Abstract
Background
Mosquito allergy is common in tropical countries but remains under-diagnosed. This may be due to the lack of knowledge and diagnostic tools for tropical mosquito allergens.
Objective
We aimed to characterize allergens from tropical mosquito species and investigate IgE reactivity in mosquito-allergic patients to the salivary gland proteins from these mosquitoes.
Methods
Salivary gland extract (SGE) from 4 mosquito species, highly distributed in the tropics, including Aedes aegypti, Aedes albopictus, Culex quinquefasciatus, and Anopheles dirus b, were studied. SGE-specific IgE and IgG ELISA were developed, and serum from 64 mosquito-allergic and 22 non-allergic healthy control subjects was assayed. Further investigations using IgE-immunoblots followed by mass spectrometry analysis were performed to identify and characterize allergens from each species.
Results
Mosquito-allergic subjects have detectable serum IgE to SGE derived from local mosquito species, while the IgE levels to Aedes communis using commercially available ELISA were mostly minimal. IgE-immunoblot analysis and mass spectrometry identified 5 novel mosquito allergens from A. albopictus (Aed al 2, Aed al 3), C. quinquefasciatus (Cul q 2.01, Cul q 3), and A. dirus b (Ano d 2). Interestingly, 4 of the 5 new allergens belong to the D7 protein family.
Conclusions & clinical relevance
Five novel allergens from 3 tropical mosquito species were characterized. The majority of mosquito-allergic subjects who live in the tropics have IgE reactivity to these allergens. Our study paves the way for the development of diagnostic tests, component-resolved diagnostics, and future immunotherapy for mosquito allergy in tropical countries.
Keywords: Allergens, Insect allergy, IgE, Mosquito, Tropical species
Introduction
Mosquito allergy is common in both tropical and subtropical countries.1 People living in the tropics are at a higher risk of developing allergic sensitization due to mosquito exposure throughout the year.1 Allergic reactions to mosquito bites are common in children and outdoor workers2 with clinical symptoms including immediate wheals and flares as well as delayed erythematous papules.3 In rare cases, severe systemic reactions to mosquito bites, known as Skeeter syndrome, can develop.4 Moreover, after the resolution of an acute lesion, secondary lesions, including chronic hyperpigmentation, can affect the quality of life.5
Female mosquitoes require mammalian blood to produce eggs,6 and allergic reactions to mosquito bites are caused by immune responses to mosquito salivary components secreted during a blood meal. Mosquito saliva contains many proteins including immunomodulating agents, enzymes, and substances that facilitate blood-feeding and parasite transmission.7 The composition of mosquito saliva differs greatly by species,8 and previous studies have shown that salivary components that serve as allergens can be shared between species or be species-specific.8 The 10 previously characterized mosquito allergens have all been isolated from Aedes aegypti (A. aegypti) (Aed a 1–11) (WHO/IUIS www.allergen.org).
Tropical countries have the highest prevalence of clinically related mosquito species.9 Previous studies have shown that commercially available mosquito-specific IgE ELISA report positive results for only one-third of mosquito allergic patients residing in the tropics.10 The low sensitivity of the commercial diagnostic kits may be due to the use of whole-body mosquito extracts (WBE), which contain a low proportion of salivary allergenic proteins.11,12 Moreover, the mosquito species used in the diagnostic kit, Aedes communis (A. communis), is endemic in the northern temperate zone, but it is not a major species in tropical countries.8,9 As mosquito allergenic proteins can vary by species, we suspect that the choice of the mosquito species could also contribute to the poor efficacy of the commercial kit in tropical patient populations.
In the current study, we investigated IgE reactivity to salivary gland extracts (SGE) from 4 widely distributed mosquito species in tropical countries, especially those in Southeast Asia: A. aegypti, Aedes albopictus (A. albopictus), Culex quinquefasciatus (C. quinquefasciatus), and Anopheles dirus b (A. dirus b) (also known as A. cracens)9,13 using in-house IgE ELISA. We demonstrated that mosquito-allergic subjects who reside in the tropics have greater IgE reactivity to tropical mosquito species SGE compared to western mosquito, A. communis WBE. The allergens from the 4 local mosquito species were further characterized using IgE immunoblot analysis and mass-spectrometry. Five new mosquito allergens from A. albopictus (Aed al 2, Aed al 3), C. quinquefasciatus (Cul q 2.01, Cul q 3), and A. dirus B (Ano d 2) were identified (WHO/IUIS www.allergen.org).
Methods
Subjects and serum samples
Sixty-four mosquito allergic subjects and 22 healthy control subjects, aged from 6 months to 33 years, were recruited after informed consent. The diagnosis of mosquito allergy was determined based on the clinical history of bite reactions. Blood samples were collected from each participant, and sera were aliquoted and stored at −20 °C until use.
Commercial mosquito-specific IgE ELISA
An aliquot of sera from each subject was tested for specific IgE antibodies to WBE from A. communis (ImmunoCAP-System, Thermo Fisher Scientific, Massachusetts, United States), following the manufacturer's protocol.
Mosquitoes and salivary gland extract (SGE) preparation
A. aegypti, A. albopictus, A. dirus b, and C. quinquefasciatus were reared in a certified insectarium. Three- to 7 day-old female mosquitoes were blood-fed and anesthetized by brief freezing at −20 °C prior to salivary gland extraction under a stereo-microscope. Salivary glands were collected in 1× PBS and homogenized with an ultra-homogenizer (BANDELIN Sonoplus) before centrifugation. Supernatants were collected and stored at −20 °C in small aliquots in the presence of 1× protease inhibitors (complete-ultra) until use. An aliquot was thawed and the protein concentration was measured using a nanodrop spectrophotometer (Thermo Scientific) before each use. The same protein concentrations of SGE were used to coat ELISA plates for standardization.
Mosquito-specific IgE/IgG ELISA
Standard ELISA protocols for IgE and IgG were established after optimization. During optimization, negative and positive control sera were used with various allergen concentrations, blocking buffers, serum dilutions, and secondary antibody dilutions. The optimal protocols were selected based on the highest signal to noise ratio.
