Abstract
To investigate whether high body mass index (BMI) had adverse effects on the postoperative outcomes for patients who underwent anterior or posterior cervical fusion procedures. A literature search were conducted in PubMed, Embase, and Web of Science. Comparative or controlled studies that examined the influences of high BMI on postoperative outcomes after cervical fusion procedures were included. Using a fixed‐effect model or random‐effect model, the effects of high BMI were determined by weight mean difference (WMD) with 95% confidence interval (95% CI) or risk ratio (RR) with 95% CI. A total of seven studies were included in this meta‐analysis. The pooled estimate showed that high BMI was associated with longer hospital stay (WMD = 1.61 days, 95% CI: 0.51, 2.71; P = 0.004), longer surgical time (WMD = 4.55, 95% CI: 1.04, 8.07; P = 0.011), higher mortality rate (RR = 3.01, 95% CI: 2.75, 3.29; P < 0.001), and higher postoperative rates of cardiac complication (RR = 1.30, 95% CI: 1.11, 1.52; P = 0.001), deep venous thromboembolism (RR = 2.29, 95% CI: 1.36, 3.86; P = 0.002), and wound complication (RR = 1.69, 95% CI: 1.26, 2.28; P < 0.001). However, there was no significant differences between high and normal BMI groups in terms of Neck Disability Index (WMD = 1.49, 95% CI: −2.34, 5.32; P = 0.447), SF‐36 Mental Component Score (MCS) (WMD = −0.87, 95% CI: −2.09, 0.35; P = 0.164), overall complications (RR = 1.18, 95% CI: 0.80, 1.76; P = 0.399), central nervous system (CNS) complications (RR = 0.68, 95% CI: 0.17, 2.76; P = 0.586), pulmonary complications (RR = 1.46, 95% CI: 0.87, 2.46; P = 0.150), and septic complications (RR = 0.87, 95% CI: 0.32, 2.38; P = 0.785).High BMI seemed to be associated with longer hospital stay, surgical time, and higher postoperative complication rates compared to normal BMI. Therefore, high BMI patients should be counseled carefully regarding the risk of postoperative complications and surgical outcomes after cervical fusion procedures.
Keywords: Body mass index, Cervical spinal fusion, Meta‐analysis, Obesity, Outcomes
Introduction
Obesity is a growing public health concern in the United States, with an estimation of 78 mn population considered to be obese (body mass index [BMI] ≥ 30 kg/m2) according to the Centers for Disease Control and Prevention1. Obesity has been associated with significant comorbidities, including heart disease, hypertension, stroke, diabetes, and obstructive sleep apnea1, 2, 3. All these not only increase the medical costs4, but also have a heavy burden on the surgical care, such as longer hospitalizations and higher rate of postoperative complications5, 6.
A high BMI has adverse effects on spinal health, and this has been confirmed by increased rates of spinal degenerative disease among obese patients7. In terms of the cervical spine, obesity increased the risk of cervical myelopathy and radiculopathy8, 9. Moreover, obesity is also a predictor of surgical outcomes, especially in those who underwent spine surgery. There were several published studies5, 10, 11, 12, 13, 14 that investigated the impact of obesity on outcomes following spine surgery; however, their results remained controversial. Some studies have demonstrated that obesity was associated with longer operative time, increased blood loss, and increased risk of complications during spinal surgery5, 10, 11, 12, 13. Whereas, another study reported opposite findings, which showed that obesity was not associated with less improvement in patient‐reported outcomes following anterior cervical discectomy and fusion (ACDF)14. In order to investigate whether high BMI had adverse effects on the surgical outcomes for patients who underwent anterior or posterior cervical fusion procedures, we conducted this meta‐analysis.
Materials and Methods
Search Strategy
This meta‐analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) statement guidelines15. Since animal experiment or human was not involved in this study, the ethical approval was not necessary.
Relevant literatures published in PubMed, Embase, and Web of Science were systematically searched from their inception to 21 February 2019. Structured search strategies were listed as followings: obese, adiposity, body mass index, BMI, cervical spinal fusion (Appendix S1). We did not impose any language limitation in the search strategy. Moreover, the reference lists of review and included studies were also checked manually to identify additional relevant articles.
Study Inclusion Criteria and Exclusion Criteria
Studies meeting the following inclusion criteria were included in this meta‐analysis: (i) population: adult patients who underwent anterior or posterior cervical fusion; (ii) variable of interest: high BMI; (iii) comparison: normal BMI; (iv) outcome: surgical time, length of hospital stay, blood loss, Neck Disability Index, SF‐36 PCS/MCS, mortality rate, and postoperative complications; and (v) study design: cohort study, case‐control study, or controlled or comparative studies. Studies were excluded that: (i) studies did not focus on topics of interest; (ii) did not involve any one of the outcomes; and/or (iii) were reviews, letters, or case reports. Disagreement between investigators were resolved by discussion and consensus.
Data Extraction
A prestandardized data extraction form was used to extract data from the included studies. Two independent investigators performed the data extraction and the accuracy was checked by a third investigator. The following information was extracted from each study: author's name, publication year, country, study design, number of patients in each group, age, gender, surgical procedures, patient characteristics, and outcome measures.
Methodological Assessment
We assessed the methodological quality of a non‐randomized trial using the modified Newcastle‐Ottawa (NOS) scale16. This method consists of three items to evaluate the quality, including patient selection, comparability of the intervention/control group, and outcome assessment16. The total score was nine points, and studies with a score of more than five points were categorized as high quality16.
Statistical Analysis
Data analysis was performed using the STATA software version 12.0 (Stata Corporation, College Station, TX, USA). We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes, and weight mean difference (WMD) with 95% CIs for continuous outcomes. Meta‐analyses were performed using a fixed‐effects model17 or a random‐effects model18, according to the heterogeneity across the included studies. Statistical heterogeneity was assessed by Cochrane Q chi‐square test and I2 statistic19, in which A P value < 0.1 or I 2 > 50% represent significant heterogeneity. For the primary outcomes, subgroup analyses were carried out according to surgical procedures (anterior or posterior cervical fusions). BMI is defined as height (in meters) divided by weight (kilograms squared). Standard categories typically included normal weight (18.0–24.9 m/kg2), overweight (25.0–29.9 m/kg2), and obese (>30.0 m/kg2). When patients were stratified by BMI, and subgroup analysis based on comparison between normal and higher BMI was present, we extracted the subgroup data for analysis. The publication bias was assessed using Begg20 and Egger test21. A P value less than 0.05 was judged as statistically significant, except where otherwise specified.
