Abstract
Objectives:
Parents living with HIV who disclose their HIV status to their children could benefit from the parental HIV disclosure. However, it is also very challenging because of persistent stigma and discrimination against HIV. This report describes the study design and protocol of the “Interactive Communication with Openness, Passion, and Empowerment (iCOPE)” randomized controlled trial aimed at assisting parents living with HIV in conducting culturally and developmentally appropriate disclosure to their uninfected children in China through trainings among both parents living with HIV and healthcare providers.
Methods:
A total of 791 parents living with HIV with children aged between 6 and 15 years and 357 healthcare providers were randomized into either the intervention group or control group. Intervention package for parents consisted of five 2-h sessions focusing on positive coping, disclosure decision making, developing a developmentally appropriate disclosure plan, and accessing social support and post-disclosure counseling. The intervention for healthcare providers was made up of two 45-min sessions organized around two primary themes: knowledge of child cognitive development and effective parent–child communication skills in the context of parental disclosure. The control group received nutritional education of either five 2-h sessions (parents) or two 45-min sessions (healthcare providers). The outcome assessments were conducted at baseline, 6, 12, 18, 24, 30, and 36 months.
Conclusion:
The iCOPE study is among the first efforts to develop and evaluate a theory-based and multi-level intervention to promote culturally and developmentally appropriate parental HIV disclosure in China. It has implications for healthcare providers, social workers, and policy makers as it will provide efficacy data on how to enhance appropriate parental HIV disclosure and will shed light on developing a clinical guideline regarding parental HIV disclosure in China and other low- and middle-income countries.
Keywords: HIV/AIDS, disclosure, parents, China, randomized controlled trial, intervention
Introduction
Because programs preventing prenatal HIV transmission are reaching a greater number of pregnant women worldwide and are successfully reducing vertical transmission, an ever-increasing majority of children born to HIV-positive mothers are uninfected. In addition, new medical innovations and increasing availability of antiretroviral therapy (ART) have improved the health and longevity of HIV-positive parents, which means they are more likely to raise their children for many years after the initial diagnosis.1 For parents living with HIV (PLH), disclosing their HIV infection to their seronegative children (“parental HIV disclosure”) becomes an increasingly important issue in terms of the well-being of parents, children, and families.
The global literature suggests that developmentally appropriate parental disclosure, particularly for young children, can have positive effects for both the parent and child, while non-disclosure and unplanned disclosure can result in negative outcomes.2–4 However, for multiple reasons including fear of stigma and the psychological burden such knowledge might place on their children, PLH struggle about whether, when, what, and how to disclose their HIV infection to children.5,6 Many of them do not disclose primarily because they lack the confidence and behavioral skills to do it appropriately and effectively.7 To date, the issues surrounding parental HIV disclosure have been understudied, particularly in low- and middle-income countries (LMICs) including China, where the HIV epidemic has been steadily expanding.
While the actual HIV seroprevalence in China remains uncertain, the current official estimate of number of people living with HIV exceeds 1.2 million with 850,000 reported cases.8 Our preliminary data confirm the global literature that disclosure of parental HIV status to children is a significant challenge for PLH.9,10 We also investigated the societal beliefs that will directly and indirectly impact the process and consequences of parental HIV disclosure to their children.4,5 First, stigmatization and perceptions of “fate” in relation to illness and death affects parental decision-making in relation to disclosure of their serostatus to their children.4,5 Within the context of familial obligations and the centrality of the family within the Chinese social structure, the dissolution of a family regardless of reason (e.g. death) is considered shameful for all family members including children.11–13 In addition, parental illness or death may signify “bad fate,” something that is at odds with the “natural” order of life and death.14
Second, sharing emotional events with children is discouraged. The family-orientated societal perspective discourages the disclosure of distressful events in general and HIV serostatus in particular.15,16 Few parents or children would openly grieve or be willing to discuss bereavement-related family issues (particularly issues associated with shame or stigma). Compounding this perspective is the widely held belief in China that children do not have emotional problems.17 A child’s needs to be aware of parental illness or to be part of the family support system are therefore more likely to be ignored or misunderstood in Chinese culture.17
In addition, respect and compliance to authority (including healthcare providers) could facilitate disclosure. Healthcare providers are generally considered authoritative in China. Professional assistance or guidance from care providers could therefore have a significant positive effect on the decision-making process of parental HIV disclosure. However, laws and institutional policy relating to HIV disclosure/notification are inconsistent in China.18 After a patient is diagnosed HIV positive, healthcare providers often struggle to decide who should be informed first: patient or family members. Some healthcare providers avoid direct notification to patients due to concerns about their ability to cope, need to protect other family members (e.g. spouse), and need for family support to the patient. Such practice may inhibit PLH from talking directly and truthfully with their children and may increase the likelihood of unplanned disclosure by other family members or through children’s own observations.
