Abstract
Ever since British Physician William Withering first described the use of foxglove extract for treatment of patients with congestive heart failure in 1785, cardiotonic steroids have been used clinically to treat heart failure and more recently atrial fibrillation. Due to their ability to bind and inhibit the ubiquitous transport enzyme sodium potassium pump, thus regulating intracellular Na+ concentration in every living cell, they are also an essential tool for research into the sodium potassium pump structure and function. Exogenous CTS have been clearly demonstrated to affect cardiovascular system through modulation of vagal tone, cardiac contraction (via ionic changes) and altered natriuresis. Reports of a number of endogenous CTS, since the 1980s, have intensified research into their physiologic and pathophysiologic roles and opened up novel therapeutic targets. Substantive evidence pointing to the role of endogenous ouabain and marinobufagenin, the two most prominent CTS, in development of cardiovascular disease has accumulated. Nevertheless, their presence, structure, biosynthesis pathways and even mechanism of action remain unclear or controversial. In this review the current state-of-the-art, the controversies and the remaining questions surrounding the role of endogenous cardiotonic steroids in health and disease are discussed.
Abbreviations: CTS, cardiotonic steroids; HF, heart failure; AF, atrial fibrillation CKD, chronic kidney disease; ESRD, end stage renal disease; NKA, sodium potassium pump; PKC, protein kinase C; ERK, mitogen-activated protein kinase; SR, sarcoplasmic reticulum; NCX, sodium calcium exchanger; ROS, reactive oxygen species; NCX, Na/Ca exchanger
Keywords: Cardiotonic steroids, Ouabain, Marinobufagenin, Digoxin, Heart failure, Atrial fibrillation, Sodium potassium pump, NKA
1. Introduction
In 1785 William Withering observed that digitalis glycoside beyond its diuretic effects also "has a power over the motion of the heart with a degree yet unobserved in any other medicine" [1]. In subsequent years, it was recognised that digitalis could also stabilise a rapid and irregular heart-beat [2] and some hundred years later, Fothergill reported that digitalis also enhanced ventricular contraction [3]. In the 1980s Hamlyn and colleagues reported the presence of endogenous sodium potassium pump inhibitors, termed cardiotonic steroids (CTS), in patients with essential hypertension [4] and healthy controls [[5], [6], [7]]. Despite almost four decades of intense research into the role of endogenous CTS in physiology and disease unanswered questions, heightened discussion and disagreement dominate the research field. Even the mere existence of endogenous, circulating CTS is questioned! Further disagreements and questions relate to their structure, biological target, interaction with digoxin, mechanism of action and function in health and disease. Digoxin, an archetypal CTS, is still used for treatment of patients with atrial fibrillation and heart failure. Research into the role of endogenous CTS continues and the clinical use of digoxin, while reduced, is unlikely to come to a halt. Aim of this review is to highlight the current state-of-the-art of the endogenous CTS in health and disease and discuss some of the key questions that remain to be addressed.
2. Sodium potassium pump is the primary target for CTS
Sodium potassium pump (NKA) is ubiquitous and as such plays a key role in many physiological processes in the body. NKA uses the free energy of hydrolysis of ATP to exchange three intracellular Na+ ions for two extracellular K+ ions, thus setting the electrochemical gradient for both Na+ and K+ across the cell membrane. NKA is therefore vital for maintaining the resting potential and Na+ and K+ gradients in almost every eukaryotic cell. These gradients ensure basic cellular homeostasis such as regulation of cell volume, essential ionic, glucose and amino acid transport processes. In excitable cells NKA activity restores the Na+ and K+ gradients following depolarisation and in the kidney its activity provides the driving force for Na+ reabsorption essential to control extracellular volume and blood pressure. Among the many Na+-dependent transmembrane transport processes in muscle cells, the activity of the NKA drives Na+/Ca2+ exchanger (NCX) and thus regulates the concentration of Ca2+ in both the cytosol and the sarcoplasmic reticulum (SR). Furthermore, recent evidence points towards a crucial role for the NKA in neurological function [8,9].
The work of Skou [10] and others on the NKA structure and function over many years identified that CTS bind to the conserved binding site and thereby inhibit NKA activity. Commonly referred to as the “ouabain binding site”, CTS binding site resides within the catalytic domain of the NKA α subunit [11], see Fig. 1. It is highly conserved across the species, suggesting an important physiological function. Mutations of the ouabain binding site, leading to CTS desensitisation, have been identified in monarch butterflies and leaf beatles that feed on plants rich in digitalis [12,13]. Similar mutations are also observed in toads, presumably developed as adaptational protection to high concentrations of CTS in their skin [14]. Remarkably, mice and rats also contain a CTS insensitive α-1 isoform [15,16]. It is unclear as to the benefits of this adaptational mutation and further studies are warranted and could shed light on the physiological and pathophysiological roles of CTS.
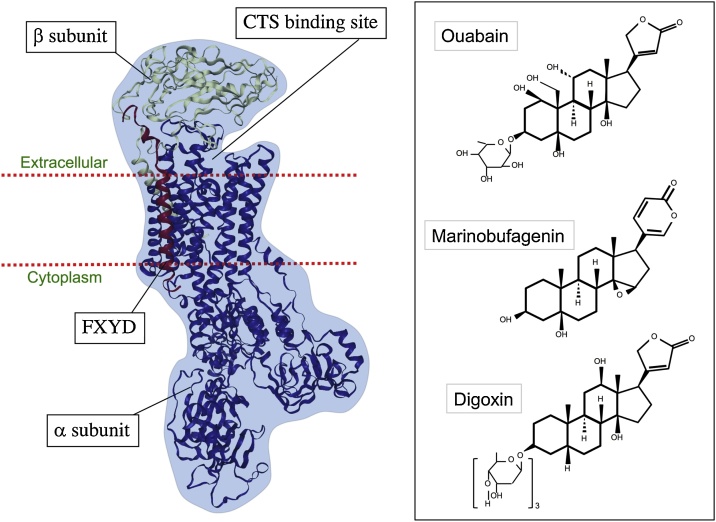
Fig. 1.
Structure of the NKA and the chemical structures of its commonly described ligands ouabain, marinobufagenin and digoxin. NKA is made up of the α and β subunits and the accessory FXYD protein. Conserved CTS binding site is found within the α subunit. Three commonly studied CTS are shown on the right. Ouabain and marinobufagenin have been detected in circulation in patients and healthy controls. Ouabain and digoxin have a five-membered lactone ring, whereas marinobufagenin contains a six-membered lactone ring.