Results
Search Strategy Results
Our initial search yielded a total of 573 records, of which 247 records remained after 326 duplicates were removed. Then 237 were excluded after title and abstract review, and three were excluded after the full‐text review. Finally, seven studies5, 14, 22, 23, 24, 25, 26 were included in this meta‐analysis. The flow diagram of the literature search process was shown in Fig. 1.
Figure 1.
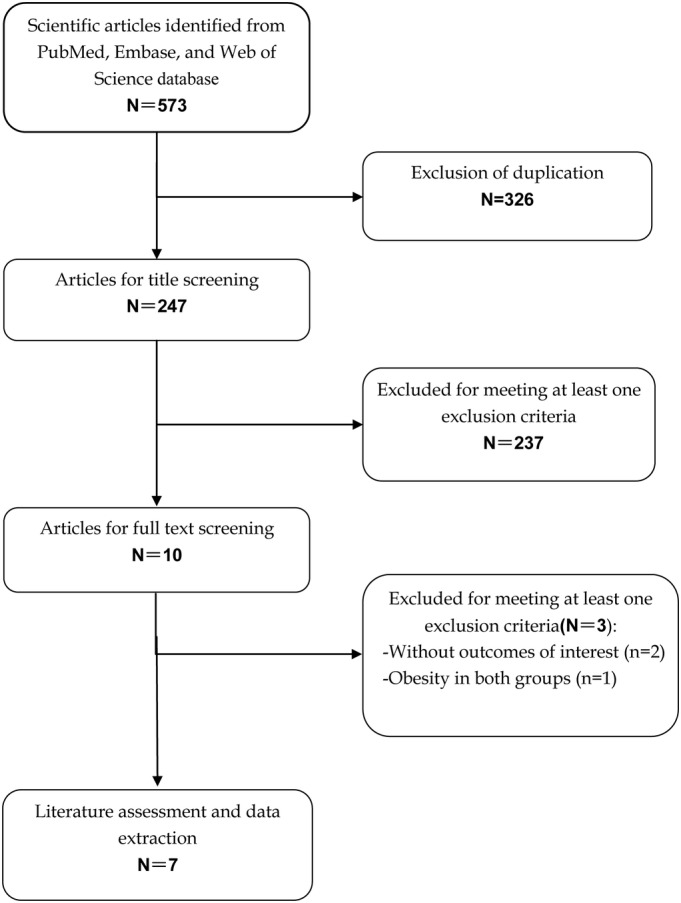
Eligibility of studies for inclusion in meta‐analysis.
Study Characteristics of Included Studies
The baseline characteristics of included studies is presented in Table 1. All the studies were conducted in the USA, and were published between 2010 and 2018. All the studies14, 22, 23, 24, 25, 26 were prospective or retrospective cohort studies except one5, which was a retrospective cross‐sectional study. Patients in three studies22, 23, 25 were categorized into the following groups according to their BMI: underweight (<18.5 kg/m2), normal weight (18.5–24.99 kg/m2), overweight (25–29.99 kg/m2), nonobese (18.5–29.9 kg/m2), obese I (30–34.9 kg/m2), obese II (35–39.9 kg/m2), or obese III (≥40 kg/m2). And in the remaining four studies5, 14, 24, 26 patients were divided into two groups, however, the definition of obesity among them varied greatly, which was >30, 35 or 40 kg/m2. Patients in four studies14, 23, 25, 26 received ACDF, one24 received posterior cervical fusion, and the remaining two5, 22 received ACDF or posterior cervical fusion.
Table 1.
Baseline characteristics of patients in the trials included in the meta‐analysis
| Study | Country | Publication year | Study design | BMI (kg/m2) | No. of patients | type of surgical procedure | Male/female | Age (mean ± SD, year) | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Kalanithi et al.5 | USA | 2012 | Retrospective cross‐sectional study | <25.0 | 83,152 | ACDF/posterior cervical fusion | NA | NA | 7 |
| ≥40 | 1455 | ACDF/posterior cervical fusion | NA | NA | |||||
| Sielatycki et al.14 | USA | 2016 | Prospective cohort | <35 | 219 | ACDF | 124/95 | 52.8 ± 10.3 | 7 |
| ≥35 | 80 | ACDF | 36/44 | 51.3 ± 9.2 | |||||
| Buerba et al.22 | USA | 2014 | Retrospective cohort | 18.5–29.9 | 2072 | ACDF | 1024/1003 | NA | 7 |
| 30–34.9 | 915 | ACDF | 508/407 | NA | |||||
| 35–39.9 | 419 | ACDF | 180/239 | NA | |||||
| ≥40 | 265 | ACDF | 97/168 | NA | |||||
| Narain et al.23 | USA | 2018 | Retrospective cohort | <25.0 | 58 | ACDF | 29/29 | 49.5 ± 11.3 | 7 |
| 25.0–29.9 | 104 | ACDF | 60/44 | 48.8 ± 9.2 | |||||
| 30.0–34.9 | 69 | ACDF | 44/25 | 51.8 ± 11.2 | |||||
| ≥35.0 | 46 | ACDF | 22/24 | 53.4 ± 11.2 | |||||
| Phan et al.24 | USA | 2017 | Retrospective cohort | ≤30 | 322 | Posterior cervical fusion | 184/138 | NA | 7 |
| >30 | 202 | Posterior cervical fusion | 103/99 | NA | |||||
| Wilson et al.25 | USA | 2017 | Retrospective cohort | <18.5 | 17 | ACDF | NA | NA | 6 |
| 18.5–24.99 | 271 | ACDF | NA | NA | |||||
| 25–29.99 | 275 | ACDF | NA | NA | |||||
| >30 | 194 | ACDF | NA | NA | |||||
| Sami Walid et al.26 | USA | 2010 | Retrospective cohort | <30 | 318 | ACDF | NA | NA | 6 |
| ≥30 | 287 | ACDF | NA | NA |
ACDF, anterior cervical discectomy and fusion; BMI, body mass index; NA, not available; SD, standard deviation.
