Abstract
Background
We investigated the tolerability and pharmacokinetic properties of various ginsenosides, including Rb1, Rb2, Rc, Rd, and compound K, after single or multiple administrations of red ginseng extract in human beings.
Methods
Red ginseng extract (dried ginseng > 60%) was administered once and repeatedly for 15 days to 15 healthy Korean people. After single and repeated administration of red ginsengextract, blood sample collection, measurement of blood pressure and body temperature, and routine laboratory test were conducted over 48-h test periods.
Results
Repeated administration of high-dose red ginseng for 15 days was well tolerated and did not produce significant changes in body temperature or blood pressure. The plasma concentrations of Rb1, Rb2, and Rc were stable and showed similar area under the plasma concentration-time curve (AUC) values after 15 days of repeated administration. Their AUC values after repeated administration of red ginseng extract for 15 days accumulated 4.5- to 6.7-fold compared with single-dose AUC. However, the plasma concentrations of Rd and compound K showed large interindividual variations but correlated well between AUC of Rd and compound K. Compound K did not accumulate after 15 days of repeated administration of red ginseng extract.
Conclusion
A good correlation between the AUC values of Rd and compound K might be the result of intestinal biotransformation of Rb1, Rb2, and Rc to Rd and subsequently to compound K, rather than the intestinal permeability of these ginsenosides. A strategy to increase biotransformation or reduce metabolic intersubject variability may increase the plasma concentrations of Rd and compound K.
Keywords: ginsenosides, pharmacokinetics, red ginseng, single and repeated administration, tolerability
Abbreviations: Hank's balanced salt solution, HBSS; apical to basal, A to B; basal to apical, B to A; apparent permeability, Papp; multiple reaction monitoring, MRM; liquid chromatography-tandem mass spectrometry, LC-MS/MS; area under the plasma concentration-time curve, AUC; maximum plasma concentration, Cmax; time to reach Cmax, Tmax; t1/2, elimination half-life; MRT, mean residence time
1. Introduction
Ginseng and specifically the roots and rhizomes of Panax ginseng are among the most popular herbal medicines in East Asian countries and worldwide. In the United States and Europe, ginseng is widely used as a medical food and pharmaceutical excipient (in vitamin pills) [1]. In recent years, the market for red ginseng products has rapidly increased because of its significant biological effects, good tolerability, and extended storage period compared with fresh and white ginseng [2], [3]. Ginsenosides, also known as steroid-like saponins, are considered as the major active pharmacological constituents of ginseng [4], [5]. To this end, numerous studies have described the immunological, antioxidant, anticoagulant, antineoplastic, neuroprotective, and hepatoprotective effects of ginseng and its associated ginsenosides [1], [5], [6], [7], [8]. However, ginsenoside content can vary depending on the method of ginseng product preparation [9]. Additionally, ginsenosides have relatively low bioavailability. The bioavailabilities of Rb1 and Rg1 were reported as 0.1 and 2%, respectively, by Odani et al. [10] and 4.3 and 18.4%, respectively, by Xu et al. [11]. Thus, the comprehensive detection of various ginsenosides after red ginseng extract administration was limited. Ginsenosides Rb1, Rb2, Rb3, Rg1, Rd, and Re have been measured in rat plasma after oral administration of the ginseng extract [11], [12]. Ginsenoside Rb1 and compound K are detectable in human plasma after a single oral dose (3 g) of the Korean Red Ginseng extract. In this study, the maximum plasma concentration of compound K was higher than that of Rb1; however, the plasma exposure [area under the plasma concentration-time curve (AUC)] and half-life of Rb1 were higher than those of compound K [13]. Additionally, Rb1 is the most abundant and stable ginsenoside in rats, exhibiting a long elimination half-life [11], [13]. In contrast, the plasma concentrations of compound K in previous studies varied according to the ginseng product used. The administration of fermented red ginseng extract resulted in a plasma concentration of compound K that was more than 10-fold that after administration of nonfermented red ginseng extract [9]. Moreover, previous studies have reported that the plasma concentration of compound K was affected by diet including prebiotic fibers and the gut microbiome [14], [15]. Accordingly, there is a need for studies identifying the relationship between pharmacological effects of ginseng and pharmacokinetic properties of its components including Rb1 and compound K. Nevertheless, quantitative pharmacokinetic data for ginsenosides Rb2, Rc, Rd, and Re as well as Rb1 and compound K detected in human plasma after ginseng administration are limited.
Therefore, the objective of this study was to examine the tolerability and pharmacokinetic properties of various ginsenosides such as Rb1, Rb2, Rc, Rd, and compound K after single or multiple administrations of red ginseng extract in human beings and to investigate the intestinal permeability of these ginsenosides in Caco-2 cell system.
2. Materials and methods
2.1. Materials
Red ginseng extract (Hong Sam Jung All day; lot no. 731902) was purchased from the Punggi Ginseng Cooperative Association (Youngjoo, Kyungpook, Republic of Korea). Ginsenosides Rb1, Rb2, Rc, Rd, Re, Rg1, Rg3, Rh1, Rh2, F1, F2, compound K, 20(s)-protopanaxadiol, and 20(s)-protopanaxatriol were purchased from the Ambo Institute (Daejeon, Republic of Korea). Berberine, used as an internal standard (IS), was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals and solvents were reagent or analytical grade.
2.2. Study participants and design
We conducted an open-label, randomized, single-sequence study in healthy male adult volunteers to investigate the pharmacokinetic profiles of ginsenosides after single-dose and repeated dose administration of red ginseng extract for 15 days. All individuals provided written informed consent before study enrollment. Healthy individuals who participated in this study were aged ≥ 19 years and with body weight ≥ 50 kg. They had no clinically significant abnormalities as confirmed by the clinical laboratory test, detailed physical examination, serology tests, 12-lead electrocardiography, and clinical history, which were conducted within 4 weeks before this study. The study was approved by the Institutional Review Board of Kyungpook National University Hospital (KNUH, Daegu, Republic of Korea) and was conducted at the KNUH Clinical Trial Center in accordance with the applicable Good Clinical Practice guidelines (CRIS registry no. KCT0002418) and the ethical standards of the Declaration of Helsinki.