NKA is composed of a catalytic α subunit, regulatory β subunit and an accessory FXYD protein [17,18]. β subunit stabilises the NKA and helps the complex traffic through the secretory pathway to the plasma membrane [19,20]. In the heart, FXYD protein named phospholemman modulates NKA activity in response to kinase/phosphatase mediated stimuli [18,[21], [22], [23], [24]]. NKA functional unit is made up of α and β macromolecular complex with four α (α1, α2, α3 and α4), three β isoforms (β1, β2 and β3) [25,26] and a FXYD protein, allowing a formation of a number of different NKA isozymes with distinct transport and pharmacological properties [27]. Whether multiple isozyme combinations exist within the same heart, whether they demarcate distinct chambers or even distinct topologies within a single cardiomyocyte, is not known and warrants investigation. It has been clearly demonstrated that CTS differentially affect alpha subunits, yet little information exists as to the biological relevance of these (see Pavlovic review [28]). Preliminary work shows that in the heart and kidneys α1 is the dominant isoform regulating bulk Na+ whereas α2 plays a more prominent role in smooth muscle contraction and has been shown to significantly contribute to regulation of T-tubular Na+ and thus modulation of cardiac contraction, via its indirect effects on the Na+/Ca2+ exchanger (NCX) [18,29]. Digoxin and ouabain have all been shown to reduce heart rate [30], and this is likely to be the primary mechanism for beneficial effects observed in patients with atrial fibrillation and heart failure. When compared to ivabradine, another rate lowering drug, digoxin performed as well in risk reduction for worsening heart failure [31]. NKA activity has been demonstrated to play an important role in the brain. Mutations in the brain α1 and α3 NKA isoforms affecting the NKA activity have been identified and linked with the neuronal defects [8,9]. Whether CTS-mediated regulation of the brain NKA plays a significant functional role in neuronal control remains to be determined.
Relevant questions:
What is the relevance of different NKA isoforms in different tissues?
Are the NKA isoforms differentially expressed in different chambers of the heart?
Why is the CTS binding site not conserved in mice and rats?
What evolutionary advantage does desensitisation of the CTS binding site confer to mice and rats?
3. Structure and biosynthesis of the endogenous CTS
Whilst the existence of an endogenous, circulating, natriuretic factor, targeting the NKA may have been suggested as early as 1960s, Hamlyn and colleagues were the first to demonstrate and identify the presence of a ouabain-like compound in human plasma [6,7]. Initial mass spectrometry studies showed the compound to be similar to ouabain, with adrenals showing 500-fold higher concentrations, possibly indicating the origins [5]. Development of an immunoassay for detection of endogenous ouabain [32] allowed multiple studies in serum of experimental animals and humans. Subnanomolar concentrations of ouabain were detected in the blood of patients with essential hypertension [[33], [34], [35]], heart failure [36,37], acute kidney injury [[38], [39], [40]], and end stage renal failure [41]. Some evidence exists for the presence of digoxin in human urine [42] and of deglycosylated congeners of digoxin in mammals [43,44]. Marinobufagenin was isolated and identified in urine of patients with myocardial infarction [45] and serum of patients with terminal renal failure [46]. Structurally related telocinobufagin, the reduced form of marinobufagenin, was identified as a constituent of human serum in patients with terminal renal failure [46], at higher concentration than that of marinobufagenin and ouabain. It is likely that other bufadienolides may also be present in circulation. Indeed there are reports of proscillaridin A- [47] and bufalin-like [48] immunoreactive substances detected in human serum. Some of the studies have relied on the use of antibody-based ELISA methods for detection of the endogenous CTS whereas others have used high-resolution mass spectrometry and NMR, though often without detailed validation. Baecher et al. have recently developed a validated ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) method for detection and quantification of ouabain, with a lower detection limit of 1.7 pmol/L [49]. Controversially, they found no ouabain in the plasma of 5 control and 25 heart failure patients and thus concluded that endogenous ouabain detected previously is an artifact. Disqualifying 30 years of work with a single study in 30 patients, though well executed, seems premature. Unsurprisingly, this precipitated a flurry of robust exchanges between the interested parties [[50], [51], [52]] with strong views being expressed on both sides. In their review [53], Hamlyn and Blaustein rightly argue for possible presence of several isomers of ouabain, and considering detection of several structurally related CTS, e.g. marinobufagenin, telocinobufagin, deglycosylatyed digoxin congeners, it is possible that several CTS species exist in circulation. No attempts have been made thus far to develop validated LC-MS/MS methods for detection and quantification of multiple CTS in plasma or serum. With the recognition that steroids do not exist nor function in isolation of others, development of methods for detection of CTS panel is necessary in order to move the field forward.
Biosynthesis pathways for the CTS are unclear, though existing evidence suggests that CTS are synthesised from cholesterol in the adrenal glands [54,55] and possibly hypothalamus [56]. Indeed, missense polymorphism (rs2254524 V642 L) in lanosterol synthase, an enzyme involved in cholesterol biosynthesis, were shown to affect ouabain biosynthesis [57]. Can this pathway be targeted pharmacologically requires investigation. The trigger for biosynthesis initiation seems complex, with serum concentrations of ouabain and marinobufagenin increasing in response to volume expansion [58], adrenocorticotropic hormone, angiotensin II, vasopressin, and phenylephrine [54,59], adding another layer of complexity.
Relevant questions:
Which CTS are present in circulation, if any?
What is the effect of multiple different CTS on cardiac, smooth muscle, neuronal and kidney function? Do they act synergistically or is there antagonism?
How do endogenous CTS get synthesised and can these biosynthetic pathways be targeted?
4. CTS mechanism of action and interaction with digoxin
Textbook model describes CTS-mediated inhibition of the cardiac NKA, thereby, raising intracellular Na+ and Ca2+ (via NCX), see Fig. 2. The effects of acutely increased Ca2+ on contractility [60] are well described and this is postulated to be the inotropic mechanism providing improved cardiac output in heart failure patients. Digoxin, and digitoxin also reduce heart rate, presumably by parasympathomimetic effects, and as such are used for rate control in patients with atrial fibrillation. Of course, it may be that bradycardic effects are the primary mechanism for improved morbidity in patients with heart failure. It is unclear, whether the effects on heart rate are mediated directly on the vagus, sino-atrial node, on cardiac conduction, or possibly on all of them. Levels required to achieve these effects in patients are around 1 nM. Considering that circulating concentrations of CTS are in the range of 0.05-0.7 nM [4,5,33,45,46,[61], [62], [63], [64], [65], [66], [67], [68]], it is questioned whether inotropic and bradycardic effects are likely to be observed. Alternative theory that does not involve modulation of intracellular Na+ and Ca2+ concentrations was presented [69]. This hypothesis proposes that the NKA acts as a receptor for the CTS and thus plays a signaling role [70], regulating early response genes associated with cell growth [71]. In this model, Lie and Xie suggest that a fraction of inactive NKA subunits are localised in the caveolae and are physically associated with other key signaling proteins such as EGFR and Src [72,73] see Fig. 2. Binding of the CTS leads to activation of hypertrophic and fibrotic signaling cascades via NKA-Src-ERK [71]. Evidence also exists for the involvement of other signaling molecules, phospholipase C, TRP proteins, PI(3)K, and PKC [69,[73], [74], [75], [76], [77]] and that the CTS induce the endocytosis of the CTS-NKA-Src-EGFR complex [[74], [75], [76]]. There is disagreement whether the activation of signaling cascades via CTS indeed occurs independently of NKA inhibition and the accompanying increases in intracellular Na+ and Ca2+ [78,79]. There is certainly substantive evidence that acute CTS inotropic effects and activation of NKA-Src-ERK signaling cascades depend on the presence of functional NCX and intracellular sodium and calcium increases [60,80,81].