The NOS score in five studies5, 14, 22, 23, 24 was seven and in two25, 26 was six, which indicated that these studies were of high quality.
Length of Hospital Stay
Three studies5, 23, 26 with nine sets of data reported the data of length of hospital stay. Pooled results showed that high BMI was associated with a significantly longer hospital stay than normal BMI (WMD = 1.61 days, 95% CI: 0.51, 2.71; P = 0.004) (Fig. 2). There was significant heterogeneity among the included studies (I2 = 50.4%, P = 0.040). When the outlier was removed, the pooled estimate changed a little (WMD = 1.81 days, 95% CI: 0.84, 2.78; P < 0.001), and the heterogeneity was still present (I2 = 42.6%, P = 0.095).
Figure 2.
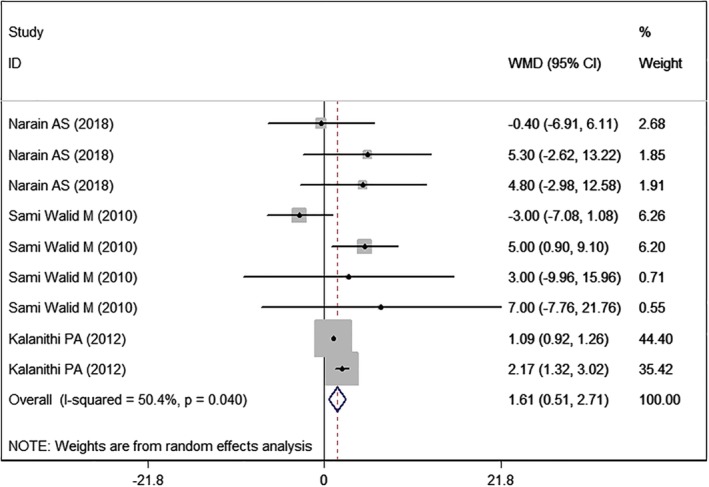
Forest plot showing the effect of high BMI on length of hospital stay.
Surgical Time
Two studies22, 23 with seven sets of data reported the data of surgical time. The pooled estimate suggested that high BMI was associated with a significantly longer surgical time than normal BMI (WMD = 4.55, 95% CI: 1.04, 8.07; P = 0.011) (Fig. 3). The test for heterogeneity was significant (I2 = 99.6%, P < 0.001). Thus, we conducted sensitivity. When the outlier was removed, the overall estimate did not change substantially (WMD = 4.94, 95% CI: 1.78, 7.66; P = 0.004), but the heterogeneity was still present (I2 = 99.3%, P < 0.001).
Figure 3.
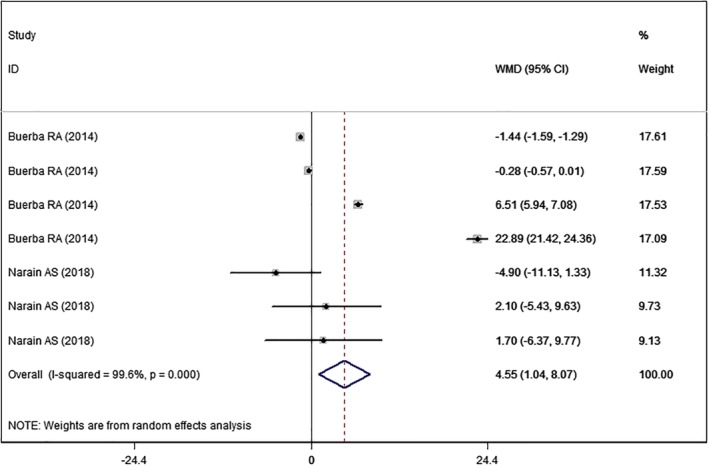
Forest plot showing the effect of high BMI on surgical time.
Neck Disability Index
Two studies14, 25 with six sets of data reported the Neck Disability Index. The pooled results showed that high BMI was associated with similar score in Neck Disability Index with normal BMI (WMD = 1.49, 95% CI: −2.34, 5.32; P = 0.447) (Fig. 4). There was significant heterogeneity among the included studies (I2 = 72.1%, P = 0.003). When we excluded the outlier, the overall estimate changed a little (WMD = 2.64, 95% CI: −1.06, 6.34; P = 0.162), but the heterogeneity was still present (I2 = 66.8%, P = 0.017).
Figure 4.
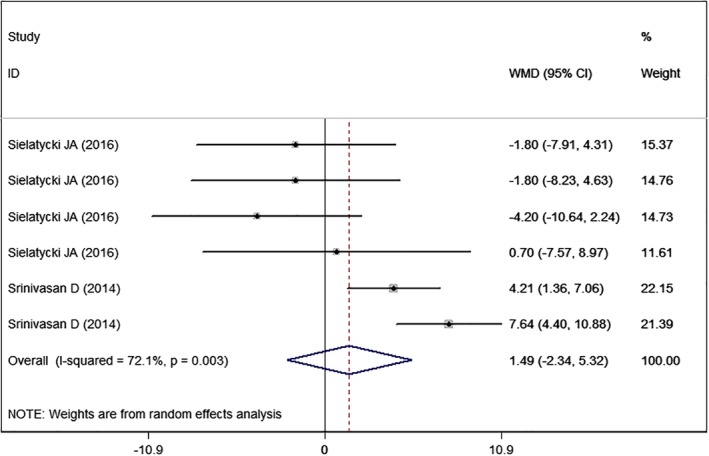
Forest plot showing the effect of high BMI on Neck Disability Index.
