Abstract
Obesity is a complex disease with multiple contributing factors. One of the most intensely studied factors during the past decade has been the gut microbiota, which is the community of all microbes in the intestinal tract. The gut microbiota, via energy extraction, inflammation, and other actions, is now recognized as an important player in the pathogenesis of obesity. Dysbiosis, or an imbalance in the microbial community, can initiate a cascade of metabolic disturbances in the host. Early life is a particularly important period for the development of the gut microbiota, and perturbations such as with antibiotic exposure can have long-lasting consequences for host health. In early life and throughout the life span, diet is one of the most important factors that shape the gut microbiota. Although diets high in fat and sugar have been shown to contribute to dysbiosis and disease, dietary fiber is recognized as an important fermentative fuel for the gut microbiota and results in the production of short-chain fatty acids that can act as signaling molecules in the host. One particular type of fiber, prebiotic fiber, contributes to changes in the gut microbiota, the most notable of which is an increase in the abundance of Bifidobacterium. This review highlights our current understanding of the role of gut microbiota in obesity development and the ways in which manipulating the microbiota through dietary means, specifically prebiotics, could contribute to improved health in the host, including musculoskeletal health.
Keywords: Gut microbiota, Musculoskeletal health, Obesity, Prebiotics
Highlights
-
•
Gut microbiota is one of the most recently recognized factors playing a critical role in obesity development.
-
•
Environmental and lifestyle factors profoundly change our gut microbiome throughout our lives, but particularly in early life.
-
•
Prebiotics improve metabolic health by lowering body weight and fat mass, reducing inflammation, improving glucose control, and increasing the health-promoting bacteria in our gut.
-
•
There is growing interest in how exercise influences our gut microbiome and how this factor, together with diet, might influence musculoskeletal health.
Graphical Abstract
1. Introduction
According to the World Health Organization, worldwide obesity has more than tripled since 1975, with more than 650 million adults living with obesity and more than 41 million children under the age of 5 considered to be overweight or obese.1 Obesity is associated with metabolic disorders affecting multiple organs and systems2 and is recognized as a major risk factor for the development of type 2 diabetes (T2D), cardiovascular diseases (heart disease and stroke), musculoskeletal disorders (osteoarthritis (OA)), and certain forms of cancer (endometrial, breast, ovarian, prostate, liver, gallbladder, kidney, and colon).1, 3
Reduced to its most simplistic nature, obesity is the consequence of greater energy intake than expenditure; however, intensive research over the past decades has uncovered obesity's extremely complex etiology, which encompasses a dynamic interplay between host genetic and environmental factors.3 One of the most recent factors to be identified as playing a critical role in obesity development is the gut microbiota. Through its role in energy harvest, metabolic signaling, and inflammation, the gut microbiota is now recognized as an important player in body weight regulation.4, 5 Strategies aimed at shifting the gut microbiota back to a “healthy state” are providing new therapeutic targets for interventions that might help to reduce the burden of obesity and its comorbidities.
2. Gut microbiota
The intestinal tract contains the human body's most densely colonized ecosystem, consisting of bacteria, archaea, viruses, and unicellular eukaryotes—the so-called gut microbiota.6 The number of microbes in the intestinal tract is approximately 100 trillion cells,7 which is estimated to be in the same order of magnitude as human cells.8 The number of bacteria increases along the length of the gut to approximately 108 bacteria per gram of content in the distal ileum and 1011 bacteria per gram in the colon.9 Bacteria are classified according to their taxonomical rank (Fig. 1). At the division level (phylum), Firmicutes (gram-positive, anaerobic, spore-forming bacteria, mainly represented by the genera Clostridium, Faecalibacterium, Blautia, Ruminococcus, and Lactobacillus10) and Bacteroidetes (gram-negative, anaerobic, non-spore-forming bacteria, mainly represented by Bacteroides and Prevotella10) are dominant and can constitute over 90% of the bacteria present in the large and small intestine.11 Even though other phyla such as Actinobacteria (Bifidobacterium), Proteobacteria (Gammaproteobacteria with Enterobacteriaceae), or Verrucomicrobia (Akkermansia) are low in numbers, they have a major impact on health.12, 13 It is clear that individuals share similar core microbiota; nevertheless, all individuals have numerous differences in their microbiota, including proportions, diversity, species, and gene functions.14 Turnbaugh et al.11 suggested that instead of sharing a core human microbiome definable by a set of abundant microbial lineages, we might share a core gut microbiome at the level of metabolic functions. The gene pool of our gut microbiota (gut microbiome) is at least 150 times larger than our own, providing us with a range of otherwise inaccessible metabolic capabilities.15 Despite the fact that a definition of a healthy microbiota remains elusive,16 it has been established that the microbiota develops and matures over the course of infancy and childhood and reaches its adult form at 3 years of life.8
Fig. 1.
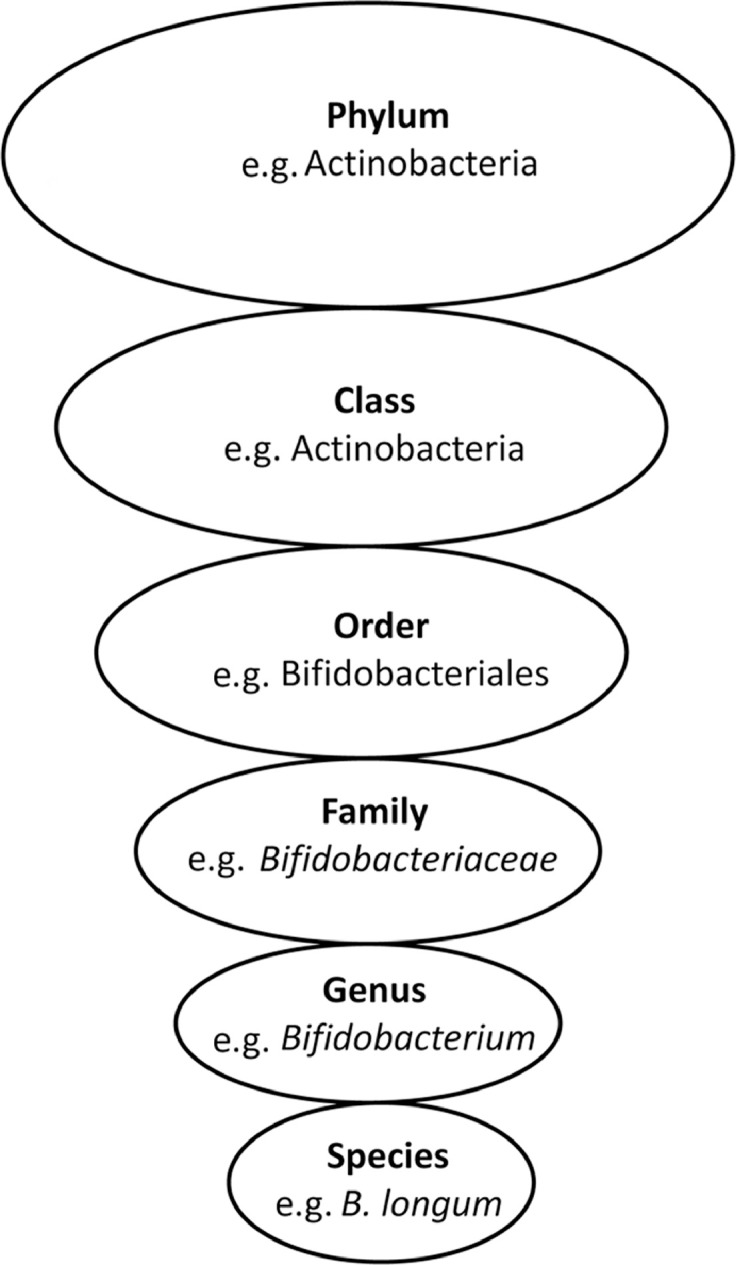
Bacterial taxonomy. Scientific classification of bacteria by rank or level.
