Abstract
Objective
Whether cervical disc arthroplasty (CDA) is superior to anterior cervical discectomy and fusion (ACDF) remains controversial, especially in relation to long‐term results. The present study aimed to evaluate the long‐term safety and efficiency of CDA and ACDF for cervical disc disease.
Methods
We performed this study according to the Cochrane methodology. An extensive search was undertaken in PubMed, Embase, and Cochrane databases up to 1 June 2019 using the following key words: “anterior cervical fusion,” “arthroplasty,” “replacement” and “artificial disc”. RevMan 5.3 (Cochrane, London, UK) was used to analyze data. Safety and efficiency outcome measures included the success rate, functional outcome measures, adverse events (AE), adjacent segment degeneration (ASD), secondary surgery, and patients’ satisfaction and recommendation rates. The OR and MD with 95% confidence interval (CI) were used to evaluate discontinuous and continuous variables, respectively. The statistically significant level was set at P < 0.05.
Results
A total of 11 randomized controlled trials with 3505 patients (CDA/ACDF: 1913/1592) were included in this meta‐analysis. Compared with ACDF, CDA achieved significantly higher overall success (2.10, 95% CI [1.70, 2.59]), neck disability index (NDI) success (1.73, 95% CI [1.37, 2.18]), neurological success (1.65, 95% CI [1.24, 2.20]), patients’ satisfaction (2.14, 95% CI [1.50, 3.05]), and patients’ recommendation rates (3.23, 95% CI [1.79, 5.80]). Functional outcome measures such as visual analog score neck pain (−5.50, 95% CI [−8.49, −2.52]) and arm pain (−3.78, 95% CI [−7.04, −0.53]), the Short Form‐36 physical component score (SF‐36 PCS) (1.93, 95% CI [0.53, 3.32]), and the Short Form‐36 mental component score (SF‐36 MCS) (2.62, 95% CI [0.95, 4.29]), revealed superiority in the CDA group. CDA also achieved a significantly lower rate of symptomatic ASD (0.46, 95% CI [0.34, 0.63]), total secondary surgery (0.50, 95% CI [0.29, 0.87]), secondary surgery at the index level (0.46, 95% CI [0.29, 0.74]), and secondary surgery at the adjacent level (0.37, 95% CI [0.28, 0.49]). However, no significant difference was found in radiological success (1.35, 95% CI [0.88, 2.08]), NDI score (−2.88, 95% CI [−5.93, 0.17]), total reported AE (1.14, 95% CI [0.92, 1.42]), serious AE (0.89, 95% CI [0.71, 1.11]), device/surgery‐related AE (0.90, 95% CI [0.68, 1.18]), radiological superior ASD (0.63, 95% CI [0.28, 1.43]), inferior ASD (0.45, 95% CI [0.19, 1.11]), and work status (1.33, 95% CI [0.78, 2.25]). Furthermore, subgroup analysis showed different results between US and non‐US groups.
Conclusion
Our study provided further evidence that compared to ACDF, CDA had a higher long‐term clinical success rate and better functional outcome measurements, and resulted in less symptomatic ASD and fewer secondary surgeries. However, worldwide multicenter RCT with long‐term follow up are still needed for further evaluation in the future.
Keywords: Adjacent segment degeneration, Anterior cervical discectomy and fusion, Cervical disc arthroplasty, Cervical disc disease, Long‐term
Introduction
Anterior cervical discectomy and fusion (ACDF) has been viewed as the gold standard procedure for cervical disc disease (CDD), including radiculopathy and myelopathy. A recent survey revealed that 84.3% of surgeons performed ACDF as the standard technique for CDD1. Even though successful clinical outcomes can be achieved with ACDF, postoperative complications such as pseudoarthrosis or non‐union, instrument failure, and adjacent segment degeneration (ASD) have been the greatest concerns2, 3, 4. Cervical fusion could lead to loss of range of motion at the index level and shift load to the adjacent level, then result in accelerating ASD2, 3, 5. Hilibrand et al. reported that annually 2.9% of the patients underwent anterior interbody fusion will most likely develop ASD requiring cervical intervention2. Thus, spinal surgeons have been attempting to find an alternative procedure to avoid these complications associated with ACDF.
A motion‐preserving procedure, cervical disc arthroplasty (CDA), seems to be a good choice. CDA was initially designed using motion‐preserving techniques to restore cervical physiologic biomechanical properties and alleviate the adjacent‐level loads, and eventually reduces or eliminates the risk of developing ASD6. Clinical data showed that preoperative motion could be maintained in the long run following CDA7. Promisingly, recent studies have proved that CDA is cost‐effective and is comparable to ACDF in long‐term follow ups8, 9, 10, 11. However, some disadvantages of CDA cannot be overlooked, such as heterotopic ossification, implant failure, and bone loss12, 13, 14. In addition, the revision burden of CDA was two times higher than that of ACDF15.
In the past 20 years, a series of randomized controlled trials (RCT) have been conducted; however, the reported results are inconsistent and have great variability. Although a few systematic reviews have been performed, researchers have failed to reach an agreement owing to varied criteria5, 16, 17, 18, 19, 20, 21, 22, 23, 24. Nevertheless, there is an absence of pooling of long‐term results in a comprehensive meta‐analysis. Therefore, this is the first study aiming at comparing CDA to ACDF with special focus on long‐term safety and efficiency. The conclusions drawn from this study could provide solid evidence for the future application of CDA.
This study was approved by the Ethics Committee of The Second Xiangya Hospital of Centeral South University.
Methods
Literature Search Strategy
We followed the Cochrane methodology guidelines to perform this meta‐analysis and searched PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CCRCT) databases up to 1 June 2019. The keywords “anterior cervical fusion,” “arthroplasty,” “replacement,” and “artificial disc” combined with “and/or” were used to identify any relevant studies.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (i) patients ≥18 years old with symptomatic CDD presenting with radiculopathy and/or myelopathy; (ii) participants were treated with either CDA or ACDF; (iii) comparison was performed between CDA and ACDF; (iv) at least one efficiency and safety outcome measurement was available; and (v) prospective RCT with a follow up ≥5 years.
Articles that met the following characteristics were excluded: (i) reviews, case reports or series, editorials, conference abstracts, and retrospective studies; (ii) duplicated data publications from the same RCT; (iii) partial results with insufficient data; and (iv) non‐English publications.
Literature Screening
Literature screening was performed by two independent investigators (Tu, ZM and Wang, QL). Any disagreement was discussed with another author (Hu, P) to reach consensus. After excluding duplicates, literature selection was carried out according to the inclusion and exclusion criteria based on title and abstract. Then, extensive screening of full‐text articles was performed. All RCT that compared the long‐term efficiency and safety of CDA and ACDF for CDD were included.
Quality Assessment of the Included Studies
Quality assessment was achieved using the criteria recommended by the Cochrane Back Review Group criteria25. The types of biases assessed are: four selection bias, four performance bias, two attrition bias, one detection bias, and one reporting bias. The articles scoring at least 6 of these 12 biases were considered as at low risk of bias. The last bia assessed is “Other,” defined as any potential bias not detected using the previous criteria.
Data Extraction
Data extraction was performed as follows: (i) general characteristics such as first author, year of publication, number of clinical trial (NCT), enrolled patients, follow‐up rate, age, sex, surgical levels, type of prosthesis, and follow‐up duration were extracted; and (ii) outcome measures, including clinical success rate (overall success, NDI success, neurological success, and radiological success), functional outcome measurements (NDI score, visual analog score [VAS] neck pain and arm pain, and SF‐36 PCS and MCS), AE (total reported AE, serious AE and device/surgery‐related AE), ASD (symptomatic ASD, radiological superior or inferior ASD), secondary surgery (total secondary surgery, secondary surgery at the index level and at the adjacent level), work status, and patients’ satisfaction and recommendation rates were extracted. This task was performed by two independent investigators (Tu, ZM and Wang, QL), who extracted the data and discussed any disagreement to reach consensus with a third investigator (Hu, P). Data‐extracting software was used to obtain data from figures when original data was not available26.
Statistical Analysis
RevMan 5.3 (Cochrane, London, UK) was used to pool extracted data into a combined analysis. The odds ratio (OR) and mean difference (MD) with 95% confidence intervals (CI) were used to evaluate discontinuous and continuous variables, respectively. Heterogeneity was assessed using a χ2‐test and an I 2‐test. A fixed effects model was used when I 2 < 50%; otherwise, a random effects model was used. Sensitivity analysis was performed by comparing two different effects models. If the statistical difference changed, the leave‐one‐out method27 and subgroup analysis was performed to find the origin of heterogeneity. Funnel plots were applied to assess for publication bias. A statistically significant difference was defined as a P‐value of less than 0.05.
Results
Literature Review
Initial database searching identified 1954 articles (PubMed: 650, Embase: 1020, CCRCT: 284) and detailed literature screening is described in the flow diagram in Figure 1. A total of 814 studies were removed because they were duplicates, 1076 studies were excluded based on their titles and abstracts, and 43 studies were excluded for other reasons. As a result, 21 studies28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 were included for further evaluation. Among them, 2 studies45, 47 were partial results of multicenter RCT and 8 studies39, 40, 41, 42, 43, 44, 46, 48 included duplicated data for publication. Ultimately, 11 articles28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 involving 3505 patients (CDA/ACDF: 1913/1592) were included in this meta‐analysis. There are 923 male and 990 female patients in the CDA group and 791 male and 801 female patients in the ACDF group. The mean age of each included population varies from 40 to 50 years in both groups. All the patients suffered from radiculopathy and/or myelopathy caused by cervical disc disease with C3‐4 to C6‐7 involvement. The basic characteristics of the included studies and patients are summarized in Table 1. Among them, 8 studies28, 29, 30, 32, 33, 34, 36, 38 compared single‐level CDD, 1 study31 compared two‐level CDD, and 2 studies35, 37 compared both single‐level and two‐level CDD independently.
