Abstract
A large gap between the number of people with end-stage kidney disease (ESKD) who received kidney replacement therapy (KRT) and those who needed it has been recently identified, and it is estimated that approximately one-half to three-quarters of all people with ESKD in the world may have died prematurely because they could not receive KRT. This estimate is aligned with a previous report that estimated that >3 million people in the world died each year because they could not access KRT. This review discusses the reasons for the differences in treated and untreated ESKD and KRT modalities and outcomes and presents strategies to close the global KRT gap by establishing robust health information systems to guide resource allocation to areas of need, inform KRT service planning, enable policy development, and monitor KRT health outcomes.
Keywords: end-stage kidney disease, global health, health information systems, health services accessibility, kidney replacement therapy, registries
Chronic kidney disease (CKD) is associated with increased risks of morbidity, mortality, and excess health care costs for individuals, families, and countries.1,2 Whereas global rates of death and disability-adjusted life years have decreased for most noncommunicable diseases over the past 2 decades, corresponding rates for CKD have overall increased.3, 4, 5 Indeed, recent data from the Global Burden of Disease study have predicted that CKD will be the 5th leading cause of death by the year 2040.6
The progression from the early stages of CKD to end-stage kidney disease (ESKD) exerts a multiplying effect on morbidity, mortality, and health care costs. Life expectancy is drastically shortened in these individuals, unless life-saving, costly kidney replacement therapy (KRT; dialysis or kidney transplantation) is initiated. A recent systematic review of data from 123 countries (representing 93% of the world’s population) estimated that 2.618 million people received KRT worldwide in 2010 and that this figure would more than double to 5.439 million people by 2030, with most of the growth occurring in low-income countries (LICs) and middle-income countries in Asia and Latin America.7
More importantly, it was reported that there was a large gap between the number of people with ESKD who received KRT and those who needed it, such that between 2.284 and 7.083 million people, representing about one-half to three-quarters of all people with ESKD in the world, may have died prematurely because they could not receive KRT.7 This estimate is aligned with a previous report8 that estimated that more than 3 million people in the world died each year because they could not access KRT. In this review, we discuss the reasons for the differences in treated and untreated ESKD and KRT modalities and outcomes, and we present strategies to decrease these gaps through the improvement of health information systems.
Global differences in KRT incidence and prevalence
Available registry data indicate that the prevalence of treated ESKD varies more than 1000-fold across the globe from close to 0 per million population (pmp) to over 2000 pmp in parts of North and East Asia, suggesting the presence of large inequities in global access to KRT.9, 10, 11, 12 The majority of all ESKD patients treated with KRT reside in North America, Japan, or Europe,9,13,14 and socioeconomic factors are likely to be a major driver for these differences, because KRT prevalence is highly correlated with countries’ wealth and investments in health care.7,14,15 Approximately 93% of individuals receiving KRT in 2010 resided in high-income countries (HICs) or upper-middle-income countries (UMICs), such that there was a 70-fold greater prevalence of KRT use in these countries compared with their LIC and lower-middle-income country (LMIC) counterparts.7,16,17 Despite this, the numbers of patients on KRT in regions such as Latin America and Asia are quickly increasing, most likely as a consequence of economic improvements and public policies that allow increased access and more universal coverage to ESKD treatment.7 HICs that provide KRT typically spend approximately 2% to 3% of their national health care budgets on patients with ESKD, despite the fact that such patients represent only 0.1% to 0.2% of the total population.5,11
Appreciable between-country variation in KRT use has also been observed according to age (particularly older people), sex, race (particularly black and indigenous peoples), and migrant populations.9,18 The reasons for these variations remain unclear, but may potentially reflect a number of factors including patient-related issues (e.g., age, presence of comorbidities, individual preference, distance of residence from a renal unit, educational and socioeconomic status, beliefs, health literacy), health care system-related factors (e.g., presence of a universal primary care system to manage diabetes, hypertension, and other risk factors; presence of a CKD care plan; presence of adequately trained workforce; broader public coverage of dialysis and transplantation; physician bias and experience; renal unit distribution) and quality of local or national registries. In some LMICs, there are emerging programs of government-funded chronic dialysis for ESKD that offer treatment to adults but not sufficient treatment for children.19