From days 1 to 15, the individuals ingested three pouches of concentrated red ginseng extract (Hong Sam Jung All day; dried ginseng > 60%, Table 1) per day. Blood samples (7 mL) were collected in a heparinized tube at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, and 48 h after dose via a saline-locked angiocatheter. Plasma was collected by centrifugation for 10 min at 3000 rpm and stored at –70°C until analysis.
Table 1.
Ginsenoside content in red ginseng extract
| Content in red ginseng extract (Hong Sam Jung All day) | |||
|---|---|---|---|
| Ginsenoside | mg/day | Composition (%) | |
| Panaxadiol | Rb1 | 23.0 ± 1.3 | 27.0 |
| Rb2 | 11.4 ± 0.6 | 13.4 | |
| Rc | 13.0 ± 1.7 | 15.3 | |
| Rd | 6.6 ± 0.5 | 7.7 | |
| Rh2 | 0.0 ± 0.0 | 0.0 | |
| Rg3 | 14.1 ± 2.8 | 16.5 | |
| F2 | 0.0 ± 0.0 | 0.0 | |
| Compound K | 0.0 ± 0.0 | 0.0 | |
| 20(s)-protopanaxadiol | 0.0 ± 0.0 | 0.0 | |
| Panaxatriol | Re | 6.2 ± 0.2 | 7.3 |
| Rh1 | 8.0 ± 1.1 | 9.4 | |
| Rg1 | 2.8 ± 1.2 | 3.3 | |
| F1 | 0.0 ± 0.0 | 0.0 | |
| 20(s)-protopanaxatriol | 0.0 ± 0.0 | 0.0 | |
Data were expressed as mean ± SD from 10 pouches of red ginseng extract
SD, standard deviation
2.3. Safety and tolerability assessments
The safety assessment was based on laboratory and clinical adverse events collected during the red ginseng administration. Vital signs (the heart rate and blood pressure) were monitored at screening, before and after red ginseng administration (at 2, 4, 8, and 12 h after dose). Body temperature was assessed at screening, before and after red ginseng administration (at 2, 4, 8, and 12 h after dose). Physical examination and routine laboratory tests (serum chemistry, urinalysis, and hematology) were performed at screening, before red ginseng administration on days 1 and 15 and at the follow-up visit (7 ± 2 days after the last dose). Twelve-lead electrocardiography was obtained at screening and at the follow-up visit. All laboratory tests were conducted at the Department of Laboratory Medicine, KNUH.
2.4. Permeability of ginsenoside in Caco-2 cells
Caco-2 cells [American Type Culture Collection (Rockville, MD, USA); passage number 41-43] were grown in Dulbecco's Modified Eagle's medium, supplemented with 20% of fetal bovine serum, 1% of nonessential amino acids, 4 mM of L-glutamine, and 1% of penicillin-streptomycin. Cells were allowed to reach 70% of confluence and then seeded in 12-Transwell plates at a density of 5 × 105 cells/insert. Cells were grown for 21 days with medium change of every 2 days [16].
Aliquots (0.5 mL) of Hank's balanced salt solution containing 10 μM of each ginsenoside (Rb1, Rb2, Rd, Rd, compound K, Rg1, and Rh1) were added to the apical side, and 1.5 mL of fresh Hank's balanced salt solution was added to the basal side. Apical to basal and basal to apical transport of ginsenoside was measured for 1 h as previously described [17]. Aliquots (50 μL) of transport samples were added to 200 μL of methanol containing berberine (0.5 ng/mL), vortexed for 10 min, and centrifuged for 5 min at 13,200 rpm. Aliquots (10 μL) of the supernatant were directly injected into a LC-MS/MS system.
2.5. Bioanalytical methods for ginsenoside Rb1, Rb2, Rc, Rd, and compound K
Fourteen ginsenoside concentrations in diluted red ginseng extract and samples for the in vitro permeability study were determined by an Agilent 6470 Triple Quadrupole LC-MS/MS system (Agilent, Wilmington, DE, USA). Separation was applied on a Synergi Polar RP column (2.0 mm × 150 mm, 4 μm particle size; Phenomenex, Torrance, CA, USA) using a mobile phase consisting of water (A) and methanol (B) containing 0.1% of formic acid at a flow rate of 0.3 mL/min. The gradient program for mobile phase was as follows: (1) 0–1.0 min, 70% B; (2) 1.0–6.5 min, 90% B; (3) 6.5–7.0 min, 80% B; and (4) 7.0–14 min, 70% B. A total of 100 mg of red ginseng extracts were diluted 50-fold with methanol, and diluted samples or samples for the in vitro permeability study (50 μL) were mixed with 200-μL methanol containing berberine (0.5 ng/mL), incubated for 10 min, and centrifuged for 5 min at 13,200 rpm. Aliquots (10 μL) of the supernatant were directly injected into the LC-MS/MS system. Quantification was carried out using multiple reaction monitoring (MRM) at m/z 1131.6 → 365.1 for Rb1 [retention time (tR) 4.1 min], m/z 1101.6 → 335.1 for Rb2 (tR 5.0 min) and Rc (tR 4.2 min), m/z 969.9 → 789.5 for Rd (tR 5.1 min) and Re (tR 1.8 min), m/z 823.5 → 365.1 for Rf (tR 2.7 min), m/z 824.0 → 643.6 for Rg1 (tR 1.9 min), m/z 807.5 → 365.1 for Rg3 (tR 6.0 min), m/z 661.5 → 203.1 for Rh1 (tR 3.2 min), m/z 645.5 → 645.5 for Rh2 (tR 6.9 min), m/z 661.5 → 203.1 for F1 (tR 3.7 min), m/z 807.5 → 627.5 for F2 (tR 6.1 min), m/z 645.5 → 203.1 for compound K (tR 6.8 min), m/z 483.4 → 483.4 for 20(s)-protopanaxadiol, m/z 499.4 → 499.4 for 20(s)-protopanaxatriol, and m/z 336.1 → 320.0 for berberine (IS) in positive ionization mode with collision energy of 30–65 eV.