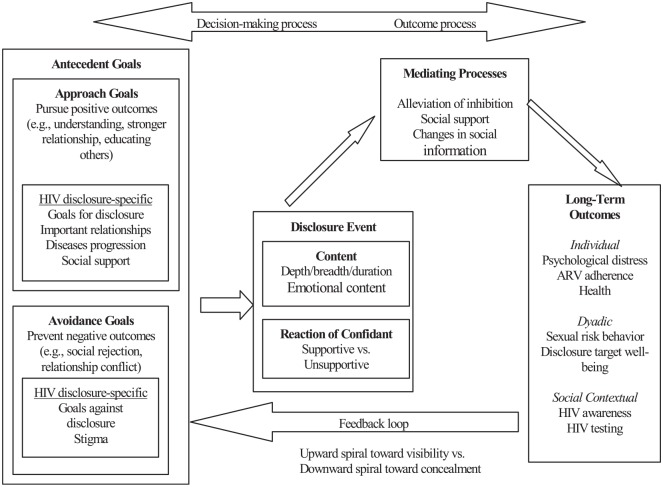
Given parental HIV disclosure is a big challenge for PLH in China and parents often need professional support in making a decision about disclosure and managing developmentally appropriate disclosure to their children, we developed the theory-based, multi-level intervention “Interactive Communication with Openness, Passion, and Empowerment” (iCOPE) based on our preliminary studies and adaptation of existing disclosure-related interventions. The iCOPE intervention design was guided by a conceptual framework (see Figure 1) adapted based on Disclosure Process Model (DPM)19 and was also consistent with the Piaget’s theory of children’s development.20 A cluster randomized controlled trial (RCT) project was conducted from 2012 to 2018 to evaluate the efficacy of the iCOPE intervention with two parallel groups (intervention and control) among PLH (either fathers or mothers) and healthcare providers. Main findings will be reported in other manuscripts soon. This current study protocol covers main items recommended by the Standard Protocol Items Recommendations for Intervention Trials (SPIRIT 2013) checklist (see supplemental materials).21
Figure 1.
Conceptual framework: disclosure process model.
This conceptual framework was adapted from Chaudoir et al.19
Methods
Study setting
The proposed intervention was conducted in Guangxi Zhuang Autonomous Region (“Guangxi”) in southern China. Guangxi is one of the regions in China that is experiencing the fastest growth of the HIV epidemic. In 1996, the first person was diagnosed with HIV in Guangxi; since then, Guangxi has witnessed an alarming increase in HIV prevalence. A total of 124,282 HIV/AIDS cases had been reported by December 2017, representing a 78.70% increase since June 2011 (69,548 HIV/AIDS cases) and placing Guangxi third among 31 Chinese provinces in terms of HIV seropositive cases.22
In Guangxi, while both local Centers for Disease Control and Prevention (CDC) and local hospitals can conduct HIV screening and counseling, there is one designated primary public hospital (specifically its HIV clinic) in each urban district/rural township that is working under the direction of the city/county CDC to conduct clinical management and semi-annual follow-ups for all HIV patients in the district/township. In collaboration with the Guangxi CDC, we ranked all 14 cities and 75 rural counties in Guangxi in terms of number of reported HIV/AIDS cases. We selected the top two cities (urban centers) and top eight rural counties with the largest number of reported HIV/AIDS cases to participate in the proposed study. In a similar fashion, the Guangxi CDC ranked urban districts in the two cities and townships in the eight rural counties and identified urban districts and rural townships with at least 200 HIV/AIDS cases. We randomly selected 40 of them as our project sites.
Participation eligibility
The inclusion criteria for PLH included (1) being at least 18 years of age, (2) having a confirmed diagnosis of HIV or AIDS, (3) living with at least one child with 6–15 years of age, and (4) having not disclosed their HIV status to their children. Both biological and non-biological parents (if they were the legal and primary guardians for the child, for example, an individual spending at least 50% of the time with the child) were eligible to participate, although the number of non-biological parents was very small. The exclusion criteria for PLH included (1) having linguistic, mental, or physical inability to respond to assessment questions or to participate in intervention; (2) being currently incarcerated or institutionalized for drug use or commercial sex; and (3) having a plan to permanently relocate outside the province within a year.
The inclusion criteria for healthcare providers included (1) being at least 18 years of age, (2) working at one of the participating HIV clinics, and (3) having regular contact with PLH. The exclusion criteria for healthcare providers included having a plan to permanently relocate outside the province within a year.