In the standard protocol, SGE from each mosquito species (25 μg/ml) in carbonate-bicarbonate buffer was used to coat ELISA plates (Nunc) at 37 °C for 2 h (or buffer alone in negative control wells). Plates were washed before blocking with 1.5% gelatin in PBST for 1 hour. Serum samples were diluted at 1:1 for IgE ELISA and 1:800 for IgG ELISA followed by incubation in the coated plates overnight at room temperature (RT). After washing, peroxidase-labeled anti-human IgE Antibody at dilution of 1:750 (KPL) or peroxidase-labeled anti-human IgG Antibody at a 1:10,000 dilution (KPL) was added for 1 hour at RT. TMB substrate (SureBlue Reserve TMB, KPL) was added, and reactions were stopped with 1 N HCl. Signals were recorded at 450 nm (Biochrom, EZ read 400). Each condition was analyzed with duplicate samples with the average OD used for further calculations. Changes in OD levels for SGE-specific IgE and -IgG were obtained by subtracting the OD reading with its negative control (no allergen). Inter-experimental controls were used in every plate to ensure the standardization of the assay. All 4 mosquitos species-specific IgE were examined for each participant. Adjusted OD for IgG analysis was calculated using normalized delta OD with inter-experimental controls.
IgE immunoblots
SGE from each mosquito species was separated by SDS-PAGE. Samples were boiled at 95 °C for 5 minutes before being separated using 14% separating and 7% stacking gels. Proteins were then transferred to PVDF membranes with a Trans-blot® Turbo™ Transfer System (Bio-Rad, USA). Membranes were blocked with 3% BSA in 0.5% PBST for 1 h and incubated with sera from patients at a dilution of 1:10 overnight at 4 °C. Membranes were then incubated with peroxidase-labeled mouse anti-Human IgE Antibody (KPL), at a dilution of 1: 10,000 for 1 hour. The IgE-binding proteins were visualized using ECL (GE Healthcare, UK).
Mass spectrometry
SGE from each mosquito species was separated using 14% SDS-PAGE, and gels were stained with 0.3% Coomassie Brilliant Blue G-250, 50% methanol, and 10% acetic acid. Gels were destained with 40% methanol and 10% acetic acid until clear. Protein bands of interest were excised from the polyacrylamide gels, and protein sizes were confirmed with IgE-immunoblotting before being applied to ESI-LC-MS/MS. For tryptic digestions, gel pieces were destained with 50% acetonitrile in 50 mM ammonium bicarbonate. Protein reduction and alkylation were performed with 4 mM dithiothreitol and 250 mM iodoacetamide. Gels were dehydrated in 100% acetonitrile, rehydrated with 10 ng/μL trypsin in 50 mM ammonium bicarbonate, and incubated at 37 °C overnight. Acetonitrile was added to the gels, and the supernatant was collected and dried using a SpeedVac (TOMY, Japan). The dried peptides were resuspended in 0.1% formic acid and subjected to reverse-phase liquid chromatography using an Ultimate 3000 nano-LC system (Dionex; Surrey, U.K.) coupled with a microToFQ II mass spectrometer (Bruker; German).
Allergenic protein identification and alignment of peptides between mosquito species
Mass spectrometric data were compared to the NCBI database using the MASCOT search engine 2.2 (Matrix Science, Ltd.). Only peptides with more than 95% confidence were reported. Alignments of allergenic proteins between mosquito species were generated using Clustal Omega.14
Statistical analysis
The Mann-Whitney U test was used to compare 2 groups of non-parametric data. Student T-test was used to compare parametric data between 2 groups. The Kruskal-Wallis test, followed by Dunn's multiple comparison test, was used to determine statistical differences among the 4 different groups (comparisons of relative IgE and IgG among four mosquito species) (GraphPad Prism version 5.0a). The Spearman ranked correlation was used to find the relationship between specific IgE levels of 2 mosquito species (GraphPad Prism version 5.0a). Logistic regression, using case/control as outcome variable and level of mosquito-specific IgE (alone in univariate analysis) and age group (pediatrics <15 years old or adults≥15 years old) (in multivariate analysis) as independent variables, was performed using STATA. The statistical methods used are indicated in the legend of each figure.
Results
Study population
The demographic data of patients and controls are summarized in Table 1. The majority of patients (87.5%) reside in Bangkok and the surrounding area, are female (54.69%), and are a pediatric population (mean age 11.40 years old). The area of residence and gender distribution of patients and controls were not statistically different. However, the average age of the patients is lower than that of the control group. This was due to ethical consideration and limited parental interest, which limited the collection of blood samples from healthy control pediatric populations.
Table 1.
Demographic data.
| Patients (n = 64) | Controls (n = 22) | p-value | |
|---|---|---|---|
| Age (years) | |||
| (mean ± S.D.) | 11.40 ± 9.99 | 22.59 ± 11.01 | 0.0003aa |
| Gender | |||
| Male (%) | 29 (45.31%) | 6 (27.27%) | NSb |
| Female (%) | 35 (54.69%) | 16 (72.73%) | NSb |
| Residency | |||
| Bangkok and perimeter (%) | 56 (87.50%) | 22 (100%) | NSb |
| Other (%) | 8 (12.5%) | 0 (0%) | NSb |
p-value < 0.05 is considered statistically significant.
Mann-Whitney test
Fisher's exact test
Mosquito-allergic subjects have detectable serum IgE specific to local tropical mosquito species
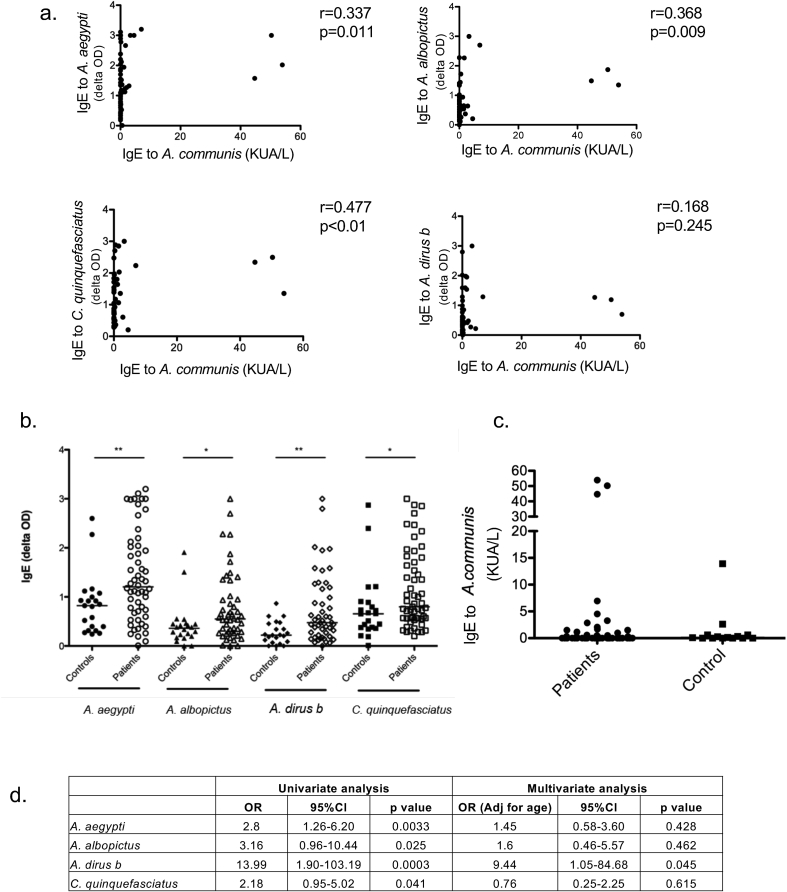
In mosquito-allergic subjects who reside in the tropics, we observed greater IgE reactivity to SGE of local mosquito species compared to those of A. communis (Fig. 1a). A significant but low level correlation was observed between IgE to A. communis and A. aegypti (r = 0.34, p = 0.01), A. albopictus (r = 0.37, p = 0.01) and C. quinquefasciatus (r = 0.48, p < 0.01) but not A. dirus b (r = 0.17, p = 0.25) (Fig. 1a).
