Abstract
Ginseng products on the market show high variability in their composition and overall quality. This becomes a challenge for both consumers and health-care professionals who are in search of high-quality, reliable ginseng products that have a proven safety and efficacy profile. The botanical extract standardization is of crucial importance in this context as it determines the reproducibility of the quality of the product that is essential for the evaluation of effectiveness and safety. This review focuses on the well-characterized and standardized ginseng extract, G115, which represents an excellent example of an herbal drug preparation with constant safety and efficacy within the herbal medicinal products. Over the many decades, extensive preclinical and clinical research has been conducted to evaluate the efficacy and safety of G115. In vitro and in vivo studies of G115 have shown pharmacological effects on physical performance, cognitive function, metabolism, and the immune system. Furthermore, a significant number of G115 clinical studies, most of them double-blind placebo-controlled, have reinforced the findings of preclinical evidence and proved the efficacy of this extract on blood glucose and lipid regulation, chronic obstructive pulmonary disease, energy, physical performance, and immune and cognitive functions. Clinical trials and 50 years of presence on the market are proof of a good safety profile of G115.
Keywords: Blood glucose and lipid regulation, Chronic obstructive pulmonary disease, Energy and physical performance, G115 standardized ginseng extract, Immune and cognitive functions
Abbreviations: 3′,5′-AMP, adenosine 3′5′ monophosphate; AMPK, 5′ AMP-activated protein kinase; ATP, adenosine triphosphate; CDR, cognitive drug research; CDRI, cognitive drug research index; CO, crossover; COPD, chronic obstructive pulmonary disease; DER, drug extract ratio; DB, double-blind; FBG, fasting blood glucose; FER, forced expiratory ratio; FEV1, forced expiration volume in one second; FVC, forced vital capacity; FEV1/FVC, ratio of FEV1/FVC; G115, standardized root extract of P. ginseng Meyer; GACPs, good agricultural and collection practices; GMPs, good manufacturing practices; HMPs, herbal medicinal products; HbAlc, glycated hemoglobin; HDL-c, high-density lipoprotein; LA, lipoic acid; LDLc, low-density lipoprotein; MVV, maximum ventilation volume; PaO2, blood oxygen pressure; PC, placebo-controlled; PEF, peak expiration flow; FEF50, forced expiratory flow50; FEF75, forced expiratory flow75; PEFR, peak expiration flow rate; PFTs, pulmonary function tests; PG, parallel group; PGC-1α, proliferator-activated receptor gamma coactivator-1α; pO2, partial oxygen pressure; PS, pilot study; R, randomized; RVIP, rapid visual information processing; SB, single-blind; SFR, saliva flow rate; SIgA, secretory immunoglobulin A; S-SIgA, SIgA secretion rate; SIRT1, sirtuin 1; TC, total cholesterol; TG, triglyceride; VLDL, very-low-density lipoprotein; VO2 max, maximal oxygen consumption; WHO, World Health Organization
1. Introduction
The root of Panax ginseng Meyer has been used medicinally for thousands of years in China, Korea, and Japan for prophylactic and curative properties in cases of decreased physical and mental capacities such as tiredness, exhaustion, and weakness and during convalescence [1], [2], [3], [4], [5]. The pharmacological properties of ginseng were first described in detail in Bencao Gangmu (本草綱目, Great Compendium of Herbs), the most comprehensive premodern herbal textbook, written by Shizhen Li (時珍, 1518–1593). In the early middle ages, ginseng was introduced into Europe by Arabian merchants; however, owing to ignorance about the “root with the human shape,” its use was banned, and it was referred to as ‘Moorish devil's” business [6]. Currently, ginseng is available on the market worldwide in a variety of forms such as fresh ginseng, dried ginseng, boiled and dried ginseng, and red ginseng or as various products based on ginseng extracts. These are sold either as food, dietary, or health supplements or as herbal medicinal products (HMPs) [7], [8]. In the last fifty years, substantial preclinical and clinical research has been conducted that has improved our understanding on both the main phytocomponents that contribute to the mechanism of action and the pharmacological properties and toxicology profile of ginseng. The main active components of ginseng were identified for the first time in 1963 [9] as ginsenosides, dammarane-type triterpene saponins, which can be further classified into 20(S)-protopanaxadiol (ginsenosides Rb1, Rb2, Rb3, Rc, and Rd) and 20(S)-protopanaxatriol (ginsenosides Re, Rg1, Rg2, and Rh1) groups according to their aglycone moieties. The quality and composition of the active components can vary according to several factors including plant species, the method of cultivation, age, and part of the plant used [10].
Owing to this complexity, work is still ongoing to understand the pharmacology of the ginsenosides. Pharmacokinetic and toxicology profiles support the observed efficacy of ginsenosides in clinical studies [11]. When ginseng is administered orally, a significant fraction of ginsenosides are metabolized by intestinal microflora into compound K, which is now considered to be the main constituent with pharmacological effects [12], [13]. Ginsenosides are primarily metabolized in the liver and enter into the enterohepatic recirculation resulting in excretion largely through feces [14], [15]. Many pharmacological effects have been investigated through in vivo and in vitro studies for different ginseng preparations. The results have provided evidence in the antioxidant effects of ginseng in various experimental models through the enhancement of the cell stress response [16], in inhibition of apoptosis in cultured neuronal cells [17], [18], and in the neuroprotective activity in animal models of ischemic stroke [19], and Parkinson [20], [21] and Alzheimer diseases [22]. Various in vivo studies have reported that the activity of ginseng on the central nervous system including learning/memory enhancement [23], improvement of cognitive deficits [24], reduction of addiction behavior, and opioid-induced hyperalgesia [25], [26]. Ginseng can inhibit proliferation and motility of cancer cell lines in vitro [27], [28], [29], and it possesses anticarcinogenic activity in animal models [30], [31], [32]. The anticarcinogenic effects of ginseng may be partly due to its ability to inhibit angiogenesis [33]. Notably, modulation of the immune response has been extensively characterized as pharmacological effects of ginseng, proving that it is able to modulate multiple immune cell types, in terms of proliferation, phagocytic activity [34], cytokine expression [35], [36], and production of antibodies [37]. Studies performed in animals have demonstrated that ginseng induces a strong immune response, protecting from both viral and bacterial infections [38], [39], [40] and enhancing the protection conferred by vaccination [41]. Pharmacological effects on the cardiovascular system have also been reported, where ginseng was able to influence nitric oxide production in vascular endothelial cells [42], [43] and to improve cardiac performance exerting cardioprotective effects in rat models [44], [45]. It has also been shown that ginseng has positive effects on both lipid and glucose metabolism in vivo [46], [47].
Preclinical toxicological studies on oral administration of ginseng extracts have reported LD50 values of 750 mg/kg in rats and 200 mg/kg in mice respectively. Chronic studies in rats and mice treated with ginseng at doses up to 5000 mg/kg for 2 years did not show any toxic effects; furthermore, no increases in the incidence of cancer or nonneoplastic lesions were detected [48].
Numerous clinical trials on ginseng-based HMPs have been conducted over the last five decades with a broad spectrum of investigated indications. More recently, a series of systematic reviews have analyzed the clinical trials that have been conducted with ginseng and the effectiveness of ginseng on cognitive function [49], [50], metabolism [51], erectile dysfunction [52], cardiovascular function [53], vitality [54], [55], and improvement of the immune system [56], [57]. The studies are heterogeneous, and in many cases, the methodological quality shows deficiencies, in particular, in the case of older studies. In general, the clinical studies indicate beneficial effects; however, because of the heterogeneity of investigated ginseng-based HMPs and study design as well as deficiencies in methodological quality and the small number of study participants, none of the systematic reviews allowed deduction of strong evidence for clinical efficacy in the respective indication.
2. Standardization: the prerequisite for pharmaceutical quality
In the last decades, the awareness and general acceptability of botanical products to replace or complement Western medicine is growing worldwide. Consumers' confidence and reliance on botanical products for self-medication are increasing because, being natural, such products are generally considered safe or are at least associated with fewer side effects than synthetic drugs. However, the enormous variety and qualitative variability of marketed products represent a great challenge for both consumers and health-care professionals when they have to use or recommend a botanical product. The quality of a plant extract is strictly related to the quality of the botanical source and the method of preparation [7], [8]. Measures of quality assurance such as good agricultural and collection practices and good manufacturing practices are mandatory requirements to obtain constant efficacy and safety profiles of such products [58], [59]. The quality of ginseng roots is related to the content of ginsenosides, which represent the major bioactive components responsible for the pharmacodynamic actions of this herbal drug [60]. Ginseng products on the market show a notable variation in saponin composition, whose constant content can be guaranteed by monitoring the manufacturing process—starting from the control of the raw material to the reproducibility of the extraction process—and pursuing a strict control on the final extract through a complete set of specifications. All these processes lead to standardization, which is essential to guarantee quality, purity, safety, and efficacy of a botanical extract. According to the World Health Organization “standardization is the process of prescribing a set of standards or inherent characteristics, constant parameters, definitive qualitative and quantitative values that carry an assurance of quality, efficacy, safety, and reproducibility” [61], [62], [63]. Only products fulfilling the basic requirements of quality have reproducible safety and efficacy profiles [8], [64].
In this context, the standardized ginseng extract G115 is regarded as different from other ginseng extracts owing to its unique manufacturing process and represents an excellent example of a standardized extract capable of providing constant safety and efficacy profiles. Quality of G115 is in compliance with the US and European pharmacopoeias [65], [66]. According to the European pharmacopoeia [66], G115 is obtained by ethanol extraction (40% V/V) of the dried roots of P. ginseng Meyer and standardized on the total content (4 %) of major ginsenosides, with a drug extract ratio of 3-7:1. G115 has been the first ginseng extract to be standardized on a defined content of ginsenosides and the first extract registered in the European market (in 1961 in Switzerland) as the active ingredient of HMPs. Currently, G115 is sold worldwide according to a different status as an over-the-counter medicine, traditional HMP, or food supplement.
The aim of the current review is to highlight the importance of how the pharmaceutical quality of a botanical product used in clinical trials is an essential requirement to ensure a reliable efficacy and safety profile. The search strategy used for this review was based on the electronic databases Scopus, PubMed, and MEDLINE accessed in May–August 2018. Key word searches were conducted by combining the terms “Panax ginseng” or “Ginseng” coupled with “G115.” Included articles were those published after 1975, written in English or at least with the abstract in English and peer-reviewed. Furthermore, only studies with G115 as a monotherapy, not in combination with other interventions (other plant extracts or drugs), were included in the review. All the preclinical and clinical studies having the aforementioned eligibility criteria on the standardized G115 extract are reported in Table 1 and Table 2, respectively.
Table 1.