Recruitment and assignment of interventions
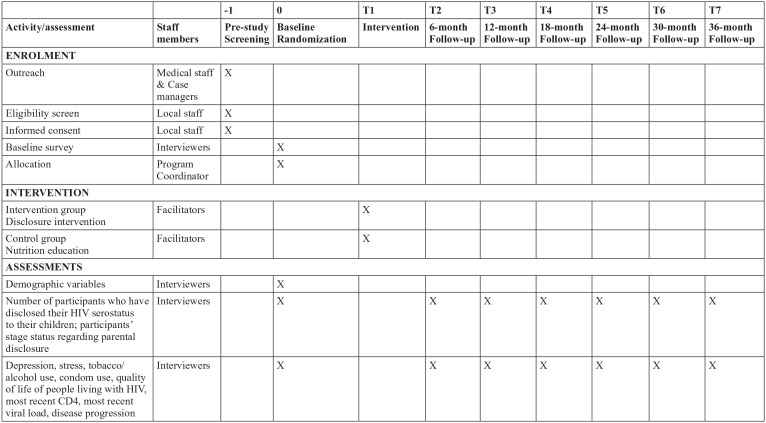
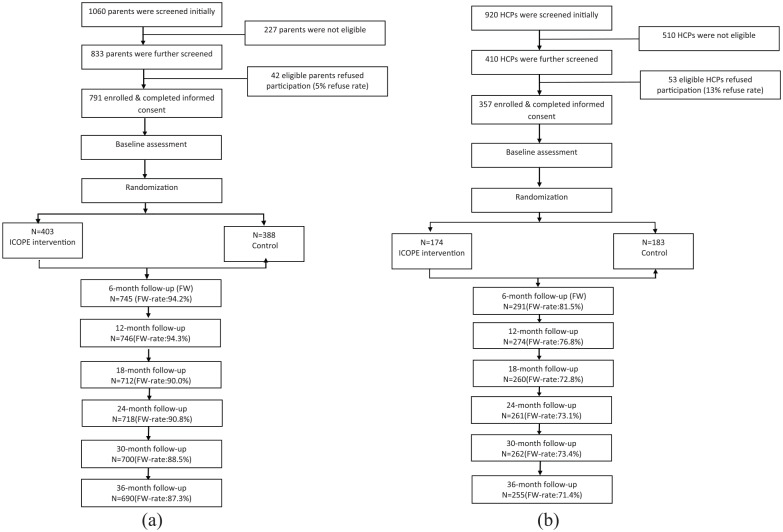
The time schedule of enrollment, interventions, and assessments for participants is demonstrated by a schematic diagram recommended by the SPIRIT 2013 (Figure 2). The sample size of the participants (both PLH and healthcare providers) at each time point is presented by the consort flowcharts (Figure 3). The PLH were recruited from the 40 participating HIV clinics (about 20 per clinic). Medical staff or case managers at HIV clinics referred potential participants to local team members who visited each clinic twice a week during the recruitment period. If both father and mother in a family were eligible, mother or physically healthier parent was invited to participate. Local team staff screened parents for eligibility and explained the study design including the potential benefits and risks and confidentiality issues. Local team staff emphasized that efforts would be taken to prevent any inadvertent direct or indirect disclosure of their serostatus to children or others during the research.
Figure 2.
Summary of schedule of enrollment, interventions, and assessments (following SPIRIT 2013).21
Figure 3.
Consort flowchart of iCOPE intervention trial: (a) for parents and (b) for healthcare providers.
The sample size of a follow-up survey may be larger than those in previous surveys because participants who completed baseline but missed a survey were allowed for future follow-ups.
The healthcare providers were recruited from the 40 clinics where we recruited PLH. Each of these HIV clinics was typically staffed with approximately 15 healthcare providers including physicians, nurses, case managers, counselors, and medical social workers. Local team members individually approached all the healthcare providers in HIV clinics (except those trained facilitators for parents intervention) as well as healthcare providers in other departments of the hospital who had regular contact with HIV patients; explained the study design including the purpose, procedure, risk and benefit, and confidentiality issues; and invited them to participate. All healthcare providers who agreed to participate provided written informed consent prior to baseline assessment.
Local program coordinators assigned the parents and healthcare providers by HIV clinics to either intervention or control condition (20 clinics each) using a stratified block randomization procedure.23 The stratified block randomization (rather than the simple randomization) was used to produce relatively comparable groups between conditions with regard to key contextual characteristics. We first stratified all 40 clinics into 10 blocks (with 4 clinics in each block) based on their similarities in (1) number of HIV-positive patients served by the clinics and (2) geographic locations (rural or urban). Then, we randomly assigned two clinics within each block to intervention and two to control. The PLH who consented for group sessions were organized into two groups in each clinic (with no more than 10 parents per group) for the delivery of intervention/control sessions.
Intervention protocol
Parent intervention
The parent intervention curriculum was modeled after the “Teaching, Raising, and Communicating with Kids (TRACK)” program24 and “Teens and Adults Learning to Communicate (TALC)” program.25 The TRACK program is composed of three sessions (children’s typical development stages and decision-making of disclosure; mother–child communication; and behavioral practice for disclosure) and we modeled after all the three sessions. The TALC is a 24-session program which can be organized into two modules for PLH alone and PLH and their adolescent children. We modified three of eight sessions (i.e. coping with illness, coping with meaning of illness, and planning for the future) in Module 1 (the module for PLH) with a focus on coping with HIV and post-disclosure adjustment. We substantially modified and adapted the intervention content and format for each intervention module based on our preliminary HIV disclosure studies to fit local cultural context.
