Highlights
-
•
Blood flow restriction without concomitant exercise seems a promising strategy to mitigate muscle strength and size reductions secondary to immobilization periods.
-
•
The high risk of bias presented by original studies limits the indications for blood flow restriction without additional exercise as an effective countermeasure against strength reduction and atrophy mediated by immobilization.
-
•
Future studies should be concerned with the gaps related to methodological aspects of blood flow restriction, such as the ideal occlusion pressure to attenuate reduction of strength and muscle mass.
Keywords: Disuse, Hypoxia, Ischemia, Muscle wasting, Rehabilitation
Abstract
Purpose
To investigate whether blood flow restriction (BFR) without concomitant exercise mitigated strength reduction and atrophy of thigh muscles in subjects under immobilization for lower limbs.
Methods
The following databases were searched: PubMed, CINAHL, PEDro, Web of Science, Central, and Scopus.
Results
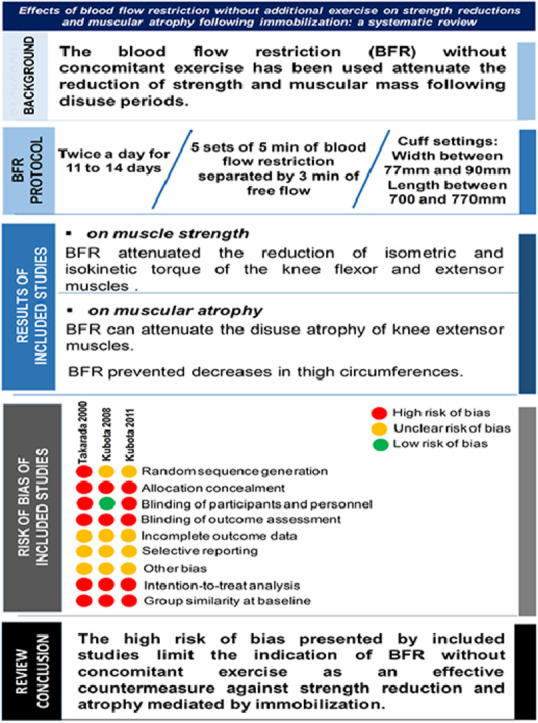
The search identified 3 eligible studies, and the total sample in the identified studies consisted of 38 participants. Isokinetic and isometric torque of the knee flexors and extensors was examined in 2 studies. Cross-sectional area of thigh muscles was evaluated in 1 study, and thigh girth was measured in 2 studies. The BFR protocol was 5 sets of 5 min of occlusion and 3 min of free flow, twice daily for approximately 2 weeks. As a whole, the included studies indicate that BFR without exercise is able to minimize strength reduction and muscular atrophy after immobilization. It is crucial to emphasize, however, that the included studies showed a high risk of bias, especially regarding allocation concealment, blinding of outcome assessment, intention-to-treat analyses, and group similarity at baseline.
Conclusion
Although potentially useful, the high risk of bias presented by original studies limits the indication of BFR without concomitant exercise as an effective countermeasure against strength reduction and atrophy mediated by immobilization.
Graphical abstract
1. Introduction
Prolonged reductions in weight-bearing activity such as that encountered during illness, injury, surgery, joint immobilization, or bed rest causes reduced strength and muscular size, along with consequent functional deficits.1, 2, 3 For instance, quadriceps strength may be decreased by 20%–60% after 30 days of bed rest,4, 5 and an average deficit between 11% and 16% has been reported for the muscle size of knee extensors after unloading with no intervention.6, 7 Additionally, a 10%–20% deficit in quadriceps strength and size can persist for years after surgical procedures, despite concentrated rehabilitation efforts.8
These negative muscle adaptations may result in prolonged hospitalization and subsequent rehabilitation,9, 10 slow one's gait speed,11 hamper balance,12 and hinder stair climbing13 and chair-rising ability.14 Since muscle strength is a predictor of physical functioning and muscle mass has an important role in metabolic homeostasis, attenuating strength and mass deficits is imperative to limit future disabilities.15, 16, 17 Furthermore, reductions in strength and muscle size were found to be a strong predictor of hip surgery in the United States, with total annual costs exceeding USD70,000 per patient.18
Exercises have been used to preserve strength and muscle mass during periods of disuse.19, 20 In situations where disuse is induced by surgery or injuries, however, muscle action with or without movement may not be prudent6 owing to immobilization, pain, local inflammatory process, or other contraindications owing to the risk of compromising surgery. In this context, the use of blood flow restriction (BFR) may be a promising strategy.