Overall Complications
All the studies5, 14, 22, 23, 24, 25, 26 reported the data of overall complications. However, only four studies provided 10 sets of available data. Pooled estimate showed that high BMI was associated with a similar complication rate with normal BMI (RR=1.18, 95% CI: 0.80, 1.76; P = 0.399) (Fig. 5).
Figure 5.
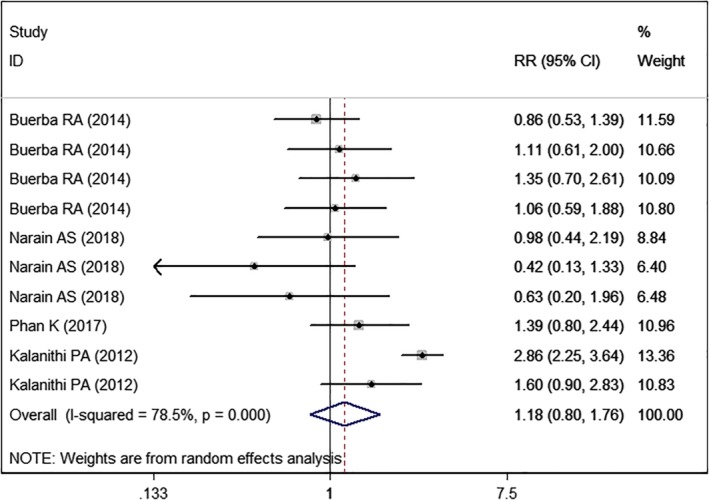
Forest plot showing the effect of high BMI on overall complications.
SF‐36 PCS
Two studies14, 25 with six sets of data reported the SF‐36 PCS. The aggregated results suggested that high BMI was associated with a significantly less score in SF‐36 PCS than normal BMI (WMD = −1.65, 95% CI: −2.91, −0.39; P = 0.010) (Fig. 6). There was no evidence of heterogeneity among the included studies (I2 = 16.2%, P = 0.309).
Figure 6.
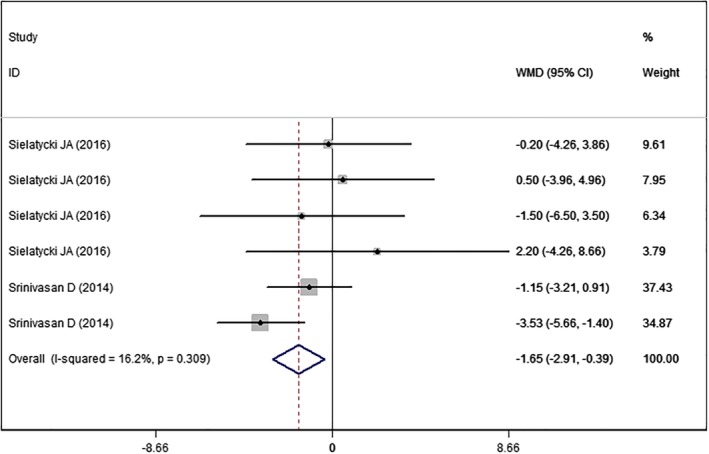
Forest plot showing the effect of high BMI on SF‐36 PCS.
SF‐36 MCS
Two studies14, 25 with six sets of data reported the SF‐36 MCS. The aggregated results showed that high BMI was associated with a similar SF‐36 MCS score as normal BMI (WMD = −0.87, 95%CI: −2.09, 0.35; P = 0.164) (Fig. 7). There was no significant heterogeneity among the included studies (I2 = 0.0%, P = 0.478).
Figure 7.
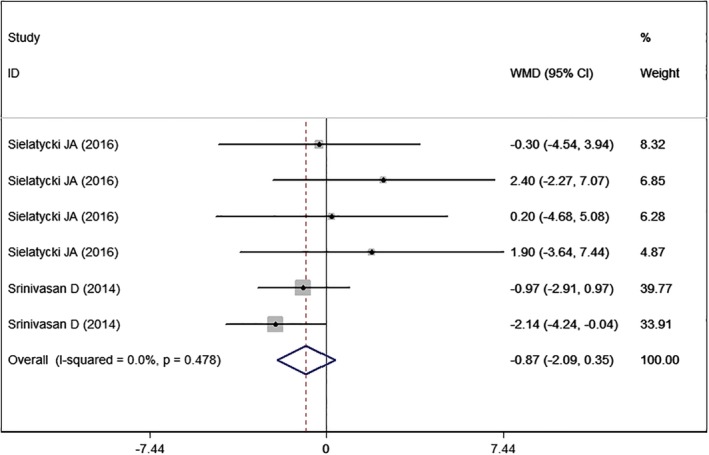
Forest plot showing the effect of high BMI on SF‐36 MCS.
Cardiac Complications
Three studies5, 22, 24 with eight sets of data reported cardiac complications. Pooled estimate showed that high BMI was associated with a significantly higher rate of cardiac complications than normal BMI (RR = 1.30, 95% CI: 1.11, 1.52; P = 0.001) (Fig. 8). The test for heterogeneity was not significant (I2 = 0.0%, P = 0.822).
Figure 8.
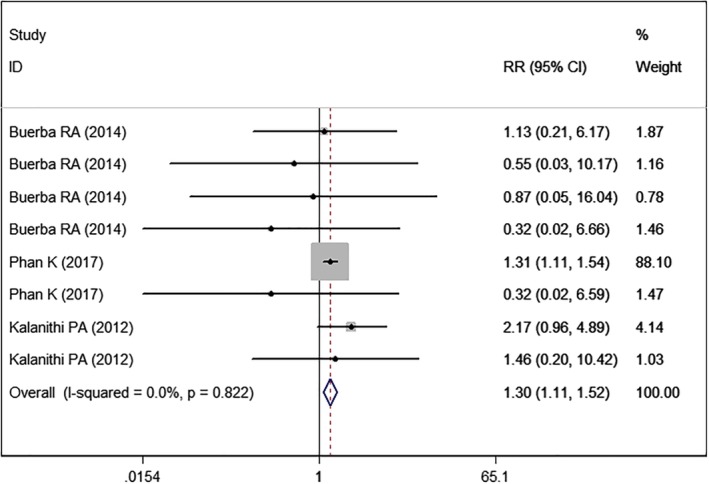
Forest plot showing the effect of high BMI on cardiac complications.