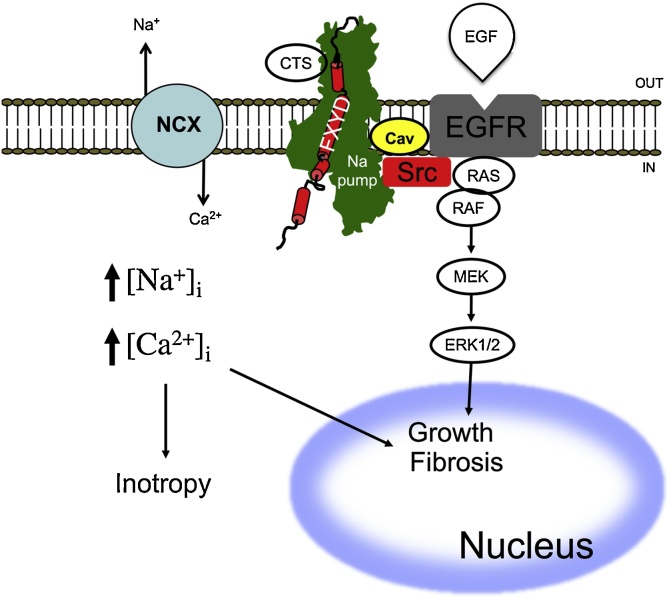
Fig. 2.
Schematic diagram of ionic versus signaling pathways for cardiotonic steroid effects. In the classic pathway (shown on the left), CTS binds and inhibits the NKA, which in turn is accompanied by changes in cytosolic [Na+]. The increase in cytosolic [Na+] then reduces NCX activity and induces an increase in cytosolic [Ca2+]. High cytosolic [Ca2+] mediates muscle contraction (inotropy) or activates a variety of signaling pathways. It is unclear whether the effects of CTS on heart rate (bradycardia) are also mediated via the ionic pathway. The signaling pathway (shown on the right hand side) occurs in the caveolar domain and involves the physical interaction with Src. When the CTS binds the NKA, SRC gets activated inducing further activation of mitogen-activated protein kinase (ERK) through activation of its mitogen-activated protein kinase kinase (MEK).
Presence of endogenous CTS raises an important therapeutic query, if endogenous CTS exist in patients treated with digoxin or digitoxin, how do these affect outcomes? It is possible that administration of digoxin to patients with already raised endogenous CTS can be detrimental. Link between mortality and elevated serum levels of CTS in heart failure patients has been clearly established. Heart failure patients treated with therapeutic doses of digoxin, where serum levels of digoxin are >1.2 ng/ml (as measured by ELISA with poor specificity), are associated with 11.8 % increase in mortality [82]. Thus, differing levels of endogenous cardiotonic steroids may partly explain the varied success of digoxin in this patient population and also the susceptibility to adverse drug reactions and overt toxicity. Surprisingly, experiments in rat arteries suggest potential antagonism of ouabain and digoxin [83], and digoxin was shown to oppose ouabain-induced hypertension [84]. This may explain the beneficial effects of digoxin therapy in heart failure patients [85], however, whether antagonism between digoxin and ouabain, and other CTS exists in the heart and what the therapeutic implications of these findings are, remains to be investigated.
Relevant questions:
Are the clinical benefits of CTS in heart failure patients primarily mediated via heart rate reduction?
Do endogenous CTS levels affect response to digoxin therapy in patients with heart failure and atrial fibrillation?
Does the antagonism between digoxin and endogenous CTS exist in the heart?
5. Endogenous CTS in physiology and pathophysiology
CTS were shown to be involved in cardiac contraction, heart rate modulation, blood pressure regulation and natriuresis. Chronic exposure to CTS can lead to remodelling of the heart, kidneys and arterial walls but also cardiac fibrosis and arrhythmias (Fig. 3). Their secretion seems to be driven by kidney dysfunction and sodium and volume status and is likely involved in a number of diseases from uremic cardiomyopathy, preeclampsia, hypertension, congestive heart failure, myocardial ischemia-induced arrhythmias and diabetes mellitus.
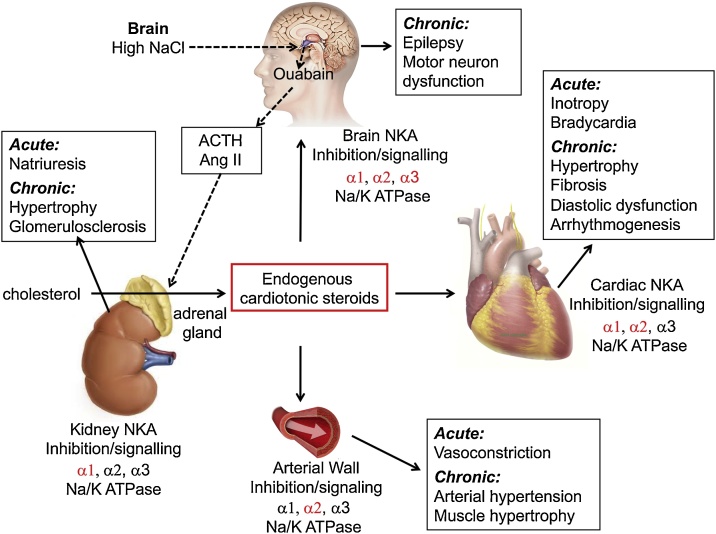
Fig. 3.
Graphical representation of the proposed biosynthesis and function of endogenous cardiotonic steroids. Synthesis of endogenous cardiotonic steroids is thought to occur in the adrenal cortex from cholesterol. Acute effects of endogenous cardiotonic steroids are mediated via inhibition of the NKA. Isoform specific effect in each tissue is shown in red. Acute and chronic effects of elevated cardiotonic steroids levels on the specific organs are shown in black boxes. Adapted from Schoner and Scheiner-Bobis [107].
Regulation of sodium content and thus blood volume is proposed as the primary physiological role of endogenous CTS. The basis of this natriuretic control is thought to be dependent on the direct inhibition of the α1 NKA isoform in renal tubular cells [86]. The most convincing evidence in support of this are the studies by Periyasami and colleagues where marinobufagenin antibodies given to salt loaded rats lowered urinary excretion and increased NKA activity [87]. Given, high similarity in chemical structures between different CTS, antibodies raised against individual CTS tend to have poor specificity [88] and thus marinobufagenin may not necessarily be the primary modulator. Indeed, increases in both endogenous ouabain and marinobufagenin are observed in states of volume expansion and volume expansion mediated hypertensive syndromes that are related to water and salt accumulation [62,86,89]. Beyond the expected inhibition of the NKA, administration of ouabain or marinobufagenin also resulted in translocation of the NKA to intracellular compartments via clathrin-dependent endocytosis [76]. This translocation required PI(3)K activation, the plasmalemmal pump to be in the context of caveola, and signaling through the Src-EGFR pathway [75]. It is therefore suggested that salt loading leads to increases in ouabain and marinobufagenin in the proximal tubules, both of which act to decrease Na+ through NKA inhibition and/or activation of a signaling mechanism mediated by NKA-Src-EGFR cascades. This decrease in renal Na+ reabsorption is expected to lead to an increase in urinary Na+ excretion. In studies of healthy individuals, salt loading for up to 6 days lead to a transient elevation in plasma ouabain and sustained increase in marinobufagenin, followed by an increase in urine Na+ excretion [58,90]. CTS are also implicated in control of blood pressure and infusion of ouabain or marinobufagenin, at concentrations comparable with in vivo plasma levels, lead to an increase in blood pressure [66,[91], [92], [93]]. Indeed, increased levels of endogenous ouabain were detected in patients with essential hypertension [33,34] and preeclampsia [[94], [95], [96]]. Hypertension induced by salt loading, ouabain infusion or preeclampsia was reduced by immune-neutralisation with anti-CTS antibodies [97,98] or CTS antagonists rostafuroxin [99] and resibufogenin [100]. It should be noted however that rostafuroxin did not reduce blood pressure in hypertensive patients enrolled in Ouabain and Adducin for Specific Intervention on Sodium in Hypertension (OASIS-HT) phase-2 Trial [101]. Whilst the evidence for the role of endogenous CTS in natriuresis and blood pressure homeostasis is mounting it is unclear which endogenous CTS are involved and whether the acute and chronic responses are differentially modulated.
