Abstract
Pseudomonas aeruginosa is an opportunistic pathogen that can form biofilms, which confer resistance to immune clearance and antibacterial treatment. Therefore, effective strategies to prevent biofilm formation are warranted. Here, 103 P. aeruginosa clinical isolates were quantitatively screened for biofilm formation ability via the tissue culture plate method. The effects of lysozyme (hydrolytic enzyme) and proteinase K (protease) on biofilm formation were evaluated at different concentrations. Lysozyme (30 μg/mL), but not proteinase K, significantly inhibited biofilm formation (19% inhibition). Treatment of 24-hour-old biofilms of P. aeruginosa isolates with 50 times the minimum inhibitory concentrations (MICs) of ceftazidime and cefepime significantly decreased the biofilm mass by 32.8% and 44%, respectively. Moreover, the exposure of 24-hour-old biofilms of P. aeruginosa isolates to lysozyme (30 μg/mL) and 50 times MICs of ceftazidime or cefepime resulted in a significant reduction in biofilm mass as compared with the exposure to lysozyme or either antibacterial agent alone. The best antibiofilm effect (49.3%) was observed with the combination of lysozyme (30 μg/mL) and 50 times MIC of cefepime. The promising antibiofilm activity observed after treatment with 50 times MIC of ceftazidime or cefepime alone or in combination with lysozyme (30 μg/mL) is indicative of a novel strategy to eradicate pseudomonal biofilms in intravascular devices and contact lenses.
1. Introduction
Biofilms are sessile microbial territories covered by an extracellular polysaccharide material, which facilitates irreversible attachment of microbial cells to the substructure or each other [1, 2]. The extracellular polysaccharide matrix subsidizes the overall microbial construction and acts as a medium for cell-to-cell and cell-to-surface interactions, which are essential for biofilm formation and arrangement [3, 4]. Hence, biofilms act as obstacles against antimicrobial agents. Biofilms may be formed on living or nonliving surfaces, water, soil, sediment, as well as soft tissues of living organisms [4].
Pseudomonas aeruginosa is an environmental pathogen that exhibits metabolic versatility [5]. It is one of the major opportunistic pathogens associated with various infections, including respiratory tract infections, implant infections, burns, wounds, and nosocomial infections [6, 7]. The ability of P. aeruginosa to form biofilms in different environments is associated with the ineffectiveness of antibiotics against many severe infections, owing to their limited penetration into the biofilm matrix and inactivation by the extracellular matrix [8, 9]. Therefore, the gram-negative bacterium P. aeruginosa has received tremendous attention for its involvement with biofilm-associated infections [8, 10].
The various strategies employed for the eradication of biofilms include prevention of microbial attachment to surfaces, suppression of biofilm development to promote antibiotic penetration, and interruption in the biofilm maturation process [11–13]. One of these strategies is the use of essential oils as natural compounds that inhibit biofilm formation without affecting cells viability [14].
Multidrug resistance is higher among biofilm producers than among biofilm nonproducers [15]. Antibiotics at concentrations 50 times their minimum inhibitory concentrations (MICs) may decrease the number of colony-forming units of some biofilm-producing species [16]. On the contrary, biofilm disruption based on enzymatic activity is thought to serve as a plausible strategy to combat persistent infections associated with biofilms, as enzymatic treatment improves the antibiotic susceptibility of microbial biofilms [17]. Lysozyme and proteinase K were reported to exhibit antibiofilm activities. Proteinase K resembles naturally produced proteases and may be used to facilitate biofilm dissemination by breaking surface proteins [18]. Antibiofilm activity of lysozyme is associated with the protective function of the innate immune system against infections with biofilms [19] and relies on the hydrolytic activity against peptidoglycan. In addition, lysozyme has an on-lytic mechanism related to its cationic and hydrophobic characteristics, which lead to bacterial autolysis [20].
Previous studies have shown the widespread of biofilm-forming bacteria in Egyptian hospitals [21, 22] that has posed a challenge for the development of novel strategies to eradicate biofilm-forming bacteria. In this work, the biofilm-forming ability of 103 P. aeruginosa clinical isolates was qualitatively and quantitatively screened, and the antibiofilm activities of lysozyme, proteinase K, and cephalosporins (cefepime and ceftazidime) were investigated.
2. Materials and Methods
2.1. Collection and Identification of P. aeruginosa Clinical Isolates
A total of 103 clinical specimens were collected from different clinical sources (wounds, urine, urinary catheter, burns, contact lenses, and sputum) at Mansoura University hospitals and private clinics over a 6-month period (May 2017 to October 2017). The clinical isolates were microscopically examined and subjected to standard biochemical tests according to the previously suggested protocols [23]. All clinical isolates were cultivated in Luria-Bertani (LB) growth medium at 37°C and stored at −80°C in the LB medium with 20% (v/v) glycerol until further analysis [24].
2.2. Ethical Approval
The experimental protocol was in accordance with the ethical guidelines adopted by the Research Ethics Committee of faculty of pharmacy, Mansoura University, Egypt (Code: 2017-20). All patients provided consent to participate in this study. All patients were above 16 years old.
