Abstract
Background
Continuous exposure to high temperatures can lead to heat stress. This stress response alters the expression of multiple genes and can contribute to the onset of various diseases. In particular, heat stress induces oxidative stress by increasing the production of reactive oxygen species. The liver is an essential organ that plays a variety of roles, such as detoxification and protein synthesis. Therefore, it is important to protect the liver from oxidative stress caused by heat stress. Korean ginseng has a variety of beneficial biological properties, and our previous studies showed that it provides an effective defense against heat stress.
Methods
We investigated the ability of Korean Red Ginseng and Korean black ginseng extracts (JP5 and BG1) to protect against heat stress using a rat model. We then confirmed the active ingredients and mechanism of action using a cell-based model.
Results
Heat stress significantly increased gene and protein expression of oxidative stress–related factors such as catalase and SOD2, but treatment with JP5 (Korean Red Ginseng extract) and BG1 (Korean black ginseng extract) abolished this response in both liver tissue and HepG2 cells. In addition, JP5 and BG1 inhibited the expression of inflammatory proteins such as p-NF-κB and tumor necrosis factor alpha-α. In particular, JP5 and BG1 decreased the expression of components of the NLRP3 inflammasome, a key inflammatory signaling factor. Thus, JP5 and BG1 inhibited both oxidative stress and inflammation.
Conclusions
JP5 and BG1 protect against oxidative stress and inflammation induced by heat stress and help maintain liver function by preventing liver damage.
Keywords: Heat stress, Inflammation, Korean ginseng extracts, Oxidative stress, Sprague–Dawley rats
1. Introduction
Continuous exposure to high temperatures can lead to psychological stress reactions, such as helplessness, irritability, and loss of concentration, and physiological stress responses, such as increased heart rate, fainting, and anorexia [1], [2], [3]. This stress response changes the expression of various genes throughout the body and can contribute to various diseases [4], [5], [6]. In addition, when body temperature is elevated due to heat stress and metabolism becomes excessive, production of reactive oxygen species (ROS) increases, leading to oxidative stress when ROS levels exceed the capacity of the body's antioxidant systems [7]. Oxidative stress, in turn, causes adverse effects such as lipid peroxidation and DNA mutations in cells and tissues by damaging cellular components such as nucleic acids, protein, and lipids [8], [9]. In addition, activation of inflammatory signals by ROS induces secretion of inflammatory cytokines and activates apoptosis [10], [11]. Therefore, suppressing excessive ROS production and inhibiting the inflammatory response is essential for preventing tissue damage and protecting the body.
The antioxidant system uses factors such as catalase, superoxide dismutase (SOD), glutathione (GSH), glutathione reductase (GR), and glutathione peroxidase (GPx) to convert ROS to water molecules and oxygen [12]. However, when ROS accumulate beyond the capacity of the system, they activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling [13], [14]. Activated NF-κB translocates into the nucleus and acts as a transcription factor to promote the secretion of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) [15]. NF-κB also promotes transcription of the mRNAs encoding pro-interleukin (IL)-1β, pro-IL-18, and components of the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome. Upon activation by ROS, the NLRP3 inflammasome converts procaspase-1 to caspase-1, which dissociates from NLRP3 and is released into the cytoplasm. Subsequently, caspase-1 converts pro-IL-1β and pro-IL-18 to their active forms, IL-1β and IL-18, promoting their secretion from the cell [16].
The liver is an essential tissue that plays a wide range of roles in the body, including detoxification, energy metabolism, and protein synthesis. In collaboration with other body systems, the liver performs up to 500 individual functions which cannot be fully reproduced by any existing artificial organs or devices [17], [18]. Excessive inflammation beyond the liver's ability to recover can cause hepatitis or cirrhosis, eventually leading to liver cancer [19], [20]. Accordingly, protection of the liver is a high priority that requires constant surveillance and management. Previously, we reported that ginseng extract inhibits lipid peroxidation and abnormal immune responses to heat stress [21], [22]. As noted above, heat stress can induce illness by adversely affecting in vivo gene expression and interfering with homeostatic mechanisms; consequently, it is important to defend the body against heat stress. Ginseng extract, a therapeutic herbal medicine with beneficial effects, such as relieving fatigue, improving memory, and improving obesity and diabetes, can help to protect the body against heat stress [23], [24], [25]. Ginseng is classified as white, red, and black depending on the manufacturing process [26], [27], [28].
The aim of this study was to investigate the effects of red and black ginseng extracts on the oxidative reaction induced by heat stress in liver tissues, identify the active ingredients, and validate the molecular mechanism of this effect using a cell-based model.