Clinical studies on muscle activity, blood glucose and lipid profile regulation, quality of life
| Author, year, literature | Number of participants | Dose of G115 | Sample | Trial design | Outcome measures | Main results |
|---|---|---|---|---|---|---|
| Hosseini et al (2013) [88] | 30 patients (15 G115 plus 15 placebo) | 300 mg daily (100 mg 3 times a day for 8 weeks) | Patients with type 2 diabetes (≥ 40 years). Inclusion criteria: FBG ≥ 126 mg/dL; ≥2 years type 2 diabetes; oral hypoglycemic drugs; no other diseases; body mass index 25–35 kg/m2 | R, PC, DB, PG | Effects on FBG levels, HbA1c, and lipid profile | Significant decrease in HbA1c (t = 2.593, p = 0.015) and FBG levels (t = 2.13, p = 0.042) for the G115 group. No improvement of lipid profiles. |
| El-Farok et al (2013) [89] | 60 patients (20 in the LA group, 20 in the G115 group, and 20 in the control group) | 200 mg daily (100 mg two times a day or 600 mg of LA daily for 8 weeks) | Patients with hypercholesterolemia (aged 40–60 years). Inclusion criteria: no coadministration of antioxidants or antihypercholesterolemic agents or any drugs that may influence the results | Open-label parallel groups | Effects on the lipid profile | LA provided a significant reduction in LDL after 4 weeks and both LDL and total cholesterol after 8 weeks. G115 after 4 or 8 weeks showed a significant reduction in LDL, TC, and LDL/HDL and Chol/HDL ratios. |
| Reay et al (2009) [93] | Study 1: 13 females and 12 males Study 2: 13 females and 5 males |
Study 1: 200 mg of G115 daily (100 mg two times a day, 1–29 days) Study 2: 200 mg of an undefined ginseng extract daily (100 mg two times a day, 30–57 days) | Healthy participants. Study 1: mean age, 35.6 years for finger prick blood glucose levels; Study 2: mean age, 33.3 years for HbAlc and mean age, 32.8 years for fasting plasma insulin | Two separate PC, DB, CO | Evaluation of HbA1c, fasting plasma insulin, fasting plasma glucose, and postprandial response (following breakfast) | No significant effects on any of the three parameters on Days 1, 29, and 57 of the study. |
| Reay et al (2006a) ; Reay et al (2006b) ; Reay et al (2005) [90], [91], [92] | Study 1: 30 participants; Study 2: 27 participants | Study 1: 200 mg, 400 mg, and placebo on separate days. Study 2: placebo (30 mg saccharin), ginseng (200 mg G115 and 30 mg saccharin), placebo-glucose (25-g oral glucose), and ginseng-glucose (200 mg G115 and 25 g oral glucose). A 7-day washout period between treatments | Healthy young adults (study 1: mean age, 22.6 years; study 2: mean age, 21.89 years) | Acute PC, DB, CO | Blood glucose levels measured at baseline after an overnight fast and then 60, 90, and 120 min after the dose | G115 significantly lowers FBG levels. A significant drink–G115 interaction was found. |
| Van Schepdael et al (1993) [87] | 43 participants (21 plus 22) | 200 mg/day (100 mg twice daily) for two consecutive training periods of 10 weeks | Males regularly competing triathlon athletes (aged 24–30 years). VO2 max, between 50 and 65 ml/kg/min. | DB, PC, CO | Endurance parameters including VO2 max, heart rate, lactic acid blood levels | Second treatment showed differences (p < 0.05). VO2 max and heart rate increase, whereas lactic acid decreases after 10 weeks in both groups. |
| Mulz et al (1990) [79] | 60 patients | Study 1: G115 plus vitamins, minerals, and trace elements Study 2: G115 treatments for two years |
Patients with psychasthenic symptoms, psychological and physical restlessness, agitation, diffused disturbances of well-being (aged 55–60 years) | Two PC | Effectiveness in the complaints | Improvement of 64% of complaints Study 1: 78% of the complaints disappeared after 2 years of treatment. No side effects reported. Study 2: no symptom was therapy resistant; 72% of the complaints disappeared with placebo and psychiatric treatment. |
| Rosenfeld (1989) [80] | 50 patients | 200 mg/day (100 mg twice daily) for 56 days | Patients suffering from asthenia, depressive syndrome, or neurovegetative disorders (aged 24–66 years, mean: 39.7 years). | DB, PC | Performance evaluated by two psychometric tests (Toulouse test and Wechsler–Bellevue test) and from the results of a comprehensive psychological questionnaire (SCAG, Sandoz Clinical Assessment Geriatric) | Significant improvement in the Toulouse test (from 148.2 to 173.2, p < 0.01) and Wechsler–Bellevue test (from 99.6 to 113.8, p < 0.01). The SCAG questionnaire score was reduced from 29.6 (Day 0) to 22.4 at the end of the treatment (p < 0.01). |
| Von Ardenne (1987) and Klemm [82] | 10 participants | 200 mg/day (100 mg twice daily) for a period of 4 weeks | Elderly participants (average age, 50 years) | PS | Acute and long-term effects on arterial and venous Hb-O2 saturation | Increase (p < 0.05) by 4.5 mm Hg in resting arterial pO2 uptake after 4 weeks. Increase O2 transport (129%). Venous pO2 decreased (4.3 mm Hg). |
| Forgo and Schimert (1985) [86] | 28 participants (14/14) | 200 mg/day (100 mg twice daily) for 9 weeks | Trained healthy male athletes (range, 20–30 years) | PC, two parallel groups | Oxygen uptake and heart rate during ergometer exercise, duration of effect | Improved maximal oxygen uptake during exercise (p < 0.01), heart rate (p < 0.001), forced expiratory volume (p < 0.01), forced vital lung capacity (p < 0.05), and visual reaction time (p < 0.01). |
| Gianoli and Riebenfeld (1984) [81] | 83 patients (24 men, 59 women) | 200 mg/day (100 mg twice daily) for 4 months | Patients with asthenia (mean age, 37.7 years) Group A (G115): 46 patients (18 men, 28 women, mean age: 40.1 years). Group B (placebo): 37 patients (6 men, 31 women, mean age: 34.6 years). | PC, DB, R | Effects on appetite, body weight. Effects on performance, general well-being (measured by means of a multiple-choice questionnaire), sleep, blood pressure, common cold symptoms | Reduction of systolic blood pressure (p < 0.005), common cold and bronchitis symptoms, of fever, cough, and otitis symptoms. Increase of appetite (p < 0.01), sleep (p < 0.025), general well-being (p < 0.005), and performance (p < 0.005) in the G115 group only. |
| Forgo (1983) [83] | 30 participants (10/10/10) | 200 mg daily of G115 plus (7% ginsenosides, 100 mg twice daily) or G115 plus vitamin E (100 mg, 200 mg, respectively, twice daily) or placebo for 9 weeks | Healthy topmost sportsmen (range: 19–31 years, mean: 24.3 years). | PC, parallel groups | Change of aerobic capacity, serum lactate, heart rate, hormone levels during ergometer exercise | Increased O2 absorption capacity (p < 0.01), decreased serum lactate and heart rate (p < 0.05, from 155 to 140 beats/min in the group G115 plus and from 155 to 142 beats/min the group G115 plus vitamin E). No hormonal changes. |
| Forgo and Kirchdorfer (1982) [85] | 30 participants (10/20) | 200 mg daily of G115 plus (7% ginsenosides, 100 mg twice daily) or G115 (4% ginsenosides, 100 mg, twice daily) or placebo for 9 weeks | Healthy topmost sportsmen (range: 19–31 years, mean: 24.3 years). | Parallel groups | Effects on physical work capacity, that is, maximum oxygen absorption capacity, lactate concentration in blood, and heart rate during effort | Increased O2 absorption capacity (p < 0.01), decreased lactate and heart rate (from 150 to 140 beats/min in the group G115 plus and from 155 to 140 beats/min in the group G115). No significant differences between the two groups. |
| Forgo et al (1981b) [84] | 120 participants | 200 mg daily (100 mg twice daily) or placebo for 12 weeks | 60 males and 60 females divided according to age (30–39 and 40–60 years), sex, and treatment | DB, PC | Effectiveness with regard to reaction time, pulmonary function, general state of health, and hormones in various age-groups after 3, 6, 9, and 12 weeks of treatment | Reduced reaction time in the group aged 40–60 years. Significant improvement of respiratory positions in the males aged 40–60 years. Unchanged levels of hormones. |
| Forgo and Kirchdorfer (1981a) [78] | 20 participants | 200 mg daily (100 mg, twice daily) for 9 weeks | Healthy topmost sportsmen (range: 18–31 years, mean: 22.8 years). | Effects on physical work capacity, that is, maximum oxygen absorption capacity, lactate concentration in blood, and heart rate during effort | Significant improvement in aerobic capacity from 4,215 ml/min to 4,895 ml/min. Reduction in blood lactate and heart rates. |
|
| Dörling (1980) [77] | 60 participants | 200 mg daily (100 mg, twice daily) | Healthy volunteers (range: 22–80 years) | DB, PC | Effects on participant's response time to light and sound, assessment of the flicker fusion threshold, two-hand coordination, recovery quotient General physical conditions assessed by a questionnaire |
Decreased reaction time in 81.6% of the G115 group. Limited effects in the placebo group. Statistically significant improvement of the flicker fusion threshold, two-hand coordination, recovery quotient. G115 was superior to placebo in the questionnaire. |
CO, crossover; DB, double-blind; FBG, fasting blood glucose, HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; TC, total cholesterol; PG, parallel group; PaO2, blood oxygen pressure; PC, placebo-controlled; PEF, peak expiration flow; PEFR, peak expiration flow rate; pO2, partial oxygen pressure; PS, pilot study; R, randomized; VO2 max, maximal oxygen consumption; VLDL, very-low-density lipoprotein
Table 2.
Clinical studies on immune response and COPD
| Author, year/literature | Number of participants | Dose of G115 | Sample | Trial design | Outcome measures | Results |
|---|---|---|---|---|---|---|
| Wu et al (2014) [106] | 10 randomly assigned to the G115 group (n = 5) or the placebo group (n = 5) | 200 mg twice daily for four weeks. Participants were enrolled for 10 weeks (2 weeks for run-in; 4 weeks for treatment; and 4 weeks for follow-up). | Patients with COPD (mean age of 64.8 years). Inclusion criteria: COPD diagnosed spirometrically, FEV1 between 20% and 79% ; FER less than 70%; not experienced an acute exacerbation of COPD for at least 4 weeks before the trial; not been hospitalized in the past 6 months. |
R, pilot study | The primary outcomes: rate of exacerbation, as a change in baseline dyspnea, St. Georges Respiratory Questionnaire, COPD Assessment Test and Short-form Health Survey (SF-36). Other outcomes: six-minute walk test, FEV1, FVC, and adverse events. |
No change in mean FEV1, FVC, or FER in the G115 or placebo groups. The small sample size and short study duration were probably related to the absence of differences in outcomes. |
| Engels et al (2003) [102] | 27 participants (15 for the G115 group and 12 for the control group) | 400 mg per day (200 mg twice a day) for 8 weeks | Active healthy adults | DB, PC, R | The SIgA secretion rate and the relation of SIgA to total protein, absolute SIgA, and salivary protein concentrations. | S-SIgA, SIgA:protein ratio, and SFR were lower after exercise at baseline (p < 0.05). Both peak and mean mechanical power output declined (p < 0.01) across consecutive Wingate tests. |
| Gross et al (2002) [104] | 92 (49 for the G115 group plus 43 for the placebo control) | 200 mg daily (100 mg twice daily) for 3 months | Inclusion criteria: COPD of moderate severity (FEV1.0); clinical stability in the six months preceding the study and ability to exercise without hemodynamic instability. | R, PC | PFTs, MVV, and MIP before treatment and every two weeks for the 3-month study period. Exercise test and VO2 max before the beginning and after six weeks and three months. | All parameters significantly increased when compared with the placebo group. FVC: 32.5%, FEV1.0: 27.0%, PEF: 27.5%, FEF50: 45.4%, FEF75: 56.9%, MVV: 40.4%, MIP: 47.0%, and VO2 max: 37.5%. |
| Scaglione et al (2001) [101] | 75 patients (37 for the G115 group plus 38 for the placebo control) | 200 mg per day (100 mg twice a day) for 9 days | Patients experiencing acute attacks of chronic bronchitis | R, comparative PS | Effects in reducing the bacterial count in the bronchial system of patients undergoing an acute attack of chronic bronchitis treated with amoxicillin and clavulanic acid twice daily | Both groups of patients responded positively to the antibacterial treatment. In the G115 group, bacterial clearance was significantly faster than in the participants receiving the antibacterial medication alone. |
| Scaglione et al (1996) [100] | 227 participants (114 for the G115 group and 113 for the placebo control) | 200 mg per day (100 mg twice a day) for 12 weeks. | Healthy participants | 12 weeks multicenter, R, PC, DB | Resistance to influenza and common cold measured as NK cell activity, at Weeks 8 and 12 in participants who have received an antiinfluenza polyvalent vaccine at Week 4 | Antibody titers at Week 8 were > after G115 treatment (272 units vs 171 units after placebo), and natural killer cell activity in the treatment group was almost twice as high as in the placebo group. |
| Gross et al (1995) [103] | 15 | 200 mg/day (100 mg twice daily) for 3 months | Patients with severe chronic respiratory diseases (mean age, 67 ± 12 years) under home oxygen treatment. | PS | FVC, FEF50, FEF75; FEV1, FEV1/FVC, PEF, MVV, arterial blood gases, and walking distance. | Significantly improved FVC, FEV1, PEFR, MVV, oxygenation. |
| Scaglione et al (1990) [98] | 60 (20 patients in each group, that is, placebo, aqueous ginseng extract, and G115) | 200 mg per day (100 mg twice a day)/ 200 mg per day of aqueous liquid extract (100 mg twice a day)/placebo for 8 weeks. | Healthy people (18-50 years). | 8 weeks, DB, PC | Immunological parameters determined on leukocytes before and the 4th and 8th weeks after the onset of the treatment. | Increase in chemotaxis of polymorphonuclear leukocytes, phagocytic index, and the total number of T3 and T4 lymphocytes after 4 and 8 weeks of G115. Increased T4:T8 ratio and the activity of natural killer cells. |
| Scaglione et al (1994) [99] | 40 (20 in each group) | 200 mg per day (100 mg twice a day) for 8 weeks | Patients suffering from chronic bronchitis | SB, PC | Function of alveolar macrophages by measuring the phagocytic activity and killing power toward Candida albicans | The phagocytosis and intracellular killing significantly increased at the eighth week of treatment with G115. |
COPD, chronic obstructive pulmonary disease; CO, crossover; DB, double-blind; FER: forced expiratory ratio, FEV1, forced expiration volume in one second; FEV1/FVC, ratio of FEV1/FVC; FVC: forced vital capacity; LA, lipoic acid; MVV, maximum ventilation volume; FEF50, forced expiratory flow50; FEF75, forced expiratory flow75; PEF, peak expiratory flow; PC, placebo-controlled; PFTs, pulmonary function tests; PS, pilot study; R, randomized; SB, single-blind; SFR, saliva flow rate; SIgA, secretory immunoglobulin A; S-SIgA, SIgA secretion rate; NK, natural killer; MIP, maximal inspiratory pressure.