2.3. Qualitative Detection of Biofilm with the Tube Method
All P. aeruginosa isolates were screened for biofilm-forming ability by the tube method as previously described [19]. An aliquot of the glycerol stock of each isolate was streaked on a nutrient agar (NA) plate, and the plate was incubated at 37°C for 24 h. A pure colony was picked up from the NA plate and inoculated in a test tube containing 10 mL tryptic soy broth supplemented with 1% glucose (TSBG), followed by overnight incubation at 37°C. A negative control containing only TSBG without bacterial inoculum was also included. After incubation, the culture from each tube was carefully aspirated, and the adhered biofilm was washed with phosphate-buffered saline (PBS, pH 7.4). The tubes were kept in an inverted position until complete dryness (30–45 min) and then stained with 1% crystal violet for 15 min. Excess of dye was rinsed off with distilled water. Biofilm formation was independently examined with naked eyes by two different observers [25, 26]. Positive results appeared as visible stained films on the walls and bottom surfaces of tubes. According to the intensity of the violet color of the stained biofilm, the biofilm-forming ability of different P. aeruginosa isolates was classified as strongly adherent, moderately adherent, weakly adherent, or nonadherent. In this work, P. aeruginosa strain PAO1 and strain PA14 were used as references for strong and weak adherent strains, respectively, where the biofilm-producing abilities for these standard strains were confirmed in previous reports [27–29].
2.4. Quantitative Assay of Biofilm Formation with the Tissue Culture Plate Method
The tissue culture plate method was used to detect biofilm formation [6, 30–32]. We screened 45 randomly selected isolates for their ability to form biofilms using 96-well microtiter plates with four different media as follows: TSBG, Mueller–Hinton broth (MHB), brain-heart infusion broth (BHIB), and LB.
Isolates streaked on NA plates were inoculated into each medium, and the plates were incubated at 37°C for 24 h. The overnight cultures of each isolate were prepared in all investigated media for the qualitative assay. Optical density (OD) of overnight cultures at 600 nm wavelength was adjusted with appropriate media to 0.2–0.25 and measured using a spectrophotometer (WPA colorwave CO7500 Colorimeter). For each isolate, aliquots of 100 μL of the OD-adjusted cultures were inoculated into four wells. A negative control containing each medium alone was included in each experiment.
After incubation at 37°C for 18–24 h, the cultures were carefully aspirated, and the adhered biofilms were washed thrice with 200 μL PBS (pH 7.4) and vigorously agitated to remove any nonadherent cells. The adherent cells were fixed with 150 μL absolute methanol for 15 min. Methanol was aspirated, and the adhered biofilms were stained with 150 μL of 1% (w/v) crystal violet for 20 min. The plates were carefully rinsed with distilled water thrice and kept inverted in air until dryness. The stained biofilm was solubilized with 150 μL of 33% (v/v) glacial acetic acid per well. After solubilization, the OD540nm value was measured using ELx808™ Absorbance Microplate Reader (BioTek Instruments Inc., Winooski, VT).
The results were analyzed as previously described [6, 30]; the mean average OD of each isolate from four wells was calculated and compared with the control cut-off OD (ODC), which is defined as three standard deviation (SD) above the mean of the negative control (3 SD + mean). The degree of adherence is an indication of biofilm mass. The stronger the adherence for each isolate, the higher was the biofilm mass and violet color intensity. The amount of biofilm formed was scored as nonadherent (ODi ≤ ODC), weakly adherent (ODC < ODi ≤ 2 ODC), moderately adherent (2 ODC < ODi ≤ 4 ODC), or strongly adherent (4 ODC < ODi). The most suitable medium that supported biofilm formation was selected to investigate the biofilm formation ability of rest of P. aeruginosa isolates.
2.5. Effects of Lysozyme and Proteinase K on Biofilm Formation
The effects of lysozyme (Carl Roth, Karlsruhe, Germany) and proteinase K (QIAGEN, cat. no. 19131) on the biofilm formation ability of the nine most strongly adherent P. aeruginosa isolates (highest values) were investigated by the biofilm quantitative assay, as mentioned in the previous section. Lysozyme stock solution (300 μg/mL) was prepared in 0.2 M PBS, while proteinase K stock solution (30 μg/mL) was prepared in the 30 mM Tris buffer. Different concentrations of lysozyme (5, 10, and 30 μg/mL) and proteinase K (2, 5, and 10 μg/mL) were adjusted in TSBG culture for each isolate and used in quadruplicates (four wells). Negative controls for each culture together with 0.2 M PBS or 30 mM Tris buffer (without the enzymes) were also included in each experiment.
2.6. Effect of Lysozyme (30 μg/mL) on Planktonic Cell Viability
To investigate the effect of lysozyme on the viability of P. aeruginosa planktonic cells, two representative strongly adherent isolates (E4 and B3 isolates) were subcultured in TSBG alone or with lysozyme (30 μg/mL). Cultures were incubated in a shaking incubator at 37°C, and their viability was investigated every hour by measuring OD600nm for a total of 16 h [19, 33].
2.7. Antimicrobial Susceptibility of Planktonic Cells
The MICs of ceftazidime and cefepime were determined for 16 P. aeruginosa isolates, according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2018) as per the broth microdilution method using the MHB medium. These selected isolates were the strongest biofilm producers.
2.8. Antibiofilm Effects of Lysozyme, Ceftazidime, and Cefepime
The biofilms of the 16 most strongly adherent P. aeruginosa isolates were established in microtiter plates containing 100 μL TSBG medium as previously mentioned. For each isolate, the biofilm was established in the presence of lysozyme (30 μg/mL). In addition, each isolate was incubated in the absence of lysozyme in TSBG containing only PBS as control. The cultures were aspirated after 24 h of biofilm establishment at 37°C without shaking. Ceftazidime and cefepime were dissolved in the TSBG medium and separately added at concentrations 50 times higher than their MICs. The plates were incubated for 24 h at 37°C without shaking. Each experiment was performed in quadruplicates. Biofilm formation was assayed as mentioned in the previous section of quantitative assay of biofilm [6, 30].
