Abstract
Objective
This study aimed to explore the predictive value of microRNA (miR)‐125a and miR‐125b for sepsis risk, and their correlations with inflammation, disease severity, and 28‐day mortality in sepsis patients.
Methods
Totally, 150 sepsis patients and 150 healthy controls (HCs) were enrolled. Plasma samples were separated from blood samples obtained from sepsis patients and HCs to detect miR‐125a and miR‐125b expressions by real‐time quantitative polymerase chain reaction. Besides, the 28‐day mortality of sepsis patients was assessed. MiR‐125a and miR‐125b expressions were elevated in sepsis patients compared with HCs, and further receiver operating characteristics (ROC) curve analysis displayed that miR‐125a (area under the curve (AUC): 0.749, 95% CI: 0.695‐0.803) and miR‐125b (AUC: 0.839, 95% CI: 0.795‐0.882) could predict sepsis risk. As for inflammation, no correlation of miR‐125a with C‐reactive protein (CRP), tumor necrosis factor‐α (TNF‐α), interleukin (IL)‐6, IL‐17, and IL‐23 was observed in sepsis patients, while miR‐125b was positively associated with CRP, TNF‐α, IL‐6, IL‐17, and IL‐23. Regarding disease severity, miR‐125a and miR‐125b were positively correlated with acute physiology and chronic health care evaluation II and sequential organ failure assessment score in sepsis patients. Besides, ROC curve analysis exhibited that miR‐125a failed to predict 28‐day mortality risk (AUC: 0.588, 95% CI: 0.491‐0.685) in sepsis patients, while miR‐125b had a potential value in predicting elevated 28‐day mortality risk (AUC: 0.699, 95% CI: 0.603‐0.795).
Conclusion
Both miR‐125a and miR‐125b predict sepsis risk, while only miR‐125b exhibits the potency for disease management and prognosis prediction in sepsis patients.
Keywords: disease severity, miR‐125a, miR‐125b, prognosis, sepsis
1. INTRODUCTION
Sepsis, a devastating and life‐threatening clinical condition, is caused by the dysregulated host immune response to a microbial infection, which can rapidly progress to organ dysfunction and death.1, 2 It is estimated to affect more than 30 million people worldwide each year according to WHO report 2018, and the mortality rate is as high as 30% for sepsis patients.3, 4 Considering the facet of rapid progression and high mortality rate of sepsis, early identification of disease severity becomes essential for the evaluation of clinical treatment and the management of sepsis.5 Acute physiology and chronic health evaluation (APACHE) II score and sequential organ failure assessment (SOFA) score are widely used for assessing sepsis risk and predicting prognosis in sepsis patients.6, 7 However, they lack adequate specificity and require multiple measurements, leading to misleading results and unnecessary administration of antibiotics under some circumstances, which contribute to prolonged hospitalization stay and antibiotic resistance.6, 8 Therefore, it is of great need to explore novel sensitive biomarkers with the potential for assisting on disease management and improving prognosis in sepsis patients.
With the wide application of high‐throughput analysis, non‐coding RNA is increasingly recognized as a regulator in various signaling pathways of inflammatory process in sepsis.9 As a class of non‐coding RNA, microRNAs (miRNAs) are endogenous small non‐coding RNA with approximately 19‐23 nucleotides in length that are involved in post‐transcriptional regulations of gene expressions via impacting both the stability and translation of mRNAs.10 As for miRNA‐125 (miR‐125) family, it is composed of miR‐125a and miR‐125b.11 Prior studies elucidate that miR‐125a and miR‐125b are involved in the regulation of inflammatory responses and organ injuries.12, 13, 14 For instance, one study that discloses the elevated expression of miR‐125a is responsible for the upregulation of cytokines and chemokines such as interleukin (IL)‐β, IL‐6 and tumor necrosis factor‐α (TNF‐α) in lupus nephritis patients.12 Another study reports on miR‐125a and miR‐125b, as hypoxia‐regulated miRNAs, is involved in several hypoxia‐induced organ dysfunctions such as heart and lung injuries through generating reactive oxygen species, protease inhibition, enzyme oxidation, and lipid peroxidation.14 Based on these aforementioned studies, we hypothesized that miR‐125a and miR‐125b might participate in the development and progression of sepsis as well. However, limited related studies have been reported yet. Therefore, this study aimed to explore the predictive value of miR‐125a and miR‐125b for sepsis risk and their correlations with disease severity, inflammation, and 28‐day mortality risk in sepsis patients.