Relevant questions:
Do endogenous CTS directly modulate natriuresis and blood pressure or are they simply by products of these homeostatic mechanisms?
Are there differences in CTS biosynthesis between the acute and chronic responses to salt loading?
Which CTS are involved in physiological volume and blood pressure control?
Endogenous CTS levels were also shown to be increased in animal models and patients with acute kidney injury [40], chronic kidney disease [66], end stage renal disease [41,46], myocardial infarction [102] and heart failure [36,103,104]. Most of the studies demonstrate increases in ouabain and marinobufagenin from circa 0.3 nM in healthy individuals to circa 0.7–1.0 nM in patients. The evidence in favor of the hypothesis that endogenous CTS drive the disease progression rather than simply act as biomarkers of pathology is growing. In healthy adult rats administration of marinobufagenin for 4 weeks led to increases in blood pressure, diastolic dysfunction and ventricular hypertrophy [66,105]. Remarkably, active and passive immunisation against marinobufagenin reversed most of these alterations [66,105]. Independent studies by Ferrandi et al. showed that infusion of 0.7 nM of ouabain for 18 weeks in healthy adult rats induced hypertension and left ventricular hypertrophy and could be reversed by ouabain antagonist PST2238 [92]. These studies demonstrate that chronic exposure to pathophysiological concentrations in healthy animals can lead to some of the cardiovascular changes observed in patients, however, utilisation of animal models with a preserved NKA sensitivity to CTS is required to provide further mechanistic insights. Whether immunisation for CTS or intravenous administration of antibodies raised against CTS (e.g. in patients on renal replacement therapy) is beneficial to these patient populations is an easily testable and attractive proposition.
Relevant questions:
Which CTS are synthesised in patients with renal and heart failure?
Is the cardiovascular disease progression mediated by chronic elevation of CTS?
Does quenching of endogenous CTS by immunisation or administration of antibodies against CTS represent a viable therapeutic option for patients with chronically high CTS levels?
6. Conclusion
Administration of CTS was conclusively shown to modulate contraction, blood pressure regulation, natriuresis and chronically can contribute to remodelling of the heart, kidneys and arterial walls. Although endogenous CTS have been detected in blood of patients with kidney and cardiovascular dysfunction as early as 1981, the chemical identities of CTS and mechanistic insight into the cardiovascular physiology and pathogenesis of these molecules remains unclear. Antibody-based assays cannot distinguish different CTS due to their high structural similarity. Furthermore there is a clear need for human studies as murine NKA shows a 1000 fold reduced sensitivity to CTS [106]. A clear barrier to further expansion of knowledge is our lack of tools to accurately detect and measure the CTS present in circulation and thus identify patients with a positive CTS status. Understanding how these endogenous molecules interact with digoxin is also important if we are to come up with novel stratified therapies, better tailored for treatment of patients with heart failure and atrial fibrillation. These studies must be carried out in either human tissue or animal models with preserved NKA sensitivity to CTS, rather than mice and rats, where NKA sensitivity is reduced.
Sources of funding
DP is supported by the British Heart Foundation (PG/17/55/33087, RG/17/15/33106, FS/19/16/34169, FS/19/12/34204) and Wellcome Trust (109604/Z/15/Z).
CRediT authorship contribution statement
Davor Pavlovic: Conceptualization, Formal analysis, Funding acquisition, Investigation, Resources, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
None.
References
- 1.Withering W. 1785. An Account of the Foxglove, and Some of Its Medical Uses: With Practical Remarks on Dropsy and Other Diseases. Birmingham, England: M. Swinney. [Google Scholar]
- 2.B.J . 1835. Traite Clinique Des Maladies Du Cour. Paris. [Google Scholar]
- 3.F.J.M . 1871. Digitalis: Its Mode of Action. London. [Google Scholar]
- 4.Hamlyn J.M., Ringel R., Schaeffer J., Levinson P.D., Hamilton B.P., Kowarski A.A., Blaustein M.P. A circulating inhibitor of (Na+ + K+)ATPase associated with essential hypertension. Nature. 1982;300:650–652. doi: 10.1038/300650a0. [DOI] [PubMed] [Google Scholar]
- 5.Hamlyn J.M., Blaustein M.P., Bova S., DuCharme D.W., Harris D.W., Mandel F., Mathews W.R., Ludens J.H. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A. 1991;88:6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamlyn J.M., Harris D.W., Clark M.A., Rogowski A.C., White R.J., Ludens J.H. Isolation and characterization of a sodium pump inhibitor from human plasma. Hypertension. 1989;13:681–689. doi: 10.1161/01.hyp.13.6.681. [DOI] [PubMed] [Google Scholar]
- 7.Hamlyn J.M., Harris D.W., Ludens J.H. Digitalis-like activity in human plasma. Purification, affinity, and mechanism. J. Biol. Chem. 1989;264:7395–7404. [PubMed] [Google Scholar]
- 8.Clapcote S.J., Duffy S., Xie G., Kirshenbaum G., Bechard A.R., Rodacker Schack V., Petersen J., Sinai L., Saab B.J., Lerch J.P., Minassian B.A., Ackerley C.A., Sled J.G., Cortez M.A., Henderson J.T., Vilsen B., Roder J.C. Mutation I810N in the alpha3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proc Natl Acad Sci U S A. 2009;106:14085–14090. doi: 10.1073/pnas.0904817106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlingmann K.P., Bandulik S., Mammen C., Tarailo-Graovac M., Holm R., Baumann M., Konig J., Lee J.J.Y., Drogemoller B., Imminger K., Beck B.B., Altmuller J., Thiele H., Waldegger S., Van’t Hoff W., Kleta R., Warth R., van Karnebeek C.D.M., Vilsen B., Bockenhauer D., Konrad M. Germline De Novo Mutations in ATP1A1 Cause Renal Hypomagnesemia, Refractory Seizures, and Intellectual Disability. Am. J. Hum. Genet. 2018;103:808–816. doi: 10.1016/j.ajhg.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skou J.C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta. 1957;23:394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa H., Shinoda T., Cornelius F., Toyoshima C. Crystal structure of the sodium-potassium pump (Na+,K+-ATPase) with bound potassium and ouabain. Proc Natl Acad Sci U S A. 2009;106:13742–13747. doi: 10.1073/pnas.0907054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holzinger F., Frick C., Wink M. Molecular basis for the insensitivity of the Monarch (Danaus plexippus) to cardiac glycosides. FEBS Lett. 1992;314:477–480. doi: 10.1016/0014-5793(92)81530-y. [DOI] [PubMed] [Google Scholar]
