Abstract
Dialysis provisions and end-stage kidney disease (ESKD) care represents an important challenge, particularly in low-resource settings. The purpose of this project was to survey nephrologists from low- and lower middle-income countries about their experiences in the following domains: (i) Dialysis funding and eligibility; (ii) dialysis-procurement mechanisms; (iii) clinical protocols for dialysis; (iv) monitoring of dialysis outcomes; and (v) barriers to care for ESKD. One hundred and twenty responses from 31 low- and middle-income countries, from 8 ISN regions, were included in the analysis. When stratified by World Bank country income status, responses were received from 7 low-income countries, 12 lower middle-income countries, and 12 upper middle-income countries. Eighty-eight documents from 18 countries were uploaded, including country or institutional guidelines, protocols, and standard operating procedures. The International Society of Nephrology aims to develop a set of guidance documents that put forward a considered approach to dialysis provisions and ESKD care within resource limitations. As an initial step in this project, local practitioners from low-resource settings were surveyed about their experiences with dialysis funding, eligibility, procurement and their use of guidance documents, and how practices and procedures may have been developed with adaptations to the local circumstances. In this manuscript we describe the methodology and the main findings from the survey using an integrated quantitative and qualitative approach.
Keywords: chronic kidney disease, dialysis, end-stage kidney disease, funding, hemodialysis, peritoneal dialysis
Between 2 and 7 million people are estimated to die of end-stage kidney disease (ESKD) globally every year, owing to the lack of access to life-sustaining treatment.1 There are many systemic and structural barriers to access to dialysis in low-resource settings, including lack of kidney disease awareness, poor access to diagnosis, limited availability of infrastructure and staffing required, and cost.2 Even among those who do gain access, unless dialysis is provided under universal health coverage (UHC), attrition rates are high because of the unsustainable financial burden on patients and families.2, 3, 4 Although sustainability of dialysis provision and ensuring access to care for all patients in such environments is a major ongoing challenge, dialysis is becoming more available in low- and middle-income countries (LMIC).5 In this context, there is a clear need to provide knowledge and support so that access to—and delivery of—dialysis and care for patients with ESKD is as equitable, safe, and sustainable as possible.
Through an integrated set of projects and consultations, the International Society of Nephrology (ISN) aims to develop a set of guidance documents that put forward a considered and informed approach to provision of dialysis and ESKD care within resource limitations. Such documents aim to outline minimum standards for safe and effective dialysis, suggest frameworks for determination of patient access to dialysis, and approaches to palliative and supportive care when dialysis may not be possible. As an initial step in this project, local practitioners from low-resource settings were surveyed about their experiences with dialysis funding, eligibility, procurement and their use of guidance documents, and how practices and procedures may have been developed with adaptations to the local circumstances. In this article, we describe the methodology and the main findings from the survey, using an integrated quantitative and qualitative approach.
Results
Descriptive analysis of the survey responses
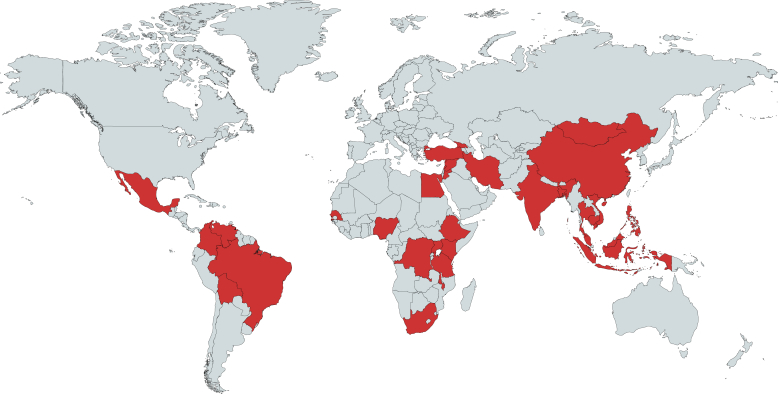
One hundred and forty-one responses were received between September 20, 2017, and February 28, 2018, of which 21 were from high-income countries (HIC). One hundred and twenty responses from LMIC, representing 31 countries from 8 ISN regions, were included in the analysis (Figure 1). The distribution of responses according to countries and ISN regions is shown in Table 1. A high proportion of responses came from Indonesia (45%) and South Africa (10%). When stratified by World Bank country income status, responses were received from 7 low-income countries (LIC), 12 LMIC, and 12 upper middle-income countries (UMIC). Response rates relative to number of countries having at least 1 ISN member in each income category were as follows: LIC, 27% (7 of 26 countries); LMIC, 38% (12 of 32 countries); and UMIC, 32% (2 of 38 countries). Eighty-eight additional documents from 18 countries were uploaded including country or institutional guidelines, protocols, and standard operating procedures. Guidelines from 4 countries were not available in English and therefore not analyzed in detail.
Figure 1.
Distribution of survey responses from low- and middle-income countries; 120 respondents from 31 countries responded to the survey. Upper middle–, lower middle–, and low-income countries are highlighted in red. Map created using mapchart.net.
Table 1.