Fig. 1.
IgE reactivity to tropical mosquito SGE and western mosquito WBE (a) Correlation between IgE level to A.communis (commercial) and each of the four tropical mosquitoes (as indicated) SGE (in-house ELISA) in mosquito-allergic subjects (Spearman correlation). Comparing IgE level between controls and mosquito-allergic patients to (b) each of the 4 local mosquito species and (c) A. communis (commercial). (Mann-Whitney U test *p < 0.05, **p < 0.01). (d) Logistic regression analysis of mosquito-specific IgE as a predictor of mosquito allergy (univariate analysis on the left, multivariate analysis adjusted for age on the right)
When compared to currently available, non-age matched, healthy controls, mosquito-allergic subjects exhibited higher IgE specific to SGE from A. aegypti (patients median OD of 1.21, IQR 0.68–2.04 vs. control median OD of 0.82, IQR 0.35–1.04, p < 0.01), A. albopictus (patients median OD of 0.55, IQR 0.28–0.97 vs. controls median OD of 0.36, IQR 0.19–0.45, p < 0.05), A. dirus b (patients median OD of 0.47, IQR 0.21–1.17 vs. controls median OD of 0.22, IQR 0.08–0.46, p < 0.01) and C. quinquefasciatus (patients median OD of 0.80, IQR 0.56–1.67 vs. controls median OD of 0.66, IQR 0.37–0.89, p < 0.05) (Fig. 1b). In contrast, IgE levels to A. communis were not statistically different between mosquito-allergic subjects (median 0.03 KUA/L, IQR 0.01–0.56) and healthy controls (median 0.12 KUA/L, IQR 0.01–0.60, p = 0.90) in our cohort (Fig. 1c). Because the average age of the control group was higher than the patient group, logistic regression analyses of mosquito-specific IgE as a predictor for mosquito allergy, using both univariate and multivariate analyses, were performed. In the univariate analysis, the odds ratio (OR) was 2.8, 3.16, 13.99 and 2.18 for A. aegypti-specific IgE, A. albopictus-specific IgE, A. dirus b-specific IgE, and C. quinquefasciatus-specific IgE, respectively, as a predictor of mosquito allergy. However, when multivariate analysis, adjusted for age was performed, the OR for each mosquito-specific IgE decreased and statistical significance was lost (Fig. 1d). This is likely due to age as a confounding factor and an insufficient number of age-matched controls in our current dataset.
Serum mosquito species-specific IgE and IgG corresponds to exposure and geographic distribution of mosquito species
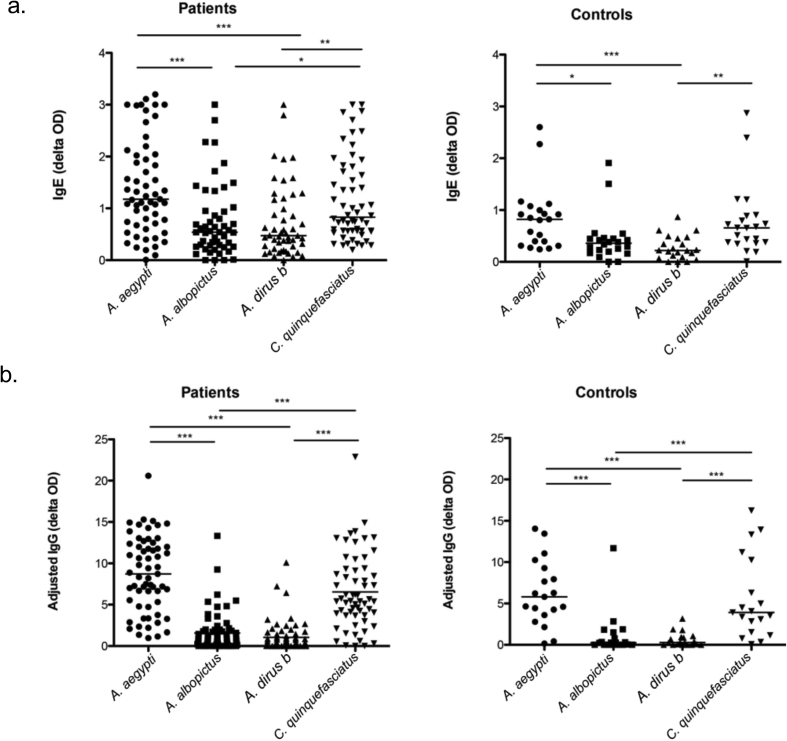
The four tropical mosquito species studied have distinct geographical distributions. A. aegypti and C. quinquefasciatus are two common mosquito species in urban and suburban areas, while A. albopictus and A. dirus b are more common in rural and forest areas.13 We examined whether our subject population, who mainly reside in urban and suburban areas (Table 1), have higher levels of IgE and IgG to the urban species, A. aegypti and C. quinquefasciatus.
The levels of specific IgE against A. aegypti (median OD of 1.21 (patients), 0.82 (controls)) and C. quinquefasciatus (median OD of 0.80 (patients), 0.66 (controls)) were significantly higher than those against the rural species, A. albopictus (median OD of 0.55 (patients), 0.36 (controls)) and A. dirus b (median OD of 0.47 (patients), 0.22 (controls)) in both healthy control subjects and mosquito allergic patients (Fig. 2a). We hypothesize that the higher IgE levels for the 2 urban species may be due to the exposure of participants to these mosquito species. The specific IgG level has been suggested to correlate with exposure to mosquitoes.15 We, therefore, measured mosquito-specific IgG against the 4 mosquito species. As expected, the highest levels of specific IgG was found against A. aegypti (median adjusted OD of 8.72 (patients), 5.80 (controls)) and C. quinquefasciatus (median adjusted OD of 5.44 (patients), 3.91 (controls)) in both healthy controls and mosquito allergic patients (Fig. 2b). In contrast, low or no IgG was found against A. albopictus (median adjusted OD of 0.78 (patients), 0.25 (controls)) and A. dirus b (median adjusted OD of 0.32 (patients), 0.28 (controls)). Based on these analyses, we conclude that the higher exposure to mosquito species distributed in the residential areas of the subjects may affect IgE sensitization in both healthy controls and mosquito allergic patients.