2.9. Statistical Analysis
Statistical analysis was performed using GraphPad prism 7® (version 7.00). A paired Student's t-test with two tailed distribution was used to evaluate differences in biofilm mass. A value of P < 0.01 was considered statistically significant.
3. Results
3.1. Bacterial Isolation and Identification
In this study, a total of 103 P. aeruginosa clinical isolates were collected from different patients in Mansoura hospitals and private clinics. These clinical isolates were purified and biochemically identified as positive for P. aeruginosa. The isolates were purified from urine (36), wounds (16), eye (13), sputum (13), burns (10), urinary catheters (9), contact lens (4), and blood (2) samples, as shown in Supplementary Material ().
3.2. Qualitative Assay for the Detection of Biofilm with the Tube Method
As per the results of qualitative assay using the tube method, 103 P. aeruginosa isolates were classified by two independent observers based on their biofilm-producing abilities in the TSBG medium as follows: 28 (27.1%) strongly adherent isolates, 23 (22.3%) moderately adherent isolates, 33 (32%) weakly adherent isolates, and 19 (18.4%) nonadherent isolates (Figure 1).
Figure 1.

Detection of biofilm formation in P. aeruginosa clinical isolates by the tube method. Negative control is a tube that contained TSBG medium alone and was exposed to the same procedure for biofilm detection.
3.3. Quantitative Assay for Biofilm Formation with the Tissue Culture Plate Method
Table 1 demonstrates the obvious increase in biofilm formation for the investigated P. aeruginosa clinical isolates (45 isolates) after cultivation in the TSBG medium, where in 44% isolates were strong biofilm producers in the tissue culture plate method. In contrast, the percentage of strong biofilm-producing isolates was lower in BHIB, LB, and MHB. Hence, the TSBG medium was selected for further quantitative screening of all clinical isolates. The results showed that 59% of 103 P. aeruginosa clinical isolates were strongly adherent in the TSBG medium, while none of the isolates were nonadherent (Table 2). The biofilm formation ability of each isolate is illustrated in Supplementary Material.
Table 1.
Quantitative assay of the effect of different media on biofilm formation by P. aeruginosa via the tissue culture plate method.
| Biofilm classification (45 isolates) | Number of isolates (%) according to biofilm formation | |||
|---|---|---|---|---|
| LB | BHIB | MHB | TSBG | |
| Strongly adherent | 7 (15.5%) | 13 (28.8%) | 6 (13.3%) | 20 (44.4%) |
| Moderately adherent | 13 (28.8%) | 20 (44.4%) | 22 (48.8%) | 18 (40%) |
| Weakly adherent | 25 (55.5%) | 12 (26.6%) | 17 (37.7%) | 7 (15.5%) |
| Nonadherent | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Table 2.
Comparative screening of P. aeruginosa clinical isolates for biofilm formation by the tube and tissue culture plate methods in the TSBG medium.
| Biofilm classification (103 isolates) | Number of isolates (%) according to biofilm formation | |
|---|---|---|
| Tube method (qualitative method) | Tissue culture plate method (quantitative method) | |
| Strongly adherent | 28 (27.1%) | 61 (59.2%) |
| Moderately adherent | 23 (22.3%) | 34 (33.0%) |
| Weakly adherent | 33 (32.0%) | 8 (7.7%) |
| Nonadherent | 19 (18.4%) | 0 (0%) |
3.4. Effect of Lysozyme and Proteinase K on Biofilm Formation
A significant reduction in biofilm mass was observed for all the investigated P. aeruginosa isolates (P < 0.01) cultured in the presence of lysozyme. The highest reduction (19%) was reported at a lysozyme concentration of 30 μg/mL; hence, we used this concentration for further experiments. As shown in Table 3, we failed to observe any significant impact of proteinase K on biofilm mass reduction at all tested concentrations (P < 0.05).
Table 3.
Biofilm reduction (%) at different concentrations of lysozyme and proteinase K.
| Isolate code | Lysozyme concentration (μg/mL) | Proteinase K concentration (μg/mL) | ||||
|---|---|---|---|---|---|---|
| 5 | 10 | 30 | 2 | 5 | 10 | |
| B3 | 16.9 | 18.1 | 18.4 | 3.5 | 20.2 | 8.8 |
| Cl3 | 17.5 | 25.8 | 30.6 | 1.3# | 2.5# | 4.2# |
| E4 | 25.8 | 34.3 | 44.8 | 2.5# | 1.3# | 3.3# |
| U7 | 8.7 | 10.4 | 12.9 | 53.4 | 69.8 | 55.4 |
| U16 | 0.8 | 0.9 | 1.5 | 33.2 | 26.9 | 41.8 |
| U19 | 5.1 | 6.5 | 7.4 | 10.4 | 10.2 | 5.6 |
| W10 | 10.5 | 11.2 | 12 | 5.2# | 6.1# | 7.3# |
| W12 | 3.4 | 6.9 | 8.2 | 15.4 | 15.4 | 15.4 |
| W14 | 28.2 | 30 | 35.9 | 28 | 30.5 | 15.9 |
| Average ± SE | 12.9 ± 1.08 | 16± | 19± | |||
| 1.29 | 19 ± 1.63 | 1.29 | 1.63 | |||
#Enhancement of biofilm formation indicated as%. The percent biofilm reduction or enhancement for each isolate was determined after normalization to negative controls, which were not treated with the enzymes; averages are the means ± standard errors (SEs) of the means from four independent experiments. B: burn; Cl: contact lens; E: eye; U: urine; W: wound.