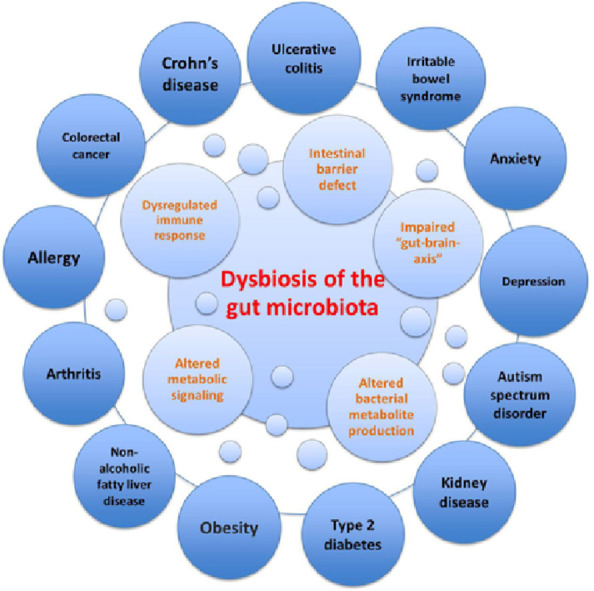
Several factors influence the microbial colonization of the infant gut, such as gestational age (term vs. preterm), mode of delivery (vaginal delivery vs. caesarean section), infant diet (breast milk vs. formula), breast-feeding patterns,17 maternal diet, genetics, sanitation, smoking during pregnancy, familial environment (rural vs. urban), home structure (large vs. small families), geography, and antibiotic treatment.18 Given the breadth of factors that influence the development of the infant's gut microbiota in the first year of life, interindividual differences in gut microbiota are significantly greater among children than among adults, even though the infant's gut microbiota is dominated by fewer bacterial genera.14 The sequence of bacterial species appearing in the first months of life is complex, and many transient species emerge owing to changes in the gut environment.11 This normal maturation can be disrupted, leading to an imbalance in the microbial community or “dysbiosis”, which can ultimately affect obesity risk5 and several other diseases (Fig. 2).19
Fig. 2.
Dysbiosis of the gut microbiota in disease. Dysbiosis of the gut microbiota impairs the intestinal barrier, immune system, metabolic functions, and bacterial metabolite production (i.e., short-chain fatty acids), as well as function/development of the central nervous system. Dysbiosis has been linked to several intestinal disorders such as inflammatory bowel disease (i.e., Crohn's disease, ulcerative colitis), irritable bowel syndrome and colorectal cancer, as well as extraintestinal disorders (i.e., obesity, type 2 diabetes, arthritis, and depression).19
2.1. Gut microbiota disruption and obesity risk
The gut microbiota of an individual with obesity may promote more efficient extraction and/or storage of energy from a certain diet, compared with gut microbiota of a lean individual. The earliest evidence supporting this hypothesis was the observation that germ-free (GF) mice are leaner when compared with conventionally raised animals and that the transplantation of gut microbiota into adult GF mice substantially increased their body fat mass despite reduced food intake.20 In addition to more efficient energy extraction from the diet, obesogenic gut microbiota also leads to intestinal inflammation contributing to the obese phenotype.21, 22, 23, 24 Specifically, proinflammatory tumor necrosis factor-α (TNF-α) messenger RNA levels in the ileum show strong correlation with the degree of weight gain, increased fat mass, and plasma glucose and insulin upon exposure to an high-fat diet (HFD).21 Furthermore, studies showed that only conventionally raised animals developed inflammation, whereas GF animals had no upregulation of TNF-α messenger RNA levels, suggesting that an HFD requires enteric bacteria to trigger intestinal inflammation. Interestingly, only obesity-prone Sprague Dawley rats and not obesity-resistant rats had increased ileal inflammation, neutrophil infiltration and innate immune Toll-like receptor 4 (TLR-4) activation once challenged with an HFD.23 In addition, obesity-prone Sprague Dawley rats displayed increased intestinal permeability, favoring increased leakage of gut-derived bacterial lipopolysaccharides (LPS) into the systemic circulation, which contributes to the chronic, low-grade inflammation associated with obesity.23, 24 It is well-established that LPS (component of the outer membrane of Gram-negative bacteria)25 and saturated fatty acids (Western diet)26 are ligands for TLR4 and can, therefore, activate the innate immune system. Upon activation of TLR4 in several tissues (intestinal epithelial cells, adipose tissue, muscle, and liver), immune cells such as proinflammatory M1 macrophages are activated and secrete proinflammatory cytokines (i.e., TNF-α and Interleukin-6).27 Proinflammatory cytokines further recruit/attract additional proinflammatory immune cells while inhibiting anti-inflammatory cells such as M2 macrophages and/or regulatory T cells.27 Chronic immune system activation and excessive production of proinflammatory cytokines in the tissues interfere with insulin signaling as demonstrated by the inhibition of insulin-stimulated glucose uptake when insulin and TNF-α were coinjected into humans.28 When mice were fed a normal diet and infused subcutaneously with LPS for 4 weeks, increased weight (whole body, liver, and adipose tissue) and inflammation (i.e., TNF-α, Interleukin-1, Interleukin-6) were seen, and the phenotype was similar in many respects to 4 weeks of high-fat feeding.29 While acute inflammation is necessary to start the healing process, there is now compelling evidence that chronic bacteria/diet-induced inflammation can contribute to obesity and the metabolic syndrome.