2. Materials and methods
2.1. Chemicals and reagents
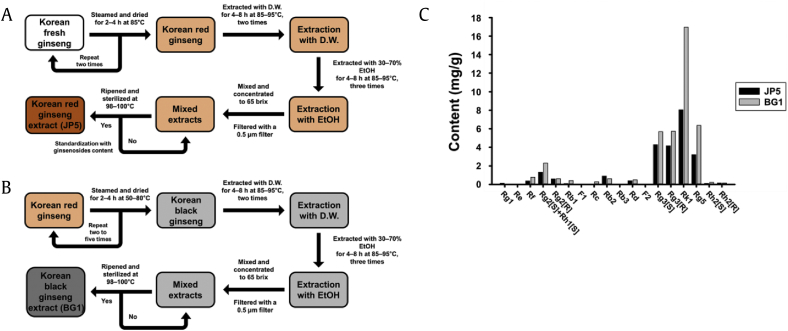
Korean fresh ginseng samples (4-year-old ginseng) were purchased from Jeonbuk Ginseng Nonghyup in Jinan province, South Korea. Korean Red Ginseng was obtained by steaming and drying white ginseng. Korean black ginseng was also obtained by steaming and drying red ginseng. Korean Red Ginseng and Korean black ginseng were extracted with deionized water and ethanol. The Korean Red Ginseng and Korean black ginseng extracts were termed JP5 and BG1, individually. Additional information on JP5 and BG1 extraction methods is described in our previous study [22]. The preparation procedures are summarized in (Fig. 1A and B), and the extracts compositions are shown in (Fig. 1C). The ginsenoside Rg5 was obtained from Korean Ginseng Research Corporation (Yangpyeong, South Korea). Antibodies against catalase, SOD2, GR, phospho-c-Jun N-terminal kinase (JNK), and GAPDH were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies against p-NF-κB, TNF-α, NLRP3, caspase-1, IL-1β, B-cell lymphoma 2 (BCL2), and JNK were purchased from Cell Signaling Technology (Beverly, MA, USA). GSH enzyme-linked immunosorbent assay (ELISA) kits were purchased from Cayman Chemicals Co. (Ann Arbor, MI, USA). Thiazolyl blue tetrazolium bromide (MTT) was purchased from Alfa Aesar Chemical Inc. (Ward Hill, MA, USA). Unless noted otherwise, all chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Fig. 1.
Preparation procedure and component of JP5 and BG1. (A) Korean Red Ginseng extract (JP5) preparation process. (B) Korean black ginseng extract (BG1) preparation process. (C) Analysis of JP5 and BG1 content by high-pressure liquid chromatography (HPLC).
2.2. Animal study design
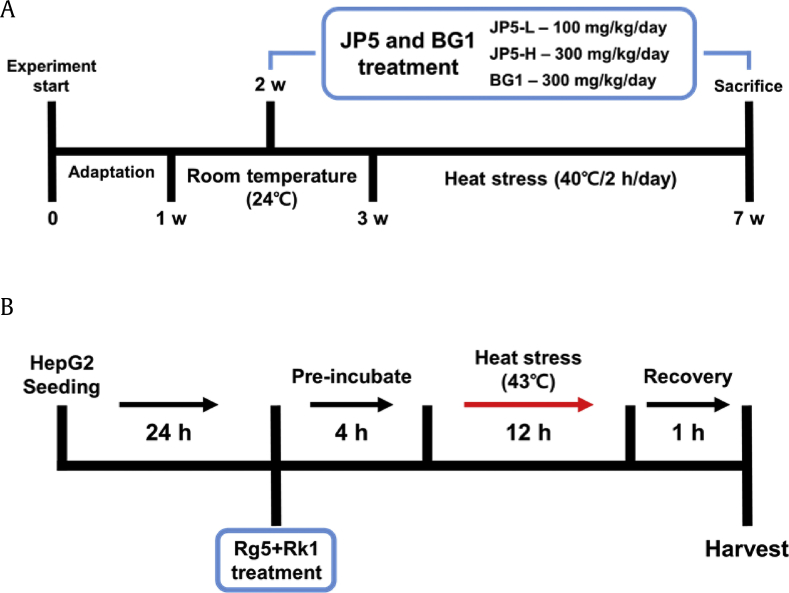
Seventy 8-weeks-old male Sprague–Dawley (SD) rats were purchased from Orient Bio (Gyeonggi-do, Korea) and housed under standard conditions (12 h light/dark cycle, 23–26°C, 50–55% humidity, light intensity of 100–200 Lux, sterilized tap water, and commercial rodent food ad libitum). All rats were used after a week of quarantine and acclimation. The animals were randomly divided into five groups (n = 14 each): room temperature (RT), heat stress (HS), heat stress + JP5 low dose (JP5-L, 100 mg/kg), heat stress + JP5 high dose (JP5-H, 300 mg/kg), and heat stress + BG1 (BG1, 300 mg/kg). RT groups were housed at room temperature for 6 weeks. The other groups were housed at room temperature for 2 weeks and then subjected to heat stress (40 ± 1°C for 2 h/day) for 4 weeks. A device for heat stress exposure (LH-10433G) was purchased from Lassele (Ansan, South Korea). Rats were treated with JP5 and BG1 from the second week until the end of the experiment. The design of the experiment to test the protective effect of JP5 and BG1 on SD rats is summarized in (Fig. 2A). After the end of the experiment, blood was harvested and stored at −20°C until analysis. Liver tissues were extracted and stored at −80°C until analysis. All experimental procedures were performed in accordance with the guidelines for animal experimentation provided by the Faculty of Agriculture, CHA University in accordance (Seongnam, Gyeonggi-do, Korea).