Human plasma samples (50 μL) were mixed with 200 μL of methanol containing berberine (0.5 ng/mL; IS) for 10 min and centrifuged for 5 min at 13,200 rpm. Aliquots (10 μL) of the supernatant were directly injected into the LC-MS/MS system for the simultaneous analysis of ginsenosides Rb1, Rb2, Rc, Rd, and compound K. The analytical conditions were identical to those described previously except for the solvent gradient; the solvent gradient program was as follows: (1) 0 min, 69% B, (1) 0-3.0 min, 85% B; (3) 3.0-6.0 min, 85% B; (4) 6.0-6.5 min, 69% B; and (5) 6.5-14 min, 69% B. The flow rate was 0.27 mL/min. Quantification was performed using MRM at m/z 1131.6 → 365.1 for Rb1 (tR 4.4 min), m/z 1101.6 → 335.1 for Rb2 (tR 5.4 min) and Rc (tR 4.6 min), m/z 969.9 → 789.5 for Rd (tR 6.2 min), m/z 645.5 → 203.1 for compound K (tR 9.5 min), and m/z 336.1 → 320.0 for berberine (IS, tR 4.1 min) in positive ionization mode with collision energy of 30–65 eV.
2.6. Data analysis
Pharmacokinetic parameters were estimated by noncompartmental methods (WinNonlin version 2.0, Pharsight Co., Certara, NJ, USA). The maximum plasma ginsenoside concentration (Cmax) and time to reach Cmax (Tmax) were obtained directly from the results. The area under the plasma concentration-time curve from 0 to the last sampling time was calculated using the trapezoidal method. Half-life (t1/2) was calculated by dividing 0.693 by the terminal elimination constant [17].
The apparent permeability (Papp) of ginsenoside was determined by dividing the transport rate of ginsenoside (V) by the ginsenoside concentration added in the donor side (C) and by the surface area of the insert (A): Papp = V/C × A [17].
2.7. Statistical analysis
Demographics, pharmacokinetic parameters, and safety data were shown as descriptive statistics. All pharmacokinetic parameters are given as the mean ± standard deviation. All statistical analyses were performed using SAS (ver. 9.4; SAS Institute Inc., Cary, NC, USA). A p-value < 0.05 was deemed to be statistically significant.
3. Results
3.1. Safety and tolerability of red ginseng extract after single and repeated administrations
The study included 15 healthy male individuals. The mean age was 24.9 years (range, 22–28 years), the mean height was 173.6 cm (range 159.9–182.3 cm), and the mean weight was 68.8 kg (range, 57.1–80.8 kg). All 15 individuals were included in the pharmacokinetic analysis of ginsenoside and safety assessment.
The ginsenoside content of the red ginseng extract provided to the participants daily for 15 days (three pouches of Hong Sam Jung All day per day) is summarized in Table 1. The most abundant ginsenoside was Rb1 (23.01%), followed by Rb2, Rc, and Rg3 (11.39–14.07%). The abundances of Rd, Re, and Rh1 were 6.21–8.03%.
Oral administration of three red ginseng pouches daily was well tolerated and did not produce any unexpected or serious adverse events. Blood pressure and body temperature was stable after single and 15-day repeated administration. Alanine transaminase and aspartate transaminase levels remained unchanged after single and 15-day repeated administrations (Table 2).
Table 2.
Safety and tolerability assessments of participants after single or repeated administrations of red ginseng extract
| >Factors | Test time | On day 1 | On day 15 |
|---|---|---|---|
| Systolic blood pressure | 0h (predose) | 121.5 ± 9.0 | 120.4 ± 9.1 |
| 2h | 125.1 ± 7.5 | 123.7 ± 6.9 | |
