Abstract
Objective
To determine the clinical and liver stiffness characteristics of a cohort of Chinese patients with Hepatocellular carcinoma in different stages of Barcelona clinic liver cancer.
Methods
Details of 1180 patients with Hepatocellular carcinoma referred from October 2014 to November 2017 were collected retrospectively. Demographic data, etiology, clinical, and biochemical details were retrospectively analyzed. The changes of liver stiffness in different etiologies and different stages of Barcelona clinic liver cancer were especially analyzed.
Results
The onset age was 60.33 ± 9.11 (range 24‐84) years, 9 cases were ≤40 years, 572 cases were 41‐60 years, males accounted for 83.92%, females accounted for 16.08%; 599 cases were ≥61 years, males accounted for 78.25%, females accounted for 21.75%. Compared with males, the proportion of females ≥61 is higher than that of men. Majority (n = 787; 66.69%) had HBV infection; second commonest cause was HCV infection (n = 217; 18.39%). More patients with HBV infection were 41‐60 years (69.06%) and were younger than HCV patients. There was no statistical difference in etiology, age, gender, and distribution of diabetes mellitus among different Barcelona clinic liver cancer stages (P > .05). The overall Hepatocellular carcinoma (HCC) was found to be positively correlated with alkaline phosphatase, γ‐glutamyltransferase, and alpha‐fetoprotein and liver stiffness measurement values from stage A to stage D (P < .05). ANOVA analysis showed that the overall liver stiffness measurement among the four BCLC stages was found to be statistically significant different in HBV‐infected and HCV‐infected HCC patients.
Conclusion
Majority (99.24%) were patients aged >40 years old. Male is a high incidence population. In etiological analysis, HBV dominates HCC occurrence, HBV‐, HCV‐, and alcohol‐associated HCC have distinct clinical and biochemical characteristics, necessitating different screening policies to optimize HCC surveillance and management.
Keywords: alcoholic liver disease, alpha‐fetoprotein, hepatocellular carcinoma, liver stiffness measurement, viral hepatitis
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is ranked worldwide as the sixth most common malignancy. It is the second leading cause of cancer related deaths and the alarming increase in incidence has made HCC a global health concern.1, 2 According to statistics, early diagnosis of HCC is difficult due to its insidious onset. The incidence of HCC in China accounts for more than 55% worldwide.3 Only 30%‐40% of HCC patients can receive radical treatment, which seriously threatens people's life and health.4
The highest liver cancer rates are found in East and Southeast Asia and in Middle and Western Africa. This difference in incidence of liver cancer between different geographical regions and countries is mainly attributed to difference in the incidence of underlying risk factors.5 A substantial amount of patients are diagnosed at a later stage of the disease, which may preclude curative treatment options. Therefore, it is very necessary to describe the clinical characteristics of HCC patients in different regions and stages, which is of great value for the early detection, early diagnosis, early treatment, reduction of morbidity, and improvement of prognosis of patients.
Liver stiffness measurement (LSM) using transient elastography has been introduced as a promising noninvasive method for assessing the degree of liver fibrosis. Moreover, transient elastic imaging detector is one of the most widely validated noninvasive tools to detect early liver cirrhosis in various chronic liver diseases. Elasticity imaging has been reported to be useful for the diagnosis and characterization of various tumors, which are usually stiffer than normal tissues. Recent studies on liver stiffness indicate that LSM may be useful in screening for HCC, and several studies have investigated the prognostic role of LSM in the noninvasive assessment of the risk for HCC development.6
In this study, we aimed to evaluate the significant clinical characteristics and to assess differences of biochemical details with HCC in different stages of Barcelona clinic liver cancer (BCLC). Moreover, the aim of this study was to investigate whether LSM assessed by transient elastography shows a significant correlation with HCC.
2. MATERIALS AND METHODS
2.1. Objects of the study
A total of 1180 patients with HCC referred to The Third Central Clinical College of Tianjin Medical University, Taiyuan Infectious Diseases Hospital and The First Hospital of Shanxi Medical University from October 2014 to November 2017 were included in the study. Inclusion criteria: (a). Patients with HCC diagnosed for the first time; (b). All patients were confirmed by clinical symptoms and signs, imaging examination (liver B‐ultrasound, MRI, CT, or hepatic angiography), quantitative examination of serum AFP, and/or liver histopathology examination; (c). Common single cause HBV infection or HCV infection or alcohol‐related; and (d). Previously diagnosed HCC without any clinical intervention. Exclusion criteria: (a). Hepatocellular carcinoma caused by autoimmune hepatitis, nonalcoholic fatty liver disease, aflatoxin, and schistosomiasis; (b). History of combined with other malignant tumor; (c). Patients less than 18 years old and those with incomplete medical history data; (d). Patients with serious heart, lung, kidney, and other diseases; and (e). Pregnant and lactating women. BCLC stage was determined in every patient with HCC at initial diagnosis according to the extent of tumor, performance status, liver function status, vascular invasion, and extra‐hepatic spread.7 The patients were divided into four groups according to BCLC stage at admission.8 The study was approved by the ethics committees of participating hospitals.