Fig. 2.
Experimental design. (A) Animal experiments: 7 weeks including 1-week adaptation period and pretreatment with JP5 and BG1 at 2 weeks. After the end of the experiment, rats were sacrificed according to animal use guidelines. (B) Cell-based experiments: cells were seeded and treated with Rg5 24 h later. After 4 h of preincubation, the cells were incubated at 43°C to induce heat stress.
2.3. Cell study design
HepG2 cells were purchased from the American Type Culture Collection. Cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) and 100 μg/mL penicillin–streptomycin and grown at 37°C in a 5% CO2 air environment. HepG2 cells were seeded at a density of 1 × 104 cells per well in 96-well plates, and the MTT assay was performed as described previously [22]. To obtain results of MTT assays and ELISA kits, absorbance was measured at 450 nm and 570 nm, respectively, on a PowerWave HT ELISA reader (BioTek, Winooski, VT, USA). As shown in (Fig. 2B), for the cell-based heat stress model, HepG2 cells were seeded in 100 mm dishes at a density of 5 × 105 cells/dish. After 24 h, the cells were treated with Rg5, incubated for an additional 4 h, and then exposed to heat stress (43°C) for 12 h. After a 1 h recovery period, the cells were harvested.
2.4. RNA isolation and reverse transcription polymerase chain reaction (RT-PCR)
Liver tissue and HepG2 cells were homogenized in 500 μL of Trizol reagent, and RNA was subsequently extracted according to the Trizol protocol. To generate cDNA, 1 μg of total RNA was subjected to RT-PCR. Primer sequences were as follows: 18S, forward (GGCCCGAAGCGTTTACTTTGAA) and reverse (GCATCGCCAGTCGGCATCGTTTAT); catalase, forward (AAGGTTTGGCCTCACAAGG) and reverse (CGGCAATGTTCTCACACAG); GPx, forward (GTGTATGCCTTCTCGGCGCG) and reverse (CGTTGCGACACACCGGAGAC); GR, forward (CAGTGGGACTCACGGAAGAT) and reverse (TTCACTGCAACAGCAAAACC); and SOD2, forward (GCACATTAACGCGCAGATCA) and reverse (AGCCTCCAGCAACTCTCCTT). PCR products were run on 1.5% agarose gels, stained with ethidium bromide, and photographed.
2.5. Western blotting
Liver tissue and HepG2 cells were washed with phosphate-buffered saline (PBS) and homogenized in 300 μL of radio immunoprecipitation assay (RIPA) buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS] supplemented with protease inhibitors (1 mM PMSF, 5 μg/mL aprotinin, and 5 μg/mL leupeptin) and phosphatase inhibitors (1 mM Na3VO4 and 1 mM NaF) and then centrifuged at 18,000 g for 10 min at 4°C. Protein concentration was determined by BCA assay (Pierce, Rockford, IL, USA) using bovine serum albumin as the standard. Proteins (20 μg/lane) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Osmonics, Minnetonka, MN, USA). The membranes were incubated with specific primary antiserum in tris-buffered saline (TBS) containing 0.05% Tween-20 (TBS-T) and 5% non-fat dry milk at 4°C overnight. After three washes with TBS-T, the blots were incubated with peroxidase-conjugated IgG for 1 h at room temperature, visualized using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA), photographed on a ChemiDoc system, and analyzed using the Image Lab Software (Bio-Rad Laboratories, Hercules, CA, USA).
2.6. Statistical analysis
Differences among groups were evaluated by one-way analysis of variance, followed by Duncan's multiple range test, using the SPSS software system (SPSS for Windows, version 20; SPSS, Inc., Chicago, IL, USA). In figures, values labeled with different letters are significantly different (p < 0.05).