Although many of the initial studies linking the gut microbiota to obesity centered around adulthood, it is now recognized that long-term metabolic perturbations could already be initiated in early life if an obesogenic gut microbiota from mothers is transferred to the infant and/or is altered in the first years of life when microbial colonization is still in progress (e.g., from antibiotic exposure or formula feeding).5 When mothers were given antibiotics during pregnancy, newborns had higher birth weights30 and children were 84% more likely to be obese at 7 years of age.31 Similarly, several other studies, including 3 large cohorts involving 28,000 mother–child pairs,32 10,000 children,33 and 6114 boys and 5948 girls,34 all reported an increased risk of being overweight when children were exposed to antibiotics in the first 12 months of life. Mechanistically, treating mice with low doses of penicillin (LDP) increased adiposity through altered gut microbiota, increased short-chain fatty acid (SCFA) levels, and altered hepatic metabolism of lipids and cholesterol.35 Cox et al.36 demonstrated that LDP enhanced the effect of HFD-induced obesity and, even though the microbial communities recovered after termination of LDP, the metabolic phenotype persisted. Microarray gene expression analysis revealed that early life exposure to broad-spectrum amoxicillin-based antibiotic delayed the maturation process of the intestine in 10%–30% of genes, downregulated the genes involved in the immune system (antimicrobial products and antigen presentation), and consequently interfered with gut barrier function.37 The weight gain observed in this study and others after early life antibiotic treatment was more pronounced in males/boys34, 35, 36 and was a consequence of reduced abundance of metabolically protective bacteria, increased availability of microbiota-derived energy, and altered hepatic metabolic signaling and/or intestinal defenses.38
In addition to antibiotics, caesarean-section (C-section) also alters early microbiota development as it bypasses exposure to vaginal microbiota during labor and exposes the child to skin and environmental microbes instead. For example, 72% of newborns’ microbiota (vaginal delivery) matched species found in the stool of their mother, whereas only 41% of these species were detected in C-section newborns, as shown by Bäckhed et al.17 To assess the associations of a C-section with body mass from birth to adolescence, 10,219 children (of which 9.06% were delivered by a C-section) were investigated.39 By 6 weeks of age, children born by C-section had a greater weight-for-length z-score, a phenotype that persisted until 15 years of age.39 Similarly, in 7-year-old children, a 46% higher obesity risk was observed in children born by C-section when compared with children delivered vaginally.31 Unlike in human C-section studies where perinatal antibiotics are used during a C-section and confound the independent effects of birth mode, Martinez et al.40 performed a study in mice to investigate the impact of antibiotic-free C-section on early life microbiota and obesity risk. Mice born via C-section gained 33% more weight at 15 weeks of age and female mice showed an even stronger phenotype (70% higher weight gain), a finding also reported in 1 birth cohort in humans.41 In addition to increased fat and body mass, microbiota development was altered in C-section mice.40 Under-represented taxa in C-section animals included Bacteroides, Ruminococcaceae, Lachnospiraceae, and Clostridiales (associated with lean phenotypes in mice36), and overrepresented taxa included S24-7, Lactobacillus, and Erysipelotrichaceae.40
2.2. Gut microbiota composition in obesity
After more than a decade of research describing the link between gut microbiota and obesity, many important questions about the host–microbiota relationship remain.42 Initially, animal studies demonstrated that obesity is associated with a change in the relative abundance of the 2 dominant bacterial phyla with a reduction in the abundance of Bacteroidetes and a proportional increase in Firmicutes.43, 44 Similar gut microbiota changes have been seen in adults45 and children with obesity,46 but some studies did not support these findings,11, 47 including 2 meta-analyses.48, 49 Accordingly, phylum-level changes in individuals with obesity are less clear, mostly because of large interpersonal variation, insufficient sample sizes, and the different methods used for the sequencing and quantifying of the taxa.49
The most consistent finding in humans appears to be a higher abundance of Escherichia coli (E. coli) and Lactobacillus in individuals with obesity.50, 51 Interestingly, there are many pathogenic strains of E. coli (in addition to the majority of harmless E. coli), whereas certain strains of Lactobacillus are commonly used as probiotics owing to their health benefits.3 This seeming discrepancy was clarified in part by Drissi et al.,52 who review evidence that the effects of Lactobacillus are age dependent and strain specific. With more than 150 Lactobacillus species identified to date, this represents a diverse group of bacteria.52 Similarly, bifidobacteria are also well-known probiotics, and lower abundance has been shown in people with a higher body mass index (BMI)53, 54 and a negative correlation was observed between Bifidobacterium and visceral adiposity.53 Likewise, lower levels of Akkermansia have been observed in individuals with a high body mass index;55, 56 however, individuals with T2D from Asia showed an increased Akkermansia muciniphila abundance.57 The authors concluded that Akkermansia could have a beneficial role in metabolic profiles depending on the environment in the gut. Since Akkermansia is a mucin-degrading bacteria, it could make the intestinal barrier thinner, thereby allowing bacterial translocation and pathogenesis of T2D.57 In line with this finding, a study in rodents showed that dietary fiber deficiency allows the mucin-degrading bacteria such as Akkermansia muciniphila to grow, express mucin-degrading enzymes, and enhance disease susceptibility.58
Regardless of inconsistencies in the precise obesogenic microbiota composition, it is clear that obesity is associated with a lower diversity and richness of the gut microbiota, which might compromise microbial function and lead to disease.3 It has been suggested that obese microbiomes can utilize a more diverse set of energy sources, resulting in greater energy harvest.59 To better understand changes in metabolism in obesity, analysis of microbial metabolites such as SCFA and bile acids can provide further insight given their role in activating signals that control appetite.60
2.2.1. Bile acids
Primary bile acids are synthesized from cholesterol by the liver and secreted into the small intestine, where Gram-positive bacteria (mostly lactobacilli and Clostridium species) convert them into secondary bile acids that can act as signaling molecules.3, 61 Insulin sensitivity, energy expenditure, lipid accumulation, and glucose homeostasis have all been shown to be modified by secondary bile acids, which act in large part via binding to receptors such as farnesoid X receptor and the G protein-coupled bile acid receptor.62 For example, secondary bile acids can bind to ileal farnesoid X receptor receptors, which in turn stimulate production of fibroblast growth factor 19 that can cross the blood–brain barrier63 and suppress activity of hypothalamic agouti-related peptide/neuropeptide Y neurons to improve energy homeostasis and glucose metabolism.60
2.2.2. SCFAs
SCFAs are the end products of bacterial polysaccharide fermentation that can be used as an energy source by the host and can, therefore, influence body weight.3 The most prominent SCFAs are butyrate, propionate, and acetate; butyrate serves as the energy substrate for the colonocytes, and propionate and acetate act as substrates for gluconeogenesis and lipogenesis in the gut and liver.64 Higher levels of SCFAs are found in the feces of obese children and adults when compared to normal weight individuals.47, 65 These higher levels likely result from increased colonic energy harvest66 rather than from reduced intestinal absorption.66, 67 The higher fecal SCFA seen in obesity appear to be at odds with the known beneficial effects of SCFA acting as signaling molecules that improve insulin sensitivity, increase satiety, and reduce inflammation in the pancreas, muscle, and adipose tissue.3, 64 Many of these benefits occur via the G protein-coupled receptors, free fatty acid receptor 2 (FFAR 2) and FFAR 3.64 For example, SCFA stimulation of FFAR 2 receptors in the gut stimulates the release of the satiety hormone glucagon-like peptide-1, while in neutrophils it suppresses inflammation.64 Given the limitations of interpreting higher concentrations of SCFA in feces in isolation from overall turnover and metabolism,64 the balance of evidence to date favors a beneficial metabolic effect for SCFA, particularly when produced from the fermentation of dietary fiber.