The clinical parameters including age, gender, etiology, diabetes, and tumor characteristics were obtained. Laboratory investigations including alanine aminotransferase (ALT), alkaline phosphatase (AKP), prothrombin time (PT), alpha‐fetoprotein (AFP), and γ‐glutamyltransferase (γ‐GT) and liver stiffness measurement (LSM), respectively, were obtained.
2.2. Statistical treatment
Epidata 3.1 dual input was used for data entry. Data were analyzed using SAS software version 9.4. Qualitative data were expressed in proportion and chi‐square test was used. Quantitative data were expressed by mean and standard deviation, and the comparison between groups and within groups was conducted by a single factor ANOVA test. A P‐value of <0.05 was considered statistically significant.
3. RESULTS
3.1. Patient inclusion and clinical characteristics
A total of 1180 patients diagnosed with HCC were included in the study. The mean age was 60.33 ± 9.11 (range 24‐84) years, with a male preponderance (n = 922; 78.25%). The overall male to female (M/F) ratio was 3.57 (922/258) (Table 1). Compared with males, females were significantly more likely to be ≥61 years (Figure 1).
Table 1.
Comparison of clinical characteristics of HCC patients (Number [%])
| Number (%) | HCC group (N = 1180) | χ 2 | P | ||||
|---|---|---|---|---|---|---|---|
|
A (N = 452) |
B (N = 465) |
C (N = 186) |
D (N = 77) |
||||
| Etiology | |||||||
| HBV | 787 | 292 (37.10) | 312 (39.64) | 129 (16.39) | 54 (6.86) | 4.75 | .576 |
| HCV | 217 | 96 (44.24) | 78 (35.94) | 30 (13.82) | 13 (5.99) | ||
| Alcohol | 176 | 64 (36.36) | 75 (42.61) | 27 (15.34) | 10 (5.68) | ||
| Age | |||||||
| ≤40 | 9 | 4 (44.44) | 5 (55.56) | 0 (0.00) | 0 (0.00) | 11.59 | .072 |
| 41‐60 | 572 | 218 (38.11) | 216 (37.76) | 88 (15.38) | 50 (8.74) | ||
| ≥61 | 599 | 230 (38.40) | 244 (40.73) | 98 (16.36) | 27 (4.51) | ||
| Gender | |||||||
| Female | 258 | 110 (42.64) | 99 (38.37) | 37 (14.34) | 12 (4.65) | 3.91 | .272 |
| Male | 922 | 342 (37.09) | 366 (39.70) | 149 (16.16) | 65 (7.05) | ||
| Diabetes | |||||||
| Negative | 928 | 361 (38.90) | 375 (40.41) | 135 (14.55) | 57 (6.14) | 6.561 | .087 |
| Positive | 252 | 91 (36.11) | 90 (35.71) | 51 (20.24) | 20 (7.94) | ||
Data are presented as percentages and numbers, and the percentages of date were calculated by BCLC standard.
Figure 1.
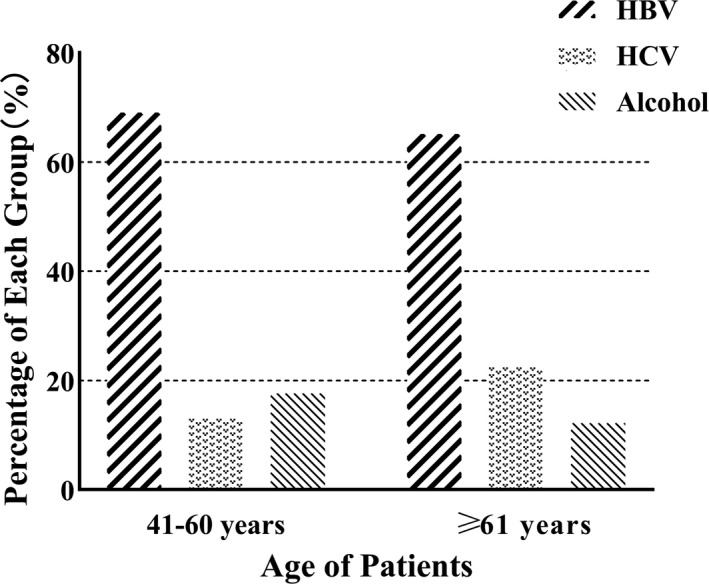
HBV‐related HCC was significantly more likely to be younger than HCV‐related HCC
There were 787 patients (66.69%) with HBV‐associated HCC and 217 patients (18.39%) with HCV‐associated HCC. Alcohol was the cause for HCC in 176 (14.92%) (Table 1).
