Abstract
Human induced pluripotent stem cells (hiPSCs) are invaluable resources for producing high-quality differentiated cells in unlimited quantities for both basic research and clinical use. They are particularly useful for studying human disease mechanisms in vitro by making it possible to circumvent the ethical issues of human embryonic stem cell research. However, significant limitations exist when using conventional flat culturing methods especially concerning cell expansion, differentiation efficiency, stability maintenance and multicellular 3D structure establishment, differentiation prediction. Embryoid bodies (EBs), the multicellular aggregates spontaneously generated from iPSCs in the suspension system, might help to address these issues. Due to the unique microenvironment and cell communication in EB structure that a 2D culture system cannot achieve, EBs have been widely applied in hiPSC-derived differentiation and show significant advantages especially in scaling up culturing, differentiation efficiency enhancement, ex vivo simulation, and organoid establishment. EBs can potentially also be used in early prediction of iPSC differentiation capability. To improve the stability and feasibility of EB-mediated differentiation and generate high quality EBs, critical factors including iPSC pluripotency maintenance, generation of uniform morphology using micro-pattern 3D culture systems, proper cellular density inoculation, and EB size control are discussed on the basis of both published data and our own laboratory experiences. Collectively, the production of a large quantity of homogeneous EBs with high quality is important for the stability and feasibility of many PSCs related studies.
Keywords: Induced pluripotent stem cells, Suspension culture, Embryoid body, Early prediction, Committed differentiation, Heterogeneity, Three-dimensional culture, Scaling-up, Quality control
Core tip:Embryoid body (EB) mediated induced pluripotent stem cell (iPSC) differentiation shows great advantages in culture scale-up, differentiation efficiency improvement, ex vivo simulation and organoid establishment. To improve the stability and feasibility of high quality EB generation, factors including iPSC pluripotency maintenance, generation of uniform morphology using micro-pattern 3D culture systems, proper cellular density inoculation and EB size control need to be considered.
INTRODUCTION
The emergence of human induced pluripotent stem cells (hiPSCs) has markedlypromoted the development of regenerative medicine. These cells are reprogrammed from differentiated human somatic cells by gene integration or non-integration methods and possess the properties ofself-proliferation and committed differentiation[1-4]. More importantly, compared to human embryonic stem cells (hESCs), the use of hiPSCs successfully avoids major immunoreactive and ethical issues[5]. As a result, hiPSCs have quickly become a critical resource for biomedical research and are expected to be used in clinical cellular transplantation, disease model establishment, and drug screening. Conventional methods, however, are usually established in flat culture systems, which impose significant limitations on cell expansion, differentiation efficiency, and multicellular 3D structure establishment. Embryoid bodies (EBs), which are cultured in a suspension system, might help to address these issues. Generally, EB is a multicellular aggregate spontaneously formed by pluripotent stem cells under suspension culture conditions, which has three germ layer structures and partially recapitulates the early embryonic development[6]. Such a multicellular 3D structure improves cell-cell contacts and intercellular communication and also enhances substance exchange[7]. Although the differentiation from iPSC to target cells is a relatively complex, time consuming, and unstable process[8], EBs have been widely used in iPSC differentiation and organoid construction because of their irreplaceable structural and functional advantages[9,10]. It has been demonstrated that a standardized EB formation procedure contributes to their high quality and improves differentiation[11,12]. Therefore, the key factors need to be carefully considered when EB-mediated differentiation is selected[9,13].
In order to understand the critical events of EB-mediated differentiation, explore better methods and solve the aforementioned problems, we recapitulated the current applications and advantages of using EBs in iPSC differentiation. Combining our own and previously published data related to EB formation and differentiation, we conducted a comparative and predictive analysis and aimed to provide a reference to create a more stable and practical way of high-quality EB generation.
APPLICATION AND ADVANTAGES OF EB USE IN IPSC DIFFERENTIATION
Scale-up of culture systems and differentiation efficiency
Clinical transplantation requires large quantities of functional target cells and most of the existing strategies are difficult to implement at a large scale or have a low differentiation efficiency, therefore posing barriers to further research. Compared to flat culture systems, EB-derived differentiation culture is kept in a relatively fixed position, which offers this method an obvious advantage in quantity and differentiation efficiency[14-16]. A variety of cell lineages have been generated from hEBs such as brain, cornea, heart, liver, and blood (Table 1). In our study, we used a suspension EB-based system to generate iPSC-derived melanocytes and achieved a significantly higher differentiation efficiency compared to that in flat culture systems and these induced melanocytes showed long-term in vivo functionality after transplantation[17]. In short, differentiation from EB to specific cell lineages is an efficient method that is likely to yield large populations of functional cells.
Table 1.