2. MATERIALS AND METHODS
2.1. Participants
Between January 2017 and May 2019, 150 sepsis patients admitted to our hospital were consecutively enrolled in this study. The inclusion criteria were as follows: (a) diagnosed as sepsis in line with the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3)15; (b) age ≥ 18 years; and (c) no history of cancers or hematological malignancies; the exclusion criteria included: (a) died within 24 hours after admission; (b) infected with human immunodeficiency virus; (c) received immunosuppressive therapy within 6 months; and (d) pregnant or lactating women. In addition, 150 healthy subjects who underwent physical examination in our hospital were enrolled as healthy controls (HCs). The screen criteria of HCs were as follows: (a) age above 18 years old; (b) had no history of sepsis or malignancies; (c) had no obvious abnormalities confirmed by physical examination; and (d) not pregnant or lactating women. This study was approved by the Ethics Committee of our hospital. All participants or their guardians provided written informed consents before enrollment.
2.2. Clinical data collection
Clinical characteristics of sepsis patients were documented after enrollment, including age, gender, body mass index (BMI), smoke status, and chronic comorbidities (such as chronic obstructive pulmonary disease (COPD), cardiomyopathy, chronic kidney failure, and cirrhosis). The laboratory indexes measured immediately after admission, such as serum creatinine (Scr), albumin, white blood cell (WBC), and C‐reactive protein (CRP), were also recorded. Besides, APACHE II score and SOFA score were documented as well, which were evaluated within 24 hours after admission and used to assess severity of sepsis and severity of organ dysfunction.
2.3. Sample collection
Blood samples of all sepsis patients were collected within 24 hours after admission, and the blood samples of HCs were collected on the enrollment. All blood samples were centrifuged at 1600 g for 10 minutes at 4℃. Subsequently, the supernatants were separated into Eppendorf tubes and further centrifuged at 12 000 g for 10 minutes at 4°C. Finally, the plasma was obtained and stored at −80°C until determination.
2.4. Inflammatory cytokines measurement
The levels of TNF‐α, IL‐6, IL‐17, and IL‐23 in plasma from AIS patients were measured using commercial human enzyme‐linked immune sorbent assay (ELISA) Kits (Abcam) in accordance with the manufacturer's protocol.
2.5. MiR‐125a and miR‐125b detection
For sepsis patients and HCs, the relative expressions of miR‐125a and miR‐125b in plasma were detected by real‐time quantitative polymerase chain reaction (qPCR). Firstly, total RNA was extracted from plasma using PureZOL RNA isolation reagent (Bio‐Rad). Then, cDNA was synthesized using RT‐PCR Quick Master Mix (Toyobo). Subsequently, qPCR was performed using SYBR Green Realtime PCR Master Mix (Toyobo). MiR‐125a and miR‐125b relative expressions were calculated by 2−ΔΔCt using U6 as internal reference.16, 17 Primers applied were as follows: MiR‐125a, forward: 5’ ACACTCCAGCTGGGTCCCTGAGACCCTTTAAC 3’, reverse: 5’ TGTCGTGGAGTCGGCAATTC 3’; miR‐125b, forward: 5’ ACACTCCAGCTGGGTCCCTGAGACCCTAACTT 3’, reverse: 5’ TGTCGTGGAGTCGGCAATTC 3’; U6, forward: 5’ CTCGCTTCGGCAGCACATATACTA 3’, reverse: 5’ ACGAATTTGCGTGTCATCCTTGC 3’.
2.6. Treatment and follow‐up
Standard treatments and resuscitation were administered to patients after the diagnosis was established, which were performed as recommended by the International Guidelines for Management of Sepsis and Septic Shock.18 All patients were followed up for 28 days, and the patients who died during the follow‐up were recorded for evaluation of 28‐day mortality. And all patients were categorized into survivors and deaths according to the survival status during 28‐day follow‐up.
2.7. Statistical analysis
Continuous variables were checked for normality by using quantile‐quantile plot (Q‐Q plot). The normally or approximately normal distributed variables were presented as mean ± standard deviation (SD), and the obviously skewed or unknown distributed variables were expressed as median and interquartile range (IQR). Categorical variables were presented as count (percentage). Comparisons of between‐groups were determined by Student's t test, chi‐square test, or Wilcoxon rank sum test. Correlations were analyzed by Spearman's rank correlation test. Receiver operating characteristic (ROC) curves were plotted, and the area under the curve (AUC) with 95% confidence interval (CI) was calculated to assess the ability of variables in discriminating different subjects or in predicting 28‐day mortality. Statistical analyses were performed using SPSS 24.0 software (IBM), and figures were made using GraphPad Prism 7.01 software (GraphPad Software). P value < .05 was considered significant.