The proportion of patients with HBV‐associated HCC in 41‐60 years was higher than in ≥61 years. The proportion of patients with HCV‐associated HCC in 41‐60 years was lower than in ≥61 years. HBV‐related HCC was significantly more likely to be younger than HCV‐related HCC (Figure 2).
Figure 2.
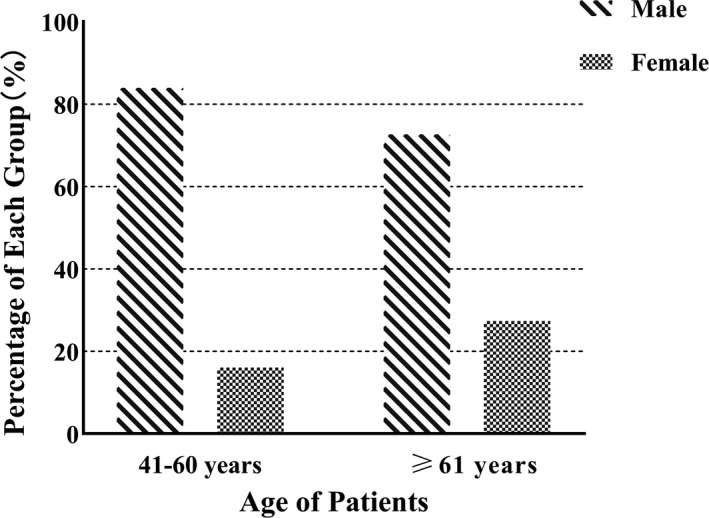
Compared with males, females were significantly more likely to be ≥61 y
In this study, all patients aged ≥18 years were enrolled as inpatients. Overall, the commonest age was ≥61 years, which were 599 patients (50.56%), followed by 41‐60 years in 572 patients (47.83%; Table 1).
In addition, in this cohort, 928 (78.25%) patients had diabetes (Table 1).
The onset age of stage A, B, C, and D were 59.97 ± 9.21, 60.90 ± 9.29, 60.74 ± 8.66, and 58.03 ± 8.15 years, respectively. There was no statistically significant difference in the four groups of the etiology, age, gender, and diabetes history (P > .05; Table 1). Tumor characteristics.
The number of patients with a single tumor was 608 (51.53%), and the remaining patients had multinodular (572 patients, 48.47%). (Table 2).
Table 2.
Tumor characteristics of HCC
| Tumor characteristics | N (%) | Tumor metastases | N (%) |
|---|---|---|---|
| Number of nodules | No extra‐hepatic metastases | 948 (80.34) | |
| A single tumor | 608 (51.53) | Lung | 112 (9.49) |
| Multinodular tumor | 572 (48.47) | Abdominal metastasis | 47 (3.98) |
| Venous invasion | 239 (20.25) | Bone | 16 (1.36) |
| Adrenal | 25 (2.12) | ||
| Metastases at other sites | 32 (2.71) |
Data are presented as numbers and percentages.
Macroscopic portal vein invasion was seen in 239 (20.25%). Nine hundred forty‐eight had tumor confined to the liver, while the rest (19.66%) had extra‐hepatic tumor spread. 112 (9.49%) had lung, 47 (3.98%) had abdominal metastasis, 16 (1.36%) had bone, 25 (2.12%) had adrenal and 32 (2.71%) had metastases in other sites (Table 2).
3.2. Etiological distribution
The age of male HCC patients according to etiology for 41‐60 years was as follows: 338 (70.42%) patients with HBV infection, 45 (9.38%) patients with HCV infection, 97 (20.21%) patients with alcohol‐related. Followed by ≥61 years, 294 cases had HBV infection (67.59%), 72 cases had HCV infection (16.55%), and 69 cases were alcohol‐related (15.86%). The least common age group was ≤40 years, including 2 cases (28.57%) of HBV infection, 3 cases (42.86%) of HCV infection, and 2 cases (28.57%) of alcohol‐related.
The major age of female HCC patients was ≥61 years, of which 96 cases (58.54%) were HBV infection, 64 cases (39.02%) were HCV infection, and 4 cases (2.44%) were alcohol‐related. Followed by 41‐60 years, 57 cases (61.96%) had HBV infection, 31 cases (33.70%) had HCV infection, and 4 cases (4.34%) were alcohol‐related. The least common age group was ≤40 years, including 2 cases (100%) of HCV infection.