3.5. Effect of Lysozyme on P. aeruginosa Planktonic Cell Viability
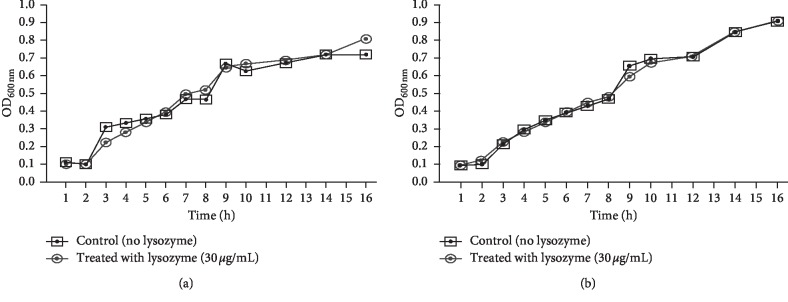
The effect of lysozyme (30 μg/mL) on the growth of P. aeruginosa isolates B3 and E4 was assayed by monitoring the absorbance of the cultures at 600 nm wavelength (Figure 2). The viability of the isolates treated with lysozyme was similar to that of the untreated control during 16 h of incubation, indicative of the inability of lysozyme to affect bacterial growth.
Figure 2.
Effect of lysozyme (30 μg/mL) on the viability of planktonic P. aeruginosa cells. Data are expressed as the mean of four different experiments.
3.6. Antimicrobial Susceptibility of P. aeruginosa Planktonic Cells
According to CLSI 2018, 11, and 13, P. aeruginosa isolates showed resistance (R) to ceftazidime and cefepime, respectively. While four isolates showed intermediate (I) resistance to ceftazidime, two isolates exhibited intermediate resistant to cefepime. Only one isolate was susceptible (S) to both the antibiotics (Table 4).
Table 4.
Resistance pattern of different P. aeruginosa clinical isolates against ceftazidime and cefepime.
| Isolate code | Ceftazidime | Cefepime |
|---|---|---|
| B3 | I | R |
| B5 | I | I |
| Cl1 | I | R |
| Cl3 | S | S |
| Cl4 | I | I |
| E4 | R | R |
| S13 | R | R |
| U16 | R | R |
| U19 | R | R |
| U25 | R | R |
| U30 | R | R |
| U7 | R | R |
| Uc2 | R | R |
| W10 | R | R |
| W12 | R | R |
| W14 | R | R |
B: burn; Cl: contact lens; E: eye; S: sputum; U: urine; Uc: urinary catheter; W: wound.
3.7. Antibiofilm Effects of Lysozyme, Ceftazidime, and Cefepime
We observed a significant reduction (P < 0.01) in the biofilm mass of P. aeruginosa clinical isolates in the presence of either lysozyme (30 μg/mL) or 50 times the MICs of ceftazidime or cefepime. The combination of lysozyme (30 μg/mL) and 50 times MIC of ceftazidime significantly inhibited (P < 0.01) biofilm formation as compared with either lysozyme or ceftazidime alone. Similarly, a significant reduction (P < 0.01) in biofilm mass was detected in the presence of the combination of lysozyme (30 μg/mL) and cefepime (50 times MIC) as compared with that observed in the presence of either the enzyme or antibiotic alone (Table 5 and Figure 3).
Table 5.
Effects of lysozyme (30 μg/mL) and 50 times MIC of ceftazidime or cefepime alone or in combination on biofilm mass reduction (%) for P. aeruginosa clinical isolates.
| Isolate code | Lysozyme (30 μg/mL) | Ceftazidime (50 × MIC) | Cefepime (50 × MIC) | Lysozyme (30 μg/mL) + ceftazidime (50 × MIC) | Lysozyme (30 μg/mL) + cefepime (50 × MIC) |
|---|---|---|---|---|---|
| B3 | 18.4 | 60.4 | 87.5 | 69.9 | 83.7 |
| B5 | 11.9 | 36.9 | 44.8 | 39.4 | 40.7 |
| Cl1 | 5.3 | 8.9 | 13.6 | 9.2 | 2.6 |
| Cl3 | 30.6 | 50.7 | 76.4 | 65 | 89.5 |
| Cl4 | 14 | 21.5 | 30.5 | 22.9 | 38.6 |
| E4 | 44.8 | 68.1 | 86.7 | 83 | 95.7 |
| S13 | 4.3 | 22.4 | 10.9 | 7.8 | 19.7 |
| U7 | 12.9 | 21.2 | 37.1 | 32 | 46.8 |
| U16 | 1.5 | 5.6 | 25.5 | 18.3 | 57.2 |
| U19 | 7.4 | 11 | 14.4 | 12.5 | 17.4 |
| U25 | 5.9 | 45.5 | 47.2 | 43.4 | 46.2 |
| U30 | 10.8 | 32.5 | 45.1 | 36.9 | 50.7 |
| Uc2 | 13.6 | 35.3 | 44.8 | 41.8 | 47.3 |
| W10 | 12 | 49.1 | 89.6 | 86 | 94.8 |
| W12 | 8.2 | 10.4 | 15 | 12.4 | 17.9 |
| W14 | 35.9 | 45.8 | 38.8 | 48.9 | 41.5 |
| Average ± SE | 14.8 ± 0.75 | 32.8 ± 1.2 | 44.2 ± 1.7 | 39.3 ± 1.61 | 49.3 ± 1.79 |
The percentage of biofilm reduction for each isolate was determined after normalization to negative controls that were not treated with lysozyme or antibiotics. Averages are the means ± standard errors of the means from four independent experiments. B: burn; Cl: contact lens; E: eye; S: sputum; U: urine; Uc: urinary catheter; W: wound.
Figure 3.