3. RESULTS
3.1. Clinical characteristics
No difference of mean age (P = .153), gender (P = .472), or mean BMI (P = .253) was observed between sepsis patients and HCs. In detail, the mean age was 56.9 ± 10.3 years in sepsis patients and 55.1 ± 11.4 years in HCs. And there were 52 (34.7%) females and 98 (65.3%) males in sepsis patients, 58 (38.7%) females and 92 (61.3%) males in HCs. Among sepsis patients, 23 (15.3%), 49 (32.7%), 18 (12.0%), and 28 (18.7%) of them had COPD, cardiomyopathy, chronic kidney failure, and cirrhosis, respectively. Meanwhile, the mean APACHE II score and mean SOFA score were 16.3 ± 6.3 and 7.3 ± 3.2 respectively in sepsis patients. Other detailed characteristics of sepsis patients and HCs were listed in Table 1.
Table 1.
Clinical characteristics of sepsis patients and HCs
| Items |
Sepsis patients (N = 150) |
HCs (N = 150) |
P value |
|---|---|---|---|
| Age (y), mean ± SD | 56.9 ± 10.3 | 55.1 ± 11.4 | 0.153 |
| Gender, No. (%) | |||
| Female | 52 (34.7) | 58 (38.7) | 0.472 |
| Male | 98 (65.3) | 92 (61.3) | |
| BMI (kg/m2), mean ± SD | 22.8 ± 4.8 | 22.3 ± 2.6 | 0.253 |
| Smoke, No. (%) | 58 (38.7) | – | – |
| Chronic comorbidities, No. (%) | |||
| COPD | 23 (15.3) | – | – |
| Cardiomyopathy | 49 (32.7) | – | – |
| Chronic kidney failure | 18 (12.0) | – | – |
| Cirrhosis | 28 (18.7) | – | – |
| Laboratory indexes, median (IQR) | |||
| Scr (mg/dL) | 1.7 (1.2‐2.4) | – | – |
| Albumin (g/L) | 27.2 (21.6‐36.8) | – | – |
| WBC (×109/L) | 14.0 (2.9‐28.1) | – | – |
| CRP (mg/L) | 98.5 (52.7‐128.8) | – | – |
| APACHE II score, mean ± SD | 16.3 ± 6.3 | – | – |
| SOFA score, mean ± SD | 7.3 ± 3.2 | – | – |
| Inflammatory cytokines, median (IQR) | |||
| TNF‐α (pg/mL) | 178.9 (120.0‐278.5) | – | – |
| IL‐6 (pg/mL) | 72.8 (42.2‐148.3) | – | – |
| IL‐17 (pg/mL) | 168.6 (76.8‐251.1) | – | – |
| IL‐23 (pg/mL) | 119.3 (56.9‐244.9) | – | – |
Comparison was determined by Student's t test or Chi‐square test.
Abbreviations: APACHE II score, acute pathologic and chronic health evaluation II score; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; HCs, healthy controls; IL, interleukin; IQR, interquartile range; Scr, serum creatinine; SD, standard deviation; SOFA score, sequential organ failure assessment score; TNF‐α, tumor necrosis factor‐α; WBC, white blood cell.
3.2. Predictive value of miR‐125a and miR‐125b for sepsis risk
MiR‐125a relative expression was higher in sepsis patients (1.781 [1.060‐2.378]) than that in HCs (0.915 [0.385‐1.433]; P < .001; Figure 1A). And miR‐125b relative expression was also increased in sepsis patients (1.778 [1.407‐3.799]) compared with HCs (0.998 [0.708‐1.407]; P < .001; Figure 1B). Further ROC curve analysis disclosed that miR‐125a (AUC: 0.749, 95% CI: 0.695‐0.803) and miR‐125b (AUC: 0.839, 95% CI: 0.795‐0.882) could distinguish sepsis patients from HCs (Figure 1C). These data suggested that miR‐125a and miR‐125b could predict sepsis risk, and the predictive value of miR‐125b for sepsis risk was superior to that of miR‐125a numerically.
Figure 1.