Chi‐square test results showed that the etiology of male patients was statistically different in different age groups (P = .001). There was no significant difference in etiology of female patients in different age groups (P = .331; Table 3).
Table 3.
Etiological distribution of HCC patients with different sex and age
| Etiology | Male (N = 922) | χ 2 | P | Female (N = 258) | χ 2 | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤40a (%) | 41‐60b (%) | ≥61c (%) | ≤40 (%) | 41‐60 (%) | ≥61 (%) | |||||
| HBV | 2 (28.57) | 338 (70.42) | 294 (67.59) | 18.56 | .001 | 0 (0.00) | 57 (61.96) | 96 (58.54) | 4.60 | .331 |
| HCV | 3 (42.86) | 45 (9.38) | 72 (16.55) | 2 (100.00) | 31 (33.70) | 64 (39.02) | ||||
| Alcohol‐related | 2 (28.57) | 97 (20.21) | 69 (15.86) | 0 (0.00) | 4 (4.34) | 4 (2.44) | ||||
a, b, and c indicate that the difference between groups is statistically significant.
Data are presented as percentages and numbers, and the percentages of date were calculated by grouping different etiologies.
3.3. Age distribution
In male patients, the majority of HBV‐induced HCC patients were 41‐60 years, accounting for 53.31%, and ≥61 years accounted for 46.37%. The majority of HCV‐induced HCC patients were in ≥61 years, accounting for 60.00%. The age of patients with alcohol‐related HCC was high in 41‐60 years (57.74%) and was 41.07% in ≥61 years.
In female patients, the majority of HBV‐induced HCC patients were in ≥61 years old, accounting for 62.75%, and 37.25% in 41‐60 years. The majority of HCV‐induced HCC patients were in ≥61 years, accounting for 65.98%. The age of patients with alcohol‐related HCC was high in ≥61 years, accounting for 50%, and 50% in 41‐60 years.
Chi‐square test results showed that male HCC patients were distributed differently in different etiological age groups (P = .001). There was no significant difference in age distribution of different etiologies among female HCC patients (P = .331; Table 4).
Table 4.
Age distribution of HCC patients with different sex and etiology
| Age | Male (N = 922) | χ 2 | P | Female (N = 258) | χ 2 | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HBVa (%) | HCVb (%) | Alcohol‐related (%)c | HBV (%) | HCV (%) | Alcohol‐related (%) | |||||
| ≤40 | 2 (0.32) | 3 (2.50) | 2 (1.19) | 18.56 | .001 | 0 (0.00) | 2 (2.06) | 0 (0.00) | 4.60 | .331 |
| 41‐60 | 338 (53.31) | 45 (37.50) | 97 (57.74) | 57 (37.25) | 31 (31.96) | 4 (50.00) | ||||
| ≥61 | 294 (46.37) | 72 (60.00) | 69 (41.07) | 96 (62.75) | 64 (65.98) | 4 (50.00) | ||||
a, b, c indicates that the difference between groups is statistically significant.
Data are presented as percentages and numbers, and the percentages of date were calculated by grouping different ages.
3.4. Sex distribution
The age at onset of HBV‐induced HCC was 41‐60 years old, of which 85.57% were males and 14.43% were females. It was followed by the age group of ≥61 years old, of which 75.38% were males and 24.62% were females. The second cause of HCC was HCV, which was more common in the age group of ≥61 years old, with 52.94% males and 47.06% females. Alcohol was the least common cause of HCC patients, most of whom were 41‐60 years old, of which 96.04% were males and 3.96% were females.
Chi‐square test results showed that the gender distribution of HBV infection in different age groups was different (P < .001). There was no significant difference in gender distribution of HCV infection and alcohol‐related in different age groups (P = .663 and P = .851; Table 3).
3.5. Comparison of clinical examination indicators
Relevant clinical indicators used to evaluate HCC patients included ALT, AKP, PT, AFP, γ‐GT, and LSM. The results of ANOVA and LSD showed that there was a statistically significant difference among the four groups in clinical examination of γ‐GT, AKP, AFP, and LSM (P < .05). There was a positively correlated increasing trend with the progression of HCC from stage A to stage D (P < .05). AKP was significantly different in HCC stages A and C (P = .002), D (P < .001), and B and D (P < .001). γ‐GT had significant difference between A and C, D (P < .001), B and C, D (P < .001). AFP was significantly different in stages A, C (P < .001) and D (P = .008). It was found that there was no significant difference between stages C and D of HCC (P = .638; Table 5).
Table 5.