BFR is usually achieved by applying a pneumatic cuff to the proximal end of a limb, partially restricting the blood flow and limiting venous return,21 and it is often combined with low-intensity resistance exercises to increase strength and muscle size.22 However, some studies have pointed out that the use of BFR without concomitant exercise can attenuate the reduction in strength and muscle size caused by disuse.23, 24
The mechanisms involved in maintaining skeletal muscle mass with BFR without exercise are still unclear.25, 26 It has been suggested that cell swelling, induced by blood-pooling accumulation of metabolites and reactive hyperemia, is detected by an intrinsic volume sensor and may consequently lead to an activation of myogenic signaling pathways, such as the mammalian target of rapamycin and mitogen-activated protein kinase.26 Additionally, the release of catecholamines (norepinephrine) stimulated by signaling through the β2-adrenoceptor27 may also have a positive effect on muscle protein metabolism.
Although individual clinical trials have identified positive effects of BFR without concomitant exercise on strength and muscular atrophy in disuse periods,24, 27, 28 no systematic review has investigated this topic. Thus, this study aimed to determine the efficacy of BFR without exercise as a countermeasure against strength reduction and muscular atrophy following periods of immobilization.
2. Methods
2.1. Study design
This systematic review was developed by using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The protocol review was prospectively registered in International prospective register of systematic reviews (Registration Number: CRD42017067934).
2.2. Identification of studies
A systematic search of the literature was carried out, without restrictions on the language of publication, from the earliest record up to January 2019. The Medline/PubMed, CINAHL, PEDro, Web of Science, Central, and Scopus databases were searched. The trial register in ClinicalTrials.gov (grey literature) and references in the included articles were also checked in order to track other promising eligible studies. The following key words were used in isolation or in combination: “blood flow restriction”, “vascular occlusion”, “kaatsu”, “immobilization”, “atrophy”, and “clinical trial”. The search strategies developed for each database are described in Supplementary Appendix.
2.3. Eligibility criteria
Articles that appeared only as short versions or that did not have full text available were not included. Trials in which exercises were performed concomitantly with BFR protocol or with insufficient data were also excluded. Studies involving participants (healthy or not) who were 18 years of age or older and who were submitted to some immobilization model for lower limbs (e.g., cast on the ankle, non-weight bearing immobilization and knee brace after anterior cruciate ligament (ACL) reconstruction) were included. The data collected about the participants included health condition, age, gender, thigh circumference, and immobilization model/period. Studies that compared BFR (without additional exercise) in the proximal end of the thigh during an immobilization period to a control group (no intervention or sham) were included. The data extracted about the intervention included the BFR protocol (periods of occlusion and rest), frequency (daily and weekly), and BFR cuff settings (width, length, placement locale, and restriction pressure).
The outcomes considered were strength and/or muscular atrophy of the knee flexors and/or extensor muscles. The extracted data for muscle strength included isokinetic (eccentric and/or concentric) and/or isometric torque (measured by dynamometer isokinetic and expressed in N·m). The extracted data for muscular atrophy included thigh girth (measured by tape and expressed in cm) and/or cross-sectional area (CSA; measured by magnetic resonance imaging (MRI) and expressed in cm2). The eligibility criteria were based on the principle of PICOS (P: participants (participants under immobilization for lower limbs); I: intervention (BFR without exercise for thigh muscles during an immobilization period); C: comparison (no intervention or sham); O: outcomes (strength and/or muscular atrophy of the knee extensors and/or flexors muscles); S: study design (randomized controlled trials)).
2.4. Study selection
The searches, data collection, and analysis of the studies were performed separately and independently by 2 reviewers (JDSN and JAMB), and differences were discussed by a 3rd evaluator (WHBV) when a consensus was not reached. The reviewers initially and independently judged the relevance of the studies by reading the titles and subsequent abstracts, according to the eligibility criteria. Then, the full text of any article whose summary had potential for eligibility or raised some questions was carefully read and analyzed.
2.5. Quality assessment of studies
The quality assessment of eligible studies was conducted using the Cochrane Risk of Bias Tool, which classifies the risk of bias as high, low, or unclear.29 The risk of bias is considered high if a methodological procedure was not described, low if the procedure was described in detail, and unclear if the description was only partially described. Information from the studies was independently extracted by both reviewers and stored in the Review Manager software Version 5.3 (The Nordic Cochrane Center, Copenhagen, Denmark) for subsequent cross-checking of the data and discussion of possible discrepancies.
3. Results
3.1. Identification and selection of studies
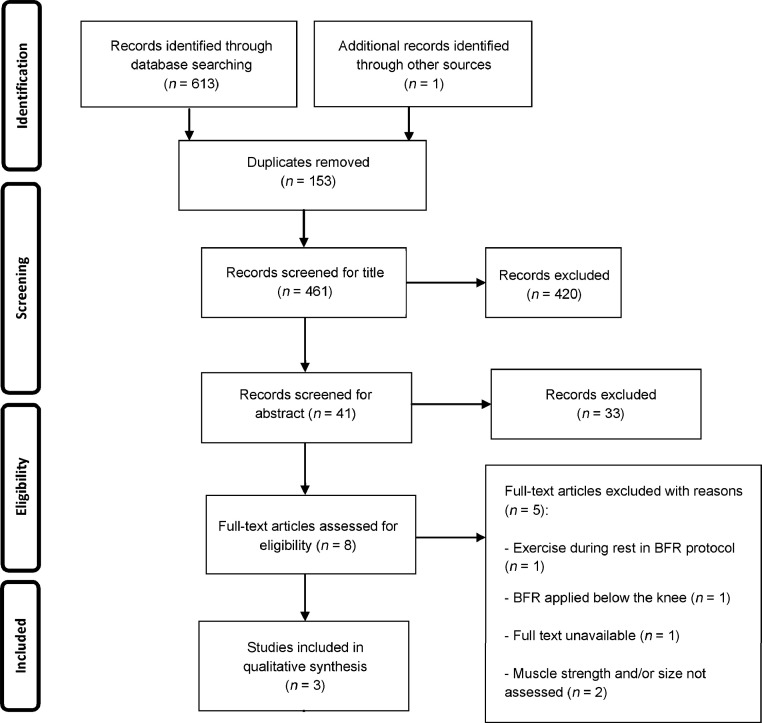
The search resulted in 614 potentially relevant articles. After removal of duplicates, the remaining articles were submitted to an analysis of title and summary. After this analysis, 8 articles were still considered to be potentially eligible. However, 5 of these articles were subsequently excluded for the following reasons: exercise concomitant with the BFR protocol,23 BFR applied below the knee,30 full text unavailable even after requested from the authors,31 and muscle strength and/or size not assessed in the pre-intervention and post-intervention period.32, 33 The remaining 3 studies24, 27, 28 were included in the systematic review (Fig. 1). The 3 articles were published between 2000 and 2011.
Fig. 1.
Flow diagram of the selection steps of the identified articles according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. BFR = blood flow restriction.
3.2. Characteristics of the included studies
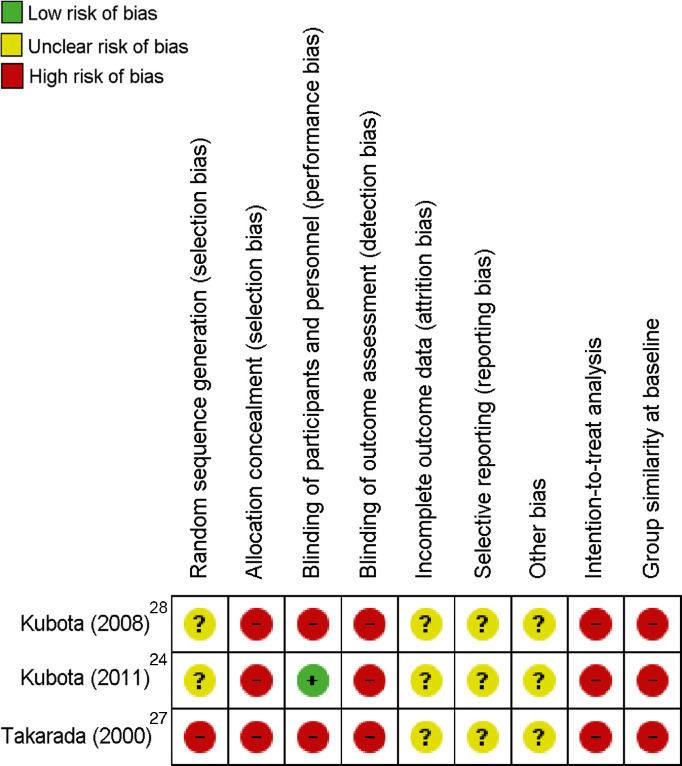
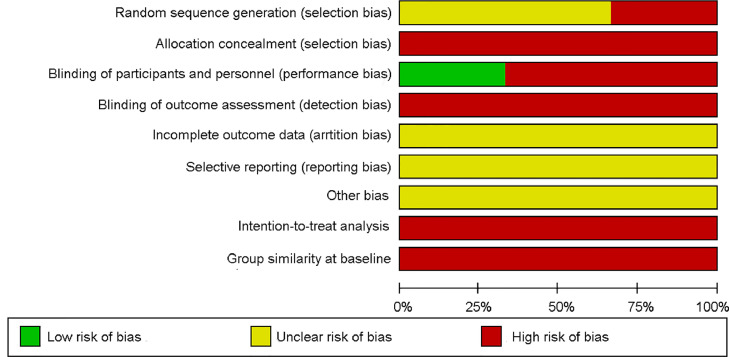
The risk of bias analysis is presented in Fig. 2, Fig. 3. The characteristics of the studies are presented in Table 1.
Fig. 2.
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Fig. 3.
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Table 1.
Summary of 3 included studies.
| Study | Participants | Immobilization model/period | Intervention | Comparison | Outcome measures |
|---|---|---|---|---|---|
| Takarada et al. (2000)27 | Patients submitted to ACL reconstruction surgery n = 16 (8 males, 8 females) Age: approximately 21–25 years Thigh girth: not informed |
Knee brace/2 weeks | BFRP: 5 sets of 5 min of occlusion and 3 min of free flow Frequency: twice a day between 3rd and 14th day after the surgery (11 days/22 sessions) OCS: Width (90 mm); Length (700 mm) Placement: Proximal end of the thigh (100 mm below the hip joint) Occlusive pressure: mean pressure of 238 mmHg |
Sham: occlusion cuff without inflation | Muscular atrophy: cross-sectional area |
| Kubota et al. (2008)28 | Healthy n = 11 males Age: approximately 21–25 years Thigh girth: approximately 45–51 cm |
Left ankle joint immobilization by cast and not weight-bearing/2 weeks | BFRP: 5 sets of 5 min of occlusion and 3 min of free flow Frequency: Twice a day for 2 weeks (14 days/28 sessions) OCS: Width (77 mm); Length (770 mm) Placement: Proximal end of the thigh Occlusive pressure: 200 mmHg |
Control: without intervention | Muscle strength: isokinetic (eccentric and concentric) and isometric torque Muscular atrophy: thigh girth |
| Kubota et al. (2011)24 | Healthy n = 11 males for muscle strength measures and n = 10 males for thigh girth measures Age: approximately 21–24 years Thigh girth: approximately 48–52 cm |
Left ankle joint immobilization by cast and not weight-bearing/2 weeks | BFRP: 5 sets of 5 min of occlusion and 3 min of free flow Frequency: Twice a day for 2 weeks (14 days/28 sessions) OCS: Width (77 mm); Length (770 mm) Placement: Proximal end of the thigh Occlusive pressure: 50 mmHg |
Control: without intervention | Muscle Strength: isokinetic (eccentric and concentric) and isometric torque Muscular Atrophy: thigh girth |
Abbreviations: ACL = anterior cruciate ligament; BFRP = blood flow restriction protocol; OCS = occlusion cuff settings.
3.3. Risk of bias
The main methodological limitation regarding randomization and allocation was lack of clarity about randomization methods (software, random numbers, or other), which constituted a high and unclear risk of bias. All included studies were classified as high risk for the allocation concealment criteria owing to nonreporting on the use of sealed and opaque envelopes. For the blinding criteria, only 1 study mentioned blinding of participants, and none of the studies mentioned blinding of collaborators, thus constituting a high risk of bias. Incomplete outcome data and selective reporting were classified as unclear in all studies, because there was little information about the exclusion of participants and data loss. In addition, none of the studies reported a prospective trial register. Regarding the intention-to-treat analysis, all studies failed to transparently report possible losses in the final analysis and thus were considered to have a high risk of bias. The similarity of the groups at baseline was not clearly described in any of the studies, which constituted a high risk of bias.
3.4. Participants
The total number of participants in the 3 studies included was 38 individuals of both genders. In 2 studies, participants were healthy and were submitted to immobilization by cast and not weight-bearing for 2 weeks.24, 28 In 1 study, the patients were submitted after ACL surgery to a usual recovery program in the hospital and immobilization by knee brace for 2 weeks.27 The participants’ thigh girth at baseline was reported in only 2 studies24, 28 and ranged from approximately 45–51 cm and approximately 48–52 cm respectively.
3.5. Intervention
All 3 included studies used an external pressure cuff to apply BFR on the proximal end of the thigh. In 2 studies,24, 28 the BFR stimulus was applied twice a day (5 sets of 5 min of occlusion and 3 min of free flow) for 14 days by cuff with 77 mm of width and 770 mm of length. In 1 study,27 the BFR stimulus was applied twice a day (5 sets of 5 min of occlusion and 3 min of free flow) for 11 days by cuff with 90 mm of width and 700 mm of length. The occlusion pressure was arbitrarily defined in all studies, with pressures of 50 mmHg,24 200 mmHg,28 and 238 mmHg.27 The comparison groups used in the included studies either received no intervention24, 28 or a sham intervention (occlusion cuff without inflation).27 Only 1 study reported the absence of BFR-related side effects after ACL reconstruction surgery.27
3.6. Outcome measures
Muscle strength was measured in only 2 studies24, 28 through isokinetic and isometric dynamometry. Isokinetic torque of knee extensors and flexors was evaluated during concentric (at 60°/s, 180°/s, and 300°/s) and eccentric (at 60°/s and 180°/s) muscle actions. Isometric torque was measured with the knee joint flexed at 60°. Thigh girth was considered as an indirect measure of muscular atrophy in 2 of the included studies.24, 28 Thigh girth was evaluated at 10 cm and 15 cm above the superior border of the patella by tape. In 1 study,27 CSA (measured by MRI) was considered as a measure for muscular atrophy outcome.
3.7. Effect of BFR on muscle strength
In 2 studies,24, 28 having a total sample of 22 participants, the effect of BFR without exercise on the muscle strength of knee flexors and extensors was reported. When compared with control (no intervention), these 2 studies concluded that there was significant attenuation in the reduction of muscle weakness.
3.8. Effect of BFR on muscular atrophy
The studies that reported the effect of BFR without concomitant exercise on thigh girth as an indirect measure of muscular atrophy24, 28 included a total sample of 21 participants. These studies concluded that BFR prevented decreases in thigh circumference. The study that evaluated muscular atrophy by MRI27 indicated that BFR can attenuate the disuse atrophy of extensor muscles.
4. Discussion
This systematic review indicated that BFR without concomitant exercises in participants under immobilization was a potential countermeasure against strength reduction and atrophy in the thigh muscles. It is important to remark that these results come from studies with a high risk of bias (mostly owing to absence of allocation concealment, no blinding of outcome assessment, no intention-to-treat analyses, and group similarity at baseline not being reported).
Previous reviews have indicated the benefits of BFR associated with exercise for strength and muscle mass gains in athletes,34 patients with musculoskeletal disorders,35 and older adults.36 However, this is the first systematic review that evaluated the quality of studies on BFR without concomitant exercise in attenuating reduction of strength and muscular atrophy in immobilization periods.
Regarding the outcome strength, the studies showed a favorable effect of BFR in mitigating the reduction in torque of the knee extensors and flexors. Regarding muscular atrophy, results of the included studies indicated a positive effect in favor of BFR for thigh girth and CSA. These results regarding CSA, however, are contrary to a report in the literature in which BFR without exercise was not able to mitigate the reduction in CSA after ACL reconstruction.23 It should be noted that in that study23 there was exercise in the interval between occlusion periods (with free flow) and that the muscular pump may have increased the venous return37 and limited the formation of muscular edema, which is suggested as one of the main mechanisms of attenuation of muscle mass loss by BFR.26 Considering the role of muscle mass in the loss of strength in the first day of disuse,38, 39 the possibility of minimizing strength reduction and atrophy with passive BFR may be useful in mitigating some negative repercussions of immobility, such as prolonged hospitalization and limitations in physical function.9, 10, 11
4.1. Gaps in original studies and future perspectives
4.1.1. Optimal restriction protocol for BFR without concomitant exercise
None of the studies included in this systematic review individually applied restriction pressure. because the restriction level is strongly influenced by the thigh girth and the cuff width,40 it is essential to avoid applying arbitrary pressures and to prioritize individualized restriction pressure to ensure similar levels of BFR among participants.
Another relevant issue is the proper restriction level for optimal results. In studies on BFR associated with exercise, pressures between 40% and 50% of the total restriction pressure are considered ideal,41 but for studies on BFR without concomitant exercise, there are insufficient data to identify an optimal restriction pressure. Regarding the restriction protocol, all included studies applied 5 sets of 5 min of occlusion and 3 min of free flow, twice daily for approximately 2 weeks, but it is unclear whether this protocol is the most effective.
Considering the possible side effects secondary to BFR (fainting, numbness, pain, discomfort, and thrombolytic events) and the higher perception of discomfort when very high pressures are used,42, 43 it is essential that studies on this topic investigate the appropriate restriction pressure and the ideal protocol (duration of cycles of occlusion and free flow and daily and weekly frequency) for the effective and safe use of BFR without concomitant exercise.
Another limitation of the included studies was the failure to investigate whether BFR overcomes the placebo effect to attenuate strength reduction. Knowing that BFR may not overcome the placebo for performance improvement,44 future studies should be concerned with this issue and consider the placebo effect evaluation of the BFR without concomitant exercise.
4.1.2. Progressive models to mitigate the consequences of disuse and enhance strength
BFR without concomitant exercise has been suggested as an initial intervention in a progressive model that avoids early losses in strength. The model then uses BFR with low-intensity exercises to improve strength and finally progresses to high-intensity exercises without BFR. This model progresses from the bed rest period to higher load resistance training.25 However, given our results, future studies having high methodological quality should initially verify the isolated effects of BFR in the rehabilitation of patients who undergo to surgical procedures or immobilization and then subsequently analyze the effects of BFR without exercise, BFR with low-intensity exercises, and high-intensity exercises without BFR.
In addition, we suggest including functional outcome assessments to achieve external validity for the results. Instead of using disuse models, such as lower limb immobilization in healthy participants,24, 28 researchers should consider disuse models that are more common in clinical practice, including bed rest and immobilization after an injury or surgery. Another option is to use BFR without concomitant exercise in a general rehabilitation program, as previously suggested for patients undergoing ACL surgery.27
4.1.3. Thigh girth as a measure of muscular atrophy
An important limitation of the studies included in this systematic review was that they inferred muscular atrophy by thigh girth, which makes it difficult to determine which muscle groups were more responsive to BFR. Furthermore, girth may not provide an accurate measure of muscular atrophy because girth measures have lower validity than other measures in estimating quadriceps muscular atrophy.45 Therefore, future studies should prioritize the use of MRI as a measure of muscular atrophy.
4.2. Risks of BFR during immobilization
Cardiovascular complications such as increased heart rate, orthostatic hypotension, and venous thromboembolism may occur during periods of immobilization.46 It is noteworthy that BFR may promote venous stasis and reduce venous return; thus, some risks may be increased with the use of BFR in patients under immobilization or prolonged bed rest. Although BFR commonly has no negative effects on prothrombin time, markers of coagulation, or fibrinolysis in healthy adults,47 some adverse cardiovascular effects may be associated with BFR.48 For example, it has been suggested that an artificial decrease of blood flow induced by external vascular compression may increase pressure reflex, leading to dangerous increases in cardiac function, blood pressure, and vascular resistance.49 In addition, the reactive hyperemia that occurs after blood flow release50 may contribute to the displacement of preexisting thrombi and clots. It is also possible to reason that the use of high pressures (as applied by Takarada et al.27) associated with the absence of the muscular pump can make it too difficult to remove metabolites and potentiate oxidative stress. Therefore, these circumstances point to a risk in applying BFR in patients with cardiovascular diseases or individuals with sympathetic hyperreactivity. To minimize the cardiovascular risks inherent to the use of BFR in periods of immobilization, we recommend the individualized determination of the restriction pressure and the application of a risk assessment tool for BFR.51
This systematic review has some limitations. First, our results only come from 3 clinical trials and a reduced sample size. Second, although the main health literature databases were searched, the Embase database was not available to the authors, but it is important to highlight that the Central and PEDro databases retrieve relevant articles from Embase.
5. Conclusion
Although BFR is potentially useful, the high risk of bias presented by original studies limits the indication of BFR without concomitant exercise as an effective countermeasure against strength reduction and atrophy mediated by immobilization. Studies with high-quality methodologies should investigate whether BFR is truly effective and safe for patients in immobilization periods and seek to fill the main knowledge gaps identified in this systematic review.
Acknowledgments
Acknowledgments
Mikhail Santos Cerqueira, Daniel Germano Maciel, and Jean Artur Mendonça Barboza would like to thank Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil), Finance code 001, for the scholarship concession.
Authors’ contributions
MSC conceived the study, participated in the design and coordination of the study, and the data interpretation, and wrote the manuscript; JDSN and JAMB participated in the design and coordination of the study and performed the literature search and study selection; DGM performed data extraction from included studies; WHBV interpreted the data. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2019.07.001.
Appendix. Supplementary materials
References
- 1.Evans W.J. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 2.Kandarian S.C., Stevenson E.J. Molecular events in skeletal muscle during disuse atrophy. Exerc Sport Sci Rev. 2002;30:111–116. doi: 10.1097/00003677-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Parry S.M., Puthucheary Z.A. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med. 2015;4:16. doi: 10.1186/s13728-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens J.E., Mizner R.L., Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21:775–779. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 5.Dudley G.A., Duvoisin M.R., Convertino V.A., Buchanan P. Alterations of the in vivo torque-velocity relationship of human skeletal muscle following 30 days exposure to simulated microgravity. Aviat Space Environ Med. 1989;60:659–663. [PubMed] [Google Scholar]
- 6.Cook S.B., Brown K.A., DeRuisseau K., Kanaley J.A., Ploutz-Snyder L.L. Skeletal muscle adaptations following blood flow-restricted training during 30 days of muscular unloading. J Appl Physiol (1985) 2010;109:341–349. doi: 10.1152/japplphysiol.01288.2009. [DOI] [PubMed] [Google Scholar]
- 7.Tesch P.A., Trieschmann J.T., Ekberg A. Hypertrophy of chronically unloaded muscle subjected to resistance exercise. J App Physiol (1985) 2004;96:1451–1458. doi: 10.1152/japplphysiol.01051.2003. [DOI] [PubMed] [Google Scholar]
- 8.Gerber J.P., Marcus R.L., Dibble L.E., Greis P.E., Burks R.T., LaStayo P.C. Effects of early progressive eccentric exercise on muscle structure after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2007;89:559–570. doi: 10.2106/JBJS.F.00385. [DOI] [PubMed] [Google Scholar]
- 9.Reardon K., Galea M., Dennett X., Choong P., Byrne E. Quadriceps muscle wasting persists 5 months after total hip arthroplasty for osteoarthritis of the hip: a pilot study. Intern Med J. 2001;31:7–14. doi: 10.1046/j.1445-5994.2001.00007.x. [DOI] [PubMed] [Google Scholar]
- 10.Christensen T., Bendix T., Kehlet H. Fatigue and cardiorespiratory function following abdominal surgery. Br J Surg. 1982;69:417–419. doi: 10.1002/bjs.1800690721. [DOI] [PubMed] [Google Scholar]
- 11.Ikezoe T., Nakamura M., Shima H., Asakawa Y., Ichihashi N. Association between walking ability and trunk and lower-limb muscle atrophy in institutionalized elderly women: a longitudinal pilot study. J Physiol Anthropol. 2015;34:31. doi: 10.1186/s40101-015-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moxley S.D., Krebs D.E., Harris B.A. Quadriceps muscle strength and dynamic stability in elderly persons. Gait Posture. 1999;10:10–20. doi: 10.1016/s0966-6362(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 13.Mizner R.L., Petterson S.C., Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005;35:424–436. doi: 10.2519/jospt.2005.35.7.424. [DOI] [PubMed] [Google Scholar]
- 14.Skelton D.A., Greig C.A., Davies J.M., Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing. 1994;23:371–377. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- 15.Thomas A.C., Stevens-Lapsley J.E. Importance of attenuating quadriceps activation deficits after total knee arthroplasty. Exerc Sport Sci Rev. 2012;40:95–101. doi: 10.1097/JES.0b013e31824a732b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petterson S.C., Mizner R.L., Stevens J.E., Raisis L., Bodenstab A., Newcomb W. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009;61:174–183. doi: 10.1002/art.24167. [DOI] [PubMed] [Google Scholar]
- 17.Nair K.S. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 18.Cui Z., Schoenfeld M.J., Bush E.N., Chen Y., Burge R. Characteristics of hip fracture patients with and without muscle atrophy/weakness: predictors of negative economic outcomes. J Med Econ. 2015;18:1–11. doi: 10.3111/13696998.2014.969433. [DOI] [PubMed] [Google Scholar]
- 19.Steffl M., Bohannon R.W., Sontakova L., Tufano J.J., Shiells K., Holmerova I. Relationship between sarcopenia and physical activity in older people: a systematic review and meta-analysis. Clin Interv Aging. 2017;12:835–845. doi: 10.2147/CIA.S132940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowen T.S., Schuler G., Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2015;6:197–207. doi: 10.1002/jcsm.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerqueira M.S., Pereira R., Rocha T., Mesquita G., Oliveira de Paiva Lima C.R., Falcão Raposo M.C. Time to failure and neuromuscular response to intermittent isometric exercise at different levels of vascular occlusion: a randomized crossover study. Int J Appl Exerc Physiol. 2017;6:55–70. [Google Scholar]
- 22.Lixandrão M.E., Ugrinowitsch C., Berton R., Vechin F.C., Conceição M.S., Damas F. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med. 2018;48:361–378. doi: 10.1007/s40279-017-0795-y. [DOI] [PubMed] [Google Scholar]
- 23.Iversen E., Røstad V., Larmo A. Intermittent blood flow restriction does not reduce atrophy following anterior cruciate ligament reconstruction. J Sport Health Sci. 2016;5:115–118. doi: 10.1016/j.jshs.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubota A., Sakuraba K., Koh S., Ogura Y., Tamura Y. Blood flow restriction by low compressive force prevents disuse muscular weakness. J Sci Med Sport. 2011;14:95–99. doi: 10.1016/j.jsams.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Loenneke J.P., Abe T., Wilson J.M., Thiebaud R.S., Fahs C.A., Rossow L.M. Blood flow restriction: an evidence based progressive model (Review) Acta Physiol Hung. 2012;99:235–250. doi: 10.1556/APhysiol.99.2012.3.1. [DOI] [PubMed] [Google Scholar]
- 26.Loenneke J.P., Fahs C.A., Rossow L.M., Abe T., Bemben M.G. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med Hypotheses. 2012;78:151–154. doi: 10.1016/j.mehy.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Takarada Y., Takazawa H., Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc. 2000;32:2035–2039. doi: 10.1097/00005768-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Kubota A., Sakuraba K., Sawaki K., Sumide T., Tamura Y. Prevention of disuse muscular weakness by restriction of blood flow. Med Sci Sports Exerc. 2008;40:529–534. doi: 10.1249/MSS.0b013e31815ddac6. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J.P.T., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark B.C., Fernhall B., Ploutz-Snyder L.L. Adaptations in human neuromuscular function following prolonged unweighting: I. Skeletal muscle contractile properties and applied ischemia efficacy. J Appl Physiol. 2006;101:256–263. doi: 10.1152/japplphysiol.01402.2005. [DOI] [PubMed] [Google Scholar]
- 31.Totsuka R., Sakuraba K., Kubota A. The effects of intermittent blood flow restriction on muscle atrophy and weakness induced by immobilization and no weight bearing. Japanese J Clin Sports Med. 2012;20:130–137. [in Japanese] [Google Scholar]
- 32.Loenneke J.P., Fahs C.A., Thiebaud R.S., Rossow L.M., Abe T., Ye X. The acute hemodynamic effects of blood flow restriction in the absence of exercise. Clin Physiol Funct Imaging. 2013;33:79–82. doi: 10.1111/j.1475-097X.2012.01157.x. [DOI] [PubMed] [Google Scholar]