3. The science behind G115
Over the last 40 years, extensive analytical, preclinical, and clinical research has been carried out to investigate the quality, efficacy, and safety of G115. The aim of this review is to examine more closely preclinical studies that have revealed the multiple molecular targets of G115 and to report all the clinical studies carried out with G115 to date.
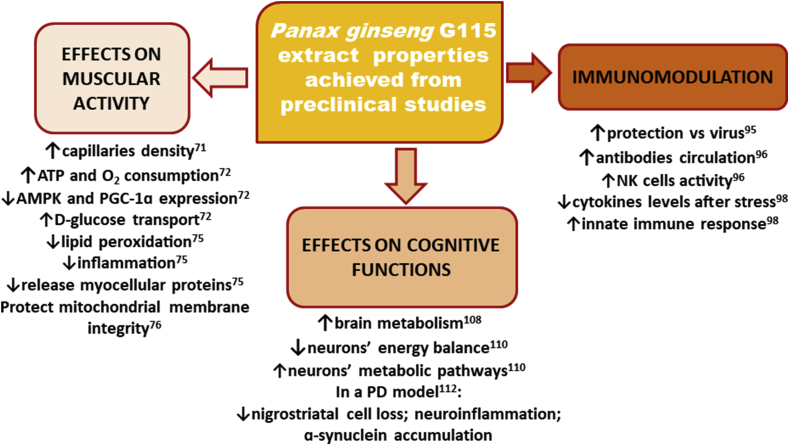
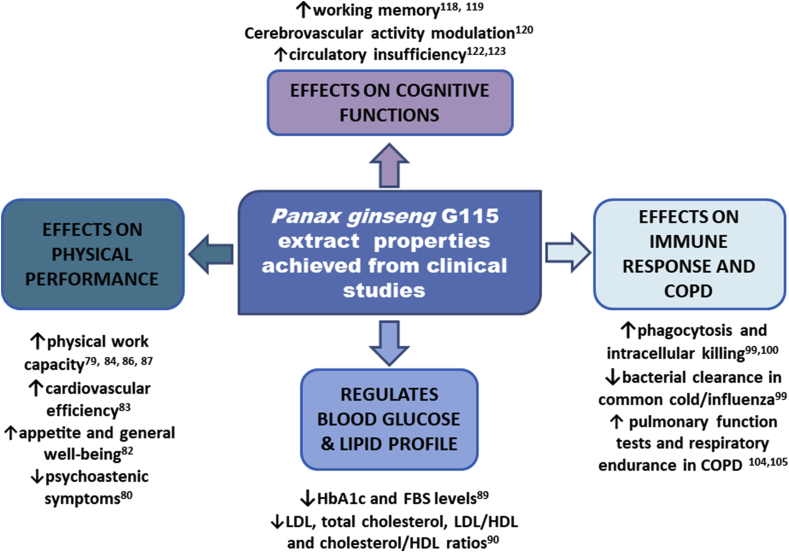
Preclinical studies have demonstrated that G115 shows many (often interconnected) properties in vitro and in vivo, revealing effects on blood glucose and lipid regulation, chronic obstructive pulmonary disease (COPD), energy, physical performance, and immune and cognitive functions. These findings have been summarized in a clinical setting (Fig. 1, Fig. 2).
Fig. 1.
Summary of main P. ginseng G115 extract properties achieved from preclinical studies.
AMPK, 5′ AMP-activated protein kinase; ATP, adenosine triphosphate; PGC-1α, proliferator-activated receptor gamma coactivator-1α.
Fig. 2.
Summary of main P. ginseng G115 extract properties achieved from clinical studies.
COPD, chronic obstructive pulmonary disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FBS, fasting blood sugars.
The clinical evaluation of the G115 extract has been a subject of review for a number of years (Table 1, Table 2, Table 3). In 2005, a review by Scaglione et al. [67] highlighted the effectiveness of G115 in increasing endurance and vitality in a number of non-good clinical practice (GCP) studies, despite several clinical studies with ginseng not having shown any significant effect on the enhancement of physical performance.
Table 3.
Clinical studies on cognitive functions
| Author, year/literature | Number of participants | Dose of G115 | Sample | Trial design | Outcome measures | Results |
|---|---|---|---|---|---|---|
| Reay et al (2010) [117] | 30 | 200 or 400 mg G115 or placebo. Acute and subchronic (8 days) testing sessions, with a 6-day washout period. |
Volunteers (mean age: 22.87 years; SD: 4.01). | DB, PC, randomized CO | Effects of G115 on subjective mood and aspects of working memory processes. Testing was on Days 1 and 8 of each treatment period at predose and 1, 2.5, and 4 h postdose. |
3-back sensitivity index scores increased with the 400-mg dose but decreased after 200 mg. The 400-mg dose also improved calmness (restricted 2.5 and 4 h on Day 1) and improved mental arithmetic on Days 1 and 8. Single doses of G115 could modulate working memory performance and improve participants' subjective self-reports on calmness. |
| Reay et al (2009) [93] | Study 1: 23 Study 2: 14 |
Study 1: placebo or G115 Study 2: placebo or ginseng extract. Two capsules (200 mg G115 or ginseng extract) every day for 57 d (on Days 1, 29, and 57). |
Healthy volunteers. Study 1: 11 females and 12 males (mean age: 35.6 years, SD: 10.8) Study 2: 5 males, 9 females (mean age: 38.4 years; SD :10.6). |
Two separate PC, DB, balance, CO studies | Effects of chronic ingestion of G115 and ginseng extract HbAlc, fasting plasma insulin, fasting plasma glucose and postprandial | No effect on indices of glucose regulation (HbAlc, fasting plasma insulin, fasting plasma glucose). No effect at the acute 3-h measurement point after a light breakfast, measured 180 min after ginseng ingestion, in healthy volunteers. |
| Reay et al (2008) [116] | 25 (placebo or 200 mg G115) for 20 weeks | 200 mg G115 or placebo for 57 days in total, with a washout period of 27 days between treatments | Twenty-five volunteers (mean age = 35.28, SD = 10.50) | PC, DB, CO | Behavior and mood effects assessed on Day 1, Day 29, and Day 57. CDR computerized assessment battery, a collection of verbal and nonverbal working memory tasks, subjective quality of life and mood. | Improvements in working memory after a single acute dose of G115. Superimposed relationship between acute and chronic ingestion was found. |
| Reay et al (2006a) [92] | 27 | 200 mg G115 or placebo and 30 minutes after a drink of glucose or placebo. Placebo; 200 mg G115/0 mg glucose; 0 mg G115/25 g glucose or 200 mg G115/25 g glucose. | 17 male and 10 female healthy young adults (mean age: 21.89 years, SD: 4.64). | Acute DB, PC, balanced CO |
Mental fatigue (self-rated), the 10-minute “cognitive demand” battery comprised a serial threes subtraction task; a serial sevens subtraction task; a RVIP task. | No synergistic relationship between G115 and glucose. Single doses of either G115 or glucose can modulate blood glucose levels, enhance cognitive performance of a mental arithmetic task (serial threes) and mental fatigue experienced by participants during sustained intense cognitive processing. |
| Reay et al (2005) [90] | 30 participants | 200 or 400 mg separate doses (G115) or placebo | Healthy young adults (16 females and 14 males, mean age: 22.6 years, SD: 5.46) | Acute DB, PC, balanced CO | Serial threes subtraction task; serial sevens task; RVIP task; “mental fatigue” visual analogue scale | G115 decreases circulating blood glucose levels (p < 0.005), enhances cognitive performance of a mentally demanding task (serial sevens), and mental fatigue experienced by participants during sustained intense cognitive processing. |
| Sünram-Lea et al (2005) [111] | 30 participants | 400 mg G115 or placebo with a 7-day washout period between treatments | 30 health young volunteers (15 males and 15 females, 18–25 years, mean age: 20 years). | Acute DB, PC, CO |
CDR testing battery followed by the Bond-Lader visual analogue scale was performed. | G115 improved the “speed of attention” component of the CDR battery from the choice reaction time task. No other measure was significant. |
| Kennedy et al (2004) [112] | 28 participants | Single doses of 200 mg G115, or 75 mg of a dried ethanolic extract of guarana (ca. 12% caffeine) and their combination (200 mg/75 mg) | Healthy young participants (18–24 years, mean age: 21.4 years). | Acute DB, PC, counterbalanced study | CDR testing battery, serial threes subtraction task, serial sevens task, sentence verification, logical reasoning, self-reported mood measures | All three treatments resulted in improved task performance throughout the day. Guarana and the combination and to a lesser extent G115 gave significant improvements in serial subtraction task performance. |
| Kennedy et al (2003) [118] | 15 participants | Single dose of 200 mg G115 or 360 mg of a ginkgo extract (EGb761) or placebo. A 7-Day “washout” period | Healthy volunteers (10 female and 5 male volunteers, mean age: 26.6 years, range: 19–39 years) | Within-participants, acute DB, PC, balanced CO | Evaluation of auditory-evoked potentials, contingent negative variation (CNV), and resting power within the delta, theta, alpha, and beta wavebands. | G115 led to a significant shortening of the latency of the P300 component of the evoked potential. Both G115 and ginkgo led to significant reductions in frontal “eyes closed” theta and beta activity, with additional reduction for G115 in the alpha waveband. |
| Kennedy et al (2002) [113] | 20 participants | Single doses of 400 mg (G115), 360 mg of a ginkgo extract (EGb761), 960 mg of a product combining the two extracts (Ginkoba M/E), or placebo, with a 7-day washout period between treatments | Healthy, young adult volunteers (21 females and 5 males, mean age: 21.1 years, SD: 3.9) | Acute DB, PC, balanced, CO |
CDR computerized assessment battery and two serial subtraction mental arithmetic tasks (serial threes and serial sevens). Bond-Lader visual analogue scales. | All treatments improved secondary memory performance on the CDR battery, and G115 improved the speed of performing memory tasks. Combination improved performance of both the serial threes and serial sevens, subtraction tasks. |
| Scholey and Kennedy (2002) [114] | 20 participants | Study 1: 120, 240, or 360 mg Ginkgo (EGb761) or placebo. Study 2: 200, 400, 600 mg G115 or placebo. Study 3: 320, 640, 960 mg their combination (Ginkoba M/E) or placebo. A 7-Day washout period between each dose. | Healthy young adults Study 1: 18 females and 11 males (mean age: 19.9, SD: 1.47) Study 2: 14 females and 6 males (mean age: 21.3, SD: 2.64) Study 3: 10 females and 10 males (mean age: 20.6, SD: 4.20) |
Acute DB, PC, CO | Serial threes and serial sevens assessed predosing and at 1, 2.5, 4, and 6 h thereafter. | Highly significant and sustained increase in the number of serial sevens responses after 320 mg of the Ginkgo–ginseng combination at all posttreatment testing times. |
| Kennedy et al (2001) [115] | 20 participants | 200, 400, 600 mg of G115 and a matching placebo, in a counterbalanced order, with a 7-day washout period between treatments. | Twenty healthy young adult volunteers (14 females and 6 males, ranging between 20 and 27 years, mean age: 21.3 years). | Acute DB, PC, balanced, CO |
“Quality of Memory,” “Speed of Memory,” “Quality of Attention” and “Speed of Attention” by CDR computerized assessment battery. Test sessions took place 1, 2.5, 4, and 6 h after the day's treatment. | Significant improvement in “Quality of Memory” and the associated “Secondary Memory” factor with 400 mg of G115.200 and 600 mg doses were associated with a significant decrement of the “Speed of Attention” factor. |
| D'Angelo et al (1986) [119] | 32 participants | 200 mg of G115 (100 mg twice a day) or placebo for 12 weeks. | Male volunteers (20–24 years, mean age: 22 years). | DB, PC | Tapping test, simple reaction time, choice reaction time, cancellation test, digit symbol substitution test, mental arithmetic test, logical deduction. | Favorable effect in attention (cancellation test), processing (mental arithmetic, logical deduction), integrated sensory–motor function (choice reaction time), and auditory reaction time. The G115 group was statistically superior to the placebo group only in mental arithmetic. |