Global differences in KRT modality
The most common (78%) form of KRT is dialysis, of which 89% is accounted for by hemodialysis (HD) and 11% by peritoneal dialysis (PD) globally.7,20 A recent evaluation of the current status of global kidney care in 124 countries, comprising 93% of the world’s population, performed by the International Society of Nephrology (ISN) Global Kidney Health Atlas (GKHA) Project observed that HD was available in all of the countries from where responses were available, but PD was available in just 80%, and in only 29% of LICs.10,11 This observation seems somewhat paradoxical, because PD has a number of features that should be attractive to LICs including fewer technical demands, greater feasibility of use in remote regions, lesser need for trained staff, and fewer management challenges in the setting of natural disasters. PD may also be an attractive modality in many countries in terms of cost, understanding that comparative cost of dialysis modality varies depending on the region, and the fact that there is no adequately performed cost-effectiveness studies comparing HD with PD in most regions.21, 22, 23 For these reasons, countries such as Thailand, Hong Kong, New Zealand, Australia, China, and USA have enacted public policies that promote and/or provide financial incentives for preferential use of PD.10,24, 25, 26, 27 Nevertheless, the relative use of PD versus HD varies markedly between countries and can be related to variability in patient factors (e.g., awareness, comorbidities, visual acuity, dexterity, mobility, cognitive ability, family support, financial status), facility factors (e.g., physician bias and experience, physician availability, surgeon availability, infrastructure support for urgent-start PD, PD and HD training processes, private vs. public), health care system factors (e.g., public vs. private models, financial incentives, clinician and particularly nursing reimbursement, dialysis policy) and industry factors (e.g., local fluid manufacture, solution costs).20,28Other models of care delivery, including point of care dialysate production (i.e., the Affordable Dialysis Project), simple technology equipment, semi- or self-care KRT, or community-based centers, will need to be considered as potential alternatives in specific regions taking into account the local reality.
The availability of dialysis does not necessarily equate with accessibility, which may be further limited within and between countries. In the GKHA survey, the key barriers to providing KRT identified by country representatives were geographical (71%—distance from care or prolonged travel time), physician-related (65%—availability, access, knowledge, and attitude), patient-related (78%—knowledge and attitude), and health care system-related (20%—availability, access, capability).10,11 These barriers were specifically identified in the ISN Collection Survey, which reported that age, comorbidities, and availability of a transplant donor were taken into account for the decision of providing access to KRT in several LMICs.29 Nevertheless, the most important barrier to KRT in LICs and LMICs is treatment cost; financial factors and out-of-pocket expenses also play a role in dialysis accessibility, because the cost of dialysis for a patient exceeds the average annual individual income in most countries30 and one-third of countries exclude dialysis from public funding.10,11 This is an even greater issue for people living in LICs where dialysis is excluded from public funding in the majority of instances.10,11
Utilization of kidney transplantation, which accounts for 22% of all KRT,7 is also highly variable within and between countries,10,11 even though this KRT modality is associated with superior survival, quality of life, and cost-effectiveness.31 According to the GKHA, kidney transplantation was not available in 21% of countries surveyed,10,11 including 64% of African countries and 88% of LICs.10,11 In those countries where it was available, transplant rates varied more than 70-fold from <1 pmp in Bangladesh to 71 pmp in the Jalisco state of Mexico.9 Rates of kidney transplantation also vary appreciably according to patient characteristics, including age, sex, race, socioeconomic status, and health insurance status.13,32, 33, 34, 35 Access to transplantation is often influenced by the affordability of transplant medications, which were reported to be publicly funded by the government and free at the point of delivery in <30% of all countries surveyed in the GKHA. In 53% of LICs, transplant medications were funded through solely private and out-of-pocket sources.10,11 Cultural background about receiving and/or donating organs from brain death donors, such as in Japan, China, and Taiwan, may also limit kidney transplantation.30,36 The proportions of living donor kidney transplants performed also vary markedly around the world, ranging from 0% in Morocco and Greece through to 100% in Iceland, Egypt, and Bangladesh.9 The majority of LICs (100%) and LMICs (62%) perform solely living donor kidney transplants.