Comparison of laboratory indicators and tumor marker
| HCC (N = 1180) | F | P | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| ALT (IU/L) | 44.04 ± 37.49 | 45.60 ± 41.08 | 49.88 ± 48.00 | 51.88 ± 48.81 | 1.81 | .144 |
| AKP (U/L) | 105.98 ± 62.39a | 110.25 ± 69.10ab | 126.86 ± 91.75b | 148.03 ± 148.36c | 8.45 | <.001 |
| γ‐GT (U/L) | 88.54 ± 102.14a | 84.38 ± 87.65a | 132.21 ± 119.07b | 122.01 ± 16.72c | 9.12 | <.001 |
| PT (s) | 14.83 ± 6.96 | 15.11 ± 7.90 | 14.82 ± 2.48 | 16.84 ± 3.13 | 2.07 | .103 |
| AFP (ug/L) | 96.34 ± 282.28a | 169.53 ± 437.40a | 244.27 ± 679.00b | 173.03 ± 347.39c | 5.60 | .001 |
| LSM (kPa) | 23.19 ± 16.32a | 28.00 ± 18.84b | 32.54 ± 21.85c | 33.06 ± 21.60c | 14.90 | <.001 |
a, b, c indicates that the difference between groups is statistically significant.
3.6. Distribution of LSM value
ANOVA analysis showed that the overall LSM values among the four BCLC stages were 23.19 ± 16.32 kPa, 28.00 ± 18.84 kPa, 32.54 ± 21.85 kPa, and 33.06 ± 21.61 kPa, respectively. LSD test showed statistically significant difference among the four stages (P < .001).
In stages A and B, the increase of LSM caused by alcohol was higher than that of HBV or HCV infection (stage A: F = 16.77, P < .001 and stage B: F = 4.84, P = .004). There was no statistically significant difference among the four BCLC stages in alcohol‐related HCC patients (F = 0.326 and P = .807). There was a statistically significant difference among the four BCLC stages in HBV‐infected HCC patients (except between groups C and D). There was a statistically significant difference among the four BCLC stages in HCV‐infected HCC patients (between stages A and B, C, D, between stages B and D). (Table 6).
Table 6.
LSM distribution in HCC groups
| HCC (n = 1180) | F | P | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| HBV infection | 21.41 ± 14.42a | 26.53 ± 19.00b | 31.93 ± 22.10c | 33.20 ± 22.03c | 13.574 | <.001 |
| HCV infection | 21.55 ± 13.26a | 27.11 ± 17.30b | 35.42 ± 19.67bc | 29.07 ± 16.00c | 21.970 | <.001 |
| Alcohol‐related | 33.77 ± 23.42 | 34.01 ± 18.71 | 32.26 ± 23.44 | 40.10 ± 25.21 | 0.326 | .807 |
| Total | 23.19 ± 16.32a | 28.00 ± 18.84b | 32.54 ± 21.85c | 33.06 ± 21.61c | 14.90 | <.001 |
| F | 16.77 | 4.84 | 0.311 | 1.03 | – | – |
| P | <.001 | .004 | .733 | .363 | – | – |
a, b and c indicate that the difference between groups is statistically significant.
4. DISCUSSION
In this retrospective, multicentre, and observational cohort study, we investigated the clinical characteristics of HCC patients, compared the incidence rate from gender, age, and etiology, and refined the epidemiological characteristics. Among the 1180 cases, it was found that the youngest individual was 24 years old, as well as with a male predominance. 9 cases were ≤40 years old, and the peak age ranged >40 years old, with 572 cases aged 41‐60 years old and 599 cases aged ≥61 years old. Previous studies have shown that this may be related to the progression of liver fibrosis with age.9 Among the studied cases, there were 395 (69.06%) cases of HBV‐related HCC patients aged 41‐60, of which 338 were males and 57 were females, and 76 (13.29%) cases of HCV‐related HCC patients, including 45 males and 31 females. There were 390 (65.11%) cases of HBV infection ≥61 years old and 136 (22.70%) cases of HCV infection. It was found that HBV‐related HCC was mostly found in 41‐60 years old, while HCV‐related HCC was mostly in ≥61 years old. This finding may be explained that HBV is transmitted vertically in the perinatal period, whereas HCV is more infected at a later stage in life, and therefore, patients with HBV‐related HCC tended to be significantly younger than patients with HCV‐related HCC.10
Our data showed that chronic viral hepatitis was the major risk factor contributing to the development of HCC and majority were related to HBV infection (66.69%). Hepatitis C (18.39%) was the second risk factors for HCC in our study. Remarkably, alcohol as a risk factor for underlying liver disease has contributed to minority of patients (14.92%). It can be seen that viral infection was the leading cause, which was consistent with the research results of other studies.11, 12 HBV/HCV infection is a process of chronic and sustained damage repair, which takes a long time to develop into HCC. Attention must be paid to the prevention of hepatitis. Although the incidence of HBV‐associated HCC has been decreasing following widespread availability of HBV vaccination, HBV infection is still the main cause of HCC at present.13 The government should further strengthen the implementation and ensure the vaccination of hepatitis B vaccine, further reduce the infection rate and the morbidity of HCC. In recent years, with the growth of the economy and the improvement of living standard, more and more alcohol is produced and consumed, and the number of people with alcohol‐related liver disease is increasing year by year. According to surveys, the output of alcohol in China rose from 7.113 million tons in 1984 to 30.6987 million tons in 2001, a fourfold increase in the past 20 years. From 1980s to 1990s, the proportion of alcoholics in the general population rose from 0.21% to 14.3%. At the beginning of the 21st century, epidemiological investigations in some provinces and cities in China have shown that the drinking population increased to 26.98%‐43.4%. Alcoholic cirrhosis rose from 3% to 7.7%, 2.3 times growth in 10 years. Alcohol is related to HCC progression. Its long‐term exposure in the body will aggravate oxidative stress,14 release a large number of harmful inflammatory factors,15 lead to malnutrition and continuous degeneration and necrosis of liver cells from large amount of intestinal toxins entering the blood, resulting in liver cirrhosis and liver cancer. Therefore, alcohol abuse should be actively controlled.