Fig. 2.
Mosquito species geographic distribution affects IgE and IgG sensitization in both patients and controls. (a) Levels of mosquito-specific IgE or (b) IgG to the 4 mosquito species in mosquito allergic patients (left panel) and controls (right panel). (Kruskal-Wallis, Dunn's multiple comparison test *p < 0.05, **p < 0.01, ***p < 0.001.)
Correlation of IgE reactivity to different tropical mosquito species
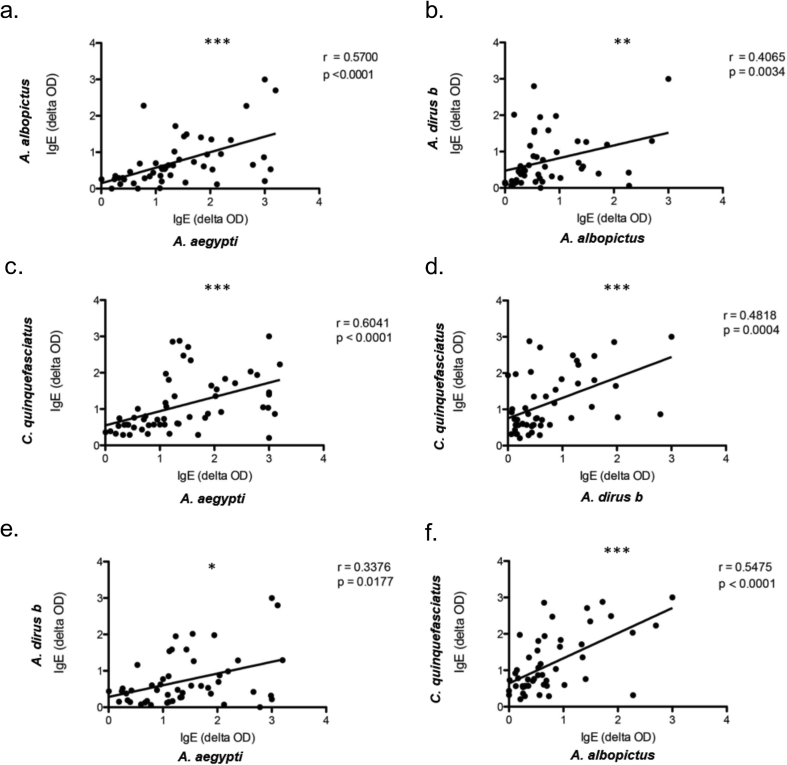
We next asked whether the levels of IgE correlate across the 4 local mosquito species examined in our study. We tested for linear correlations between pairs of the 4 species (Fig. 3a–f). The highest correlation (r = 0.60, p < 0.0001) was observed between A. aegypti and C. quinquefasciatus (Fig. 3c), the 2 species which are co-distributed in urban and suburban areas.13 While the second highest correlation was found between A. aegypti and A. albopictus (r = 0.57, p < 0.0001), 2 species that belong to the same genus (Fig. 3a). Interestingly, significant positive correlations were also found in every pair tested (Fig. 3a–f), despite differences in mosquito taxa or geographic distribution, suggesting that some IgE-binding proteins in the SGE may be conserved or are cross-reactive across the 4 species. In general, the levels of pairwise correlations of IgE levels to the 4 tropical mosquito species were higher than the correlation between IgE to A. communis and each of the 4 tropical species (Fig. 1a).
Fig. 3.
Pairwise correlations among IgE levels against the four mosquito species. (a) (b) (c) (d) (e) (f). (Spear-Mann ranked correlation *p < 0.05, **p < 0.01, ***p < 0.001)
Major and minor salivary gland allergens from the 4 mosquito species
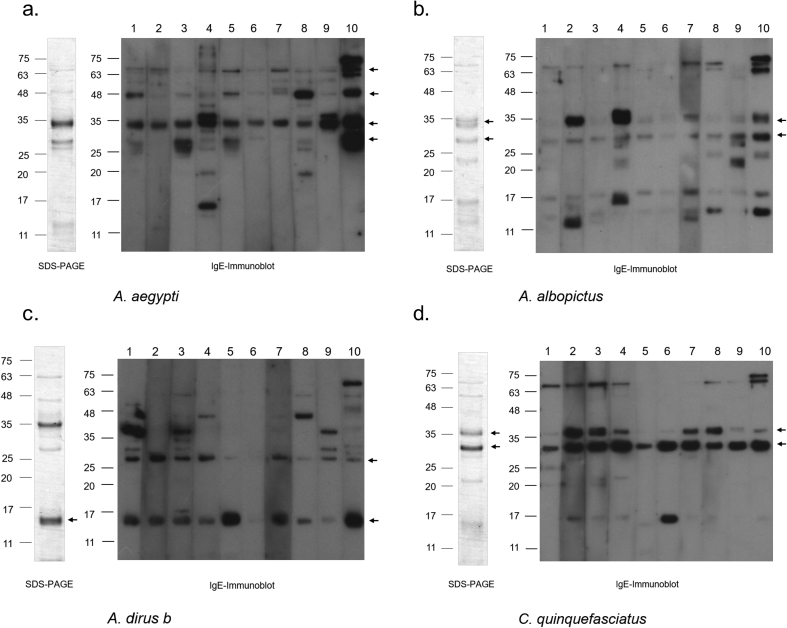
To investigate the allergenic proteins present in the salivary gland from each of the four mosquito species, IgE immunoblot analyses, with serum from 15 mosquito-allergic subjects, was performed (10 representative results are shown in Fig. 4). In agreement with previous reports, IgE-reactive bands of A. aegypti were 68 kD (consistent with Aed a 1) (found in 60% of patients), 35 kD (Aed a 2) (100% of patients) and 30 kD (Aed a 3) (40% of patients) proteins8,12 (Fig. 4a). Interestingly, 60% of patients have IgE that binds to a yet-to-be-defined 48 kD protein. Unfortunately, we could not obtain a sufficient quantity of the 48kD protein for accurate mass spectrometry analysis.
Fig. 4.