Although the evidence indicates that the majority of patients with ESKD in the world do not receive KRT, it is unclear to what extent in each country this reflects unrecognized ESKD (thereby preventing KRT from being offered), difficulty accessing KRT due to the various patient and health care–related factors described previously, or medically informed patient choice to receive comprehensive conservative care (also known as nondialysis supportive care). Whereas comprehensive conservative care has been widely recognized and provided,37, 38, 39, 40 the incidence or prevalence of this treatment pathway has been poorly studied.41 A survey of comprehensive conservative care practice patterns in relation to older patients (≥75 years old) was administered to all 71 adult renal units in the UK in 2013 and demonstrated that comprehensive conservative care was practiced in almost all units but varied markedly in its scale and approach between centers.42 In a community-based cohort study of over 1.8 million adults in Alberta, Canada, the incidence of treated and untreated ESKD was 0.18% and 0.17%, respectively, over a median follow-up period of 4.4 years.43 Compared with young adults, rates of untreated ESKD were over 5-fold higher in patients ≥85 years old.43 Similar findings were observed in a population-based study in Australia.44 As dialysis registries are patchy around the world and as most dialysis registries do not capture patients who do not access dialysis for whatever reason, the precise frequency of occurrence and reasons for ESKD not being treated are unknown, particularly in LICs.
Thus, global access to KRT is both poor and inequitable, and the majority of people with ESKD in the world die prematurely because of lack of access to KRT. In the following sections, we discuss the importance of and define potential actions to improve the capturing and monitoring of global differences in the incidence and prevalence of ESKD, ESKD care, and modality of ESKD care.
The importance of health information systems in ESKD care planning and delivery
It is difficult to manage a problem unless it can be measured. Therefore, a cornerstone of closing the global KRT gap is establishing robust health information systems in each country to define CKD and ESKD burdens, guide resource allocation to areas of need, identify KRT access blocks, capture costs and funding of treatment, inform KRT service planning, enable policy development, and monitor KRT health outcomes, particularly following interventions.
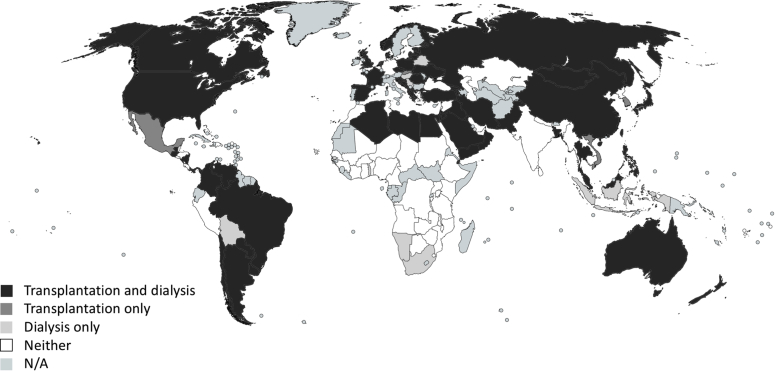
According to the GKHA survey, only 62% of countries could estimate their prevalence of kidney disease10,11 through KRT registries. This figure fell to 24% in LICs.10,11 Only 8% of countries had a national nondialysis CKD registry.10,11 With respect to dialysis registries, 64% of countries had a national or regional dialysis registry (HICs 89%, UMICs 73%, LMICs 50%, LICs 18%).10,11 African (35%) and South Asian (40%) countries had the lowest frequencies of dialysis registries. Similar findings were observed for kidney transplantation, for which only 58% of countries had a national or regional registry (HICs 89%, UMICs 67%, LMICs 44%, LICs 0%).10,11 Only 19% of African countries had a transplant registry. The Latin American Dialysis and Renal Transplant Registry (LADRTR) captures data on KRT (including dialysis and transplantation) combining national registries from 20 Latin American–affiliated countries since 1991, covering 95% of the population for this region. However, the registry has some limitations as most nations do not have formal registries and in most of those that have them, reporting to the existing registry is not mandatory, generating potential information gaps.45 Several heavily populated countries, including India, Germany, and a number of African countries, collectively representing over 20% of the world’s population, do not have any CKD, KRT, or acute kidney injury registries (Figure 1).46
Figure 1.
Availability of renal (dialysis and transplantation) registries across the globe. Reprinted with permission from See EJ, Alrukhaimi M, Ashuntantang GE, et al. Global coverage of health information systems for kidney disease: availability, challenges, and opportunities for development. Kidney Int Suppl. 2018;8:74–81.46 N/A, information not available.