- 13.Labeyrie E., Dobler S. Molecular adaptation of Chrysochus leaf beetles to toxic compounds in their food plants. Mol. Biol. Evol. 2004;21:218–221. doi: 10.1093/molbev/msg240. [DOI] [PubMed] [Google Scholar]
- 14.Morris J.F., Ismail-Beigi F., Butler V.P., Jr., Gati I., Lichtstein D. Ouabain-sensitive Na+,K(+)-ATPase activity in toad brain. Comp. Biochem. Physiol. A Physiol. 1997;118:599–606. doi: 10.1016/s0300-9629(96)00465-3. [DOI] [PubMed] [Google Scholar]
- 15.Akera T., Larsen F.S., Brody T.M. The effect of ouabain on sodium- and potassium-activated adenosine triphosphatase from the hearts of several mammalian species. J. Pharmacol. Exp. Ther. 1969;170:17–26. [PubMed] [Google Scholar]
- 16.Berry R.G., Despa S., Fuller W., Bers D.M., Shattock M.J. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase alpha1 and alpha2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc. Res. 2007;73:92–100. doi: 10.1016/j.cardiores.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Sweadner K.J., Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics. 2000;68:41–56. doi: 10.1006/geno.2000.6274. [DOI] [PubMed] [Google Scholar]
- 18.Pavlovic D., Fuller W., Shattock M.J. Novel regulation of cardiac Na pump via phospholemman. J. Mol. Cell. Cardiol. 2013;61:83–93. doi: 10.1016/j.yjmcc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Geering K. The functional role of the beta-subunit in the maturation and intracellular transport of Na,K-ATPase. FEBS Lett. 1991;285:189–193. doi: 10.1016/0014-5793(91)80801-9. [DOI] [PubMed] [Google Scholar]
- 20.Horisberger J.D., Jaunin P., Good P.J., Rossier B.C., Geering K. Coexpression of alpha 1 with putative beta 3 subunits results in functional Na+/K+ pumps in Xenopus oocytes. Proc. Natl. Acad. Sci. U.S.A. 1991;88:8397–8400. doi: 10.1073/pnas.88.19.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastug-Ozel Z., Wright P.T., Kraft A.E., Pavlovic D., Howie J., Froese A., Fuller W., Gorelik J., Shattock M.J., Nikolaev V.O. Heart failure leads to altered beta2-adrenoceptor/cyclic adenosine monophosphate dynamics in the sarcolemmal phospholemman/Na,K ATPase microdomain. Cardiovasc. Res. 2019;115:546–555. doi: 10.1093/cvr/cvy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boguslavskyi A., Pavlovic D., Aughton K., Clark J.E., Howie J., Fuller W., Shattock M.J. Cardiac hypertrophy in mice expressing unphosphorylatable phospholemman. Cardiovasc. Res. 2014;104:72–82. doi: 10.1093/cvr/cvu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavlovic D., Hall A.R., Kennington E.J., Aughton K., Boguslavskyi A., Fuller W., Despa S., Bers D.M., Shattock M.J. Nitric oxide regulates cardiac intracellular Na(+) and Ca(2)(+) by modulating Na/K ATPase via PKCepsilon and phospholemman-dependent mechanism. J. Mol. Cell. Cardiol. 2013;61:164–171. doi: 10.1016/j.yjmcc.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Armouche A., Wittkopper K., Fuller W., Howie J., Shattock M.J., Pavlovic D. Phospholemman-dependent regulation of the cardiac Na/K-ATPase activity is modulated by inhibitor-1 sensitive type-1 phosphatase. FASEB J. 2011;25:4467–4475. doi: 10.1096/fj.11-184903. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 26.Sweadner K.J. Isozymes of the Na+/K+-ATPase. Biochim. Biophys. Acta. 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 27.Crambert G., Hasler U., Beggah A.T., Yu C., Modyanov N.N., Horisberger J.D., Lelievre L., Geering K. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J. Biol. Chem. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- 28.Pavlovic D. The role of cardiotonic steroids in the pathogenesis of cardiomyopathy in chronic kidney disease. Nephron Clin. Pract. 2014;128:11–21. doi: 10.1159/000363301. [DOI] [PubMed] [Google Scholar]
- 29.Blaustein M.P. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am. J. Physiol. 1977;232:C165–173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- 30.Ten Eick R.E., Hoffman B.F. Chronotropic effect of cardiac glycosides in cats, dogs, and rabbits. Circ. Res. 1969;25:365–378. doi: 10.1161/01.res.25.4.365. [DOI] [PubMed] [Google Scholar]
- 31.Castagno D., Petrie M.C., Claggett B., McMurray J. Should we SHIFT our thinking about digoxin? Observations on ivabradine and heart rate reduction in heart failure. Eur. Heart J. 2012;33:1137–1141. doi: 10.1093/eurheartj/ehs004. [DOI] [PubMed] [Google Scholar]
- 32.Harris D.W., Clark M.A., Fisher J.F., Hamlyn J.M., Kolbasa K.P., Ludens J.H., DuCharme D.W. Development of an immunoassay for endogenous digitalislike factor. Hypertension. 1991;17:936–943. doi: 10.1161/01.hyp.17.6.936. [DOI] [PubMed] [Google Scholar]
- 33.Manunta P., Stella P., Rivera R., Ciurlino D., Cusi D., Ferrandi M., Hamlyn J.M., Bianchi G. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension. 1999;34:450–456. doi: 10.1161/01.hyp.34.3.450. [DOI] [PubMed] [Google Scholar]
- 34.Pierdomenico S.D., Bucci A., Manunta P., Rivera R., Ferrandi M., Hamlyn J.M., Lapenna D., Cuccurullo F., Mezzetti A. Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am. J. Hypertens. 2001;14:44–50. doi: 10.1016/s0895-7061(00)01225-5. [DOI] [PubMed] [Google Scholar]
- 35.Tentori S., Messaggio E., Brioni E., Casamassima N., Simonini M., Zagato L., Hamlyn J.M., Manunta P., Lanzani C. Endogenous ouabain and aldosterone are coelevated in the circulation of patients with essential hypertension. J. Hypertens. 2016;34:2074–2080. doi: 10.1097/HJH.0000000000001042. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb S.S., Rogowski A.C., Weinberg M., Krichten C.M., Hamilton B.P., Hamlyn J.M. Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation. 1992;86:420–425. doi: 10.1161/01.cir.86.2.420. [DOI] [PubMed] [Google Scholar]