Figure 1.
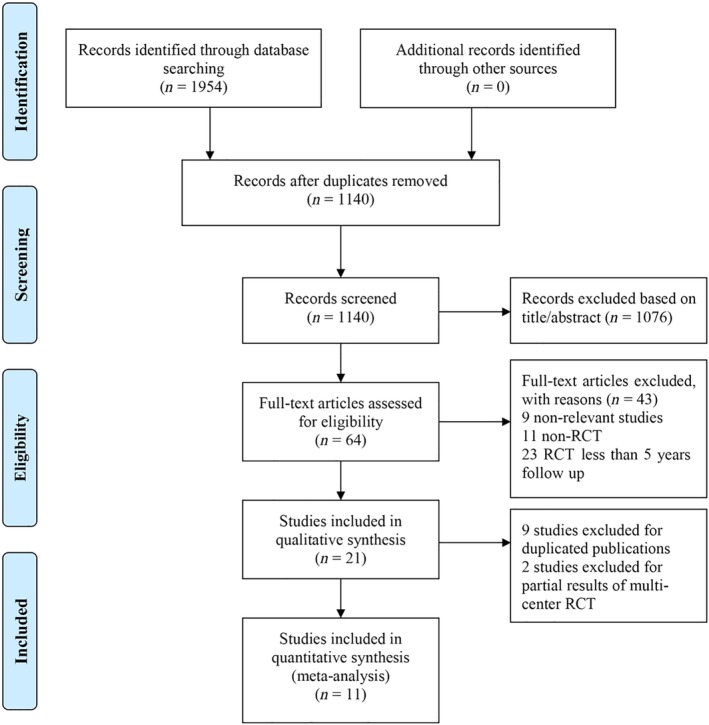
Flow diagram for study selection.
Table 1.
Characteristics of included studies
| Study | Region | Number of clinical trial | Design | Enrolled patients (CDA/ACDF) | Follow‐up rate (CDA/ACDF) | Age (CDA/ACDF) | Sex (female) | BMI (CDA/ACDF) | Level | Prosthesis | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Burkus 201428 | USA | NCT00642876 | RCT, 31‐sites | 276/265 | 76.8%/69.1% | 43.3/43.9 | 148/142 | / | 1 | Prestige ST | 7 years |
| Coric 201829 | USA | NCT00374413 | RCT, 21‐sites | 136/133 | 68.4%/62.4% | 43.7(7.76)/43.9(7.39) | 74/74 | 27.5(5.0)/28.7(5.7) | 1 | Kineflex|C | 5 years |
| Donk 201730 | Netherlands | ISRCTN41681847 | RCT, single‐site | 50/47 | 98.0%/97.9% | 44.1(6.4)/43.1(7.5) | 26/22 | / | 1 | Bryan | 9 years |
| Gornet 201931 | USA | NCT00637156 | RCT, 30‐sites | 209/188 | 86.0%/84.9% | 47.1(8.3)/47.3(7.7) | 117/98 | 28.2(5.6)/28.6(4.9) | 2 | Prestige LP | 10 years |
| Hou 201632 | China | Unknown | RCT, single‐site | 56/51 | 91.1%/94.1% | 46.3(7.8)/48.5(8.3) | 30/28 | 21(3.2)/22(2.5) | 1 | Mobi‐C | 5 years |
| Janssen 201533 | U.S. | NCT00291018 | RCT, 13‐sites | 103/106 | 92%/92% | 42.1(8.42)/43.5(7.15) | 55/54 | 26.44(5.32)/27.34(5.54) | 1 | ProDisc‐C | 7 years |
| Lavelle 201834 | USA | NCT00437190 | RCT, 38‐sites | 242/221 | 100%/100% | 44.4/44.7 | 132/108 | 26.6(4.8)/27.6(5.0) | 1 | BRYAN | 10 years |
| MacDowall 201935 | Sweden | ISRCTN44347115 | RCT, 3‐sites | 83/70 | 89.2%/87.1 | 46.9 (6.8)/47.0 (6.9) | 42/33 | 26/26 | 1–2 | Discover | 5 years |
| Phillips 201536 | USA | Unknown | RCT, 24‐sites | 218/185 | 74.8%/70.3% | 49.3(5.0)/43.7(8.3) | 105/89 | 28.2(4.6)/27.4(4.8) | 1 | PCM | 5 years |
| Radcliff 2017a37 * | USA | NCT00389597 | RCT, 24‐sites | 164/81 | 80.1%/74.3% | 43.3(9.2)/44.0(8.2) | 78/36 | 27.3 (4.4)/27.4 (4.2) | 1 | Mobi‐C | 7 years |
| Radcliff 2017b37 | USA | NCT00389597 | RCT, 24‐sites | 225/105 | 84.4%/75% | 44.3(8.1)/46.2(8.0) | 113/45 | 27.6(4.5)/28.1(4.2) | 2 | Mobi‐C | 7 years |
| Vaccaro 201838 | USA | Unknown | RCT, 18‐sites | 151/140 | 86.1%/84.2% | 43.4(7.50)/44.4(7.86) | 70/72 | 28.9(5.53)/29.0(5.47) | 1 | SECURE‐C | 7 years |
Radcliff (2017a) and Radcliff (2017b) are from the same study.
ACDF, anterior cervical discectomy and fusion; BMI, body mass index; CDA, cervical disc arthroplasty.
Quality Assessment of the Included Studies
Methodological quality assessment of the 11 eligible studies is shown in Fig. 2. Nine studies28, 30, 31, 32, 33, 34, 35, 36, 37 were adequately randomized, but 1 study29 did not provide detailed information of randomization, and 1 study38 failed to achieve adequate randomization. Only 4 studies30, 32, 33, 35 provided a clear statement regarding avoiding allocation concealment. In addition, all included RCT28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 failed to achieve blinding to patients and care providers due to the specialty of this kind of trial. The patients were informed immediately after surgery about the type of surgical procedure they had been underwent, and care providers were aware of which kind of surgery was to be performed during surgery28, 30, 31, 32, 33, 34, 35, 36, 37. Almost all the studies described the dropout rate and 2 studies28, 29 with a follow‐up rate below 70% were considered as having high risk of bias. All included studies were scored above seven and were rated as having low risk of bias.
Figure 2.
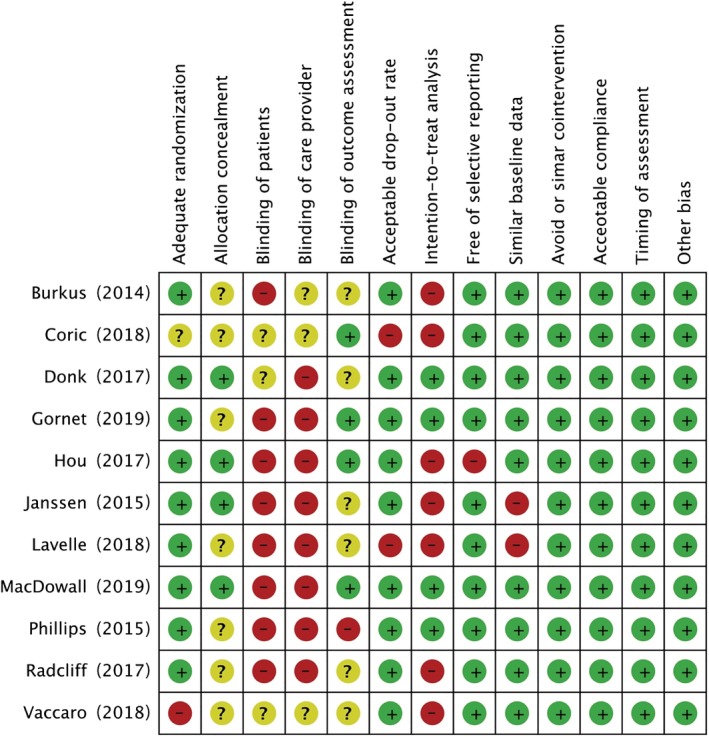
Risk bias of included studies.
Heterogeneity Analysis
Of all the parameters identified for meta‐analysis, 6 studies compared overall success28, 29, 31, 34, 37, 38 and NDI success28, 31, 34, 36, 37, 38, 7 studies compared neurological success28, 31, 33, 34, 36, 37, 38, 3 studies compared radiological success31, 36, 37, 7 studies compared NDI score28, 32, 33, 35, 36, 37, 38, 5 studies compared neck pain score33, 35, 36, 37, 38, 4 studies compared arm pain score 33, 35, 36, 37, 5 studies compared SF‐36 PCS28, 30, 33, 36, 38, 4 studies compared SF‐36 MCS30, 33, 36, 38, 8 studies compared any AE28, 29, 30, 31, 33, 36, 37, 38, 4 studies compared serious AE31, 36, 37, 38, 6 studies compared device/surgery‐related AE29, 31, 33, 36, 37, 38 and symptomatic ASD30, 33, 34, 35, 37, 38, 2 studies compared radiological superior and inferior ASD37, 38, 8 studies compared total secondary surgeries28, 29, 30, 32, 33, 35, 36, 37 and secondary surgeries at the index level28, 30, 31, 33, 35, 36, 37, 38, 9 studies compared secondary surgeries at the adjacent level28, 30, 31, 33, 34, 35, 36, 37, 38, 2 studies compared work status28, 34, 4 studies compared patients’ satisfaction rate31, 36, 37, 38, and 2 studies compared patients’ recommendation rate36, 37.