The lack of comprehensive health information systems, particularly in LICs, to accurately capture CKD or ESKD burden and KRT provision needs to be addressed as a matter of priority, given that KRT is associated with substantial health care costs. The current data pertaining to ESKD incidence or prevalence (or both) and the gap between KRT need and use are based on modeling projections from limited regional or national (or both) registry data pertaining to dialysis with or without kidney transplantation and adjusted for limited variables, such as country income and life expectancy.7 Data collection methodologies and analyses also vary considerably between countries. Consequently, the degree and reliability of within- and between-country variation in the prevalence of treated ESKD remain uncertain. Data pertaining to the number of ESKD patients requiring KRT are even less certain. In the study by Liyanage et al.,7 low and high estimates were respectively developed from age-specific KRT data from 16 HICs with incomplete KRT uptake and 4 HICs (Japan, Singapore, Taiwan, and the USA) in which KRT was presumed to be provided to almost all individuals needing it. The uncertainties regarding the true incidence and prevalence of treated and untreated ESKD are greatest in LICs and LMICs.
Initiatives to implement KRT registries in LMICs
Some LMICs across the world have managed to establish dialysis registries. Africa, Chad, Guinea, and Niger have recently implemented KRT registries. Indeed, Guinea additionally has nondialysis CKD and acute kidney injury registries. The experiences of these countries could be drawn on to help establish registries in other African countries. Other successful African renal registries, such those established in Tunisia in 199047 and South Africa,48 demonstrate the powerful potential for registries to highlight inequitable KRT access and help inform policy decisions in favor of providing additional resources for treating ESKD.47 Similar experiences have been reported following the establishment of renal registries in other countries, such as Thailand,49 Malaysia,50 and Latin America.51
A number of LMICs are in the process of setting up national dialysis registries. The LADRTR was created in 1991, and since then, it has published several reports.52 As the registry is not mandatory, it has required a sustained effort from the Latin American nephrology community. Twenty countries participated in the surveys (>90% of the region). In principle, a wide variability in kidney disease is observed in the region. The mean prevalence of KRT is 776 patients pmp (ranging from 199 in Paraguay to 1881 in Puerto Rico), the mean incidence is 162 pmp (23 Paraguay to 420 Mexico) and the mean rate of kidney transplantation is 26 pmp (0.6 Honduras to 58 Mexico). The mean number of nephrologists is 16 pmp (2 Colombia to 53 Uruguay). Noncommunicable diseases are the main cause of CKD in Latin America, with 36% of KRT patients developing CKD due to diabetes (∼70% in Mexico and Puerto Rico). Besides the traditional causes of CKD, Mesoamerican nephropathy is an incompletely known form of CKD, affecting men working under disadvantaged conditions in agricultural areas of Central America. Although advances have been made, there is a need to support local and regional research on this topic to further understand how to prevent and treat this and other forms of the disease.
The South African Renal Registry (SARR) is, thus far, the only regularly published and updated report of KRT in sub-Saharan Africa. The SARR was first published in 2014 (based on 2012 data) with the data demonstrating a significant inequality in the distribution of KRT across provinces and between the public and private health sectors in South Africa. A more recent SARR report (based on 2015 data) showed an ESKD treatment rate of 71.9 pmp in the public sector (representing a population of 46.2 million) versus 799.3 pmp in the private sector (representing a population of 8.8 million). It is hoped that these reports will influence governments to respond with measures to improve the care of patients with kidney diseases. The SARR reports have influenced the African Association of Nephrology (AFRAN) to adapt its methods and use the same platform to develop an African Renal Registry. However, only 4 countries (Ghana, Burundi, Zambia, and South Africa) are currently participating in data collection (R. Davids, personal communication, 2019). Low participation in the African Renal Registry could limit the usefulness of reported data.
Initiatives to support the implementation of KRT registries in LMICs
Consideration could also be given to incorporating resource-limited countries into existing registries, as has happened on occasion with North African countries contributing to the European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) Registry48,53 and the United States Renal Data System (USRDS) registry.9 Pacific Island countries, such as Fiji, could leverage the infrastructure and collaborative expertise of the Australian and New Zealand Dialysis and Transplant (ANZDATA) Registry. Recently, an agreement was reached between AFRAN and the African Paediatric Nephrology Association (AFPNA) to establish the African Renal Registry, which will use the shared web-based technology platform and common data dictionary of the SARR.48
Moreover, the Latin American Society of Nephrology and Hypertension (SLANH), which coordinates the LADRTR, is promoting action in the area of training and capacity building, namely courses and training opportunities for national registries (A.M. Cueto-Manzano, personal communication, 2019). The aim is to create more national ESKD registries (to have one in every country of the region) and increase their quality as a key step in determining the true burden of kidney disease and outcomes for patients. SLANH has established an active training program in conjunction with Pan American Health Organization (PAHO), the national societies of nephrology and the ministries of health of every country, to develop regional and national registries. An important challenge is the lack of a normative policy frame for the registry functioning. In most countries, the registry is voluntary and only administered by its national society of nephrology (no participation of the ministry of health), without human or economic resources. Countries with more developed registries have institutionalized their functioning: they have a committee with all the relevant stakeholders, have a normative frame, reporting is mandatory, and health authorities are actively involved in providing technology infrastructure, as well as economic guarantees and human resources.