| 4h | 126.5 ± 9.4 | 123.1 ± 9.2 | |
| 8h | 121.8 ± 10.6 | 128.0 ± 9.4 | |
| 12h | 124.6 ± 8.1 | 126.6 ± 9.0 | |
| Dystolic blood pressure | 0h (predose) | 78.9 ± 7.8 | 77.7 ± 8.8 |
| 2h | 81.2 ± 6.0 | 76.2 ± 5.3 | |
| 4h | 76.4 ± 8.5 | 76.5 ± 8.4 | |
| 8h | 71.3 ± 8.8 | 71.9 ± 7.1 | |
| 12h | 72.7 ± 8.9 | 71.5 ± 7.2 | |
| Body temperature | 0h (predose) | 36.3 ± 0.1 | 36.3 ± 0.3 |
| 2h | 36.3 ± 0.2 | 36.4 ± 0.2 | |
| 4h | 36.5 ± 0.3 | 36.5 ± 0.2 | |
| 8h | 36.7 ± 0.2 | 36.7 ± 0.2 | |
| 12h | 36.7 ± 0.2 | 36.6 ± 0.2 | |
| Alanine transaminase | 22.1 ± 9.8 | 17.0 ± 8.8 | |
| Aspartate transaminase | 19.9 ± 8.7 | 21.6 ± 9.1 |
Data were expressed as mean ± SD of 15 participants
SD, standard deviation
3.2. Chromatograms of ginsenoside Rb1, Rb2, Rd, Rd, and compound K in plasma samples
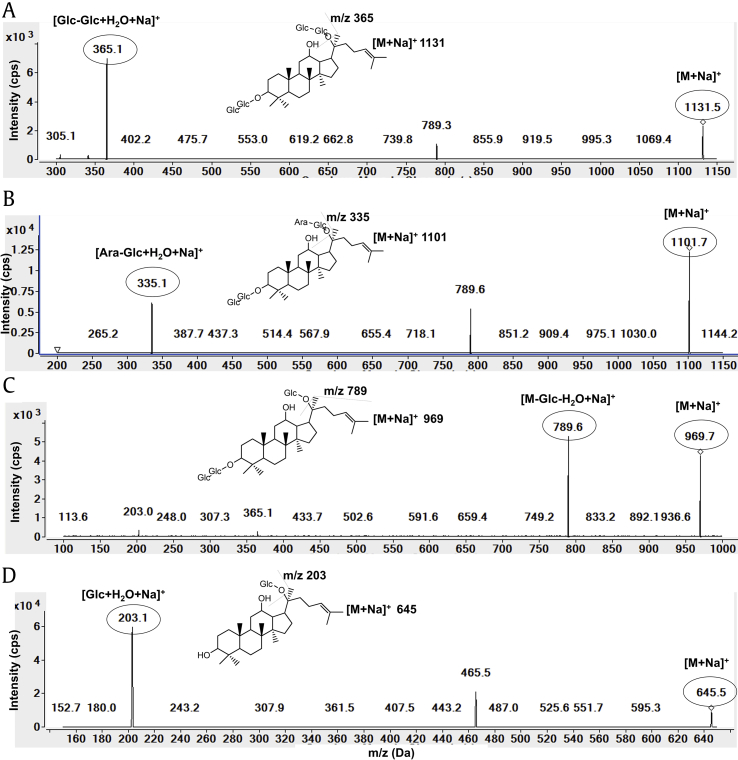
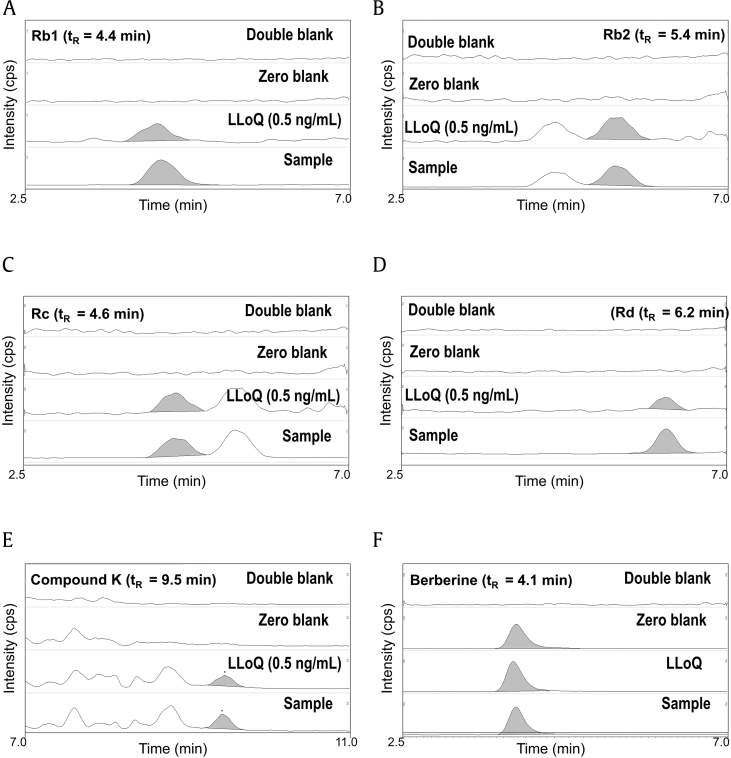
Of the 14 ginsenosides examined, only five ginsenosides (Rb1, Rb2, Rc, Rd, and compound K) were detected in our plasma samples. Therefore, we developed an analytical method for the simultaneous detection of ginsenosides Rb1, Rb2, Rd, Rd, and compound K in human plasma samples using a LC-MS/MS system and previously described methods with some modifications [18], [19], [20]. Product ion scan results are shown in Fig. 1, and the representative MRM chromatograms for the five identified ginsenosides are shown in Fig. 2. Collectively, five ginsenosides and IS peaks were well separated with no interfering endogenous peaks, and the resolution between the peaks of ginsenoside Rb2 and Rc was calculated to be more than 1.2. The concentrations of ginsenosides Rb1, Rb2, Rc, and compound K in plasma were analyzed in the range of 0.5–100 ng/mL while that of Rd was analyzed in the range of 0.25–100 ng/mL. Interday and intraday precision and accuracy for the analysis of ginsenosides Rb1, Rb2, Rd, Rd, and compound K are shown in Table 3. For interday assays, the precision and accuracy ranged from 2.1 to 12.1% and from 91.1 to 111.4%, respectively (Table 3). In intraday assay validation, the precision and accuracy ranged from 3.5 to 9.3% and from 87.1 to 109.1%, respectively (Table 3).
Fig. 1.
Product ion spectra of ginsenosides (A) Rb1, (B) Rb2/Rc, (C) Rd, and (D) compound K.
Fig. 2.
Representative MRM chromatograms of ginsenosides (A) Rb1, (B) Rb2, (C) Rc, (D) Rd, (E) compound K, and (F) berberine (IS) in human double blank plasma, zero blank plasma, blank plasma samples spiked with ginsenosides of lower limit of quantitation (LLoQ), and plasma samples after single administration of red ginseng extracts. MRM, multiple reaction monitoring; IS, internal standard; tR, retention time.
Table 3.
Intraday and interday precision and accuracy of ginsenosides Rb1, Rb2, Rc, Rd, and compound K
| Ginsenosides | Concentration (ng/mL) | Interday (n = 3) |
Intraday (n = 3) |
||||
|---|---|---|---|---|---|---|---|
| Calculated (ng/mL) | Precision (%) | Accuracy (%) | Calculated (ng/mL) | Precision (%) | Accuracy (%) | ||
| Rb1 | 0.75 | 0.8 | 12.1 | 102.1 | 0.8 | 6.7 | 109.1 |
| 4 | 3.9 | 7.9 | 98.4 | 3.8 | 4.5 | 96.2 | |
| 40 | 37.7 | 5.3 | 94.3 | 36.3 | 5.4 | 90.8 | |
| Rb2 | 0.75 | 0.7 | 11.3 | 92.2 | 0.7 | 9.3 | 99.8 |
| 4 | 3.9 | 6.3 | 98.7 | 3.7 | 3.5 | 92.3 | |
| 40 | 39.3 | 4.7 | 98.2 | 37.9 | 5.1 | 94.7 | |
| Rc | 0.75 | 0.8 | 6.3 | 104.2 | 0.8 | 7.2 | 107.0 |
| 4 | 3.9 | 2.9 | 96.3 | 3.8 | 4.7 | 95.6 | |
| 40 | 37.3 | 2.1 | 93.2 | 37.1 | 4.6 | 92.7 | |