The heterogeneity test showed that I 2 < 50% for overall success, NDI success, neurological success, radiological success, VAS neck pain and arm pain, SF‐36 PCS and MCS, total reported AE, serious AE, device/surgery‐related AE, symptomatic ASD, secondary surgery at the adjacent level, and patients’ satisfaction and recommendation rates. This indicates that there is low heterogeneity among these parameters and a fix effects model could be applied for combined statistics. In contrast, the heterogeneity test showed I 2 > 50% for NDI score, radiological superior and inferior ASD, total secondary surgery, secondary surgery at the index level, and work status, which indicates significant or large heterogeneity. Therefore, a random effects model could be applied for combined statistics. The results of the heterogeneity test are summarized in Table 2.
Table 2.
The heterogeneity test and meta‐analysis of outcome measurements
| Outcome measurements | Included studies | Participants | I 2 | Statistic effect model | Effect estimate | P‐value |
|---|---|---|---|---|---|---|
| Overall success | 6 | 1734 | 0% | OR (M‐H, Fixed, 95% CI) | 2.10 [1.70, 2.59] | <0.00001 |
| NDI success | 6 | 1972 | 20% | OR (M‐H, Fixed, 95% CI) | 1.73 [1.37, 2.18] | <0.00001 |
| Neurological success | 7 | 1982 | 16% | OR (M‐H, Fixed, 95% CI) | 1.65 [1.24, 2.20] | 0.0006 |
| Radiological success | 3 | 1002 | 0% | OR (M‐H, Fixed, 95% CI) | 1.35 [0.88, 2.08] | 0.17 |
| NDI score | 7 | 1885 | 68% | MD (IV, Random, 95% CI) | −2.88 [−5.93, 0.17] | 0.06 |
| VAS neck pain | 5 | 1366 | 33% | MD (IV, Fixed, 95% CI) | −5.50 [−8.49, −2.52] | 0.0003 |
| VAS arm pain | 4 | 1134 | 0% | MD (IV, Fixed, 95% CI) | −3.78 [−7.04, −0.53] | 0.02 |
| SF‐36 PCS | 4 | 1149 | 0% | MD (IV, Fixed, 95% CI) | 1.93 [0.53, 3.32] | 0.007 |
| SF‐36 MCS | 3 | 761 | 0% | MD (IV, Fixed, 95% CI) | 2.62 [0.95, 4.29] | 0.002 |
| Total reported AE | 8 | 2872 | 46% | OR (M‐H, Fixed, 95% CI) | 1.14 [0.92, 1.42] | 0.22 |
| Serious AE | 4 | 1756 | 13% | OR (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.11] | 0.29 |
| Device/surgery‐related AE | 6 | 2317 | 2% | OR (M‐H, Fixed, 95% CI) | 0.90 [0.68, 1.18] | 0.43 |
| Symptomatic ASD | 6 | 1628 | 29% | OR (M‐H, Fixed, 95% CI) | 0.46 [0.34, 0.63] | <0.00001 |
| Radiological superior ASD | 2 | 659 | 83% | OR (M‐H, Random, 95% CI) | 0.63 [0.28, 1.43] | 0.27 |
| Radiological inferior ASD | 2 | 474 | 78% | OR (M‐H, Random, 95% CI) | 0.45 [0.19, 1.11] | 0.08 |
| Total secondary surgery | 8 | 2058 | 64% | OR (M‐H, Random, 95% CI) | 0.50 [0.29, 0.87] | 0.01 |
| Secondary surgery at the index level | 8 | 2712 | 55% | OR (M‐H, Random, 95% CI) | 0.46 [0.29, 0.74] | 0.001 |
| Secondary surgery at the adjacent level | 9 | 2937 | 18% | OR (M‐H, Fixed, 95% CI) | 0.37 [0.28, 0.49] | <0.00001 |
| Work status | 2 | 622 | 53% | OR (M‐H, Random, 95% CI) | 1.33 [0.78, 2.25] | 0.29 |
| Patients’ satisfaction rate | 4 | 1224 | 0% | OR (M‐H, Fixed, 95% CI) | 2.14 [1.50, 3.05] | <0.0001 |
| Patients’ recommendation rate | 2 | 727 | 0% | OR (M‐H, Fixed, 95% CI) | 3.23 [1.79, 5.80] | <0.0001 |
AE, adverse event; ASD, adjacent segment degeneration; CI, confidence interval; MD, mean difference; NDI, neck disability index; OR, odds ratio; VAS, visual analog score.
Results of the Meta‐Analysis
We pooled all extracted data comparing CDA with ACDF for CDD in this meta‐analysis. The combined results are shown in Table 2 and Figs 3, 4, 5, 6, 7, 8.
Figure 3.
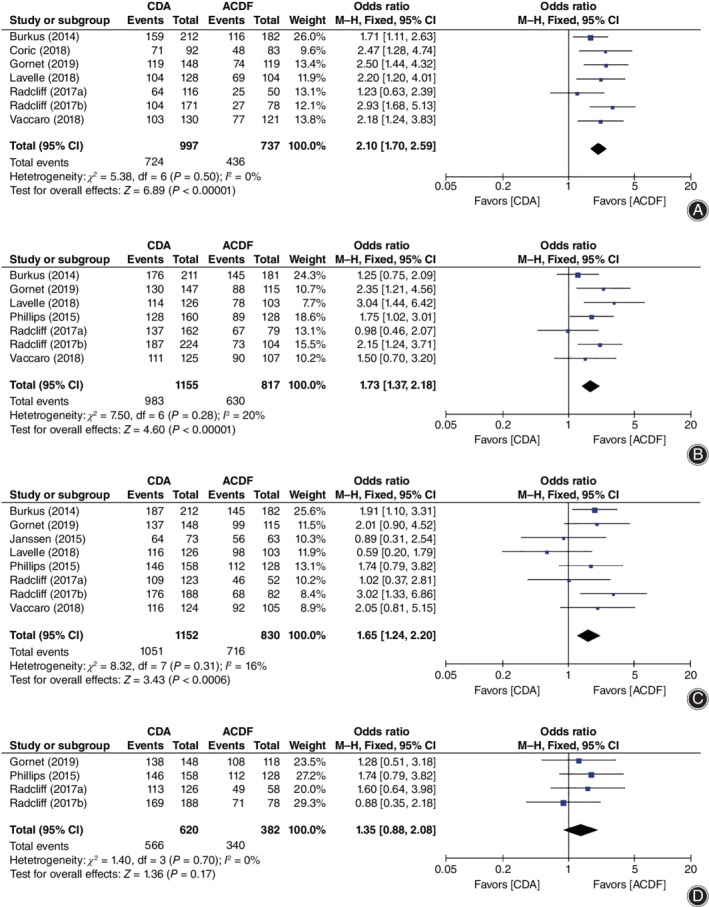
Forest plot comparing clinical success rate between cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF). (A) Overall success. (B) Neck disability index (NDI) success. (C) Neurological success. (D) Radiological success. CI, confidence interval.
Figure 4.
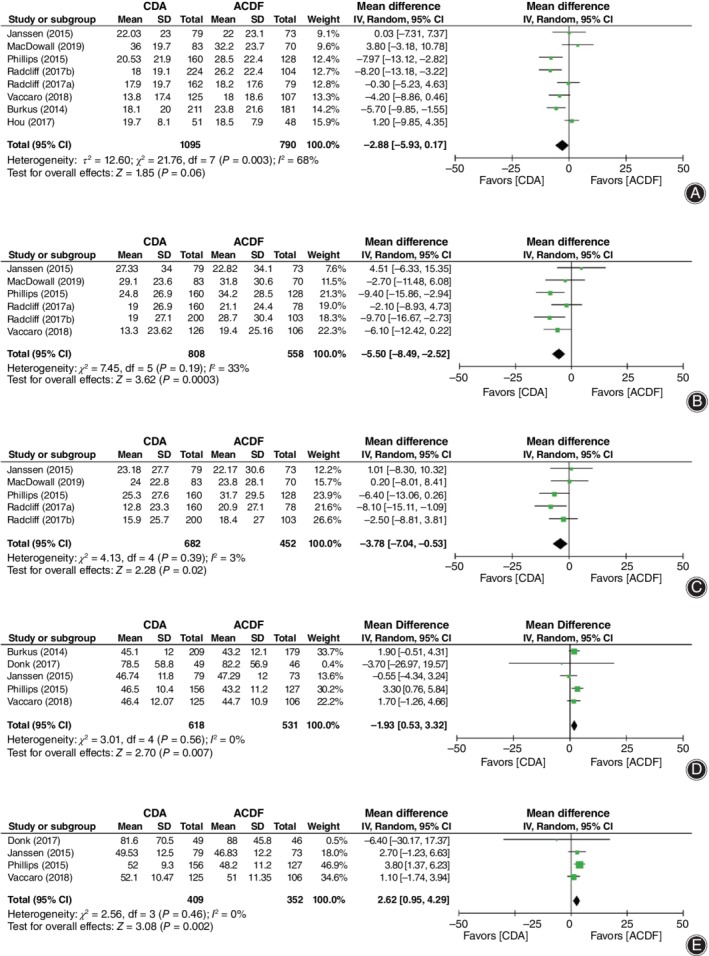
Forest plot comparing functional outcome measurements between cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF). (A) Neck disability index (NDI) score. (B) Visual analog score (VAS) neck pain. (C) VAS arm pain. (D) Short Form‐36 physical component score (SF‐36 PCS). (E) Short Form‐36 mental component score (SF‐36 MCS). CI, confidence interval.