3. Results
3.1. Body weight, food intake, water consumption, and liver weight
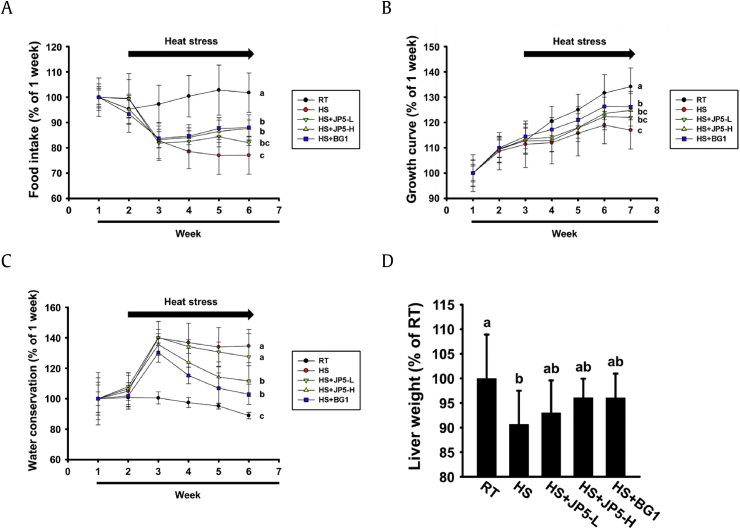
The change in body weight, food intake, and water conservation according to heat stress in each group was expressed as a percentage change compared with the mean value at 1 week. We observed no significant difference in body weight, food intake, and water conservation changes in all groups prior to heat stress but saw a dramatic difference in the HS group after application of heat stress. As shown in (Fig. 3), food intake decreased 24% after the end of heat stress, and body weight decreased by about 18%. Growth rate relative to the first week was 134% in the RT group, but only 116% in the HS group. However, in the JP5- and BG1-treated groups, food intake increased by 10% and 11% relative to the HS group (Fig. 3A), and body weights increased by 8% and 10%, respectively (Fig. 3B). In addition, water intake increased 46% in the HS group relative to the RT group, but only 23% and 14% in the JP5- and BG1-treated groups, respectively (Fig. 3C). After the end of the experiment, liver weights were measured by dissection to determine the effects of heat stress, JP5, and BG1 on the liver. In the HS group, liver weight decreased by 10% relative to the RT group, but this decrease in liver weight was suppressed in the JP5- and BG1-treated groups (Fig. 3D).
Fig. 3.
Effects of heat stress, JP5 and BG1 on food intake, growth curve, water conservation, and liver weight in rats. Rats were reared at room temperature (RT) for 2 weeks and then exposed to heat stress (HS) (2 h/day) for 4 weeks. (A) Food intake, (B) growth curve, and (C) water conservation were monitored weekly and calculated as a percentage relative to the value at 1 week. (D) Wet liver weight after sacrifice. Results are expressed as means ± standard deviation (n = 7, repeated). Values labeled with different letters are significantly different (p < 0.05).
3.2. Effect of heat stress and JP5 and BG1 on genes related to oxidative stress
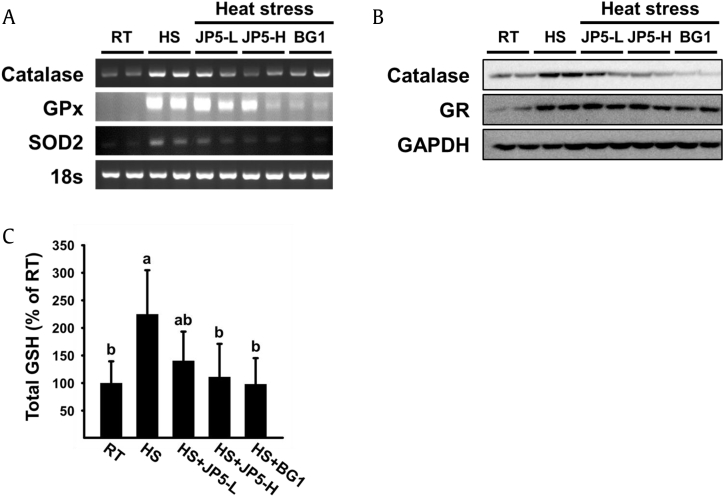
To investigate the effect of JP5 and BG1 on oxidative stress induced by ROS generation, we monitored the expression of genes related to oxidative stress in the liver. Expression levels of catalase, GPx, and SOD2, which are involved in ROS removal, were significantly increased by heat stress. Both the protein and mRNA levels of catalase followed the same trends. However, the expression of all three genes was dramatically reduced in groups treated with JP5 and BG1 (Fig. 4A). GR, another ROS scavenging factor, was upregulated at the protein level in the HS group but downregulated in groups treated with JP5 and BG1 (Fig. 4B). Serum analysis revealed that GSH secretion was increased by heat stress, whereas GSH secretion of JP5 and BG1-treated groups was suppressed to a similar degree as in the RT group (Fig. 4C).
Fig. 4.