Updated summary of the formation of human embryoid bodies
| Target cells or organoids | Culture vessels | Cell separation (cell suspension) | Culture period (d) | Media and supplements for EB formation | Ref. |
| Human brain organoids | Micropillar array plates | Accutase (single-) | 11 | KSR, Y27632, NEAA, GlutaMAX, bFGF | Zhu et al[65], 2017 |
| Midbrain-like organoids | Low adhesion 96-well V-bottom multi-well plates | NA (single-) | 4 | mTeSR1, Y27632, Matrigel | Jo et al[21], 2016 |
| Corneal epithelial cells | Static suspension culture | NA (non-single-) | 14 | E6 medium, Y27632, IWP-2, bFGF | Martínez García de la Torreet al[66], 2017 |
| Osteoblast-like cells | Collagenase type IV, trypsin–EDTA (non-single-) | 6 | Human ES medium, thiazovivin | Ochiai-Shinoet al[67], 2014 | |
| Cardiac cells | NA (non-single-) | 6 | DMEM/F-12, NEAA, GlutaMAX, FBS, DMSO | Hoque et al[68], 2018 | |
| Neurospheres | Accutase (single-) | 5 | KSR, Y27632, FGF-2, NEAA, L-Glutamine | Pauly et al[69], 2018; | |
| Cardiomyocytes | Type IV collagenase (single-) | 10 | DMEM, FBS, NEAA, GlutaMAX | Chauveau et al[70], 2017; | |
| Osteoprogenitors | Dispase (non-single-) | 7 | DMEM, FBS, Glutamax, ABAM | Roberts et al[71], 2017 | |
| Chondrocytes | NA (single-) | 6 | Aggrewell medium, TeSR-E8 medium | Nam et al[72], 2017 | |
| Hepatocytes | Trypsin and collagenase IV (non-single-) | 5 | Knockout DMEM, NEAA, L-glutamine, FBS | Tomotsune et al[17], 2016 | |
| Oligodendrocytes | Trypsin-EDTA (non-single-) | 2 | KnockOut-DMEM, KSR, NEAA, SB431542, glutamine, ITS, Dorsomorphin | Espinosa-Jeffreyet al[73], 2016 | |
| Hematopoietic stem cells | Dispase (non-single-) | 7 | KnockOut-DMEM, KSR, Y27632, GlutaMAX, NEAA | Phondeechareon et al[74], 2016 | |
| Neural precursor cells | EDTA (non-single-) | 5 | DMEM/F12, KSR, NEAA, SB431542, GlutaMAX, Recombinant Noggin | Plaisted et al[75], 2016 | |
| Mesenchymal stem cells (MSCs) | Collagenase type IV (non-single-) | 10 | DMEM/F12, KSR, MEF conditioned medium, NEAA, bFGF, glutamine | Tang et al[76], 2014 | |
| Chondrocyte-like cells | Non-adherent 96-well plate | Collagenase IV (non-single-) | 7 | DMEM F12, L-glutamine, FBS, KSR, NEAA | Suchorska et al[77], 2017 |
| Chondrocyte-like cells | Low adhesion 96-well C-bottom multi-well plate | Trypsin-EDTA (single-) | 1 | DMEM/F12, Y27632, KSR, NEAA | Lach et al[78], 2018 |
| Retinal tissue | 96-well non-adherent U-bottom plate | Accutase (single-) | 12 | mTeSR1, Y27632 | Hunt et al[79], 2017 |
| Insulin-secreting cells | Agarose round-bottom microwell plate | Accutase (single-) | 10 | IMDM/F-12, FBS, insulin transferring selenium-A, monothioglycerol | Pettinato et al[52], 2014 |
| Neural precursors | Static or dynamic suspension culture method (orbital shaker) | EDTA (non-single-) | 6-9 | mTeSR1, Y27632; special equipment: orbital shaker | Miranda et al[80], 2016 |
| Macrophages | AggreWellTM, 96-well round-bottomed multi-well plate | Collagenase-dispase mixture (single-) | 4 | DMEM/F12, Y27632, KSR, L-glutamine | Mukherjee et al[81], 2018 |
| Primordial germ cell-likecells | AggreWell microwells | Accutase (single-) | 1 | KnockOut DMEM, Y27632, KSR, NEAA, bovine pancreas insulin, LIF, TGF-β, CHIR99021, PD0325901, BIRB796, SP600125 | Mitsunaga et al[41], 2017 |
| Forebrain cortical cells | Collagenase (non-single-) | 4 | DMEM/F12, KSR, glutamine, NEAA | Muratore et al[49], 2014 | |
| Melanocytes | 24-well ElplasiaTMmicrowell plates (round bottom) | Accutase (single-) | 7 | mTeSR1, Y27632 | Liu et al[82], 2019 |
“NA” means enzyme deficiency or mechanical dissection of the iPSC clones. E6 medium: Essential 6 medium; IMDM/F-12: Iscove's Modified Dulbecco's Medium/Nutrient Mixture F-12; DMEM/F-12: Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12; mTeSR1: Standardized medium for the feeder-independent maintenance of hESCs and hiPSCs; TeSR-E8 medium: A feeder-free, animal component-free culture medium for hESCs and hiPSCs; MEF: Mouse embryonic fibroblast; FBS: Fetal bovine serum; LIF: Leukemia inhibitory factor; TGF-β: Transforming growth factor-beta; bFGF: Basic fibroblast growth factor; Y27632: Rho-associated kinas inhibitor; KSR: Knockout serum replacement; NEAA: Non-essential amino acids; DMSO: Dimethyl sulphoxide; IWP-2: Inhibitor of the WNT pathway; ITS: Insulin-Transferrin-Selenium; SB431542: Inhibitor of the activin type I receptor ALK4 and the nodal type I receptor ALK7; CHIR99021: Inhibitor of glycogen synthase kinase 3β (GSK3β); PD0325901: Inhibitor of the mitogen-activated protein kinase (MEK) pathway; BIRB796: Inhibitor of the mitogen-activated protein kinases (MAPK); SP600125: Inhibitor of c-Jun N-terminal kinase (JNK).
Ex vivo simulation and organoids establishment
Recently, due to the features of their three germ layer structure[18], hEBs have become useful models for developing human ESC- and iPSC-derived organoids by stimulation with a cocktail of biological agents (Table 1). These EB-derived organoids not only contain some cell subtypes but also show distinct stratification which is similar to the in vivo structure of the tissues or organs developing[19,20]. For example, Jo et al[21] observed an identical organization structure in 3D cultured human midbrain-like organoids (hMLOs) compared with human postmortem midbrain tissue under the electron microscope. Furthermore, these EB-derived organoids are functional. Qian et al[22]found that EB-derived midbrain organoids not only expressed a wider range of characteristic markers common to normal midbrain tissue compared with direct differentiation from iPSCs, but also demonstrated firing action potentials in response to current injection which can be used to establish a disease model of microcephaly[22]. These EB-derived organoids could be used to understand unique features of specific human organs and to gain insights into different disorders.