| Rd | 0.75 | 0.7 | 9.6 | 98.8 | 0.7 | 5.5 | 95.2 |
| 4 | 3.8 | 7.4 | 94.7 | 3.7 | 3.5 | 92.2 | |
| 40 | 40.0 | 3.3 | 100.0 | 39.2 | 3.7 | 97.9 | |
| Compound K | 0.75 | 0.7 | 9.0 | 96.5 | 0.7 | 7.6 | 97.0 |
| 4 | 3.6 | 7.2 | 91.1 | 3.5 | 3.6 | 87.1 | |
| 40 | 44.5 | 8.1 | 111.4 | 40.8 | 4.3 | 101.9 | |
3.3. Pharmacokinetics of ginsenosides Rb1, Rb2, Rc, Rd, and compound K after single or multiple administrations of red ginseng
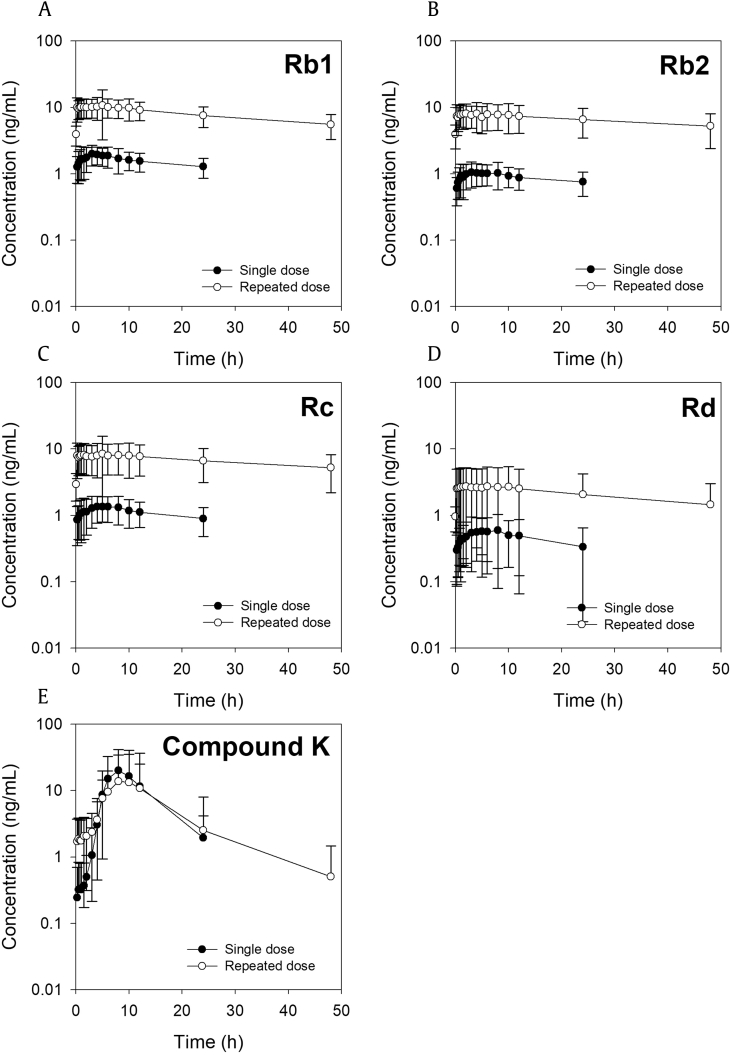
As shown in Fig. 3, plasma Rb1 concentrations were very stable over time, and Rb1 had a long terminal half-life. As a result, the plasma concentration and AUC of Rb1 after repeated administration for 15 days (23.0 mg of Rb1 per day) were significantly higher than those after a single oral administration of the same dose. The accumulation factor (calculated by dividing AUC of repeated administration by the AUC of single administration) was 4.55 (Table 4). In contrast, Tmax, t1/2, and mean residence time were not changed by repeated administration, suggesting that repeated administration of red ginseng extract did not alter the absorption pattern of Rb1. The plasma concentrations of Rb2 and Rc showed similar characteristics to that of Rb1; however, the plasma concentration of Rd and related parameters such as Cmax and AUC were lower than those for Rb1, Rb2, and Rc (Fig. 3 and Table 4). The accumulation factors of Rb1, Rb2, Rc, and Rd were similar.
Fig. 3.
Mean ± standard deviation plasma concentration-time profiles for ginsenosides (A) Rb1, (B) Rb2, (C) Rc, (D) Rd, and (E) compound K after single (●) or repeated (○) administrations of red ginseng extract.
Table 4.
Pharmacokinetic parameters of ginsenosides Rb1, Rb2, Rc, Rd, and compound K after single or repeated administrations of red ginseng extract
| Ginsenoside | PK parameters | Single dose | Repeated dose for 15 day | Accumulation factor |
|---|---|---|---|---|
| Rb1 | Tmax (h) | 3.53 ± 1.25 | 2.12 ± 1.76 | |
| Cmax (ng/mL) | 2.19 ± 0.73 | 12.20 ± 7.34∗ | ||
| AUC (ng∙h/mL) | 82.68 ± 26.26 | 375.93 ± 128.17∗ | 4.55 | |
| t1/2 (h) | 35.05 ± 8.46 | 51.01 ± 16.71∗ | ||
| MRT (h) | 26.83 ± 1.59 | 20.74 ± 1.06∗ | ||
| Rb2 | Tmax (h) | 3.87 ± 2.47 | 3.02 ± 3.08 | |
| Cmax (ng/mL) | 1.26 ± 0.39 | 8.83 ± 3.68∗ | ||
| AUC (ng∙h/mL) | 47.12 ± 16.40 | 316.96 ± 145.92∗ | 6.73 | |
| t1/2 (h) | 38.19 ± 20.33 | 68.69 ± 26.92∗ | ||
| MRT (h) | 26.80 ± 1.74 | 21.72 ± 1.08∗ | ||
| Rc | Tmax (h) | 3.68 ± 1.70 | 2.08 ± 2.76 | |
| Cmax (ng/mL) | 1.50 ± 0.63 | 9.69 ± 7.08∗ | ||
| AUC (ng∙h/mL) | 59.40 ± 22.95 | 323.21 ± 169.61∗ | 5.44 | |
| t1/2 (h) | 35.63 ± 14.79 | 59.09 ± 14.96∗ | ||
| MRT (h) | 27.39 ± 2.34 | 21.58 ± 0.78∗ | ||
| Rd | Tmax (h) | 4.87 ± 1.92 | 3.47 ± 3.06 | |
| Cmax (ng/mL) | 0.70 ± 0.46 | 3.14 ± 2.79∗ | ||
| AUC (ng∙h/mL) | 22.58 ± 18.31 | 101.42 ± 101.00∗ | 4.49 | |
| t1/2 (h) | 23.96 ± 15.26 | 45.82 ± 15.87 | ||
| MRT (h) | 24.67 ± 2.23 | 20.50 ± 1.41∗ | ||
| Compound K | Tmax (h) | 7.87 ± 2.03 | 6.93 ± 2.46 | |
| Cmax (ng/mL) | 24.80 ± 23.25 | 18.24 ± 27.11 | ||
| AUC (ng∙h/mL) | 247.50 ± 269.49 | 210.88 ± 400.44 | 0.85 | |
| t1/2 (h) | 9.95 ± 4.91 | 7.58 ± 4.12 | ||
| MRT (h) | 13.31 ± 3.72 | 10.51 ± 3.16 |
Tmax, time to reach Cmax; Cmax, maximum plasma ginsenoside concentration; AUC, area under the plasma concentration-time curve; t1/2, half-life; MRT, mean residence time
p < 0.05 statistically significant from single-dose group by unpaired t-test.