SDS-PAGE of SGE proteins and IgE Immunoblots of mosquito-allergic patient sera to SGE of the four mosquito species. SDS-PAGE of SGE proteins (left), as well as IgE immunoblots (right) from 10 representative patient samples for each of the indicated mosquito species, are shown. Arrows on IgE immunoblots indicate major IgE reactive bands. An arrow on each SDS-PAGE indicates the corresponding protein band cut and subjected to mass spectrometry for the identification of novel allergen
Major IgE-reactive protein bands from SGE of A. albopictus were 30 kDa (found in 11/15, 73% of the patients), and 33 kDa (10/15, 67% of patients) (Fig. 4b). The major IgE reactive bands from A. dirus b were 15 kDa (9/15, 60% of patients) and 27 kDa (11/15, 73% of patients) (Fig. 4c). In C. quinquefasciatus, the most common IgE reactive bands among allergic patients were proteins with molecular weights of 33 kDa (15/15, 100% of patients) and 35 kDa (11/15, 73% of patients) (Fig. 4d). For each mosquito species, the bands indicated on SDS-PAGE (arrows on Fig. 4a–d, SDS-PAGE) were cut and subjected to mass spectrometry.
Characterization of 5 novel tropical mosquito allergens
Among the mosquito SGE allergens identified using IgE immunoblots, major IgE reactive bands were selected for further characterization by mass spectrometry. The major allergens from A. aegypti identified in the current study were Apyrase (68 kDa), the long-form D7 protein (35 kDa), and a salivary gland allergen (30 kDa), which correspond with the previously characterized Aed a 1, Aed a 2 and Aed a 3, respectively (www.allergen.org).
The WHO/IUIS allergen nomenclature subcommittee assigned the 5 new mosquito allergens from three other mosquito species (www.allergen.org) from this study as Aed al 2 (33 kDa), Aed al 3 (30 kDa), Ano d 2 (15 kDa), Cul q 2.01 (33 kDa), and Cul q 3 (35 kDa) (Fig. 4b–d arrows indicate novel allergens on SDS-PAGE and IgE Immunoblot, Table 2). Interestingly, 4 out of 5 novel allergens, Aed al 2, Ano d 2, Cul q 2.01, and Cul q 3 were found to be different forms of D7 proteins. While Aed al 2, Cul q 2.01, and Cul q 3 are long forms of the D7 protein, Ano d 2 was identified as a D7 short form. Aed al 3 from Ae. albopictus is a salivary protein with high sequence similarity to the Ae. aegypti 30 kD allergen, Aed a 3.
Table 2.
Summary of five new allergens from three mosquito species identified by mass spectrometry.
| Allergen name | Species | Accession number | Biochemical name | Mascot score | Coverage (%) | Experimental MW (kDa) | Theoretical MW (kDa) | Identified peptides |
|---|---|---|---|---|---|---|---|---|
| Aed al 2 | Aedes albopictus | AAA29348-like | D7 like salivary odorant-binding protein | 70 | 8.7 | 33 | 36.897 | SWHYYK QSYFEFCENK KSYFEFCENKa CMEDNLEDGPNR |
| Aed al 3 | Aedes albopictus | AAV90693 | 30 kDa salivary protein | 154 | 18.5 | 30 | 28.314 | GKLSPITSK NPVVEAISR VVAILDKDTK VDNIQSEYLR SALNNDLQSEVR |
| Ano d 2 | Anophels dirus B | AAP68775 | Odorant-binding protein | 74 | 14.3 | 15 | 21.854 | NAVDYNELLK ANTFYTCFLGTSSSPAFKa |
| Cul q 2.01 | Culex quinquefasciatus | AAL16047 | Salivary odorant-binding protein | 196 | 40.9 | 33 | 35.967 | FQQAVQALGTIDSADCLKa YGPVHAQFTDVQR NVYFGKK EITDKIYNSDSTVKa SNFKDGSEELCTLR TGITTNNNHLDCLFR NGNINPDEIK DLHFINVK DKDAAVDNALNNCK YFIENTDPYDVAK |
| Cul q 3 | Culex quinquefasciatus | AAL16046 | Salivary odorant-binding protein 2 | 56 | 13.8 | 35 | 35.179 | GFIQVNNANKGVLEKa IYLLDSSVRa NGEMDESAILRa LDYIEVR |
Unique peptide
Similarity of allergenic proteins between mosquito species
Amino acid sequence alignment of D7 proteins from different mosquito species was performed. Aed a 2 sequence was used as the reference sequence as it is the most well-characterized D7 protein known to be a mosquito allergen. Furthermore, our Aed al 2 peptide fragments from mass spectrometry matched 100% with Aed a 2, while it contains one mismatch with the two currently available A. albopictus D7 proteins in the database (Supplementary Fig. 1). The D7 proteins of A. albopictus, D712 Alb1 and D712 Alb 2, have 68.85 and 69.47% sequence similarity with the Aed a 2 respectively (Supplementary Fig.1). When aligned with, Sequence similarity between Aed a 2 and Cul q 2, Cul q 3 and Ano d 2 were 34.11%, 29.80%, and 20.42%, respectively (Fig. 5a). Although they derive from the same species, Cul q 2 and Cul q 3 contain only 34.11% sequence similarity. Not surprisingly, Ano d 2, the only short-form D7, contains the least sequence similarity with the other D7 proteins in this analysis (14.97–20.42%) (Fig. 5a). In addition, high sequence similarity (65.22%) was observed between Aed al 3 and Aed a 3, (Fig. 5b).
Fig. 5.
Amino acid alignment of mosquito allergen groups between mosquito species (a) D7 proteins (Cul q 2, Cul q 3 and Ano d 2) aligned with Aed a 2. (b) Aed a 3 and Aed al 3 alignment. The similarity percentage between each pair is shown in the table below each subfigure. Peptide fragments from mass spectrometry are highlighted in gray.
Discussion
Despite being a very common condition, information on mosquito allergy from tropical countries is scarce. We showed here that increased IgE reactivity to salivary gland proteins of local mosquito species is observed in mosquito-allergic subjects who reside in the tropics. In addition, we further characterized these novel allergens. Our results will help pave the way for improvement in diagnostic tests and future therapeutics for mosquito allergy in tropical countries, where this condition is most prevalent.