In this study, in HCC patients aged 41‐60 years old, there were 480 (83.92%) males and 92 (16.08%) females, and in HCC patients ≥61 years old, there were 435 (72.62%) males and 164 (27.38%) females. This study revealed that HCC was more prevalent in males, which is in agreement with previous studies,16, 17 as well as other local and regional studies.18, 19In addition, the finding that females were significantly older than males might also refect a course in disease progression. Previous studies have shown that the liver fibrosis status of women changes with age, which may be due to changes in the reproductive status,20 providing some hints for the result. The study showed that male patients had different etiology distribution (hepatitis B, hepatitis C, and alcohol‐associated) and the etiologies were diversified. However, alcohol‐related HCC in female patients was similar among the four stages, which might be related to the basic knowledge of low alcohol consumption by women.
Hepatocellular carcinoma grows continuously and can infiltrate the neighboring vasculature, including the portal vein and less frequently the hepatic veins, which is associated with poor disease outcome. Macroscopic portal vein invasion was found in 20.25% of our patients. Portal vein thrombosis was documented in 15.3% of the cases in European studies. They observed that male patients tend to have higher rate of portal vein thrombosis. Portal vein thrombosis is a critical issue that can deteriorate the prognosis of HCC because it can lead to wide dissemination of tumors through the liver and cause a marked deterioration of hepatic function.21 The incidence of extra‐hepatic metastases has been reported in 19.66% in our studies and occurred mainly to the lungs. According to reports in the literature that macroscopic venous invasion precludes most of effective treatments available. Presence of extra‐hepatic metastases and portal vein invasion has made palliative care the only option for a significant proportion of our patients at the time of presentation.22 Therefore, less treatment measures will affect the prognosis of patients. We again recommend regular follow‐up of patients with liver disease to improve early diagnosis.
AFP is a useful diagnostic marker for HCC, and roughly 50%‐70% of adults with HCC have increased levels of AFP.23, 24 In this study, AFP was found to be related to HCC progression from stage A to stage D and was basically positively correlated, which suggests that higher serum levels of AFP were more likely to present with advanced stage HCC with severe liver dysfunction and compromised performance status. γ‐GT mainly exists in liver cell membrane and microsome. Data have indicated that γ‐GT serum level can be used to evaluate liver fibrosis and injury as a sensitive and accurate biomarker.25 γ‐GT increases moderately or highly in patients with viral hepatitis, liver cirrhosis, alcoholic hepatitis, and primary or metastatic liver cancer. In this study, it is found that γ‐GT had an increasing trend with advanced stage HCC and had statistical significance. In addition, SandraL 26, 27 and other studies have found that AKP is closely related to liver fibrosis. This study showed that AKP level gradually increased with advanced stage HCC from stage A to stage D, with statistical differences.
Many literatures have shown that liver cirrhosis is the main risk factor for liver cancer.28, 29 Transient elastography (TE) is a new noninvasive diagnostic technique for liver fibrosis in recent years, of which the most widely used is FibroScan developed by French company Echosens, which can evaluate the degree of liver fibrosis when liver lesions occur. LSM measured by FibroScan can not only accurately diagnose liver fibrosis, but also predict liver cancer effectively.30 The results of this study showed that the LSM value gradually increased in the progression of HCC patients from stage A to stage D. Further comparison showed that the LSM value of patients with alcohol‐associated HCC was significantly higher than that of HBV‐associated group and HCV‐associated group, whether in stage A or stage B. However, the difference of LSM values between BCLC stages was not significant in patients with alcohol‐associated HCC. In contrast, in patients with viral hepatitis, no matter HCC was caused by hepatitis B or hepatitis C, LSM value increased with the progress of HCC stage, and it was statistically significant. Therefore, patients with viral hepatitis still need active etiological treatment to minimize or even eliminate viral replication.