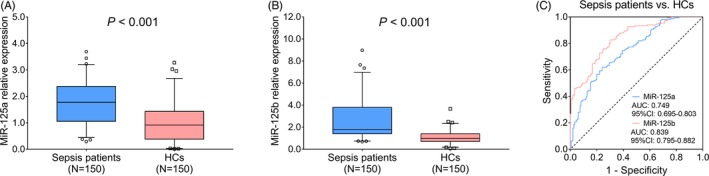
The value of miR‐125a and miR‐125b for predicting sepsis risk. The comparison of miR‐125a relative expression between sepsis patients and HCs (A). The comparison of miR‐125b relative expression between sepsis patients and HCs (B). The ability of miR‐125a/b in differentiating sepsis patients from HCs (C). Comparisons of miR‐125a and miR‐125b relative expressions between sepsis patients and HCs were determined by Wilcoxon rank sum test. P < .05 was considered significant. The performance of miR‐125a and miR‐125b for predicting sepsis risk was assessed by ROC curve and AUC with 95% CI. AUC, area under the curve; CI, confidence interval; HCs, healthy controls; miR‐125a, microRNA‐125a; miR‐125b, microRNA‐125b; ROC, receiver operating characteristic
3.3. Correlation of miR‐125a with miR‐125b in sepsis patients
In sepsis patients, the miR‐125a relative expression was positively correlated with miR‐125b relative expression (P < .001, r = .509; Figure 2).
Figure 2.
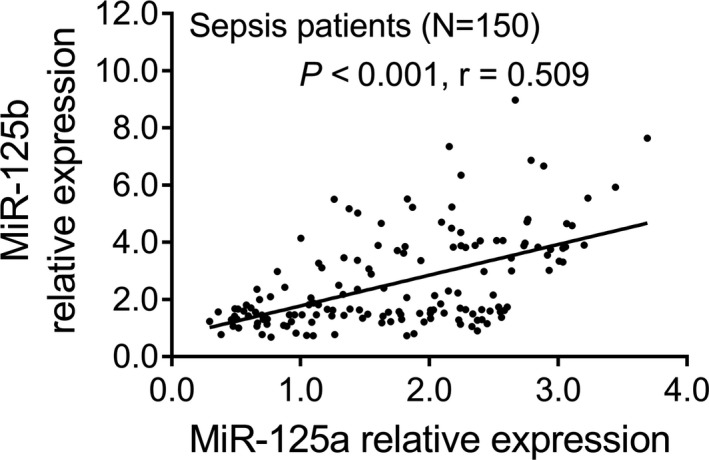
Positive correlation of miR‐125a relative expression with miR‐125b relative expression in sepsis patients. The association of miR‐125a relative expression with miR‐125b relative expression was analyzed by the Spearman's rank correlation test. P < .05 was considered significant. MiR‐125a, microRNA‐125a; miR‐125b, microRNA‐125b
3.4. Correlation of miR‐125a and miR‐125b with APACHE II and SOFA scores in sepsis patients
In sepsis patients, miR‐125a relative expression was positively correlated with APACHE II score (P < .001, r = .287; Figure 3A) and SOFA score (P = .010, r = .209; Figure 3B). Meanwhile, miR‐125b relative expression was positively associated with APACHE II score (P < .001, r = .351; Figure 3C) and SOFA score (P < .001, r = .278; Figure 3D) in sepsis patients. These data implied that both miR‐125a and miR‐125b relative expressions were positively associated with disease severity in sepsis patients.
Figure 3.
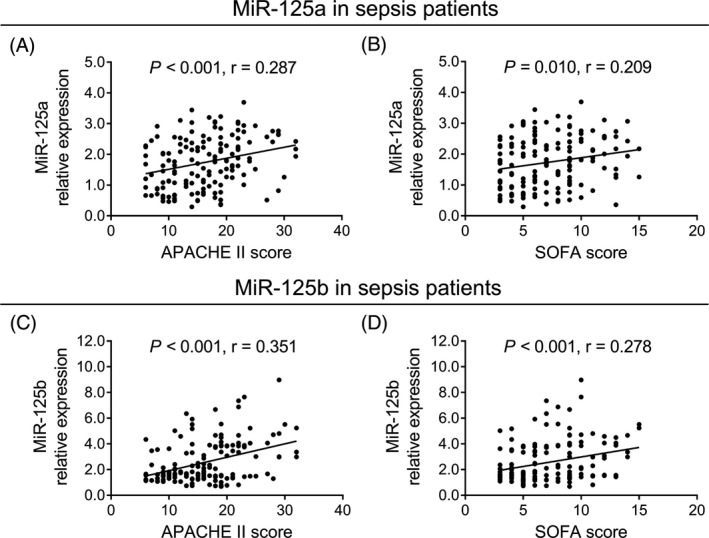
Positive correlation of miR‐125a and miR‐125b relative expressions with disease severity in sepsis patients. The correlation of miR‐125a relative expression with APACHE II score (A) and SOFA score (B) in sepsis patients. And the association of miR‐125b relative expression with APACHE II score (C) and SOFA score (D) in sepsis patients. The associations of miR‐125a and miR‐125b relative expressions with APACHE II and SOFA scores in sepsis patients were analyzed by the Spearman's rank correlation test. P < .05 was considered significant. APACHE II, acute physiology and chronic health evaluation II; MiR‐125a, microRNA‐125a; miR‐125b, microRNA‐125b; SOFA, sequential organ failure assessment
3.5. Correlation of miR‐125a and miR‐125b with inflammatory indexes in sepsis patients
In sepsis patients, no correlation of miR‐125a relative expression with CRP (P = .074, r = .147; Figure 4A), TNF‐α (P = .930, r = .007; Figure 4B), IL‐6 (P = .368, r = .074; Figure 4C), IL‐17 (P = .619, r = .041; Figure 4D), or IL‐23 (P = .535, r = .051; Figure 4E) was observed, while miR‐125b relative expression was positively correlated with CRP (P < .001, r = .414; Figure 4F), TNF‐α (P = .002, r = .253; Figure 4G), IL‐6 (P < .001, r = .291; Figure 4H), IL‐17 (P < .001, r = .305; Figure 4I), and IL‐23 (P = .004, r = .233; Figure 4J) in sepsis patients. These data indicated that there was no association of miR‐125a relative expression with inflammation in sepsis patients, while miR‐125b relative expression was positively associated with inflammation.