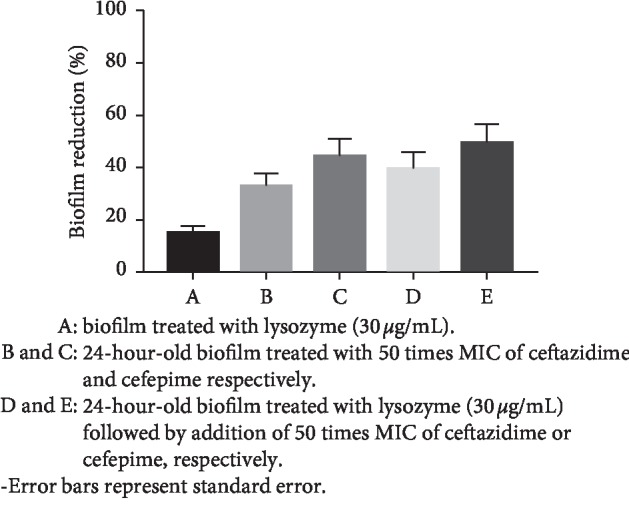
Effect of lysozyme (30 μg/mL) and 50 times MIC of ceftazidime or cefepime alone or in combinations on the average reduction in percent biofilm mass of P. aeruginosa clinical isolates. The percent reduction in biofilm mass for each isolate was normalized to that for the control, which was free from the enzyme and antibiotics (set as 100% biofilm formation).
4. Discussion
P.aeruginosa is an opportunistic human pathogen associated with chronic lung infections and cystic fibrosis [34, 35]. P. aeruginosa colonizes the lung tissue by forming biofilms in the alveoli. Biofilm-forming bacteria are highly resistant to high doses of antibiotics and are protected from polymorphonuclear bactericidal activity [1, 36]. Therefore, we aimed to study the ability of lysozyme, proteinase K, and some cephalosporins (ceftazidime and cefepime) to eradicate biofilms of clinical isolates of P. aeruginosa detected in Egypt. A total of 103 clinical isolates were included in this study.
Tube and tissue culture plate methods are commonly used as qualitative and quantitative assays, respectively, for the detection of biofilms [6, 19, 30, 37, 38]. We qualitatively screened 103 P. aeruginosa clinical isolates for their biofilm formation ability using the tube method and found that 27% isolates were strongly adherent, while 54% were either moderately or weakly adherent; about 18% isolates were nonadherent. These results are similar to the previously reported findings on the biofilm formation ability of P. aeruginosa [39].
Some studies have evaluated the effect of medium composition on biofilm formation [30, 40]. Tryptic soy broth with 1% glucose is considered to enhance biofilm formation as compared with other media such as LB and BHI. To perform the quantitative assay with P. aeruginosa clinical isolates, it is essential to select the best medium that could enhance the biofilm formation ability in the quantitative assay. Among the 45 clinical isolates, 20 (44%), 13 (28%), 7 (15%), and 6 (13%) isolates were strongly adherent in TSBG, BHIB, LB, and MHB media, respectively, as shown in Table 2. Therefore, the TSBG medium was used for the investigation of the remaining clinical isolates. As a result, about 59% of 103 clinical isolates were found to be strongly adherent, while none (0%) of the isolates were nonadherent in TSBG.
The tube method is considered to be less sensitive than the tissue culture plate method, as some clinical isolates that were nonadherent, weak, or moderate biofilm producers in the tube method showed strong biofilm patterns in the tissue culture plate method [41, 42]. Using the tube method, we found that 84 (81.5%) isolates were biofilm producers and 19 (18.5%) were nonbiofilm producers (Table 3). On the contrary, all isolates were biofilm producers when quantitatively assayed using the tissue culture plate method. Thus, 19 isolates were false negative in the tube method owing to its low accuracy, as reported in many studies [40]. It is hard to accurately differentiate between moderately adherent, weakly adherent, and nonbiofilm producers by the tube method; therefore, several studies recommend the analysis of biofilm production via the tube method by different observers [25, 43].
Lysozyme and proteinase K are capable of inhibiting the biofilm formation ability of many bacterial species [19, 33, 44]. Lysozyme is present in physiological secretions at concentrations between 10 and 30 μg/mL [33]. Nonphysiological concentrations of lysozyme (greater than 30 μg/mL) was shown to enhance biofilm formation of P. aeruginosa [19] and Candida albicans [33]. Therefore, we investigated the effect of lysozyme on biofilm formation at concentrations ≤30 μg/mL.
A previous study investigated the effect of lysozyme on P. aeruginosa [19]. However, this study screened the antibiofilm ability of lysozyme against only two strains of P. aeruginosa. The highest antibiofilm activity of lysozyme was noted at a concentration of 30 μg/mL [19]. In our study, a significant reduction in biofilm formation ability of P. aeruginosa with lysozyme was observed at different concentrations. Our results (Table 3) show that lysozyme is a promising biofilm inhibitor, especially at 30 μg/mL concentration (19% reduction in biofilm formation).
To confirm that the observed inhibitory effect of lysozyme enzyme on biofilm formation is solely due to its antibiofilm activity and not due to growth inhibition of P. aeruginosa cells, we investigated the effect of the enzyme on the viability of planktonic cells. For this purpose, two clinical isolates (E4 and B3) were selected for such experiment. The E4 and B3 isolates were representative isolates for strong (44% reduction) and moderate (18% reduction) antibiofilm effect by lysozyme (30 μg/mL), respectively.