Figure 5.
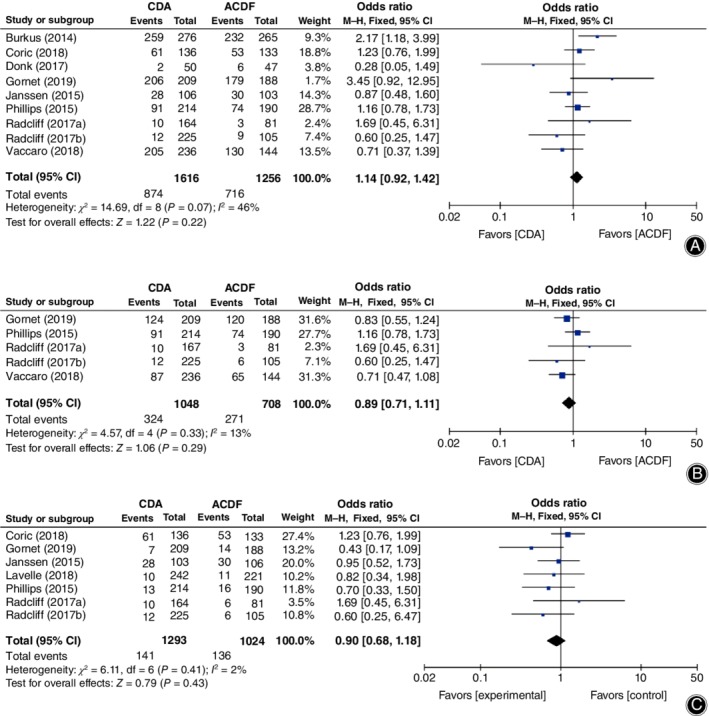
Forest plot showing a comparison of the frequency of adverse events (AE) between cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF). (A) Total reported AE. (B) Serious AE. (C) Device/surgery‐related AE. CI, confidence interval.
Figure 6.
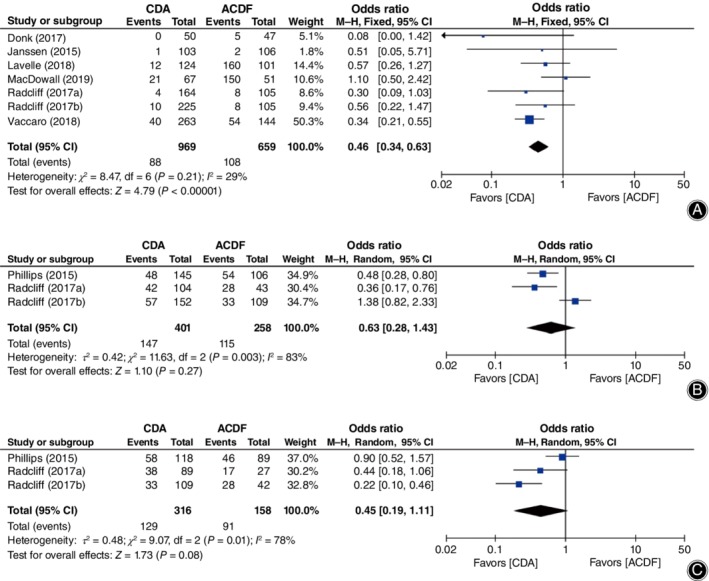
Forest plot comparing the incidence of adjacent segment degeneration (ASD) between cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF). (A) Symptomatic ASD. (B) Radiological superior ASD. (C) Radiological inferior ASD. CI, confidence interval.
Figure 7.
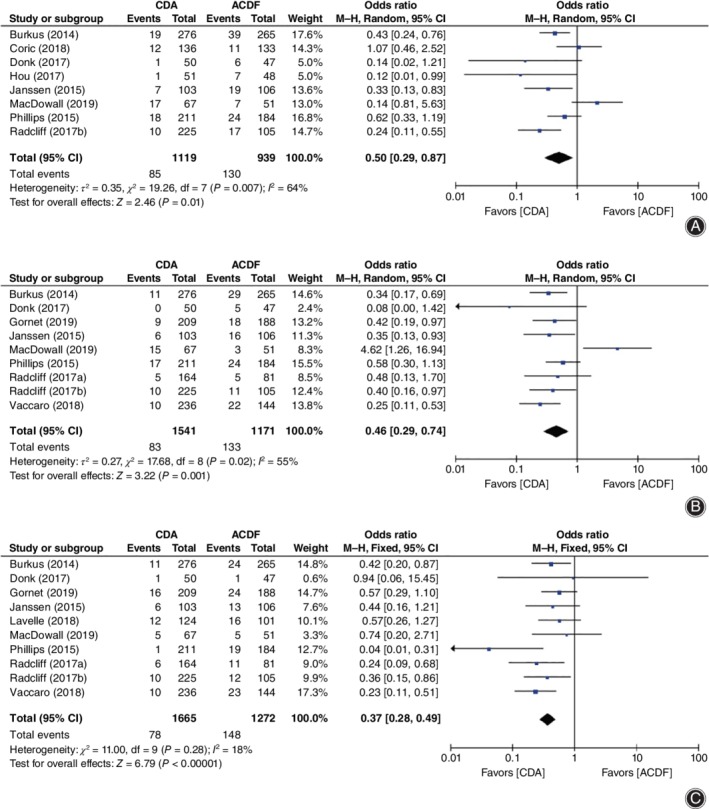
Forest plot showing a comparison of secondary surgery rate between cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF). (A) Total secondary surgery. (B) Secondary surgery at the index level. (C) Secondary surgery at the adjacent level. CI, confidence interval.
Figure 8.
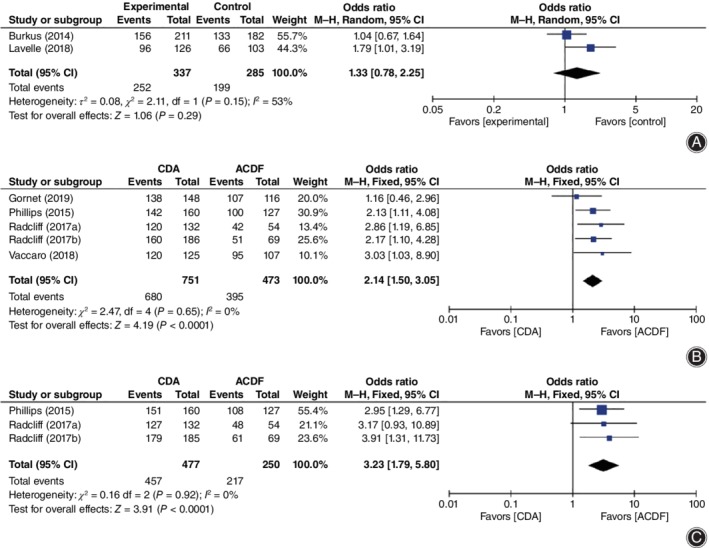
Forest plot comparing work status (A), patients’ satisfaction (B), and patients’ recommendation (C) between cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF). CI, confidence interval.
For clinical success rate, CDA showed significant superiority in overall success (OR = 2.10, 95% CI [1.70, 2.59], P < 0.00001, Fig. 3A), NDI success (OR = 1.73, 95% CI [1.37, 2.18], P < 0.00001; Fig. 3B), and neurological success (OR = 1.65, 95% CI [1.24, 2.20], P = 0.0006; Fig. 3C), while no superiority was found in radiological success (OR = 1.35, 95% CI [0.88, 2.08], P = 0.17; Fig. 3D).
Functional outcome measurements showed superiority in CDA except for NDI score. The NDI score (WMD = −2.88, 95% CI [−5.93, 0.17]), P = 0.06; Fig. 4A) was found to be lower in CDA without statistical difference. However, the combined results that favored CDA were identified in neck pain score (WMD = −5.50, 95% CI [−8.49, −2.52], P = 0.0003; Fig. 4B), arm pain score (WMD = −3.78, 95% CI [−7.04, −0.53], P = 0.02; Fig. 4C), SF‐36 PCS (WMD = 1.93, 95% CI [0.53, 3.32], P = 0.0007; Fig. 4D), and SF‐36 MCS (WMD = 2.62, 95% CI [0.95, 4.29], P = 0.002; Fig. 4E).
No superiority was showed in AE. Total reported AE (OR = 1.14, 95% CI [0.92, 1.42], P = 0.22, Fig. 5A), serious AE (OR = 0.89, 95% CI [0.71, 1.11], P = 0.29, Fig. 5B), and device/surgery‐related AE (OR = 0.90, 95% CI [0.68, 1.18], P = 0.43; Fig. 5C) were similar between CDA and ACDF.
As for ASD, the incidence of symptomatic ASD (OR = 0.46, 95% CI [0.34, 0.63]), P < 0.00001; Fig. 6A) was significantly lower in CDA; however, radiologically superior ASD (OR = 0.63, 95% CI [0.28, 1.43], P = 0.27; Fig. 6B) and inferior ASD (OR = 0.45, 95% CI [0.19, 1.11], P = 0.08; Fig. 6C) were not significantly different between groups.