Early prediction of differentiation potential
There is a remarkable difference in differentiation capability in distinct iPSC lineages, and it is urgently necessary to predict the differentiation potential in an early stage of the long differentiation period to avoid unnecessary resource losses. Because EBs can potentially develop into three germ layer tissues for multi-directional differentiation[23,24], it can be hypothesized that they may be suitable candidates for predicting iPSC differentiation potential. Kim et al[25]classified hESCs-derived EBs into several types including cystic, bright cavity and dark cavity according to their morphology. By detecting the expression of specific markers, they showed that most of the cells in cystic EBs are endoderm-lineage populations and both bright and dark cavity EBs own cells from all of the three germ layers. Besides the morphology, EB size can also be used determine the differentiation direction of iPSC. For example, the larger EBs (450 μm diameter) often contained cells of the cardiovascular system, while endothelial cells were usually generated in smaller EBs (150 μm diameter) and such differentiation potentials are mainly caused by the noncanonical WNT pathway[26]. Moreover, gene expression profiles of hPSCs and EBs recovered by high-throughput RNA sequencing can also be used for differentiation prediction, and the results is parallel to the germ layer development in vivo[27].
The novel methods described above have some limitations. Simple morphology or size-dependent prediction system is somewhat subjective, and it is also closely related to the viability of the cells inside EB[28,29], whereas high-throughput RNA sequencing is expensive and complicated. In our study, we developed a simple and practical system and identified a set of parameters combining EB formation, maintenance, and germ layer-specific gene expression in EB stage to predict the differentiation tendency of the various iPSC lines. The validity of this evaluation system was finally confirmed by differentiating these iPSC lines into melanocytes[24]. Recently, we summarized the current methods related to iPSC differentiation prediction and we also postulated that the use of EBs could be more efficient when combined with other modified detection methods such molecular probes[34].Thus, due to their special morphology and functionality, EBs have played an important role in the differentiation prediction related studies.
Multiple-cell environment and cell communication
The remarkable functionality and application prospects of EBs are a result of their unique 3D microstructure and cell communication. Frequent passage in the adherent culture system limits the cell–cell communication in both cell number and time. The suspension culture system for EBs provides extensive material exchange and increases both cell–cell interactions and cell–matrix communication in the aggregate, which is conducive to the transmission and action of cell signals[35,36]. In general, intercellular communication is increased both in space and time at the 3D level. Additionally, differentiation of EBs is an asynchronous process and 3D culture produces distinct layers of spheroids (EBs) as a result of concentration gradients in the culture medium[37]. The differentiation of the outer endoderm of EB caused by growth factors in the medium will further induce endoderm differentiation by producing signals[38]. This unbalanced phenomenon also explains the heterogeneity throughout the differentiation process in EBs. Moreover, more intricate gene profiles could be expressed in a 3D culture system, reflecting original expression in vivo more accurately[16]. Jo et al[21] found that gene profiles detected by RNA sequencing analysis are analogously expressed between hMLOs and the human prenatal midbrain. However, there is a significant difference between the 2D dopamine neurons and normal tissue. The integrity of gene expression is a considerable advantage of the 3D culture system, providing a better foundation for future structural and functional studies. Meanwhile, for the hiPSC-based differentiation, the cadherin-β-catenin-Wnt pathway was found to be involved in the development of EBs by intercellular adhesion[39]. In addition, the activation or inhibition of relevant signaling pathways can yield a definitive lineage differentiation[22,40,41]. Therefore, controlling the dose and duration of cytokines regulates the differentiation of iPSCs by activating various pathways.
Compared with a flat culture, microgravity has been found to be a subsidiary factor that can regulate cell proliferation and survival in a 3D culture system[29-32]. Therefore, bioreactors and random positioning machines have been used to simulate states of microgravity and weightless condition for EB-derived differentiation[42,43].
In conclusion, EBs have shown great application potential in the generation of various functional cells and organoids at a large scale and also in the prediction of iPSC differentiation capability. These EB-derived cells and organoids highly resemble the in vivo status in both gene expression and function display as a result of the structure and microenvironment in the EB.
QUALITY CONTROL OF EB
Considering the wide range of applications of EBs in iPSC differentiation and their potential advantages, a comprehensive quality control of the EB formation process would help to improve the stability and feasibility of their application.
Pluripotency maintenancein iPSCs
To generate high quality EBs, the initial step is the pluripotency maintenance in iPSCs where the addition of various cytokines and inhibitors is essential. Two studies[15,44]demonstrated that basic fibroblast growth factor (bFGF) induced hES cell derived fibroblast-like cells produce insulin-like growth factor II and transforming growth factor β family of factors and indirectly establish a balanced stem cell niche of hESCs, contributing to maintain the undifferentiated state of hESCs. In addition, Y-27632 was confirmed to benefit the expression of POU class 5 homeobox 1 (OCT4) in EB formation[45-48]. Compared with the conventional methods, single cell culture system can reduce the complexity caused by feeder cells. However, it is more difficult to maintain undifferentiated status of iPSC when single-cell passage is used. Tsutsui et al[45]developed a unique combination of inhibition of Rho-associated kinase, glycogen synthase kinase and mitogen-activated protein kinase with bFGF for hES cells maintenance and they found these cells to present good pluripotency and genetic integrity in long-term maintenance (> 20 passages).