Expression of oxidative stress–related genes and proteins in liver tissue and serum. (A) Analysis of catalase, GPx, and SOD2 gene expression by RT-PCR. 18S rRNA was used as a loading control. (B) Analysis of catalase and GR protein expression by Western blot. GAPDH was used as a loading control.
HS, heat stress; RT, room temperature; SOD, superoxide dismutase; GR, glutathione reductase; GPx, glutathione peroxidase; GSH, glutathione; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
3.3. Effect of heat stress and Rg5 on cell viability and oxidative stress–related genes in HepG2 cells
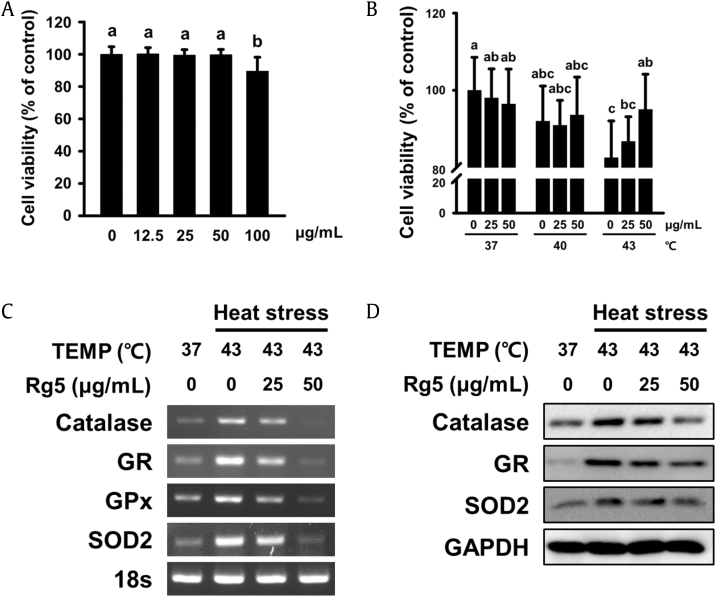
We then conducted cell culture experiments to further investigate the protective effects of the active components of JP5 and BG1 against heat stress and oxidative stress. Analysis of the components of the JP5 and BG1 used in the animal experiments confirmed that our extracts had a higher content of Rg5 and Rk1 than general Korean ginseng extracts (Fig. 1B). Based on this observation, we predicted that Rg5 and Rk1 would have protective effects against oxidative stress. To test this idea, we treated the liver cell-line HepG2 with Rg5. The MTT assay revealed that Rg5 was not toxic at doses up to 50 μg/mL (Fig. 5A). Cell viability was measured at 37, 40, and 43°C to determine the appropriate stress levels for cells. Cell viability was significantly reduced at 43°C (Fig. 5B). Therefore, final Rg5 concentrations of 25 μg/mL and 50 μg/mL were used at 43°C. When HepG2 cells were exposed to heat stress, expression levels of catalase, GR, GPx, and SOD2 (all of which are involved in ROS clearance) increased, as in the animal experiments. However, in the Rg5-treated group, the increase in gene expression was inhibited in a dose-dependent manner (Fig. 5C). Both mRNA and protein expression levels exhibited the same trends (Fig. 5D).
Fig. 5.
Effects of heat stress and Rg5 on cell viability and expression of oxidative stress–related factors in HepG2 cells. (A) Dose-dependent cell viability was measured by MTT assay. (B) Cell viability was measured by MTT assay at three temperatures (37, 40, and 43°C) and three concentrations of Rg5 (0, 25, and 50 μg/mL). (C) Analysis of catalase, GR, GPx, and SOD2 gene expression by RT-PCR. 18S rRNA was used as a loading control. (D) Analysis of catalase, GR, and SOD2 protein expression by Western blot. GAPDH was used as a loading control. Values labeled with different letters are significantly different (p < 0.05).
MTT, Thiazolyl blue tetrazolium bromide; SOD, superoxide dismutase; GR, glutathione reductase; GPx, glutathione peroxidase.