Strikingly, when compared to ACDF, our results revealed that CDA had significant superiority in total secondary surgery (OR = 0.50, 95% CI [0.29, 0.87], P = 0.01, Fig. 7A), secondary surgery at the index level (OR = 0.46, 95% CI [0.29, 0.74], P = 0.001, Fig. 7B), and secondary surgery at the adjacent level (OR = 0.37, 95% CI [0.28, 0.49], P < 0.00001; Fig. 7C).
Finally, work status (OR = 1.33, 95% CI [0.78, 2.25], P = 0.29, Fig. 8A) was similar at the last follow up between CDA and ACDF. CDA achieved a higher rate of patient satisfaction (OR = 2.14, 95% CI [1.50, 3.05], P = 0.0002; Fig. 8B) and patients’ recommendation (OR = 3.23, 95% CI [1.79, 5.80], P < 0.00001; Fig. 8C).
Sensitivity Analysis
Combined OR or MD with 95% CI using fixed and random effects for all outcome measures are showed in Table 3. The consistency of the combined results was identified in overall success, NDI success, neurological success, radiological success, VAS neck pain and arm pain, SF‐36 PCS and MCS, total reported AE, serious AE, device/surgery‐related AE, symptomatic ASD, total secondary surgery, secondary surgery at the index level and at the adjacent level, and patients’ satisfaction and recommendation rates. This means that these results are stable and reliable. However, the situation was quite different for NDI score, and radiological superior and inferior ASD, indicating that the combined results were unreliable. Therefore, further analysis was performed.
Table 3.
Comparison of the combined results from fixed and random effects model
| Fixed effects model | Random effects model | |||
|---|---|---|---|---|
| Outcome measures | Effect estimated | P‐value | Effect estimated | P‐value |
| Overall success | 2.10 [1.70, 2.59] | <0.00001 | 2.10 [1.70, 2.59] | <0.00001 |
| NDI success | 1.73 [1.37, 2.18] | <0.00001 | 1.73 [1.33, 2.26] | <0.00001 |
| Neurological success | 1.65 [1.24, 2.20] | 0.0006 | 1.64 [1.19, 2.27] | 0.003 |
| Radiological success | 1.35 [0.88, 2.08] | 0.17 | 1.36 [0.87, 2.10] | 0.17 |
| NDI score | −2.67 [−4.33, −1.01] | 0.002 | −2.88 [−5.93, 0.17] | 0.06 |
| VAS neck pain | −5.50 [−8.49, −2.52] | 0.0003 | −5.21 [−8.91, −1.51] | 0.006 |
| VAS arm pain | −3.78 [−7.04, −0.53] | 0.02 | −3.77 [−7.08, −0.46] | 0.03 |
| SF‐36 PCS | 1.93 [0.53, 3.32] | 0.007 | 1.93 [0.53, 3.32] | 0.007 |
| SF‐36 MCS | 2.62 [0.95, 4.29] | 0.002 | 2.62 [0.95, 4.29] | 0.002 |
| Total reported AE | 1.14 [0.92, 1.42] | 0.22 | 1.12 [0.80, 1.55] | 0.51 |
| Serious AE | 0.89 [0.71, 1.11] | 0.29 | 0.88 [0.69, 1.13] | 0.32 |
| Device/surgery‐related AE | 0.90 [0.68, 1.18] | 0.43 | 0.89 [0.67, 1.18] | 0.42 |
| Symptomatic ASD | 0.46 [0.34, 0.63] | <0.00001 | 0.49 [0.32, 0.76] | 0.001 |
| Radiological superior ASD | 0.69 [0.50, 0.95] | 0.02 | 0.63 [0.28, 1.43] | 0.27 |
| Radiological inferior ASD | 0.53 [0.36, 0.78] | 0.001 | 0.45 [0.19, 1.11] | 0.08 |
| Total secondary surgery | 0.52 [0.39, 0.69] | <0.00001 | 0.50 [0.29, 0.87] | 0.01 |
| Secondary surgery at the index level | 0.46 [0.34, 0.61] | <0.00001 | 0.46 [0.29, 0.74] | 0.001 |
| Secondary surgery in the adjacent level | 0.37 [0.28, 0.49] | <0.00001 | 0.39 [0.28, 0.55] | <0.00001 |
| Work status | 1.28 [0.90, 1.82] | 0.17 | 1.33 [0.78, 2.25] | 0.29 |
| Patients’ satisfaction rate | 2.14 [1.50, 3.05] | <0.0001 | 2.14 [1.50, 3.06] | <0.0001 |
| Patients’ recommendation rate | 3.23 [1.79, 5.80] | <0.0001 | 3.25 [1.81, 5.82] | <0.0001 |
AE, adverse event; ASD, adjacent segment degeneration; CI, confidence interval; NDI, neck disability index; VAS, visual analog score.
Then, we performed sensitivity analysis based on the leave‐one‐out method27. For NDI score, we found that the combined result changed significantly when removing the study from Hou et al.32 or MacDowall et al.35, with the P‐value reduced from 0.06 to 0.02. Thus, we performed a subgroup analysis (Table 4) and found that the heterogeneity was 40% and 0% in the US and non‐US subgroups, respectively, indicating that the heterogeneity originated from the studies from different regions. In addition, for radiological superior ASD, after we excluded the data from Radicliff et al. (2017)37, I 2 decreased from 83% to 0%, and the statistical significance changed. For radiological inferior ASD, after we excluded the study from Phillips et al.36, I 2 decreased from 78% to 28%, and the statistical significance also changed. This indicates that they were the source of heterogeneity for radiological superior and inferior ASD, respectively.
Table 4.
The combined results of subgroup analysis based on regions
| Outcome measurements | Included studies | Participants | I 2 | Statistic effect model | Effect estimate | P‐value | |
|---|---|---|---|---|---|---|---|
| NDI score | US | 6 | 1633 | 40% | MD (IV, Random, 95% CI) | −4.71 [−7.38, −2.04] | 0.0005 |
| Non‐US | 2 | 252 | 0% | MD (IV, Fixed, 95% CI) | 1.64 [−1.23, 4.51] | 0.26 | |
| Symptomatic ASD | US | 5 | 1413 | 0% | OR (M‐H, Fixed, 95% CI) | 0.40 [0.28, 0.58] | <0.00001 |
| Non‐US | 2 | 215 | 68% | OR (M‐H, Random, 95% CI) | 0.42 [0.03, 5.57] | 0.51 | |
| Total secondary surgery | US | 5 | 1744 | 47% | OR (M‐H, Fixed, 95% CI) | 0.48 [0.35, 0.66] | <0.00001 |
| Non‐US | 3 | 314 | 79% | OR (M‐H, Random, 95% CI) | 0.39 [0.04, 3.49] | =0.40 | |
| Secondary surgery at the index level | US | 7 | 2497 | 0% | OR (M‐H, Fixed, 95% CI) | 0.39 [0.29, 0.53] | <0.00001 |
| Non‐US | 2 | 215 | 85% | OR (M‐H, Random, 95% CI) | 0.73 [0.01, 46.24] | =0.88 | |
| Secondary surgery at the adjacent level | US | 8 | 2722 | 27% | OR (M‐H, Fixed, 95% CI) | 0.35 [0.26, 0.47] | <0.00001 |
| Non‐US | 2 | 215 | 0% | OR (M‐H, Fixed, 95% CI) | 0.77 [0.24, 2.51] | =0.67 | |
ASD, adjacent segment degeneration; CI, confidence interval; MD, mean difference; NDI, neck disability index; OR, odds ratio.
Subgroup Analysis
First, we performed subgroup analysis based on different regions. The included studies were classified into US and non‐US subgroups. The combined results of NDI score, symptomatic ASD, total secondary surgery, and secondary surgery at the index level and at the adjacent level are shown in Table 4. Surprisingly, the combined results showed that CDA was superior to ACDF, with significant difference in all these outcome measures in the US subgroup. However, in the non‐US subgroup, all these combined results were similar without statistical difference.
Second, we performed subgroup analysis based on the number of surgical levels. The combined results of overall success, neurological success, NDI success, radiological success, total reported AE, serious AE, device/surgery‐related AE, secondary surgery at the index level and at the adjacent level, and patients’ satisfaction rate are showed in Table 5. The combined results showed significantly less device/surgery‐related AE of CDA in the two‐level CDD group, with no statistical difference in single‐level CDD. In contrast, patients’ satisfaction favored CDA in single‐level CDD (P = 0.0002), while in two‐level CDD (P = 0.05), further studies are needed to identify the superiority. The residual outcome measures are similar for single‐level and two‐level CDD.
Table 5.