Formation and uniform morphology
In general, EBs can be spontaneously generated with hiPSC clusters[8,46] or single cells[41,49] in 3D culture systems using EB-specific medium. When conventional methods such as static suspension culture and the hanging drop method[50,51]are chosen, EB-derived differentiation seems unstable, which is in part associated with the heterogeneity of EBs[52,53]. Therefore, it is necessary to control the generation of uniform EBs for stable and reproducible differentiation. Recently, different types of micro-pattern plates have been developed and widely applied for the generation of EBs with uniform size and morphology using single hiPSCs (Table 1) and on this basis, a variety of culture systems such as the micro-space system[50,51,54], spinner flasks[22], and the National Aeronautics and Space Administration developed rotary cell culture system (NASA rotary system)[55] have also been designed for scale-up.
Cellular density and EB size
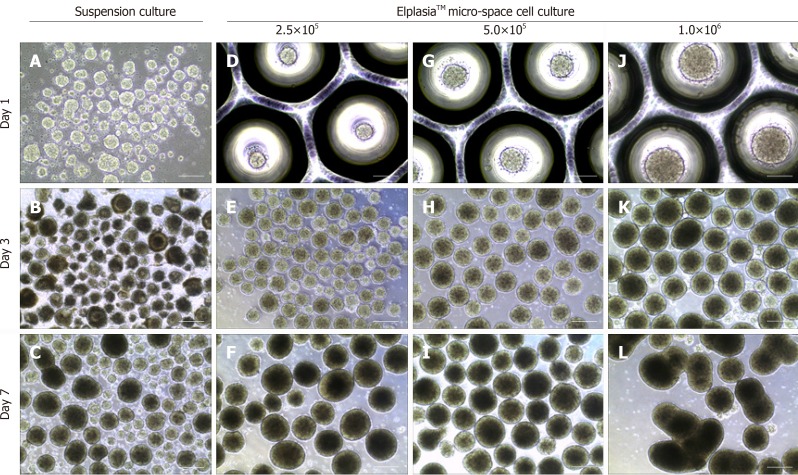
Besides morphological uniformity, the efficiency and direction of differentiation are also affected by EB size[56-58] and which size is more appropriate depends on the specific cell lineages. It has been demonstrated that a larger amount of cardiomyocytes was found in hESC-derived differentiation using EBs with100 μm diameter when compared to larger ones (300 μm)[59]. However, we found that 300-400 μm might be a more suitable diameter for melanocytes differentiation compared to those bigger or smaller. Since the size and quality of EB during culture maintenance are affected by the number of inoculated cells, micro-pattern plates can be used to control EB size by adjusting cellular density. However, inoculated cell density also depends on the EB culture plate type and culture duration before differentiation. In a study of iPSC differentiation into cardiomyocytes, input of 1.5 × 104 to 4.0 × 104 cells per EB was determined to be the proper density to form homogenous and synchronized EBs. By contrast, we found that 5.0 × 102 to 1.0 × 103 cells were enough for each EB generation using an ElplasiaTM micro-well plate and this density tended to yield stable and intact EBs (Figure 1) which could be maintained over a relatively long term for differentiation. Overly high cell density (2.0 × 103 cells/EB) will lead to breakage or fusion in a short time (Figure 1). In short, EBs within a certain size range are more conducive to differentiation which can be modified by cell density adjustment. However, size usually varies across different differentiation assays and needs to be optimized in accordance to the specific conditions.
Figure 1.
Static suspension culture and 24-well ElplasiaTM micro-well culture with human induced pluripotent stem cells. A-C:Induced pluripotent stem cell (iPSC)-derived embryoid bodies (EBs) formed under a suspension system and cultured with mTeSR™, respectively, on day 1 (A), day 3 (B), and day 7 (C); D-L: iPSC-derived EBs formed with single hiPSCs in micro-space cell culture plates at different cell densities: D-F: 2.5 × 105 cells/mL; G–I: 5.0 × 105 cells/mL; and J–L: 1.0 × 106 cells/mL, respectively, on day 1 (D, G, and J), day 3 (E, H, and K), and day 7 (F, I, and L). Scale bars: A, D, G, and J 100 μm; resident graphs, 250 μm.
FUTURE PERSPECTIVES AND CONCLUSION
EBs can effectively mimic the in vivo differentiation process and have shown excellent advantages in the field of iPSC-derived differentiation, especially in organoid establishment. However, a gap between these products and normal organs is still inevitable because the inclusion of a cocktail of factors and additives only provides a somewhat analogous survival environment rather than completely simulating the in vivo conditions and this difference generally leads to a temporal and spatial distinction[60,61]. To reduce these differences as much as possible, many studies have been carried out with the aim to optimize the culture system, including culture duration, plate type, dose of growth factors and small molecules, matrix proteins and even the order of the additives[35,36]based on research aims and differentiated cell types. In terms of iPSC-derived EB formation, the initial inoculation density is more critical than additives and centrifugation (forcing cell aggregation), and more of such insights can likely be obtained from tumor sphere related studies[62]. For example, magnetic levitation, a new method for spheroid formation, has been used to establish a levitation profile for distinct tumor cells[63]. Whether this novel technology can also be adopted for EB related differentiation and cell purification remains to be explored.
Overall, an EB mediated culture system is more amenable to improvement in differentiation efficiency and simulation of the human microenvironment compared to 2D differentiation. Cell proliferation, cell–cell interaction, and material metabolism partially undergo the influence of microgravity, achieving more intricate gene expression and epigenetic profiles. More importantly, most of the iPSC related studies require a relatively long observation time which inevitably leads to instability. EBs with good quality control could provide a stable and feasible system to better present the functional integrity of tissues and organs[64], underlining their potential in the exploration of promising drugs and precision medicine where classical 2D cell assays might fail.