The ISN is supporting these efforts through the Sharing Expertise to support the set-up of Renal Registries (SharE-RR) project (https://www.theisn.org/advocacy/share-rr), which is developing a resource available to kidney health advocates in countries wishing to establish or develop a renal registry to support advocacy, quality assurance, and research. This project will facilitate sharing of registry policies, procedures, governance structures, databases, datasets, technology platforms, files, and consent processes. Surveys of existing registries will be conducted to help inform the establishment of a minimum dataset to be collected by all renal registries to permit benchmarking between registries and to monitor the quality of care and outcomes. A minimum dataset will help health care professionals and people with ESKD make better informed treatment decisions and capture serious health-related suffering in people with ESKD for inclusion in global health reports.
Additional strategies to optimize the information capture from health information systems
Registry data linked with geographic information systems can monitor variations in ESKD prevalence and identify hot spots and areas where there are major mismatches between KRT supply and demand.54 Registry output and reports should be freely accessible online to maximize reach, transparency, and impact.48 A systematic review of renal registries reported that “public accessibility to annual reports, publications, or basic data was good for 17 (35%) registries.”55
Benchmarking will be facilitated by developing standardized definitions and terminology (data dictionary) for registries. For example, ESKD is variously defined around the world, ranging from requirement for KRT to estimated glomerular filtration rate <15 ml/min per 1.73 m2. This definition and other important variables collected in registries need to be revised and harmonized. Defining conservative care for capture by registries has proven challenging, with very limited data available on numbers treated conservatively and the components and quality of that care. It is necessary to define the initiation of conservative care if registries are to measure it and report on quality of care. For similar reasons, it is also necessary to agree on a definition for discontinuation of dialysis.
Data captured in ESKD registries could be supplemented with data from observational studies. The implementation of representative cohort studies in LMICs, such as Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) in Thailand and Colombia, CKDOPPS in Brazil, and DOPPS in China are examples of how observational studies can be useful in generating information related to the kidney care in LMICs where registries are not available. In Brazil, the implementation of a locally and nationally representative observational study (BRAZPD) provided information about the reality of PD treatment in a country where a PD registry was not available.56 Granular information on patient characteristics, local practice, and factors associated with clinical outcomes, usually not captured in registries, can be a valuable (and complementary) addition to the information from registries.
Finally, innovative information technologies could improve the capturing of data through automated extraction from electronic health records, data linkage from multiple sources, and simplified collection of patient level data using mobile phones or personal computers and web-based platforms.
Final considerations
To capture, monitor, and map the journeys of patients’ kidney disease, ESKD registries should be linked to primary and secondary care to map patient flow, the impact of improved CKD care, and outcomes of interventions. Key patient-centered performance indicators and outcome measures in KRT modalities (including conservative care) should be captured to measure quality of care and patients’ preferences. In addition, registries should be integrated within countries and regions, such that pediatric and adult registries are combined and all forms of ESKD care (including dialysis, transplantation, comprehensive conservative care, and choice-restricted conservative care) are captured and supplemented with observational studies. Ideally, the focus of renal registries should shift from modality-centered to person-centered. Finally, registries should be utilized to help design and implement clinical trials. Initiatives to provide support for registry implementation and training and sharing experiences could help in the development and implementation of local registries.
Disclosure
Publication of this article was supported by the International Society of Nephrology.