Compound K exhibited distinct pharmacokinetic properties compared with Rb1, Rb2, Rc, and Rd. Cmax and AUC values were not increased by repeated administration of red ginseng extract, resulting in a low accumulation factor of 0.85, which was attributed to the relatively short half-life of compound K.
3.4. Caco-2 permeability study
We next examined whether the identified ginsenosides differed in terms of gastrointestinal permeability. For this purpose, we used Caco-2 cells as a representative experimental model of intestinal permeability [16], [17]. All the studied ginsenosides showed low permeability (<10−6 cm/s; Table 5), which indicated limited intestinal absorption. The identified ginsenosides also showed similar absorptive permeability (Papp of the apical to basal direction) except for compound K, which had a twofold higher value.
Table 5.
Apparent permeability (Papp) of ginsenosides Rb1, Rb2, Rc, Rd, Rg3, Rh1, and compound K in Caco-2 cells
| Ginsenosides | Papp (☓10−6 cm/s) |
||
|---|---|---|---|
| A to B | B to A | ER | |
| Rb1 | 0.27 ± 0.03 | 0.30 ± 0.08 | 1.12 |
| Rb2 | 0.28 ± 0.02 | 0.24 ± 0.05 | 0.88 |
| Rc | 0.28 ± 0.09 | 0.33 ± 0.06 | 1.28 |
| Rd | 0.22 ± 0.02 | 0.31 ± 0.04 | 1.45 |
| Compound K | 0.47 ± 0.05 | 0.53 ± 0.03 | 1.15 |
| Rg3 | 0.05 ± 0.01 | 0.14 ± 0.06 | 2.97 |
| Rh1 | 0.22 ± 0.04 | 1.35 ± 0.14 | 6.27 |
Data were expressed as mean ± SD from triplicated measurements
SD, standard deviation; A to B, apical to basal; B to A, basal to apical
3.5. Correlation between pharmacokinetic parameters of ginsenoside Rd and compound K
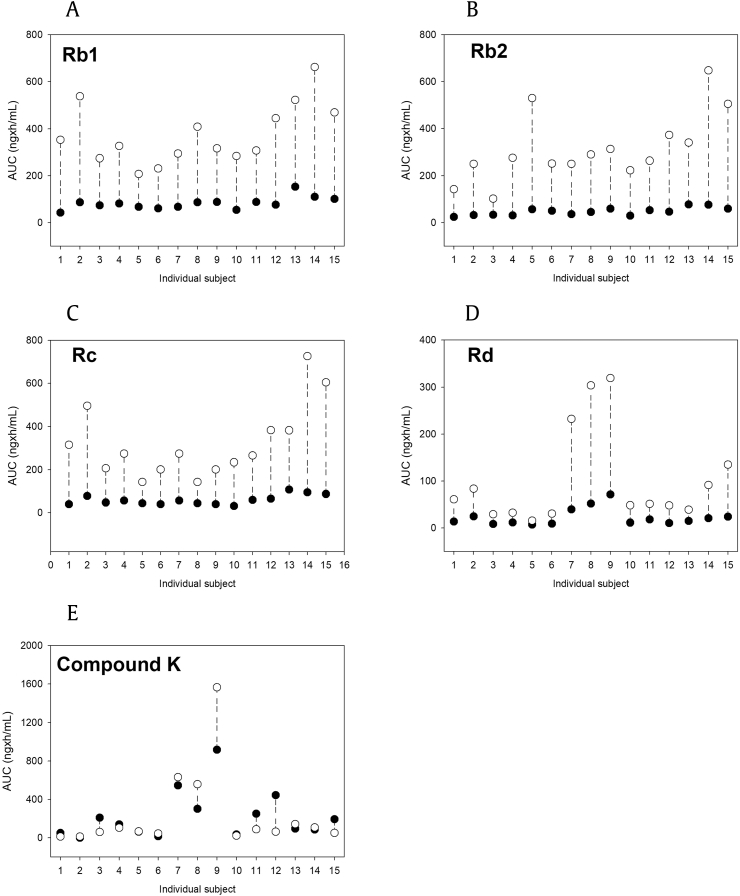
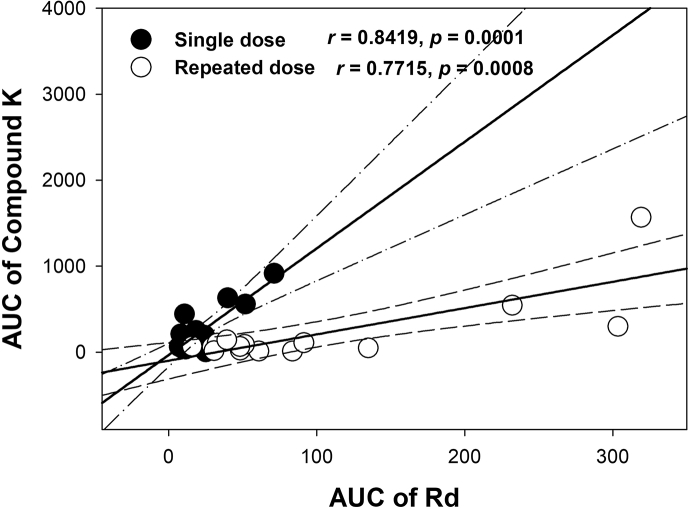
When comparing the individual plasma concentrations of Rb1, Rb2, Rc, Rd, and compound K, it was noted that Rd and compound K concentrations exhibited substantial variability. Three individuals (participant no. 7–9) showed higher plasma concentrations of Rd and compound K and higher Rd accumulation after red ginseng extract administration than the remaining 12 individuals, suggesting that these three individuals had higher conversion of other ginsenosides into Rd and compound K. Correlation analyses on the pharmacokinetic parameters of Rb1, Rb2, Rc, Rd, and compound K revealed a good correlation between the AUC values of Rd and compound K, independent of the dosing paradigm (i.e., single or repeated administration for 15 days; Fig. 4). These results also suggested the upstream biotransformation of ginsenosides before intestinal absorption, consistent with the previous reports on the intestinal biotransformation of ginsenosides mediated by the gut microbiome [15], [21], [22].