The primary goal of the parent intervention was to prepare and assist PLH to make developmentally appropriate disclosure (or an articulated plan for such a disclosure) based on children’s age and psychosocial maturity, family dynamics, and clinical outcomes of parental HIV. The parent curriculum consisted of five interactive training sessions (120 min each session for 10 h total) with three specific focuses: understanding the stages of childhood cognitive development in the context of parental illness (Session 1 “Child’s readiness for disclosure”); improving the parents’ cognitive and behavioral skills related to parental HIV disclosure (Session 2 “Benefits and risks of disclosure,” Session 3 “How to tell and what to tell,” and Session 4 “Disclosure is an ongoing process”); and improving parental psychosocial well-being in adapting to living with HIV/AIDS (Session 5 “Cope with my infection/illness”). The curriculum addressed the issues of child and family strengths and community support across sessions.
Healthcare provider intervention
Tasker’s four-phase model (FPM) of disclosure emphasizes the importance of professional support in each of the four phases of a planned disclosure process (secrecy phase, exploratory phase, readiness phase, and disclosure phase).26 Although not all disclosures automatically start at the secrecy stage, the model suggests a critical role of healthcare providers and parent–provider relationship at different time points leading to disclosure. Guided by Tasker’s FPM, the goal of healthcare provider intervention was to train healthcare providers to assist PLH in creating an appropriate disclosure plan that could meet the individual needs of the children and families and provid PLH with continuous support during the disclosure process. The intervention curriculum consisted of two 45-min sessions organized around two primary themes: (1) knowledge of child cognitive development and (2) effective parent–child communication skills in the context of parental disclosure (e.g. how to help children understand HIV). These two sessions were developed by modifying (and shortening) similar components in the parent curriculum. The first component also contained a short clinical guide that was modeled after a single-page “Step by Step Guide for Conversation with Children (Toward Disclosure)” developed by an interdisciplinary team of clinicians and researchers in South Africa (“SA Guide”).27 Although we were not aware of evaluation data regarding its efficacy, the SA Guide was based on the principles of child cognitive development and was originally designed for use by healthcare professionals and counselors working with HIV-positive children and their caregivers. The SA Guide analyzed the developmental level of the children and recommended level of disclosure (no disclosure, early disclosure, partial disclosure, and full disclosure), content of disclosure, and the aim of the disclosure for four age groups of children (0–4 years, 5–7 years, 8–11 years, 12–14 years). We culturally adapted the SA Guide in China and used it in training healthcare providers in China to help PLH to make appropriate disclosure to their uninfected children.
Control group protocol
The attention control condition for parents was five 2-h sessions of nutrition education curriculum. The nutrition curriculum was modeled after the “Simply Good Eating” curriculum developed at University of Minnesota.28 The Minnesota curriculum was modified in accordance with current “Dietary Guidelines for Chinese Residents.”29 The modified curriculum consisted of five 2-h interactive training sessions with aims to increase parents’ knowledge of nutrition (Session 1: Food variety; Session 2: Food for growing child), healthy diets and cooking practice (Session 3: Fat, salt, and sugar; Session 4: Fruits, vegetables, and minerals), and food safety (Session 5: Food safety). The control curriculum for healthcare providers was a shortened version (90 min) of the nutrition education curriculum with two components: food for growing child and food safety.
Staff training
Intervention facilitators (two nurses or other paraprofessionals from each of the 40 intervention trial sites) were trained and certified to deliver the parent sessions (with separate training for intervention and control facilitators). Training for parent intervention was a 4-day retreat including 3 days on content of the sessions and facilitator skills and 1 day on research ethics. Eight (four pairs) health educators from Guangxi CDC were trained to deliver the care provider sessions through a 2-day training retreat (1 day for intervention content and 1 day for research ethics). Survey interviewers (two CDC staff in each study site) also received 2-day training (1 day for survey study and 1 day for research ethics). In the training workshops, we used the drafts of intervention manuals (for both PLH and healthcare provider interventions) and survey protocol and then finalized the manuals and protocol based on the feedback collected from the training workshops. The intervention manuals were not “word for word,” but covered all the guidelines, knowledge points, instructions of intervention activities, and supplemental materials.