RPF reports grant support from Fresenius Medical Care. JD reports consulting fees from the International Society of Nephrology. DCHH reports grant support from the National Health and Medical Research Council. AC reports wages and stock options as employee of NxStage Medical Inc., now Fresenius Medical Care North America. BG reports lecture fees from Baxter, Fresenius Medical Care, Sanofi, and Kwoya Kirin and grant support from Baxter and Kwoya Kirin. MS reports consulting and lecture fees from Boehringer Ingelheim and current grant support from Merit Telangana. RTK reports lecture fees from Baxter. RCW reports grant support from Lotteries Health Research Grant and Baxter Health Care (Clinical Evidence Council [CEC] Research Grant). QY reports wages from Baxter Healthcare. DWJ reports future consulting fees from Astra-Zeneca, lecture fees from Baxter Healthcare and Fresenius Medical Care, and grant support from Baxter Extramural and Baxter CEC grants. All other authors declared no competing interests.
Acknowledgments
This manuscript emerged as an individual product of the International Society of Nephrology’s 2nd Global Kidney Health Summit held in Sharjah, United Arab Emirates, in March 2018, and portions of the material in this document have been published in the full report from the summit (Harris et al.12). In addition to the International Society of Nephrology, support of the summit was provided through unrestricted grants from Baxter and BBraun. We thank Mohamed Osman and Feng Ye for their assistance with figures and analyses.
Footnotes
The views expressed in this commentary are solely the responsibility of the authors and they do not necessarily reflect the views, decisions, or policies of the institutions with which they are affiliated.
References
- 1.Couser W.G., Remuzzi G., Mendis S. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 2.Neuen B.L., Chadban S., Demaio A.R. Chronic kidney disease and the global NCDs agenda. BMJ Global Health. 2017;2 doi: 10.1136/bmjgh-2017-000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jager K.J., Fraser S.D.S. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant. 2017;32(suppl 2):ii121–ii128. doi: 10.1093/ndt/gfw330. [DOI] [PubMed] [Google Scholar]
- 4.BD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luyckx V.A., Miljeteig I., Ejigu A.M. Ethical challenges in the provision of dialysis in resource-constrained environments. Semin Nephrol. 2017;37:273–286. doi: 10.1016/j.semnephrol.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Foreman K.J., Marquez N., Dolgert A. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liyanage T., Ninomiya T., Jha V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 8.Anand S., Bitton A., Gaziano T. The gap between estimated incidence of end-stage renal disease and use of therapy. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.USRDS . USRDS; Bethesda, MD: 2017. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 10.Bello A.K., Levin A., Tonelli M. Assessment of global kidney health care status. JAMA. 2017;317:1864–1881. doi: 10.1001/jama.2017.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bello A.K., Levin A., Tonelli M. International Society of Nephrology; Brussels, Belgium: 2017. Global Kidney Health Atlas: A Report by the International Society of Nephrology on the Current State of Organization and Structures for Kidney Care Across the Globe. [Google Scholar]
- 12.Harris D.C.H., Davies S.J., Finkelstein F.O. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int. 2019;95:S1–S33. doi: 10.1016/j.kint.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 13.White S.L., Chadban S.J., Jan S. How can we achieve global equity in provision of renal replacement therapy? Bull World Health Organ. 2008;86:229–237. doi: 10.2471/BLT.07.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schieppati A., Remuzzi G. Chronic renal diseases as a public health problem: epidemiology, social, and economic implications. Kidney Int Suppl. 2005:S7–S10. doi: 10.1111/j.1523-1755.2005.09801.x. [DOI] [PubMed] [Google Scholar]
- 15.Caskey F.J., Kramer A., Elliott R.F. Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant. 2011;26:2604–2610. doi: 10.1093/ndt/gfq781. [DOI] [PubMed] [Google Scholar]
- 16.Wetmore J.B., Collins A.L. Global challenges imposed by the growth of end-stage renal disease. Renal Repl Ther. 2016;2:15. [Google Scholar]
- 17.Coresh J., Jafar T.H. Disparities in worldwide treatment of kidney failure. Lancet. 2015;385:1926–1928. doi: 10.1016/S0140-6736(14)61890-0. [DOI] [PubMed] [Google Scholar]