Mortality
Three studies5, 22, 24 with seven sets of data reported the mortality rate. Pooled estimate showed that high BMI was associated with a significantly higher mortality rate than normal BMI (RR = 3.01, 95% CI: 2.75, 3.29; P < 0.001) (Fig. 9). There was no significant heterogeneity (I2 = 46.0%, P = 0.085).
Figure 9.
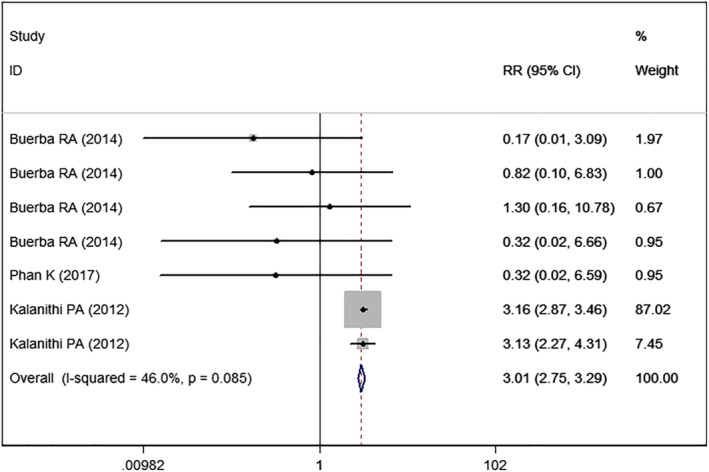
Forest plot showing the effect of high BMI on mortality.
Central Nervous System (CNS) Complications
Three studies5, 22, 24 with eight sets of data reported the CNS complications. Pooled estimate suggested that high BMI was associated with a similar rate of CNS complications than normal BMI (RR = 0.68, 95% CI: 0.17, 2.76; P = 0.586) (Fig. 10). The test for heterogeneity was significant (I2 = 88.0%, P < 0.005).
Figure 10.
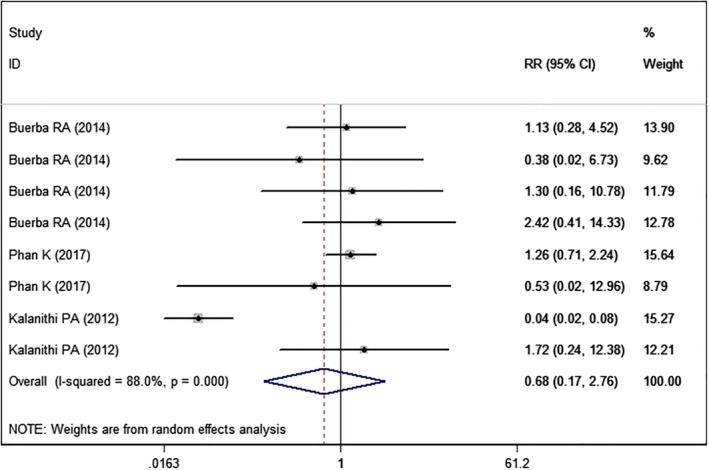
Forest plot showing the effect of high BMI on CNS complications.
Deep Venous Thromboembolism
Three studies5, 22, 24 with seven sets of data reported DVT. The pooled estimate showed that high BMI was associated with a higher rate of DVT than normal BMI (RR = 2.29, 95% CI: 1.36, 3.86; P = 0.002) (Fig. 11). The test for heterogeneity was significant (I2 = 51.8%, P = 0.053).
Figure 11.
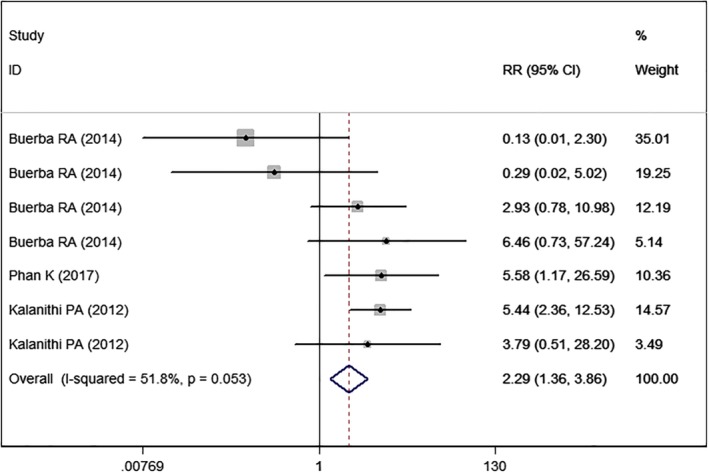
Forest plot showing the effect of high BMI on deep venous thromboembolism.
Pulmonary Complications
Three studies5, 22, 24 with eight sets of data reported pulmonary complications. The pooled results demonstrated that high BMI was associated with a similar rate of pulmonary complications (RR = 1.46, 95% CI: 0.87, 2.46; P = 0.150) (Fig. 12). The test for heterogeneity was significant (I2 = 65.2%, P = 0.001).
Figure 12.
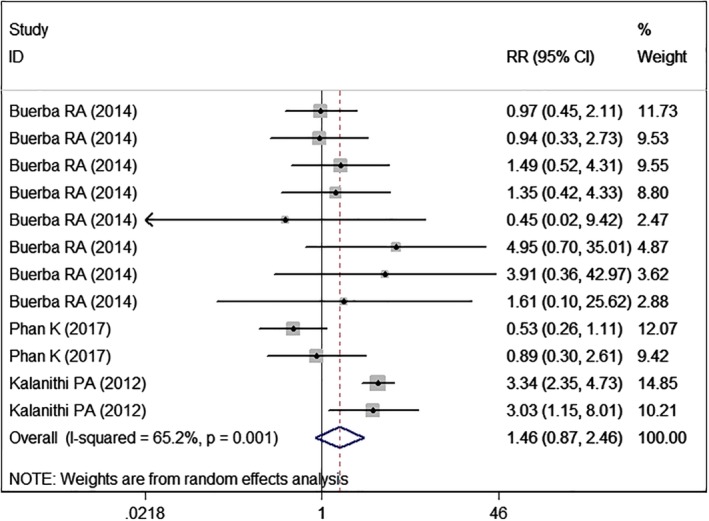
Forest plot showing the effect of high BMI on pulmonary complications.