| Quiroga (1982) [121] | 45 patients | 200 mg of G115 (100 mg twice a day) or placebo and 3 mg Hydergin per day or placebo for 90 days. | Patients with cerebrovascular deficit (23 males and 22 females, aged between 40 and 76 years, mean: 58 years). | Comparative DB, four test groups | Rheoencephalographic controls before the treatment and after 30, 60, and 90 days of treatment. | G115 improved quotients of 30% to 45% with respect to the pretreatment values. Hydergin improved quotients >50%. In the placebo group, no improvement was observed. |
| Quiroga and Imbriano (1979) [120] | 200 | G115 (500 mg twice a day) for the first month and then 500 mg per day for the following two months up to 90 days. 20 patients received placebo. |
Patients: 155 males and 41 females, aged 40–71 years. 157 patients had arteriosclerotic cerebrovascular circulatory. | No data | Clinical, hematological, radiological, and rheographic examination before the treatment and after 7, 15, 30, 60, and 90 days. | 36% of patients experienced an improvement more than 60% in circulatory insufficiency. 54% of patients experienced an improvement (ca. 30%) of cerebral flow. 33% of patients failed to attend the final examination after 90 days. |
CDR, cognitive drug research; CO, crossover; DB, double-blind; HbAlc, glycated hemoglobin; RVIP, rapid visual information processing; PC, placebo-controlled; SD, standard deviation
The review reports that administration of G115 as an acute multiple dose is associated with enhancements in cognitive function; G115 can potentiate vaccination against the common cold and/or influenza syndrome, and it is effective in reducing the bacterial count in patients undergoing an acute attack of chronic bronchitis [67].
Recently, a review by Neale et al has focused on the neurocognitive effects of modafinil and two standardized botanical extracts: G115 and a well-characterized extract of Bacopa monnieri, CDRI 08. The study investigated the effects of G115 on healthy human participants in a double-blind, placebo-controlled trial. G115 effect sizes (Cohen's d) were 0.86, confirming that neurocognitive enhancement from well-characterized extracts can produce cognition-enhancing effects of similar magnitude to those from pharmaceutical interventions (0.77 for modafinil) [68].
3.1. Effect of G115 on muscle activity, blood glucose and lipid profile regulation, and quality of life
The impact of G115 on several hematological parameters during aerobic exercise has been investigated in rats. A clear physiological response was associated with G115 administration, reproducing many of the effects obtained after long-term exercise, such as increased erythrocyte count and hemoglobin levels [69]. Additional studies have suggested that prolonged treatment with G115 in rat models increases the capillary density and the oxidative capacity of the muscles in a manner similar to that observed with increased physical activity [70].
G115 has also been shown to improve energy balance in C2C12 myotubes, dose and time dependently, and it increases adenosine triphosphate production and oxygen consumption. An increased gene expression of the metabolic sensors, 5′ AMP-activated protein kinase and sirtuin 1, and the master regulator of mitochondria proliferator-activated receptor gamma coactivator-1α is also reported during acute exercise in mice treated with G115. In addition, G115 was able to counteract the inhibition of gene expression of 5′ AMP-activated protein kinase and proliferator-activated receptor gamma coactivator-1α induced by prolonged exercise [71].
G115 stimulates in vivo D-glucose transport into cells [72] and inhibits the formation of free radicals generated by physical stress dose dependently [73]. G115 is also effective in reducing lipid peroxidation, inflammation, and release of myocellular proteins [74]. Further analyses revealed that G115 was effective in preserving mitochondrial membrane integrity and reducing nitrate concentration and carbonyl contents in vastus and rectus muscles [75], and it was able to protect the muscle from exercise-induced oxidative stress irrespective of the fiber type [76].
In conclusion, preclinical studies have demonstrated that G115 is able to modulate the expression of genes involved in cellular energy expenditure, reproducing many of the effects obtained after long-term exercise, including changes at the hematological and muscular level, and exerting protective effects from exercise-induced tissue damage through its free radical scavenging abilities.
Clinical studies conducted with G115 (Table 1) have validated several of the preclinical findings including the impact on metabolic activity, cognitive function, and immunomodulatory effects. Twelve studies have been published evaluating the effects of G115 on muscular activity, focusing on endurance and vitality [77], [78], psychasthenia [79], [80], [81], and psychomotor functions [82], [83], [84], [85], [86], [87], [88].
Studies conducted by Dörling et al. [77] demonstrated that chronic administration of 100 mg G115 twice daily provided beneficial effects on subjective physical condition and physical fitness parameters in healthy volunteers. Visual and auditory improvement (i.e., shorting of the reaction time) was observed in 81.6% of those in the G115-treated group, with only 6.6% showing no change and 11.6% having a decrease in function after 3 months. The flicker fusion threshold gave a 59.4% improvement in the G115-treated group and 29.7% improvement in the placebo group. In the G115-treated group, two-hand coordination was enhanced by 38.0%, compared with 16.6% in the placebo control group. Significant improvement in the recovery quotient of 97% was only observed in the G115-treated group. This study was followed by a series of clinical studies by Forgo and Kirchdorfer [79,86], Forgo [84], and Forgo and Schimert [87], evaluating the impact of G115 (200 mg/day) on the physical work capacity in a group of healthy young athletes. In one study, a significant improvement in aerobic capacity from 4,215 mL/min to 4,895 mL/min with a reduction in blood lactate and heart rates was observed in the G115-treated group compared with the placebo control. In the other small studies, there were reports of G115-induced increases in the oxygen absorption capacity and reduction of serum lactate and heart rate versus the placebo control. This finding was supported by a study indicating these positive effects lasted at least 3 weeks after treatment [86] but was in slight contradiction to a study demonstrating that there were no significant differences detected between the G115 and placebo groups [85]. In a further study, cardiovascular efficiency was evaluated in an older population (mean age, 50 years) after both acute and chronic administration of G115 (200 mg/day) by measuring the difference between Hb-O2 saturation parameters. A significant increase (p < 0.05) by 4.5 mm Hg in resting arterial pO2 uptake and a 4.3 mm Hg decrease in venous pO2 were found in the G115-treated group compared with the placebo control after 4 weeks. This translated to an intensification of O2 transport into the organs and tissues of the body, from 100% before to 129% after G115 treatment. G115 treatment in combination with oxygen treatment resulted in an additive increase in resting arterial pO2 uptake to 10.1 mm Hg [82].
Van Schepdael et al have demonstrated that G115 can prevent the loss of physical performance that is typically associated with end-of-season fatigue in habitually competing athletes. Athletes treated with 200 mg/day of G115 for 10 weeks showed an improved maximal oxygen consumption (57.9 mL to 60.4 mL), and the energy (in watts) for a heart rate of 170/min showed a related increase. Lactic acid levels were reduced significantly at the end of the first 10 weeks in both groups, with only a very small diminution in the second period of treatment. The results from the study suggested that G115 could be used as a nondoping “adaptogen” [87].
Adaptogenic properties of G115 were supported by another study in a larger group of patients suffering from asthenia. In this study, treatment with G115 resulted in both a rise in appetite and general well-being with respect to placebo. Only the G115-treated group showed a statistically significant decrease in systolic blood pressure, of both common cold and bronchitis symptoms, with a reduction of fever, cough, otitis symptoms, sleep amelioration, and augmentation of performance. This study also demonstrated that G115 was well tolerated, with only one patient of eighty-three reporting to have experienced nausea during treatment [81].
In longitudinal studies conducted by Mulz et al., G115 was evaluated for its ability to alleviate psychasthenic symptoms in a >50-year patient cohort. The patients were treated with either G115 alone or G115 in combination with vitamins and minerals versus the placebo control. Both treatments demonstrated an improvement in psychasthenic symptoms after 2 years of treatment, with 78% of patients experiencing alleviation of symptoms with G115 treatment alone and 72% of patients demonstrating a response to therapy in the G115 combination treatment compared with the placebo. Although treatment with G115 alone was well tolerated, treatment with the combination resulted in 2 of 28 patients experiencing minor adverse events [79].
A study conducted by Forgo et al investigated the effect of G115 on pulmonary health and general well-being in a cohort of healthy patients. The patients in this study were grouped according to age (30–39 and 40–60 years) and gender and received either G115 (200 mg/day) or the placebo control for 12 weeks. A significant improvement was observed in the pulmonary function in the male 40- to 60-year cohort; however, the levels of testosterone and luteinizing hormones in plasma and of free cortisol in urine were unchanged for the treatment group compared with the placebo control [84].