The combined results of subgroup analysis based on surgical level
| Outcome measurements | Included studies | Participants | I 2 | Statistic effect model | Effect estimate | P‐value | |
|---|---|---|---|---|---|---|---|
| Overall success | Single‐level | 5 | 1218 | 0% | OR (M‐H, Fixed, 95% CI) | 1.89 [1.47, 2.42] | <0.00001 |
| Two‐level | 2 | 516 | 0% | OR (M‐H, Fixed, 95% CI) | 2.70 [1.83, 4.00] | <0.00001 | |
| NDI success | Single‐level | 5 | 1382 | 27% | OR (M‐H, Fixed, 95% CI) | 1.55 [1.17, 2.05] | 0.002 |
| Two‐level | 2 | 590 | 0% | OR (M‐H, Fixed, 95% CI) | 2.23 [1.46, 3.40] | 0.0002 | |
| Neurological success | Single‐level | 6 | 1449 | 9% | OR (M‐H, Fixed, 95% CI) | 1.46 [1.04, 2.03] | 0.03 |
| Two‐level | 2 | 533 | 0% | OR (M‐H, Fixed, 95% CI) | 2.44 [1.37, 4.34] | 0.003 | |
| Radiological success | Single‐level | 2 | 470 | 0% | OR (M‐H, Fixed, 95% CI) | 1.68 [0.92, 3.05] | 0.09 |
| Two‐level | 2 | 532 | 0% | OR (M‐H, Fixed, 95% CI) | 1.06 [0.56, 2.00] | 0.87 | |
| Total reported AE | Single‐level | 7 | 2145 | 40% | OR (M‐H, Fixed, 95% CI) | 1.14 [0.91, 1.43] | 0.24 |
| Two‐level | 2 | 727 | 79% | OR (M‐H, Random, 95% CI) | 1.34 [0.24, 7.49] | 0.74 | |
| Serious AE | Single‐level | 3 | 1029 | 43% | OR (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.26] | 0.72 |
| Two‐level | 2 | 727 | 0% | OR (M‐H, Fixed, 95% CI) | 0.79 [0.54, 1.14] | 0.20 | |
| Device/surgery‐related AE | Single‐level | 5 | 1590 | 0% | OR (M‐H, Fixed, 95% CI) | 1.02 [0.75, 1.38] | 0.91 |
| Two‐level | 2 | 727 | 0% | OR (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.96] | 0.04 | |
| Secondary surgery at the index level | Single‐level | 6 | 1867 | 0% | OR (M‐H, Fixed, 95% CI) | 0.37 [0.26, 0.52] | <0.00001 |
| Two‐level | 2 | 727 | 0% | OR (M‐H, Fixed, 95% CI) | 0.41 [0.22, 0.75] | 0.004 | |
| Secondary surgery at the adjacent level | Single‐level | 7 | 2092 | 29% | OR (M‐H, Fixed, 95% CI) | 0.31 [0.22, 0.45] | <0.00001 |
| Two‐level | 2 | 727 | 0% | OR (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.82] | 0.007 | |
| Patients’ satisfaction rate | Single‐level | 3 | 705 | 0% | OR (M‐H, Fixed, 95% CI) | 2.48 [1.55, 3.96] | 0.0002 |
| Two‐level | 2 | 519 | 12% | OR (M‐H, Fixed, 95% CI) | 1.73 [1.00, 3.00] | 0.05 | |
AE, adverse event; CI, confidence interval; NDI, neck disability index; OR, odds ratio
Assessment of Publication Bias
The funnel plot was applied to detect publication bias. As for neurological success (Fig. 9A), the funnel plots appeared symmetric and all studies were included inside, indicating that no publication bias existed. However, for secondary surgery at the adjacent level (Fig. 9B), the funnel plots appeared symmetric and 1 study was not included inside, indicating that publication bias existed.
Figure 9.
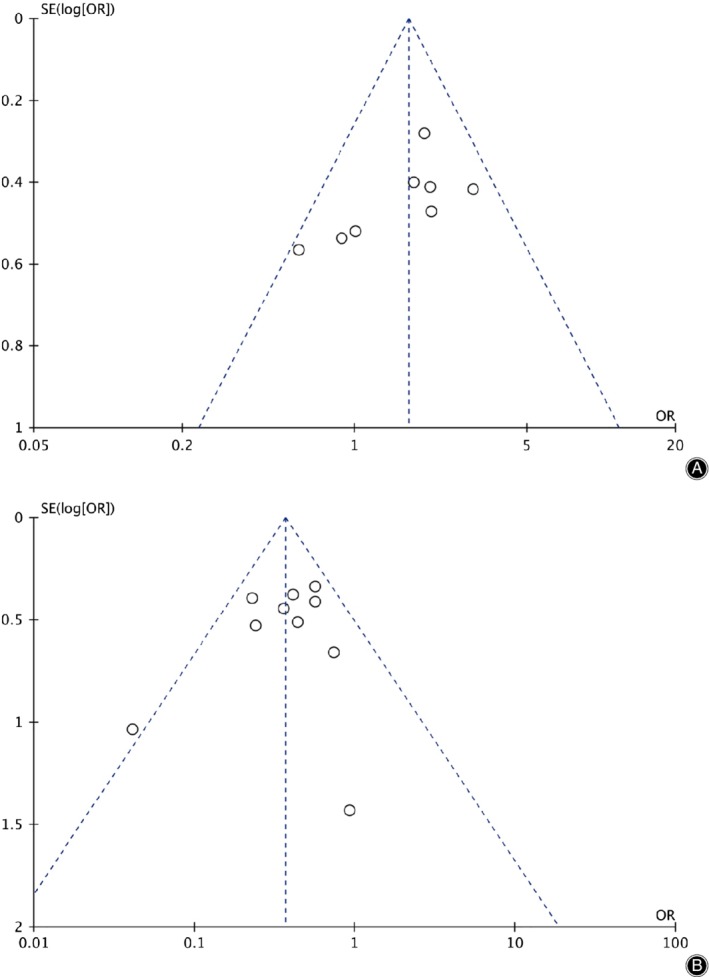
The funnel plot of neurological success (A) and secondary surgery at the adjacent level (B). CI, confidence interval.
Discussion
Up to now, CDA application in spinal practice has remained controversial. Whether CDA is superior to ACDF has not been established in the long run ASD is always associated with the length of follow up. Therefore, it is crucial to evaluate the safety and efficiency of CDA in the long run. To our knowledge, there have been several meta‐analyses comparing CDA with ACDF. Most of them have included partial long‐term results, but they were mixed up with short‐term and mid‐term results5, 16, 17, 18, 19, 20, 21, 22, 23. Therefore, given the availability of newly published long‐term results28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, we performed this study. This is the first time comparing the safety and efficiency of CDA with ACDF only focusing on long‐term follow‐ups.
In our meta‐analysis, 11 RCT with more than 5 years’ follow‐up were identified. Based on the quality assessment criteria recommended by the Cochrane Back Review Group25, all the studies were rated as low risk of bias. However, blinding to patients and care providers was not appropriately achieved in any studies. In addition, only 4 studies29, 31, 32, 35 achieved blinding to outcome evaluators. This may result in reporting bias. Heterogeneity definitely existed in the included studies. First, various different types of CDA devices were used in the 11 RCT, including Kineflex|C29, Bryan30, 34, Discover35 Secure‐C38, Prestige28, 31, Mobi‐C32, 37, ProDisc‐C33, and PCM36, differing in design and biomechanical properties. Second, the surgical level was different among studies. A total of 8 studies compared one‐level CDD28, 29, 30, 32, 33, 34, 36, 38, 1 study compared two‐level CDD31, and 2 studies compared both one‐level and two‐level CDD35, 37. Third, the region of studies was also different. Eight studies28, 29, 31, 33, 34, 36, 37, 38 were conducted in the US and just 3 studies30, 32, 35 were out of the USA. Fourth, evaluation criteria of outcome measures varied among studies. Thus, we performed a sensitivity analysis including comparing two different effect models, using the leave‐one‐out method27 and subgroup analysis to find the origin of heterogeneity. The combined results of radiological superior and inferior ASD were not stable and reliable and should be considered with caution. One possible reason is that only 2 studies reported this outcome36, 37. Although no publication bias existed in neurological success, publication bias existed in secondary surgery at the adjacent level.
After 5 years’ follow up or more, our study revealed that CDA achieved a higher rate of clinical success and better functional outcome measurements with statistical significance, except for NDI score. A mid‐term to long‐term meta‐analysis conducted by Hu et al.17 compared 4–7 years’ clinical results, pooling data from 8 RCT, and showed that CDA achieved a significantly higher clinical success rate and better functional outcome. Similarly, Gao et al.5 compared 2–5 years’ clinical results, pooling data for 14 RCT for analysis, and found that CDA was superior in VAS pain scores and neurological success, but NDI scores remained similar. In addition, major functional outcome measurements of CDA proved to have no obvious benefits when pooling 1–2 years’ data into the analysis24. This difference may originate from the different follow‐up duration. Theoretically, CDA shares the same procedure of discectomy, endplate preparing, and decompression. VAS arm pain should be similar. However, VAS arm pain score was favored for CDA at the final follow up.
Adverse events are another major concern when applying CDA. Our results showed no statistical difference in total reported AE, serious AE, and device or surgery‐related serious AE. This finding is consistent with some previous meta‐analyses5, 18, 23 but contrary to others17. This difference can be explained by the different inclusion criteria for each study. Our study was focused on the long‐term data and only enrolled RCT with more than 5 years’ follow‐up. Undeniably, pseudoarthrosis would not occur after CDA, but heterotopic ossification and bone loss became new problems12, 14. A recent systematic review14 showed that the long‐term heterotopic ossification rate after CDA was 53.6% and the severe (grade 3 and 4) heterotopic ossification rate was 47.5%. In addition, the severe heterotopic ossification rate was significantly associated with follow‐up time, with a 0.63% increase per month growth14. Bone loss was as high as 60.4%, although it did not affect mid‐term to long‐term clinical outcomes12. This might be the reason why surgeons did not feel confident recommending CDA as a standard option30. Moreover, it could explain the similar incidence of AE between CDA and ACDF.