- 37.Simonini M., Pozzoli S., Bignami E., Casamassima N., Messaggio E., Lanzani C., Frati E., Botticelli I.M., Rotatori F., Alfieri O., Zangrillo A., Manunta P. Endogenous Ouabain: An Old Cardiotonic Steroid as a New Biomarker of Heart Failure and a Predictor of Mortality after Cardiac Surgery. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/714793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iatrino R., Lanzani C., Bignami E., Casamassima N., Citterio L., Meroni R., Zagato L., Zangrillo A., Alfieri O., Fontana S., Macrina L., Delli Carpini S., Messaggio E., Brioni E., Dell’Antonio G., Manunta P., Hamlyn J.M., Simonini M. Lanosterol synthase genetic variants, endogenous ouabain, and both acute and chronic kidney injury. Am. J. Kidney Dis. 2019;73:504–512. doi: 10.1053/j.ajkd.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Simonini M., Lanzani C., Bignami E., Casamassima N., Frati E., Meroni R., Messaggio E., Alfieri O., Hamlyn J., Body S.C., Collard C.D., Zangrillo A., Manunta P., Investigators C.G. A new clinical multivariable model that predicts postoperative acute kidney injury: impact of endogenous ouabain. Nephrol. Dial. Transplant. 2014;29:1696–1701. doi: 10.1093/ndt/gfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bignami E., Casamassima N., Frati E., Lanzani C., Corno L., Alfieri O., Gottlieb S., Simonini M., Shah K.B., Mizzi A., Messaggio E., Zangrillo A., Ferrandi M., Ferrari P., Bianchi G., Hamlyn J.M., Manunta P. Preoperative endogenous ouabain predicts acute kidney injury in cardiac surgery patients. Crit. Care Med. 2013;41:744–755. doi: 10.1097/CCM.0b013e3182741599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stella P., Manunta P., Mallamaci F., Melandri M., Spotti D., Tripepi G., Hamlyn J.M., Malatino L.S., Bianchi G., Zoccali C. Endogenous ouabain and cardiomyopathy in dialysis patients. J. Intern. Med. 2008;263:274–280. doi: 10.1111/j.1365-2796.2007.01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goto A., Ishiguro T., Yamada K., Ishii M., Yoshioka M., Eguchi C., Shimora M., Sugimoto T. Isolation of a urinary digitalis-like factor indistinguishable from digoxin. Biochem. Biophys. Res. Commun. 1990;173:1093–1101. doi: 10.1016/s0006-291x(05)80898-8. [DOI] [PubMed] [Google Scholar]
- 43.Qazzaz H.M., Goudy S.L., Valdes R., Jr. Deglycosylated products of endogenous digoxin-like immunoreactive factor in mammalian tissue. J. Biol. Chem. 1996;271:8731–8737. doi: 10.1074/jbc.271.15.8731. [DOI] [PubMed] [Google Scholar]
- 44.Qazzaz H.M., Valdes R., Jr. Simultaneous isolation of endogenous digoxin-like immunoreactive factor, ouabain-like factor, and deglycosylated congeners from mammalian tissues. Arch. Biochem. Biophys. 1996;328:193–200. doi: 10.1006/abbi.1996.0160. [DOI] [PubMed] [Google Scholar]
- 45.Bagrov A.Y., Fedorova O.V., Dmitrieva R.I., Howald W.N., Hunter A.P., Kuznetsova E.A., Shpen V.M. Characterization of a urinary bufodienolide Na+,K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension. 1998;31:1097–1103. doi: 10.1161/01.hyp.31.5.1097. [DOI] [PubMed] [Google Scholar]
- 46.Komiyama Y., Dong X.H., Nishimura N., Masaki H., Yoshika M., Masuda M., Takahashi H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 2005;38:36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Sich B., Kirch U., Tepel M., Zidek W., Schoner W. Pulse pressure correlates in humans with a proscillaridin A immunoreactive compound. Hypertension. 1996;27:1073–1078. doi: 10.1161/01.hyp.27.5.1073. [DOI] [PubMed] [Google Scholar]
- 48.Oda M., Kurosawa M., Numazawa S., Tanaka S., Akizawa T., Ito K., Maeda M., Yoshida T. Determination of bufalin-like immunoreactivity in serum of humans and rats by time-resolved fluoroimmunoassay for using a monoclonal antibody. Life Sci. 2001;68:1107–1117. doi: 10.1016/s0024-3205(00)01013-4. [DOI] [PubMed] [Google Scholar]
- 49.Baecher S., Kroiss M., Fassnacht M., Vogeser M. No endogenous ouabain is detectable in human plasma by ultra-sensitive UPLC-MS/MS. Clin. Chim. Acta. 2014;431:87–92. doi: 10.1016/j.cca.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 50.Blaustein M.P. Reply to “Letter to the editor: comments on Blaustein (2018):’ the pump, the exchanger, and the holy spirit: origins and 40-year evolution of ideas about the ouabain-Na(+) pump endocrine system’”. Am. J. Physiol., Cell Physiol. 2018;314:C641–C642. doi: 10.1152/ajpcell.00069.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaustein M.P. The pump, the exchanger, and the holy spirit: origins and 40-year evolution of ideas about the ouabain-Na(+) pump endocrine system. Am. J. Physiol., Cell Physiol. 2018;314:C3–C26. doi: 10.1152/ajpcell.00196.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogeser M. Letter to the editor: Comments on Blaustein (2018): “The pump, the exchanger, and the holy spirit: origins and 40-year evolution of ideas about the ouabain-Na(+) pump endocrine system”. Am. J. Physiol., Cell Physiol. 2018;314:C640. doi: 10.1152/ajpcell.00034.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamlyn J.M., Blaustein M.P. Endogenous ouabain: recent advances and controversies. Hypertension. 2016;68:526–532. doi: 10.1161/HYPERTENSIONAHA.116.06599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laredo J., Hamilton B.P., Hamlyn J.M. Secretion of endogenous ouabain from bovine adrenocortical cells: role of the zona glomerulosa and zona fasciculata. Biochem. Biophys. Res. Commun. 1995;212:487–493. doi: 10.1006/bbrc.1995.1996. [DOI] [PubMed] [Google Scholar]
- 55.Dmitrieva R.I., Bagrov A.Y., Lalli E., Sassone-Corsi P., Stocco D.M., Doris P.A. Mammalian bufadienolide is synthesized from cholesterol in the adrenal cortex by a pathway that is independent of cholesterol side-chain cleavage. Hypertension. 2000;36:442–448. doi: 10.1161/01.hyp.36.3.442. [DOI] [PubMed] [Google Scholar]
- 56.Murrell J.R., Randall J.D., Rosoff J., Zhao J.L., Jensen R.V., Gullans S.R., Haupert G.T., Jr. Endogenous ouabain: upregulation of steroidogenic genes in hypertensive hypothalamus but not adrenal. Circulation. 2005;112:1301–1308. doi: 10.1161/CIRCULATIONAHA.105.554071. [DOI] [PubMed] [Google Scholar]
- 57.Lanzani C., Gatti G., Citterio L., Messaggio E., Delli Carpini S., Simonini M., Casamassima N., Zagato L., Brioni E., Hamlyn J.M., Manunta P. Lanosterol synthase gene polymorphisms and changes in endogenous ouabain in the response to low sodium intake. Hypertension. 2016;67:342–348. doi: 10.1161/HYPERTENSIONAHA.115.06415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manunta P., Hamilton B.P., Hamlyn J.M. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men, American journal of physiology. Regulatory, integrative and comparative physiology. 2006;290:R553–559. doi: 10.1152/ajpregu.00648.2005. [DOI] [PubMed] [Google Scholar]
- 59.Shah J.R., Laredo J., Hamilton B.P., Hamlyn J.M. Effects of angiotensin II on sodium potassium pumps, endogenous ouabain, and aldosterone in bovine zona glomerulosa cells. Hypertension. 1999;33:373–377. doi: 10.1161/01.hyp.33.1.373. [DOI] [PubMed] [Google Scholar]