Figure 4.
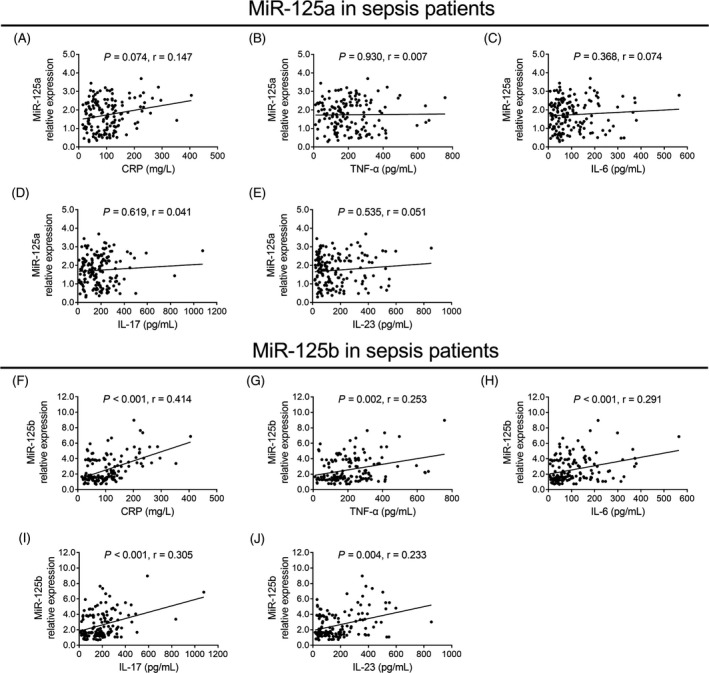
Association of miR‐125a and miR‐125b relative expressions with inflammation in sepsis patients. The correlation of miR‐125a relative expression with CRP (A), TNF‐α (B), IL‐6 (C), IL‐17 (D) and IL‐23 (E) in sepsis patients. And the correlation of miR‐125b relative expression with CRP (F), TNF‐α (G), IL‐6 (H), IL‐17 (I), and IL‐23 (J) in sepsis patients. The correlations of miR‐125a and miR‐125b relative expressions with CRP, TNF‐α, IL‐6, IL‐17, and IL‐23 were evaluated by the Spearman's rank correlation test. P < .05 was considered significant. CRP, C‐reactive protein; IL‐17, interleukin‐17; IL‐23, interleukin‐23; IL‐6, interleukin‐6; MiR‐125a, microRNA‐125a; miR‐125b, microRNA‐125b; TNF‐α, tumor necrosis factor‐α
3.6. Predictive value of miR‐125a and miR‐125b for 28‐day mortality risk in sepsis patients
Sepsis patients were categorized into survivors and deaths according to 28‐day mortality status. As for miR‐125a relative expression, there was no difference between survivors (1.682 [0.921‐2.372]) and deaths (1.868 [1.412‐2.465]; P = .097; Figure 5A), while miR‐125b relative expression was elevated in deaths (3.450 [1.563‐4.709]) compared with survivors (1.646 [1.295‐3.091]; P < .001; Figure 5B). Furthermore, ROC curve analysis showed that miR‐125a could not predict 28‐day mortality risk in sepsis patients (AUC: 0.588, 95% CI: 0.491‐0.685; Figure 5C), while miR‐125b (AUC: 0.699, 95% CI: 0.603‐0.795), CRP (AUC: 0.720, 95% CI: 0.624‐0.815), APACHE II score (AUC: 0.755, 95% CI: 0.675‐0.836), and SOFA score (AUC: 0.739, 95% CI: 0.655‐0.823) were valuable for predicting increased 28‐day mortality risk in sepsis patients. By numerical comparison, the predictive value of miR‐125b for 28‐day mortality risk was similar to that of CRP, APACHE II, and SOFA score. These data suggested that miR‐125a lacked a predictive value for 28‐day mortality risk in sepsis patients, while miR‐125b presented a potential value for predicting increased 28‐day mortality risk.