Significantly, EBs are also expected to reveal the development of early human embryos, avoiding ethical issues. EB formation and lineage commitment have become the focus of research in terms of the structure and composition of differentiated cells. RNA sequencing of single EB cells will help to clarify the evolution or/and unknown event of early embryos and the fate development of individual cells. In conclusion, the production of a large quantity of homogeneous EBs with high quality is important for many PSCs related studies, including scaling up culturing, organoid formation, and differentiation potential prediction.
Footnotes
Conflict-of-interest statement: No potential conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: April 3, 2019
First decision: August 23,2019
Article in press:December 13, 2019
Specialty type: Cell and tissue engineering
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Stocco G S-Editor:Dou Y L-Editor:A E-Editor:Xing YX
Contributor Information
Ning-Ning Guo, Institute of Regenerative Medicine, Affiliated Hospital of Jiangsu University, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
Li-Ping Liu, Institute of Regenerative Medicine, Affiliated Hospital of Jiangsu University, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
Yun-Wen Zheng, Institute of Regenerative Medicine, Affiliated Hospital of Jiangsu University, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China; Department of Gastrointestinal and Hepato-Biliary-Pancreatic Surgery, University of Tsukuba Faculty of Medicine, Tsukuba, Ibaraki 305-8575, Japan; Yokohama City University School of Medicine, Yokohama, Kanagawa 234-0006, Japan; Division of Regenerative Medicine, Center for Stem Cell Biology and Regenerative Medicine, The Institute of Medical Science, the University of Tokyo, Tokyo 108-8639, Japan. ywzheng@md.tsukuba.ac.jp.
Yu-Mei Li, Institute of Regenerative Medicine, Affiliated Hospital of Jiangsu University, Jiangsu University, Zhenjiang 212001, Jiangsu Province, China.
References
- 1.Zhao Y, Zhao T, Guan J, Zhang X, Fu Y, Ye J, Zhu J, Meng G, Ge J, Yang S, Cheng L, Du Y, Zhao C, Wang T, Su L, Yang W, Deng H. A XEN-like State Bridges Somatic Cells to Pluripotency during Chemical Reprogramming. Cell. 2015;163:1678–1691. doi: 10.1016/j.cell.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 4.Miyoshi N, Ishii H, Nagano H, Haraguchi N, Dewi DL, Kano Y, Nishikawa S, Tanemura M, Mimori K, Tanaka F, Saito T, Nishimura J, Takemasa I, Mizushima T, Ikeda M, Yamamoto H, Sekimoto M, Doki Y, Mori M. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8:633–638. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Menon S, Shailendra S, Renda A, Longaker M, Quarto N. An Overview of Direct Somatic Reprogramming: The Ins and Outs of iPSCs. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 7.Saltzman WM, Kyriakides TR. Chapter 20 - Cell Interactions with Polymers. In: Principles of Tissue Engineering (Fourth Edition). Lanza R, Langer R, Vacanti J, Editors. 2014, Academic Press: Boston, 385-406 [Google Scholar]
- 8.Chen KG, Mallon BS, Johnson KR, Hamilton RS, McKay RD, Robey PG. Developmental insights from early mammalian embryos and core signaling pathways that influence human pluripotent cell growth and differentiation. Stem Cell Res. 2014;12:610–621. doi: 10.1016/j.scr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J. 2008;3:1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 10.Breslin S, O'Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today. 2013;18:240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Froehlich K, Haeger JD, Heger J, Pastuschek J, Photini SM, Yan Y, Lupp A, Pfarrer C, Mrowka R, Schleußner E, Markert UR, Schmidt A. Generation of Multicellular Breast Cancer Tumor Spheroids: Comparison of Different Protocols. J Mammary Gland Biol Neoplasia. 2016;21:89–98. doi: 10.1007/s10911-016-9359-2. [DOI] [PubMed] [Google Scholar]
- 12.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24:344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 13.Burridge PW, Metzler SA, Nakayama KH, Abilez OJ, Simmons CS, Bruce MA, Matsuura Y, Kim P, Wu JC, Butte M, Huang NF, Yang PC. Multi-cellular interactions sustain long-term contractility of human pluripotent stem cell-derived cardiomyocytes. Am J Transl Res. 2014;6:724–735. [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Pérez J, Ballesteros P, Cerdán S. Microscopic images of intraspheroidal pH by 1H magnetic resonance chemical shift imaging of pH sensitive indicators. MAGMA. 2005;18:293–301. doi: 10.1007/s10334-005-0013-z. [DOI] [PubMed] [Google Scholar]
- 15.Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One. 2008;3:e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Tomotsune D, Hirashima K, Fujii M, Yue F, Matsumoto K, Takizawa-Shirasawa S, Yokoyama T, Sasaki K. Enrichment of Pluripotent Stem Cell-Derived Hepatocyte-Like Cells by Ammonia Treatment. PLoS One. 2016;11:e0162693. doi: 10.1371/journal.pone.0162693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brickman JM, Serup P. Properties of embryoid bodies. Wiley Interdiscip Rev Dev Biol. 2017:6. doi: 10.1002/wdev.259. [DOI] [PubMed] [Google Scholar]