3.4. Effect of JP5, BG1, and Rg5 on inflammatory response by heat stress
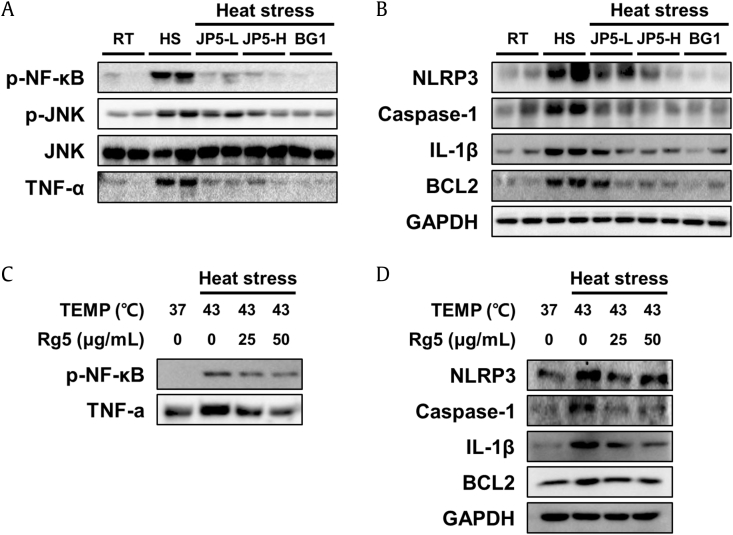
To investigate the effects of heat stress on the inflammatory response in the liver and the effect of JP5 and BG1, we monitored the expression of inflammatory factors. In general, phosphorylation of NF-κB, a key inflammatory factor, affects secretion of cytokines and cell survival [29]. As shown in (Fig. 6A), heat stress increased NF-κB phosphorylation in liver tissue, but phosphorylation was inhibited in the JP5- and BG1-treated groups. The same trend was observed for phosphorylation of JNK, a downstream factor of MAPK. In addition, the inflammatory cytokine TNF-α was also elevated by heat stress but inhibited by JP5 and BG1. Especially in JP5-H and BG1, levels of phosphorylation and protein expression were similar to those in the RT group. (Fig. 6B) shows that NLRP3 and caspase-1, which constitute the NLRP3 inflammasome [16], were both upregulated in the HS group, and expression of IL-1β was also elevated. In addition, BCL2, an apoptosis-related factor induced by inflammatory reactions [30], exhibited the same tendency. In cells, phosphorylation of NF-κB and the expression of TNF-α were increased by heat stress, but Rg5 inhibited this increase (Fig. 6C). As in animal tissues, NLRP3, caspase-1, IL-1β, and BCL2 exhibited the same tendencies (Fig. 6D).
Fig. 6.
Expression of inflammatory proteins in liver tissue and HepG2 cells. (A) Expression of proteins related to inflammation, including p-NF-κB, p-JNK, JNK, and TNF-α analyzed by Western blot. (B and D) Expression of proteins related to the NLRP3 inflammasome and apoptosis, including NLRP3, caspase-1, IL-1β, and BCL2, in (B) liver tissue and (D) HepG2 cells. (C)The effects of Rg5 and heat stress on expression of inflammatory factors p-NF-κB and TNF-α were confirmed in HepG2 cells. GAPDH was used as a loading control.
TNF-α, tumor necrosis factor-alpha; RT, room temperature; HS, heat stress.
4. Discussion
Oxidation plays important roles in signal transduction in the body [31]. However, problems arise when ROS produced by high levels of oxidation reactions exceed the capacity of antioxidant protection systems. Oxidative stress induced by heat stress is also caused by excessive oxidation, which can cause many problems in the body. Antioxidant systems increase the expression of antioxidant enzymes such as catalase, SOD2, GR, and GPx to eliminate ROS caused by heat stress [32], [33], [34].
We found that when JP5 and BG1 were administered to heat-stressed cells, the expression of the antioxidant enzymes did not increase, suggesting that ROS had been effectively removed via the antioxidant effect of ginsenoside, a major component of ginseng. In addition, as reported in our previous studies, JP5 and BG1 help maintain homeostasis and effectively protect against the stress response to high temperature. We assume that this protective effect involves inhibition of ROS generation or elimination of the ROS produced in response to heat stress.
Korean ginseng contains various kinds of ginsenosides, which have different functions depending on the species [35]. JP5 and BG1, extracted from Korean Red Ginseng and black ginseng, have high levels of Rg5 and Rk1. Therefore, we examined the antioxidant effects of JP5 and BG1 by administering Rg5 and Rk1 to cultured cells. The results revealed that Rg5 and Rk1 exert antioxidant and antiinflammatory effects in the liver. Because Rg5 and Rk1 interconvert under normal conditions, we used a mixture of Rg5 and Rk1 in our cell-based experiments. Previous studies showed that Rg3 also has an antioxidant effect [36], [37]. Because JP5 and BG1 also have a high content of Rg3, they may also exhibit synergistic effects with Rg5 in terms of antioxidant function. We plan to investigate this possibility in future studies.
In a previous study, we showed that antioxidant function is activated by ROS and inhibited by Korean ginseng [24]. In addition, we found that JP5 and BG1 prevent activation of NF-κB by ROS and its downstream effects on cytokine secretion. Especially, it helped to reveal that affect the NLRP3 inflammasome-related factors and signaling pathways.