More than 3500 mosquito species are distributed worldwide, with a large diversity of species present in tropical regions. Climate conditions in tropical areas, especially warm temperatures, allow for high mosquito density throughout the year. Following a mosquito bite, local skin reactions are almost universal11,16; however, some people suffer from large local or even systemic reactions. Current diagnostic tests for mosquito allergy are limited.17 The mosquito bite test is the gold standard, although this procedure is not practical for clinical use. Previous studies have demonstrated a correlation between mosquito-specific IgE to an immediate skin reaction in the mosquito bite test.18 Further, passive transfer of IgE anti-mosquito saliva antibodies can induce cutaneous mosquito-bite reaction and basophil histamine release.19,20 Taken together, these data suggested that mosquito-specific IgE causes an immediate skin reaction and might be used as a surrogate to test for mosquito allergy. Commercially available skin prick tests and IgE ELISA are prepared using mosquito whole body extracts, which contain minute amounts of the relevant salivary allergens. In addition, they contain non-salivary gland allergens, such as tropomyosin, that could be cross-reactive to other species such as house dust mite21 or Cte f 2 that could cross-react with cat flea.22 Moreover, the commercial test is based on A. communis which is not distributed in tropical areas.8,9 These A. communis WBE-based commercial tests are insensitive and give positive findings for less than 30% of the patients in the tropics.10 Improved sensitivity of IgE ELISA was observed in studies from North America that utilized salivary gland, saliva, or recombinant salivary proteins.2,11,17 Our analysis showed high IgE reactivity to SGE of tropical mosquito species and suggests that future development of diagnostic tests based on these SGE might also be helpful in tropical patient populations.
The primary goal of this study was to identify tropical mosquito allergens and IgE reactivity to tropical mosquito species among mosquito allergic subjects. The current investigation was not designed to develop a mosquito allergy diagnostic test. Our case-control comparison has a limitation in that the average age of the control group is higher than the patients, due to ethical consideration and the lack of parental interest in allowing blood sample collections from healthy children. IgE recognition of insect allergens causing skin hypersensitivity, including those from mosquito, has been reported to decrease with age23,24 and this is in agreement with the acquisition of tolerance that characterizes this disease.3 Thus, IgE levels in adults and children may differ, and additional study with more age-matched controls is needed. Furthermore, IgE was shown to correlate with immediate skin reaction in mosquito allergy but not delayed-type hypersensitivity where cell-mediated response plays a more important role.18,25 Therefore, mosquito-allergic patients with delayed-type hypersensitivity might not exhibit high levels of IgE.18 Some non-mosquito allergic healthy controls, especially children, may have high mosquito-specific IgE, due to the higher IgE response in children.23,24 Children are in a sensitization phase prior to the development of clinical mosquito allergy20 or may exhibit cross-reactivity between mosquito allergens and allergens from other sources.1 With these considerations, we cannot establish a cut-off point, or predict test sensitivity or specificity with currently available data. Therefore, future developments towards diagnostic tests are needed, especially the inclusion of more age-matched controls, classification of patients into immediate and delayed reaction groups, improvement in assay quantification by serum endpoint titration, construction of standard curves, and additional assay standardization for production in a large scale.
This is the first report in the WHO/IUIS allergen nomenclature database of mosquito allergens derived from mosquito species beyond A. aegypti. To the best of our knowledge, all of the 10 reported mosquito allergens in the WHO/IUIS database have been identified from A. aegypti. Here, we report 5 novel salivary gland allergens from 3 other mosquito species. The most well-characterized A. aegypti mosquito salivary allergens are apyrase (Aed a 1),26 a D7 family protein (Aed a 2)27, a 30 kDa salivary protein (Aed a 3)28, and alpha-glucosidase (Aed a 4).29 In addition, 6 non-salivary allergenic proteins were recently characterized: Aed a5 (sarcoplasmic Ca + binding protein), Aed a6 (Porin3), Aed a 7, Aed a 8 (HSC-70), Aed a 10 (Tropomyosin),30 and Aed a 11 (lysosomal aspartic protease).31 The novel salivary allergens identified in this study from A. albopictus are Aed al 2 (33 kDa), a D7 family protein, and Aed al 3 (30 kDa), which has high sequence similarity to Aed a 3. Allergens from the other 2 mosquito species include Anopheles dirus b, Ano d 2 (15 kDa), a short form of D7, and C. quinquefasciatus D7 family proteins, Cul q 2.01 (33 kDa) and Cul q 3 (35 kDa).
Various forms of D7 proteins were found in our study to be the major allergenic proteins across the 4 mosquito species. Approximately 80%–100% of participants exhibited reactivity to these proteins. Interestingly, peptide fragments from the 33 kD band of A. albopictus was a 100% match to Aed a 2 but contained 1 mismatch to the previously reported D7 proteins of A. albopictus. This might be due to high sequence similarity between D7 of A. aegypti and A. albopictus and incomplete deposition of D7 peptides from A. albopictus in the database. A search for D7 proteins-encoding genes from A. albopictus, AALF024477 identified a D7 protein homologous to Aed a 2, with one transcript (splice variant) and 5 paralogues.32 Thus, there are likely multiple D7 proteins with high amino acid sequence similarity encoded within the genome of A. albopictus, whose roles in mosquito allergy remain to be further characterized. The low number of unique peptides in Aed al 2 (and Aed al 3) is also probably due to high sequence similarity between A. aegypti and A. albopictus, as they are in the same genus.
The D7 proteins belong to the odorant-binding protein (OBP) superfamily and are one of the most abundant proteins in the saliva of Aedes, Culex, and Anopheles mosquitoes.33, 34, 35 Aedes and Culex mosquitoes express a long form of D7, approximately 32 kDa, containing two OBP domains. In contrast, A. gambiae D7 was found to have a single OBP domain (short form, 15 kDa) with structural homology to the C-terminus of Aedes D7.33, 34, 35, 36 The C-terminal domain of Aedes D7 binds to biogenic amine while the N-terminus interacts with leukotriene.36 The association of D7 with these molecules is believed to neutralize their activity, hampering the host immune defenses as well as acting to prevent defensive behavior, such as scratching, that could interrupt feeding. Polyclonal rabbit anti-rAeda2 was shown to bind to salivary proteins from A. albopictus and C. quinquefasciatus27; however, further study using serum from diverse patient populations is needed. In addition, inhibition tests with different forms of recombinant D7, against various mosquito species, are required to confirm if the full-length D7 or the C terminal domain are cross-reactive between species, including Anopheles. If confirmed, the D7 protein could serve as a good candidate for the development of recombinant allergens for diagnostic and therapeutic use as species-shared allergens.