Adjacent segment degeneration is the most important factor to be considered. The initial purpose of designing CDA was to prevent ASD after surgery. The biomechanical advantages have been well established3, 49. A recent meta‐analysis showed that there was no statistically significant difference in ASD between CDA and ACDF within 24‐months’ follow‐up period, but ASD was significantly lower with an increase of follow‐up duration in CDA16. In contrast, Xu et al.21 and Zhu et al.23 found that CDA was superior in reducing the ASD incident rate when compared with ACDF, and this superiority became more apparent over time21. Although these 3 studies16, 21, 23 attempted to evaluate ASD and symptomatic ASD separately, the follow‐up period was not separated clearly, and long‐term results were weak. Our results show that CDA has significantly lower symptomatic ASD. However, when we pooled all data together, there was no statistical difference in radiological superior ASD between CDA and ACDF. Interestingly, Ren et al.20 found that ASD was not significantly different between CDA and ACDF with a smaller sample. Nunley et al.50 (2018) summarized biomechanical and clinical evidence from worldwide application of CDA and concluded that CDA decreased the rate of radiographic adjacent segment pathology by alleviating adjacent‐level stress. However, the reason why subgroup analysis showed no significant difference in the non‐US group is still difficult to explain.
Increased attention has been focused on the secondary surgery rate. Ghobrial et al.40 found that fewer patients with the Bryan disc required surgery for symptomatic ASD when compared with ACDF without statistical significance at 10 years’ follow‐up. However, they performed combined analysis using Bryan and Prestige artificial discs and found significant differences in symptomatic ASD requiring surgery as early as after 7 years40. Surprisingly, MacDowall et al.51 conducted a retrospective study based on a Swedish database and found that CDA had a similar secondary surgery rate at the adjacent level but a higher secondary surgery rate at the index level with significant difference. However, based on our long‐term results, CDA had a significantly lower rate of total secondary surgery, secondary surgery at the adjacent level, and secondary surgery at the index level, which is consistent with mid‐term to long‐term results17 However, this finding is contrary to the short‐term to mid‐term result reported by Zhang et al.52 that the secondary surgery rate at the adjacent level showed no significant difference. It seems that CDA exhibited superiority in reducing secondary surgery through restoring favorable physiological biomechanical properties in the long‐term follow‐up. However, it is important to note that our subgroup analysis also showed no statistical difference in the secondary surgery rate in the non‐US group.
Several limitations may exist in this study. First, due to our focus on long‐term results, only 11 RCT were included and 8 of them were conducted in the USA. Therefore, our study may not reflect the worldwide results and may result in bias. In addition, larger size samples are needed in future studies. Second, although all included studies were rated as low risk of bias based on the Cochrane Back Review Group, all of them failed to achieve sufficient blinding and the allocation concealment was rarely clearly described. Third, high heterogeneity exists in NDI score, radiological superior ASD and inferior ASD. Our sensitivity analysis results revealed that radiological superior ASD and inferior ASD were not stable and, therefore, should be considered with caution. Finally, subgroup analysis showed different results for NDI score, symptomatic ASD, total secondary surgery, secondary surgery at the index level, and secondary surgery at the adjacent level between US and non‐US regions. Therefore, well‐designed worldwide multi‐center RCTs with long‐term follow‐ups are still needed for further evaluation in the future.
Conclusion
Our study provided further evidence that CDA is superior in achieving long‐term clinical outcomes such as overall success, NDI success and neurological success, VAS neck pain and arm pain, SF‐36 PCS and MCS, symptomatic ASD, total secondary surgery, and secondary surgery at the index level and at the adjacent level. However, no clear benefit could be identified in regard to NDI score, total reported AE, serious AE, device/surgery‐related AE, and radiological superior and inferior ASD. Well‐designed worldwide RCT with long‐term follow up are still necessary for further evaluation in the future.
Acknowledgments
No funding support was received for this study.
References
- 1. Chin‐See‐Chong TC, Gadjradj PS, Boelen RJ, Harhangi BS. Current practice of cervical disc arthroplasty: a survey among 383 AOSpine international members. Neurosurg Focus, 2017, 42: E8. [DOI] [PubMed] [Google Scholar]
- 2. Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion?. Spine J, 2004, 4: 190S–194S. [DOI] [PubMed] [Google Scholar]
- 3. Eck JC, Humphreys SC, Lim TH, et al Biomechanical study on the effect of cervical spine fusion on adjacent‐level intradiscal pressure and segmental motion. Spine (Phila Pa 1976), 2002, 27: 2431–2434. [DOI] [PubMed] [Google Scholar]
- 4. Park DH, Ramakrishnan P, Cho TH, et al Effect of lower two‐level anterior cervical fusion on the superior adjacent level. J Neurosurg Spine, 2007, 7: 336–340. [DOI] [PubMed] [Google Scholar]
- 5. Gao F, Mao T, Sun W, et al An updated meta‐analysis comparing artificial cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) for the treatment of cervical degenerative disc disease (CDDD). Spine (Phila Pa 1976), 2015, 40: 1816–1823. [DOI] [PubMed] [Google Scholar]
- 6. Duggal N, Pickett GE, Mitsis DK, Keller JL. Early clinical and biomechanical results following cervical arthroplasty. Neurosurg Focus, 2004, 17: E9. [DOI] [PubMed] [Google Scholar]
- 7. Walraevens J, Demaerel P, Suetens P, et al Longitudinal prospective long‐term radiographic follow‐up after treatment of single‐level cervical disk disease with the Bryan cervical disc. Neurosurgery, 2010, 67: 679–687. [DOI] [PubMed] [Google Scholar]
- 8. McAnany SJ, Overley S, Baird EO, et al The 5‐year cost‐effectiveness of anterior cervical discectomy and fusion and cervical disc replacement: a Markov analysis. Spine (Phila Pa 1976), 2014, 39: 1924–1933. [DOI] [PubMed] [Google Scholar]
- 9. Ament JD, Yang Z, Nunley P, Stone MB, Lee D, Kim KD. Cost utility analysis of the cervical artificial disc vs fusion for the treatment of 2‐level symptomatic degenerative disc disease: 5‐year follow‐up. Neurosurgery, 2016, 79: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JS, Dowdell J, Cheung ZB, et al The seven‐year cost‐effectiveness of anterior cervical discectomy and fusion versus cervical disc arthroplasty: a Markov analysis. Spine (Phila Pa 1976), 2018, 43: 1543–1551. [DOI] [PubMed] [Google Scholar]
- 11. Overley SC, McAnany SJ, Brochin RL, Kim JS, Merrill RK, Qureshi SA. The 5‐year cost‐effectiveness of two‐level anterior cervical discectomy and fusion or cervical disc replacement: a Markov analysis. Spine J, 2018, 18: 63–71. [DOI] [PubMed] [Google Scholar]
- 12. Heo DH, Lee DC, Oh JY, Park CK. Bone loss of vertebral bodies at the operative segment after cervical arthroplasty: a potential complication?. Neurosurg Focus, 2017, 42: E7. [DOI] [PubMed] [Google Scholar]
- 13. Wagner SC, Kang DG, Helgeson MD. Implant migration after Bryan cervical disc arthroplasty. Spine J, 2014, 14: 2513–2514. [DOI] [PubMed] [Google Scholar]
- 14. Kong L, Ma Q, Meng F, Cao J, Yu K, Shen Y. The prevalence of heterotopic ossification among patients after cervical artificial disc replacement: a systematic review and meta‐analysis. Medicine (Baltimore), 2017, 96: e7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saifi C, Fein AW, Cazzulino A, et al Trends in resource utilization and rate of cervical disc arthroplasty and anterior cervical discectomy and fusion throughout the United States from 2006 to 2013. Spine J, 2018, 18: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 16. Dong L, Xu Z, Chen X, et al The change of adjacent segment after cervical disc arthroplasty compared with anterior cervical discectomy and fusion: a meta‐analysis of randomized controlled trials. Spine J, 2017, 17: 1549–1558. [DOI] [PubMed] [Google Scholar]
- 17. Hu Y, Lv G, Ren S, Johansen D. Mid‐ to long‐term outcomes of cervical disc arthroplasty versus anterior cervical discectomy and fusion for treatment of symptomatic cervical disc disease: a systematic review and meta‐analysis of eight prospective randomized controlled trials. PLoS One, 2016, 11: e0149312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kan SL, Yuan ZF, Ning GZ, Liu FF, Sun JC, Feng SQ. Cervical disc arthroplasty for symptomatic cervical disc disease: traditional and Bayesian meta‐analysis with trial sequential analysis. Int J Surg, 2016, 35: 111–119. [DOI] [PubMed] [Google Scholar]
- 19. Muheremu A, Niu X, Wu Z, Muhanmode Y, Tian W. Comparison of the short‐ and long‐term treatment effect of cervical disk replacement and anterior cervical disk fusion: a meta‐analysis. Eur J Orthop Surg Traumatol, 2015, 25: S87–S100. [DOI] [PubMed] [Google Scholar]