In conclusion, JP5 and BG1 inhibit induction of ROS production by heat stress and thus prevent oxidative stress from damaging the body. At a molecular level, Rg5 inhibits NF-κB signaling by ROS and the activity of the NLRP3 inflammasome, a sub-signal of the NF-κB pathway, thereby suppressing the inflammatory response to oxidative stress. Therefore, JP5 and BG1 can prevent oxidative damage caused by heat stress in liver tissue. Although pharmaceuticals may cause hepatotoxicity, ginseng extract has no such side-effects and therefore represents a potentially useful therapeutic agent against oxidative stress. We anticipate that our JP5 and BG1 could be used as a functional food to defend against heat stress and oxidative stress in high-temperature environments.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was partially supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1A09917209) and by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Export Promotion Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (316014032HD020).
References
- 1.Myers S.S., Bernstein A. The coming health crisis: indirect health effects of global climate change. F1000 Biol Rep. 2011;3:3. doi: 10.3410/B3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGeehin M.A., Mirabelli M. The potential impacts of climate variability and change on temperature-related morbidity and mortality in the United States. Environ Health Perspect. 2001;109(Suppl 2):185–189. doi: 10.1289/ehp.109-1240665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulman F.G., Danon A., Pfeifer Y., Tal E., Weller C.P. Urinalysis of patients suffering from climatic heat stress (Sharav) Int J Biometeorol. 1970;14:45–53. doi: 10.1007/BF01440676. [DOI] [PubMed] [Google Scholar]
- 4.Keim S.M., Guisto J.A., Sullivan J.B., Jr. Environmental thermal stress. Ann Agric Environ Med. 2002;9:1–15. [PubMed] [Google Scholar]
- 5.Jian B., Hsieh C.H., Chen J., Choudhry M., Bland K., Chaudry I., Raju R. Activation of endoplasmic reticulum stress response following trauma-hemorrhage. Biochim Biophys Acta. 2008;1782:621–626. doi: 10.1016/j.bbadis.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonna L.A., Wenger C.B., Flinn S., Sheldon H.K., Sawka M.N., Lilly C.M. Exertional heat injury and gene expression changes: a DNA microarray analysis study. J Appl Physiol (1985) 2004;96:1943–1953. doi: 10.1152/japplphysiol.00886.2003. [DOI] [PubMed] [Google Scholar]
- 7.Ando M., Katagiri K., Yamamoto S., Wakamatsu K., Kawahara I., Asanuma S., Usuda M., Sasaki K. Age-related effects of heat stress on protective enzymes for peroxides and microsomal monooxygenase in rat liver. Environ Health Perspect. 1997;105:726–733. doi: 10.1289/ehp.97105726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 9.Gutteridge J.M., Halliwell B. Comments on review of free radicals in biology and medicine. In: Halliwell Barry, Gutteridge John M.C., editors. 2nd ed. vol. 12. 1992. pp. 93–95. (Free radic biol med). [Google Scholar]
- 10.Xia F., Wang C., Jin Y., Liu Q., Meng Q., Liu K., Sun H. Luteolin protects HUVECs from TNF-alpha-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-kappaB and MAPK pathways. J Atheroscler Thromb. 2014;21:768–783. doi: 10.5551/jat.23697. [DOI] [PubMed] [Google Scholar]
- 11.Jiang F., Gao Y., Dong C., Xiong S. ODC1 inhibits the inflammatory response and ROS-induced apoptosis in macrophages. Biochem Biophys Res Commun. 2018;504(4):734–741. doi: 10.1016/j.bbrc.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Andersen H.R., Nielsen J.B., Nielsen F., Grandjean P. Antioxidative enzyme activities in human erythrocytes. Clin Chem. 1997;43:562–568. [PubMed] [Google Scholar]
- 13.Wang H., Wang L., Li N.L., Li J.T., Yu F., Zhao Y.L., Wang L., Yi J., Wang L., Bian J.F. Subanesthetic isoflurane reduces zymosan-induced inflammation in murine Kupffer cells by inhibiting ROS-activated p38 MAPK/NF-kappaB signaling. Oxid Med Cell Longev. 2014;2014:851692. doi: 10.1155/2014/851692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J., Min J.S., Kim B., Chae U.B., Yun J.W., Choi M.S., Kong I.K., Chang K.T., Lee D.S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci Lett. 2015;584:191–196. doi: 10.1016/j.neulet.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci. 2012;33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Misih S.R., Bloomston M. Liver anatomy. Surg Clin North Am. 2010;90:643–653. doi: 10.1016/j.suc.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brockmoller J., Roots I. Assessment of liver metabolic function. Clinical implications. Clin Pharmacokinet. 1994;27:216–248. doi: 10.2165/00003088-199427030-00005. [DOI] [PubMed] [Google Scholar]