Fig. 4.
Individual exposure of ginsenosides (A) Rb1, (B) Rb2, (C) Rc, (D) Rd, and (E) compound K after single (●) or repeated (○) administration of red ginseng extract. AUC, area under the plasma concentration-time curve.
4. Discussion
Our study investigated the comparative pharmacokinetic properties of various, ginsenosides including Rb1, Rb2, Rc, Rd, and compound K after single or repeated administration of red ginseng extract for 15 days in healthy human beings for the first time. Repeated administration of red ginseng extract was generally safe, well tolerated, and unassociated with any changes in blood pressure, body temperature, alanine transaminase, or aspartate transaminase, suggesting that the study dose was safe and tolerable.
The results of the pharmacokinetic analysis revealed that five of 14 ginsenosides (i.e., Rb1, Rb2, Rc, Rd, and compound K) were detected in human plasma samples after oral ingestion of red ginseng extract. Notably, panaxadiol ginsenosides, which are present in high content in red ginseng extract (i.e., Rb1, Rb2, Rc, and Rd), were identified in human plasma, whereas panaxatriol ginsenosides such as Re, Rh1, and Rg1 could not be found. To understand the absorption property of these ginsenosides, we measured the Caco-2 permeability of major ginsenosides. Rg3 showed the lowest permeability (0.05☓10−6 cm/s) when compared with other panaxadiol ginsenosides such as Rb1, Rb2, Rd, and Rd (0.22-0.28☓10−6 cm/s), potentially accounting for the absence of Rg3 in human plasma samples. Rb1, Rb2, Rc, and Rd showed similar efflux ratios less than 2, although Rh1 and Rg3 had an efflux ratio of 6.27 and 2.97, which suggested the involvement of efflux transporters in the absorption process of Rh1 and Rg3 [17]. This also potentially explained the limited detection of Rh1 and Rg3 in human plasma.
The distinctive pharmacokinetic characteristics of ginsenosides in this study could be summarized as follows: (1) plasma concentrations of Rb1, Rb2, Rc, and Rd but not compound K showed accumulation after 15 days of repeated administration. That is, accumulation factors of Rb1, Rb2, Rc, and Rd except for compound K were 4.5–6.7; (2) we found a significant correlation between the AUC values of Rd and compound K (Fig. 5); and (3) in addition, AUCs for Rd and compound K showed high intersubject variability, regardless of the dosing paradigm (Fig. 4).
Fig. 5.
Correlations between the area under the plasma concentration-time curve (AUC) values for ginsenosides Rd and compound K after single dose (●) or 15 days of repeated administrations (○) of red ginseng extract. Lines were generated from a linear regression analysis, and dotted lines represent 95% confidence intervals around the geometric mean value.
Several studies have described the biotransformation of Rb1 to compound K by intestinal microflora and its effects on plasma concentrations of compound K [18], [23], [24], [25]. However, previous studies failed to demonstrate a correlation between the biotransformation of Rb1 and compound K [13], [15]. Considering the biotransformation pathway of Rb1, Rb2, and Rc → Rd → compound K [10], [21], [22] and the similar intersubject variability in the plasma concentrations of Rd and compound K in our study, the good correlation between the AUC values of Rd and compound K would be reasonable rather than the correlation between Rb1 and compound K.
Previous studies have indicated that the intestinal biotransformation of ginsenosides to Rd and compound K is a very slow process [21], and Rb1, Rb2, Rc, and Rd were stable over the enzymatic and alkaline hydrolysis [19], [26]. This may be the reason why Rb1, Rb2, Rc, and Rd were all detected in plasma in addition to compound K. In addition to the intestinal stability, the comparable intestinal permeability of Rb1, Rb2, Rc, and Rd and slow elimination rate of Rb1, Rb2, Rc, and Rd from the plasma (t1/2 greater than 45 h) also contributed to the pharmacokinetic characteristics of Rb1, Rb2, Rc, Rd, and compound K.
Despite F2 is an intermediate metabolite between Rd and compound K [10], [21], [22], F2 was not detectable in human plasma samples in our study. This could be attributed to the difference between the transformation rate from Rd to F2 and that from F2 to compound K, which should be further investigated. Taken together, we concluded that the intestinal biotransformation of ginsenosides (Rb1, Rb2, and Rc) to Rd and subsequently transformed to compound K played a critical role in the observed pharmacokinetics.
In numerous clinical studies, the dosage of red ginseng extract ranged from 0.5 to 3.0 g/day. When the red ginseng extract dose was expressed as ginsenoside contents, red ginseng extract usually contained 50–100 mg of ginsenosides/day [3], [9], [27], [28], [29], [30]. For example, in a clinical study of herb–drug interaction between red ginseng extract and drug metabolizing enzymes, 14 healthy male individuals were administered red ginseng extract containing 100-mg ginsenosides/day for 2 weeks [3], [27]. In another clinical study by Malati et al, 500 mg of ginseng powder containing 5% of ginsenoside was administered to 12 healthy individuals twice daily (50-mg ginsenosides/day) for 28 days [28]. Sixty diabetic patients received 5-g red ginseng extract for 12 weeks (82.9 ginsenosides/day) and showed a significant decrease or trends toward a decrease in both serum insulin and blood glucose levels [30]. In another study, red ginseng extract containing 77.7 mg of ginsenosides/3 g of extract was used to investigate the antidiabetic efficacy in streptozotocin-induced diabetic mice [29]. In our study, oral administration of red ginseng extract (dried ginseng > 60%; 85.1 mg/day as sum of Rb1, Rb2, Rc, Rd, Re, Rg1, Rg3, and Rh1) to 15 healthy individuals once daily for 15 days were comparable to the previously reported dose of red ginseng or ginsenosides. Therefore, the pharmacokinetic properties of various ginsenosides, including Rb1, Rb2, Rc, Rd, and compound K, after single or repeated administration of red ginseng extract for 15 days in our study could provide valuable information to understand the role of ginsenosides in the pharmacokinetic properties and therapeutic efficacy of red ginseng extract.