Aed al 3 is similar in size and sequences to Aed a 3 and likely has a similar biological function. Aed a 3 or aegyptin binds to collagen and inhibits platelet aggregation. This protein regulates mosquito probing time and the success of blood feedings.37 Recombinant Aed a 3 is useful in mosquito allergy diagnosis with skin prick tests and IgE ELISA.28
In addition to the improvement of diagnostic tests, knowledge of mosquito salivary allergens has other applications including immunotherapy and mosquito saliva-based vaccine development. Immunotherapy with whole-body mosquito extract was found to successfully prevent future reactions.38 The use of SGE or recombinant allergens have the potential to improve the standardization, safety, and efficacy of the mosquito immunotherapy. As mosquitoes are an important vector for several arboviruses, the in-depth knowledge of mosquito salivary proteins might also aid the development of mosquito saliva-based vaccines for these arboviruses, such as dengue, Zika, chikungunya, and West Nile viruses.39
Our results could serve as the foundation for the development of effective mosquito allergy diagnostic kits for patients in the tropical areas using local mosquito SGE or species-shared recombinant allergens. Furthermore, we provided an in-depth analysis of tropical mosquito salivary allergens that will be valuable in the future development of recombinant allergens for diagnosis and therapeutic purposes, as well as component-resolved diagnostics and mosquito-based vaccine design.
Funding
This work is supported by a research collaborative grant between Faculty of Science, Mahidol University and Faculty of Medicine, Ramathibodi Hospital, Mahidol University.
Consent for publication
All authors provided consent for publication.
Ethics approval
This study was approved by the committee on human rights related to research involving human subjects, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand (certificate number: MURA2013/259S1,Dec16).
Declaration of Competing Interest
Authors declared no conflict of interest.
Acknowledgments
We appreciate Ms. Saifon Sudjai and Dr. Jetsumon Sattabongkot Prachumsri, Mahidol Vivax Research Unit, for assistance with mosquito salivary gland dissection, Dr. Pitipol Choopong for statistical analysis using STATA, Ms. Wilawan Chan-in for part of figure preparation and Dr. Laran Jensen for critical review and English editing of the manuscript. The proof reading of this manuscript was supported by the Editorial Office, Faculty of Graduate Studies, Mahidol University. We thank the staff at the Pediatric Allergy Unit, Ramathibodi Hospital for collecting patient samples and express our thanks to all subjects and their parents for participation in this study. We thank Faculty of Science, Mahidol University for providing Central Instrument Facility (CIF) support, Faculty development grant (to PM) and research assistantship support (to WT). We appreciate an award from Anandamahidol foundation (to PM) and Mahidol Medical Scholar Program scholarship (to AO).
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100099.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cantillo J.F., Puerta L., Lafosse-Marin S. Allergens involved in the cross-reactivity of Aedes aegypti with other arthropods. Ann Allergy Asthma Immunol. 2017 Jun;118(6):710–718. doi: 10.1016/j.anai.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Peng Z., Simons F.E. Advances in mosquito allergy. Curr Opin Allergy Clin Immunol. Aug 2007;7(4):350–354. doi: 10.1097/ACI.0b013e328259c313. [DOI] [PubMed] [Google Scholar]
- 3.Kulthanan K., Wongkamchai S., Triwongwaranat D. Mosquito allergy: clinical features and natural course. J Dermatol. Dec 2010;37(12):1025–1031. doi: 10.1111/j.1346-8138.2010.00958.x. [DOI] [PubMed] [Google Scholar]
- 4.Simons F.E., Peng Z. Skeeter syndrome. J Allergy Clin Immunol. Sep 1999;104(3 Pt 1):705–707. doi: 10.1016/s0091-6749(99)70348-9. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez R.G., Cohen B.A. Insect bite-induced hypersensitivity and the SCRATCH principles: a new approach to papular urticaria. Pediatrics. Jul 2006;118(1):e189–e196. doi: 10.1542/peds.2005-2550. [DOI] [PubMed] [Google Scholar]
- 6.Woke P.A., Ally M.S., Rosenberger J.C.R. The numbers of eggs developed related to the quantities of human blood ingested in Aedes aegypti (L.) (Diptera: Culicidae) Ann Entomol Soc Am. 1956;49(5):435–441. [Google Scholar]
- 7.James A.A., Rossignol P.A. Mosquito salivary glands: parasitological and molecular aspects. Parasitol today. Oct 1991;7(10):267–271. doi: 10.1016/0169-4758(91)90092-3. [DOI] [PubMed] [Google Scholar]
- 8.Peng Z., Li H., Simons F.E. Immunoblot analysis of salivary allergens in 10 mosquito species with worldwide distribution and the human IgE responses to these allergens. J Allergy Clin Immunol. Apr 1998;101(4 Pt 1):498–505. doi: 10.1016/S0091-6749(98)70357-4. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer M.U., Sinka M.E., Duda K.A. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife. Jun 30 2015;4 doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manuyakorn W., Itsaradisaikul S., Benjaponpitak S. Mosquito allergy in children: clinical features and limitation of commercially-available diagnostic tests. Asian Pac J Allergy Immunol. Dec 2017;35(4):186–190. doi: 10.12932/AP0842. [DOI] [PubMed] [Google Scholar]
- 11.Peng Z., Simons F.E. Comparison of proteins, IgE, and IgG binding antigens, and skin reactivity in commercial and laboratory-made mosquito extracts. Ann Allergy Asthma Immunol : Off Pub Am Coll Allergy Asthma Immunol. Nov 1996;77(5):371–376. doi: 10.1016/S1081-1206(10)63335-2. [DOI] [PubMed] [Google Scholar]
- 12.Wongkamchai S., Khongtak P., Leemingsawat S. Comparative identification of protein profiles and major allergens of saliva, salivary gland and whole body extracts of mosquito species in Thailand. Asian Pac J Allergy Immunol. Jun-Sep 2010;28(2-3):162–169. [PubMed] [Google Scholar]
