSION
The infection of UU, CT, or NG is associated with urogenital disease, additional STI syndromes and complications, and adverse pregnancy outcome in women. This study provides information on the prevalence of UU, CT, and NG in gynecological outpatients in Taizhou, Zhejiang Province, southeast China. In China, few large‐scale studies have been published regarding the prevalence of UU, CT, NG, and their relationship. In this study, UU was detected in 62.04% of the 6190 enrolled women, CT was found in 10.20% of the 11 242 enrolled women, and NG was isolated in 4.09% of the 8289 enrolled women. Compared with region‐based data on the worldwide population, the infection rate of UU (62.04%) in the present study was higher than rates reported in a meta‐analysis (1%‐62%),9 but was similar to the rate in Changzhou (60.11%),10 which is another region in China. Because of the high infection rate of UU in Taizhou area, clinicians should carry out routine testing for UU in high‐risk women, including women infected with other STI agents, symptomatic women with cervicitis, urethritis and other gynecological diseases, women planning for pregnancy, and sex workers. However, part of women colonized with UU would not develop disease, and routine treatment in these asymptomatic women for UU might result in antimicrobial resistance; therefore, clinicians are more difficult to make therapy decision for asymptomatic women. The infection rate of CT (10.20%) in the present study was lower than that in Iranian women (15%),11 but was similar to that in Shanghai (10.13%),12 southeast China. The proportion of women infected with NG in this study was within the range reported in a previous meta‐analysis (0.7%‐9.9%).13 The prevalence of UU, CT, and NG was wide‐ranging because of the variations of the clinical and laboratory methods of the studies (culture, PCR, or antigen) and the different sources of sample (cervix, or peritoneum, amniotic fluid).14, 15 Likewise, the different economic conditions, genetic variations, cultural diversity, and different lifestyles also affect the prevalence.12, 16
Moreover, our results illustrated that the infection rates of UU, CT, and NG were related to age. Our study further confirmed that the younger age group (<25 years) had the highest prevalence rate of UU and CT, which had also been reported in recent studies.1, 4, 17, 18 It might be due to the fact that women aged <25 years were just after sexual debut, and the genital tissues were still immature. In addition, this study found that the age‐specific NG distribution presented a bimodal curve, the younger age group (<25 years) had the higher prevalence rate, the middles ages had a lower prevalence rate, and women aged ≥40 years had the highest susceptibility to NG infection. The distinct increase in the NG prevalence rate in older women (≥40 years) might be associated with the poor hygiene of older women and host susceptibility. The distribution of NG according to age groups had a marked variation in previous Chinese studies. Xu WH et al12 found that women aged 21‐30 had the highest prevalence rate of NG in Shanghai, while Huai P et al16 and Luo ZZ et al19 reported that the prevalence differences of NG infection between age groups were not statistically significant. Comparing our findings with different areas in China is difficult because of a variety in ethnic and social populations. Moreover, large differences in regular screening, treatment, and control programs may also influence the distribution characteristics. Our study reflected the natural history of NG infection in Taizhou area.
From 2013 to 2018, the overall UU, CT, or NG infection rate revealed no upward trend. However, we failed to show any decline in the prevalence of UU, CT, or NG, and the overall UU, CT, and NG prevalence found here was distinctly higher than estimates from high‐income countries.20, 21 Comprehensive screening guidelines and treatment policies should be conducted to control the population prevalence.
Based on our results, the UU‐CT co‐infection pattern predominated in the study population, and CT colonization seemed to contribute to UU or NG infection. To date, to the best of our knowledge, this is the first study in which we find relationship between the infection of CT and UU (or NG); however, the specific mechanisms were not clear, thus further research might give new answers for the very limited understanding of the STIs.
Some limitations should be addressed when interpreting our results. First of all, it is based on a random population of women who visited the gynecologic outpatient service of Taizhou First People's Hospital. The annual amount of gynecological outpatients in Taizhou First People's Hospital exceeds 100 000 people and may represent the prevalence of UU, CT, and NG in Taizhou area, which is a city with a population of 6 000 000. However, as a single‐center study, it might have introduced some biases in the present study. Second, this was a record‐based study. Some included women did not detect UU, CT, and NG at the same time. Furthermore, it was a study designed for outpatient; thus, detailed information of patient connected with clinical significance was insufficient. Third, using gynecologic outpatient as our target may cause some biases in the study; nevertheless, it is a real‐world and hospital‐based study in the clinical practice. Our study reflected the infection rates of UU, CT, and NG in real world for gynecological outpatients. The infection rates of UU, CT, and NG reported in our paper may be slightly higher than those in health check people. However, our study is of guiding significance for people visiting the hospital for various reasons and may give more advices for clinicians to make diagnosis and therapy decision. Fourth, the conclusions of our study might not be applicable to all areas in Zhejiang Province, China.
In conclusion, our study retrospectively analyzed the prevalence of UU, CT, and NG in women from gynecological outpatients regardless of symptom status. The prevalence and distribution of UU, CT, and NG seem to be age‐related, UU and CT being significantly more often found in younger and NG in older women. CT is distinctly associated with the infection of UU or NG. More well‐designed large‐scale studies investigating the lifestyle, demographic, and behavioral characteristics of STIs are warranted in the future.