- 19.Pavesi A, Adriani G, Rasponi M, Zervantonakis IK, Fiore GB, Kamm RD. Controlled electromechanical cell stimulation on-a-chip. Sci Rep. 2015;5:11800. doi: 10.1038/srep11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simmons CS, Petzold BC, Pruitt BL. Microsystems for biomimetic stimulation of cardiac cells. Lab Chip. 2012;12:3235–3248. doi: 10.1039/c2lc40308k. [DOI] [PubMed] [Google Scholar]
- 21.Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Göke J, Tan ZY, Saw TY, Tan CP, Lokman H, Lee Y, Kim D, Ko HS, Kim SO, Park JH, Cho NJ, Hyde TM, Kleinman JE, Shin JH, Weinberger DR, Tan EK, Je HS, Ng HH. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell. 2016;19:248–257. doi: 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 24.Guo NN, Liu LP, Zhang YX, Cai YT, Guo Y, Zheng YW, Li YM. Early prediction of the differentiation potential during the formation of human iPSC-derived embryoid bodies. Biochem Biophys Res Commun. 2019;516:673–679. doi: 10.1016/j.bbrc.2019.06.081. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, Moon SH, Lee SG, Cho YJ, Hong KS, Lee JH, Lee HJ, Chung HM. Assessment of differentiation aspects by the morphological classification of embryoid bodies derived from human embryonic stem cells. Stem Cells Dev. 2011;20:1925–1935. doi: 10.1089/scd.2010.0476. [DOI] [PubMed] [Google Scholar]
- 26.Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 2009;106:16978–16983. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han X, Chen H, Huang D, Chen H, Fei L, Cheng C, Huang H, Yuan GC, Guo G. Mapping human pluripotent stem cell differentiation pathways using high throughput single-cell RNA-sequencing. Genome Biol. 2018;19:47. doi: 10.1186/s13059-018-1426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. J Clin Invest. 2010;120:995–1003. doi: 10.1172/JCI41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller-Klieser W. Method for the determination of oxygen consumption rates and diffusion coefficients in multicellular spheroids. Biophys J. 1984;46:343–348. doi: 10.1016/S0006-3495(84)84030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jha R, Wu Q, Singh M, Preininger MK, Han P, Ding G, Cho HC, Jo H, Maher KO, Wagner MB, Xu C. Simulated Microgravity and 3D Culture Enhance Induction, Viability, Proliferation and Differentiation of Cardiac Progenitors from Human Pluripotent Stem Cells. Sci Rep. 2016;6:30956. doi: 10.1038/srep30956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unsworth BR, Lelkes PI. Growing tissues in microgravity. Nat Med. 1998;4:901–907. doi: 10.1038/nm0898-901. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Liu P, Chen L, Wang Y, Wang Z, Zhang B. The effects of spheroid formation of adipose-derived stem cells in a microgravity bioreactor on stemness properties and therapeutic potential. Biomaterials. 2015;41:15–25. doi: 10.1016/j.biomaterials.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Pardo SJ, Patel MJ, Sykes MC, Platt MO, Boyd NL, Sorescu GP, Xu M, van Loon JJ, Wang MD, Jo H. Simulated microgravity using the Random Positioning Machine inhibits differentiation and alters gene expression profiles of 2T3 preosteoblasts. Am J Physiol Cell Physiol. 2005;288:C1211–C1221. doi: 10.1152/ajpcell.00222.2004. [DOI] [PubMed] [Google Scholar]
- 34.Liu LP, Zheng YW. Predicting differentiation potential of human pluripotent stem cells: Possibilities and challenges. World J Stem Cells. 2019;11:375–382. doi: 10.4252/wjsc.v11.i7.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Liu M, Yang ST. Dendritic cells derived from pluripotent stem cells: Potential of large scale production. World J Stem Cells. 2014;6:1–10. doi: 10.4252/wjsc.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodge AJ, Zhong J, Lipke EA. Enhanced stem cell-derived cardiomyocyte differentiation in suspension culture by delivery of nitric oxide using S-nitrosocysteine. Biotechnol Bioeng. 2016;113:882–894. doi: 10.1002/bit.25849. [DOI] [PubMed] [Google Scholar]
- 37.Bratt-Leal AM, Carpenedo RL, McDevitt TC. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25:43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burdsal CA, Flannery ML, Pedersen RA. FGF-2 alters the fate of mouse epiblast from ectoderm to mesoderm in vitro. Dev Biol. 1998;198:231–244. [PubMed] [Google Scholar]
- 39.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 40.Mori S, Sakakura E, Tsunekawa Y, Hagiwara M, Suzuki T, Eiraku M. Self-organized formation of developing appendages from murine pluripotent stem cells. Nat Commun. 2019;10:3802. doi: 10.1038/s41467-019-11702-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitsunaga S, Odajima J, Yawata S, Shioda K, Owa C, Isselbacher KJ, Hanna JH, Shioda T. Relevance of iPSC-derived human PGC-like cells at the surface of embryoid bodies to prechemotaxis migrating PGCs. Proc Natl Acad Sci U S A. 2017;114:E9913–E9922. doi: 10.1073/pnas.1707779114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.England LS, Gorzelak M, Trevors JT. Growth and membrane polarization in Pseudomonas aeruginosa UG2 grown in randomized microgravity in a high aspect ratio vessel. BBA - General Subjects. 2003;1624:76–80. doi: 10.1016/j.bbagen.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 43.van Loon J. Some history and use of the random positioning machine, RPM, in gravity related research. Advances in Space Research. 2007;39:1161–1165. [Google Scholar]