- 33.Karabulut M., Mccarron J., Abe T., Sato Y., Bemben M. The effects of different initial restrictive pressures used to reduce blood flow and thigh composition on tissue oxygenation of the quadriceps. J Sports Sci. 2011;29:951–958. doi: 10.1080/02640414.2011.572992. [DOI] [PubMed] [Google Scholar]
- 34.Scott B.R., Loenneke J.P., Slattery K.M., Dascombe B.J. Blood flow restricted exercise for athletes: a review of available evidence. J Sci Med Sport. 2016;19:360–367. doi: 10.1016/j.jsams.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Hughes L., Paton B., Rosenblatt B., Gissane C., Patterson S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2017;51:1003–1011. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- 36.Centner C., Wiegel P., Gollhofer A., König D. Effects of blood flow restriction training on muscular strength and hypertrophy in older individuals: a systematic review and meta-analysis. Sports Med. 2019;49:95–108. doi: 10.1007/s40279-018-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kropp A.T., Meiss A.L., Guthoff A.E., Vettorazzi E., Guth S., Bamberger C.M. The efficacy of forceful ankle and toe exercises to increase venous return: a comprehensive Doppler ultrasound study. Phlebology. 2018;33:330–337. doi: 10.1177/0268355517706042. [DOI] [PubMed] [Google Scholar]
- 38.Cros N., Muller J., Bouju S., Piétu G., Jacquet C., Léger J.J. Upregulation of M-creatine kinase and glyceraldehyde3-phosphate dehydrogenase: two markers of muscle disuse. Am J Physiol. 1999;276:R308–R316. doi: 10.1152/ajpregu.1999.276.2.R308. [DOI] [PubMed] [Google Scholar]
- 39.Lieber R.L. 2nd ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2010. Skeletal muscle structure, function, and plasticity: the physiological basis of rehabilitation. [Google Scholar]
- 40.Loenneke J.P., Fahs C.A., Rossow L.M., Sherk V.D., Thiebaud R.S., Abe T. Effects of cuff width on arterial occlusion: implications for blood flow restricted exercise. Eur J Appl Physiol. 2012;112:2903–2912. doi: 10.1007/s00421-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loenneke J.P., Kim D., Fahs C.A., Thiebaud R.S., Abe T., Larson R.D. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve. 2014;51:713–721. doi: 10.1002/mus.24448. [DOI] [PubMed] [Google Scholar]
- 42.Brandner C.R., May A.K., Clarkson M.J., Warmington S.A. Reported side-effects and safety considerations for the use of blood flow restriction during exercise in practice and research. Tech Ortho. 2018;33:114–121. [Google Scholar]
- 43.Mattocks K.T., Jessee M.B., Counts B.R., Buckner S.L., Grant Mouser J., Dankel S.J. The effects of upper body exercise across different levels of blood flow restriction on arterial occlusion pressure and perceptual responses. Physiol Behav. 2017;171:181–186. doi: 10.1016/j.physbeh.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Sabino-Carvalho J.L., Lopes T.R., Obeid-Freitas T., Ferreira T.N., Succi J.E., Silva A.C. Effect of ischemic preconditioning on endurance performance does not surpass placebo. Med Sci Sports Exerc. 2017;49:124–132. doi: 10.1249/MSS.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 45.Doxey G. Assessing quadriceps femoris muscle bulk with-girth measurements in subjects with patellofemoral pain. J Orthop Sports Phys Ther. 1987;9:177–183. doi: 10.2519/jospt.1987.9.5.177. [DOI] [PubMed] [Google Scholar]
- 46.Dittmer D.K., Teasell R. Complications of immobilization and bed rest. Part 1: musculoskeletal and cardiovascular complications. Can Fam Physician. 1993;39:1428–1437. [PMC free article] [PubMed] [Google Scholar]
- 47.Clark B.C., Manini T.M., Hoffman R.L., Williams P.S., Guiler M.K., Knutson M.J. Relative safety of 4 weeks of blood flow-restricted resistance exercise in young, healthy adults. Scand J Med Sci Sports. 2011;21:653–662. doi: 10.1111/j.1600-0838.2010.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patterson S.D., Brandner C.R. The role of blood flow restriction training for applied practitioners: a questionnaire-based survey. J Sports Sci. 2018;36:123–130. doi: 10.1080/02640414.2017.1284341. [DOI] [PubMed] [Google Scholar]
- 49.Spranger M.D., Krishnan A.C., Levy P.D., O'Leary D.S., Smith S.A. Blood flow restriction training and the exercise pressor reflex: a call for concern. Am J Physiol Heart Circ Physiol. 2015;309:H1440–H1452. doi: 10.1152/ajpheart.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gundermann D.M., Fry C.S., Dickinson J.M., Walker D.K., Timmerman K.L., Drummond M.J. Reactive hyperemia is not responsible for stimulating muscle protein synthesis following blood flow restriction exercise. J Appl Physiol(1985) 2012;112:1520–1528. doi: 10.1152/japplphysiol.01267.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kacin A., Rosenblatt B., Žargi T.G., Biswas A. Safety considerations with blood flow restricted resistance training. Annales Kinesiol. 2015;6:2–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.