3. Modulation of gut microbiota in obesity with diet (specifically prebiotics) and exercise
While our individual host genome does not change over time, many environmental and lifestyle factors can profoundly change our gut microbiome throughout our lives.68 One of the characteristics of the gut microbiota that make it an opportune target for new obesity treatments is the relative ease by which it can be manipulated with dietary agents. Interestingly, some gut microbes can remember past diets and exhibit a so-called hysteresis that reflects those prior diets.69 For example, when mice were put on a chow diet between 2 bouts of a high-fat lard-based diet, accelerated weight regain was seen after the second exposure to the Western diet.70 The authors were able to identify a gut microbiome signature that persisted after successful dieting in the obese mice and contributed to faster weight regain upon re-exposure to the HFD.70 Experiments in so-called “humanized mice” (GF mice colonized with human fecal samples) also provide similar evidence in that the dietary history of the human donor determines the response to the diet intervention in mice.71 This effect is transmittable across generations. When “humanized mice” were exposed to a low-fiber diet, reduced microbial diversity/function was seen and the effects were transmitted to future generations.72 Microbiota diversity loss was greater with each subsequent generation (4 in total) with an additional loss of microbial fiber-degrading capacity.72 Exposing the 4th generation of mice to a high-fiber diet could not correct the loss of diversity and function. Recapturing this function could only be achieved through the reintroduction of lost bacteria with a fecal microbiota transplant from control mice.72 After the fecal transplant and a switch to a high-fiber diet, 110 taxa were restored and the differences between the low-fiber and high-fiber diet groups were no longer detectable.72 These studies demonstrate the importance of a high-fiber diet to prevent the loss of microbial taxa and function seen with consumption of a low-fiber Western diet.73
3.1. Prebiotics
When Gibson and Roberfroid74 first defined prebiotics in 1995, only a few compounds fit the definition, including short- and long-chain β-fructans (fructo-oligosaccharides and inulin), galacto-oligosaccharides, and lactulose. The most recent definition of prebiotics is that they are a substrate that is selectively utilized by host microorganisms conferring a health benefit.75 Changes in the definition from its inception have enabled more compounds, such as resistant starches, pectin, arabinoxylan, whole grains, and noncarbohydrate compounds (polyphenols), to be considered as candidate or confirmed prebiotics.75, 76 Interestingly, not all dietary fibers can be classified as prebiotics since consumption of prebiotics must result in a health benefit for the host.76 For example, soluble dextrin fibers from corn failed to be classified as prebiotics even though microbial changes in the gut were detected along with a lower secretion of proinflammatory and immunoregulatory cytokines.77 Nevertheless, no improvement in histological colonic inflammation was seen.77 It might be that the dose administered was too low to improve health, since a dose-dependent effect of prebiotics on disease risk has been described, with higher doses displaying more health benefits.78
Intake of prebiotics has been associated with improvements in metabolic health that have included lower body weight and fat mass, improved glucose control, a reduction in inflammation, and an increase in health-promoting bacteria.75, 79 For example, in infants, breast milk is a rich source of human milk oligosaccharides (candidate prebiotics), which stimulate the growth of commensal bacteria (Bifidobacterium and Bacteroides spp.) and restrict the adhesion of pathogens such as E. coli, Campylobacter jejuni, and Helicobacter pylori.80 As early as 1935, a report from Massachusetts General Hospital convincingly showed benefits of breast-feeding.81 In an analysis of 20,000 patients, breast-fed infants had a lower incidence of mortality and morbidity, especially of enteric disease, otitis media, and respiratory infection, when compared to exclusively formula-fed infants.81 It is plausible that the microbiota, at least in part, is involved in these improved infant outcomes.
Several studies have reported a correlation between a low abundance of Bifidobacterium spp. and obesity,82, 83 along with an increased capacity of obesogenic gut microbiota to produce SCFAs47, 67, 84; however, both studies were modified with a prebiotic approach. One study showed that a 3-month supplementation with oligofructose-enriched inulin (16 g/day) increased the abundance of health-promoting Bifidobacterium spp. and decreased total fecal SCFA concentration in 44 women with obesity; however, no significant reduction in BMI was observed.85 Oligofructose-enriched inulin provides a blend of long-chain (inulin) and short-chain (oligofructose) fructans that ferment at different rates in the colon; oligofructose ferments more rapidly. Intervention studies that exposed normal-weight, healthy adolescents to oligofructose-enriched inulin (8 g/day) for 1 year86 and adults with overweight or obesity to oligofructose (21 g/day) for 3 months87 reported decreased body weight gain and fat mass. The study in adults also reported a decrease in energy intake and an increase in satiety hormones, thus showing additional positive effects of prebiotics relevant to obesity management. Similarly, a sample of children (7–12 years of age) who were administered 8 g/day of oligofructose-enriched inulin for 16 weeks had reduced body fat88 and improved appetite control89 compared to children given a placebo. Prebiotic consumption normalized childhood weight gain, reduced total and trunk body fat, altered primary fecal bile acids, and changed microbiota composition by increasing Bifidobacterium species.88 Mechanistically, several animal studies have provided insight into prebiotic-mediated outcomes noted in human studies. In rodents, prebiotic intake led to the following positive outcomes: increased number/activity of enteroendocrine L-cells responsible for the production of satiety hormones and improved glucose homeostasis,90, 91 recovery of gut barrier function through increased Bifidobacterium spp.,91, 92 and expression/activity of tight junction proteins with a subsequent decrease in circulatory LPS levels,92, 93 reduced hepatic accumulation of triglycerides and cholesterol,94, 95 and improved weight maintenance and weight loss.90, 91 Thus, the positive effects of prebiotic use likely go beyond weight loss, since all of the benefits described also contribute to the improvement of overall host health.