A number of clinical trials have been conducted to evaluate the impact of G115 on metabolic activity, with particular focus on blood glucose levels and lipid profiles. A brief summary of the key findings from these studies is discussed in the following section.
Hosseini et al evaluated the effects of G115 on fasting blood sugar levels, glycated hemoglobin, and lipid profiles in patients with type 2 diabetes. The patients treated with G115 (300 mg/day for 8 weeks) had a significant reduction in glycated hemoglobin and fasting blood glucose levels, but there was no enhancement of lipid profiles compared with the placebo control [88].
El-Farok et al investigated the effects of G115 in patients with hypercholesterolemia consuming low-fat diet and performing one-hour daily exercise. A significant reduction in low-density lipoprotein, total cholesterol, low-density lipoprotein/high-density lipoprotein, and cholesterol/high-density lipoprotein ratios, higher alpha lipoic acid, was found [89].
A series of studies by Reay et al [90], [91], [92] investigated the effects of G115 on blood glucose levels. Two studies reported significant decreases in fasting blood glucose levels, measured one hour after a single dose of G115 (200 or 400 mg/day) in healthy volunteers, but no effect on postprandial glucose (after a 25-g glucose drink) response. In a study, there was a significant drink–G115 interaction, suggesting that in presence of high blood glucose levels, the administration of G115 leads to a further increase in blood glucose [90], [91], [92]. A study on healthy patients demonstrated no effect on any glucoregulatory parameter investigated on Days 1, 29, and 57 of the study. Therefore, the benefits to glucose regulation associated with long-term ginseng use may only be present in populations with compromised glucose control [93].
3.2. Effect of G115 on immune response and COPD
Several in vitro and in vivo studies have identified an immunomodulatory effect of G115. In studies conducted in mouse models, G115 demonstrated protective effect against experimental viral infection, protecting 34–40% of mice infected with the Semliki Forest virus [94]. G115 was also capable of improving antibody plaque-forming cell response and circulating antibody titer against sheep erythrocytes, immunity against the Semliki Forest virus, and natural killer cell activity in mice [95]. G115 and several fractions of the extract possess anticomplement and mitogen activities in mice spleen cell cultures, with the strongest anticomplement activity obtained with the crude polysaccharide fraction [96]. A more recent study has proposed that G115 modulates the immune response by reducing the peak of cytokine release after a few weeks of stress and stimulates the innate immune response, gradually facilitating host defense and potentiating the response against bacterial or pathogenic challenge [97].
Diverse clinical studies have provided evidence to support the in vitro and in vivo studies, and the key findings are summarized in Table 2. Pivotal clinical studies are discussed in the following section. In a study conducted by Scaglione et al, it was found that treatment of patients with G115 extract (200 mg/day) stimulates the immune system in humans and it is more active than an aqueous ginseng (200 mg/day) extract. Analysis of blood samples from patients treated with G115 at the 4th and 8th week revealed an increase in chemotaxis of polymorphonuclear leukocytes, the phagocytic index, and the total number of T3 and T4 lymphocytes when compared with the placebo control. The G115-treated cohort also demonstrated an increase in the T4:T8 ratio and the activity of natural killer cells [98]. In a further study performed by the same authors, a significant increase in phagocytosis and intracellular killing was also observed in patients treated with G115 (200 mg/day) compared with the placebo control [99]. In addition, G115 (200 mg/day) was found to potentiate the immunity response against the common cold and/or influenza syndrome. Frequency of influenza or colds was significantly reduced (p < 0.0001) in the G115-treated group compared with the placebo control. Antibody titers at Week 8 were highest after G115 treatment, and natural killer cell activity in the treatment group was almost twice than that seen in the placebo group [100]. Moreover, G115 (200 mg/day) was more effective in reducing the bacterial clearance than in the participants receiving only antibiotics. Statistically significant differences between treatment groups were observed on Days 4, 5, 6, and 7 (p = 0.0049, p = 0.0104, p = 0.0175, and p = 0.0182, respectively), whereas a borderline trend was seen on Day 8 (p = 0.0554) [101]. Finally, Engels et al proved that intake of G115 (400 mg/day) did not affect mucosal immunity, physical performance, and heart rate recovery. Before and after the intervention, each participant performed three consecutive 30-s Wingate tests interspersed with 3-min recovery periods under controlled laboratory conditions. The secretory immunoglobulin A secretion rate, secretory immunoglobulin A:protein ratio, and saliva flow rate were lower after exercise at baseline (P < 0.05) in the G115 group [102]. Salient outcome measures and results of the cited clinical studies are reported in Table 2.
Two studies by Gross et al [103], [104] demonstrated that G115 (200 mg/day) improved pulmonary function tests and respiratory endurance in patients with COPD. In the first study [103], forced vital capacity (FVC) changed from 32.1% to 67.3% (p < 0.05) and to 72.8% (p < 0.01) after 1.5 and 3 months, respectively, whereas forced expiration volume in one second (FEV1) improved from 34.75% to 44.5% (p < 0.05) and to 47.3% (p < 0.05) for the same period. The peak expiration flow rate enhanced from 37.8 ± 5% to 46.6 ± 5% (p < 0.01) and to 47.2 ± 5%, respectively. Maximum ventilation volume, an index of the endurance test, was changed from 32.7 ± 4% to 43.3 ± 4% (p < 0.01) and to 50.6 ± 4% (p < 0.01), respectively. The index of respiratory strength was improved from an maximal inspiratory pressure (MIP) of 37.3 ± 4 cmH2O to 44.7 ± 5 (p < 0.01) and to 51.2 ± 5 cmH2O (p < 0.01), respectively. At the same time, a significant improvement in oxygenation was clearly demonstrated with PaO2 that changed from 47.0 ± 4 torr to 65.3 ± 3 torr (p < 0.05) and subsequently to 69.3 ± 4 torr (p < 0.01), respectively [103]. These findings were validated in a second study where it was observed that for G115-treated patients, all respiratory function parameters significantly increased above baseline compared with the placebo control [104]. The observed increases were as follows: FVC, 32.5%; FEV1, 0–27.0%; peak expiration flow, 27.5%; forced expiratory flow50, 45.4%; forced expiratory flow75, 56.9%; maximum ventilation volume, 40.4%; MIP, 47.0%; and maximal oxygen consumption, 37.5% [104]. An optimized protocol study based on previous randomized controlled trials (RCTs) and systematic reviews on Quality of Life (QoL) improvements using ginseng for COPD was developed [105]. Finally, a clinical study [106] conducted on patients with COPD confirmed the lack of exacerbations and efficacy of G115 (400 mg/day) for the improvement of quality of life and pulmonary parameters such as the FEV1/FVC ratio [i.e., the percent of the lung size (FVC) that can be exhaled in one second], respectively.
3.3. Effect of G115 on cognitive function
Numerous old preclinical studies have investigated the effects of G115 on cognitive function. An in vivo study conducted by Petkov [107] demonstrated that administration of G115 improved learning and memory that correlated with an increase in the 14C-phenylalanine transport across the blood–brain barrier. The biochemical analysis of monoamines, the adenosine 3′5′ monophosphate system, and activity of phosphodiesterase and adenylate cyclase after intraperitoneal injection of G115 in the brain stem and brain cortex was carried out and revealed that G115 had an impact on brain metabolism [107]. Additional experiments in rats reported that G115 exerted the most favorable effects on learning and memory in cases where these processes had decayed because of either senescence or individual specificities [108].
Experiments using rabbit brain tissues showed a dose-related significant increase in glucose uptake with simultaneous significant reduction of lactate, pyruvate, and the lactate/pyruvate ratio in the presence of G115, indicating changes in the metabolic pathways and improving the energy balance of neuronal cells. Furthermore, G115 was found to have a stimulant, desynchronizing action on the electrocorticogram of the conscious rabbit brain [109].
The positive influences of G115 on the central nervous system are not limited to the modulation of brain metabolism but extend toward neuroprotection. In fact, G115 treatment significantly blocked tyrosine hydroxylase–positive cell loss in the substantia nigra and reduced the appearance of locomotor dysfunction in two rodent models of Parkinson disease [21]. In an additional study, oral administration of G115 significantly reduced dopaminergic (nigrostriatal) cell loss, prevented neuroinflammation, microgliosis, and accumulation of α-synuclein aggregates. Furthermore, G115 administration fully prevented the development of locomotor deficits [110].
Neale et al [68] have recently reviewed some relevant clinical studies on the effects of G115 on cognitive functions, namely, studies by Reay et al [93], Reay et al [92], Reay et al [90], Sünram-Lea et al [111], Kennedy et al [112], Kennedy et al [113], Scholey and Kennedy [114], and Kennedy et al [115]. This review assessed that G115 enhances cognition effects comparable with those from modafinil therapy. G115 possesses acute positive effects on secondary memory and working memory tasks. Neurocognitive G115 functions include glucoregulation, modulation of cholinergic and dopaminergic activity, and increasing nitric oxide synthesis [68]. A complete report of all the clinical studies carried out in the last 40 years, and discussed in the present review, is summarized in Table 3.
Reay et al investigated the behavioral and mood effects of G115 in a 20-week treatment period. The study revealed improvements in working memory after a single dose of G115 (200 mg/day), whereas after chronic dosing, the results revealed both improvement and decrement in aspects of cognition and mood [116]. In a further study, the same research group investigated the effects of treatment with G115 on subjective mood and aspects of working memory processes after a dose of 200 or 400 mg of G115 [117]. In this study, dose-related treatment effects (p < 0.05) were found. Two hundred milligrams reduced a fall in mood at 2.5 and 4 h on Day 1 and at 1 and 4 h on Day 8 but decreased the replay on a mental arithmetic task on Day 1 and at 1 and 2.5 h on Day 8. Reaction time on 3-back reaction time was decreased after the 400-mg dose, but reaction time increased with 200 mg; 3-back sensitivity index scores improved with the 400-mg dose but diminished after a dose of 200 mg. G115 enhanced self-reported mood levels. The 400-mg dose also improved calmness (restricted 2.5 and 4 h on Day 1) and mental arithmetic on Days 1 and 8. The study revealed that 7 consecutive days of G115 ingestion had no effect on mood or cognitive performance [117].
Kennedy et al [118] investigated the electroencephalograph effects of G115 (200 mg/day) or a ginkgo extract (EGb761, 360 mg/day). The results showed that G115 led to a significant shortening of the latency of the P300 component of the evoked potential. Furthermore, significant reductions in frontal “eyes closed” theta, beta, and alpha activity were observed. These findings showed that G115 could directly modulate cerebroelectrical activity [118].
The psychomotor performance of G115 (200 mg/day) was assessed in a study by D'Angelo et al. A positive G115 effect to baseline performance was observed in attention, processing, integrated sensory–motor function and auditory reaction time. The G115 group was superior statistically to the placebo group only in mental arithmetic. No adverse effects were found, and laboratory parameters remained in the normal values [119].
Quiroga and Imbriano [120] investigated the effects of G115 (1 g/day followed by 500 mg/day) on patients with circulatory insufficiency (Table 3). An enhancement of more than 60% in circulatory insufficiency compared with the pretreatment value was experienced by 36% of patients (36%), with a complete recovery after 30 days of treatment, which remained constant after 60 days and 90 days. About half of the patients experienced an enhancement of approximately 30% in the cerebral flow in comparison with the pretreatment values, and about 10% of patients showed no or only short-lasting improvement [120]. In a further study by Quiroga [121], the performance of 200 mg of G115 was compared with 3-mg Hydergin per day during 90 days. G115 treatment improved quotients between 30% and 45% with respect to the pretreatment values and Hydergin enhanced quotients of more than 50%, whereas in the placebo group, no improvement was observed [121].