Septic Complications
Two studies22, 24 with five sets of data reported the septic complications. The pooled results showed that the septic complication rate was comparable between higher BMI and normal BMI groups (RR = 0.87, 95% CI: 0.32, 2.38; P = 0.785) (Fig. 13). The test for heterogeneity was not significant (I2=0.0%, P = 0.755).
Figure 13.
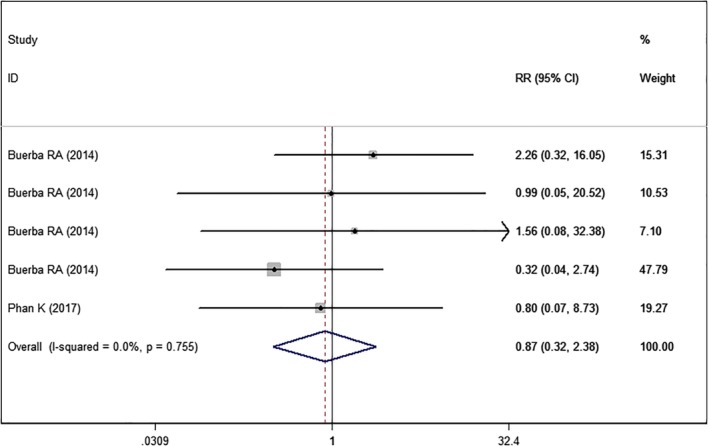
Forest plot showing the effect of high BMI on septic complications.
Wound Complications
Three studies5, 22, 24 with seven sets of data reported the wound complications. Pooled estimate suggested that patients with high BMI had a significantly higher rate of wound complications than those with normal BMI (RR = 1.69, 95% CI: 1.26, 2.28; P < 0.001) (Fig. 14). The test for heterogeneity was not significant (I2 = 38.2%, P = 0.138).
Figure 14.
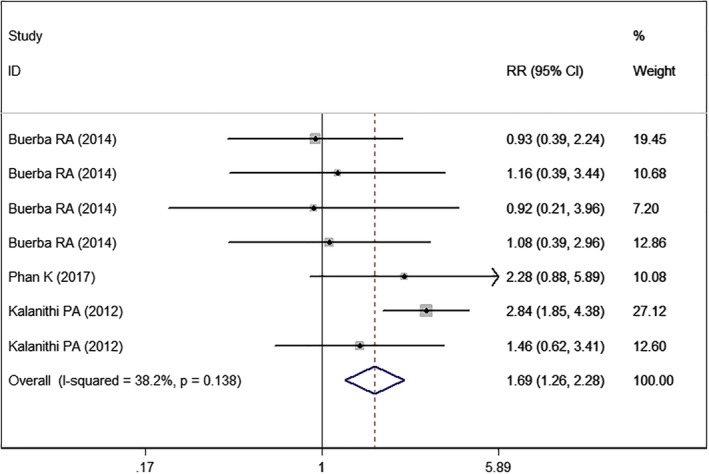
Forest plot showing the effect of high BMI on wound complications.
Subgroup Analysis
We conducted subgroup analysis based on the type of surgical procedure. In patients who underwent ACDF, high BMI had no influence on the surgical outcomes, including hospital stay (WMD = 1.53 days, 95% CI: −0.46, 3.53; P = 0.131), surgical time (WMD = 0.94, 95% CI: −1.78, 3.66; P = 0.497), cardiac complications (RR = 1.56, 95% CI: 0.79, 3.11; P = 0.204), CNS complications (RR = 0.35, 95% CI: 0.04, 3.22; P = 0.351), morality rate (RR = 0.56, 95% CI: 0.16, 1.99; P = 0.37), DVT (RR = 1.55, 95% CI: 0.84, 2.84; P = 0.161), pulmonary complications (RR = 1.76, 95% CI: 0.93, 3.32; P = 0.082), wound complications (RR = 1.48, 95% CI: 0.74, 2.98; P = 0.268), and septic complications (RR = 1.70, 95% CI: 0.41, 7.10; P = 0.464).
Whereas, in patients who underwent posterior cervical fusion, high BMI had adverse effects on hospital stay (WMD = 2.17 days, 95% CI: 1.32, 3.02; P < 0.001), surgical time (WMD = 22.89, 95% CI: 21.42, 24.36; P < 0.001), cardiac complications (RR = 1.28, 95% CI: 1.09, 1.50; P = 0.003), and DVT (RR = 5.49, 95% CI: 1.80, 16.73; P = 0.003). For other outcomes, such as cardiac complications (RR = 1.28, 95% CI: 1.09, 1.50; P = 0.003), CNS (RR = 1.33, 95% CI: 0.79, 2.24; P = 0.280), morality (RR = 0.32, 95% CI: 0.04, 2.73; P = 0.298), pulmonary complications (RR = 1.15, 95% CI: 0.56, 2.37; P = 0.705), wound complications (RR = 1.54, 95% CI: 0.90, 2.64; P = 0.113), and septic complications (RR = 0.48, 95% CI: 0.10, 2.20; P = 0.330), high BMI had no effect.
We conducted subgroup analysis based on the obesity categories. Compared with nonobese patients, obese III patients had a longer surgical time (WMD = 6.49, 95% CI: 5.92, 7.05; P < 0.001), whereas obese I (WMD = −1.44, 95% CI: −1.59, 0.29; P = 0.074) and obese II (WMD = −0.28, 95% CI: −0.57, 0.01; P = 0.063) patients did not. There was no significant differences between nonobese and obese I/II/III groups in overall complication rate (RR = 0.89, 95% CI: 0.58, 1.34, P = 0.566; RR = 0.90, 95% CI: 0.53, 1.50, P = 0.678; RR = 1.10, 95% CI: 0.63, 1.93, P = 0.747).