3.2. Exercise
Diet and exercise are often prescribed together, and in combination form the cornerstone for lifestyle modifications aimed at maintaining health and managing chronic diseases. Given the profound role that diet plays in shaping the gut microbiota,96 the question soon emerged whether or not exercise also influenced gut microbiota composition. One of the first indications that exercise might influence gut microbiota composition came from Clarke et al.97 in 2014, when they showed that, compared to more sedentary control subjects, professional rugby athletes had a higher diversity of gut microorganisms, a characteristic often associated with a “healthy” gut microbiota. More recently, this same group has shown that the differences observed between the elite athletes and more sedentary controls at the microbial composition level are even greater when considered at the functional and metabolic levels (using metagenomics to examine the microbial genes and metabolomics to examine metabolites).98 Importantly, the elite athletes had higher levels of SCFAs than the controls, which can influence such important actions as intestinal barrier integrity, brain function, and immunity.98 This finding is consistent the findings of Estaki et al.,99 who also observed increased butyrate-producing bacteria and higher microbiota diversity in healthy participants with higher cardiorespiratory fitness (measured with peak oxygen uptake) compared with those who were less fit. Although our understanding of the mechanisms by which exercise and the gut microbiota interact to provide health benefits is still in its infancy, the mechanisms may include the aforementioned SCFAs production, microbiota-mediated changes in immune function, and enhanced gut barrier function.100
Some of the first animal work to examine the effect of exercise on gut microbiota used a 6-week, low-intensity treadmill running protocol in normal and diabetic (db/db) mice, with running occurring 5 days/week.101 After adjusting for the mice's body weight and blood glucose, the exercise protocol reduced Bacteroides/Prevotella spp. and Methanobrevibacter spp. and increased Clostridium cluster I in all animals; however, the abundance of the health-promoting Bifidobacterium increased only in nondiabetic mice, suggesting that exercise may not exert the same effects on gut microbiota in a healthy host versus a host with diabetes.101 In another study, nondiabetic rats also showed an increase in the health-promoting Bifidobacterium following exercise.102 In contrast to these studies, Evans et al.103 saw a reduction in Bifidobacteriaceae in mice that were fed a low-fat diet and used a non load-bearing hamster wheel for 12 weeks. The discrepancy between the study by Evans et al.103 and other work requires further investigation, but it is possible that in metabolically challenged states (diabetes/HFD), a typical increase in Bifidobacterium with exercise is overridden by the disease or precipitating diet, given that an HFD is known to have a suppressive effect on Bifidobacterium.92 Interestingly, exercise was able to alter the gut microbial composition and lean mass to a greater extent in juvenile versus adult rats, highlighting the importance of also considering the developmental stage and vulnerability/instability of early-life microbiota in future investigations.104
4. Potential for gut microbiota in musculoskeletal disorders
OA is a highly prevalent, debilitating joint disorder commonly associated with obesity.105 The risk imposed by obesity is not just due to the mechanical burden on joints, but also due to the metabolic and inflammatory derangements associated with obesity.106 Given that microbial dysbiosis is associated with obesity, there is growing interest in determining if modifying the gut microbiota signature could in turn improve pain and physical function in patients with OA and obesity. Indications that this might be possible come from 2 large-cohort studies investigating knee OA in the United States.107, 108 Using data from the Osteoarthritis Initiative and the Framingham Offspring Osteoarthritis Study, Dai et al.107 found that total dietary fiber was inversely associated with the risk of symptomatic knee OA. Using data from only the Osteoarthritis Initiative, Dai et al.108 also showed that a higher intake of total dietary fiber or cereal grain fiber (e.g., whole-grain wheat and bran cereals) was inversely associated with the likelihood of developing moderate to severe knee pain over an 8-year time course. Dietary fiber is one of the most important fuels for the gut microbiota.109 Therefore, although no randomized clinical trials examining the effect of microbiota-altering diets (e.g., high fiber or high prebiotic) on knee OA have been published to date, there is good reason to initiate these trials in the near future. Additional support for such trials also comes from very promising studies in rodents.
In mice, the prebiotic oligofructose was protective against the detrimental effect of obesity induced by an HFD on trauma-induced OA.110 Importantly, obesity markedly reduced beneficial Bifidobacterium microbes that coincided with increased macrophage presence in the knee capsule and accelerated joint degeneration, including cartilage loss.110 Improving the composition of gut microbiota with dietary oligofructose was, in fact, able to completely rescue these obesity-associated detriments. The joint damage seen in mice is consistent with the effects of a high-fat or high-sucrose diet on knee and shoulder joints in rats.111 Somewhat surprisingly, the derangements associated with a high-fat or high-sucrose diet appear to very rapidly (in as few as 3 days) alter muscle integrity, inflammation, and the gut microbiota in rats.112 Early changes in muscle integrity due to obesity or poor diet, including muscle loss, intramuscular lipid accumulation, or deposition of connective tissue, may precipitate further downstream damage to tendons, bone, cartilage, and joints.113 For a full review of the role of inflammation and muscle integrity on musculoskeletal-related conditions (e.g., osteoporosis, OA, tendinopathy), see Collins et al.113 Important for designing future translational studies in humans is our recent demonstration that prebiotic oligofructose supplementation, aerobic exercise, and the combination of the 2 completely prevent knee damages associated with obesity induced through high-fat or high-sucrose diets in rats.114 Normalization of insulin resistance, dyslipidemia and endotoxemia (LPS) accompanied the protection of the knee joint.114
5. Conclusion
The environment determines bacterial growth; therefore, it is not surprising that external factors such as diet and physical activity drive our gut microbial composition and function. Diet has the potential to outweigh the effect of host genetics, immunity, and early-life disruptors (antibiotics and C-section). Unfortunately, a Western diet, with an abundance of highly processed foods that are low in fiber and rich in fat and sugar, is a major threat to our gut microbial community. This threat may not be strictly confined to the generation that consumes it, but could perpetuate dysbiosis across multiple generations. The hope of researchers in the field is that we will be able to identify personalized effective dietary strategies, such as prebiotics and other targeted interventions, that will positively modify the gut microbiota from early life onwards and ultimately reduce the burden of obesity worldwide.