- 18.Hecking M., Bieber B.A., Ethier J. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS) PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bamgboye E.L. The challenges of ESRD care in developing economies: sub-Saharan African opportunities for significant improvement. Clin Nephrol. 2016;86:18–22. doi: 10.5414/CNP86S128. [DOI] [PubMed] [Google Scholar]
- 20.Li P.K., Chow K.M., Van de Luijtgaarden M.W. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13:90–103. doi: 10.1038/nrneph.2016.181. [DOI] [PubMed] [Google Scholar]
- 21.Mehrotra R., Devuyst O., Davies S.J. The current state of peritoneal dialysis. J Am Soc Nephrol. 2016;27:3238–3252. doi: 10.1681/ASN.2016010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karopadi A.N., Mason G., Rettore E. The role of economies of scale in the cost of dialysis across the world: a macroeconomic perspective. Nephrol Dial Transplant. 2014;29:885–892. doi: 10.1093/ndt/gft528. [DOI] [PubMed] [Google Scholar]
- 23.Wang V., Maciejewski M.L., Coffman C.J. Impacts of geographic distance on peritoneal dialysis utilization: refining models of treatment selection. Health Serv Res. 2017;52:35–55. doi: 10.1111/1475-6773.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P.K., Chow K.M. Peritoneal dialysis-first policy made successful: perspectives and actions. Am J Kidney Dis. 2013;62:993–1005. doi: 10.1053/j.ajkd.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 25.Sedor J.R., Watnick S., Patel U.D. ASN End-Stage Renal Disease Task Force: perspective on prospective payments for renal dialysis facilities. J Am Soc Nephrol. 2010;21:1235–1237. doi: 10.1681/ASN.2010060656. [DOI] [PubMed] [Google Scholar]
- 26.Liu F.X., Gao X., Inglese G. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit Dial Int. 2015;35:406–420. doi: 10.3747/pdi.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tantivess S., Werayingyong P., Chuengsaman P. Universal coverage of renal dialysis in Thailand: promise, progress, and prospects. BMJ. 2013;346:f462. doi: 10.1136/bmj.f462. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer F., Borzych-Duzalka D., Azocar M. Impact of global economic disparities on practices and outcomes of chronic peritoneal dialysis in children: insights from the International Pediatric Peritoneal Dialysis Network Registry. Perit Dial Int. 2012;32:399–409. doi: 10.3747/pdi.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luyckx V.A., Smyth B., Harris D.C.H., Pecoits-Filho R. Dialysis funding, eligibility, procurement, and protocols in low- and middle-income settings: results from the International Society of Nephrology collection survey. Kidney Int Suppl. 2020;10:e10–e18. doi: 10.1016/j.kisu.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teerawattananon Y., Luz A., Pilasant S. How to meet the demand for good quality renal dialysis as part of universal health coverage in resource-limited settings? Health Res Policy Syst. 2016;14:21. doi: 10.1186/s12961-016-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolfe R.A., Ashby V.B., Milford E.L. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 32.Pruthi R., Curnow E., Roderick P. UK Renal Registry 17th Annual Report: chapter 11: Centre variation in access to renal transplantation in the UK (2008–2010) Nephron. 2015;129(suppl 1):247–256. doi: 10.1159/000370281. [DOI] [PubMed] [Google Scholar]
- 33.Ghods A.J., Nasrollahzadeh D. Gender disparity in a live donor renal transplantation program: assessing from cultural perspectives. Transplant Proc. 2003;35:2559–2560. doi: 10.1016/j.transproceed.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Lawton P.D., McDonald S.P., Snelling P.L. Organ transplantation in Australia: inequities in access and outcome for indigenous Australians. Transplantation. 2017;101:e345–e346. doi: 10.1097/TP.0000000000001911. [DOI] [PubMed] [Google Scholar]
- 35.Hod T., Goldfarb-Rumyantzev A.S. The role of disparities and socioeconomic factors in access to kidney transplantation and its outcome. Ren Fail. 2014;36:1193–1199. doi: 10.3109/0886022X.2014.934179. [DOI] [PubMed] [Google Scholar]
- 36.Irving M.J., Tong A., Jan S. Factors that influence the decision to be an organ donor: a systematic review of the qualitative literature. Nephrol Dial Transplant. 2012;27:2526–2533. doi: 10.1093/ndt/gfr683. [DOI] [PubMed] [Google Scholar]