- 20. Ren C, Song Y, Xue Y, Yang X. Mid‐ to long‐term outcomes after cervical disc arthroplasty compared with anterior discectomy and fusion: a systematic review and meta‐analysis of randomized controlled trials. Eur Spine J, 2014, 23: 1115–1123. [DOI] [PubMed] [Google Scholar]
- 21. Xu S, Liang Y, Zhu Z, Qian Y, Liu H. Adjacent segment degeneration or disease after cervical total disc replacement: a meta‐analysis of randomized controlled trials. J Orthop Surg Res, 2018, 13: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu RS, Kan SL, Cao ZG, Jiang ZH, Zhang XL, Hu W. Secondary surgery after cervical disc arthroplasty versus fusion for cervical degenerative disc disease: a meta‐analysis with trial sequential analysis. Orthop Surg, 2018, 10: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Y, Zhang B, Liu H, Wu Y, Zhu Q. Cervical disc arthroplasty versus anterior cervical discectomy and fusion for incidence of symptomatic adjacent segment disease: a meta‐analysis of prospective randomized controlled trials. Spine (Phila Pa 1976), 2016, 41: 1493–1502. [DOI] [PubMed] [Google Scholar]
- 24. Bartels RH, Donk R, Verbeek AL. No justification for cervical disk prostheses in clinical practice: a meta‐analysis of randomized controlled trials. Neurosurgery, 2010, 66: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 25. Furlan AD, Malmivaara A, Chou R, et al Updated method guideline for systematic reviews in the Cochrane Back and neck group. Spine (Phila Pa 1976), 2015, 40: 1660–1673. [DOI] [PubMed] [Google Scholar]
- 26. Jelicic Kadic A, Vucic K, Dosenovic S, Sapunar D, Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J Clin Epidemiol, 2016, 74: 119–123. [DOI] [PubMed] [Google Scholar]
- 27. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between‐study heterogeneity in meta‐analysis: proposed metrics and empirical evaluation. Int J Epidemiol, 2008, 37: 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burkus JK, Traynelis VC, Haid RW Jr, Mummaneni PV. Clinical and radiographic analysis of an artificial cervical disc: 7‐year follow‐up from the prestige prospective randomized controlled clinical trial: Clinical article. J Neurosurg Spine, 2014, 21: 516–528. [DOI] [PubMed] [Google Scholar]
- 29. Coric D, Guyer RD, Nunley PD, et al Prospective, randomized multicenter study of cervical arthroplasty versus anterior cervical discectomy and fusion: 5‐year results with a metal‐on‐metal artificial disc. J Neurosurg Spine, 2018, 28: 252–261. [DOI] [PubMed] [Google Scholar]
- 30. Donk RD, Verbeek ALM, Verhagen WIM, Groenewoud H, Hosman AJF, Bartels R. What's the best surgical treatment for patients with cervical radiculopathy due to single‐level degenerative disease? A randomized controlled trial. PLoS One, 2017, 12: e0183603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gornet MF, Lanman TH, Burkus JK, et al Two‐level cervical disc arthroplasty versus anterior cervical discectomy and fusion: 10‐year outcomes of a prospective, randomized investigational device exemption clinical trial. J Neurosurg Spine, 2019, 31: 508–518. [DOI] [PubMed] [Google Scholar]
- 32. Hou Y, Nie L, Pan X, et al Effectiveness and safety of Mobi‐C for treatment of single‐level cervical disc spondylosis: a randomised control trial with a minimum of five years of follow‐up. Bone Joint J, 2016, 98: 829–833. [DOI] [PubMed] [Google Scholar]
- 33. Janssen ME, Zigler JE, Spivak JM, Delamarter RB, Darden BV 2nd, Kopjar B. ProDisc‐C Total disc replacement versus anterior cervical discectomy and fusion for single‐level symptomatic cervical disc disease: seven‐year follow‐up of the prospective randomized U.S. Food and Drug Administration investigational device exemption study. J Bone Joint Surg Am, 2015, 97: 1738–1747. [DOI] [PubMed] [Google Scholar]
- 34. Lavelle WF, Riew KD, Levi AD, Florman JE. Ten‐year outcomes of cervical disc replacement with the BRYAN cervical disc: results from a prospective, randomized, controlled clinical trial. Spine (Phila Pa 1976), 2019, 44: 601–608. [DOI] [PubMed] [Google Scholar]
- 35. MacDowall A, Canto Moreira N, Marques C, et al Artificial disc replacement versus fusion in patients with cervical degenerative disc disease and radiculopathy: a randomized controlled trial with 5‐year outcomes. J Neurosurg Spine, 2019, 30: 323–331. [DOI] [PubMed] [Google Scholar]
- 36. Phillips FM, Geisler FH, Gilder KM, Reah C, Howell KM, McAfee PC. Long‐term outcomes of the US FDA IDE prospective, randomized controlled clinical trial comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. Spine (Phila Pa 1976), 2015, 40: 674–683. [DOI] [PubMed] [Google Scholar]
- 37. Radcliff K, Davis RJ, Hisey MS, et al. Long‐term evaluation of cervical disc arthroplasty with the Mobi‐C(c) cervical disc: a randomized, prospective, Multicenter clinical trial with seven‐year follow‐up. Int J Spine Surg, 2017, 11: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vaccaro A, Beutler W, Peppelman W, et al Long‐term clinical experience with selectively constrained SECURE‐C cervical artificial disc for 1‐level cervical disc disease: results from seven‐year follow‐up of a prospective, randomized, controlled investigational device exemption clinical trial. Int J Spine Surg, 2018, 12: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Delamarter RB, Zigler J. Five‐year reoperation rates, cervical total disc replacement versus fusion, results of a prospective randomized clinical trial. Spine (Phila Pa 1976), 2013, 38: 711–717. [DOI] [PubMed] [Google Scholar]
- 40. Ghobrial GM, Lavelle WF, Florman JE, Riew KD, Levi AD. Symptomatic adjacent level disease requiring surgery: analysis of 10‐year results from a prospective, randomized, clinical trial comparing cervical disc arthroplasty to anterior cervical fusion. Neurosurgery, 2019, 84: 347–354. [DOI] [PubMed] [Google Scholar]
- 41. Hisey MS, Zigler JE, Jackson R, et al Prospective, randomized comparison of one‐level Mobi‐C cervical Total disc replacement vs. anterior cervical discectomy and fusion: results at 5‐year follow‐up. Int J Spine Surg, 2016, 10: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jackson RJ, Davis RJ, Hoffman GA, et al Subsequent surgery rates after cervical total disc replacement using a Mobi‐C cervical disc prosthesis versus anterior cervical discectomy and fusion: a prospective randomized clinical trial with 5‐year follow‐up. J Neurosurg Spine, 2016, 24: 734–745. [DOI] [PubMed] [Google Scholar]
- 43. Lanman TH, Burkus JK, Dryer RG, Gornet MF, McConnell J, Hodges SD. Long‐term clinical and radiographic outcomes of the prestige LP artificial cervical disc replacement at 2 levels: results from a prospective randomized controlled clinical trial. J Neurosurg Spine, 2017, 27: 7–19. [DOI] [PubMed] [Google Scholar]
- 44. Loumeau TP, Darden BV, Kesman TJ, et al A RCT comparing 7‐year clinical outcomes of one level symptomatic cervical disc disease (SCDD) following ProDisc‐C total disc arthroplasty (TDA) versus anterior cervical discectomy and fusion (ACDF). Eur Spine J, 2016, 25: 2263–2270. [DOI] [PubMed] [Google Scholar]
- 45. Miller J, Sasso R, Anderson P, Riew KD, McPhilamy A, Gianaris T. Adjacent level degeneration: Bryan Total disc arthroplasty versus anterior cervical discectomy and fusion. Clin Spine Surg, 2018, 31: E98–E101. [DOI] [PubMed] [Google Scholar]
- 46. Radcliff K, Coric D, Albert T. Five‐year clinical results of cervical total disc replacement compared with anterior discectomy and fusion for treatment of 2‐level symptomatic degenerative disc disease: a prospective, randomized, controlled, multicenter investigational device exemption clinical trial. J Neurosurg Spine, 2016, 25: 213–224. [DOI] [PubMed] [Google Scholar]
- 47. Sasso WR, Smucker JD, Sasso MP, Sasso RC. Long‐term clinical outcomes of cervical disc arthroplasty: a prospective, randomized, Controlled Trial. Spine (Phila Pa 1976), 2017, 42: 209–216. [DOI] [PubMed] [Google Scholar]
- 48. Zigler JE, Delamarter R, Murrey D, Spivak J, Janssen M. ProDisc‐C and anterior cervical discectomy and fusion as surgical treatment for single‐level cervical symptomatic degenerative disc disease: five‐year results of a Food and Drug Administration study. Spine (Phila Pa 1976), 2013, 38: 203–209. [DOI] [PubMed] [Google Scholar]
- 49. DiAngelo DJ, Roberston JT, Metcalf NH, McVay BJ, Davis RC. Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech, 2003, 16: 314–323. [DOI] [PubMed] [Google Scholar]
- 50. Nunley PD, Coric D, Frank KA, Stone MB. Cervical disc arthroplasty: current evidence and real‐world application. Neurosurgery, 2018, 83: 1087–1106. [DOI] [PubMed] [Google Scholar]
- 51. MacDowall A, Skeppholm M, Lindhagen L, et al Artificial disc replacement versus fusion in patients with cervical degenerative disc disease with radiculopathy: 5‐year outcomes from the National Swedish Spine Register. J Neurosurg Spine, 2018, 30: 159–167. [DOI] [PubMed] [Google Scholar]
- 52. Zhang Y, Liang C, Tao Y, et al Cervical total disc replacement is superior to anterior cervical decompression and fusion: a meta‐analysis of prospective randomized controlled trials. PLoS One, 2015, 10:e0117826. [DOI] [PMC free article] [PubMed] [Google Scholar]