Acknowledgments
Acknowledgments
This work was supported by a research grant from the Canadian Institutes of Health Research (PJT-159626). Teja Klancic is supported by a Vanier Canada Graduate Scholarship, Alberta Innovates Health Solutions Doctoral Scholarship and Eye's High Doctoral Scholarship.
Author contributions
Both authors contributed to the writing and editing of the manuscript. Both authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
Raylene A. Reimer reports that she received honoraria from Beneo GmbH for work related to the subject of this article (i.e., prebiotics). Teja Klancic has not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Appendix. Supplementary materials
References
- 1.World Health Organization. Obesity and overweight. Available at:http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [accessed 17.07.2018]
- 2.Everard A., Cani P.D. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol. 2013;27:73–83. doi: 10.1016/j.bpg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Moran-Ramos S., López-Contreras B.E., Canizales-Quinteros S. Gut microbiota in obesity and metabolic abnormalities: a matter of composition or functionality? Arch Med Res. 2017;48:735–753. doi: 10.1016/j.arcmed.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez M., Panahi S., Tremblay A. Childhood obesity: a role for gut microbiota? Int J Environ Res Public Health. 2014;12:162–175. doi: 10.3390/ijerph120100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox L.M., Blaser M.J. Pathways in microbe-induced obesity. Cell Metab. 2013;17:883–894. doi: 10.1016/j.cmet.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vos W.M. Microbial biofilms and the human intestinal microbiome. NPJ Biofilms Microbiomes. 2015;1:15005. doi: 10.1038/npjbiofilms.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dave M., Higgins P.D., Middha S., Rioux K.P. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res J Lab Clin Med. 2012;160:246–257. doi: 10.1016/j.trsl.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Blaser M.J., Dominguez-Bello M.G. The Human Microbiome before Birth. Cell Host Microbe. 2016;20:558–560. doi: 10.1016/j.chom.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Hooper L.V., Midtvedt T., Gordon J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 10.Tap J., Mondot S., Levenez F., Pelletier E., Caron C., Furet J.P. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11:2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 11.Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everard A., Lazarevic V., Derrien M., Girard M., Muccioli G.M., Neyrinck A.M. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60:2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willing B.P., Dicksved J., Halfvarson J., Andersson A.F., Lucio M., Zheng Z. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–1854. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Matamoros S., Gras-Leguen C., Le Vacon F., Potel G., de La Cochetiere M.F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013;21:167–173. doi: 10.1016/j.tim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeney K.M., Yurist-Doutsch S., Arrieta M.C., Finlay B.B. Effects of antibiotics on human microbiota and subsequent disease. Annu Rev Microbiol. 2014;68:217–235. doi: 10.1146/annurev-micro-091313-103456. [DOI] [PubMed] [Google Scholar]
- 17.Bäckhed F., Roswall J., Peng Y., Feng Q., Jia H., Kovatcheva-Datchary P. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Munyaka P.M., Khafipour E., Ghia J.E. External influence of early childhood establishment of gut microbiota and subsequent health implications. Front Pediatr. 2014;2:1–9. doi: 10.3389/fped.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carding S., Verbeke K., Vipond D.T., Corfe B.M., Owen L.J. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding S., Chi M.M., Scull B.P., Rigby R., Schwerbrock N.M., Magness S. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karin M., Lin A. NF-κB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 23.de La Serre C.B., Ellis C.L., Lee J., Hartman A.L., Rutledge J.C., Raybould H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. AJP Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cani P.D., Osto M., Geurts L., Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–288. doi: 10.4161/gmic.19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 26.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid–induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bleau C., Karelis A.D., St-Pierre D.H., Lamontagne L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab Res Rev. 2015;31:545–561. doi: 10.1002/dmrr.2617. [DOI] [PubMed] [Google Scholar]
- 28.Rask-Madsen C., Domínguez H., Ihlemann N., Hermann T., Køber L., Torp-Pedersen C. Tumor necrosis factor-alpha inhibits insulin's stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circulation. 2003;108:1815–1821. doi: 10.1161/01.CIR.0000091406.72832.11. [DOI] [PubMed] [Google Scholar]
- 29.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 30.Jepsen P., Skriver M.V., Floyd A., Lipworth L., Schønheyder H.C., Sørensen H.T. A population-based study of maternal use of amoxicillin and pregnancy outcome in Denmark. Br J Clin Pharmacol. 2003;55:216–221. doi: 10.1046/j.1365-2125.2003.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller N.T., Whyatt R., Hoepner L., Oberfield S., Dominguez-Bello M.G., Widen E.M. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes. 2015;39:665–670. doi: 10.1038/ijo.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ajslev T.A., Andersen C.S., Gamborg M., Sørensen T.I., Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes. 2011;35:522–529. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 33.Trasande L., Blustein J., Liu M., Corwin E., Cox L.M., Blaser M.J. Infant antibiotic exposures and early-life body mass. Int J Obes. 2013;37:16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saari A., Virta L.J., Sankilampi U., Dunkel L., Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135:617–626. doi: 10.1542/peds.2014-3407. [DOI] [PubMed] [Google Scholar]
- 35.Cho I., Yamanishi S., Cox L., Methé B.A., Zavadil J., Li K. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox L.M., Yamanishi S., Sohn J., Alekseyenko A.V., Leung J.M., Cho I. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumann A., Nutten S., Donnicola D., Comelli E.M., Mansourian R., Cherbut C. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol Genomics. 2005;23:235–245. doi: 10.1152/physiolgenomics.00057.2005. [DOI] [PubMed] [Google Scholar]