Proteinase K was shown to exert antibiofilm effects against Staphylococcus aureus at a concentration of 2 μg/mL [44, 45]. However, we failed to observe any significant effect of proteinase K on the biofilm formation ability of P. aeruginosa at different concentrations (2, 5, and 10 μg/mL). Moreover, the effect of proteinase K was biphasic; i.e., it promoted or inhibited P. aeruginosa biofilms at different concentrations. For example, the antibiofilm activity of proteinase K in the isolates B3 and W14 (Table 3) was greater with the concentration of 5 μg/mL in comparison with that of 10 μg/mL. A reason for such unexpected result could be the relatively unspecific cleavage of this protease [46]. It should be mentioned that proteinase K was reported to have no antibiofilm activity against P. aeruginosa in a previous study [47]. Therefore, proteinase K was not included in our subsequent experiments.
Cephalosporins are broad-spectrum antibiotics that are effective against gram-negative and gram-positive bacteria. Ceftazidime and cefepime are highly effective and the most commonly used agents for the treatment of P. aeruginosa infections [48, 49]. Therefore, we selected these antibiotics and investigated their inhibitory effects on the biofilm formation ability of P. aeruginosa. A previous study revealed that isolates with a higher ability to produce biofilm and had a tendency to produce higher rates of resistance to many antimicrobial agents [28]. Antibiotics at concentrations higher than their MICs do not induce killing effects on bacteria in biofilm communities [16, 40]. For instance, the MIC of ceftazidime was found to increase by 15-fold for P. aeruginosa PAO 579 in a chronic lung infection rat model [50]. Therefore, several studies have investigated the effects of antibiotics on biofilm formation by different bacteria at concentrations 25, 50, or even 104 times the MIC values [16, 51–53]. According to our knowledge, the effect of ceftazidime and cefepime at concentrations 50 times their MICs on biofilm formation has not been evaluated.
We screened the effect of 50 times MICs of ceftazidime and cefepime on the biofilm formation ability of 16 representative isolates of P. aeruginosa that were strongly adherent in the tissue culture plate method. We observed a significant inhibition in biofilm formation with both ceftazidime (32.8% reduction) and cefepime (44% reduction) at these tested concentrations. A previous study had shown that cefotaxime, a member of the cephalosporin family, failed to significantly inhibit biofilm formation of P. aeruginosa [16]. However, it should be mentioned that ceftazidime and cefepime are the only recommended members of cephalosporins for the treatment of P. aeruginosa infections according to CLSI (2018).
The promising antibiofilm activity of lysozyme (30 μg/mL) and 50 times MIC of ceftazidime and cefepime motivated us to investigate the antibiofilm effects of the combination of the enzyme (30 μg/mL) and each antibiotic (50 times MIC). Interestingly, ceftazidime or cefepime at 50 times MICs significantly inhibited the 24-hour-old biofilm formed in the presence of lysozyme (30 μg/mL) as compared with individual antibiotic treatment (Table 3). The highest inhibitory effect (49.3% reduction) was observed with the combination of cefepime (50 times its MIC) and lysozyme (30 μg/mL).
5. Conclusions
In conclusion, to the best of our knowledge, this is the first study to demonstrate the effects of 50 times MICs of ceftazidime and cefepime alone or in combination with lysozyme on the biofilm mass of P. aeruginosa. Therefore, the promising antibiofilm activities observed with 50 times MIC of ceftazidime and cefepime alone and in combinations with lysozyme (30 μg/mL) may serve as a plausible strategy for the eradication of P. aeruginosa biofilms in catheters, contact lenses, intravascular devices, or ventilator tubes. Future studies should investigate the effects of direct immobilization of lysozyme and cephalosporins on these items.
Acknowledgments
The research project was funded by the authors as well as the Faculty of Pharmacy, Mansoura University, Egypt.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Supplementary materials include data about the quantitative assay of biofilm formation for all clinical isolates, in addition to their clinical and geographical sources.
References
- 1.Donlan R. M., Costerton J. W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 2002;15(2):167–193. doi: 10.1128/cmr.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karatan E., Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiology and Molecular Biology Reviews. 2009;73(2):310–347. doi: 10.1128/mmbr.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branda S. S., Vik Å., Friedman L., Kolter R. Biofilms: the matrix revisited. Trends in Microbiology. 2005;13(1):20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Flemming H.-C., Wingender J., Szewzyk U., Steinberg P., Rice S. A., Kjelleberg S. Biofilms: an emergent form of bacterial life. Nature Reviews Microbiology. 2016;14(9):563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 5.Yang L., Liu Y., Wu H., Høiby N., Molin S., Song Z. J. Current understanding of multi—species biofilms. International Journal of Oral Science. 2011;3(2):74–81. doi: 10.4248/ijos11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lima J., Alves L. R., Pereira da Paz J. N., et al. Analysis of biofilm production by clinical isolates of Pseudomonas aeruginosa from patients with ventilator-associated pneumonia. Revista Brasileira de Terapia Intensiva. 2017;29(3):310–316. doi: 10.5935/0103-507x.20170039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmadi Jalali Moghadam M., Honarmand H., Asfaram Meshginshahr S. Contamination of hospital water supplies in Gilan, Iran, with Legionella pneumophila, Escherichia coli, and Pseudomonas aeruginosa. Interdisciplinary Perspectives on Infectious Diseases. 2015;2015:7. doi: 10.1155/2015/809842.809842 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. International Journal of Antimicrobial Agents. 2010;35(35):322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Taylor P. K., Yeung A. T. Y., Hancock R. E. W. Antibiotic resistance in Pseudomonas aeruginosa biofilms: towards the development of novel anti-biofilm therapies. Journal of Biotechnology. 2014;191:121–130. doi: 10.1016/j.jbiotec.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Alves M. J., Barreira J. C. M., Carvalho I., et al. Propensity for biofilm formation by clinical isolates from urinary tract infections: developing a multifactorial predictive model to improve antibiotherapy. Journal of Medical Microbiology. 2014;63(3):471–477. doi: 10.1099/jmm.0.071746-0. [DOI] [PubMed] [Google Scholar]