- 60.Altamirano J., Li Y., DeSantiago J., Piacentino V., 3rd, Houser S.R., Bers D.M. The inotropic effect of cardioactive glycosides in ventricular myocytes requires Na+-Ca2+ exchanger function. J. Physiol. (Paris) 2006;575:845–854. doi: 10.1113/jphysiol.2006.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fedorova L.V., Raju V., El-Okdi N., Shidyak A., Kennedy D.J., Vetteth S., Giovannucci D.R., Bagrov A.Y., Fedorova O.V., Shapiro J.I., Malhotra D. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: implication of epithelial-to-mesenchymal transition. Am. J. Physiol. Renal Physiol. 2009;296:F922–934. doi: 10.1152/ajprenal.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fedorova O.V., Doris P.A., Bagrov A.Y. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clin. Exp. Hypertens. 1998;20:581–591. doi: 10.3109/10641969809053236. [DOI] [PubMed] [Google Scholar]
- 63.Gallice P.M., Kovacic H.N., Brunet P.J., Berland Y.F., Crevat A.D. A non ouabain-like inhibitor of the sodium pump in uremic plasma ultrafiltrates and urine from healthy subjects. Clin. Chim. Acta. 1998;273:149–160. doi: 10.1016/s0009-8981(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 64.Gonick H.C., Ding Y., Vaziri N.D., Bagrov A.Y., Fedorova O.V. Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clin. Exp. Hypertens. 1998;20:617–627. doi: 10.3109/10641969809053240. [DOI] [PubMed] [Google Scholar]
- 65.Harwood S., Mullen A.M., McMahon A.C., Dawnay A. Plasma OLC is elevated in mild experimental uremia but is not associated with hypertension. Am. J. Hypertens. 2001;14:1112–1115. doi: 10.1016/s0895-7061(01)02219-1. [DOI] [PubMed] [Google Scholar]
- 66.Kennedy D.J., Vetteth S., Periyasamy S.M., Kanj M., Fedorova L., Khouri S., Kahaleh M.B., Xie Z., Malhotra D., Kolodkin N.I., Lakatta E.G., Fedorova O.V., Bagrov A.Y., Shapiro J.I. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 67.Li S., Liu G., Jia J., Miao Y., Gu S., Miao P., Shi X., Wang Y., Yu C. Therapeutic monitoring of serum digoxin for patients with heart failure using a rapid LC-MS/MS method. Clin. Biochem. 2010;43:307–313. doi: 10.1016/j.clinbiochem.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 68.Periyasamy S.M., Chen J., Cooney D., Carter P., Omran E., Tian J., Priyadarshi S., Bagrov A., Fedorova O., Malhotra D., Xie Z., Shapiro J.I. Effects of uremic serum on isolated cardiac myocyte calcium cycling and contractile function. Kidney Int. 2001;60:2367–2376. doi: 10.1046/j.1523-1755.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- 69.Liu J., Tian J., Haas M., Shapiro J.I., Askari A., Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J. Biol. Chem. 2000;275:27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- 70.Pierre S.V., Xie Z., Na T. K-ATPase receptor complex: its organization and membership. Cell Biochem. Biophys. 2006;46:303–316. doi: 10.1385/cbb:46:3:303. [DOI] [PubMed] [Google Scholar]
- 71.Li Z., Xie Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers Arch. 2009;457:635–644. doi: 10.1007/s00424-008-0470-0. [DOI] [PubMed] [Google Scholar]
- 72.Liang M., Tian J., Liu L., Pierre S., Liu J., Shapiro J., Xie Z.J. Identification of a pool of non-pumping Na/K-ATPase. J. Biol. Chem. 2007;282:10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 73.Wang H., Haas M., Liang M., Cai T., Tian J., Li S., Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J. Biol. Chem. 2004;279:17250–17259. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 74.Liu J., Liang M., Liu L., Malhotra D., Xie Z., Shapiro J.I. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 2005;67:1844–1854. doi: 10.1111/j.1523-1755.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 75.Liu J., Kesiry R., Periyasamy S.M., Malhotra D., Xie Z., Shapiro J.I. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004;66:227–241. doi: 10.1111/j.1523-1755.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 76.Liu J., Periyasamy S.M., Gunning W., Fedorova O.V., Bagrov A.Y., Malhotra D., Xie Z., Shapiro J.I. Effects of cardiac glycosides on sodium pump expression and function in LLC-PK1 and MDCK cells. Kidney Int. 2002;62:2118–2125. doi: 10.1046/j.1523-1755.2002.00672.x. [DOI] [PubMed] [Google Scholar]
- 77.Tian J., Liu J., Garlid K.D., Shapiro J.I., Xie Z. Involvement of mitogen-activated protein kinases and reactive oxygen species in the inotropic action of ouabain on cardiac myocytes. A potential role for mitochondrial K(ATP) channels, Molecular and cellular biochemistry. 2003;242:181–187. [PubMed] [Google Scholar]
- 78.Peng M., Huang L., Xie Z., Huang W.H., Askari A. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J. Biol. Chem. 1996;271:10372–10378. doi: 10.1074/jbc.271.17.10372. [DOI] [PubMed] [Google Scholar]
- 79.Dong X.H., Komiyama Y., Nishimura N., Masuda M., Takahashi H. Nanomolar level of ouabain increases intracellular calcium to produce nitric oxide in rat aortic endothelial cells. Clin. Exp. Pharmacol. Physiol. 2004;31:276–283. doi: 10.1111/j.1440-1681.2004.03995.x. [DOI] [PubMed] [Google Scholar]
- 80.Reuter H., Henderson S.A., Han T., Ross R.S., Goldhaber J.I., Philipson K.D. The Na+-Ca2+ exchanger is essential for the action of cardiac glycosides. Circ. Res. 2002;90:305–308. doi: 10.1161/hh0302.104562. [DOI] [PubMed] [Google Scholar]
- 81.Andrikopoulos P., Baba A., Matsuda T., Djamgoz M.B., Yaqoob M.M., Eccles S.A. Ca2+ influx through reverse mode Na+/Ca2+ exchange is critical for vascular endothelial growth factor-mediated extracellular signal-regulated kinase (ERK) 1/2 activation and angiogenic functions of human endothelial cells. J. Biol. Chem. 2011;286:37919–37931. doi: 10.1074/jbc.M111.251777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rathore S.S., Curtis J.P., Wang Y., Bristow M.R., Krumholz H.M. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. doi: 10.1001/jama.289.7.871. [DOI] [PubMed] [Google Scholar]
- 83.Song H., Karashima E., Hamlyn J.M., Blaustein M.P. Ouabain-digoxin antagonism in rat arteries and neurones. J. Physiol. (Lond.) 2014;592:941–969. doi: 10.1113/jphysiol.2013.266866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song H., Karashima E., Hamlyn J.M., Blaustein M.P. Ouabain-digoxin antagonism in rat arteries and neurones. J. Physiol. (Paris) 2013 doi: 10.1113/jphysiol.2013.266866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahmed A., Rich M.W., Love T.E., Lloyd-Jones D.M., Aban I.B., Colucci W.S., Adams K.F., Gheorghiade M. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur. Heart J. 2006;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fedorova O.V., Kolodkin N.I., Agalakova N.I., Lakatta E.G., Bagrov A.Y. Marinobufagenin, an endogenous alpha-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension. 2001;37:462–466. doi: 10.1161/01.hyp.37.2.462. [DOI] [PubMed] [Google Scholar]