Figure 5.
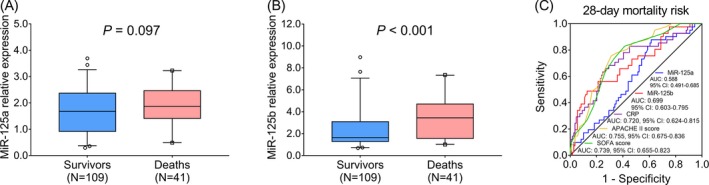
The value of miR‐125a and miR‐125b for predicting 28‐day mortality risk in sepsis patients. The comparison of miR‐125a relative expression between survivors and deaths in sepsis patients (A). And the comparison of miR‐125b relative expression between survivors and deaths in sepsis patients (B). Besides, the ability of miR‐125a, miR‐125b, CRP, APACHE II score, and SOFA score in predicting 28‐day mortality risk in sepsis patients (C). The comparisons of miR‐125a and miR‐125b relative expressions between survivors and deaths in sepsis patients were performed by Wilcoxon rank sum test. P < .05 was considered significant. And the performances of miR‐125a, miR‐125b, CRP, APACHE II score, and SOFA score for predicting 28‐day mortality risk in sepsis patients were assessed by ROC curve and AUC with 95% CI. APACHE II, acute physiology and chronic health evaluation; AUC, area under the curve; CI, confidence interval; CRP, C‐reactive protein; MiR‐125a, microRNA‐125a; miR‐125b, microRNA‐125b; ROC, receiver operating characteristic; SOFA, sequential organ failure assessment
4. DISCUSSION
In the present study, we have discovered that: (a) MiR‐125a and miR‐125b could predict sepsis risk, and miR‐125b exhibited a better predictive value for sepsis risk compared with miR‐125a numerically. (b) No association of miR‐125a with inflammation was observed in sepsis patients, while miR‐125b was positively associated with inflammation. And miR‐125a as well as miR‐125b were positively correlated with disease severity in sepsis patients. (c) MiR‐125a failed to predict 28‐day mortality risk in sepsis patients, while miR‐125b exhibited a potential predictive value for 28‐day mortality risk.
MiR‐125a and miR‐125b have been implied in regulating innate and adaptive immunity during the etiology of different inflammatory and autoimmune diseases.5, 11 Regarding miR‐125a, it is located on chromosome 19 in a cluster with miR‐99B and miR‐7E, which enhances alternative activation of macrophages to assist inflammation.19, 20 As for miR‐125b, it promotes macrophage‐mediated inflammation and upregulates co‐stimulatory factor expression via suppressing interferon regulatory factor 4.21 Evidence exhibit that miR‐125a and miR‐125b participate in regulating inflammatory responses and organ injuries.14, 22 For instance, one study reveals that miR‐125a predicts active disease status and is correlated with inflammation in patients with Crohn's disease.22 Another study illuminates that miR‐125b mediates the hypoxia‐induced pulmonary hypertension through regulating apoptosis of airway epithelial cells, resulting in lung injury.14 In sepsis, the potential source of blood miR‐125a/b might from vital organs such as liver, lung, and heart since sepsis is a disorder involved multiple organ dysfunction and failures. Considering that sepsis is a severe infected disease, we hypothesized that miR‐125a and miR‐125b expressions might be dysregulated and play an important role in the development of sepsis. Our study disclosed that miR‐125a (AUC: 0.749, 95% CI: 0.695‐0.803) and miR‐125b (AUC: 0.839, 95% CI: 0.795‐0.882) could distinguish sepsis patients from HCs. The possible reasons were as follows: (a) The upregulation of miR‐125a and miR‐125b might enhance the activation of macrophages and trigger immune as well as inflammatory responses, thus, leading to the development of sepsis. (b) MiR‐125a and miR‐125b might be involved in the damage and necrosis of tissues via its downstream pathway, thus, accelerating organ injury, multiple organ dysfunction, and higher sepsis risk. However, this hypothesize needed further exploration.