Publication Bias
Since the number of included studies was less than 10, the assessment of publication bias was not performed.
Discussion
The major purpose of present meta‐analysis was to evaluate whether high BMI had adverse effects on the surgical outcomes for patients after cervical fusion procedures. Our results suggested that high BMI was associated with longer hospital stay and surgical time as compared with normal BMI. Additionally, high BMI was associated with increased complication rates, including cardiac complications, mortality, DVT and wound complications. Furthermore, there was no significant differences among high and normal BMI patients in regard to outcomes of cervical fusion procedures, including Neck Disability Index, SF‐36 MCS, overall complications, CNS complications, pulmonary complications, and septic complications. Our results indicated that high BMI might have adverse effects in surgical outcomes in patients who underwent cervical fusion procedures.
To our knowledge, this is the first systematic review and meta‐analysis that investigates the influence of high BMI on the surgical outcome in patients who underwent cervical fusion procedures. There were two systematic review and meta‐analyses13, 27 that examined the effects of obesity on surgical and postoperative outcomes after spine surgery. The findings of the two meta‐analyses were consistent with ours. In the meta‐analysis conducted by Jiang et al.13 the authors included 32 studies with 8576 patients who underwent spine surgery. Their results demonstrated that obesity was associated with higher risk of surgical site infection (odds ratio (OR) = 2.33, 95% CI: 1.94, 2.79), venous thromboembolism (OR = 3.15, 95% CI: 1.92, 5.17), mortality (OR = 2.6, 95% CI: 1.50, 4.49), revision rate (OR = 1.43, 95% CI: 1.05, 1.93), longer operation time (OR = 14.55, 95% CI: 10.03, 19.07), and more blood loss (mean difference = 28.89, 95% CI: 14.20, 43.58)13. These results were in agreement with ours. However, whether this positive relationship could also be seen in the patients undergoing cervical spinal surgery was still unclear since the authors did not separate their results by the level of the spine. Another systematic review27 examined several relevant studies, and also reported similar results to ours. In that study, the authors demonstrated that obese patients who underwent spine surgery were more likely to develop postoperative complications, particularly surgical site infection and venous thromboembolism27.
In the present meta‐analysis, we found significant heterogeneity in several outcomes among the included studies. Although we performed sensitivity analysis, no significant improvement was found in heterogeneity, which indicated that the pooled estimate in our study was stability. The reliability of our findings regarding the positive relationship between high BMI and adverse outcomes does not seem diminished. This is because, in a meta‐analysis that had small number of included studies and large heterogeneity, the robustness of the finding is best assessed with sensitivity analysis28.
In the present study, our results suggested that high BMI was associated with longer hospital stay as compared with normal BMI. Our findings were consistent with the results of Kalanithi et al.5 but contradicted that of Narain et al.23. Kalanithi et al.5 conducted a retrospective cross‐sectional study of 84,607 patients undergoing spinal fusion, and 1455 of them were morbidly obese (≥40 kg/m2). Their results demonstrated that hospital stay was significantly longer in the morbid obesity group than normal weight group (4.8 days vs 3.5 days, P < 0.001)5. Conversely, in a study of 277 patients who underwent 1‐ to 2‐lelev ACDF produces, the authors demonstrated similar length of hospital stay between the higher and normal BMI groups23. In that study, the authors stratified patients by BMI into nonobese (<25 kg/m2), obese I (25.0–29.9 kg/m2), obese II (30.0–34.9 kg/m2), and obese III (≥35.0 kg/m2) groups. The mean length of hospital stay in these groups was 31.9 ± 22.5, 31.5 ± 16.6, 37.2 ± 22.9, and 35.7 ± 18.0 h, respectively23. There were no significant differences between the obese and nonobese groups.
Surgical time was significantly longer in the high BMI group than in the normal BMI group in this present study. This finding is in agreement with studies regarding the effects of higher BMI (obese III) after cervical fusion procedures. In a retrospective cohort study of 3671, of which 400 patients underwent anterior or posterior cervical fusion, Buerba et al.22 demonstrated that surgical time was significantly longer in obese class III patients who underwent anterior (138.75 ± 4.69 vs 132.24 ± 1.53) or posterior cervical fusion (200.24 ± 8.04 vs 177.35 ± 5.86) than in nonobese patients. However, in obese class I or II patients, this difference was not seen.
In the present study, we found that high BMI was associated with increased postoperative complications, including cardiac complication, DVT, and wound complications. This indicates that patients with high BMI should be counseled carefully about the risks of postoperative complications. Our findings were consistent with much of the literature regarding the effects of obesity on cervical fusion procedures5, 14, 24. Kalanithi et al.5 performed a retrospective cross‐sectional study on 84,607 patients who underwent spinal fusion. They discovered that morbid obesity was associated with 97% higher complication rates than normal weight patients (13.6% vs 6.9%), and this was seen in all complication types (cardiac, renal, pulmonary, wound complications)5. The most common complication type in morbidly obese patients was wound complication (6.0%) and pulmonary complication (5.8%)5. Phan et al.24 also reported similar results, which demonstrated that obese patients (BMI > 30) who underwent posterior cervical fusion surgery were at an increased risk of VTE. In that study, the authors prospectively collected data from the American College of Surgeons National Surgical Quality Improvement Program (ACSNSQIP), and then analyzed them by univariate analysis and multivariate logistic regression24. The multivariate analysis showed that obesity was an independent predictor for VTE (OR = 6.15, 95% CI: 1.26, 30.20; P = 0.02)24, which was in consistent with our findings.