- 38.Cox L.M., Blaser M.J. Antibiotics in early life and obesity. Nat Rev Endocrinol. 2015;11:182–190. doi: 10.1038/nrendo.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blustein J., Attina T., Liu M., Ryan A.M., Cox L.M., Blaser M.J. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes (Lond) 2013;37:900–906. doi: 10.1038/ijo.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez K.A., 2nd, Devlin J.C., Lacher C.R., Yin Y., Cai Y., Wang J. Increased weight gain by C-section: functional significance of the primordial microbiome. Sci Adv. 2017;3:eaao1874. doi: 10.1126/sciadv.aao1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barros A.J., Santos L.P., Wehrmeister F., Motta J.V, Matijasevich A., Santos I.S. Caesarean section and adiposity at 6, 18 and 30 years of age: results from three Pelotas (Brazil) birth cohorts. BMC Public Health. 2017;17:256. doi: 10.1186/s12889-017-4165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turnbaugh P.J. Microbes and diet-induced obesity: fast, cheap, and out of control. Cell Host Microbe. 2017;21:278–281. doi: 10.1016/j.chom.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turnbaugh P.J., Backhed F., Fulton L., Gordon J.I. Marked alterations in the distal gut microbiome linked to diet-induced obesity. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parnell J.A., Reimer R.A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr. 2012;107:601–613. doi: 10.1017/S0007114511003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 46.Bervoets L., Van Hoorenbeeck K., Kortleven I., Van Noten C., Hens N., Vael C. Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog. 2013;5:10. doi: 10.1186/1757-4749-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwiertz A., Taras D., Schäfer K., Beijer S., Bos N.A., Donus C. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 48.Walters W.A., Xu Z., Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223–4233. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome. MBio 2019;7: pii: e01018-16. doi:10.1128/mBio.01018-16 [DOI] [PMC free article] [PubMed]
- 50.Million M., Angelakis E., Maraninchi M., Henry M., Giorgi R., Valero R. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes (Lond) 2013;37:1460–1466. doi: 10.1038/ijo.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Armougom F., Henry M., Vialettes B., Raccah D., Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in lactobacillus in obese patients and methanogens in anorexic patients. PLoS One. 2009;4:e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drissi F., Raoult D., Merhej V. Metabolic role of lactobacilli in weight modification in humans and animals. Microb Pathog. 2017;106:182–194. doi: 10.1016/j.micpath.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Pallister T., Jackson M.A., Martin T.C., Glastonbury C.A., Jennings A., Beaumont M. Untangling the relationship between diet and visceral fat mass through blood metabolomics and gut microbiome profiling. Int J Obes. 2017;41:1106–1113. doi: 10.1038/ijo.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakayama J., Watanabe K., Jiang J., Matsuda K., Chao S.H., Haryono P. Diversity in gut bacterial community of school-age children in Asia. Sci Rep. 2015;5:8397. doi: 10.1038/srep08397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falony G., Joossens M., Vieira-Silva S., Wang J., Darzi Y., Faust K. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 56.Fu J., Bonder M.J., Cenit M.C., Tigchelaar E.F., Maatman A., Dekens J.A.M. The gut microbiome contributes to a substantial proportion of the variation in blood lipids: novelty and significance. Circ Res. 2015;117:817–824. doi: 10.1161/CIRCRESAHA.115.306807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 58.Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenblum S., Turnbaugh P.J., Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc Natl Acad Sci U S A. 2012;109:594–599. doi: 10.1073/pnas.1116053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Del Chierico F., Abbatini F., Russo A., Quagliariello A., Reddel S., Capoccia D. Gut microbiota markers in obese adolescent and adult patients: age-dependent differential patterns. Front Microbiol. 2018;9:1210. doi: 10.3389/fmicb.2018.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joyce S.A., Gahan C.G. Disease-associated changes in bile acid profiles and links to altered gut microbiota. Dig Dis. 2017;35:169–177. doi: 10.1159/000450907. [DOI] [PubMed] [Google Scholar]
- 63.Hsuchou H., Pan W., Kastin A.J. Fibroblast growth factor 19 entry into brain. Fluids Barriers CNS. 2013;10:32. doi: 10.1186/2045-8118-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nehra V., Allen J.M., Mailing L.J., Kashyap P.C., Woods J.A. Gut microbiota: modulation of host physiology in obesity. Physiology. 2016;31:327–335. doi: 10.1152/physiol.00005.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernandes J., Su W., Rahat-Rozenbloom S., Wolever T.M., Comelli E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahat-Rozenbloom S., Fernandes J., Gloor G.B., Wolever T.M. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes (Lond) 2014;38:1525–1531. doi: 10.1038/ijo.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 68.Velasquez-Manoff M. Gut microbiome: the peacekeepers. Nature. 2015;518(Suppl. 1):S3–11. doi: 10.1038/518S3a. [DOI] [PubMed] [Google Scholar]
- 69.Carmody R.N., Gerber G.K., Luevano J.M., Gatti D.M., Somes L., Svenson K.L. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17:72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thaiss C.A., Itav S., Rothschild D., Meijer M.T., Levy M., Moresi C. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 2016;540:544–551. doi: 10.1038/nature20796. [DOI] [PubMed] [Google Scholar]
- 71.Griffin N.W., Ahern P.P., Cheng J., Heath A.C., Ilkayeva O., Newgard C.B. Prior dietary practices and connections to a human gut microbial metacommunity alter responses to diet interventions. Cell Host Microbe. 2017;21:84–96. doi: 10.1016/j.chom.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sonnenburg E.D., Smits S.A., Tikhonov M., Higginbottom S.K., Wingreen N.S., Sonnenburg J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bindels L.B., Delzenne N.M., Cani P.D., Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12:303–310. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- 74.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 75.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 76.Valcheva R., Dieleman L.A. Prebiotics: definition and protective mechanisms. Best Pract Res Clin Gastroenterol. 2016;30:27–37. doi: 10.1016/j.bpg.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 77.Valcheva R., Hotte N., Gillevet P., Sikaroodi M., Thiessen A., Madsen K.L. Soluble dextrin fibers alter the intestinal microbiota and reduce proinflammatory cytokine secretion in male IL-10–deficient mice. J Nutr. 2015;145:2060–2066. doi: 10.3945/jn.114.207738. [DOI] [PubMed] [Google Scholar]
- 78.Valcheva R., Koleva P., Meijer B.J., Walter J., Gänzle M., Dieleman L.A. 1091a Beta-fructans reduce inflammation in mild to moderate ulcerative colitis through specific microbiota changes associated with improved butyrate formation and MUC2 expression. Gastroenterology. 2012;142(Suppl. 1):S-196. [Google Scholar]
- 79.Delzenne N.M., Neyrinck A.M., Cani P.D. Gut microbiota and metabolic disorders: how prebiotic can work? Br J Nutr. 2013;109(Suppl. 2):S81–S85. doi: 10.1017/S0007114512004047. [DOI] [PubMed] [Google Scholar]
- 80.Newburg D.S. Oligosaccharides in human milk and bacterial colonization. J Pediatr Gastroenterol Nutr. 2000;30(Suppl. 2):S8–17. [PubMed] [Google Scholar]