Proportions of survey responses by International Society of Nephology region
| ISN region | n | Response (n) | % of Responses | |
|---|---|---|---|---|
| Africa | 10 | 33 | 27.5 | |
| Eastern and Central Europe | 1 | 2 | 1.7 | |
| Latin America and the Caribbean | 5 | 7 | 5.8 | |
| Middle East | 4 | 7 | 5.8 | |
| Russia and newly independent states | 1 | 1 | 0.8 | |
| North and East Asia | 2 | 3 | 2.5 | |
| Oceania and South East Asia | 6 | 60 | 50.0 | |
| South Asia | 2 | 7 | 5.8 | |
| Total | 31 | 120 | 100 |
ISN, International Society of Nephrology.
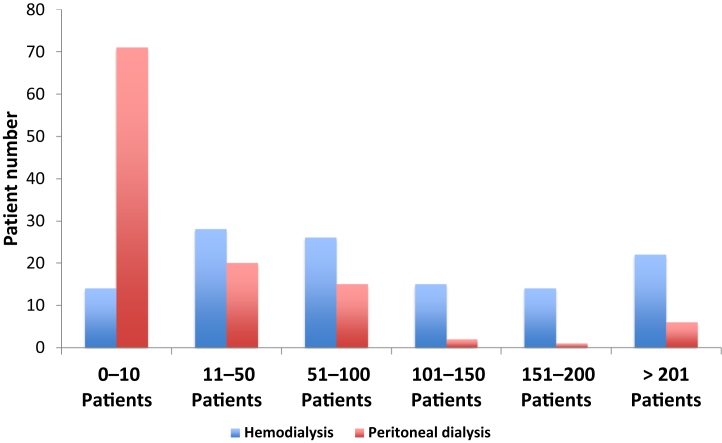
Overall, most respondents primarily practiced nephrology in teaching, public, or private hospitals, although dialysis clinics, medical societies, and foundations were also represented (Table 2). Respondents represented hemodialysis (HD) clinics with a wide range of patient numbers: 11.8% clinics treating 10 or fewer HD patients and 18.5% clinics treating more than 200 patients (Figure 2). In contrast, most (61.7%) of the clinics treated less than 10 peritoneal dialysis (PD) patients (Figure 2).
Table 2.
Respondent institutions or affiliations
| Institution | n | % of Responses | Weighted %a |
|---|---|---|---|
| National Medical Society | 5 | 4.2 | 10.0 |
| Hospital | |||
| University hospital | 32 | 26.7 | 38.8 |
| Private hospital | 26 | 21.7 | 11.2 |
| Public hospital | 45 | 37.5 | 26.8 |
| University-based dialysis clinic | 3 | 2.5 | 5.3 |
| Private dialysis clinic | 7 | 5.8 | 7.1 |
| Kidney/health care foundation | 2 | 1.7 | 0.9 |
| Total respondents | 120 |
Given an imbalance in responses (with some countries represented by multiple respondents), a weighted analysis was also performed in which each response was assigned a weight inversely proportional to the number of responses from that country.
Figure 2.
Numbers of patients receiving hemodialysis or peritoneal dialysis in respondents’ institutions. Distribution of numbers of patients receiving hemodialysis or peritoneal dialysis followed by survey respondents.
Methods
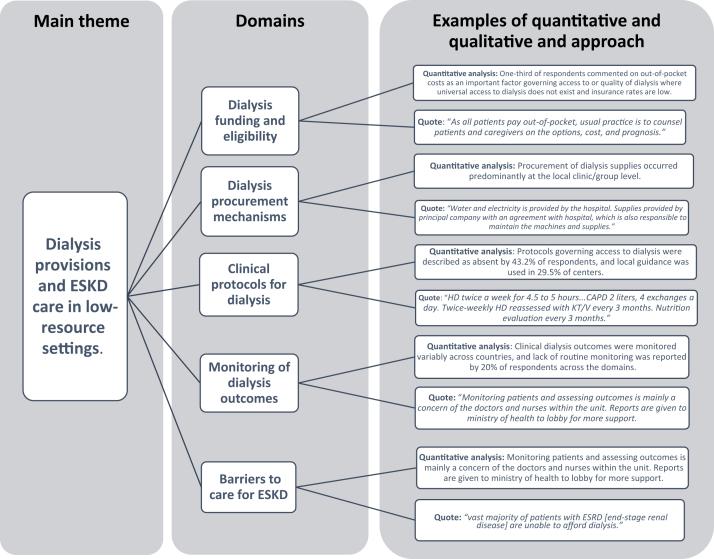
All active members of ISN from LMIC (as defined by the World Bank6) were contacted by e-mail using the ISN Regional Boards as points of dissemination. Individuals were requested to share information on their dialysis practice and how decisions around the provision of dialysis and ESKD care are made in their institution/dialysis units. The primary domains of the survey included the following: (i) dialysis funding and eligibility; (ii) dialysis-procurement mechanisms; (iii) clinical protocols for dialysis; (iv) monitoring of dialysis outcomes; and (v) barriers to care for patients with ESKD. Respondents were allocated sequential numbers as identifiers and were invited to supplement their multiple-choice answers with commentary entered as free text. The quotes were transferred from the research electronic data capture (REDCap) survey database to an Excel spreadsheet and coded manually by an expert in the subject (VAL) before the analysis. To allow clarity and provide focus to the qualitative description of the results, authors (VAL and RP-F) selected the quotes from free text that were more representative of the quantitative trends observed for each domain. A mixed-methods research approach was applied, integrating quantitative (descriptive analysis of the countries’ characteristics, proportion of answers in each domain) and qualitative results (identification of representative quotes for each domain) (Figure 3).