Therapeutic approaches for HCC include partial hepatectomy, liver transplantation, and interventional methods such as transarterial chemoembolization (TACE), selective internal radiotherapy (SIRT), or local ablative methods. For advanced and metastatic HCC, pharmacological treatment options have largely expanded over recent years.28 Surgical treatments, TACE, and Radiofrequency ablation (RFA) are best options to achieve optimal survival rates in the long‐term, and there is still a need for improvement of current surveillance methods for earlier detection of HCC to facilitate those curative treatments for most of the patients.
In summary, the peak age of incidence of HCC was found to be >40 years old. HBV‐associated HCC patients are often younger than HCV‐associated HCC patients. Male is a high incidence population, but with the increase of age, the number of HCC female patients tends to increase despite of the etiology. In etiological analysis, HBV dominates HCC occurrence, and alcohol‐associated liver diseases account for a certain proportion of male patients, which cannot be ignored. In clinical examination, γ‐GT, AKP, AFP, and LSM values gradually increase with advanced stage HCC from stage A to stage D, showing positive correlation. Primary prevention of chronic hepatitis, including universal HBV vaccination, identification of the at‐risk population (patients with HCV or HBV or alcohol) by mass screening of the general population, prevention of liver disease progression and hepatic dysfunction by providing antiviral treatment, minimization of alcohol exposure, implementation of HCC surveillance among the population at risk for the disease, and establishment of centers of excellence for HCC treatment are essential components of attempts (both ongoing and future) to curb the morbidity and mortality from HCC.
The results of this study are limited by its retrospective design and need to be further confirmed by wider prospective and multi‐center studies.
ACKNOWLEDGMENTS
The author thanks the data support from The Third Central Clinical College of Tianjin Medical University, Taiyuan Infectious Disease Hospital and the First Hospital of Shanxi Medical University and thanks the financial support from the National Natural Science Foundation of China 81870429 and from the 2017 National 13th 5‐Year Plan for Hepatitis Research 2017ZX10203201‐007.
Zhao H, Zhu P, Han T, et al. Clinical characteristics analysis of 1180 patients with hepatocellular carcinoma secondary to hepatitis B, hepatitis C and alcoholic liver disease. J Clin Lab Anal. 2020;34:e23075 10.1002/jcla.23075
Ping Zhu contributed equally as that of first author
REFERENCES
- 1. Hartke J, Johnson M, & Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diaqn Pathol. 2017;34(2):153‐159. [DOI] [PubMed] [Google Scholar]
- 2. Bakouny Z, Assi T, El Rassy E, Nasr F. Second‐line treatments of advanced hepatocellular carcinoma: systematic review and network meta‐analysis of randomized controlled trials. J Clin Gastroenterol. 2019;53(4):251‐261. [DOI] [PubMed] [Google Scholar]
- 3. Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24(35):4000‐4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhuang BW, Li W, Xie XH, Hu HT, Lu MD, Xie XY. Sorafenib versus hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: a systematic review and meta‐analysis. Jpn J Clin Oncol. 2019;7:1‐11. [DOI] [PubMed] [Google Scholar]
- 5. Abdel‐Rahman O, Helbling D, Schöb O, et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: an updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10(4):245‐254. [DOI] [PubMed] [Google Scholar]
- 6. He C, Zhou Z, Xiao Z, Wang J. Treatment strategy for huge hepatocellular carcinoma with intrahepatic metastasis and macrovascular invasion: a case report and literature review. J Cancer Res Ther. 2018;14(Supplement):S1233‐S1236. [DOI] [PubMed] [Google Scholar]
- 7. Jung YK, Jung CH, Seo YS, Kim JH, Kim TH, Yoo YJ. BCLC stage B is a better designation for single large hepatocellular carcinoma than BCLC stage A. J Gastroenterol Hepatol. 2016;31(2):467‐474. [DOI] [PubMed] [Google Scholar]
- 8. Cai BB, Shi KQ, Li P, et al. A nomogram integrating hepatic reserve and tumor characteristics for hepatocellular carcinoma following curative liver resection. Clin Chim Acta. 2018;485:187‐194. [DOI] [PubMed] [Google Scholar]