- 19.Pikarsky E., Porat R.M., Stein I., Abramovitch R., Amit S., Kasem S., Gutkovich-Pyest E., Urieli-Shoval S., Galun E., Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 20.Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401–435. doi: 10.1007/978-3-0348-0837-8_16. [DOI] [PubMed] [Google Scholar]
- 21.Kim K.J., Hong H.D., Lee O.H., Lee B.Y. The effects of Acanthopanax senticosus on global hepatic gene expression in rats subjected to heat environmental stress. Toxicology. 2010;278:217–223. doi: 10.1016/j.tox.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Song J.-H., Kim K.-J., Choi S.-Y., Koh E.-J., Park J., Lee B.-Y. Korean ginseng extract ameliorates abnormal immune response through the regulation of inflammatory constituents in Sprague Dawley rat subjected to environmental heat stress. Journal of Ginseng Research. 2018 doi: 10.1016/j.jgr.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh E.J., Kim K.J., Choi J., Jeon H.J., Seo M.J., Lee B.Y. Ginsenoside Rg1 suppresses early stage of adipocyte development via activation of C/EBP homologous protein-10 in 3T3-L1 and attenuates fat accumulation in high fat diet-induced obese zebrafish. J Ginseng Res. 2017;41:23–30. doi: 10.1016/j.jgr.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K.J., Yoon K.Y., Hong H.D., Lee B.Y. Role of the red ginseng in defense against the environmental heat stress in Sprague Dawley rats. Molecules. 2015;20:20240–20253. doi: 10.3390/molecules201119692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou J., Xue J., Lee M., Liu L., Zhang D., Sun M., Zheng Y., Sung C. Ginsenoside Rh2 improves learning and memory in mice. J Med Food. 2013;16:772–776. doi: 10.1089/jmf.2012.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang J.-B., Ha J.-H., Hawer W.-D., Nahmgung B., Lee B.-Y. Ginsenoside contents of Korean white ginseng and taegeuk ginseng with various sizes and cultivation years. Korean Journal of Food Science and Technology. 2005;37:508–512. [Google Scholar]
- 27.Lee B.-Y., Kim E.-J., Park D.-J., Hong S.-I., Chun H.-S. Composition of saponin and free sugar of some white ginsengs with processing conditions. Korean Journal of Food Science and Technology. 1996;28:922–927. [Google Scholar]
- 28.Kim S.-N., Kang S.-J. Effects of black ginseng (9 times-steaming ginseng) on hypoglycemic action and changes in the composition of ginsenosides on the steaming process. Korean Journal of Food Science and Technology. 2009;41:77–81. [Google Scholar]
- 29.Gonzalez-Ramos R., Defrere S., Devoto L. Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril. 2012;98:520–528. doi: 10.1016/j.fertnstert.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Glover M., Soni S., Ren Q., Maclennan G.T., Fu P., Gupta S. Influence of chronic inflammation on Bcl-2 and PCNA expression in prostate needle biopsy specimens. Oncol Lett. 2017;14:3927–3934. doi: 10.3892/ol.2017.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosentino-Gomes D., Rocco-Machado N., Meyer-Fernandes J.R. Cell signaling through protein kinase C oxidation and activation. Int J Mol Sci. 2012;13:10697–10721. doi: 10.3390/ijms130910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabuncuoglu S., Eken A., Aydin A., Ozgunes H., Orhan H. Cofactor metals and antioxidant enzymes in cisplatin-treated rats: effect of antioxidant intervention. Drug Chem Toxicol. 2015;38:375–382. doi: 10.3109/01480545.2014.974107. [DOI] [PubMed] [Google Scholar]
- 33.Sakaida I., Okita K. The role of oxidative stress in NASH and fatty liver model. Hepatol Res. 2005;33:128–131. doi: 10.1016/j.hepres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan S.K., Thangaraj K.R., Eapen C.E., Ramachandran A., Mukhopadhya A., Mathai M., Seshadri L., Peedikayil A., Ramakrishna B., Balasubramanian K.A. Liver injury in acute fatty liver of pregnancy: possible link to placental mitochondrial dysfunction and oxidative stress. Hepatology. 2010;51:191–200. doi: 10.1002/hep.23245. [DOI] [PubMed] [Google Scholar]
- 35.Mohanan P., Subramaniyam S., Mathiyalagan R., Yang D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res. 2018;42:123–132. doi: 10.1016/j.jgr.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Chen L., Wang T., Jiang X., Zhang H., Li P., Lv B., Gao X. Ginsenoside Rg3 antagonizes adriamycin-induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomedicine. 2015;22:875–884. doi: 10.1016/j.phymed.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Wei X., Su F., Su X., Hu T., Hu S. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia. 2012;83:636–642. doi: 10.1016/j.fitote.2012.01.006. [DOI] [PubMed] [Google Scholar]