- 44.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 45.Tsutsui H, Valamehr B, Hindoyan A, Qiao R, Ding X, Guo S, Witte ON, Liu X, Ho CM, Wu H. An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat Commun. 2011;2:167. doi: 10.1038/ncomms1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 47.Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khoo ML, McQuade LR, Smith MS, Lees JG, Sidhu KS, Tuch BE. Growth and differentiation of embryoid bodies derived from human embryonic stem cells: effect of glucose and basic fibroblast growth factor. Biol Reprod. 2005;73:1147–1156. doi: 10.1095/biolreprod.104.036673. [DOI] [PubMed] [Google Scholar]
- 49.Muratore CR, Srikanth P, Callahan DG, Young-Pearse TL. Comparison and optimization of hiPSC forebrain cortical differentiation protocols. PLoS One. 2014;9:e105807. doi: 10.1371/journal.pone.0105807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandrasekaran A, Avci HX, Ochalek A, Rösingh LN, Molnár K, László L, Bellák T, Téglási A, Pesti K, Mike A, Phanthong P, Bíró O, Hall V, Kitiyanant N, Krause KH, Kobolák J, Dinnyés A. Comparison of 2D and 3D neural induction methods for the generation of neural progenitor cells from human induced pluripotent stem cells. Stem Cell Res. 2017;25:139–151. doi: 10.1016/j.scr.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Hemmi N, Tohyama S, Nakajima K, Kanazawa H, Suzuki T, Hattori F, Seki T, Kishino Y, Hirano A, Okada M, Tabei R, Ohno R, Fujita C, Haruna T, Yuasa S, Sano M, Fujita J, Fukuda K. A massive suspension culture system with metabolic purification for human pluripotent stem cell-derived cardiomyocytes. Stem Cells Transl Med. 2014;3:1473–1483. doi: 10.5966/sctm.2014-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettinato G, Wen X, Zhang N. Formation of well-defined embryoid bodies from dissociated human induced pluripotent stem cells using microfabricated cell-repellent microwell arrays. Sci Rep. 2014;4:7402. doi: 10.1038/srep07402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messana JM, Hwang NS, Coburn J, Elisseeff JH, Zhang Z. Size of the embryoid body influences chondrogenesis of mouse embryonic stem cells. J Tissue Eng Regen Med. 2008;2:499–506. doi: 10.1002/term.125. [DOI] [PubMed] [Google Scholar]
- 54.Draper JS, Moore HD, Ruban LN, Gokhale PJ, Andrews PW. Culture and characterization of human embryonic stem cells. Stem Cells Dev. 2004;13:325–336. doi: 10.1089/scd.2004.13.325. [DOI] [PubMed] [Google Scholar]
- 55.Lei XH, Ning LN, Cao YJ, Liu S, Zhang SB, Qiu ZF, Hu HM, Zhang HS, Liu S, Duan EK. NASA-approved rotary bioreactor enhances proliferation of human epidermal stem cells and supports formation of 3D epidermis-like structure. PLoS One. 2011;6:e26603. doi: 10.1371/journal.pone.0026603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moon SH, Ju J, Park SJ, Bae D, Chung HM, Lee SH. Optimizing human embryonic stem cells differentiation efficiency by screening size-tunable homogenous embryoid bodies. Biomaterials. 2014;35:5987–5997. doi: 10.1016/j.biomaterials.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Koay EJ, Hoben GM, Athanasiou KA. Tissue engineering with chondrogenically differentiated human embryonic stem cells. Stem Cells. 2007;25:2183–2190. doi: 10.1634/stemcells.2007-0105. [DOI] [PubMed] [Google Scholar]
- 58.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, Thomson JA, Lyons GE, Palecek SP, Kamp TJ. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31:1885–1893. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kempf H, Olmer R, Kropp C, Rückert M, Jara-Avaca M, Robles-Diaz D, Franke A, Elliott DA, Wojciechowski D, Fischer M, Roa Lara A, Kensah G, Gruh I, Haverich A, Martin U, Zweigerdt R. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Reports. 2014;3:1132–1146. doi: 10.1016/j.stemcr.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otsuji TG, Bin J, Yoshimura A, Tomura M, Tateyama D, Minami I, Yoshikawa Y, Aiba K, Heuser JE, Nishino T, Hasegawa K, Nakatsuji N. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Reports. 2014;2:734–745. doi: 10.1016/j.stemcr.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen YC, Zhang Z, Fouladdel S, Deol Y, Ingram PN, McDermott SP, Azizi E, Wicha MS, Yoon E. Single cell dual adherent-suspension co-culture micro-environment for studying tumor-stromal interactions with functionally selected cancer stem-like cells. Lab Chip. 2016;16:2935–2945. doi: 10.1039/c6lc00062b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Durmus NG, Tekin HC, Guven S, Sridhar K, Arslan Yildiz A, Calibasi G, Ghiran I, Davis RW, Steinmetz LM, Demirci U. Magnetic levitation of single cells. Proc Natl Acad Sci U S A. 2015;112:E3661–E3668. doi: 10.1073/pnas.1509250112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeziorowska D, Fontaine V, Jouve C, Villard E, Dussaud S, Akbar D, Letang V, Cervello P, Itier JM, Pruniaux MP, Hulot JS. Differential Sarcomere and Electrophysiological Maturation of Human iPSC-Derived Cardiac Myocytes in Monolayer vs. Aggregation-Based Differentiation Protocols. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18061173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Y, Wang L, Yu H, Yin F, Wang Y, Liu H, Jiang L, Qin J. In situ generation of human brain organoids on a micropillar array. Lab Chip. 2017;17:2941–2950. doi: 10.1039/c7lc00682a. [DOI] [PubMed] [Google Scholar]