- 37.Morton R.L., Turner R.M., Howard K. Patients who plan for conservative care rather than dialysis: a national observational study in Australia. Am J Kidney Dis. 2012;59:419–427. doi: 10.1053/j.ajkd.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 38.Da Silva-Gane M., Wellsted D., Greenshields H. Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol. 2012;7:2002–2009. doi: 10.2215/CJN.01130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Luijtgaarden M.W., Noordzij M., van Biesen W. Conservative care in Europe—nephrologists' experience with the decision not to start renal replacement therapy. Nephrol Dial Transplant. 2013;28:2604–2612. doi: 10.1093/ndt/gft287. [DOI] [PubMed] [Google Scholar]
- 40.Seah A.S., Tan F., Srinivas S. Opting out of dialysis—exploring patients' decisions to forego dialysis in favour of conservative non-dialytic management for end-stage renal disease. Health Expect. 2015;18:1018–1029. doi: 10.1111/hex.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murtagh F.E., Burns A., Moranne O. Supportive care: comprehensive conservative care in end-stage kidney disease. Clin J Am Soc Nephrol. 2016;11:1909–1914. doi: 10.2215/CJN.04840516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamoto I., Tonkin-Crine S., Rayner H. Conservative care for ESRD in the United Kingdom: a national survey. Clin J Am Soc Nephrol. 2015;10:120–126. doi: 10.2215/CJN.05000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemmelgarn B.R., James M.T., Manns B.J. Rates of treated and untreated kidney failure in older vs younger adults. JAMA. 2012;307:2507–2715. doi: 10.1001/jama.2012.6455. [DOI] [PubMed] [Google Scholar]
- 44.The Australian Institute of Health and Welfare . Australian Institute of Health and Welfare; Canberra, Australia: 2016. Incidence of end-stage kidney disease in Australia 1997–2013. Contract No.: Catalogue number PHE211. [Google Scholar]
- 45.Rosa-Diez G., Gonzalez-Bedat M., Ferreiro A. Burden of end-stage renal disease (ESRD) in Latin America. Clin Nephrol. 2016;86:29–33. doi: 10.5414/CNP86S105. [DOI] [PubMed] [Google Scholar]
- 46.See E.J., Alrukhaimi M., Ashuntantang G.E. Global coverage of health information systems for kidney disease: availability, challenges, and opportunities for development. Kidney Int Suppl. 2018;8:74–81. doi: 10.1016/j.kisu.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Counil E., Cherni N., Kharrat M. Trends of incident dialysis patients in Tunisia between 1992 and 2001. Am J Kidney Dis. 2008;51:463–470. doi: 10.1053/j.ajkd.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 48.Davids M.R., Caskey F.J., Young T. Strengthening renal registries and ESRD research in Africa. Semin Nephrol. 2017;37:211–223. doi: 10.1016/j.semnephrol.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Noppakun K., Ingsathit A., Pongskul C. A 25-year experience of kidney transplantation in Thailand: report from the Thai Transplant Registry. Nephrology (Carlton) 2015;20:177–183. doi: 10.1111/nep.12378. [DOI] [PubMed] [Google Scholar]
- 50.Lim T.O., Goh A., Lim Y.N. Review article: use of renal registry data for research, health-care planning and quality improvement: what can we learn from registry data in the Asia-Pacific region? Nephrology (Carlton) 2008;13:745–752. doi: 10.1111/j.1440-1797.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez-Bedat M., Rosa-Diez G., Pecoits-Filho R. Burden of disease: prevalence and incidence of ESRD in Latin America. Clin Nephrol. 2015;83(suppl 1):3–6. doi: 10.5414/cnp83s003. [DOI] [PubMed] [Google Scholar]
- 52.Registro LatinoAmericano de Dialysis y Translante Renal. INFORME 2015–2016. Available at: https://slanh.net/wp-content/uploads/2018/10/INFORME-2015-2016.pdf. Accessed January 20, 2020.
- 53.Davids M.R., Eastwood J.B., Selwood N.H. A renal registry for Africa: first steps. Clin Kidney J. 2016;9:162–167. doi: 10.1093/ckj/sfv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez R.A., Hotchkiss J.R., O'Hare A.M. Geographic information systems and chronic kidney disease: racial disparities, rural residence and forecasting. J Nephrol. 2013;26:3–15. doi: 10.5301/jn.5000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu F.X., Rutherford P., Smoyer-Tomic K. A global overview of renal registries: a systematic review. BMC Nephrol. 2015;16:31. doi: 10.1186/s12882-015-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Moraes T.P., Figueiredo A.E., de Campos L.G. Characterization of the BRAZPD II cohort and description of trends in peritoneal dialysis outcome across time periods. Perit Dial Int. 2014;34:714–723. doi: 10.3747/pdi.2013.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]