Intervention delivery
Parent sessions
The five 2-h parent intervention and control sessions were delivered one session per week for 5 weeks in the clinics where the parents were recruited or nearby community space if the spaces in some of the clinics were inadequate for conducting the group sessions. The sessions were delivered in either group sessions (for parents who felt comfortable doing so) or tailored for one-on-one sessions (for parents who preferred this option). Two trained facilitators delivered the materials through discussions, role-play, exercise, and/or games (for group sessions). The same two facilitators were assigned to deliver all the five sessions in each clinic to increase group cohesion and/or rapport with parents. The day of each session was scheduled at least 1 week in advance with periodic reminders to the parents, including a reminder by the facilitator on the day before the scheduled session. Refreshments were served at each session.
Healthcare provider sessions
The 90-min healthcare provider training (both intervention and control conditions) consisted of two 45-min modules that were delivered individually or in a small group (three to five healthcare providers) in the clinic setting by trained facilitators (e.g. health educators from the provincial CDC). Ideally, two modules were delivered on the same day. However, given the variation of clinical schedules among healthcare providers in a clinic, the delivery schedule was flexible and individually tailored (e.g. two modules could be given on two different days or over multiple short sessions). Immediately following randomization, the intervention facilitators reviewed the situation of the clinics and contextualized each session as necessary based on the healthcare providers’ workload and clinic schedule. Facilitators also worked with each healthcare provider during the consent process to develop an individualized plan/schedule for assessment, training, and follow-up.
Intervention fidelity
The following measures were used to assure fidelity to content and delivery of the intervention protocol: (1) institution of monitoring actual intervention implementation. The assistant intervention facilitators completed the fidelity process form for each session including content delivered, time allocated, participation rate, and main activities covered by the sessions. The local team staff collected and checked the fidelity process forms promptly. If any discrepancy between the protocol and the implementation emerged, the intervention facilitators would be informed in a timely manner and necessary steps would be taken to prevent deviations from the intervention protocol; and (2) audio-recording all the sessions. After each session, the facilitators uploaded the recording to a designated USB flash drive. The Chinese investigators randomly selected and reviewed 20% of the sessions and completed the fidelity process form for these sessions. These “independent” process measures were compared with the ones completed by the facilitators and feedback concerning fidelity would be provided to the facilitators promptly.
Outcome measures
Primary outcomes for parent measures included the number of participants who have disclosed their HIV serostatus to their children and participants’ stage status regarding parental disclosure. Other key outcomes included perceptions and plans for parental disclosure, Derlega’s scales on reasons for disclosure or non-disclosure,30 Delaney’s scale for child’s reaction to the disclosure,31 depressive symptoms (Center for Epidemiologic Studies Depression Scale (CES-D)),32,33 stress (Perceived Stress Scale),34 substance use (tobacco use, alcohol use (alcohol use disorders identification test (AUDIT)),35 and other drug use), sexual behavior and reproductive health, HIV-related quality of life (Medical Outcomes Study HIV Health Survey (MOS-HIV)),36 medical adherence (treatment history, knowledge about ART and adherence, and adherence to care and medications), and HIV clinical and immunologic status (most recent CD4 count, viral load, and disease progression).
Primary outcomes for healthcare provider included experience in HIV notification and disclosure. Other key outcomes included knowledge of child cognitive development, knowledge of effective parent–child communication skills, perceived roles of healthcare providers in the disclosure process, and perceived self-efficacy and self-readiness of assisting their clients in parental disclosure.
Most of the demographic, psychosocial, and behavioral measurements we used in the project were valid and reliable measures with acceptable psychometric properties. The questionnaires used in this study were also pilot tested among 20 parents and 10 healthcare providers to get participants’ perspectives on the clarity, cultural sensitivity, and developmental appropriateness of relevant measures.
Data collection procedure
Parent survey
Interviewers (who were blinded to the intervention assignment) administered the baseline and six follow-up surveys (every 6 months up to 36 months post-intervention) to the parents. The parent surveys were administered in a private room (e.g. doctor’s office) at district/township hospitals where these parents were recruited. Interviewers administered the questionnaire orally to a parent one-on-one. The interviewer read each question in the questionnaire, and the participant gave an oral response to the interviewer. By using this method, we could ensure that varying degrees of literacy did not affect the individual’s ability to understand the items. Clarifications were provided by the interviewers as needed. The survey usually took 60 min for parents. Participants were offered a short break after 30 min of assessment or as needed.
Healthcare provider survey
The participating healthcare providers were asked to complete baseline and six follow-up surveys (6-, 12-, 18-, 24-, 30-, and 36-months post-intervention) in HIV clinics where they were recruited. The questionnaires were self-administered and interviewers were presented during the survey to provide necessary clarification. The survey took about 15 min to complete.
Data management and data protection
A data manager/biostatistician at University of South Carolina (UofSC) developed the data management procedures and data entry modules for all quantitative data and trained the data manager at Guangxi CDC. All data were double-entered into SPSS data station and backed up daily by skilled research assistants (the SPSS file can be directly read into SAS or covert to seven other different formats for analysis if needed). All the hard copies of the assessment instruments were kept in a secured space at the collaborators’ offices at Guangxi CDC. Participants’ confidentiality was maintained through the use of arbitrary identification numbers (ID) on questionnaires and in databases. Upon completion of the survey, the completed questionnaire, which could be linked to the participant only by a unique numerical ID, was put in a sealed envelope and returned to the project office at Guangxi CDC for data entry. A master list of IDs and name of research participants was kept in a locked file cabinet and password-protected file at principal investigator’s (PI) office at UofSC and at in-country PI’s office at Guangxi CDC.
Sample size and power analysis
Because of the absence of empirical data on the effect of parental disclosure intervention in China or any other LMICs, we conservatively assumed a “smaller-than-medium” effect of our proposed intervention on the primary parent outcomes (e.g. rate of parental disclosure or plan to disclosure). According to the range of effect size established by Cohen,37 Cohen’s d = 0.20 represents a “small effect” and d = 0.50 represents a “medium effect.” To be conservative in our power analysis, we assumed a smaller-than-medium effect size (Cohen’s d = 0.35) for the long-term effect (i.e. 36-month follow-up) of our proposed intervention. In addition, we assumed a two-tailed test at alpha = 0.05.
The sample size of the participants by 36-month follow-up was 690. However, the unit of randomization in this study was not the individual parents, but rather the clinics; the sample size calculation, assuming that the unit of randomization was the individual, needs to be adjusted for clustering effect, for example, intraclass correlation (ICC). Donner et al.23 developed a procedure to determine an “effective sample size (ESS)” in such situation: ESS = kn/[1 + (n – 1)ICC] (where k is the number of clusters, n is the number of individuals in each cluster, and kn is the actual sample size if the unit of randomization is the individual). Because there was no clinic level ICC data available in the literature, we conservatively assumed a large ICC of 0.10 for clustering effect (for a total of 40 clinics and two groups of parents in each clinic with a maximum of 10 parents per group). According to Donner’s procedure,23 a sample size of 690 yields an ESS of 363 with an ICC of 0.10.
According to the sample size estimation procedure developed by Cohen,37 when the sample size was 360 (or 180 participants in each intervention cell) and the effect size was 0.35 (Cohen’s d), the power of analysis would be 0.91. Thus, our sample of 791 at baseline (or 690 at 36-month follow-up) provides adequate power to test the efficacy of this intervention.
Discussion
We hypothesize that the iCOPE intervention will demonstrate efficacy in helping PLH to make developmental appropriate disclosure to children or make a developmentally appropriate plan of disclosure; as a result, the intervention will demonstrate short-, medium-, and long-term efficacy in improving well-being of parents, children, and families; likewise, the intervention will increase provider awareness, willingness, and confidence in their role in assisting PLH with disclosure to children.
The proposed study has important innovations and potentials to advance scientific knowledge in several ways. First, it will provide new cross-cultural evidence to support theory-based intervention. The proposed study addresses the dearth of targeted interventions supporting parental efforts in disclosing their HIV status to their children in resource-poor settings. Although there is a growing interest in parental HIV disclosure among researchers and practitioners, the theo-retical frameworks guiding the disclosure research and the development of culturally and developmentally appropriate interventions are limited. Although some theoretical/conceptual models have been developed and tested for HIV disclosure,38,39 they have not been explicitly applied to parental disclosure to uninfected children. The innovative use of the child development stage theory and DPM in this research will enable us to address the developmental and cognitive aspects of parental HIV disclosure and generate novel insights and valuable data that will inform the development of evidence-based intervention strategies to facilitate parental HIV disclosure among PLH in low-resource settings worldwide.
Second, the proposed study suggests an innovative paradigm shift. Disclosure has been treated as a single event (disclosed vs non-disclosed) in many studies without taking into consideration the child’s cognitive development stage.4 The iCOPE intervention is centered on child developmental theory with a shift of conceptualization of parental disclosure from a “discrete event” to a “gradient process” that is aligned with a child’s cognitive development. This study will emphasize a child’s cognitive ability to understand information of an emotion-laden nature during the process of disclosure as well as the importance of such disclosure to their normal development and maturation.
Last but not least, this study applies a novel intervention approach. While available studies suggested high desire from PLH to obtain professional guidance and support during the disclosure process due to complexity of parental HIV disclosure to children,40 intervention studies related to parental HIV disclosure to children were limited worldwide with no evidence-based intervention or clinical guideline existing in low-resource settings.41 The proposed intervention takes a system perspective to engage both parents and care providers, examines the potential role of gender in parental disclosure by including both mothers and fathers, and takes a “task-shifting” approach by training a large number of nurses or other paraprofessionals to deliver the parent intervention so that the intervention can be implemented and sustained in typical HIV care settings.
The iCOPE intervention is among the first efforts to innovatively develop and test a multi-level theory-based parent HIV disclosure to promote developmental appropriate disclosure to children or make a developmentally appropriate plan of disclosure, and to improve psychological well-being and quality of life of parents, children, and families. The intervention also aims to increase provider awareness, willingness, and confidence in their role in assisting PLH with disclosure to children. Once proven efficacious, iCOPE could be potentially adapted and tailored to other settings in China and LMICs where HIV disclosure remains a big challenge for parents living with HIV. We will report the intervention efficacy when we finish data analysis and the impact evaluation.
Supplemental Material
Supplemental material, parents_survey_english_sage_open_1 for iCOPE, a multi-level, cluster randomized, 36-month, parallel-group study to assess the efficacy of HIV disclosure intervention in HIV parental disclosure among parents living with HIV in China by Xiaoming Li, Shan Qiao and Yuejiao Zhou in SAGE Open Medicine
Acknowledgments
We acknowledge the inputs of members in the iCOPE project including but not limited by Dr Wei Liu, Dr Zhiyong Shen, Dr Weimin Yang, and Ms Wenbin Chen from Guangxi CDC; Dr Danhua Lin from Beijing Normal University; Drs George Tam, Wendi Da, and Xueying Yang at University of South Carolina. We appreciate the assistance of Ms Joni Zwemer during the manuscript preparation.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Wayne State University (052112B3F), University of South Carolina (CR00018564), and Guangxi Center for Disease Control and Prevention (IRB00001584) institutional review boards.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIH/NICHD R01HD074221. The funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Informed consent: Written informed consent was obtained from all subjects before the study. Written informed consent was obtained from the legally authorized representatives (parents in most cases) of all the minor subjects (including assent from them) prior to the data collection and the intervention implementation.
Trial registration: Clinical Trials.gov Register Number NCT04051177 Protocol ID:10006371
ORCID iD: Shan Qiao  https://orcid.org/0000-0001-9685-0277
https://orcid.org/0000-0001-9685-0277
Supplemental material: Supplemental material for this article is available online.
References
- 1. Enger C, Graham N, Peng Y, et al. Survival from early, intermediate, and late stages of HIV infection. JAMA 1996; 275(17): 1329–1334. [PubMed] [Google Scholar]
- 2. Hawk ST. Disclosures of maternal HIV infection to seronegative children: a literature review. J Soc Personal Relat 2007; 24: 657–673. [Google Scholar]
- 3. Murphy DA. HIV-positive mothers’ disclosure of their serostatus to their young children: a review. Clin Child Psychol Psychiatry 2008; 13(1): 105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qiao S, Li X, Stanton B. Disclosure of parental HIV infection to children: a systematic review of global literature. AIDS Behav 2013; 17(1): 369–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaudoir SR, Fisher JD, Simoni JM. Understanding HIV disclosure: a review and application of the disclosure processes model. Soc Sci Med 2011; 72(10): 1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thorne C, Newell ML, Peckham CS. Disclosure of diagnosis and planning for the future in HIV-affected families in Europe. Child Care Health Dev 2000; 26(1): 29–40. [DOI] [PubMed] [Google Scholar]
- 7. Corona R, Beckett MK, Cowgill BO, et al. Do children know their parent’s HIV status? Parental reports of child awareness in a nationally representative sample. Ambul Pediatr 2006; 6(3): 138–144. [DOI] [PubMed] [Google Scholar]
- 8. Xinhua .Net. China reports HIV/AIDS numbers, zero infections by blood transfusions, Yang Y. (editor), 2018, http://www.xinhuanet.com/english/2018-09/29/c_137501310.htm
- 9. Faithfull J. HIV-positive and AIDS-infected women: challenges and difficulties of mothering. Am J Orthopsychiatry 1997; 67(1): 144–151. [DOI] [PubMed] [Google Scholar]
- 10. Pilowsky DJ, Sohler N, Susser E. Reasons given for disclosure of maternal HIV status to children. J Urban Health 2000; 77(4): 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chin P. Chinese Americans. In: Lipson JG, Dibble SL, Minarik PA. (eds) Culture & nursing care: a pocket guide. San Francisco, CA: UCSF Nursing Press, 1996, pp. 74–81. [Google Scholar]
- 12. Kim BS, Yang PH, Atkinson DR, et al. Cultural value similarities and differences among Asian American ethnic groups. Cultur Divers Ethnic Minor Psychol 2001; 7(4): 343–361. [DOI] [PubMed] [Google Scholar]
- 13. Tong KL, Spicer BJ. The Chinese palliative patient and family in North America: a cultural perspective. J Palliat Care 1994; 10(1): 26–28. [PubMed] [Google Scholar]
- 14. Zhao G, Li X, Fang X, et al. Care arrangements, grief and psychological problems among children orphaned by AIDS in China. AIDS Care 2007; 19(9): 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tse C-Y, Chong A, Fok SY. Breaking bad news: a Chinese perspective. Palliat Med 2003; 17(4): 339–343. [DOI] [PubMed] [Google Scholar]
- 16. Yoshioka MR, Schustack A. Disclosure of HIV status: cultural issues of Asian patients. AIDS Patient Care STDS 2001; 15(2): 77–82. [DOI] [PubMed] [Google Scholar]
- 17. Tseng W-S, Wu DY. Chinese culture and mental health. Cambridge, MA: Academic Press, 2013. [Google Scholar]
- 18. Li L, Lin C, Wu Z, et al. To tell or not to tell: HIV disclosure to family members in China. Develop World Bioethic 2008; 8: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaudoir SR, Fisher JD, Simoni JM. Understanding HIV disclosure: a review and application of the Disclosure Processes Model. Soc Sci Med 2011; 72(10): 1618–1629. DOI: S0277-9536(11)00191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piaget J. Piaget’s Theory, Handbook of Child Psychology. New York: John Wiley & Sons, 1983. [Google Scholar]
- 21. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. Bmj 2013; 346: e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guangxi Center of Disease Control and Prevention. Know your status, embrace health. 2018; http://www.gxcdc.com/zxdt/2018/1204/9594.html (accessed 10 December 2018). [Google Scholar]
- 23. Donner A, Birkett N, Buck C. Randomization by cluster: sample size requirements and analysis. Am J Epidemiol 1981; 114(6): 906–914. [DOI] [PubMed] [Google Scholar]
- 24. Murphy DA, Armistead L, Marelich WD, et al. Pilot trial of a disclosure intervention for HIV+ mothers: the TRACK program. J Consult Clin Psychol 2011; 79(2): 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rotheram-Borus MJ, Lee MB, Gwadz M, et al. An intervention for parents with AIDS and their adolescent children. Am J Public Health 2001; 91(8): 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tasker M. How can I tell you? Secrecy and disclosure with children when a family member has AIDS. London: ERIC, 1992. [Google Scholar]
- 27. Naidoo K, Melvin D, Houghton J. Step by Step guide for conversation with children (toward disclosure), 2010, http://www.kznhealth.gov.za/arv/Disclosure_Poster.pdf
- 28. Gromberg J, Wells D. Simply good eating users’ guide. Minneapolis, MN: University of Minnesota, 1997. [Google Scholar]
- 29. Chinese Nutrition Society. Dietary guidelines for Chinese residents 2007. Lahsa, Tibet: Tibetan People’s Publishing House, 2008. [Google Scholar]
- 30. Derlega VJ, Winstead BA, Greene K, et al. Reasons for HIV disclosure/nondisclosure in close relationships: testing a model of HIV–disclosure decision making. J Soc Clin Psychol 2004; 23: 747–767. [Google Scholar]
- 31. Delaney RO, Serovich JM, Lim JY. Reasons for and against maternal HIV disclosure to children and perceived child reaction. AIDS Care 2008; 20(7): 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lv Y, Zhao Q, Li X, et al. Depression symptoms among caregivers of children in HIV-affected families in rural China. AIDS Care 2010; 22(6): 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1: 385–401. [Google Scholar]
- 34. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Social Behav 1983; 24: 385–396. [PubMed] [Google Scholar]
- 35. Saunders JB, Aasland OG, Babor TF, et al. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993; 88(6): 791–804. [DOI] [PubMed] [Google Scholar]
- 36. Hsiung P-C, Fang C-T, Lee K-L, et al. Validation of the medical outcomes study HIV (MOS-HIV) health survey among HIV-infected patients in Taiwan. Qual Life Res 2011; 20(2): 281–286. [DOI] [PubMed] [Google Scholar]
- 37. Cohen J. Statistical power analysis for the behavioral sciences. Abingdon: Routledge, 2013. [Google Scholar]
- 38. Qiao S, Li X, Stanton B. Theoretical models of parental HIV disclosure: a critical review. AIDS Care 2013; 25(3): 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Serovich JM. A test of two HIV disclosure theories. AIDS Educ Prev 2001; 13(4): 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nöstlinger C, Bartoli G, Gordillo V, et al. Children and adolescents living with HIV positive parents: emotional and behavioural problems. Vulnerable Child Youth Stud 2006; 1: 29–43. [Google Scholar]
- 41. Qiao S, Li X. Disclosure of parental illness to children: examples from HIV/AIDS. In: Morley D, Li X, Jenkinson C. (eds) Children and young people’s response to parental illness: a handbook of assessment and practice. Boca Raton, FL: CRC Press, 2016, pp. 171–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, parents_survey_english_sage_open_1 for iCOPE, a multi-level, cluster randomized, 36-month, parallel-group study to assess the efficacy of HIV disclosure intervention in HIV parental disclosure among parents living with HIV in China by Xiaoming Li, Shan Qiao and Yuejiao Zhou in SAGE Open Medicine