- 66.Martínez García de la Torre RA, Nieto-Nicolau N, Morales-Pastor A, Casaroli-Marano RP. Determination of the Culture Time Point to Induce Corneal Epithelial Differentiation in Induced Pluripotent Stem Cells. Transplant Proc. 2017;49:2292–2295. doi: 10.1016/j.transproceed.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 67.Ochiai-Shino H, Kato H, Sawada T, Onodera S, Saito A, Takato T, Shibahara T, Muramatsu T, Azuma T. A novel strategy for enrichment and isolation of osteoprogenitor cells from induced pluripotent stem cells based on surface marker combination. PLoS One. 2014;9:e99534. doi: 10.1371/journal.pone.0099534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoque A, Sivakumaran P, Bond ST, Ling NXY, Kong AM, Scott JW, Bandara N, Hernández D, Liu GS, Wong RCB, Ryan MT, Hausenloy DJ, Kemp BE, Oakhill JS, Drew BG, Pébay A, Lim SY. Mitochondrial fission protein Drp1 inhibition promotes cardiac mesodermal differentiation of human pluripotent stem cells. Cell Death Discov. 2018;4:39. doi: 10.1038/s41420-018-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pauly MG, Krajka V, Stengel F, Seibler P, Klein C, Capetian P. Adherent vs. Free-Floating Neural Induction by Dual SMAD Inhibition for Neurosphere Cultures Derived from Human Induced Pluripotent Stem Cells. Front Cell Dev Biol. 2018;6:3. doi: 10.3389/fcell.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chauveau S, Anyukhovsky EP, Ben-Ari M, Naor S, Jiang YP, Danilo P, Jr, Rahim T, Burke S, Qiu X, Potapova IA, Doronin SV, Brink PR, Binah O, Cohen IS, Rosen MR. Induced Pluripotent Stem Cell-Derived Cardiomyocytes Provide In Vivo Biological Pacemaker Function. Circ Arrhythm Electrophysiol. 2017;10:e004508. doi: 10.1161/CIRCEP.116.004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roberts CL, Chen SS, Murchison AC, Ogle RA, Francis MP, Ogle RC, Sachs PC. Preferential Lineage-Specific Differentiation of Osteoblast-Derived Induced Pluripotent Stem Cells into Osteoprogenitors. Stem Cells Int. 2017;2017:1513281. doi: 10.1155/2017/1513281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nam Y, Rim YA, Jung SM, Ju JH. Cord blood cell-derived iPSCs as a new candidate for chondrogenic differentiation and cartilage regeneration. Stem Cell Res Ther. 2017;8:16. doi: 10.1186/s13287-017-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Espinosa-Jeffrey A, Blanchi B, Biancotti JC, Kumar S, Hirose M, Mandefro B, Talavera-Adame D, Benvenisty N, de Vellis J. Efficient Generation of Viral and Integration-Free Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes. Curr Protoc Stem Cell Biol. 2016;38:2D.18.1–2D.18.27. doi: 10.1002/cpsc.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phondeechareon T, Wattanapanitch M, U-Pratya Y, Damkham C, Klincumhom N, Lorthongpanich C, Kheolamai P, Laowtammathron C, Issaragrisil S. Generation of induced pluripotent stem cells as a potential source of hematopoietic stem cells for transplant in PNH patients. Ann Hematol. 2016;95:1617–1625. doi: 10.1007/s00277-016-2756-1. [DOI] [PubMed] [Google Scholar]
- 75.Plaisted WC, Zavala A, Hingco E, Tran H, Coleman R, Lane TE, Loring JF, Walsh CM. Remyelination Is Correlated with Regulatory T Cell Induction Following Human Embryoid Body-Derived Neural Precursor Cell Transplantation in a Viral Model of Multiple Sclerosis. PLoS One. 2016;11:e0157620. doi: 10.1371/journal.pone.0157620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang M, Chen W, Liu J, Weir MD, Cheng L, Xu HH. Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng Part A. 2014;20:1295–1305. doi: 10.1089/ten.tea.2013.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suchorska WM, Augustyniak E, Richter M, Trzeciak T. Comparison of Four Protocols to Generate Chondrocyte-Like Cells from Human Induced Pluripotent Stem Cells (hiPSCs) Stem Cell Rev Rep. 2017;13:299–308. doi: 10.1007/s12015-016-9708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lach MS, Kulcenty K, Jankowska K, Trzeciak T, Richter M, Suchorska WM. Effect of cellular mass on chondrogenic differentiation during embryoid body formation. Mol Med Rep. 2018;18:2705–2714. doi: 10.3892/mmr.2018.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hunt NC, Hallam D, Karimi A, Mellough CB, Chen J, Steel DHW, Lako M. 3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development. Acta Biomater. 2017;49:329–343. doi: 10.1016/j.actbio.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 80.Miranda CC, Fernandes TG, Diogo MM, Cabral JM. Scaling up a chemically-defined aggregate-based suspension culture system for neural commitment of human pluripotent stem cells. Biotechnol J. 2016;11:1628–1638. doi: 10.1002/biot.201600446. [DOI] [PubMed] [Google Scholar]
- 81.Mukherjee C, Hale C, Mukhopadhyay S. A Simple Multistep Protocol for Differentiating Human Induced Pluripotent Stem Cells into Functional Macrophages. Methods Mol Biol. 2018;1784:13–28. doi: 10.1007/978-1-4939-7837-3_2. [DOI] [PubMed] [Google Scholar]
- 82.Liu LP, Li YM, Guo NN, Li S, Ma X, Zhang YX, Gao Y, Huang JL, Zheng DX, Wang LY, Xu H, Hui L, Zheng YW. Therapeutic Potential of Patient iPSC-Derived iMelanocytes in Autologous Transplantation. Cell Rep. 2019;27:455–466.e5. doi: 10.1016/j.celrep.2019.03.046. [DOI] [PubMed] [Google Scholar]