4. Safety evidence of G115
The safety profile of G115 has been well established both from preclinical toxicology/safety studies and clinical studies in healthy volunteers and in patients with defined health conditions.
In vivo dose escalation or maximum tolerated dose studies with G115 have established a LD50 value higher than 5000 mg/kg/day and 1000 mg/kg intraperitoneally for rats and mice, respectively. The same extract given to rats at doses of 4000 mg/kg/day for 20 days resulted in normal levels of both hematological and histological biomarkers. G115 administered to beagle dogs in doses up to 15 mg/kg/day for 90 days did not cause any subchronic toxicity. G115 was also investigated on growth, reproduction, lactation, and maturation of male and female Sprague–Dawley rats. At doses ranging from 1.5 to 15 mg/kg/day, G115 did not show adverse effects on the reproductive parameters evaluated or treatment-related effects on animal behavior, physical appearance, or food consumption. No chronic carcinogenetic studies of ginseng on experimental animals have been found in the literature [123].
High tolerability of G115 is evidenced in human clinical trials. Although rare, reported adverse effects from G115 treatment are mild and mainly gastrointestinal or sleep-related, including stomach discomfort, nausea, vomiting, diarrhea, constipation, headache, and insomnia. Furthermore, hypersensitivity reactions such as urticaria and itching as well as eye burning have been reported in a mild and transient way [2], [3], [4], [18]. This is in agreement with a recent review that has highlighted that no significant side effects have been observed in the supplementation with diverse preparations of ginseng [123]. However, drug interactions can occur, in particular, in anticoagulants such as warfarin [122]. G115 has also been used and marketed as a medical product worldwide for decades, but no serious adverse events have been reported. Epidemiological studies, postmarketing surveillance trials, and spontaneous reporting schemes clearly correlated with the ingestion of G115 [2], [3], [4]. Taken together, these data support the claim that G115 is a well-tolerated natural medicine.
5. Conclusions
This review focuses on the studies of the well-characterized ginseng extract G115, providing evidence that quality is essential to define constant efficacy and safety profiles in pharmaceutical interventions. It is well known that poor standardization causes difficulties in the evaluation of data obtained from animal experiments and clinical studies, and consequently, data from different trials cannot be compared. This partially explains the inconsistent findings obtained from some clinical trials over the past decades, which prevent any firm conclusions being drawn on the efficacy of ginseng. By contrast, products fulfilling the basic requirements of quality have reproducible safety and efficacy profiles [63], [64]. This is the case of the standardized extract G115. In fact, numerous clinical studies, most of them double-blind, placebo-controlled trials of scientific relevance, have proved the efficacy of G115 on blood glucose and lipid regulation, COPD, energy, physical performance, and immune and cognitive functions. Moreover, the safety profile is also recognized to be very good with less frequent and milder side effects than those of other therapies. Clinical trials and 50 years of presence on the market are proof of a good safety profile of G115.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- 1.Sandberg F., Corrigan D. Taylor & Francis; London: 2001. Natural remedies: their origins and uses. [Google Scholar]
- 2.World Health Organization . vol. 1. WHO; Geneva: 1999. pp. 168–182. (WHO monographs on selected medicinal plants). [Google Scholar]
- 3.European Scientific Cooperative on Phytotherapy . Thieme; Germany: 2003. ESCOP monographs. The scientific foundation for herbal medicinal products. [Google Scholar]
- 4.European Medicines Agency . WHO; London: 2013. Community herbal monograph on Panax ginseng CA Meyer, radix. [Google Scholar]
- 5.Park H.J., Kim D.H., Park S.J., Kim J.M., Ryu J.H. Ginseng in traditional herbal prescriptions. J Ginseng Res. 2012;36(3):225–241. doi: 10.5142/jgr.2012.36.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37(1):1. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenthal M. Thieme; New York: 2003. The ABC clinical guide to herbs; pp. 211–225. [Google Scholar]
- 8.Bilia A.R. Science meets regulation. J Ethnopharmacol. 2014;158(Pt B):487–494. doi: 10.1016/j.jep.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Shibata S., Fujita M., Itokawa H., Tanako O., Ishii T. Studies on the constituents of Japanese and Chinese Crude Drugs. XI. Panaxadiol, a sapogenin of ginseng roots. Chem Pharm Bull. 1963;11:759–761. doi: 10.1248/cpb.11.759. [DOI] [PubMed] [Google Scholar]
- 10.Leung K., Wong S. Pharmacology of ginsenosides: a literature review. Chinese Medicine. 2010;5:20–34. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W., Choi H.K., Huang L. State of Panax ginseng research: a global analysis. Molecules. 2017;22:1518–1525. doi: 10.3390/molecules22091518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H.Y., Qi L.W., Wang C.Z., Li P. Bioactivity enhancement of herbal supplements by intestinal microbiota focusing on ginsenosides. Am J Chin Med. 2011;39:1103–1115. doi: 10.1142/S0192415X11009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J., Lee E., Kim D., Lee J., Yoo J., Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009;122:143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Qi L.W., Wang C.Z., Du G.J., Zhang Z.Y., Calway T., Yuan C.S. Metabolism of ginseng and its interactions with drugs. Curr Drug Metab. 2011;12:818–822. doi: 10.2174/138920011797470128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui J.F., Björkhem I., Eneroth P. Gas chromatographic-mass spectrometric determination of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol for study on human urinary excretion of ginsenosides after ingestion of ginseng preparations. J Chromatogr B Biomed Sci Appl. 1997;689:349–355. doi: 10.1016/s0378-4347(96)00304-0. [DOI] [PubMed] [Google Scholar]
- 16.Park S.H., Jang J.H., Chen C.Y., Na H.K., Surh Y.J. A formulated red ginseng extract rescues PC12 cells from PCB-induced oxidative cell death through Nrf2- mediated upregulation of heme oxygenase-1 and glutamate cysteine ligase. Toxicology. 2010;278:131–139. doi: 10.1016/j.tox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Kim S., Lee Y., Cho J. Korean red ginseng extract exhibits neuroprotective effects through inhibition of apoptotic cell death. Biol Pharm Bull. 2014;37(6):938–946. doi: 10.1248/bpb.b13-00880. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y.L., Huang C.L., Lee Y.C., Liao W.C., Lai W.L., Lin Y.J., Huang N.K. Mechanisms of Panax ginseng in preventing rat pheochromocytoma cells from apoptosis. J Ethnopharmacol. 2009;125(1):10–15. doi: 10.1016/j.jep.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y.O., Kim H.J., Kim G.S., Park H.G., Lim S.J., Seong N.S., Ham Y.W., Lee S.D., Jang K.H., Jung K.H. Panax ginseng protects against global ischemia injury in rat hippocampus. J Med Food. 2009;12(1):71–76. doi: 10.1089/jmf.2007.0614. [DOI] [PubMed] [Google Scholar]
- 20.Van Kampen J.M., Baranowski D.B., Shaw C.A., Kay D.G. Panax ginseng is neuroprotective in a novel progressive model of Parkinson's disease. Exp Gerontol. 2014;50:95–105. doi: 10.1016/j.exger.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Van Kampen J., Robertson H., Hagg T., Drobitch R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson's disease. Exp Neurol. 2003;184(1):521–529. doi: 10.1016/j.expneurol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Choi J.G., Kim N., Huh E., Lee H., Oh M.H., Park J.D., Pyo M.K., Oh M.S. White ginseng protects mouse hippocampal cells against amyloid-beta oligomer toxicity. Phytother Res. 2017;31(3):497–506. doi: 10.1002/ptr.5776. [DOI] [PubMed] [Google Scholar]
- 23.Dong L., Wang Y., Lv J., Zhang H., Jiang N., Lu C., Xu P., Liu X. Memory enhancement of fresh ginseng on deficits induced by chronic restraint stress in mice. Nutr Neurosci. 2017;15:1–8. doi: 10.1080/1028415X.2017.1373928. [DOI] [PubMed] [Google Scholar]
- 24.Tan X., Gu J., Zhao B., Wang S., Yuan J., Wang C., Chen J., Liu J., Feng L., Jia X. Ginseng improves cognitive deficit via the RAGE/NF-κB pathway in advanced glycation end product-induced rats. J Ginseng Res. 2015;39(2):116–124. doi: 10.1016/j.jgr.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yayeh T., Yun K., Jang S., Oh S. Morphine dependence is attenuated by red ginseng extract and ginsenosides Rh2, Rg3, and compound K. J Ginseng Res. 2016;40(4):445–452. doi: 10.1016/j.jgr.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H.S., Jang C.G., Lee M.K. Antinarcotic effects of the standardized ginseng extract G115 on morphine. Planta Med. 1990;56(2):158–163. doi: 10.1055/s-2006-960915. [DOI] [PubMed] [Google Scholar]
- 27.Kim C., Lee J.H., Baek S.H., Ko J.H., Nam D., Ahn K.S. Korean red ginseng extract enhances the anticancer effects of sorafenib through abrogation of CREB and c-jun activation in renal cell carcinoma. Phytother Res. 2017;31(7):1078–1089. doi: 10.1002/ptr.5829. [DOI] [PubMed] [Google Scholar]
- 28.Seo E.Y., Kim W.K. Red ginseng extract reduced metastasis of colon cancer cells in vitro and in vivo. J Ginseng Res. 2011;35(3):315–324. doi: 10.5142/jgr.2011.35.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho Y.L., Li K.C., Chao W., Chang Y.S., Huang G.J. Korean red ginseng suppresses metastasis of human hepatoma SK-Hep1 cells by inhibiting matrix metalloproteinase-2/-9 and urokinase plasminogen activator. Evid Based Complement Alternat Med. 2012;2012:965846. doi: 10.1155/2012/965846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong V.K., Cheung S.S., Li T., Jiang Z.H., Wang J.R., Dong H., Yi X.Q., Zhou H., Liu L. Asian ginseng extract inhibits in vitro and in vivo growth of mouse lewis lung carcinoma via modulation of ERK-p53 and NF-κB signaling. J Cell Biochem. 2010;111(4):899–910. doi: 10.1002/jcb.22778. [DOI] [PubMed] [Google Scholar]
- 31.Lee S.D., Park S.K., Lee E.S., Kim H.M., Lee C.W., Lee K., Lee K.H., Kang M.R., Lee K.S., Lee J. A lipid-soluble red ginseng extract inhibits the growth of human lung tumor xenografts in nude mice. J Med Food. 2010;13(1):1–5. doi: 10.1089/jmf.2009.1142. [DOI] [PubMed] [Google Scholar]
- 32.Bespalov V.G., Alexandrov V.A., Semenov A.L., Kovan'Ko E.G., Ivanov S.D. Anticarcinogenic activity of alpha-difluoromethylornithine, ginseng, eleutherococcus, and leuzea on radiation-induced carcinogenesis in female rats. Int J Radiat Biol. 2014;90(12):1191–1200. doi: 10.3109/09553002.2014.932937. [DOI] [PubMed] [Google Scholar]