- 9. Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The obsvirc, metavir, clinivir, and dosvirc groups. Lancet. 1997;349(9055):825‐832. [DOI] [PubMed] [Google Scholar]
- 10. Borzio M, Dionigi E, Vitale A, et al. Management and prognosis of HCC in the elderly: results of an in‐field multicenter cohort study. Liver Int. 2017;37(8):1184‐1192. [DOI] [PubMed] [Google Scholar]
- 11. Torre LA, Bray F, Seal RL, Ferlay J, Loret‐Tieulent J, Jemal A. Global Cancer Statistics. CA cancer J Clin. 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 12. Yang H, Chen K, Wei Y, et al. Treatment of spontaneous ruptured hepatocellular carcinoma: a single‐center study. Pak J Med Sci. 2014;30(3):472‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mogul DB, Ling SC, Murray KF, Schwarzenberg SJ, Rudzinski ER, Schwarz KB. Characteristics of hepatitis B virus–associated hepatocellular carcinoma in children: a multi‐center study. J Pediatr Gastroenterol Nutr. 2018;67(4):437‐440. [DOI] [PubMed] [Google Scholar]
- 14. Zhu H, Jia ZQ, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012;13(3):133‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide‐induced inflammation are linked to IRAK‐M in human monocytes. J Immunol. 2009;183(2):1320‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelen GD, Green GB, Purcell RH, et al. Hepatitis B and Hepatitis C in emergency department patients. N Engl J Med. 1992;326(21):1399‐1404. [DOI] [PubMed] [Google Scholar]
- 17. El‐Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745‐750. [DOI] [PubMed] [Google Scholar]
- 18. Alswat KA, Sanai FM, Altuwaijri M, et al. Clinical characteristics of patients with hepatocellular carcinoma in a middle eastern population. Hepat Mon. 2013;13(5):e7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaka H, Mshana SE, Rambau PF, Masalu N, Chalya PL, Kalluvya SE. Hepatocellular carcinoma: clinicopathological profile and challenges of management in a resource‐limited setting. World J Surg Oncol. 2014;12:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sagnelli E, Stroffolini T, Sagnelli C, et al. Gender differences in chronic liver diseases in two cohorts of 2001 and 2014 in Italy. Infection. 2018;46(1):93‐101 [DOI] [PubMed] [Google Scholar]
- 21. Zhan T, Sollors J, Steinbrunner N, et al. Pharmacological treatment of hepatocellular carcinoma with cavoatrial tumor thrombus–case series and literature review. Z Gastroenterol. 2019;57(4):501‐507. [DOI] [PubMed] [Google Scholar]
- 22. Bulathsinhala BKS, Siriwardana RC, Gunetilleke MB, Niriella MA, Dassanayake A. Clinical characteristics and outcomes of hepatocellular carcinoma: results from prospective study, from a tertiary referral center in Sri Lanka. Ceylon Med J. 2018;63:133‐138. [DOI] [PubMed] [Google Scholar]
- 23. Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCC. Liver Cancer. 2015;4(2):126‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yao MJ, Zhao JM, Lu FM. Alpha‐fetoprotein still is a valuable diagnostic and prognosis predicting biomarker in hepatitis B virus infection‐related hepatocellular carcinoma. Oncotarget. 2016;7(4):3702‐3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang N, Zhang Y, Liu Z, Ai X, Liang Q. Correlation of four potential biomarkers of liver fibrosis with liver function and grade of hepatic fibrosis in a neonatal cholestatic rat model. Mol Med Rep. 2017;16(1):415‐421. [DOI] [PubMed] [Google Scholar]
- 26. Loyal S, Rock L, Silva A, Frearia J, Dinis‐Oliveira RJ, Sá SI. Evaluation of progressive hepatic histopathology in long‐term tamoxifen therapy. Pathol Res Pract. 2018;214(12):2115‐2120. [DOI] [PubMed] [Google Scholar]
- 27. Tarantino G, Finelli C, Colao A, et al. Are hepatic steatosis and carotid intima media thickness associated in obese patients with normal or slightly elevated gamma‐glutamyl‐transferase. J Transl Med. 2012;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hulin A, Stocco J, Bouattour M. Clinical pharmacokinetics and pharmacodynamics of transarterial chemoembolization and targeted therapies in hepatocellular carcinoma. Clin Pharmacokinet. 2019;58(8):983‐1014. [DOI] [PubMed] [Google Scholar]
- 29. Masuzaki R, Tateishi R, Yoshida H, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49(6):1954‐1961. [DOI] [PubMed] [Google Scholar]
- 30. Stefanescu H, Procopet B. Noninvasive assessment of portal hypertension in cirrhosis: Liver stiffness and beyond. World J Gastroenterol. 2014;20(45):16811‐16819. [DOI] [PMC free article] [PubMed] [Google Scholar]