It is reported that miR‐125b expression is involved in the regulating inflammation in RA and acute exacerbations of COPD (AECOPD).14, 23 For instance, one study illustrates that miR‐125b expression is positively associated with TNF‐α, IL‐6, and IL‐1β in RA patients.23 Another study displays that miR‐125b expression is correlated with TNF‐α, IL‐1β, IL‐8, and leukotriene B4 in patients with AECOPD.14 However, limited study reports the association of miR‐125b expression with inflammation in sepsis patients. Our study reported that miR‐125b expression was positively associated with disease severity (APACHE II score and SOFA score) and inflammation (CRP, TNF‐α, IL‐6, IL‐17, and IL‐23) in sepsis patients. These might be explained that: (a) MiR‐125b enhanced macrophage‐mediated inflammation via upregulating the expression of co‐stimulatory factors,21 which contributed to elevated inflammation and disease severity in sepsis patients and (b) MiR‐125b might cause the necrosis of several organs and followed multiple organ failure, thus, resulting in increased disease severity in sepsis patients. Besides, our study also illuminated that no correlation of miR‐125a expression with inflammation was observed in sepsis patients, and miR‐125a expression was positively associated with disease severity. While, the correlation efficient of miR‐125a with disease severity was below 0.3, which indicated that the correlation of miR‐125a with disease severity was weak. The possible explanations might be as follows: MiR‐125a might influence the disease severity via other pathways (such as lung injury) rather than inflammation in sepsis patients, which contributed to no correlation of miR‐125a with inflammation and the weak correlation efficient of miR‐125a with disease severity.13, 14
In the present, further analysis of the role of miR‐125a and miR‐125b in predicting prognosis in sepsis patients reported that miR‐125a lacked a value in predicting elevated 28‐day mortality risk in sepsis patients (AUC: 0.588, 95% CI: 0.491‐0.685), and miR‐125b exhibited a fair value in predicting increased 28‐day mortality risk (AUC: 0.699, 95% CI: 0.603‐0.795), among which miR‐125b presented a better value for predicting higher 28‐day mortality risk in sepsis patients. The results might be explained by that: (a) Since miR‐125a expression was not correlated with inflammation and the correlation of miR‐125a expression with disease severity was weak, miR‐125a failed to predict 28‐day mortality risk in sepsis patients, (b) Since no correlation of miR‐125a with inflammation was observed and miR‐125b was positively correlated with pro‐inflammatory effects, thereby, miR‐125b had a superior predictive value for 28‐day mortality risk than that of miR‐125a in sepsis patients, (c) MiR‐125b amplified the inflammatory response by exerting positive effect on macrophage‐trigger inflammation, which was responsible for higher disease severity and 28‐mortality risk in sepsis patients, and (d) MiR‐125b was positively correlated with inflammation and organ dysfunction, thus, led to elevated disease severity and 28‐day mortality risk in sepsis patients. In addition, we also exhibited that the predictive value of miR‐125b for 28‐day mortality risk was similar compared with CRP, APACHE II score, and SOFA score. CRP, APACHE II score, and SOFA score are commonly clinically applied biomarkers and scoring systems for predicting mortality in sepsis patients; however, they lack adequate sensitivity and specificity.6, 7 The findings in our study implied that the miR‐125b might assist on improving the sensitivity and specificity of CRP, APACHE II score, and SOFA score for predicting prognosis in sepsis patients.
There were some limitations in our study: (a) The sample size of enrolled sepsis patients was relatively small, which might influence the statistic power, (b) Only 28‐day mortality was evaluated, thus, the long‐term effect of miR‐125a and miR‐125b on prognosis should be studied further, (c) The detailed mechanism of miR‐125a and miR‐125b in regulating the development and progression of sepsis were not investigated, thus, further experiments in exploring the detailed mechanism of miR‐125a and miR‐125b in sepsis should be carried out, and (d) Sepsis patients who died within 24 hours after admission were excluded, which might cause selection bias and limit the generalizability of findings.
In conclusion, miR‐125a and miR‐125b both predict increased sepsis risk, while only miR‐125b correlates with elevated inflammation, disease severity, and 28‐day mortality risk in sepsis patients. These findings might offer some clues for sepsis risk identification, disease management, and prognosis prediction in sepsis patients in clinical practice.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
None.