There were several potential limitations to this study. First, our results were analyzed based on seven studies, some of which had a small sample size. Compared with larger trials, smaller trials were more likely to overestimate the treatment effect. Second, all of the included studies were prospective or retrospective observational trials rather than randomized controlled trials. The inherent nature of observational trials may be associated with selective bias, which might have influences on our results. Third, we tried to conduct subgroup analysis based on obesity categories for all the outcomes. However, only for surgical time, several studies provided available data, and for the remaining outcomes there was paucity of data. Therefore, the subgroup analysis for other outcomes was not performed.
In conclusion, the present meta‐analysis examined the influence of high BMI on the surgical outcomes in patients who underwent cervical fusion procedures. Our study demonstrated significant differences in length of hospital stay, surgical time, mortality rate, cardiac complications, DVT, and wound complications among the high BMI group and normal BMI group after the cervical fusion procedures. Our results suggested that high BMI seemed to increase the rate of adverse outcomes after cervical fusion surgeries.
Supporting information
Appendix S1 Search strategy
Disclosure: The authors have nothing to declare.
References
- 1. Witek AM, Benzel EC. Obesity in cervical spine surgery. World Neurosurg, 2014, 82: e147–e148. [DOI] [PubMed] [Google Scholar]
- 2. Porhomayon J, Leissner KB, El‐Solh AA, Nader ND. Strategies in postoperative analgesia in the obese obstructive sleep apnea patient. Clin J Pain, 2013, 29: 998–1005. [DOI] [PubMed] [Google Scholar]
- 3. Schug SA, Raymann A. Postoperative pain management of the obese patient. Best Pract Res Clin Anaesthesiol, 2011, 25: 73–81. [DOI] [PubMed] [Google Scholar]
- 4. Popkin BM, Kim S, Rusev ER, Du S, Zizza C. Measuring the full economic costs of diet, physical activity and obesity‐related chronic diseases. Obes Rev, 2006, 7: 271–293. [DOI] [PubMed] [Google Scholar]
- 5. Kalanithi PA, Arrigo R, Boakye M. Morbid obesity increases cost and complication rates in spinal arthrodesis. Spine (Phila Pa 1976), 2012, 37: 982–988. [DOI] [PubMed] [Google Scholar]
- 6. Walid MS, Robinson JS Jr. Economic impact of comorbidities in spine surgery. J Neurosurg Spine, 2011, 14: 318–321. [DOI] [PubMed] [Google Scholar]
- 7. Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976), 2015, 40: E675–E693. [DOI] [PubMed] [Google Scholar]
- 8. Nilsen TI, Holtermann A, Mork PJ. Physical exercise, body mass index, and risk of chronic pain in the low back and neck/shoulders: longitudinal data from the Nord‐Trondelag Health Study. Am J Epidemiol, 2011, 174: 267–273. [DOI] [PubMed] [Google Scholar]
- 9. Teraguchi M, Yoshimura N, Hashizume H, et al Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population‐based cohort: the Wakayama Spine Study. Osteoarthritis Cartilage, 2014, 22: 104–110. [DOI] [PubMed] [Google Scholar]
- 10. Mehta AI, Babu R, Karikari IO, et al Young Investigator Award winner: the distribution of body mass as a significant risk factor for lumbar spinal fusion postoperative infections. Spine (Phila Pa 1976), 2012, 2012: 1652–1656. [DOI] [PubMed] [Google Scholar]
- 11. Senker W, Meznik C, Avian A, Berghold A. Perioperative morbidity and complications in minimal access surgery techniques in obese patients with degenerative lumbar disease. Eur Spine J, 2011, 20: 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao J, Kong L, Meng F, Zhang Y, Shen Y. Impact of obesity on lumbar spinal surgery outcomes. J Clin Neurosci, 2016, 28: 1–6. [DOI] [PubMed] [Google Scholar]
- 13. Jiang J, Teng Y, Fan Z, Khan S, Xia Y. Does obesity affect the surgical outcome and complication rates of spinal surgery? A meta‐analysis. Clin Orthop Relat Res, 2014, 472: 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sielatycki JA, Chotai S, Kay H, Stonko D, McGirt M, Devin CJ. Does obesity correlate with worse patient‐reported outcomes following elective anterior cervical discectomy and fusion? Neurosurgery, 2016, 79: 69–74. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ, 2009, 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wells G, Shea B, O'connell D, Peterson J, Welch V. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. 3rd Symposium on Systematic Reviews: Beyond the Basics, 2000. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 05 January 2019).
- 17. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst, 1959, 22: 719–748. [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials, 1986, 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ, 2003, 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics, 1994, 50: 1088–1101. [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ, 1997, 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buerba RA, Fu MC, Grauer JN. Anterior and posterior cervical fusion in patients with high body mass index are not associated with greater complications. Spine J, 2014, 14: 1643–1653. [DOI] [PubMed] [Google Scholar]
- 23. Narain AS, Hijji FY, Haws BE, et al Impact of body mass index on surgical outcomes, narcotics consumption, and hospital costs following anterior cervical discectomy and fusion. J Neurosurg Spine, 2018, 28: 160–166. [DOI] [PubMed] [Google Scholar]
- 24. Phan K, Kothari P, Lee NJ, Virk S, Kim JS, Cho SK. Impact of obesity on outcomes in adults undergoing elective posterior cervical fusion. Spine (Phila Pa 1976), 2017, 42: 261–266. [DOI] [PubMed] [Google Scholar]
- 25. Wilson JR, Tetreault LA, Schroeder G, et al Impact of elevated body mass index and obesity on long‐term surgical outcomes for patients with degenerative cervical myelopathy: analysis of a combined prospective dataset. Spine (Phila Pa 1976), 2017, 42: 195–201. [DOI] [PubMed] [Google Scholar]
- 26. Sami Walid M, Zaytseva NV. The impact of chronic obstructive pulmonary disease and obesity on length of stay and cost of spine surgery. Indian J Orthop, 2010, 44: 424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackson KL 2nd, Devine JG. The effects of obesity on spine surgery: a systematic review of the literature. Global Spine J, 2016, 6: 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fanelli D. How many scientists fabricate and falsify research? A systematic review and meta‐analysis of survey data. PLoS One, 2009, 4: e5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy


