Abstract
Background
The non-saponin fraction of Korean Red Ginseng has been reported to have many biological activities. However, the effect of this fraction on anti-diabetic activity has not been elucidated in detail. In this study, we investigated the effects of KGC05P0, a non-saponin fraction of Korean Red Ginseng, on anti-diabetic activity in vitro and in vivo.
Methods
We measured the inhibition of commercially obtained α-glucosidase and α-amylase activities in vitro and measured the glucose uptake and transport rate in Caco-2 cells. C57BL/6J mice and C57BLKS/Jdb/db (diabetic) mice were fed diets with or without KGC05P0 for eight weeks. To perform the experiments, the groups were divided as follows: normal control (C57BL/6J mice), db/db control (C57BLKS/Jdb/db mice), positive control (inulin 400 mg/kg b.w.), low (KGC05P0 100 mg/kg b.w.), medium (KGC05P0 200 mg/kg b.w.), and high (KGC05P0 400 mg/kg b.w.).
Results
KGC05P0 inhibited α-glucosidase and α-amylase activities in vitro, and decreased glucose uptake and transport rate in Caco-2 cells. In addition, KGC05P0 regulated fasting glucose level, glucose tolerance, insulin, HbA1c, carbonyl contents, and proinflammatory cytokines in blood from diabetic mice and significantly reduced urinary glucose excretion levels. Moreover, we found that KGC05P0 regulated glucose production by down-regulation of the PI3K/AKT pathway, which inhibited gluconeogenesis.
Conclusion
Our study thereby demonstrated that KGC05P0 exerted anti-diabetic effects through inhibition of glucose absorption and the PI3K/AKT pathway in in vitro and in vivo models of diabetes. Our results suggest that KGC05P0 could be developed as a complementary food to help prevent T2DM and its complications.
Keywords: Diabetes mellitus, Korean Red Ginseng, Non-saponin fraction, Glucose regulation
1. Introduction
Diabetes mellitus (DM) is the third most common chronic disease in the world and causes serious complications such as nephropathy, neuropathy, and retinopathy [1,2]. This disease is classified into type Ⅰ DM, known as childhood diabetes or insulin-dependent type, and type Ⅱ DM, known as adult diabetes or insulin-dependent type [3]. A number of patients on most diabetes medications have difficulty in maintaining long-term glycemic control and experience adverse effects. Therefore, the development of natural product-derived substances with insulin-like activity is a promising approach.
One of the characteristics of DM is the change in liver metabolism. The liver plays a very important role in the regulation of systemic metabolism of energy nutrients. When the target tissue for insulin is damaged, insulin resistance occurs because the response function is lowered, and the liver can then no longer control glucose homeostasis [4]. Most of the body's production of glucose occurs in the liver and kidneys, and the liver acutely responds to insulin by reducing the production of glucose [5]. In the fed state, insulin binds to its receptor (IR), which then begins phosphorylation of its substrates. This in turn causes the activation of phosphatidylinositol-3-kinase (PI3K) and leads to the phosphorylation of PI3K. This pathway activates protein kinase B (PKB/AKT) and causes the phosphorylation of AKT, which leads to the suppression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), which are known as hepatic gluconeogenic genes [[6], [7], [8]].
The db/db mouse model is the most widely used model for type Ⅱ DM studies and is characterized by leptin deficiency. Deficiency of leptin secretion in the hypothalamus leads to persistent excess appetite and obesity, resulting in hyperglycemia and abnormalities of renal function [9,10].
Korean Red Ginseng (RG, Panax ginseng Meyer) is an herb that has been traditionally used for thousands of years in Asian countries [1]. RG is produced by repeatedly heating and drying the roots of ginseng. It has strong pharmacological effects in several diseases, such as diabetes, cancer, and inflammatory diseases [[11], [12], [13]]. In particular, saponin, which is known to be an active ingredient of red ginseng, showed benefits in studies of diabetes, apoptosis, cardiac damage, and obesity [[14], [15], [16], [17]]. However, there have been few evaluations of the pharmacological activity in diabetes of the non-saponin fraction of Korean Red Ginseng.
In this study, we first investigated whether the non-saponin fraction of Korean Red Ginseng (KGC05P0) inhibits α-glucosidase and α-amylase activities in vitro. Second, we assessed the effects of KGC05P0 on glucose uptake and transport rate in Caco-2 cells, and third, we assessed its effects on glycemic control through down-regulation of the PI3K/AKT pathway in diabetic db/db mice.
2. Materials and methods
2.1. Preparation of the non-saponin fraction of Korean Red Ginseng
The non-saponin fraction of Korean Red Ginseng (KGC05P0) was obtained from Korea Ginseng Corporation Research Institute (Daejeon, Korea). This is a powder sample in which only saponin was removed from red ginseng concentrate through non-saponin fractionation procedure. This procedure was carried out after filling Diaion (HP-20, Mitsubishi Chemical Industries, Ltd.) into the fractionation apparatus, adding 80% alcohol, and washing it with purified water until no alcohol remained. The concentrate was diluted to 10% in purified water and then filtered, and water fraction and 30% ethanol fraction were respectively performed. The two fractions were combined, concentrated, and spray dried to complete the sample preparation. KGC05P0 was sealed to protect from air and light and stored at −20°C until use.
2.2. High-performance liquid chromatography (HPLC) analysis
KGC05P0 (1.0 g) was weighted in a 50 mL volumetric flask, and 50 mL of deionized water was added and extracted by an ultrasonic method at room temperature for 30 min. Ethanol (4 mL) was added to the extract solution (1 mL), and the precipitate was collected and diluted with water (10 mL). The solution (0.5 mL) was condensed under air at 60°C. 2M TFA (trifluoroacetic acid, 1 mL) was added to hydrolyze, reacted in an oven at 121°C for 90 min, and then condensed under air at 60°C and PMP derivatization was performed by adding 0.3M NaOH (100 uL) and 0.5M PMP (1-phenyl-3-methyl-5-pyrazolone, 120 uL) for 1 h at 70°C in an oven. After neutralizing with 0.3M HCl (100 uL), the mixture was shaken by adding water (1 mL) and chloroform (1 mL). Then, the solution was injected into the HPLC system after getting the filtered water layer. HPLC analysis was performed with a Waters alliance performance liquid chromatography instrument (Waters, USA) equipped with a photo diode array detector (Waters, USA). A Discovery C18 column (4.6 mm × 250 mm, 5 μm particles) was used for separation. The column temperature was 35°C, the flow rate was 1.0 mL/min, and the injection volume was 10 uL. The mobile phase consisted of 0.01M phosphate buffer (pH 6.7) (A) and acetonitrile (B). HPLC gradient conditions were as follows: 0–35 min (15% B), 35–45 min (15–20% B), 45–55 min (20% B), 55–56 min (20–15% B), and 60 min (15% B). The detection wavelength was set at 250 nm.
2.3. Measurement of α-glucosidase and α-amylase inhibitory activities
α-Glucosidase and α-amylase activity colorimetric assay kits were purchased from Biovision (Milpitas Blvd., Milpitas, CA, USA). α-Glucosidase, α-amylase, and acarbose were purchased from Sigma-Aldrich (St. Louis, MO, USA). KGC05P0 (0–2000 μg/mL), inulin (0–2000 μg/mL), and acarbose (1000 μg/mL) were added to 96-well plates. For the α-glucosidase inhibitory activity assay, 10 μL of α-glucosidase (1.0 U/mL) was added to each well, and assay buffer was added for a total volume of 50 μL/well. A volume of 50 μL of reaction mix containing 3 μL of α-glucosidase substrate mix was added to each well, and the absorbance was read with an iMARK™ Microplate Reader (Bio-Rad Laboratories, Hercules, CA, USA) at 410 nm. For the α-amylase inhibitory activity assay, a volume of 5 μL of α-amylase (3 U/mL) was added to each well, and the volume was adjusted to 50 μL/well with sterile distilled water. A volume of 100 μL of reaction mix containing 50 μL of substrate mix was added to each well, and absorbance readings were recorded at 405 nm. The experiments were conducted according to Bio-Rad's protocol.
2.4. Caco-2 cell culture and glucose transport measurement
The human colon adenocarcinoma cell line (Caco-2) was purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in a 95% air/5% CO2 atmosphere at 37°C. Cells were grown in DMEM, supplemented with 20% FBS, 1% P/S, 1% sodium pyruvate, and 1% NEAA mixture. The glucose uptake colorimetric assay kit used in the experiment was purchased from Biovision. Cells were seeded into a transwell plate (Corning® Transwell®, 24 mm diameter, pore size 0.4 μm) at a density of 1 × 104 cells/cm2 and cultured for 21 days. The electrical resistance of the cell monolayers was measured to confirm that cell differentiation had occurred, and the medium was replaced with serum-free medium 24 h before the permeability study. For the experiment, the medium was discarded, 2 mL of Krebs-Ringer-Phosphate-HEPES (KRPH) buffer (Biosolution Co., Seoul, Korea) containing 2% BSA was added to each well, and the plate was incubated for 40 min at 37°C. After washing, KGC05P0 (0–500 μg/mL) samples were added to the plate, which was then incubated for 3 h at 37°C. A volume of 10 μL of 10mM 2-DG was added to each well, and the plate was incubated for 20 min. After the washing, NADPH generation, NADP degradation, and recycling amplification reaction steps, the absorbance was read with an iMARK™ Microplate Reader (Bio-Rad) at 412 nm. The experiment was conducted according to Bio-Rad's protocol.
2.5. HepG2 cell culture and treatments
The human hepatocellular carcinoma cell (HepG2) was purchased from ATCC and cultured in a 95% air/5% CO2 atmosphere at 37°C. HepG2 cells were grown in DMEM, supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, 1% sodium pyruvate, and 1% NEAA mixture. Cells were seeded at 2 × 105 cells/well in a 6-well culture plate. After incubation for 15 h, the cells were treated with 100 nM insulin or KGC05P0 (0–500 μg/mL) for 6 h and extracted to perform RT-PCR.
2.6. Experimental animals and treatments
The animal experiment was approved by the Institutional Animal Care and Use Review Committee of Kyung Hee University (KHUASP[SE]-17-026). Male C57BL/6J mice (6 weeks old, 22±1g) and C57BLKS/Jdb/db (db/db, 6 weeks old, 34.5±4g) mice (60 total mice) were purchased from Saeronbio, Inc. (Uiwang, Korea). The mice were controlled in specific pathogen-free barrier facilities at 23 ± 3°C and 55% humidity with a 12 h light/dark cycle. The mice were acclimatized for the first week and provided a standard chow diet before experimentation, and then they were divided into six groups as follows: one C57BL/6J mice group (normal control, NC, AIN93G diet) and 5 C57BLKS/Jdb/db mice groups (untreated control, C; positive control, PC, inulin 400 mg/kg b.w.; low, L, KGC05P0 100 mg/kg b.w.; medium, M, KGC05P0 200 mg/kg b.w.; and high, H, KGC05P0 400 mg/kg b.w.). All the experimental diets were based on the AIN93G diet. The experiment was conducted for eight weeks. The body weight, food intake, and the fasting blood glucose level were recorded weekly. Mouse urine was collected using a metabolic cage three days before the end of the experiment. After 4 h of fasting on the last day of the experiment, all mice were sacrificed and the livers were stored at −80°C until the analysis.
2.7. Oral glucose tolerance tests (OGTT)
One week before the end of the experiment, all mice were fasted for 4 h and OGTT were performed. A glucose solution (2 g/kg b.w.) was orally administered to the mice, blood samples were taken from the tail vein at 0, 30, 60, 90, and 120 min, and blood glucose levels were measured using a G. Doctor Blood Glucose Monitoring System (Allmedicus, Anyang, Korea).
2.8. ELISA assay of biochemical factors in blood, urine, and liver
A portion of the whole blood collected at the sacrifice of all experimental animals was used for hemoglobin A1c (HbA1c) measurement, using a Mouse Hemoglobin A1c Kit (Crystal Chem Inc., USA). The remaining whole blood was centrifuged (3,000 rpm at 4°C for 20 min) to collect serum samples. Glucose Assay Kits, Insulin (Mouse) ELISA Kits, Protein Carbonyl Content Assay Kit (Biovision), and a TNF-α and IL-1β R&D Duoset ELISA kit (R&D Systems, Minneapolis, MN, USA) were used for serum or urine analysis.
The isolated liver was homogenized and lipid peroxidation was measured using the Lipid Peroxidation Colorimetric Assay Kit (Biovision). All experiments were conducted according to the specifications of the manufacturers.
2.9. Western blotting
Twenty-five–milligram samples of liver were homogenized using 0.5 mL of CelLytic™ MT Cell Lysis Reagent (Sigma-Aldrich) with Halt™ Protease and Phosphatase inhibitor Cocktail (Thermo Fisher Scientific, Rockford, IL, USA). The homogenized samples were centrifuged at 14,000 rpm at 4°C for 15 min. The protein content was measured using the Bradford assay. Protein samples (20 μg each) were loaded into 10% Mini-PROTEAN® TGX™ Precast Gels (Bio-Rad) and transferred to PVDF membranes using the Trans-Blot® Turbo™ Transfer system (Bio-Rad). The membranes were blocked with blocking buffer (5% skim milk in Tris buffered saline with 1% Tween® 20) for 1 h at room temperature. After washing, the membranes were probed overnight at 4°C with primary antibodies against β-actin, IRS-1, P-IRS-1, AKT, phospho-AKT, PI3K, and phospho-PI3K (Cell Signaling Technology, 1:1000). After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, 1:2000) for 1 h at room temperature. After washing, the membranes were visualized with EzWestLumi plus (ATTO, Tokyo, Japan) using an Ez-Capture Ⅱ (ATTO), and analyzed using the CS Analyzer 3.0 software (ATTO).
2.10. Real-time polymerase chain reaction (PCR)
Total RNA of hepG2 cells and homogenized liver tissues were extracted using an RNeasy extraction kit (QIAGEN, Gaithersburg, MD, USA) or chloroform (Sigma-Aldrich) following the manufacturer's specifications. Purified total RNA was quantified by using a Q5000 UV-Vis spectrophotometer (Quawell, San Jose, CA, USA). cDNA was synthesized from purified total RNA by using the iScript cDNA Synthesis kit (Bio-Rad). Real-time PCR was performed by using a CFX Connect™ Real-Time System (Bio-Rad) with the iQ™ SYBR® Green Supermix (Bio-Rad), cDNA, and custom-designed primers. Then, the cDNA was amplified with 40 cycles of denaturation (95°C for 15 s), annealing (56°C for 30 s), and extension (72°C for 30 s). Data analysis was conducted by using the CFX Manager Software 3.1 (Bio-Rad). The gene sequences are shown in Table 1.
Table 1.
Primer sequences used in real-time PCR quantification of mRNA
| Gene | Human sequences | Murine sequences |
|---|---|---|
| IRS | F 5′-AAGAAGTGGCGGCACAAGTC-3′ | F 5′-CTGTGTGTGGTTTGGTTTATCATCT-3′ |
| R 5′-CAGCCCGCTTGTTGATGTT-3′ | R 5′-CATGCGTCGGTCTTTTGTACA-3′ | |
| PI3K | F 5′-AGCAAATGAGGCGACCAGAT-3′ | F 5′-GGGTAGAATTGGCTCCATTGG-3′ |
| R 5′-ATGAGCAGGGTTTAGAGGAGACA-3′ | R 5′-GCTATCTCATGGCGACAAGCT-3′ | |
| Akt1 | F 5′-CTGAGACGCCCGGTACATG-3′ | F 5′-GCTCAACCCCATGTGTCTGA-3′ |
| R 5′-CCCCTGCCGGTCTGAATC-3′ | R 5′-GCACCCCCGCAGTGAAG-3′ | |
| FoxO1 | F 5′-GTGTTGCCCAACCAAAGCTT-3′ | F 5′-GAACCAGCTCAAATGCTAGTACCA-3′ |
| R 5′-CTCAGCCTGACACCCAGCTAT-3′ | R 5′-GTCCCCATCTCCCAGGTCAT-3′ | |
| PEPCK | F 5′-TGGGCTCGCCTCTGTCA-3′ | F 5′-TTGCGGGAAACAAGGACAAC-3′ |
| R 5′-CCACCACGTAGGGTGAATCC -3′ | R 5′-CAGCCACTAGATTCTGGATAACTATAC-3′ | |
| G6Pase | F 5′-GAGTGGAGTGGCACGATCTTG-3′ | F 5′-CAACCGCCATGCAAAGG-3′ |
| R 5′-GACATGAGAATCGCTTGAACCA-3′ | R 5′-CTGGCCTCACAATGGGTTTC-3′ | |
| GLUT2 | F 5′-TGGCAGCTGCTCAACTAATCA-3′ | F 5′-GGATTAAGAGGACAATTCCACACA-3′ |
| R 5′-CCAACTGCAAAGCTGGATACAG-3′ | R 5′-AGCCAAGGTTCCGGTGATC-3′ | |
| GCK | F 5′-CCGCAGCGAGGACGTAAT-3′ | F 5′-GCTTTTGAGACCCGTTTTGTG-3′ |
| R 5′-CTTGTACACGGAGCCATCCA-3′ | R 5′-GCCTTCGGTCCCCAGAGT-3′ | |
| PFK | F 5′-GATGCCGCATACATTTTCGA-3′ | F 5′-CGACCGAATCCTGAGTAGCAA-3′ |
| R 5′-CTCCACGTTGGACTGCAGATC-3′ | R 5′-TGTCAGGCGTGGCCTCTAG-3′ | |
| ACC | F 5′-TGGCCGGGACCCTACTCTA-3′ | F 5′-TGTCCGCACTGACTGTAACCA-3′ |
| R 5′-CTACTTTGGCATTGGTGGTCTTACT-3′ | R 5′-TGCTCCGCACAGATTCTTCA-3′ | |
| GAPDH | F 5′-CAAGGCTGTGGGCAAGGT-3′ | F 5′-CATGGCCTTCCGTGTTCCTA-3′ |
| R 5′-GGAAGGCCATGCCAGTGA-3′ | R 5′-GCGGCACGRCAGATCCA-3′ |
IRS, Insulin receptor substrate; PI3K, Phosphoinositide 3-kinases; Akt, Protein kinase B; FoxO, Forkhead box; PEPCK, Phosphoenolpyruvate carboxykinase; G6Pase, Glucose 6-phosphatase; GLUT2, glucose transporter protein type 2; GCK, glucokinase; PFK, phosphofructokinase; ACC, acetyl-coA carboxylase; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase
2.11. Statistical analysis
All experimental results are expressed as mean ± standard deviation (SD). The significance of differences was determined with a one-way analysis of variance (ANOVA) and Duncan's multiple-range test using the SPSS PASW Statistic 22.0 (SPSS, Inc., Chicago, IL, USA). Values of P < 0.05 were considered statistically significant.
3. Results
3.1. HPLC analysis of KGC05P0
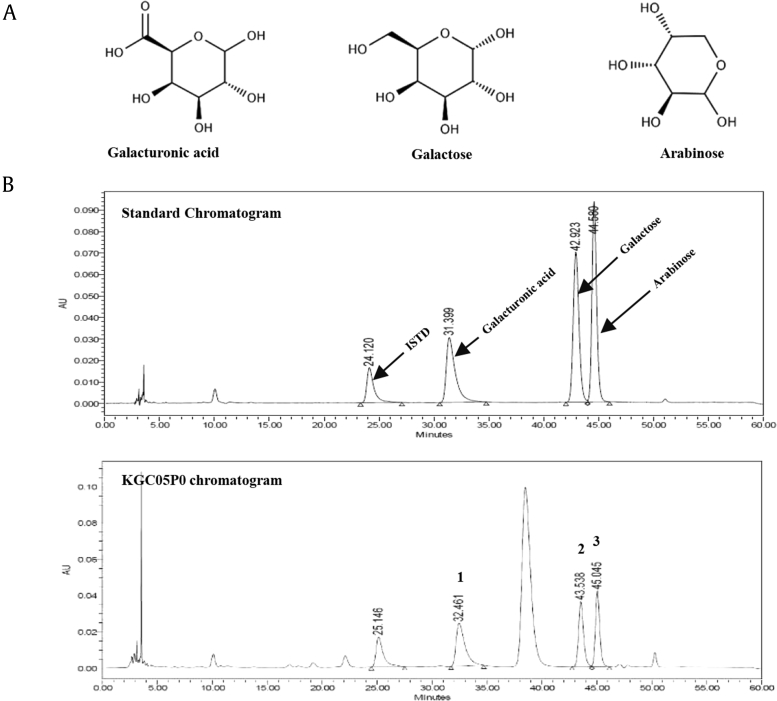
The HPLC analysis of the KGC05P0 revealed three peaks matching those of the commercial standards galacturonic acid, galactose, and arabinose, with retention times of approximately 32.5 min, 43.5 min, 45.0 min, respectively (Fig. 1). The KGC05P0 contained 23.62 mg/g galacturonic acid, 15.93 mg/g galactose, and 14.08 mg/g arabinose.
Fig. 1.
HPLC chromatogram of a mixture of three standards (A) and KGC05P0 at (B) at 250 nm. Galacturonic acid (1), galactose (2), and arabinose (3) appeared with retention times of approximately 32.5 min, 43.5 min, 45.0 min, respectively.
3.2. Inhibitory effects of KGC05P0 on α-glucosidase and α-amylase in vitro
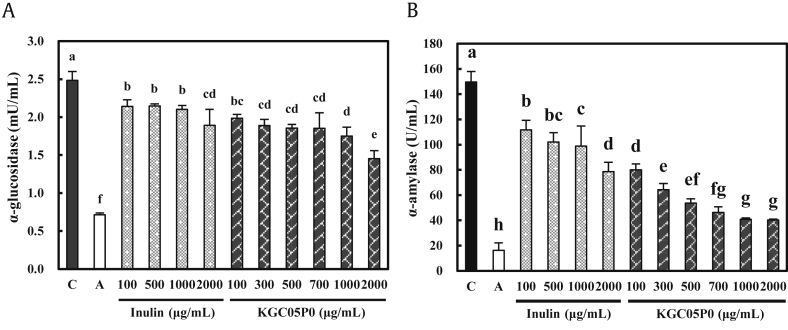
KGC05P0 inhibited α-glucosidase activity in a dose-dependent manner by 20.1, 23.9, 25.3, 25.3, 29.5, and 41.5% compared with the control group at 100, 300, 500, 700, 1000, and 2000 μg/mL (P < 0.05) (Fig. 2A). KGC05P0 inhibited α-amylase activity in a dose-dependent manner by 46.5, 57.05, 64.15, 69.2, 72.7, and 73.1% compared with the control group at 100, 300, 500, 700, 1000, and 2000 μg/mL (P < 0.05) (Fig. 2B). Furthermore, the KGC05P0 was more effective than inulin at the same concentration in terms of α-glucosidase and α-amylase inhibition. In addition, the inhibitory activity of both enzymes did not exceed the inhibitory effect of acarbose, a commercially obtained inhibitor.
Fig. 2.
Inhibitory effects of KGC05P0 on α-glucosidase and α-amylase. (A) α-Glucosidase. (B) α-Amylase. Values are presented as mean ± SD (n = 3), and different alphabets indicate significance at P < 0.05. C, control; A, acarbose (1000 μg/mL).
3.3. Effects of KGC05P0 on glucose uptake and transport rate in Caco-2 cells
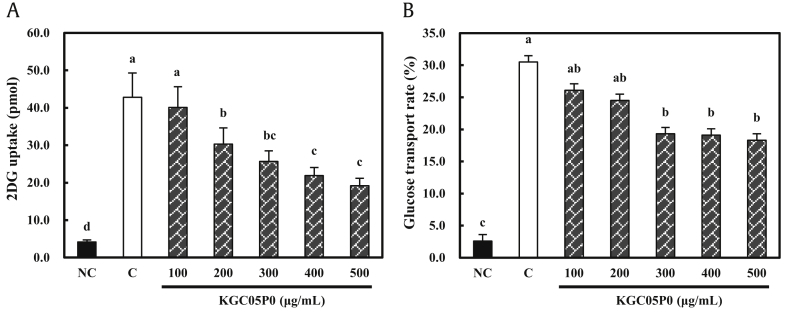
Prior to the experiment, the viability of Caco-2 cells treated with KGC05P0 was tested; KGC05P0 was not cytotoxic up to a concentration of 500 μg/mL (data not shown). KGC05P0 caused a statistically significant reduction of glucose uptake in a dose-dependent manner: 29.2, 40.0, 48.8, and 55.1% compared to the control group at 200, 300, 400, and 500 μg/mL, respectively, in Caco-2 cells (P < 0.05) (Fig. 3A). In addition, the glucose permeability rate of Caco-2 cells was significantly decreased: 36.7, 37.4, and 40.0% compared to the control group at 300, 400, and 500 μg/mL, respectively (P < 0.05) (Fig. 3B).
Fig. 3.
Effects of KGC05P0 on glucose transport by Caco-2 cells under sodium-dependent conditions. (A) 2DG uptake. (B) Transport rate. Values are presented as mean ± SD (n = 3), and different alphabets indicate significance at P < 0.05. NC, normal control; C, control.
3.4. Down-regulation effects of KGC05P0 on gluconeogenesis pathway-related mRNA expression in HepG2 cell
Prior to the experiment, cell viability of KGC05P0 on HepG2 cells was tested and was not cytotoxic up to a concentration of 500 μg/mL (data not shown). The mRNA expressions of IRS1 of KGC05P0 groups were increased in a dose-dependent manner compared to the control group, but was significantly higher in 300 and 500 groups. The mRNA expressions of PI3K, AKT, and forkhead box 1 (FoxO1) of KGC05P0 groups were significantly increased in a dose-dependent manner compared to the control group. The mRNA expressions of PEPCK and G6Pase of KGC05P0 groups were significantly decreased in a dose-dependent manner compared to the control group. The mRNA expressions of glucose transporter protein type 2 (GLUT-2) of KGC05P0 groups were significantly increased in a dose-dependent manner compared to the control group. The mRNA expressions of GCK, PFK, and ACC of KGC05P0 groups were significantly increased in a dose-dependent manner compared to the control group. In particular, KGC05P0 showed a greater effect at the same dose compared to the inulin groups (P < 0.05) (Table 2).
Table 2.
Effects of KGC05P0 on basal and insulin action on gluconeogenesis pathway-related mRNA levels in HepG2 cells
|
Gene description |
NC | C | PC | KGC05P0 |
||
|---|---|---|---|---|---|---|
| 100 | 300 | 500 | ||||
| IRS1 | 1.00 ± 0.08c | 1.42 ± 0.27b | 1.83 ± 0.04a | 1.59 ± 0.11b | 1.87 ± 0.11a | 2.05 ± 0.06a |
| PI3K | 1.00 ± 0.09e | 1.42 ± 0.10d | 1.86 ± 0.08ab | 1.59 ± 0.07c | 1.75 ± 0.07bc | 1.96 ± 0.12a |
| AKT | 1.00 ± 0.20c | 1.06 ± 0.10c | 1.48 ± 0.07ab | 1.33 ± 0.06bc | 1.45 ± 0.01ab | 1.60 ± 0.11a |
| FoxO1 | 1.00 ± 0.13d | 1.18 ± 0.09c | 1.37 ± 0.05b | 1.39 ± 0.04b | 1.60 ± 0.03a | 1.68 ± 0.07a |
| PEPCK | 1.00 ± 0.05a | 0.90 ± 0.06a | 0.54 ± 0.05c | 0.73 ± 0.06b | 0.51 ± 0.08c | 0.40 ± 0.05d |
| G6Pase | 1.00 ± 0.26a | 0.83 ± 0.13ab | 0.49 ± 0.05cd | 0.64 ± 0.05bc | 0.35 ± 0.04de | 0.23 ± 0.04e |
| GLUT2 | 1.00 ± 0.13a | 0.65 ± 0.05c | 0.75 ± 0.04hc | 0.75 ± 0.03bc | 0.82 ± 0.01b | 0.95 ± 0.05a |
| GCK | 1.00 ± 0.12d | 2.10 ± 0.16c | 2.36 ± 0.12bc | 2.37 ± 0.11bc | 2.68 ± 0.16b | 3.24 ± 0.36a |
| PFK | 1.00 ± 0.20b | 1.28 ± 0.07b | 1.78 ± 0.44a | 1.84 ± 0.04a | 2.19 ± 0.20a | 2.20 ± 0.17a |
| ACC | 1.00 ± 0.29d | 1.23 ± 0.01c | 1.46 ± 0.07bc | 1.53 ± 0.02b | 1.64 ± 0.02ab | 1.84 ± 0.12a |
All groups were stimulated with 100 nM insulin for 6 h except normal control group. Insulin receptor substrate (IRS1), Phosphoinositide 3-kinases (PI3K), Protein kinase B (AKT), Forkhead box 1 (FoxO1), Phosphoenolpyruvate carboxykinase (PEPCK), Glucose 6-phosphatase (G6Pase), glucose transporter protein type 2 (GLUT2), Glucokinase (GCK), Phosphofructokinase (PFK), and Acetyl-CoA carboxylase (ACC) mRNA expressions were assessed by RT-PCR. Values are presented as mean ± SD (n = 3), and different alphabets indicate significance at P < 0.05. NC, normal control; C, control; PC, positive control (inulin 500 μg/mL); KGC05P0 (100, 300, 500 μg/mL)
3.5. Effects of KGC05P0 on body weight, food intake, food efficiency rate, water intake, and urine excretion of diabetic mice
This study showed a significant increase in weight gain in the C57BLKS/Jdb/db groups compared to the normal control (5.43 ± 1.03 g) group. In particular, the weight gains of the KGC05P0 100, 200, and 400 groups (11.69 ± 4.50 g, 9.99 ± 4.20 g, and 8.53 ± 2.22 g) were significantly decreased compared to the control (15.43 ± 4.63 g) group. There were also no significant differences in food efficiency rate among the C57BLKS/Jdb/db groups. Also, the amounts of water intake and urine excretion were significantly increased in the C57BLKS/Jdb/db groups compared to the normal control and were significantly decreased in a dose-dependent manner in the groups fed KGC05P0-containing diets compared to the normal control group (P < 0.05) (Table 3).
Table 3.
Effects of KGC05P0 on weight gain, food intake and FER and parameters in blood and urine of C57BLKS/Jdb/db mice
|
C57BLKS/Jdb/db |
||||||
|---|---|---|---|---|---|---|
| NC | C | PC | L | M | H | |
| Variables | ||||||
| Weight gain (g)1 | 5.43 ± 1.03c | 15.43 ± 4.63a | 12.31 ± 4.51ab | 11.69 ± 4.50ab | 9.99 ± 4.20bc | 8.53 ± 2.22bc |
| Food intake (g/day/mouse) | 2.32 ± 0.13f | 4.25 ± 0.40a | 3.90 ± 0.33e | 3.96 ± 0.35d | 4.17 ± 0.58b | 4.08 ± 0.27c |
| Water intake (mL/day) | 3.00 ± 1.00d | 18.00 ± 2.65a | 6.33 ± 2.89c | 15.33 ± 0.58a | 12.00 ± 1.73b | 9.33 ± 0.58bc |
| Urine excretion (mL/day) | 0.50 ± 0.23e | 15.33 ± 1.53a | 5.78 ± 2.20d | 11.67 ± 0.58b | 9.00 ± 1.00c | 7.33 ± 0.58cd |
| FER2 | 4.34 ± 0.82bc | 6.93 ± 2.82a | 6.30 ± 0.31ab | 5.50 ± 2.12abc | 4.39 ± 1.85bc | 3.89 ± 1.01c |
| Blood | ||||||
| Glucose (mg/dL) | 172.67 ± 24.35d | 559.00 ± 35.94a | 367.60 ± 32.28c | 433.25 ± 5.38b | 395.20 ± 41.81bc | 367.33 ± 51.18c |
| Insulin (μU/mL) | 7.36 ± 0.48a | 4.06 ± 0.35d | 6.41 ± 0.66bc | 4.45 ± 0.21d | 6.08 ± 0.44c | 6.62 ± 0.51b |
| HOMA-IR3 | 3.14 ± 0.49c | 5.66 ± 0.51a | 5.64 ± 0.35a | 4.76 ± 0.25b | 5.53 ± 0.35a | 5.37 ± 0.98ab |
| HbA1c (%) | 5.91 ± 0.91d | 11.32 ± 1.49a | 6.88 ± 0.38cd | 9.93 ± 0.71b | 7.50 ± 0.42c | 6.61 ± 0.71cd |
| Carbonyl (nmol/mg prot.) | 6.09 ± 1.17d | 12.16 ± 0.70a | 7.56 ± 0.66c | 11.17 ± 1.02a | 9.22 ± 0.84b | 7.14 ± 0.52cd |
| TNF-α (pg/mL) | 113.08 ± 22.45e | 441.75 ± 22.39a | 288.25 ± 7.20c | 325.58 ± 8.76b | 302.25 ± 14.47c | 249.75 ± 15.49d |
| IL-1β (pg/mL) | 7.32 ± 0.65e | 14.75 ± 1.07a | 12.21 ± 0.65c | 13.17 ± 0.97b | 11.71 ± 0.35c | 10.65 ± 0.43d |
| Urine | ||||||
| Glucose (mg/dL) | 0.34 ± 0.18d | 9.88 ± 0.94a | 6.68 ± 0.15c | 8.63 ± 0.37b | 7.88 ± 0.99b | 7.90 ± 0.74b |
Values are presented as mean ± standard deviation (n = 8), and different superscript letters indicate significance at P < 0.05
NC, normal control; C, control; PC, positive control (inulin 400 mg/kg b.w.); L (KGC05P0 100 mg/kg b.w.); M (KGC05P0 200 mg/kg b.w.); H (KGC05P0 400 mg/kg b.w.); HbA1c, glycated hemoglobin; TNF-α, tumor necrosis factor alpha; IL-1β, interleukin-1 beta
Weight gain (g/12 weeks) = final body weight (g) – initial body weight (g).
FER (food efficiency rate) = weight gain (g)/total food consumption (g) × 100.
HOMA-IR (homeostasis model assessment of insulin resistance) = fasting insulin (μU/mL) × fasting glucose (mg/dL)/405.
3.6. Effects of KGC05P0 on glucose level and glucose tolerance in diabetic mice over eight weeks
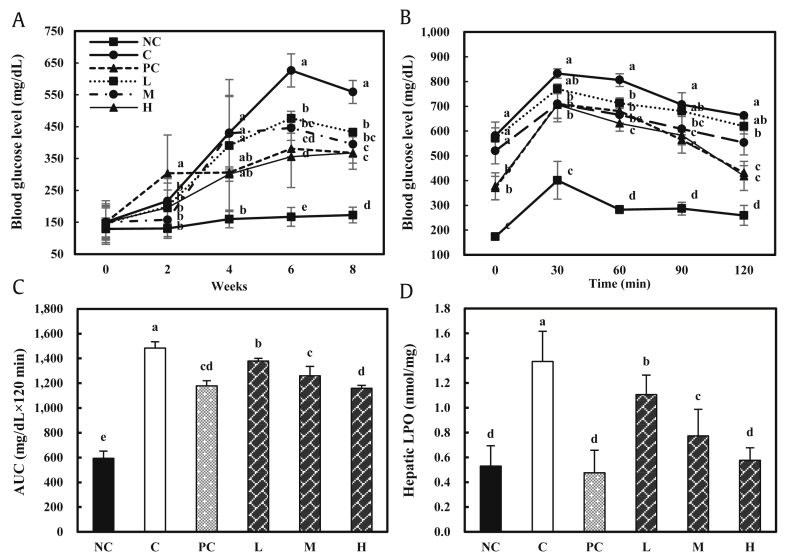
The blood glucose levels of the C57BLKS/Jdb/db groups gradually increased until eight weeks compared with the normal control group. Also, the blood glucose levels measured at eight weeks decreased in a dose-dependent manner in the groups fed with the KGC05P0-containing diet compared to the control group (559.00 ± 35.94 mg/dL) (P < 0.05) (Fig. 4A).
Fig. 4.
Effects of KGC05P0 on glucose levels and hepatic profile in C57BL/6J and C57BLKS/Jdb/db mice. (A) Weekly glucose level. (B) Oral glucose tolerance tests (OGTT). (C) Total area under the curve (AUC). (D) Hepatic lipid peroxidation (LPO). Values are presented as mean ± SD (n = 10), and different alphabets indicate significance at P < 0.05. NC, normal control; C, control; PC, positive control (Inulin, 400 mg/kg b.w.); L, low (KGC05P0 100 mg/kg b.w.); M, medium (KGC05P0 200 mg/kg b.w.); H, high (KGC05P0 400 mg/kg b.w.).
As shown by OGTT, the blood glucose level of the control group was highest at 30 min after oral glucose administration (832.33 ± 18.93 mg/dL) compared to the fasting state, and the blood glucose level gradually decreased over the following 90 min, but the control group still had the highest value (662.33 ± 5.51 mg/dL) at 120 min post challenge compared with the other groups. In the groups fed with KGC05P0-containing diets, the blood glucose levels measured at 30 min after oral glucose administration increased significantly compared to the normal control group, but were lower than that of the control group. In addition, the blood glucose levels gradually decreased in a dose-dependent manner through 120 min compared with the control group (P < 0.05) (Fig. 4B).
The total area under the curve (AUC) was calculated by the trapezoid rule during the OGTT. The AUC in the groups fed with KGC05P0-containing diets significantly decreased in a dose-dependent manner compared to the control group (P < 0.05) (Fig. 4C).
3.7. Effects of KGC05P0 on blood biochemical factors in diabetic mice
The effects of KGC05P0 on blood biochemical factors in mice are shown in Table 3. The level of insulin in the control group was significantly decreased compared to the normal control group, and the levels in the groups fed KGC05P0-containing diets were significantly increased in a dose-dependent manner compared to the control group (all P < 0.05). The homeostasis model assessment of insulin resistance (HOMA-IR) was significantly increased in the C57BLKS/Jdb/db groups compared to the normal control group (P < 0.05), and the HOMA-IR of the groups fed with KGC05P0-containing diets were decreased compared to the control group. However, there were no significant differences among the groups fed KGC05P0-containing diets.
The levels of HbA1c and carbonyl in the control group (11.32 ± 1.49% and 12 ± 0.70 nmol/mg, respectively) were significantly increased compared to the normal control group (5.91 ± 0.91% and 6.09 ± 1.17 nmol/mg, respectively), and the groups fed with KGC05P0-containing diets were significantly decreased in a dose-dependent manner compared to the control group (all P < 0.05).
The levels of proinflammatory cytokines (TNF-α and IL-1β) in the control group (441.75 ± 22.39 pg/mL and 14.75 ± 1.07 pg/mL, respectively) were significantly increased compared to the normal control group (113.08 ± 22.45 pg/mL and 7.32 ± 0.65 pg/mL, respectively), and those of the groups fed with KGC05P0-containing diets were significantly decreased in a dose-dependent manner compared to the control group (all P < 0.05).
3.8. Effects of KGC05P0 on urine glucose levels in diabetic mice
The level of urine glucose in the control group (9.88 ± 0.94 mg/dL) was significantly increased compared to the normal control group (0.34 ± 0.18 mg/dL), and those of the groups fed with KGC05P0-containing diets were significantly decreased compared to the control group (all P < 0.05). However, there were no significant differences among the groups fed with KGC05P0-containing diets (Table 3).
3.9. Effects of KGC05P0 on hepatic lipid peroxidation (LPO) in diabetic mice
The level of hepatic LPO in the control group (1.37 ± 0.12 nmol/mg) was significantly increased compared to the normal control group (0.53 ± 0.10 nmol/mg), and those of the groups fed with KGC05P0-containing diets were significantly decreased in a dose-dependent manner compared to the control group (P < 0.05). In particular, the level of LPO in the H group (0.58 ± 0.07 nmol/mg) was the lowest among the groups fed with KGC05P0-containing diets, and it was not significantly different from the positive control (0.48 ± 0.07 nmol/mg) and normal control groups (Fig. 4D).
3.10. Down-regulation effects of KGC05P0 on gluconeogenesis pathway-related protein and mRNA expression in the livers of diabetic mice
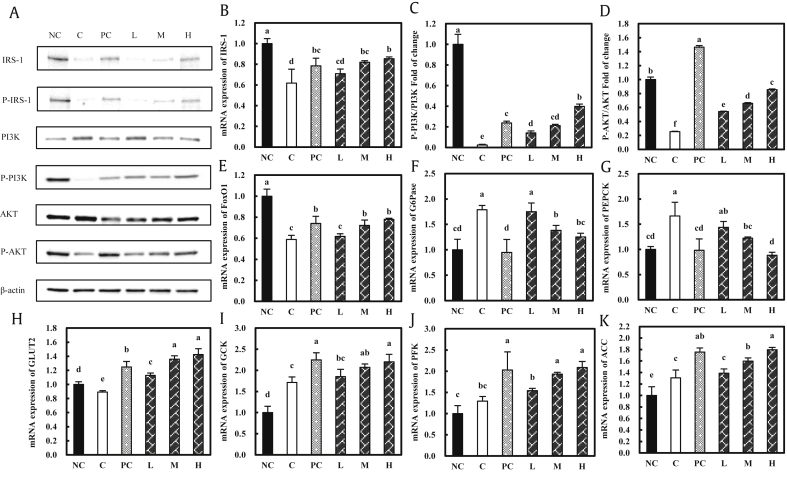
The expression levels of IRS-1 and p-IRS-1 were significantly inhibited in the control group compared to the normal control group, and those in the groups fed with the KGC05P0-containing diets were increased in a dose-dependent manner compared to the control group at the protein and mRNA levels (all P < 0.05) (Fig. 5A and B). The phospho-PI3K/PI3K ratio and phospho-AKT/AKT ratio were significantly decreased in the control group compared to the normal group, and those in the groups fed with KGC05P0-containing diets were significantly increased in a dose-dependent manner compared to the control group, at the protein and mRNA levels (mRNA data not shown) (P < 0.05) (Fig. 5A, C, and D). The expression of FoxO1 was significantly decreased in the control group compared to the normal control group, and those of the groups fed with KGC05P0-containing diets were increased in a dose-dependent manner compared to the control group, at the mRNA level (P < 0.05) (Fig. 5E). The expression levels of G6Pase and PEPCK were significantly increased in the control group compared to the normal control group, and those in the groups fed with KGC05P0-containing diets were decreased in a dose-dependent manner compared to the control group, at the mRNA level (P < 0.05) (Fig. 5F and G). The expression of GLUT2 was significantly decreased in the control group compared to the normal control group, and those of the groups fed with KGC05P0-containing diets were increased in a dose-dependent manner compared to the control group, at the mRNA level (P < 0.05) (Fig. 5H). The expression levels of GCK, PFK, and ACC were significantly increased in the control group compared to the normal control group, and those in the groups fed with KGC05P0-containing diets were increased in a dose-dependent manner compared to the control group, at the mRNA level (P < 0.05) (Fig. 5I, J, and K).
Fig. 5.
Effects of KGC05P0 on protein and mRNA expression in liver from C57BL/6J and C57BLKS/Jdb/db mice. (A) Protein expressions, (B) mRNA expression of IRS-1, (C) the ratio of phosphorylated/total PI3K protein expression, (D) the ratio of phosphorylated/total AKT protein expression, (E) FoxO1 mRNA expression, (F) G6Pase mRNA expression, (G) PEPCK mRNA expression, (H) GLUT2 mRNA expression, (I) glucokinase mRNA expression, (J) phosphofructokinase mRNA expression, and (K) acetyl-CoA carboxylase mRNA expressions. Values are presented as mean ± SD, and different alphabets indicate significance at P < 0.05. NC, normal control; C, control; PC, positive control (inulin, 400 mg/kg b.w.); L, low (KGC05P0 100 mg/kg b.w.); M, medium (KGC05P0 200 mg/kg b.w.); H, high (KGC05P0 400 mg/kg b.w.).
4. Discussion
A healthy diet is an essential factor in lowering the risk of developing T2DM. T2DM, a chronic metabolic disease, is characterized by insulin resistance and insufficient insulin secretion [18]. Some medications for T2DM have several limitations, such as adverse effects and high secondary failure rates [19]. Therefore, interest in food materials without adverse effects is increasing, and the market for health functional foods is increasing year by year.
RG, a popular functional food among Asian people, and saponin, its active ingredient, have been reported to have anti-diabetic effects such as controlling blood glucose and insulin resistance [[20], [21], [22]]. However, the absolute content of saponin in the thick root (main root), which is recognized as an important medicinal site of ginseng, is significantly less than that of the fine root. This suggests that the non-saponin components include an active ingredient. The non-saponin components include polysaccharides, maltulosyl arginine, protein fraction, polypeptides, polyacetylene compounds, phenol compounds, alkaloids, and lignans [23]. In particular, many studies have shown that polysaccharides of natural products have anti-diabetic effects [24]. Among them, galacturonic acid, galactose, and arabinose are present in various plants [[25], [26], [27]]. Our HPLC analysis showed that KGC05P0 contained galacturonic acid, galactose, and arabinose. In addition, there are several studies on the effects of the non-saponin fraction on platelet aggregation, learning deficits, immunomodulation, and multi-drug resistance [[28], [29], [30], [31]], but little is known about its anti-diabetic effect.
In this study, we focused on whether KGC05P0, a non-saponin fraction of Korean Red Ginseng, has anti-diabetic activity in vitro and in vivo. KGC05P0 showed stronger inhibitory activity against both α-glucosidase and α-amylase compared with inulin, a functional food, but its activity was not stronger than that of acarbose (1000 μg/mL), a carbohydrate digestive enzyme inhibitor [32]. Acarbose (IC50 = 1000 μg/mL) is frequently used for the treatment of T2DM and has been tested at concentrations up to 1000 μg/mL in several studies [33,34]. It has been reported that polysaccharides obtained from Camellia sinensis leaves and flowers have inhibitory activity against α-glucosidase and α-amylase and thus may prevent diabetes [35]. In addition, phenolic compounds, alkaloids, and polypeptides are known to act as inhibitors of α-glucosidase and α-amylase [[36], [37], [38]]. The inhibition of α-glucosidase and α-amylase activities in the digestive tract was reported to inhibit diabetes by reducing the absorption of glucose degraded from starch [32].
In addition, glucose uptake in the digestive tract controls blood glucose levels, and repeated high postprandial glucose levels are associated with severe metabolic disease and an increased risk of T2DM [39]. In this study, KGC05P0 significantly reduced the glucose uptake and glucose transport rate compared to the control group in Caco-2 cells. Caco-2 cells have been widely used in dietary polyphenol transport and metabolism studies, and are suitable for glucose uptake and transport studies because of their abundant expression of glucose transport proteins and sodium-dependent glucose transporters [39].
Glucose transport is the most fundamental process in energy metabolism, and the permeation of glucose into small intestinal cells plays a key role in metabolic regulation. Recently, it has been reported that polyphenols and phenolic acids, which are bioactive compounds, can affect the uptake, transport, and blood level of glucose [40,41]. In addition, more studies are investigating the interaction of transporters with enzymes and polyphenols of importance to glucose uptake and metabolism [42,43]. Therefore, it is worthwhile to confirm the uptake and transport level of glucose after treatment with KGC05P0, a non-saponin fraction of Korean Red Ginseng, and further experiments should be conducted to confirm the expression of glucose transport proteins and sodium-dependent glucose transporters.
OGTT is one of the most important criteria for assessing hypoglycemic effects [44]. KGC05P0 is expected to increase glucose utilization because it significantly lowers blood glucose levels and significantly inhibits its increase during OGTT in diabetic mice. The serum insulin level in the KGC05P0-treated diabetic mice was significantly controlled compared to the control group. In addition, KGC05P0 significantly decreased HbA1c, carbonyl contents, TNF-α, and IL-1β levels compared to the control group among diabetic mice. HbA1c is a crucial biomarker that shows the severity of hyperglycemia. HbA1c levels are a useful measure of overall blood glucose control because they reflect accumulated glycation over the lifetime of red blood cells [45]. Hyperglycemia leads to the production of glycosylated hemoglobin through non-enzymatic glycation and oxidation of proteins such as hemoglobin and insulin. When additional denaturation occurs thereafter, irreversible products of final glycation are formed, leading to insulin resistance and diabetic complications. The production of glycated hemoglobin and the final glycation end product is also highly correlated with production of inflammatory factors. The proinflammatory cytokines TNF-α and IL-1β induce structural changes in insulin and promote the formation of glycated hemoglobin, and also cause the production of the advanced glycation end products [46]. In addition, increased urinary glucose, a typical symptom of T2DM, indicates the occurrence of postprandial hyperglycemia and hepatic glucose output, as they lead to an increase in fasting glucose and urinary glucose excretion [47]. Urinalysis studies have demonstrated that KGC05P0 significantly reduces urinary glucose excretion compared to the control group.
The liver plays an important role in maintaining glucose levels by lowering the glucose level in circulation in the post-meal state and supplying glucose through gluconeogenesis and glycogenolysis in the fasting state [48]. However, abnormal glucose metabolism in the liver is the main characteristic of diabetes; abnormal activation of the glucose production pathway leads to elevated glucose levels in the blood and insulin resistance in the liver [48,49]. In our study, KGC05P0 significantly inhibited the PI3K/AKT pathway and increased the expression of G6Pase and PEPCK in the livers of diabetic mice. The binding of insulin to the IR leads to an increase in IRS-1/-2 expression and stimulates the PI3K/AKT pathway, which are needed for the suppression of gluconeogenic genes such as G6Pase and PEPCK in the liver [4,50]. PI3K is a secondary messenger that plays a crucial role in cellular signaling. PI3K comprises a regulatory subunit p85 and a catalytic subunit p110, and quantitative imbalance of the PI3K subunits can induce insulin resistance. AKT is an important downstream node of PI3K, and activation of AKT inhibits the expression of G6Pase and PEPCK through FoxO1 phosphorylation [51,52]. Padiya and Liu confirmed that the phosphorylated forms of PI3K and AKT in the diabetic model decreased compared to the total expression level and increased again by Galic or Irisin treatment [53,54]. Several studies have shown that (−)-epicatechin, phenolic extract, and naringenin stimulate the AKT pathway in HepG2 cells [[55], [56], [57]], and polyphenols of green tea also increased the expression of the PI3K/AKT levels in the livers in rat models of insulin resistance [58]. G6Pase and PEPCK are important factors controlling hepatic glucose output and AG-dieckol is known to decrease their activities in db/db mice [[59], [60], [61]].
GLUT-2 is primarily located in the liver cell membrane. There are many studies that showed that GLUT-2 is needed for glucose-stimulated insulin secretion, and glycemic controlling in response to dietary intake [[62], [63], [64]]. In our study, KGC05P0 significantly increased GLUT-2 mRNA expression in vitro and in vivo and the result shows that KGC05P0 may facilitate the transport of blood glucose into the liver. In glycolysis pathway, it is shown that the GCK, PFK enzyme activities and their mRNA levels are decreased in diabetic liver [65]. ACC is an enzyme involved in converting glucose into fat after pyruvate generates energy through the TCA cycle [66,67]. Interestingly, in this study we confirm that the mRNA levels of GCK, PFK, and ACC were significantly increased in the KGC05P0 groups compared to the control group. Taken together, the increased mRNA levels of these observed in this study implicate that KGC05P0 enhances glycolysis to regulate glucose level and not only converts glucose into fat, but also improve insulin resistance in the diabetic liver.
5. Conclusion
In conclusion, we observed evidence that KGC05P0, isolated from the non-saponin fraction of Korean Red Ginseng, can inhibit the digestion and absorption of glucose through inhibition of α-glucosidase, α-amylase, glucose uptake, and glucose transport in vitro. In addition, our study shows that KGC05P0 has anti-diabetic activities in C57BLKS/Jdb/db mice. KGC05P0 regulated hyperglycemia and glucose tolerance and the levels of insulin, HbA1c, carbonyl contents, and proinflammatory cytokines in blood. It also decreased glucose excretion in the urine in diabetic mice. In addition, we found that KGC05P0 regulates glucose level by down regulating the PI3K/AKT pathway and enhancing glycolysis. Although further experiments are required to define the precise role of KGC05P0 in relation to the glucose transport pathway, KGC05P0 is known to contain abundant polysaccharides, phenol compounds, and other active substances, which can be useful as hyperglycemic foods.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by research funding from the Korea Ginseng Corporation.
Contributor Information
Ok-Kyung Kim, Email: 20woskxm@jnu.ac.kr.
Jeongmin Lee, Email: jlee2007@khu.ac.kr.
References
- 1.Oh M.J., Kim H.J., Park E.Y., Ha N.H., Song M.G., Choi S.H., Chun B.G., Kim D.H. The effect of Korean Red Ginseng extract on rosiglitazone-induced improvement of glucose regulation in diet-induced obese mice. J Ginseng Res. 2017:52–59. doi: 10.1016/j.jgr.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn J., Um M.Y., Lee H., Jung C.H., Heo S.H., Ha T.Y. Eleutheroside E, an active component of Eleutherococcus senticosus, ameliorates insulin resistance in type 2 diabetic db/db mice. Evid Based Complement Alternat Med. 2013:934183. doi: 10.1155/2013/934183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwon H.O., Lee M.H., Kim Y.J., Kim E., Kim O.K. Beneficial effects of Acanthopanax senticocus extract in type Ⅱ diabetes animal model via down-regulation of advanded glycated hemoglobin and glycosylation end products. J Korean Soc Food Sci. 2016;45(7):929–937. [Google Scholar]
- 4.Cordero-Herrera I., Martin M.A., Bravo L., Goya L., Ramos S. Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol Nutr Food Res. 2013;57:974–985. doi: 10.1002/mnfr.201200500. [DOI] [PubMed] [Google Scholar]
- 5.Elieen L.W., Han C., Birnbaum M.J. Role of Akt protein kinase B in metabolism. Trends in Endocrinol Metab. 2002;13(10):444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 6.Shephered P.R., Withers D.J., Siddle L. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333(3):471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakae J., Kitamura T., silver D.L., Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phospahatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puigserver P., Rhee J., Donovan J., Walkey C.J., Toon J.C., Oriente F., Kitamura Y., Altomonte J., Dong H., Accili D. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 9.Alpers C.E., Hudkins K.L. Mouse models of diabetic nephropathy. Curr Opin Nephrol Hypertens. 2011;20:278–284. doi: 10.1097/MNH.0b013e3283451901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma K., McCue P., Dunn S.R. Diabetic kidney disease in the db/db mouse. An J Physiol Renal Physiol. 2003;284:F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 11.Yang H.N., Son W.S., Park H.R., Lee S.E., Park Y.S. Effect of Korean red ginseng treatment on the gene expression profile of diabetic rat retina. J Ginseng Res. 2016;40:1–8. doi: 10.1016/j.jgr.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun T.K., Zheng S., Choi S.Y., Cai S.R., Lee Y.S., Liu X.Y., Cho K.J., Park K.Y. Non-organ-specific preventive effect of long-term administration of Korean red ginseng extract on incidence of human cancers. J Med Food. 2010;13(3):489–494. doi: 10.1089/jmf.2009.1275. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel S.B., Santani D., Patel V., Shah M. Anti-diabetic effects of ethanol extract of AMPK compensatory effects for ER stress-mediated beta-cell dysfunction during the progression of type-2 diabetes. Cell Signal. 2013;25:2348–2361. doi: 10.1016/j.cellsig.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.Y., Kim K. Protective effect of ginseng on cytokine-induced apoptosis in pancreatic beta-cells. J Agric Food Chem. 2007;55:2816–2823. doi: 10.1021/jf062577r. [DOI] [PubMed] [Google Scholar]
- 16.Lim K.H., Cho J.Y., Kim B., Bae B.S., Kim J.H. Red ginseng (Panax ginseng) decreases isoproterenol-induced cardiac injury via antioxidant properties in porcine. J Med Food. 2014;17:111–118. doi: 10.1089/jmf.2013.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuate D., Kengne A.P., Biapa C.P., Azantsa B.G., Abdul Manan Bin Wan Muda W. Tetrapleura tetraptera spice attenuates high-carbohydrate, high-fat diet induced obese and type 2 diabetic rats with metabolic syndrome features. Lipids Health Dis. 2015;14:50. doi: 10.1186/s12944-015-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishina P.M., Naggert J.K., Verstuyft J., Paigen B. Atherosclerosis in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism. 1994;5:554–558. doi: 10.1016/0026-0495(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee S.H., Min K.H., Han J.S., Lee D.H., Park D.B., Jung W.K., Park P.J., Jeon B.T., Kim S.K., Jeon Y.J. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem Toxicol. 2012;50:575–582. doi: 10.1016/j.fct.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 20.Vuksan V., Sung M.K., Sievenpiper J.L., Stravro P.M., Jenkins A.L., Buono M.D., Lee K.S., Leiter L.A., Nam K.Y., Arnason J.T. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metabol Cardiovasc Dis. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Bang H.J., Kwak J.H., Ahn H.Y., Shin D.Y., Lee J.H. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food. 2014;17(1):128–134. doi: 10.1089/jmf.2013.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng T., Shu G., Yang Z., Mo S., Zhao Y., Mei Z. Antidiabetic effect of total saponins from Entada phaseoloides (L.) Merr. In type 2 diabetic rats. J Ethnopharmacol. 2012;139:814–821. doi: 10.1016/j.jep.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Nam K.Y., Ko S.R., Choi K.J. Relationship of saponin and non-saponin for the quality of ginseng. J Ginseng Res. 1998;22(4):274–283. [Google Scholar]
- 24.Wang P.C., Zhao S., Yang B.Y., Wang Q.H., Kuang H.X. Anti-diabetic polysaccharides from natural sources: a review. Carbohydrate Polymers. 2016;148:86–97. doi: 10.1016/j.carbpol.2016.02.060. [DOI] [PubMed] [Google Scholar]
- 25.Zou S., Zhang X., Yao W., Niu Y., Gao X. Structure characterization and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Carbohydrate Polymers. 2010;80(4):1161–1167. [Google Scholar]
- 26.Zhang S., Li X.Z. Inhibition of α-glucosidase by polysaccharides from the fruit hull of Camellia oleifera Abel. Carbohydrate Polymers. 2015;115:38–43. doi: 10.1016/j.carbpol.2014.08.059. [DOI] [PubMed] [Google Scholar]
- 27.Jiang S., Du P., An L., Yuan G., Sun Z. Anti-diabetic effect of Coptis Chinensis polysaccharide in high-fat diet with STZ-induced diabetic mice. International Journal of Biological Macromolecules. 2013;55:118–122. doi: 10.1016/j.ijbiomac.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Park H.J., Rhee M.H., Park K.M., Nam K.Y., Park K.H. Effect of non-saponin fraction from Panax ginseng on cGMP and thromboxane A2 in human platelet aggregation. J Ethnopharmacol. 1995;49:157–162. doi: 10.1016/0378-8741(95)01317-2. [DOI] [PubMed] [Google Scholar]
- 29.Kurimoto H., Nishijo H., Uwano T., Yamaguchi H., Zhong Y.M., Kawanishi K., Ono T. Effects of nonsaponin fraction of red ginseng on learning deficits in aged rats. Physiol Behav. 2004;82:345–355. doi: 10.1016/j.physbeh.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Sohn E.H., Kim J.H., Choi H.S., Park H.J., Kim B.O., Rhee D.K., Pyo S.N. Immunomodulatory effects of non-saponin red ginseng components on innate immune cells. J Ginseng Res. 2008;32(1):67–72. [Google Scholar]
- 31.Kim E.H., Park J.D., Pyo S.N., Rhee D.K. Effects of non-saponin red ginseng components on multi-drug resistance. J Ginseng Res. 2007;31(2):74–78. [Google Scholar]
- 32.Lee S.H., Park M.H., Heo S.J., Kang S.M., Ko S.C., Han J.S., Jeon Y.J. Dieckol isolated from Echlonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postproandial hyperglycemia in streptozotocin-induced diabetic mice. Food and Chemical Toxicology. 2010;48:2633–2637. doi: 10.1016/j.fct.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Shan X., Liu X., Hao J.J., Cai C., Fan F., Dun Y., Zha X., Liu X., Li C., Yu G. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int J Biol Macromol. 2016;82:249–255. doi: 10.1016/j.ijbiomac.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Kasabri V., Afifi F.U., Hamdan I. In vitro and in vivo acute antihyperglycemic effects of of five selected indigenous plants from Jordan used in traditional medicine. J Ethnopharmacol. 2011;133:888–896. doi: 10.1016/j.jep.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Yang Z., Wei X. Sugar compositions, α-glucosidase inhibitory and α-amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int J Biol Macromol. 2010;47:534–539. doi: 10.1016/j.ijbiomac.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S., Narwal S., Kumar V., Prakash O. α-glucosidase inhibitors from plants: a natural approach to treat diabetes. Phcog Rev. 2011;5(9):19–29. doi: 10.4103/0973-7847.79096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamid H.A., Yusoff M.M., Liu M., Karim M.R. α-glucosidase and α-amylase inhibitory constituents of Tinospora crispa: isolation and chemical profile confirmation by ultra-high performance liquid chromatography-quadrupole time-of-flight/mass spectrometry. J Funct Foods. 2015;16:74–80. [Google Scholar]