- 33.Sagar S.M., Yance D., Wong R.K. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 1. Curr Oncol. 2006;13:14–26. [PMC free article] [PubMed] [Google Scholar]
- 34.Kang K.A., Kang J.H., Yang M.P. Ginseng total saponin enhances the phagocytic capacity of canine peripheral blood phagocytes in vitro. Am J Chin Med. 2008;36(2):329–341. doi: 10.1142/S0192415X08005801. [DOI] [PubMed] [Google Scholar]
- 35.Jang H.I., Shin H.M. Wild Panax ginseng (Panax ginseng C.A. Meyer) protects against methotrexate-induced cell regression by enhancing the immune response in RAW 264.7 macrophages. Am J Chin Med. 2010;38(5):949–960. doi: 10.1142/S0192415X10008378. [DOI] [PubMed] [Google Scholar]
- 36.Larsen M.W., Moser C., Høiby N., Song Z., Kharazmi A. Ginseng modulates the immune response by induction of interleukin-12 production. APMIS. 2004;112(6):369–373. doi: 10.1111/j.1600-0463.2004.apm1120607.x. [DOI] [PubMed] [Google Scholar]
- 37.Park H.Y., Lee S.H., Lee K.S., Yoon H.K., Yoo Y.C., Lee J., Choi J.E., Kim P.H., Park S.R. Ginsenoside Rg1 and 20(S)-Rg3 induce IgA production by mouse B cells. Immune Netw. 2015;15(6):331–336. doi: 10.4110/in.2015.15.6.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhuo X., Sun H., Wang S., Guo X., Ding H., Yang Y., Shan Y., Du A. Ginseng stem-and-leaf saponin (GSLS)-Enhanced protective immune responses induced by toxoplasma gondii heat shocked protein 70 (HSP70) against toxoplasmosis in mice. J Parasitol. 2017;103(1):111–117. doi: 10.1645/16-54. [DOI] [PubMed] [Google Scholar]
- 39.Silvestrini P., Beccaria C., Pereyra E.A.L., Renna M.S., Ortega H.H., Calvinho L.F., Dallard B.E., Baravalle C. Intramammary inoculation of Panax ginseng plays an immunoprotective role in Staphylococcus aureus infection in a murine model. Res Vet Sci. 2017;115:211–220. doi: 10.1016/j.rvsc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Lee J.S., Lee Y.N., Lee Y.T., Hwang H.S., Kim K.H., Ko E.J., Kim M.C., Kang S.M. Ginseng protects against respiratory syncytial virus by modulating multiple immune cells and inhibiting viral replication. Nutrients. 2015;7(2):1021–1036. doi: 10.3390/nu7021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu M.L., Kim H.J., Choi Y.R., Kim H.J. Intake of Korean red ginseng extract and saponin enhances the protection conferred by vaccination with inactivated influenza a virus. J Ginseng Res. 2012;36(4):396–402. doi: 10.5142/jgr.2012.36.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim Y.M., Namkoong S., Yun Y.G., Hong H.D., Lee Y.C., Ha K.S., Lee H., Kwon H.J., Kwon Y.G., Kim Y.M. Water extract of Korean red ginseng stimulates angiogenesis by activating the PI3K/Akt-dependent ERK1/2 and eNOS pathways in human umbilical vein endothelial cells. Biol Pharm Bull. 2007;30:1674–1679. doi: 10.1248/bpb.30.1674. [DOI] [PubMed] [Google Scholar]
- 43.Yu J., Eto M., Akishita M., Kaneko A., Ouchi Y., Okabe T. Signaling pathway of nitric oxide production induced by ginsenoside Rb1 in human aortic endothelial cells: a possible involvement of androgen receptor. Biochem Biophys Res Commun. 2007;353:764–769. doi: 10.1016/j.bbrc.2006.12.119. [DOI] [PubMed] [Google Scholar]
- 44.Luo P., Dong G., Liu L., Zhou H. The long-term consumption of ginseng extract reduces the susceptibility of intermediate-aged hearts to acute ischemia reperfusion injury. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144733. e0144733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim K.H., Ko D., Kim J.H. a. Cardioprotective potential of Korean Red Ginseng extract on isoproterenol-induced cardiac injury in rats. J Ginseng Res. 2013;37:273–282. doi: 10.5142/jgr.2013.37.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin S.S., Yoon M. Korean red ginseng (Panax ginseng) inhibits obesity and improves lipid metabolism in high fat diet-fed castrated mice. J Ethnopharmacol. 2017;210:80–87. doi: 10.1016/j.jep.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 47.Liu C., Hu M.Y., Zhang M., Li F., Li J., Zhang J., Li Y., Guo H.F., Xu P., Liu L. Association of GLP-1 secretion with anti-hyperlipidemic effect of ginsenosides in high-fat diet fed rats. Metabolism. 2014 Oct;63(10):1342–1351. doi: 10.1016/j.metabol.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 48.National Toxicology Program Toxicology and carcinogenesis studies of ginseng (CAS No. 50647-08-0) in F344/N rats and B6C3F1 mice (gavage studies) Natl Toxicol Program Tech Rep Ser. 2011;2011(567):1–149. [PubMed] [Google Scholar]
- 49.Geng J., Dong J., Ni H., Lee M.S., Wu T., Jiang K., Wang G., Zhou A.L., Malouf R. Ginseng for cognition. Cochrane Database Syst Rev. 2010;12:CD007769. doi: 10.1002/14651858.CD007769.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Lee M.S., Yang E.J., Kim J.I., Ernst E. Ginseng for Cognitive function in Alzheimer’s Disease: a systematic review. J Alzheimers Dis. 2009;18(2):339–344. doi: 10.3233/JAD-2009-1149. [DOI] [PubMed] [Google Scholar]
- 51.Shishtar E., Sievenpiper J.L., Djedovic V., Cozma A.I., Ha V., Jayalath V.H., Jenkins D.J., Meija S.B., de Souza R.J., Jovanovski E. The effect of ginseng (the genus panax) on glycemic control: a systematic review and meta-analysis of randomized controlled clinical trials. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107391. e107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang D.J., Lee M.S., Shin B.C., Lee Y.C., Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin Pharmacol. 2008;66(4):444–450. doi: 10.1111/j.1365-2125.2008.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang Y.Z.1, Shang H.C., Gao X.M., Zhang B.L. Systematic review of the effects of ginseng on cardiovascular risk factors quality of life. Phytother Res. 2008;22(7):851–858. doi: 10.1002/ptr.2384. [DOI] [PubMed] [Google Scholar]
- 54.Lee N.-H., Son C.-G. Systematic review of randomised controlled trials evaluating the efficacy and safety of ginseng. J Acupunct Meridian Stud. 2011;4(2):85–97. doi: 10.1016/S2005-2901(11)60013-7. [DOI] [PubMed] [Google Scholar]
- 55.Vogler B.K., Pittler M.H., Ernst E. The Efficacy of ginseng. A systematic review of randomised controlled trials. Eur J Clin Pharmacol. 1999;55(8):567–575. doi: 10.1007/s002280050674. [DOI] [PubMed] [Google Scholar]
- 56.Seida J.K., Durec T., Kuhle S. North American (Panax quinquefolius) and Asian Ginseng (Panax ginseng) preparations for prevention of the common cold in healthy adults: a systematic review and chronic respiratory diseases. Evid Based Complement Alternat Med. 2011;2011:282151. doi: 10.1093/ecam/nep068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.An X., Zhang A.L., Yang A.W., Lin L., Wu D., Guo X., Shergis J.L., Thien F.C., Worsnop C.J., Xue C.C. Oral ginseng formulae for stable chronic obstructive pulmonary disease: a systematic review. Respir Med. 2015;105(2):165–176. doi: 10.1016/j.rmed.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Bilia A.R. Herbal medicinal products versus botanical-food supplements in the European market: state of art and perspectives. Nat Prod Commun. 2015;100:125–131. [PubMed] [Google Scholar]
- 59.Van Breemen R.B., Fong H.H.S., Farnsworth N.R. The role of quality assurance and standardization in the safety of botanical dietary supplements. Chem Res Toxicol. 2007:577–582. doi: 10.1021/tx7000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization . WHO; Geneva: 1992. Quality control methods for medicinal plant materials. [Google Scholar]
- 61.World Health Organization . vol. 2. WHO; Geneva: 1996. (Quality assurance of pharmaceuticals: a compendium of guidelines and related materials. Good manufacturing practices and inspection). [Google Scholar]
- 62.World Health Organization . WHO; Geneva: 1996. Guidelines for the assessment of herbal medicines. [Google Scholar]
- 63.European Medicines Agency . EMA; London: 2011. Guidelines on quality of herbal medicinal product/traditional herbal medicinal products. [Google Scholar]
- 64.World Health Organization . WHO; Geneva: 2000. General guidelines for methodologies on research and evaluation of traditional medicine. [Google Scholar]
- 65.United States Pharmacopoeial Convention . 2012. Powered Asian ginseng extract; pp. 1185–1186. [Google Scholar]
- 66.Council of Europe . 2013. Ginseng dry extract; pp. 1374–1375. [Google Scholar]
- 67.Scaglione F., Pannacci M., Petrini O. The standardised G115® Panax ginseng CA Meyer extract. Evidence-based Integrative Medicine. 2005;2(4):195–206. [Google Scholar]
- 68.Neale C., Camfield D., Reay J., Stough C., Scholey A. Cognitive effects of two nutraceuticals Ginseng and Bacopa benchmarked against modafinil: a review and comparison of effect sizes. British J Clin Pharm. 2012;75:728–737. doi: 10.1111/bcp.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferrando A., Vila L., Voces J.A., Cabral A.C., Alvarez A.I., Prieto J.G. Effects of ginseng extract on various hematological during aerobic exercise in the rat. Planta Med. 1999;65(3):288–290. doi: 10.1055/s-2006-960783. [DOI] [PubMed] [Google Scholar]
- 70.Ferrando A., Vila L., Voces J.A., Cabral A.C., Alvarez A.I., Prieto J.G. Effects of a standardized Panax ginseng extract on the skeletal muscle of the rat: a comparative study in animals at rest and under exercise. Planta Med. 1999;65(3):239–244. doi: 10.1055/s-1999-14081. [DOI] [PubMed] [Google Scholar]
- 71.Pannacci M., Lucini V., Dugnani S., Ciraci R., Zangara A., Scaglione F. Activity of Panax ginseng CA Meyer on energy production in mammals. Biochem Pharmacol. 2016;5(5):221. [Google Scholar]
- 72.Yamasaki K., Murakami C., Ohtani K., Kasai R., Kurokawa T., Ishibashi S., Soldati F., Stöckli M., Mulz D. Effects of the standardized Panax ginseng extract G115 on the D-glucose transport by Ehrlich ascites tumour cells. Phytother Res. 1993;7(2):200–202. [Google Scholar]
- 73.Voces J., Alvarez A.I., Vila L., Ferrando A., Cabral de Oliveira C., Prieto J.G. Effects of administration of the standardized Panax ginseng extract G115 on hepatic antioxidant function after exhaustive exercise. Comp Biochem Physiol Part C. 1999;123(2):175–184. doi: 10.1016/s0742-8413(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 74.Cabral de Oliveira A.C., Perez A.C., Merino G., Prieto J.G., Alvarez A.I. Protective effects of Panax ginseng on muscle injury and inflammation after eccentric exercise. Comp Biochem Physiol Part C. 2002;130(3):369–377. doi: 10.1016/s1532-0456(01)00262-9. [DOI] [PubMed] [Google Scholar]
- 75.de Oliveira A.C.C., Perez A.C., Prieto J.G., Duarte I.D.G., Alvarez A.I. Protection of Panax ginseng in injured muscles after eccentric exercise. J Ethnopharmacol. 2005;97(2):211–214. doi: 10.1016/j.jep.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 76.Voces J., Cabral de Oliveira A.C., Prieto J.G., Vila L., Perez A.C., Duarte I.D., Alvarez A.I. Ginseng administration protects skeletal muscle from oxidative stress induced by acute exercise in rats. Braz J Med Biol Res. 2004;37(12):1863–1871. doi: 10.1590/s0100-879x2004001200012. [DOI] [PubMed] [Google Scholar]