- 87.Periyasamy S.M., Liu J., Tanta F., Kabak B., Wakefield B., Malhotra D., Kennedy D.J., Nadoor A., Fedorova O.V., Gunning W., Xie Z., Bagrov A.Y., Shapiro J.I. Salt loading induces redistribution of the plasmalemmal Na/K-ATPase in proximal tubule cells. Kidney Int. 2005;67:1868–1877. doi: 10.1111/j.1523-1755.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 88.Pullen M.A., Brooks D.P., Edwards R.M. Characterization of the neutralizing activity of digoxin-specific Fab toward ouabain-like steroids. J. Pharmacol. Exp. Ther. 2004;310:319–325. doi: 10.1124/jpet.104.065250. [DOI] [PubMed] [Google Scholar]
- 89.Fedorova O.V., Kolodkin N.I., Agalakova N.I., Namikas A.R., Bzhelyansky A., St-Louis J., Lakatta E.G., Bagrov A.Y. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. J. Hypertens. 2005;23:835–842. doi: 10.1097/01.hjh.0000163153.27954.33. [DOI] [PubMed] [Google Scholar]
- 90.Anderson D.E., Fedorova O.V., Morrell C.H., Longo D.L., Kashkin V.A., Metzler J.D., Bagrov A.Y., Lakatta E.G. Endogenous sodium pump inhibitors and age-associated increases in salt sensitivity of blood pressure in normotensives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1248–1254. doi: 10.1152/ajpregu.00782.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dostanic-Larson I., Van Huysse J.W., Lorenz J.N., Lingrel J.B. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15845–15850. doi: 10.1073/pnas.0507358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferrandi M., Molinari I., Barassi P., Minotti E., Bianchi G., Ferrari P. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J. Biol. Chem. 2004;279:33306–33314. doi: 10.1074/jbc.M402187200. [DOI] [PubMed] [Google Scholar]
- 93.Pamnani M.B., Chen S., Yuan C.M., Haddy F.J. Chronic blood pressure effects of bufalin, a sodium-potassium ATPase inhibitor, in rats. Hypertension. 1994;23:I106–109. doi: 10.1161/01.hyp.23.1_suppl.i106. [DOI] [PubMed] [Google Scholar]
- 94.Lopatin D.A., Ailamazian E.K., Dmitrieva R.I., Shpen V.M., Fedorova O.V., Doris P.A., Bagrov A.Y. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J. Hypertens. 1999;17:1179–1187. doi: 10.1097/00004872-199917080-00018. [DOI] [PubMed] [Google Scholar]
- 95.Adair C.D., Buckalew V.M., Graves S.W., Lam G.K., Johnson D.D., Saade G., Lewis D.F., Robinson C., Danoff T.M., Chauhan N., Hopoate-Sitake M., Porter K.B., Humphrey R.G., Trofatter K.F., Amon E., Ward S., Kennedy L., Mason L., Johnston J.A. Digoxin immune fab treatment for severe preeclampsia. Am. J. Perinatol. 2010;27:655–662. doi: 10.1055/s-0030-1249762. [DOI] [PubMed] [Google Scholar]
- 96.Lam G.K., Hopoate-Sitake M., Adair C.D., Buckalew V.M., Johnson D.D., Lewis D.F., Robinson C.J., Saade G.R., Graves S.W. Digoxin antibody fragment, antigen binding (Fab), treatment of preeclampsia in women with endogenous digitalis-like factor: a secondary analysis of the DEEP Trial. Am. J. Obstet. Gynecol. 2013;209(119):e111–116. doi: 10.1016/j.ajog.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Fedorova O.V., Zhuravin I.A., Agalakova N.I., Yamova L.A., Talan M.I., Lakatta E.G., Bagrov A.Y. Intrahippocampal microinjection of an exquisitely low dose of ouabain mimics NaCl loading and stimulates a bufadienolide Na/K-ATPase inhibitor. J. Hypertens. 2007;25:1834–1844. doi: 10.1097/HJH.0b013e328200497a. [DOI] [PubMed] [Google Scholar]
- 98.Fedorova O.V., Talan M.I., Agalakova N.I., Lakatta E.G., Bagrov A.Y. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride--dependent hypertension. Circulation. 2002;105:1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 99.Ferrari P., Ferrandi M., Valentini G., Bianchi G. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+-K+- ATPase alterations in ouabain and adducin-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R529–535. doi: 10.1152/ajpregu.00518.2005. [DOI] [PubMed] [Google Scholar]
- 100.Horvat D., Severson J., Uddin M.N., Mitchell B., Puschett J.B. Resibufogenin prevents the manifestations of preeclampsia in an animal model of the syndrome. Hypertension in pregnancy : official journal of the International Society for the Study of Hypertension in Pregnancy. 2010;29:1–9. doi: 10.3109/10641950802629709. [DOI] [PubMed] [Google Scholar]
- 101.Staessen J.A., Thijs L., Stolarz-Skrzypek K., Bacchieri A., Barton J., Espositi E.D., de Leeuw P.W., Dluzniewski M., Glorioso N., Januszewicz A., Manunta P., Milyagin V., Nikitin Y., Soucek M., Lanzani C., Citterio L., Timio M., Tykarski A., Ferrari P., Valentini G., Kawecka-Jaszcz K., Bianchi G. Main results of the ouabain and adducin for Specific Intervention on Sodium in Hypertension Trial (OASIS-HT): a randomized placebo-controlled phase-2 dose-finding study of rostafuroxin. Trials. 2011;12:13. doi: 10.1186/1745-6215-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bagrov A., Fedorova O.V., Maslova M.N., Roukoyatkina N.I., Ukhanova M.V., Zhabko E.P. Endogenous plasma Na,K-ATPase inhibitory activity and digoxin like immunoreactivity after acute myocardial infarction. Cardiovasc. Res. 1991;25:371–377. doi: 10.1093/cvr/25.5.371. [DOI] [PubMed] [Google Scholar]
- 103.Kennedy D.J., Shrestha K., Sheehey B., Li X.S., Guggilam A., Wu Y., Finucan M., Gabi A., Medert C.M., Westfall K., Borowski A., Fedorova O., Bagrov A.Y., Tang W.H. Elevated plasma marinobufagenin, an endogenous cardiotonic steroid, is associated with right ventricular dysfunction and nitrative stress in heart failure. Circ. Heart Fail. 2015;8:1068–1076. doi: 10.1161/CIRCHEARTFAILURE.114.001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pitzalis M.V., Hamlyn J.M., Messaggio E., Iacoviello M., Forleo C., Romito R., de Tommasi E., Rizzon P., Bianchi G., Manunta P. Independent and incremental prognostic value of endogenous ouabain in idiopathic dilated cardiomyopathy. Eur. J. Heart Fail. 2006;8:179–186. doi: 10.1016/j.ejheart.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 105.Haller S.T., Drummond C.A., Yan Y., Liu J., Tian J., Malhotra D., Shapiro J.I. Passive immunization against marinobufagenin attenuates renal fibrosis and improves renal function in experimental renal disease. Am. J. Hypertens. 2013 doi: 10.1093/ajh/hpt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lingrel J.B. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu. Rev. Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schoner W., Scheiner-Bobis G. Role of endogenous cardiotonic steroids in sodium homeostasis. Nephrol. Dial. Transplant. 2008;23:2723–2729. doi: 10.1093/ndt/gfn325. [DOI] [PubMed] [Google Scholar]