- 38.Zuraini A., Zamhuri K.F., Yaacob A., Siong C.H., Selvarajah M., Ismail A., Hakim M.N. In vitro anti-diabetic activities and chemical analusis of polypeptide-k and oil isolated from seeds of Momordica charantia (Bitter Gourd) Molecules. 2012;17:9531–9640. doi: 10.3390/molecules17089631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston K., Sharp P., Clifford M., Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Letters. 2005;579:1653–1657. doi: 10.1016/j.febslet.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 40.Manzano S., Williamson G. Plyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol Nutr Food Res. 2010;54:1773–1780. doi: 10.1002/mnfr.201000019. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi Y., Suzuki M., Satsu H., Arai S., Hara Y., Suzuki K., Miyamoto Y., Shimizu M. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem. 2000;48:5618–5623. doi: 10.1021/jf0006832. [DOI] [PubMed] [Google Scholar]
- 42.Wang A., Clifford M.N., Sharp P. Analysis of chlorogenic acids in beverages prepared from Chinese health foods and investigation, in vitro, of effects on glucose absorption in cultured Caco-2 cells. Food Chem. 2008;108:369–373. [Google Scholar]
- 43.Andrade-Cetto A., Vazquez R.C. Gluconeogenesis inhibition and phytochemical composition of two Cercropia species. J Ethnopharmacol. 2010;130:93–97. doi: 10.1016/j.jep.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Alberti K.G., Zimmet P.Z. New diagnostic criteria and classification of diabetes-again? Diabet Med. 1998;7:535–536. doi: 10.1002/(SICI)1096-9136(199807)15:7<535::AID-DIA670>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 45.Zheng W., Welihinda A., Mechanic J., Ding H., Zhu L., Lu Y., Deng Z., Sheng Z., Lv B., Chen Y. EGT1442, a potent and selective SGLT2 inhibitor, attenuates blood glucose and HbA1c levels in db/db mic and prolongs the survival of stroke-prone rats. Pharmacol Res. 2011;63:284–293. doi: 10.1016/j.phrs.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Tan K.C.B., Shiu S.W.M., Wong Y., Tam X. Serum advanced glycation end products (AGEs) are associated with insulin resistance. Diabetes Metab Res Rev. 2011;27:488–492. doi: 10.1002/dmrr.1188. [DOI] [PubMed] [Google Scholar]
- 47.Jo R., Min J.R., Jeong S.H. Anti-diabetic effects of water extracts of Rehmannia glutinosa libosch root in 3T3-L1 adipocytes and C57BL/KsJ-db/db mice. J Korean Soc Food Sci Nutr. 2018;47(10):957–965. [Google Scholar]
- 48.Klover P.J., Mooney R.A. Hepatocytes: critical for glucose homeostasis. Int J Biochem Cell Biol. 2004;36:753–758. doi: 10.1016/j.biocel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Basu A., Basu R., Shah P., Vella A., Johnson C.M., Nair K.S., Jensen M.D., Schwenk W.F., Rizza R.A. Type 2 diabetes impairs splanchnic uptake of glucose but does not alter intestinal glucose absorption during enteral glucose feeding: additional evidence for a defect in hepatic glucokinase activity. Diabetes. 2001;50:1351–1362. doi: 10.2337/diabetes.50.6.1351. [DOI] [PubMed] [Google Scholar]
- 50.Huang X., Liu G., Guo J., Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14(11):1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan F., Zhang J., Zhang L., Zheng X. Mulberry anthocyanin extract regulates glucose metabolism by promotion of glycogen synthesis and reduction of gluconeogenesis in human HepG cell. Food Funct. 2016;7:425. doi: 10.1039/c5fo00841g. [DOI] [PubMed] [Google Scholar]
- 52.Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World Journal of Diabetes. 2010;1(3):68–75. doi: 10.4239/wjd.v1.i3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padiya R., Chowdhury D., Borkar R., Srinivas R., Bhadra M.P., Banerjee S.K. Galic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLOS ONE. 2014;9(5) doi: 10.1371/journal.pone.0094228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu T.Y., Shi C.X., Gao R., Sun H.J., Xiong X.Q., Ding L., Chen L., Li Y.H., Wang J.I., Kang Y.M. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clinical Science. 2015;129:839–850. doi: 10.1042/CS20150009. [DOI] [PubMed] [Google Scholar]
- 55.Granado-Serrano A.B., Martin M.A., Goya L., Bravo L., Ramos S. Time-course regulation of survival pathways by epicatechin on HepG2 cells. J Nutr. 2010;103:168–179. doi: 10.1016/j.jnutbio.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Martin M.A., Granado-Serrano A.B., Ramos S., Izquierdo Pulido M., Bravo L., Goya L. Cocoa flavonoids up-regulate antioxidant enzyme activity via the ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J Nutr Biochem. 2010;21:196–205. doi: 10.1016/j.jnutbio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 57.Borradaile N.M., de Dreu L.E., Huff M.W. Inhibition of ne HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes. 2003;52:2554–2561. doi: 10.2337/diabetes.52.10.2554. [DOI] [PubMed] [Google Scholar]
- 58.Cao H., Hininger-Favier I., Kelly M.A., Benaraba R., Dawson H.D., Coves S., Roussel A.M., Anderson R.A. Green tea polyphenol extract regulates the expression of genes involved in glucose uptake and insulin signaling in rats fed a high fructose diet. J Agric Food Chem. 2007;55:6372–6378. doi: 10.1021/jf070695o. [DOI] [PubMed] [Google Scholar]
- 59.Nordlie R., Bode A.M., Foster J.D. Recent advances in gepatic glucose-6-phosphate regulation and function. Proc Soc Exp Biol Med. 1993;3:274–285. doi: 10.3181/00379727-203-43600. [DOI] [PubMed] [Google Scholar]
- 60.Mithieux G. New knowledge regarding glucose-6-phosphatase gene and proein and their roles in the regulation of glucose metabolism. Eur J Endocrinol. 1997;136:137–145. doi: 10.1530/eje.0.1360137. [DOI] [PubMed] [Google Scholar]
- 61.Davies G.F., Khandelwal R.L., Wu L., Juurlink B.H., Roesler W.J. Inhibition of phosphoenolpyruvate carboxykinase (PEPCK) gene expression by troglitazone: a peroxisome proliferators-activated receptor-gamma (PPAR gamma)- independent, antioxidant-related mechanism. Biochem Pharmacol. 2001;62:1071–1079. doi: 10.1016/s0006-2952(01)00764-x. [DOI] [PubMed] [Google Scholar]
- 62.Kim H.S., Noh J.H., Hong S.H., Hwang Y.C., Yang T.Y., Lee M.S., Kim K.W., Lee M.K. Rosiglitazone stimulates the release and synthesis of insulin by enhancing GLUT-2, glucokinase and BETA2/NeuroD expression. Biochem Biophys Res Commun. 2008;367:623–629. doi: 10.1016/j.bbrc.2007.12.192. [DOI] [PubMed] [Google Scholar]
- 63.Ohtsubo K., Takamatsu S., Minowa M.T., Yoshida A., Takeuchi M., Marth J.D. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;123:1307–1321. doi: 10.1016/j.cell.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 64.Valera A., solanes G., Fernández-Alvarez J., Pujol A., Ferrer J., Asins G., Gomis R., Bosch F. Expression of GLUT-2 antisense RNA in beta cells of transgenic mice leads to diabetes. J Biol Chem. 1994;269:28543–28546. [PubMed] [Google Scholar]
- 65.Zhang F., Xu X., Zhang Y., Zhou B., He Z., Zhai Q. Gene expression profile analysis of type 2 diabetic mouse liver. PLOS ONE. 2013;8(3) doi: 10.1371/journal.pone.0057766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davies S.P., Sim A.T., Hardie D.G. Location and function of three sites phosphorylated on rat acetyl-CoA carboxylase by the AMP-activated protein kinase. Eur J Biochem. 1990;187:183–190. doi: 10.1111/j.1432-1033.1990.tb15293.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X.D., Yan J.W., Yan G.R., Sun X.Y., Ji J., li Y.M., Hu Y.H., Wang H.Y. Pharmacological inhibition of diacylglycerol acyltransferase 1 reduces body weight gain, hyperlipidemia, and hepatic steatosis in db/db mice. Acta Pharmacol Sin. 2010;31:1470–1477. doi: 10.1038/aps.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]