- 77.Dörling E., Kirchdorfer A.M., Rückert K.H. Do ginsenosides influence the performance? Notab Med. 1980;10(5):241–246. [Google Scholar]
- 78.Forgo I., Kirchdorfer A.M. On the question of influencing the performance of top sportsmen by means of biologically active substances. Aerztl Prax. 1981;33(44):1784–1786. [Google Scholar]
- 79.Mulz D., Scardigli G., Jans G., Degenring F.H. Long-term treatment of psychasthenia in the second half of life. Pharm Rundsch. 1990;12 86-86. [Google Scholar]
- 80.Rosenfeld M.S. Evaluation of the efficacy of a standardized ginseng extract in patients with psychophysical asthenia and neurological disorder. Sem Med. 1989;173(9):148–154. [Google Scholar]
- 81.Gianoli A.C., Riebenfeld D. A double-blind study to assess the tolerability and efficacy of the standardized ginseng extract G115 with special regard to its effect on the resistance of the organism to external influences. Cytobiol Rev. 1984;8(3):177–186. [Google Scholar]
- 82.Von Ardenne M., Klemm W. Measurements of the increase in the difference between the arterial and venous Hb-O2 saturation obtained with daily administration of 200 mg standardized ginseng extract G115 for four weeks. Long-term increase of the =2 transport into the organs and tissues of the organism through biologically active substances. Panminerva Med. 1987;29(2):143–150. [PubMed] [Google Scholar]
- 83.Forgo I. Effect of drugs on physical performance and hormone system of sportsmen. Munch Med Wschr. 1983;125(38):822–824. [PubMed] [Google Scholar]
- 84.Forgo I., Kayasseh L., Staub J.J. Effect of a standardized ginseng extract on general well-being, reaction capacity, pulmonary function and gonadal hormones. Med Welt. 1981;32(19):751–756. [PubMed] [Google Scholar]
- 85.Forgo I., Kirchdorfer A.M. The effect of different ginsenoside concentrations on physical work capacity. Notab Med. 1982;12(9):721–727. [Google Scholar]
- 86.Forgo I., Schimert G. The duration of the effect of the standardized ginseng extract G115 in healthy competitive athletes. Notab Med. 1985;15(9):636–640. [Google Scholar]
- 87.Van Schepdael P. The effects of ginseng G115 on physical performance of endurance athletes. Acta Ther. 1993;19(4):337–347. [Google Scholar]
- 88.Hosseini S.A., Ehsanpour A., Asgari M., Malihi R. Evaluation of standardized ginseng extract Panax (G115®) effect on fasting blood glucose levels, glycated hemoglobin and lipid profile in patients with diabetes type 2. Jundishapur J Chron Dis Care. 2013;2(3):26–32. [Google Scholar]
- 89.El-Farok D.H.M., El-Denshry E.S., Mahmoud M., Asaaf N., Emam M. Lipid lowering effect of ginseng and alpha-lipoic acid in hypercholesterolemic patients. Global J Pharmacol. 2013;7(3):298–306. [Google Scholar]
- 90.Reay J.L., Kennedy D.O., Scholey A.B. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol. 2005;19(4):357–365. doi: 10.1177/0269881105053286. [DOI] [PubMed] [Google Scholar]
- 91.Reay J.L., Kennedy D.O., Scholey A.B. The glycaemic effects of single doses of Panax ginseng in young healthy volunteers. Br J Nutr. 2006;96(4):639–642. [PubMed] [Google Scholar]
- 92.Reay J.L., Kennedy D.O., Scholey A.B. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained ‘mentally demanding’ tasks. J Psychopharmacol. 2006;20(6):771–781. doi: 10.1177/0269881106061516. [DOI] [PubMed] [Google Scholar]
- 93.Reay J.L., Scholey A.B., Milne A., Fenwick J., Kennedy D.O. Panax ginseng has no effect on indices of glucose regulation following acute or chronic ingestion in healthy volunteers. Br J Nutr. 2009;101:1673–1678. doi: 10.1017/S0007114508123418. [DOI] [PubMed] [Google Scholar]
- 94.Singh V.K., George C.X., Singh N., Agarwal S.S., Gupta B.M. Combined treatment of mice with Panax ginseng extract and interferon inducer. Planta Med. 1983;47(4):234–236. doi: 10.1055/s-2007-969995. [DOI] [PubMed] [Google Scholar]
- 95.Singh V.K., Agarwal S.S., Gupta B.M. Immunomodulatory activity of Panax ginseng extract. Planta Med. 1984;50(6):462–465. doi: 10.1055/s-2007-969773. [DOI] [PubMed] [Google Scholar]
- 96.Yamada H., Otsuka H., Kiyohara H. Fractionation and characterization of anticomplementary and mitogenic substances from Panax ginseng G115. Phytother Res. 1995;9(4):264–269. [Google Scholar]
- 97.Pannacci M., Lucini V., Colleoni F., Martucci C., Grosso S., Sacerdote P., Scaglione F. Panax ginseng CA Mayer G115 modulates pro-inflammatory cytokine production in mice throughout the increase of macrophage toll-like receptor 4 expression during physical stress. Brain Behav Immun. 2006;20(6):546–551. doi: 10.1016/j.bbi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 98.Scaglione F., Ferrara F., Dugnani S., Falchi M., Santoro G., Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng CA Meyer. Drugs Exp Clin Res. 1990;16(10):537–543. [PubMed] [Google Scholar]
- 99.Scaglione F., Cogo R., Cocuzza C., Arcidiacono M., Beretta A. Immunomodulatory effects of Panax ginseng CA Meyer (G115) on alveolar macrophages from patients suffering with chronic bronchitis. Int J Immunother. 1994;10(1):21–24. [Google Scholar]
- 100.Scaglione F., Cattaneo G., Alessandria M., Cogo R. Efficacy and safety of the standardized ginseng extract G115 for potentiating vaccination against common cold and/or influenza syndrome. Drugs Exp Clin Res. 1996;22(2):65–72. [PubMed] [Google Scholar]
- 101.Scaglione F., Weiser K., Alessandria M. Effects of the standardised ginseng extract G115 in patients with chronic bronchitis. Clin Drug Invest. 2001;21(1):41–45. [Google Scholar]
- 102.Engels H., Fahlman M.M., Wirth J.C. Effects of ginseng on secretory IgA, performance, and recovery from interval exercise. Med Sci Sports Exerc. 2003;35(4):690–696. doi: 10.1249/01.MSS.0000058363.23986.D2. [DOI] [PubMed] [Google Scholar]
- 103.Gross D., Krieger D., Efrat R., Dayan M. Ginseng extract G115® in the treatment of chronic respiratory diseases. Schweiz Z Ganzheitsmed. 1995;1:29–33. [Google Scholar]
- 104.Gross D., Shenkman Z., Bleiberg B., Dayan M., Gittelson M., Efrat R. Ginseng improves pulmonary functions and exercise capacity in patients with COPD. Monaldi Arch Chest Dis. 2002;57(5–6):242–246. [PubMed] [Google Scholar]
- 105.Xue C.C., Shergis J.L., Zhang A.L., Worsnop C., Fong H., Story D., Da Costa C., Thien F.C. Panax ginseng CA Meyer root extract for moderate COPD: study protocol for a randomised controlled trial. Trials. 2011;12(1):164. doi: 10.1186/1745-6215-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu L., Zhang A.L., Di Y.M., Shergis J.L., Chen Y., Guo X., Wen Z., Thien F., Worsnop C., Lin L. Panax ginseng therapy for chronic obstructive pulmonary disease: a clinical trial protocol and pilot study. Chin Med-UK. 2014;9(1):20. doi: 10.1186/1749-8546-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petkov V. Effects of ginseng on the brain biogenic monoamines and 3‘,5‘-AMP-system. Arznei-Forschung. 1978;28(3):388. [PubMed] [Google Scholar]
- 108.Petkov V.D., Mosharrof A.H. Age-and individual-related specificities in the effects of standardized ginseng extract on learning and memory (experiments on rats) Phytother Res. 1987;1(2):80–84. [Google Scholar]
- 109.Samira M.M., Attia M.A., Allam M., Elwan O. Effect of the standardized ginseng extract G115® on the metabolism and electrical activity of the rabbit‘s brain. J Int Med Res. 1985;13(6):342–348. doi: 10.1177/030006058501300608. [DOI] [PubMed] [Google Scholar]
- 110.Van Kampen J.M., Baranowski D.B., Shaw C.A., Kay D.G. Panax ginseng is neuroprotective in a novel progressive model of Parkinson's disease. Exp Gerontol. 2014;50:95–105. doi: 10.1016/j.exger.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 111.Sünram-Lea S.I., Birchall R.J., Wesnes K.A., Petrini O. The effect of acute administration of 400 mg of Panax ginseng on cognitive performance and mood in healthy young volunteers. Curr Top Nutraceut R. 2005;3(1):65–74. [Google Scholar]
- 112.Kennedy D.O., Haskell C.F., Wesnes K.A., Scholey A.B. Improved cognitive performance in human volunteers following administration of guarana (Paullinia cupana) extract: comparison and interaction with Panax ginseng. Pharmacol Biochem Behav. 2004;79(3):401–411. doi: 10.1016/j.pbb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 113.Kennedy D.O., Scholey A.B., Wesnes K.A. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physiol Behav. 2002;75:739–751. doi: 10.1016/s0031-9384(02)00665-0. [DOI] [PubMed] [Google Scholar]
- 114.Scholey A.B., Kennedy D.O. Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Hum Psychopharmacol. 2002;17(1):35–44. doi: 10.1002/hup.352. [DOI] [PubMed] [Google Scholar]
- 115.Kennedy D.O., Scholey A.B., Wesnes K.A. Dose dependent changes in cognitive performance and mood following acute administration of Ginseng to healthy young volunteers. Nutr Neurosci. 2001;4(4):295–310. doi: 10.1080/1028415x.2001.11747370. [DOI] [PubMed] [Google Scholar]
- 116.Reay J.L., Kennedy D.O., Scholey A.B. The behavioural and mood effects of Panax ginseng (G115): a 20 week chronic trial. Appetite. 2008;50(2):564. [Google Scholar]
- 117.Reay J.L., Scholey A.B., Kennedy D.O. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum Psychopharmacol. 2010;25(6):462–471. doi: 10.1002/hup.1138. [DOI] [PubMed] [Google Scholar]
- 118.Kennedy D.O., Scholey A.B., Drewery L., Marsh V.R., Moore B., Ashton H. Electroencephalography effects of single doses of Ginkgo biloba and Panax ginseng in healthy young volunteers. Pharmacol Biochem Behav. 2003;75(3):701–709. doi: 10.1016/s0091-3057(03)00120-5. [DOI] [PubMed] [Google Scholar]
- 119.D’Angelo L., Grimaldi R., Caravaggi M., Marcoli M., Perucca E., Lecchini S. A double-blind, placebo-controlled clinical study on the effect of a standardized Ginseng extract on psychomotor performance in healthy volunteers. J Ethnopharmacol. 1986;16(1):15–22. doi: 10.1016/0378-8741(86)90063-2. [DOI] [PubMed] [Google Scholar]
- 120.Quiroga H.A., Imbriano A.E. The effect of Panax ginseng extract on cerebrovascular deficits. Orientacion Med. 1979;28:86–87. [Google Scholar]
- 121.Quiroga H.A. A comparative double-blind study on the effect of Ginsana G115 and Hydergin on cerebrovascular deficits. Orientacion Med. 1982;31:201–202. [Google Scholar]
- 122.Cheng T.O. Ginseng and other herbal medicines that interact with warfarin. Int J Cardiol. 2005;104(2):227. doi: 10.1016/j.ijcard.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 123.Mancuso C., Santangelo R. Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food Chem Toxicol. 2017;107(Pt A):362–372. doi: 10.1016/j.fct.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]