Zhao D, Li S, Cui J, Wang L, Ma X, Li Y. Plasma miR‐125a and miR‐125b in sepsis: Correlation with disease risk, inflammation, severity, and prognosis. J Clin Lab Anal. 2020;34:e23036 10.1002/jcla.23036
REFERENCES
- 1. Reorganized text. JAMA Otolaryngol Head Neck Surg. 2015;141(5):428. [DOI] [PubMed] [Google Scholar]
- 2. Coburn B, Morris AM, Tomlinson G, et al. Does this adult patient with suspected bacteremia require blood cultures? JAMA. 2012;308(5):502‐511. [DOI] [PubMed] [Google Scholar]
- 3. Kumar S, Tripathy S, Jyoti A, Singh SG. Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review. Biosens Bioelectron. 2019;124‐125:205‐215. [DOI] [PubMed] [Google Scholar]
- 4. Andriolo BN, Andriolo RB, Salomao R, Atallah ÁN. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev. 2017;1:CD010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang TN, Li D, Xia J, et al. Non‐coding RNA: a potential biomarker and therapeutic target for sepsis. Oncotarget. 2017;8(53):91765‐91778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oeschger T, McCloskey D, Kopparthy V, Singh A, Erickson D. Point of care technologies for sepsis diagnosis and treatment. Lab Chip. 2019;19(5):728‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giamarellos‐Bourboulis EJ, Norrby‐Teglund A, Mylona V, et al. Risk assessment in sepsis: a new prognostication rule by APACHE II score and serum soluble urokinase plasminogen activator receptor. Crit Care. 2012;16(4):R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sinha M, Jupe J, Mack H, Coleman TP, Lawrence SM, Fraley SI. Emerging technologies for molecular diagnosis of sepsis. Clin Microbiol Rev. 2018;31(2):pii: e00089‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borderud SP, Li Y, Burkhalter JE, Sheffer CE, Ostroff JS. Electronic cigarette use among patients with cancer: Characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer. 2015;120(5):800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Essandoh K, Li Y, Huo J, et al. MiRNA‐mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. 2016;46(2):122‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun YM, Lin KY, Chen YQ. Diverse functions of miR‐125 family in different cell contexts. Journal of Hematol Oncol. 2013;6(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li H, Ding G. Elevated serum inflammatory cytokines in lupus nephritis patients, in association with promoted hsa‐miR‐125a. Clin Lab. 2016;62(4):631‐638. [DOI] [PubMed] [Google Scholar]
- 13. Liu G, Mei H, Chen M, et al. Protective effect of agmatine against hyperoxia‐induced acute lung injury via regulating lncRNA gadd7. Biochem Biophys Res Commun. 2019;516(1):68‐74. [DOI] [PubMed] [Google Scholar]
- 14. Hu HL, Nie ZQ, Lu Y, et al. Circulating miR‐125b but not miR‐125a correlates with acute exacerbations of chronic obstructive pulmonary disease and the expressions of inflammatory cytokines. Medicine. 2017;96(51):e9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315(8):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jia P, Wu X, Dai Y, et al. MicroRNA‐21 is required for local and remote ischemic preconditioning in multiple organ protection against sepsis. Crit Care Med. 2017;45(7):e703‐e710. [DOI] [PubMed] [Google Scholar]
- 17. Wu X, Yang J, Yu L, et al. Plasma miRNA‐223 correlates with risk, inflammatory markers as well as prognosis in sepsis patients. Medicine. 2018;97(27):e11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304‐377. [DOI] [PubMed] [Google Scholar]
- 19. Lehmann TP, Korski K, Ibbs M, et al. rs12976445 variant in the pri‐miR‐125a correlates with a lower level of hsa‐miR‐125a and ERBB2 overexpression in breast cancer patients. Oncol Lett. 2013;5(2):569‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banerjee S, Cui H, Xie N, et al. miR‐125a‐5p regulates differential activation of macrophages and inflammation. J Biol Chem. 2013;288(49):35428‐35436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaudhuri AA, So AY, Sinha N, et al. MicroRNA‐125b potentiates macrophage activation. J Immunol. 2011;187(10):5062‐5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun CM, Wu J, Zhang H, et al. Circulating miR‐125a but not miR‐125b is decreased in active disease status and negatively correlates with disease severity as well as inflammatory cytokines in patients with Crohn's disease. World J Gastroenterol. 2017;23(44):7888‐7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang B, Wang LS, Zhou YH. Elevated microRNA‐125b promotes inflammation in rheumatoid arthritis by activation of NF‐kappaB pathway. Biomed Pharmacother. 2017;93:1151‐1157. [DOI] [PubMed] [Google Scholar]