Figure 3.
Thematic diagram summarizing the key themes, domains, and examples of the mixed-research approach. ESKD, end-stage kidney disease.
In addition, respondents were asked to upload any relevant supporting documents: in particular, any protocols, practice guidelines, or other institutional guidance documents used in the provision of the relevant aspect of ESKD care. Documents were uploaded in the original languages and translated by the authors using Internet tools to capture the high-level information. If specific documents were not available, we requested a brief explanation of the usual practice as free text. The survey was developed and administered using REDCap tools,7 hosted at The George Institute for Global Health, Sydney, Australia. Data were analyzed descriptively using Excel (Microsoft, Redmond, WA) and Stata IC 15.1 (StataCorp LP, College Station, TX). Given an imbalance in responses (with some countries represented by multiple respondents), a weighted analysis was also performed in which each response was assigned a weight inversely proportional to the number of responses from that country. For example, if 3 responses were received from 1 country, each of those 3 responses was assigned a weight of 1 of 3. Survey participation was voluntary. Because of the characteristics of the survey, ethics approval was not necessary.
Mixed-methods approach to survey responses
Domain 1: dialysis funding and eligibility
The majority of respondents (87.5%) reported partial or total funding for dialysis by the government. Contributions from insurance (76%) and out-of-pocket expenses (45.8%) were frequent (Table 3). Thirty-three of 61 respondents explicitly reported that mixed models of funding were used in their setting.
Table 3.
Sources of dialysis funding
| Source of dialysis funding | n | % of Responsesa | Weighted % responsesb | Overall % funding contribution |
|---|---|---|---|---|
| Government-funded | 105 | 87.5 | 84.3 | 37.6 |
| Private insurance | 50 | 41.7 | 30.7 | 18.3 |
| Employment-based insurance | 42 | 35.0 | 33.8 | 15.1 |
| Charity | 22 | 18.3 | 27.2 | 7.9 |
| Out-of-pocket payment | 55 | 45.8 | 46.6 | 19.7 |
| Other | 4 | 3.3 | 1.8 | 1.4 |
| Total responses | 278a | 100 | ||
| Total respondents | 120 |
Total greater than 100% as multiple options could be chosen.
Given an imbalance in responses (with some countries represented by multiple respondents), a weighted analysis was also performed in which each response was assigned a weight inversely proportional to the number of responses from that country.
Limitations to access to dialysis (for otherwise medically eligible patients) were reported in 17 of the 31 countries responding to the survey (Table 4). No limitations in access to dialysis were reported in a majority of systems where dialysis was funded by government (54.4%), private insurance (67.4%), or out-of-pocket (70.6%), whereas when dialysis was funded by charities, there were more limitations (57.7%). Taken together, the most common limitations concerned dialysis modality choice (HD vs. PD, 31.6% of limitations) and dialysis only if transplant eligible (18.8% of limitations). Although the majority of respondents stating that there was a limitation in dialysis access to patients eligible for a transplant were from South Africa, some other countries responded that there was a limitation in access to dialysis dependent on the availability of a living donor. Age and comorbidities were limitations cited in 11.1% and 12.8% of responses, respectively. Two or more limitations were described by 54.2% of respondents. Limitations in access to dialysis were reported from respondents in all countries. In 29 of 31 countries, respondents reported at least 1 limitation, and 2 or more limitations were reported by respondents from 13 of 31 countries.
Table 4.
Limitations on access to dialysis for otherwise clinically suitable patients
| Limitations on dialysis access | Government n (% respondents) | Weighted % responsesa | Private n (% respondents) |
Weighted % responsesa | Out-of-pocket n (% respondents) |
Weighted % responsesa | Charity n (% respondents) | Weighted % responsesa |
|---|---|---|---|---|---|---|---|---|
| No limitations | 60 (57.1) | 54.4 | 34 (68.0) | 67.4 | 39 (70.9) | 70.6 | 10 (45.5) | 42.3 |
| Limitations on modality (e.g., PD only or HD only) | 24 (22.9) | 26.7 | 4 (8.0) | 11.0 | 6 (10.9) | 14.6 | 3 (13.6) | 14.1 |
| Transplant-eligible patients only | 15 (14.3) | 7.3 | 3 (6.0) | 1.3 | 3 (5.5) | 1.7 | 1 (4.6) | 1.3 |
| Limitations based on age | 8 (7.6) | 4.6 | 1 (2.0) | 3.9 | 4 (7.3) | 6.3 | 0 (0.0) | 0.0 |
| Limitations based on comorbidities or cause of renal disease | 8 (7.6) | 4.1 | 3 (6.0) | 9.0 | 2 (3.6) | 3.1 | 2 (9.1) | 15.4 |
| Other limitations | 15 (14.3) | 16.4 | 5 (5.0) | 11.3 | 4 (7.3) | 12.5 | 6 (27.3) | 26.9 |
| Total responsesb | 130 | 50 | 58 | 22 | ||||
| Total respondents | 105 | 50 | 55 | 22 |
HD, hemodialysis; PD, peritoneal dialysis.
Given an imbalance in responses (with some countries represented by multiple respondents), a weighted analysis was also performed in which each response was assigned a weight inversely proportional to the number of responses from that country.
Totals are greater than 100%, as multiple options could be chosen.
As a main finding captured in this domain, one-third of respondents commented on out-of-pocket costs as an important factor governing access to or quality of dialysis where universal access to dialysis does not exist and insurance rates are low. The following quotes illustrate the need for out-of-pocket contributions:
Nigeria (R48): “As all patients pay out-of-pocket, usual practice is to counsel patients and caregivers on the options, cost, and prognosis.”
Uganda (R134): “Most patients get their initial CKD diagnosis as emergency at the time they require dialysis. The physicians assess patients for dialysis indication. The rest is determined by if patient can afford the subsidized fee of 100-200 USD per week.”
Domain 2: dialysis procurement mechanisms
Procurement of dialysis supplies occurred predominantly at the local clinic/group level (54.1% of responses). Competitive bidding or tenders were reported in 48.1% of responses, whereas use of contracts without defined criteria was reported by 33.6% of respondents. The following quotes highlight the important variability both within and between countries with regard to dialysis procurement mechanisms:
South Africa (R10): “Government hospital: there is a regional or national tender system to providers for PD and acute HD services.”
South Africa (R15): “At our hospital for HD we use a private company that gives us a fixed rate for public patients and bill funded patients directly. For PD, companies supply equipment through a tender process.”
Indonesia (R35): “Water and electricity is provided by the hospital. Supplies provided by principal company with an agreement with hospital which is also responsible to maintain the machines and supplies.”
Domain 3: clinical protocols for dialysis and transplantation
Access to dialysis and modality choice
A large variety of sources was used across countries for guidance across the spectrum of dialysis practice as shown in Table 5 and Supplementary Table S1. Dialysis infrastructure and staffing requirements were most often determined at the health authority level. Examples of staffing ratios are outlined in Supplementary Table S2. Protocols governing access to dialysis were described as absent by 43.2% of respondents, and local guidance was used in 29.5% of centers. Only 17.5% of respondents reported official regulation or legislation governing access to dialysis. Patient choice of dialysis modality was variable across countries, as described in the narrative examples below:
Bangladesh (R46): “Modality choice is dependent on patients. Our first choice is CAPD [continuous ambulatory peritoneal dialysis].”
India (R127): “People with living donor in the family are given the option of pre-emptive transplantation. Choice of RRT [renal replacement therapy] is often left to the family's wish.”
South Africa (R20): “PD first because of limited HD slots. All patients must be transplantable.”
Table 5.
Source of protocols governing dialysis services
| Source of protocols | Infrastructure |
Staffing |
Access to dialysis, initiation |
Dialysis access |
Dialysis prescription |
Patient monitoring |
Infection surveillance |
Transplant work-up/transplant |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Wt %a | n | Wt %a | n | Wt %a | n | Wt %a | n | Wt %a | n | Wt %a | n | Wt %a | n | Wt %a | |
| Institution | 52 | 28.9 | 56 | 33.5 | 48 | 29.5 | 46 | 22.7 | 42 | 30.7 | 36 | 34.4 | 42 | 38 | 35 | 36.5 |
| Network/chain | 10 | 5.8 | 8 | 4.8 | 2 | 1.7 | 6 | 1.9 | 5 | 1.9 | 5 | 3.4 | 5 | 3.4 | 5 | 2.3 |
| Regional/provincial society | 12 | 1.6 | 10 | 1.3 | 7 | 1 | 7 | 0.4 | 7 | 0.7 | 7 | 0.7 | 7 | 0.7 | 7 | 1.1 |
| National society | 44 | 19.5 | 39 | 17.2 | 36 | 13.3 | 39 | 12.7 | 50 | 17.3 | 46 | 14 | 45 | 15 | 30 | 10.9 |
| International society | 12 | 13.9 | 4 | 6.5 | 7 | 13 | 8 | 8.7 | 9 | 10.6 | 15 | 17 | 12 | 12.2 | 9 | 10.4 |
| Adapted from international guidelines | 17 | 11.6 | 10 | 11.2 | 16 | 13.5 | 21 | 23.1 | 23 | 22.6 | 27 | 24.6 | 19 | 18.5 | 11 | 12.7 |
| Statute/legislation | 7 | 7.2 | 4 | 6.6 | 2 | 3.3 | 3 | 6.5 | 1 | 0.1 | 1 | 0.1 | 2 | 1.7 | 4 | 5 |
| Health authority/regulation | 47 | 43.8 | 39 | 41.7 | 23 | 14.2 | 18 | 16.8 | 15 | 13.5 | 12 | 5.4 | 20 | 17 | 21 | 19.4 |
| Other | 1 | 0.5 | 1 | 0.5 | 2 | 0.7 | 3 | 0.8 | 6 | 8.9 | 4 | 5.2 | 2 | 0.8 | 1 | 0.3 |
| None exist | 12 | 19.5 | 19 | 21.5 | 32 | 43.2 | 23 | 34.2 | 11 | 19.4 | 17 | 25 | 11 | 17 | 30 | 22.9 |
| Unsure | 4 | 4.6 | 1 | 0.1 | 1 | 1.1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3.7 | 4 | 6.5 |
| Total responsesa | 218 | 191 | 176 | 174 | 169 | 170 | 167 | 157 | ||||||||
| Total participants (n) | 118 | 117 | 118 | 116 | 115 | 114 | 114 | 115 | ||||||||
Wt %, weighted percentage of total responses.
Guidelines used are listed in Supplementary Table S1.
Total greater than 100%, as multiple options could be chosen.
Dialysis initiation
Most respondents reported initiation of dialysis once patients were symptomatic, especially if glomerular filtration rate (GFR) < 15 ml/min; some reported dialysis initiation with a GFR < 10 ml/min even in asymptomatic patients. Respondents from South Africa, Indonesia, China, and Iran specifically reported that conservative care is given to those not eligible for dialysis, who cannot afford it, are too frail for dialysis, or who are older than 70 years of age. No specific guidelines for conservative care were mentioned. One-third of respondents did not report any defined dialysis access (vascular or peritoneal) protocols (Table 5). Representative narratives indicated that access creation decisions often depended on patient’s ability to pay or on whether there was government or private insurer coverage:
Tanzania (R126): “Most of our patients have acute catheter. We use sterile procedures. The catheter is used as long as it’s working.”
South Africa (R10): “In government, HD access is predominantly via tunneled or temporary catheter due to inadequate surgical support. In Private, fistulae are created preferentially. In government, PD catheters are preferentially inserted by nephrologists at the bedside. In Private, the catheters are inserted laparoscopically.”
Nigeria (R48): “Usual practice is to have patients have a fistula in place before initiation of dialysis. However, less than 5% can even afford the cost of fistula creation.”
Bangladesh (R27): “[majority] of the patients underwent fistula or catheter at different parts of the country and come here (near to their residence) for HD.”
Use of clinical protocols: dialysis frequency
Protocols guiding clinical practice of dialysis and transplantation were mostly defined at the institution level (Table 5). Frequency of dialysis was highly variable across countries. For routine HD, 6 free-text respondents reported 3 times a week for 4 hours, 8 reported 2 times a week for 4 to 5 hours, 2 reported 1 to 3 times a week, depending on ability to pay. For routine PD, 7 free-text respondents reported 4 × 2 liter exchanges per day, 2 reported 3 × 2 liters per day. In several countries, frequency of dialysis was largely dependent on institutional or patient resources:
India (R23): “[dialysis frequency is] driven by patient affordability.”
Nigeria (R146): “1 to 3 times per week, depending on patient ability to pay for dialysis; 3 to 4 hours per session.”
Indonesia (R35): “HD twice a week for 4.5 to 5 hours...CAPD 2 liters, 4 exchanges a day. Twice-weekly HD reassessed with KT/V every 3 months. Nutrition evaluation every 3 months.”
Use of clinical protocols: laboratory testing and medication
Frequency of laboratory testing was seldom described by respondents beyond referring to established guidelines, but from the free text responses it appears this is variable and, at times, dependent on cost (Table 6; Supplementary Table S1). Several units reported monitoring hemoglobin 1 to 3 times per month, monitoring calcium, phosphate 3 to 6 times per month and parathyroid hormone, if measured, once per 6 months. Access to medication was also reported to be out-of-pocket in several settings, which is highlighted in the following quotes:
Nigeria (R146): “EPO [erythropoietin] for patients who can afford [it].”
Uganda (R134): “Patients pay for meds: erythropoietin, calcium supplements, phosphate binders, and blood pressure medications. So achieving targets is hampered by affordability.”
India (R23): “regular blood work, iron, erythropoietin, are administered to the patients, also driven by affordability.”
Table 6.
Routine monitoring of dialysis outcomes
| Dialysis outcome | Respondents | Institution | Network/chain | Region/province | National | Not monitored | Total responses |
|---|---|---|---|---|---|---|---|
| Mortality | n | 69 | 9 | 14 | 51 | 10 | 111 |
| Overall % | 62.2 | 8.1 | 12.6 | 46 | 9 | ||
| Weighted % | 67.9 | 3.5 | 4.2 | 37.2 | 16.1 | ||
| Peritonitis | n | 57 | 12 | 11 | 25 | 22 | 100 |
| Overall % | 57 | 12 | 11 | 25 | 22 | ||
| Weighted % | 62.4 | 5.7 | 5 | 19 | 26.1 | ||
| Vascular catheter-related infections | n | 71 | 9 | 9 | 26 | 16 | 110 |
| Overall % | 64.6 | 8.2 | 8.2 | 23.6 | 14.6 | ||
| Weighted % | 63.8 | 1.4 | 0.8 | 11.5 | 27.7 | ||
| Proportion with fistula, graft, or catheter | n | 70 | 11 | 11 | 38 | 16 | 111 |
| Overall % | 63.1 | 9.9 | 9.9 | 34.2 | 14.4 | ||
| Weighted % | 64.6 | 2.3 | 1.5 | 31.4 | 19.8 | ||
| Vascular access dysfunction | n | 69 | 10 | 9 | 19 | 20 | 110 |
| Overall % | 62.7 | 9.1 | 8.2 | 17.3 | 18.2 | ||
| Weighted % | 63.4 | 2.2 | 1.4 | 9.2 | 26.3 | ||
| Hospitalization | n | 69 | 7 | 7 | 26 | 21 | 110 |
| Overall % | 62.7 | 6.4 | 6.4 | 23.6 | 19.1 | ||
| Weighted % | 64 | 1.8 | 1.2 | 22.5 | 24 | ||
| Fluid balance, blood pressure | n | 72 | 6 | 8 | 21 | 18 | 108 |
| Overall % | 66.7 | 5.6 | 7.4 | 19.4 | 16.7 | ||
| Weighted % | 66.7 | 2.6 | 1.3 | 11.9 | 27 | ||
| Disorders of PTH, PO4, bone | n | 70 | 6 | 4 | 21 | 22 | 108 |
| Overall % | 64.8 | 5.6 | 3.7 | 19.4 | 20.4 | ||
| Weighted % | 65.7 | 1.2 | 1.8 | 18.3 | 22.4 | ||
| Anemia | n | 74 | 7 | 6 | 33 | 13 | 110 |
| Overall % | 67.3 | 6.4 | 5.5 | 30 | 11.8 | ||
| Weighted % | 65.9 | 1.3 | 2 | 19.3 | 21.6 | ||
| Dialysis adequacy | n | 72 | 10 | 11 | 30 | 16 | 111 |
| Overall % | 64.9 | 9 | 9.9 | 27 | 14.4 | ||
| Weighted % | 62.8 | 3.8 | 4 | 22.6 | 22.7 |
PO4, phosphate; PTH, parathyroid hormone.
Blood cultures and antibiotics for treatment were used when indicated. In some settings, blood cultures and antibiotics for dialysis-associated infections were paid for out-of-pocket. Viral infection surveillance was reported in all countries. HIV, hepatitis B (HBV), and hepatitis C (HCV) were almost universally tested before initiation of dialysis, although there were exceptions:
Malawi (R60): “By the time they come to us, all the patients would have had an HIV test, but we very rarely have hepatitis B/C test kits.”
Many countries followed national guidelines for surveillance and repeated testing every 3 to 6 months. In most countries, patients with HBV, and at times HCV, were dialyzed on separate machines and in separate areas, although, in some settings, such patients were not accepted for dialysis or referred elsewhere.
Indonesia (R107): “We don’t have a machine for HBsAg + [HBV surface antigen positive] patients, so the patient is always referred to another hospital.”
Tanzania (R126): “We don’t treat patients with HIV, HBV, HCV.”
Use of clinical protocols: transplantation
Among those who commented regarding transplantation facilities, 4 respondents reported that none was available, 3 reported reliance on out-of-pocket payments for transplantation, 5 reported within-country referral for transplantation, and 3 reported sending patients to other countries for transplantation. The costs of transplantation in settings without universal coverage for ESKD care were borne out-of-pocket, which is reinforced by the following statements from responders:
India (R23): “Related, spousal, and deceased donor. Patients have to pay from out-of-pocket.”
Tanzania (R126): “Now the ministry of health does their own transplantation, before patients travelled to India. Sometimes government pays and sometimes patients themselves. Money for drugs after transplant, the patients need to get themselves.”
Uganda (R134): “We're currently in the process of developing policies and laws to govern transplant as a country. Most patients go for transplant in India...affordability for individual patients determines who will get transplanted.”
Domain 4: Monitoring of dialysis outcomes
Clinical dialysis outcomes were monitored variably across countries, and lack of routine monitoring was reported by 20% of respondents across the domains (Table 6). Monitoring of dialysis quality was inconsistently mentioned. Some respondents cited a Kt/V measurement every 3 months as a standard. Some form of institutional patient outcome monitoring occurred in approximately two-thirds of countries (Table 6), and collection of registry data was reported from Indonesia, Malaysia, South Africa, Philippines, Egypt, and India. Reporting data to document outcomes was valued by the responders and used for advocacy and learning, according to the following representative quote:
Uganda (R134): “Monitoring patients and assessing outcomes is mainly a concern of the doctors and nurses within the unit. Reports are given to ministry of health to lobby for more support. The renal team has started monthly meetings for all involved in the care of kidney patients—private and public—so they now share information, discuss outcomes, and borrow working ideas from each other.”
Domain 5: Barriers to care for ESKD
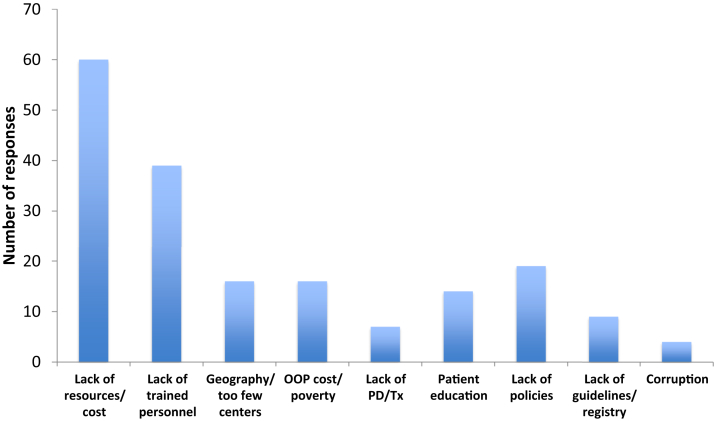
Ninety-six respondents entered free-text descriptions of barriers and limitations to care in their settings, and approximately half of the responses directly mentioned cost as a barrier (Figure 4), as emphasized in the following narrative:
Cambodia (R12): “No money, no life. Life and treatment are only limited by money.”
India (R127): “vast majority of patients with ESRD [end-stage renal disease] are unable to afford dialysis.”
Vietnam (R44): “Recipient must provide the proof of poverty.”
Figure 4.
Most common barriers to end-stage kidney disease care cited by respondents. Frequency of reporting of specific barriers by respondents in free-text entries to the survey. OOP, out-of-pocket; PD, peritoneal dialysis; Tx, transplantation.
With settings in which patients relied on out-of-pocket payments, access to dialysis was driven by ability to pay. With settings in which dialysis is available, access to treatment was limited by insufficient infrastructure. When insurance is in place, access to appropriate therapy may still be limited by restrictions placed by funders or incomplete reimbursement of the medications and laboratory testing required for delivery of quality dialysis:
India (R127): “Government funding is largely limited to supporting hemodialysis.”
South Africa (R19): “Dialysis is being paid by the private funders. Each funder has its own set of rules/goals that has nothing to do with the nephrologist (myself) goals as set by international guidelines.”
Philippines (R22): “HD patients transfer units looking for more affordable HD sessions and become difficult to follow-up; hence outcomes are difficult to report. Monitoring on a regular basis can be costly. Government funding for dialysis does not generally include cost of laboratories for monitoring, much less outcomes.”
Additional narratives considered relevant, but not directly related to the quantitative data analyses, are displayed in Supplementary Table S3.
Additional deliverable from the project: the ISN collection library
A further relevant output of this project was the generation of a library of original documents from different countries (total of 88 documents, several in the local language) ranging from clinical protocols to policies, from adapted guidelines to standard operating procedures, and definitions of minimal quality standards (Supplementary Table S4). We requested authorization to the responders to share the documents in this library, and in cases when authorization was granted, documents could be shared with other countries to provide relevant examples of actions and projects that may be relevant to their local needs.
Discussion
The ISN collection survey aimed to collate current experience of local practice, policies, protocols, and guidelines about the provision of HD and PD in LIC and MIC around the world and to identify common barriers to care. The findings, presented in a mixed (quantitative and qualitative)-methods research approach highlighted here are not new or surprising but serve to reinforce the urgent need for better planning and implementation of dialysis services, especially where resources are limited (summarized in Figure 3).
The low response rate is a common challenge in global surveys, particularly in low-resource settings, and represents an important limitation of the current study. However, the large number of responses and time taken to upload documents and enter free-text commentary is testament to the high engagement of nephrology professionals globally and their desire to advocate for patient needs through better policy development, more sustainable financing strategies, and better quality of care. Most responses to the survey reflected experience in adult dialysis care, and a large knowledge gap remains in terms of pediatric ESKD care in low-resource settings, where it is known that children have far less access to care and specialist availability is more scarce.8
Even though ESKD care, including dialysis and transplantation, is available in vast majority of LMIC according to the Global Kidney Health Atlas (GKHA),9 important barriers in access to ESKD care were identified in this survey. However, there is a striking disparity between availability and access to dialysis treatment within and among countries, which points to important barriers to the implementation of kidney replacement therapy, particularly in low-resource areas. Overall cost to the patient or the health system was the most cited barrier to access to ESKD care and to the provision of quality care. These data confirm findings of the GKHA, in which the public funding structures for all forms of kidney replacement therapy (hemodialysis, peritoneal dialysis, and kidney transplantation) were less prevalent in LMIC. Importantly, out-of-pocket expenses related to ESKD were more common in low-resource areas in contrast to higher-income countries, where public or private insurance funding was more frequent.9 Lack of infrastructure, staff, and training were also important frequently cited barriers. Because of cost, staffing, and infrastructure constraints, dialysis quality may be limited by reduction in weekly delivered dose of dialysis, unaffordability of concomitant medications required for optimal health of dialysis patients (e.g., erythropoietin, phosphate binders, antibiotics), limited use or availability of routine laboratory testing to monitor dialysis quality and complications, and unaffordability or lack of expertise for optimal vascular access.
Many centers rely on local institutional protocols or practice or adapt local guidelines from elsewhere. Despite the multiple barriers that may hinder equitable access to dialysis, most centers did not report using formal dialysis eligibility criteria. Lack of such criteria may be an important source of moral distress for nephrologists and nurses, as life and death decisions (dialysis: yes or no) devolve to the individual bedside instead of the responsibility being shared by policy makers and society.10 A major limitation to the survey was its voluntary nature. We cannot exclude uncertainty about systematic bias among those who responded (e.g., those more prone to advocacy or those struggling the most), and therefore the true representativeness of the findings is not known. However, the relatively consistent description of the challenges across diverse settings serves to support the essential validity of the findings. Some inconsistencies suggest that further in-depth contextual inquiry is required. For example, at face value it may appear that there is discordance between the relative lack of limitations to ESKD care cited in Table 4 (no limitations in around 60% of responses) and the large number of limitations cited in the free-text responses (96 of 120 respondents). It is possible that the formal limitation responses focused more on clinical limitations and limitations in patients who do have funding (government coverage, private insurance, or ability to pay), whereas the free-text responses describe the more general barriers experienced. Such discrepancies are not possible to resolve through this survey; however, they suggest that further qualitative study to investigate the drivers of inequity in access to dialysis care is required.
The ISN is well placed to lead a comprehensive program of support for providers of ESKD care worldwide. Such a program should include local capacity building; knowledge sharing and training; advocacy for sustainable planning of dialysis programs; advocacy for fair pricing of dialysis supplies and medication; engagement with policy makers, patients, and civil society; and fostering research on prevention and treatment of kidney disease as well as patient and provider experiences from diverse settings.