- 81.Grulee C.G., Sanford H.N., Schwartz H. Breast and artificially fed infants: a study of the age incidence in the morbidity and mortality in twenty thousand cases. JAMA. 1935;104:1986–1988. [Google Scholar]
- 82.Collado M.C., Isolauri E., Laitinen K., Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894–899. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 83.Kalliomäki M., Collado M.C., Salminen S., Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 84.Ley R.E., Bäckhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salazar N., Dewulf E.M., Neyrinck A.M., Bindels L.B., Cani P.D., Mahillon J. Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women. Clin Nutr. 2014;34:501–507. doi: 10.1016/j.clnu.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 86.Abrams S.A., Griffin I.J., Hawthorne K.M., Ellis K.J. Effect of prebiotic supplementation and calcium intake on body mass index. J Pediatr. 2007;151:293–298. doi: 10.1016/j.jpeds.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 87.Parnell J.A., Reimer R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89:1751–1759. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nicolucci A.C., Hume M.P., Martínez I., Mayengbam S., Walter J., Reimer R.A. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017;153:711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 89.Hume M.P., Nicolucci A.C., Reimer R.A. Prebiotic supplementation improves appetite control in children with overweight and obesity: a randomized controlled trial. Am J Clin Nutr. 2017;105:790–799. doi: 10.3945/ajcn.116.140947. [DOI] [PubMed] [Google Scholar]
- 90.Reimer R.A., Maurer A.D., Eller L.K., Hallam M.C., Shaykhutdinov R., Vogel H.J. Satiety hormone and metabolomic response to an intermittent high energy diet differs in rats consuming long-term diets high in protein or prebiotic fiber. J Proteome Res. 2012;11:4065–4074. doi: 10.1021/pr300487s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hallam M.C., Reimer R.A. Postnatal prebiotic fiber intake in offspring exposed to gestational protein restriction has sex-specific effects on insulin resistance and intestinal permeability in rats. J Nutr. 2014;144:1556–1563. doi: 10.3945/jn.114.194142. [DOI] [PubMed] [Google Scholar]
- 92.Cani P.D., Neyrinck A.M., Fava F., Knauf C., Burcelin R.G., Tuohy K.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 93.Neyrinck A.M., Van Hée V.F., Piront N., De Backer F., Toussaint O., Cani P.D. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes. 2012;2:e28. doi: 10.1038/nutd.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parnell J.A., Reimer R.A. Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: a dose–response study in JCR:LA-cp rats. Br J Nutr. 2010;103:1577–1584. doi: 10.1017/S0007114509993539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parnell J.A., Raman M., Rioux K.P., Reimer R.A. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012;32:701–711. doi: 10.1111/j.1478-3231.2011.02730.x. [DOI] [PubMed] [Google Scholar]
- 96.Reimer R.A. Establishing the role of diet in the microbiota–disease axis. Nat Rev Gastroenterol Hepatol. 2019;16:86–87. doi: 10.1038/s41575-018-0093-7. [DOI] [PubMed] [Google Scholar]
- 97.Clarke S.F., Murphy E.F., O'Sullivan O., Lucey A.J., Humphreys M., Hogan A. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 98.Barton W., Penney N.C., Cronin O., Garcia-Perez I., Molloy M.G., Holmes E. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67:625–633. doi: 10.1136/gutjnl-2016-313627. [DOI] [PubMed] [Google Scholar]
- 99.Estaki M., Pither J., Baumeister P., Little J.P., Gill S.K., Ghosh S. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4:42. doi: 10.1186/s40168-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Monda V., Villano I., Messina A., Valenzano A., Esposito T., Moscatelli F. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lambert J.E., Myslicki J.P., Bomhof M.R., Belke D.D., Shearer J., Reimer R.A. Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiol Nutr Metab. 2015;40:749–752. doi: 10.1139/apnm-2014-0452. [DOI] [PubMed] [Google Scholar]
- 102.Queipo-Ortuño M.I., Seoane L.M., Murri M., Pardo M., Gomez-Zumaquero J.M., Cardona F. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8:e65465. doi: 10.1371/journal.pone.0065465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evans C.C., LePard K.J., Kwak J.W., Stancukas M.C., Laskowski S., Dougherty J. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 2014;9:e92193. doi: 10.1371/journal.pone.0092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mika A., Treuren W.V., González A., Herrera J.J., Knight R., Fleshner M. Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile versus adult male F344 rats. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhuo Q., Yang W., Chen J., Wang Y. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol. 2012;8:729–737. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 106.Felson D.T., Lawrence R.C., Dieppe P.A., Hirsch R., Helmick C.G., Jordan J.M. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 107.Dai Z., Niu J., Zhang Y., Jacques P., Felson D.T. Dietary intake of fibre and risk of knee osteoarthritis in two US prospective cohorts. Ann Rheum Dis. 2017;76:1411–1419. doi: 10.1136/annrheumdis-2016-210810. [DOI] [PubMed] [Google Scholar]
- 108.Dai Z., Lu N., Niu J., Felson D.T., Zhang Y. Dietary fiber intake in relation to knee pain trajectory. Arthritis Care Res (Hoboken) 2017;69:1331–1339. doi: 10.1002/acr.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han M., Wang C., Liu P., Li D., Li Y., Ma X. Dietary fiber gap and host gut microbiota. Protein Pept Lett. 2017;24:388–396. doi: 10.2174/0929866524666170220113312. [DOI] [PubMed] [Google Scholar]
- 110.Schott E.M., Farnsworth C.W., Grier A., Lillis J.A., Soniwala S., Dadourian G.H. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight. 2018;3:95997. doi: 10.1172/jci.insight.95997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Collins K.H., Hart D.A., Seerattan R.A., Reimer R.A., Herzog W. High-fat/high-sucrose diet-induced obesity results in joint-specific development of osteoarthritis-like degeneration in a rat model. Bone Jt Res. 2018;7:274–281. doi: 10.1302/2046-3758.74.BJR-2017-0201.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Collins K.H., Paul H.A., Hart D.A., Reimer R.A., Smith I.C., Rios J.L. A high-fat high-sucrose diet rapidly alters muscle integrity, inflammation and gut microbiota in male rats. Sci Rep. 2016;6:37278. doi: 10.1038/srep37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Collins K.H., Herzog W., MacDonald G.Z., Reimer R.A., Rios J.L., Smith I.C. Obesity, metabolic syndrome, and musculoskeletal disease: common inflammatory pathways suggest a central role for loss of muscle integrity. Front Physiol. 2018;9:112. doi: 10.3389/fphys.2018.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rios J.L., Bomhof MR, Reimer R.A., Hart D.A., Collins K.H., Herzog W. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep. 2019;9:3893. doi: 10.1038/s41598-019-40601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.