Cai S, Pan J, Duan D, Yu C, Yang Z, Zou J. Prevalence of Ureaplasma urealyticum, Chlamydia trachomatis, and Neisseria gonorrhoeae in gynecological outpatients, Taizhou, China. J Clin Lab Anal. 2020;34:e23072 10.1002/jcla.23072
Cai and Pan contributed equally to this work.
Contributor Information
Zaixing Yang, Email: yangzaixingdiyi@163.com.
Jinyan Zou, Email: wyzoujinyan@163.com.
REFERENCES
- 1. Verteramo R, Patella A, Calzolari E, et al. An epidemiological survey of Mycoplasma hominis and Ureaplasma urealyticum in gynaecological outpatients, Rome, Italy. Epidemiol Infect. 2013;141(12):2650‐2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanski L, Vuorela P. Lead discovery strategies for identification of chlamydia pneumoniae inhibitors. Microorganisms. 2016;4(4):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmadi A, Ramazanzadeh R, Sayehmiri K, Sayehmiri F, Amirmozafari N. Association of Chlamydia trachomatis infections with preterm delivery; a systematic review and meta‐analysis. BMC Pregnancy Childbirth. 2018;18(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hussen S, Wachamo D, Yohannes Z, Tadesse E. Prevalence of chlamydia trachomatis infection among reproductive age women in sub Saharan Africa_ a systematic review and meta‐analysis. BMC Infect Dis. 2018;18(1):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newman L, Rowley J, Hoorn SV, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE. 2015;10(12):e0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wadsworth CB, Arnold BJ, Sater M, Grad YH. Azithromycin resistance through interspecific acquisition of an epistasis‐dependent efflux pump component and transcriptional regulator in neisseria gonorrhoeae. MBio. 2018;9(4): e01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitsunari M, Yoshida S, Deura I, et al. Cervical Ureaplasma urealyticum colonization might be associated with increased incidence of preterm delivery in pregnant women without prophlogistic microorganisms on routine examination. J Obstet Gynaecol Res. 2005;31(1):16‐21. [DOI] [PubMed] [Google Scholar]
- 8. Salfa MC, Suligoi B; Italian STI Laboratory‐based Surveillance Working Group . Prevalence of chlamydia trachomatis, trichomonas vaginalis and neisseria gonorrhoeae based on data collected by a network of clinical microbiology laboratories, in Italy. Adv Exp Med Biol. 2016;901:47‐57. [DOI] [PubMed] [Google Scholar]
- 9. Kletzel HH, Rotem R, Barg M, Michaeli J, Reichman O. Ureaplasma urealyticum: the role as a pathogen in women's health, a systematic review. Curr Infect Dis Rep. 2018;20(9):33. [DOI] [PubMed] [Google Scholar]
- 10. Zhu C, Liu J, Ling Y, et al. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Chinese women with genital infectious diseases. Indian J Dermatol Venereol Leprol. 2012;78(3):406‐407. [DOI] [PubMed] [Google Scholar]
- 11. Roshani D, Ramazanzadeh R, Farhadifar F, et al. A PRISMA systematic review and meta‐analysis on Chlamydia trachomatis infections in Iranian women (1986–2015). Medicine. 2018;97(16):e0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu WH, Chen JJ, Sun Q, et al. Chlamydia trachomatis, Ureaplasma urealyticum and Neisseria gonorrhoeae among Chinese women with urinary tract infections in Shanghai_ A community‐based cross‐sectional study. J Obstet Gynaecol Res. 2018;44(3):495‐502. [DOI] [PubMed] [Google Scholar]
- 13. Tamarelle J, Thiébaut A, de Barbeyrac B, Bébéar C, Ravel J, Delarocque‐Astagneau E. The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections_ a systematic review and meta‐analysis. Clin Microbiol Infect. 2019;25(1):35-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Capoccia R, Greub G, Baud D. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis. 2013;26(3):231‐240. [DOI] [PubMed] [Google Scholar]
- 15. Yoneda N, Yoneda S, Niimi H, et al. Polymicrobial amniotic fluid infection with mycoplasma/ureaplasma and other bacteria induces severe intra‐amniotic inflammation associated with poor perinatal prognosis in preterm labor. Am J Reprod Immunol. 2016;75(2):112‐125. [DOI] [PubMed] [Google Scholar]
- 16. Huai P, Li F, Li Z, et al. Prevalence, risk factors, and medical costs of Chlamydia trachomatis infections in Shandong Province, China_ a populationbased, cross‐sectional study. BMC Infect Dis. 2018;18(1):534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmadi MH, Mirsalehian A, Bahador A. Prevalence of genital in Iran: a systematic review and meta‐analysis. Pathog Glob Health. 2015;109(6):290‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dielissen PW, Teunissen DA, Lagro‐Janssen AL. Chlamydia prevalence in the general population: is there a sex difference? A systematic review. BMC Infect Dis. 2013;13(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luo ZZ, Li W, Wu QH, et al. Population‐based study of chlamydial and gonococcal infections among women in Shenzhen, China: Implications for programme planning. PLoS ONE. 2018;13(5):e0196516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heijne J, Broek I, Bruisten SM, Bergen J, Graaf HD, Benthem B. National prevalence estimates of chlamydia and gonorrhoea in the Netherlands. Sexually Transm Infect. 2019;95(1):53-59. [DOI] [PubMed] [Google Scholar]
- 21. Pam S, Soazig C, Simon B, et al. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet. 2013;382(9907):1795‐1806. [DOI] [PMC free article] [PubMed] [Google Scholar]