- 11.Masák J., Čejková A., Schreiberová O., Řezanka T. Pseudomonasbiofilms: possibilities of their control. FEMS Microbiology Ecology. 2014;89(1):1–14. doi: 10.1111/1574-6941.12344. [DOI] [PubMed] [Google Scholar]
- 12.Rasamiravaka T., Labtani Q., Duez P., El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. BioMed Research International. 2015;2015:17. doi: 10.1155/2015/759348.759348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Aziz S. M., Aeron A. Bacterial biofilm: dispersal and inhibition strategies. Scholarena Journal of Biotechnology. 2014;1(1):p. 105. doi: 10.18875/2375-6713.1.105. [DOI] [Google Scholar]
- 14.Artini M., Patsilinakos A., Papa R., et al. Antimicrobial and antibiofilm activity and machine learning classification analysis of essential oils from different mediterranean plants against Pseudomonas aeruginosa. Molecules. 2018;23(2):p. 482. doi: 10.3390/molecules23020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maharjan G., Khadka P., Shilpakar G. S., Chapagain G., Dhungana G. R. Catheter-associated urinary tract infection and obstinate biofilm producers. Canadian Journal of Infectious Diseases and Medical Microbiology. 2018;2018:7. doi: 10.1155/2018/7624857.7624857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tetz G. V., Artemenko N. K., Tetz V. V. Effect of DNase and antibiotics on biofilm characteristics. Antimicrobial Agents and Chemotherapy. 2009;53(3):1204–1209. doi: 10.1128/aac.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lister J. L., Horswill A. R. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Frontiers in Cellular and Infection Microbiology. 2014;4:p. 178. doi: 10.3389/fcimb.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar Shukla S., Rao T. S. Dispersal of Bap-mediated Staphylococcus aureus biofilm by proteinase K. The Journal of Antibiotics. 2013;66(2):55–60. doi: 10.1038/ja.2012.98. [DOI] [PubMed] [Google Scholar]
- 19.Hukic M., Seljmo D., Ramovic A., et al. The effect of lysozyme on reducing biofilms by Staphylococcus aureus, Pseudomonas aeruginosa, and Gardnerella vaginalis: an in vitro examination. Microbial Drug Resistance. 2018;24(4):353–358. doi: 10.1089/mdr.2016.0303. [DOI] [PubMed] [Google Scholar]
- 20.Humann J., Lenz L. L. Bacterial peptidoglycan-degrading enzymes and their impact on host muropeptide detection. Journal of Innate Immunity. 2009;1(2):88–97. doi: 10.1159/000181181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Shaer S., Shaaban M., Barwa R., Hassan R. Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl β-naphthylamide. Journal of Medical Microbiology. 2016;65(10):1194–1204. doi: 10.1099/jmm.0.000327. [DOI] [PubMed] [Google Scholar]
- 22.El-Baz R., Rizk D. E., Barwa R., Hassan R. Virulence characteristics and molecular relatedness of methicillin resistant Staphylococcus aureus harboring different staphylococcal cassette chromosome mec. Microbial Pathogenesis. 2017;113:385–395. doi: 10.1016/j.micpath.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Brown V. I., Lowbury E. J. L. Use of an improved cetrimide agar medium and other culture methods for Pseudomonas aeruginosa. Journal of Clinical Pathology. 1965;18(6):752–756. doi: 10.1136/jcp.18.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaBauve A. E., Wargo M. J. Growth and laboratory maintenance of Pseudomonas aeruginosa. Current Protocols in Microbiology. 2012;25(1) doi: 10.1002/9780471729259.mc06e01s25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singhai M., Malik A., Shahid M., Malik M., Goyal R. A study on device-related infections with special reference to biofilm production and antibiotic resistance. Journal of Global Infectious Diseases. 2012;4(4):193–198. doi: 10.4103/0974-777x.103896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen G. D., Simpson W. A., Younger J. J., et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. Journal of Clinical Microbiology. 1985;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez L. R. R., Costa M. C. N., Freitas A. L. P., Barth A. L. Evaluation of biofilm production by Pseudomonas aeruginosa isolates recovered from cystic fibrosis and non-cystic fibrosis patients. Brazilian Journal of Microbiology. 2011;42(2):476–479. doi: 10.1590/s1517-83822011000200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez L. R. R., Barth A. L. Biofilm production using distinct media and antimicrobial susceptibility profile of Pseudomonas aeruginosa. Brazilian Journal of Infectious Diseases. 2011;15(4):301–304. doi: 10.1590/s1413-86702011000400001. [DOI] [PubMed] [Google Scholar]
- 29.Di Domenico E. G., Toma L., Provot C., et al. Development of an in vitro assay, based on the BioFilm ring test®, for rapid profiling of biofilm-growing bacteria. Frontiers in Microbiology. 2016;7:p. 1429. doi: 10.3389/fmicb.2016.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stepanovic S., Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. Journal of Microbiological Methods. 2000;40(2):175–179. doi: 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 31.Wilson C., Lukowicz R., Merchant S., et al. Quantitative and qualitative assessment methods for biofilm growth: a mini-review. Research & Reviews. Journal of Engineering and Technology. 2017;6(4) [PMC free article] [PubMed] [Google Scholar]