Conflicts of interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Export Promotion Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No 316017-3), Republic of Korea.
References
- 1.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of korean panax ginseng c a meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of korean red ginseng (panax ginseng meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim M.G., Kim Y., Jeon J.Y., Kim D.S. Effect of fermented red ginseng on cytochrome p450 and p-glycoprotein activity in healthy subjects, as evaluated using the cocktail approach. Br J Clin Pharmacol. 2016;82:1580–1590. doi: 10.1111/bcp.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ru W., Wang D., Xu Y., He X., Sun Y.E., Qian L., Zhou X., Qin Y. Chemical constituents and bioactivities of panax ginseng (c. A. Mey.) Drug Discov Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 5.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yun T.K., Choi S.Y., Yun H.Y. Epidemiological study on cancer prevention by ginseng: are all kinds of cancers preventable by ginseng? J Korean Med Sci. 2001;16(Suppl):S19–S27. doi: 10.3346/jkms.2001.16.S.S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gui Q.F., Xu Z.R., Xu K.Y., Yang Y.M. The efficacy of ginseng-related therapies in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e2584. doi: 10.1097/MD.0000000000002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park T.Y., Hong M., Sung H., Kim S., Suk K.T. Effect of korean red ginseng in chronic liver disease. J Ginseng Res. 2017;41:450–455. doi: 10.1016/j.jgr.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi I.D., Ryu J.H., Lee D.E., Lee M.H., Shim J.J., Ahn Y.T., Sim J.H., Huh C.S., Shim W.S., Yim S.V. Enhanced absorption study of ginsenoside compound k (20-o-beta-(d-glucopyranosyl)-20(s)-protopanaxadiol) after oral administration of fermented red ginseng extract (hyfrg) in healthy korean volunteers and rats. Evid Base Compl Alternat Med. 2016;2016:3908142. doi: 10.1155/2016/3908142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odani T., Tanizawa H., Takino Y. Studies on the absorption, distribution, excretion and metabolism of ginseng saponins. Iv. Decomposition of ginsenoside-rg1 and -rb1 in the digestive tract of rats. Chem Pharm Bull (Tokyo) 1983;31:3691–3697. doi: 10.1248/cpb.31.3691. [DOI] [PubMed] [Google Scholar]
- 11.Xu Q.F., Fang X.L., Chen D.F. Pharmacokinetics and bioavailability of ginsenoside rb1 and rg1 from panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–192. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 12.Liu H., Yang J., Du F., Gao X., Ma X., Huang Y., Xu F., Niu W., Wang E., Mao Y. Absorption and disposition of ginsenosides after oral administration of panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- 13.Kim H.K. Pharmacokinetics of ginsenoside rb1 and its metabolite compound k after oral administration of korean red ginseng extract. J Ginseng Res. 2013;37:451–456. doi: 10.5142/jgr.2013.37.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akao T., Kida H., Kanaoka M., Hattori M., Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound k in rat plasma after oral administration of ginsenoside rb1 from panax ginseng. J Pharm Pharmacol. 1998;50:1155–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee J., Lee E., Kim D., Lee J., Yoo J., Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009;122:143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Choi M.K., Song I.S. Characterization of efflux transport of the pde5 inhibitors, vardenafil and sildenafil. J Pharm Pharmacol. 2012;64:1074–1083. doi: 10.1111/j.2042-7158.2012.01498.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi Y.A., Yoon Y.H., Choi K., Kwon M., Goo S.H., Cha J.S., Choi M.K., Song I.S. Enhanced oral bioavailability of morin administered in mixed micelle formulation with pluronicf127 and tween80 in rats. Biol Pharm Bull. 2015;38:208–217. doi: 10.1248/bpb.b14-00508. [DOI] [PubMed] [Google Scholar]
- 18.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 19.Zheng M.M., Xu F.X., Li Y.J., Xi X.Z., Cui X.W., Han C.C., Zhang X.L. Study on transformation of ginsenosides in different methods. Biomed Res Int. 2017;2017:8601027. doi: 10.1155/2017/8601027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian T., Cai Z. Biotransformation of ginsenosides rb1, rg3 and rh2 in rat gastrointestinal tracts. Chin Med. 2010;5:19. doi: 10.1186/1749-8546-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park S.E., Na C.S., Yoo S.A., Seo S.H., Son H.S. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. J Ginseng Res. 2017;41:36–42. doi: 10.1016/j.jgr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang A., Zhang S., Zhu D., Dong Y., Shan J., Xie T., Wen H., Di L. Gut microbiota in the pharmacokinetics and colonic deglycosylation metabolism of ginsenoside rb1 in rats: contrary effects of antimicrobials treatment and restraint stress. Chem Biol Interact. 2016;258:187–196. doi: 10.1016/j.cbi.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 24.Kang K.S., Kim H.Y., Yamabe N., Nagai R., Yokozawa T. Protective effect of sun ginseng against diabetic renal damage. Biol Pharm Bull. 2006;29:1678–1684. doi: 10.1248/bpb.29.1678. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa H., Sung J.H., Benno Y. Role of human intestinal prevotella oris in hydrolyzing ginseng saponins. Planta Med. 1997;63:436–440. doi: 10.1055/s-2006-957729. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto E., Odashima S., Kitagawa I., Tsuji A. Stability kinetics of ginsenosides in aqueous solution. J Pharm Sci. 1984;73:409–410. doi: 10.1002/jps.2600730334. [DOI] [PubMed] [Google Scholar]
- 27.Kim D.S., Kim Y., Jeon J.Y., Kim M.G. Effect of red ginseng on cytochrome p450 and p-glycoprotein activities in healthy volunteers. J Ginseng Res. 2016;40:375–381. doi: 10.1016/j.jgr.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malati C.Y., Robertson S.M., Hunt J.D., Chairez C., Alfaro R.M., Kovacs J.A., Penzak S.R. Influence of panax ginseng on cytochrome p450 (cyp)3a and p-glycoprotein (p-gp) activity in healthy participants. J Clin Pharmacol. 2012;52:932–939. doi: 10.1177/0091270011407194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J.H., Pan J.H., Cho H.T., Kim Y.J. Black ginseng extract counteracts streptozotocin-induced diabetes in mice. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bang H., Kwak J.H., Ahn H.Y., Shin D.Y., Lee J.H. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food. 2014;17:128–134. doi: 10.1089/jmf.2013.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]