Informed by the results of this survey, and in response to needs expressed by multiple lower-resource countries and the World Health Organization (WHO), the ISN aims to develop a set of guidance documents that put forward a considered approach to provision of dialysis and ESKD care within resource limitations. This will begin with discussion of the importance of priority setting and realistic estimation of need; costs and opportunity costs to maximize sustainability of dialysis; frameworks to consider in determining patient access to dialysis; outlines of—and minimum acceptable standards for—safe and effective dialysis provision, as well as supportive and palliative care. Important allied considerations will be the prevention; early diagnosis; and treatment of CKD, which are proven to be cost effective overall;11 support for progress toward living and deceased donor kidney transplantation, which are cost effective relative to dialysis; and ensuring that holistic approaches to palliative and supportive care are in place when dialysis may not be possible.
Disclosure
Publication of this article was supported by the International Society of Nephrology.
BS reports travel support from Roche. DCHH reports grant support from the National Health and Medical Research Council. RP-F reports grant support from Fresenius Medical Care. The other author declared no competing interests.
Acknowledgments
The authors thank the International Society of Nephrology’s staff members, Luisa Strani and Kelly Hendricks, and Rebecca Mandell from Arbor Research Collaborative for Health for her valuable contributions to the qualitative approach.
The following individuals contributed to the survey (some names are not listed as they were not clearly stated, or the individuals declined to be named): Bangladesh: Islam M, Nessa A, Rahman M, and Rashid HU; Bolivia: Claure-Del Granado R; Cambodia: Hyod T; China: Liu L; Colombia: Gomez R; Congo Brazzaville: Gandzali-Ngabe PE; Democratic Republic of the Congo: Sumaili Kiswaya E; Ethiopia: Melkie Ejigu A and Tadesse Mengistu Y; Georgia: Tchokhonelidze I; India: Abraham G, Effendi I, Parameswaran S, and Rai PK; Indonesia: Alatas H, Armelia L, Candra JM, Fitri I, Gede S, Gofur A, Harjadi E, Harsoyo S, Irianto A, Iryaningrum MR, Kurniawan E, Loekman JS, Lubis AR, Maman A, Naya R, Prasanto H, Situmorang CA, Surachno R, Widiana IGR, and Zakaria R; Islamic Republic of Iran: Argani H, Hashemi SH, and Ossareh S; Jordan: Ghnaimat M; Lebanon: Najem R; Malawi: Dreyer G; Malaysia: Bavanandan S and Seong HL; Mexico: Correa-Rotter R and Obrador GT; Nigeria: Effa E, Eke F, and Liman H; Philippines: Dimacali CT; Senegal: Niang A; South Africa: Bapoo N, Bhimma R, Cullis B, Davids R, Freercks R, Gerntholtz T, Meyers A, Moosa MR, Nourse P, Schubert C, Stead P, Stratling A, and Swanepoel C; Syrian Arab Republic: Saeed B; Tanzania: Nygård HT; Turkey: Arici M and Kazancıoğlu RT; Uganda: Atuhe DM, Batte A, Kalyesubula R, Makanga G, Mirembe BD, and Muhindo R; Venezuela: Domínguez M and Marquez V; and Vietnam: An HPH.
Footnotes
The views expressed in this commentary are solely the responsibility of the authors and they do not necessarily reflect the views, decisions, or policies of the institutions with which they are affiliated.
Table S1. Source of guidance from free text responses.
Table S2. Examples of staffing ratios.
Table S3. Multiple additional factors and notes on how to overcome the limitation in the provision of dialysis identified in the free text.
Table S4. Uploaded documents and countries.
Supplementary Material
References
- 1.Liyanage T., Ninomiya T., Jha V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 2.Ashuntantang G., Osafo C., Olowu W.A. Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2017;5:e408–e417. doi: 10.1016/S2214-109X(17)30057-8. [DOI] [PubMed] [Google Scholar]
- 3.Kaur G., Prinja S., Ramachandran R. Cost of hemodialysis in a public sector tertiary hospital of India. Clin Kidney J. 2018;11:726–733. doi: 10.1093/ckj/sfx152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazhar F., Nizam N., Fatima N. Problems Associated with access to renal replacement therapy: experience of the Sindh Institute of Urology and Transplantation. Exp Clin Transplant. 2017;15(suppl 1):46–49. doi: 10.6002/ect.mesot2016.O27. [DOI] [PubMed] [Google Scholar]
- 5.Wetmore J.B., Collins A.J. Meeting the world's need for maintenance dialysis. J Am Soc Nephrol. 2015;26:2601–2603. doi: 10.1681/ASN.2015060660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The World Bank . The World Bank Group; 2018. World Bank Country and Lending Groups.https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Available at: [Google Scholar]
- 7.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap):a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niang A., Iyengar A., Luyckx V.A. Hemodialysis versus peritoneal dialysis in resource-limited settings. Curr Opin Nephrol Hypertens. 2018;27:463–471. doi: 10.1097/MNH.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 9.Bello A.K., Levin A., Tonelli M. Global kidney health atlas: a report by the International Society of Nephrology on the current state of organization and structures for kidney care across the globe. 2017. https://www.theisn.org/images/ISN_Biennial_Report_2011-2013/GKHAtlas_Linked_Compressed1.pdf Available at.
- 10.Luyckx V.A., Miljeteig I., Ejigu A.M. Ethical challenges in the provision of dialysis in resource-constrained environments. Semin Nephrol. 2017;37:273–286. doi: 10.1016/j.semnephrol.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Vanholder R., Annemans L., Brown E. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol. 2017;13:393–409. doi: 10.1038/nrneph.2017.63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.