- 32.Magana M., Sereti C., Ioannidis A., et al. Options and limitations in clinical investigation of bacterial biofilms. Clinical Microbiology Reviews. 2018;31(3):e00084–16. doi: 10.1128/cmr.00084-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sebaa S., Hizette N., Boucherit-Otmani Z., Courtois P. Dose-dependent effect of lysozyme upon Candida albicans biofilm. Molecular Medicine Reports. 2017;15(3):1135–1142. doi: 10.3892/mmr.2017.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sriramulu D. D., Lünsdorf H., Lam J. S, Römling U. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. Journal of Medical Microbiology. 2005;54(7):667–676. doi: 10.1099/jmm.0.45969-0. [DOI] [PubMed] [Google Scholar]
- 35.Peek M. E., Bhatnagar A., McCarty N. A., Zughaier S. M. Pyoverdine, the major siderophore in Pseudomonas aeruginosa, evades NGAL recognition. Interdisciplinary Perspectives on Infectious Diseases. 2012;2012:10. doi: 10.1155/2012/843509.843509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjarnsholt T., Jensen P. Ø., Burmølle M., et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology. 2005;151(2):373–383. doi: 10.1099/mic.0.27463-0. [DOI] [PubMed] [Google Scholar]
- 37.Mathur T., Singhal S., Khan S., Upadhyay D., Fatma T., Rattan A. Detection of biofilm formation among the clinical isolates of staphylococci: an evaluation of three different screening methods. Indian Journal of Medical Microbiology. 2006;24(1):25–29. doi: 10.4103/0255-0857.19890. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J., Bi S., Chen H., et al. Anti-biofilm and antivirulence activities of metabolites from Plectosphaerella cucumerina against Pseudomonas aeruginosa. Frontiers in Microbiology. 2017;8:p. 769. doi: 10.3389/fmicb.2017.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena S., Banerjee G, Garg R, Singh M. Comparative study of biofilm formation in Pseudomonas aeruginosa isolates from patients of lower respiratory tract infection. Journal of Clinical and Diagnostic Research. 2014;8(5):DC09–11. doi: 10.7860/jcdr/2014/7808.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haney E., Trimble M., Cheng J., Vallé Q., Hancock R. Critical assessment of methods to quantify biofilm growth and evaluate antibiofilm activity of host defence peptides. Biomolecules. 2018;8(2):p. 29. doi: 10.3390/biom8020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdel Halim R. M., Kassem N. N., Mahmoud B. S. Detection of biofilm producing Staphylococci among different clinical isolates and its relation to methicillin susceptibility. Open Access Macedonian Journal of Medical Sciences. 2018;6(8):1335–1341. doi: 10.3889/oamjms.2018.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gawish S., Abbass A., Abaza A. Occurrence and biofilm forming ability of Pseudomonas aeruginosa in the water output of dental unit waterlines in a dental center in Alexandria, Egypt. Germs. 2019;9(2):71–80. doi: 10.18683/germs.2019.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rishpana M. S., Kabbin J. S. Candiduria in catheter associated urinary tract infection with special reference to biofilm production. Journal of Clinical and Diagnostic Research. 2015;9(10):DC11–DC13. doi: 10.7860/jcdr/2015/13910.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shukla S. K., Rao T. S. Staphylococcus aureus biofilm removal by targeting biofilm-associated extracellular proteins. Indian Journal of Medical Research. 2017;146(7):S1–S8. doi: 10.4103/ijmr.ijmr_410_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ming D., Wang D., Cao F., et al. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Frontiers in Microbiology. 2017;8:p. 2263. doi: 10.3389/fmicb.2017.02263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrett A. J., Woessner J. F., Rawlings N. D. Handbook of Proteolytic Enzymes. Vol. 1. Amsterdam, Netherlands: Elsevier; 2012. [Google Scholar]
- 47.Papa R., Selan L., Parrilli E., et al. Anti-biofilm activities from marine cold adapted bacteria against Staphylococci and Pseudomonas aeruginosa. Frontiers in Microbiology. 2015;6:p. 1333. doi: 10.3389/fmicb.2015.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacVane S. H., Kuti J. L., Nicolau D. P. Clinical pharmacodynamics of antipseudomonal cephalosporins in patients with ventilator-associated pneumonia. Antimicrobial Agents and Chemotherapy. 2014;58(3):1359–1364. doi: 10.1128/aac.01463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bassetti M., Vena A., Croxatto A., Righi E., Guery B. How to manage Pseudomonas aeruginosa infections. Drugs in Context. 2018;7:1–8. doi: 10.7573/dic.212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bagge N., Ciofu O., Skovgaard L. T., Hoiby N. Rapid development in vitro and in vivo of resistance to ceftazidime in biofilm-growing Pseudomonas aeruginosa due to chromosomal β-lactamase. APMIS. 2000;108(9):589–600. doi: 10.1034/j.1600-0463.2000.d01-102.x. [DOI] [PubMed] [Google Scholar]
- 51.Yassien M., Khardori N., Ahmedy A., Toama M. Modulation of biofilms of Pseudomonas aeruginosa by quinolones. Antimicrobial Agents and Chemotherapy. 1995;39(10):2262–2268. doi: 10.1128/aac.39.10.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tetz V. V., Tetz G. V. Effect of extracellular DNA destruction by DNase I on characteristics of forming biofilms. DNA and Cell Biology. 2010;29(8):399–405. doi: 10.1089/dna.2009.1011. [DOI] [PubMed] [Google Scholar]
- 53.Habash M. B., Goodyear M. C., Park A. J., et al. Potentiation of tobramycin by silver nanoparticles against Pseudomonas aeruginosa biofilms. Antimicrobial Agents and Chemotherapy. 2017;61(11) doi: 10.1128/aac.00415-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials include data about the quantitative assay of biofilm formation for all clinical isolates, in addition to their clinical and geographical sources.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.