- 13.Thongsripong P., Green A., Kittayapong P., Kapan D., Wilcox B., Bennett S. Mosquito vector diversity across habitats in central Thailand endemic for dengue and other arthropod-borne diseases. PLoS Neglected Trop Dis. 2013;7(10) doi: 10.1371/journal.pntd.0002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madeira F., Park Y.M., Lee J. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47(W1):W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontaine A., Pascual A., Orlandi-Pradines E. Relationship between exposure to vector bites and antibody responses to mosquito salivary gland extracts. PloS One. 2011;6(12) doi: 10.1371/journal.pone.0029107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oka K. Correlation of Aedes albopictus bite reaction with IgE antibody assay and lymphocyte transformation test to mosquito salivary antigens. J Dermatol. Oct 1989;16(5):341–347. doi: 10.1111/j.1346-8138.1989.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 17.Crisp H.C., Johnson K.S. Mosquito allergy. Ann Allergy Asthma Immunol : Off Pub Am Coll Allergy Asthma Immunol. Feb 2013;110(2):65–69. doi: 10.1016/j.anai.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Peng Z., Yang M., Simons F.E. Immunologic mechanisms in mosquito allergy: correlation of skin reactions with specific IgE and IgG antibodies and lymphocyte proliferation response to mosquito antigens. Ann Allergy Asthma Immunol : Off Pub Am Coll Allergy Asthma Immunol. Sep 1996;77(3):238–244. doi: 10.1016/S1081-1206(10)63262-0. [DOI] [PubMed] [Google Scholar]
- 19.Reunala T., Brummer-Korvenkontio H., Rasanen L., Francois G., Palosuo T. Passive transfer of cutaneous mosquito-bite hypersensitivity by IgE anti-saliva antibodies. J Allergy Clin Immunol. Nov 1994;94(5):902–906. doi: 10.1016/0091-6749(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 20.Reunala T., Brummer-Korvenkontio H., Palosuo T. Are we really allergic to mosquito bites? Ann Med. Aug 1994;26(4):301–306. doi: 10.3109/07853899409147906. [DOI] [PubMed] [Google Scholar]
- 21.Cantillo J.F., Puerta L., Fernandez-Caldas E. Tropomyosins in mosquito and house dust mite cross-react at the humoral and cellular level. Clin Exp Allergy. 2018 Oct;48(10):1354–1363. doi: 10.1111/cea.13229. [DOI] [PubMed] [Google Scholar]
- 22.Sabogal P., Lozano A., Mercado D. Cellular and humoral responses to Cte f 2, a cat flea allergen, in children with papular urticaria. Int Arch Allergy Immunol. 2019;179(2):89–101. doi: 10.1159/000496743. [DOI] [PubMed] [Google Scholar]
- 23.Cuéllar A., Rodríguez A., Halpert E. Specific pattern of flea antigen recognition by IgG subclass and IgE during the progression of papular urticaria caused by flea bite. Allergol Immunopathol. Jul-Aug 2010;38(4):197–202. doi: 10.1016/j.aller.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Peng Z., Ho M.K., Li C., Simons F.E. Evidence for natural desensitization to mosquito salivary allergens: mosquito saliva specific IgE and IgG levels in children. Ann Allergy Asthma Immunol. 2004 Dec;93(6):553–556. doi: 10.1016/s1081-1206(10)61262-8. [DOI] [PubMed] [Google Scholar]
- 25.Karppinen A., Rantala I., Vaalasti A., Palosuo T., Reunala T. Effect of cetirizine on the inflammatory cells in mosquito bites. Clin Exp Allergy: J Br Soc Allergy and Clin Immunol. Jun 1996;26(6):703–709. [PubMed] [Google Scholar]
- 26.Peng Z., Xu W., James A.A. Expression, purification, characterization and clinical relevance of rAed a 1-a 68-kDa recombinant mosquito Aedes aegypti salivary allergen. Int Immunol. Dec 2001;13(12):1445–1452. doi: 10.1093/intimm/13.12.1445. [DOI] [PubMed] [Google Scholar]
- 27.Peng Z., Xu W., Lam H., Cheng L., James A.A., Simons F.E. A new recombinant mosquito salivary allergen, rAed a 2: allergenicity, clinical relevance, and cross-reactivity. Allergy. Apr 2006;61(4):485–490. doi: 10.1111/j.1398-9995.2006.00985.x. [DOI] [PubMed] [Google Scholar]
- 28.Peng Z., Xu W.W., Sham Y. Mosquito salivary allergen Aed a 3: cloning, comprehensive molecular analysis, and clinical evaluation. Allergy. May 2016;71(5):621–628. doi: 10.1111/all.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Z., Caihe L., Beckett A.N., Guan Q., James A.A., Simons F.E. rAed a 4: a new 67-kDa Aedes aegypti mosquito salivary allergen for the diagnosis of mosquito allergy. Int Arch Allergy Immunol. 2016;170(3):206–210. doi: 10.1159/000448587. [DOI] [PubMed] [Google Scholar]
- 30.Cantillo J.F., Puerta L., Lafosse-Marin S., Subiza J.L., Caraballo L., Fernandez-Caldas E. Identification and characterization of IgE-binding tropomyosins in Aedes aegypti. Int Arch Allergy Immunol. 2016;170(1):46–56. doi: 10.1159/000447298. [DOI] [PubMed] [Google Scholar]
- 31.Cantillo J.F., Puerta L., Puchalska P., Lafosse-Marin S., Subiza J.L., Fernández-Caldas E. Allergenome characterization of the mosquito Aedes aegypti. Allergy. 2017;72(10):1499–1509. doi: 10.1111/all.13150. [DOI] [PubMed] [Google Scholar]
- 32.https://www.vectorbase.org/Aedes_albopictus/Gene/Compara_Paralog?db=core;g=AALF024477;r=JXUM01S006260:56950-58160;t=AALF024477-RA
- 33.Valenzuela J.G., Pham V.M., Garfield M.K., Francischetti I.M., Ribeiro J.M. Toward a description of the sialome of the adult female mosquito Aedes aegypti. Insect Biochem Mol Biol. Sep 2002;32(9):1101–1122. doi: 10.1016/s0965-1748(02)00047-4. [DOI] [PubMed] [Google Scholar]
- 34.Choumet V., Carmi-Leroy A., Laurent C. The salivary glands and saliva of Anopheles gambiae as an essential step in the Plasmodium life cycle: a global proteomic study. Proteomics. Sep 2007;7(18):3384–3394. doi: 10.1002/pmic.200700334. [DOI] [PubMed] [Google Scholar]
- 35.Kalume D.E., Okulate M., Zhong J. A proteomic analysis of salivary glands of female Anopheles gambiae mosquito. Proteomics. Sep 2005;5(14):3765–3777. doi: 10.1002/pmic.200401210. [DOI] [PubMed] [Google Scholar]
- 36.Calvo E., Mans B.J., Ribeiro J.M., Andersen J.F. vol. 106. Mar 10 2009. Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein; pp. 3728–3733. (Proceedings of the National Academy of Sciences of the United States of America). 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chagas A.C., Ramirez J.L., Jasinskiene N. vol.111. May 13 2014. Collagen-binding protein, Aegyptin, regulates probing time and blood feeding success in the dengue vector mosquito, Aedes aegypti; pp. 6946–6951. (Proceedings of the National Academy of Sciences of the United States of America). 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beaudouin E., Kanny G., Renaudin J.-M., Moneret-Vautrin D.A. Allergen-specific immunotherapy to mosquitoes. Allergy. 2001;56(8) doi: 10.1034/j.1398-9995.2001.056008787.x. 787-787. [DOI] [PubMed] [Google Scholar]
- 39.Manning J.E., Morens D.M., Kamhawi S., Valenzuela J.G., Memoli M. Mosquito saliva: the hope for a universal arbovirus vaccine? J Infect Dis. 2018;218(1):7–15. doi: 10.1093/infdis/jiy179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.