Abstract
Right ventricular (RV) function strongly associates with mortality in patients with pulmonary arterial hypertension (PAH). Current methods to determine RV function require temporal measurements of pressure and volume. The aim of the study was to investigate the feasibility of using right heart catheterization (RHC) measurements to estimate systolic and diastolic RV function. RV pressure and volume points were fit to P = α(eβV-1) to assess diastolic stiffness coefficient (β) and end-diastolic elastance (Eed). Single-beat methods were used to assess RV contractility (Ees). The effects of a non-zero unstressed RV volume (V0), RHC-derived stroke volume (SVRHC), and normalization of the end-diastolic volume (EDV) on estimates of β, Eed, and Ees were tested using Bland–Altman analysis in an incident PAH cohort (n = 32) that had both a RHC and cardiac magnetic resonance (CMR) test. RHC-derived measures of RV function were used to detect the effect of prostacyclin therapy in an incident PAH cohort and the severity of PAH in prevalent PAH (n = 21). A non-zero V0 had a minimal effect on β with a small bias and limits of agreement (LOA). Stroke volume (SV) significantly influenced estimates of β and Ees with a large LOA. Normalization of EDV had minimal effect on both β and Eed. RHC-derived β and Eed increased due to the severity of PAH and decreased due to three months of prostacyclin therapy. It is feasible to detect therapeutic changes in specific stiffness and elastic properties of the RV from signal-beat pressure-volume loops by using RHC-derived SV and normalizing RV EDV.
Keywords: pulmonary hypertension, right ventricle, diastolic stiffness, single-beat, pressure-volume loop
Right ventricular (RV) function is the main determinant of prognosis in pulmonary arterial hypertension (PAH).1 High RV diastolic stiffness associates with increased mortality and disease progression in patients with pulmonary hypertension (PH).2,3 While a right heart catheterization (RHC) remains the standard for diagnosing PH, risk stratification strategies for assessing prognosis in PH demonstrate that functional measurements like 6-min walking distance (6MWD), World Health Organization (WHO) functional class (FC), brain natriuretic peptide, and clinical worsening outperform standard hemodynamics in predicting mortality.4,5 More sophisticated measurements may be needed to thoroughly investigate the adaptive process of the right ventricle to increased afterload6,7 including the evaluation of RV diastolic and systolic function. Current methods to determine RV systolic/diastolic function and RV-pulmonary artery coupling require temporal measurements of RV pressure from a RHC and RV volume from cardiac magnetic resonance (CMR) or three-dimensional (3D) echocardiographic imaging. CMR-derived and thermodilution-derived stroke volumes (SV) have a strong correlation,8 but CMR-derived SV does not always reflect pulmonary SV in PAH patients due to the large variability in measurements from serial cine imaging in the right ventricle and the presence of tricuspid regurgitation.9 Due to limited access to CMR or 3D echocardiographic imaging as part of standard clinical care or a long-time interval (>48 h) between the RHC and imaging, accurate assessment of RV function in retrospective cohort studies can be limited. A long interval between the RHC and cardiac imaging can result in a change in patient status, i.e. different heart rate or change in fluid load, that could mask the signal due to noise. Current clinical assessment of RV function from a standard RHC is generally limited to systolic RV pressure, end-diastolic pressure (EDP), and RV stroke work.
Ventricular contractility and relaxation have been assessed using the maximum and minimum of the pressure velocity or the first derivative of pressure (max dP/dt and min dP/dt)10–13 in animal models of PH. Few clinical studies14 have reported on changes in max dP/dt and min dP/dt because it is not part of standard catheterization reports. It is feasible to use re-digitized RV pressure waveforms to calculate dP/dt from retrospective RHCs.11 A non-invasive global measure of systolic and diastolic function called the Myocardial Performance Index (MPI or Tei index) has been shown to be of prognostic importance.15 A high MPI or the ratio of the isovolumetric phases (contraction and relaxation) to ejection time associates with worse ventricular performance.16 MPI is independent of heart rate and cardiac morphology but highly sensitive to changes in loading conditions.
The aim of the study was to investigate the feasibility of using RHC measured RV pressure waveforms and thermodilution/ direct Fick measured cardiac output to estimate RV function (systolic and diastolic) and demonstrate that clinically relevant measures beyond systolic RV pressure, EDP, and RV stroke work are attainable from a standard RHC. We hypothesize that the simplification of single-beat pressure-volume loop method has reduced the required data points to those that are obtainable from a standard RHC without a loss in the accuracy of the measured RV function. Our results demonstrate the feasibility of assessing advanced parameters of RV function in patients from retrospective RHCs and report on changes in RV function in mild and severe prevalent PAH patients. We also investigated the longitudinal change and the ability of the parameters to detect changes in cardiopulmonary status in incident PAH three months after initiation of therapy.
Methods
Participants
Two cohorts of patients with PH were included. In the first cohort, treatment-naïve PAH adult patients with a same-day RHC and cardiac magnetic resonance imaging (cMRI) were included in the study (incident PAH, n = 32).17 RV function was assessed three months after the initiation of parenteral treprostinil therapy. The second cohort included prevalent PAH patients that were identified from the University of Arizona Pulmonary Hypertension registry (UA-PH registry, n = 24). All participants gave informed consent through the Respiratory-Related Disease Patient Registry (UA PH registry), which was approved by the institutional review board at the University of Arizona (IRB Protocol no. 1100000621).
Right heart catheterization
All patients underwent a RHC according to standard clinical guidelines. Briefly, a pulmonary artery catheter was advanced via the antecubital vein into the right atrium, right ventricle, and the pulmonary artery. Right atrial pressure (RAP), RV pressure, pulmonary arterial pressure (PAP), and pulmonary arterial wedge pressure were recorded. Cardiac output (CO) was measured by thermodilution or direct Fick methods. RHC-derived stroke volume (SVRHC) was calculated from the ratio of CO and heart rate. Routine clinical measurements of pressure waveforms were digitally stored at a sampling rate of 250 Hz and full pressure waveforms were retrieved for post-processing from the Philips Xper system (Philips North America Corporation, Andover, MA, USA).
Measurement of RV volumes
Incident PAH patients underwent CMR to measure RV end-diastolic volume (EDV) and end-systolic volume (ESV). Standard volumetric measurements were made from multiphase-multislice short-axis cine projections for CMR. Volumes were calculated by manually tracing the endocardial surface from the tricuspid valve to the apex including the moderator band and large trabeculations. Stroke volume (SVCMR) was calculated as EDV – ESV.
Identification of isovolumetric-contraction and -relaxation regions from RV pressure waveforms
The right ventricle has relatively low pressure during the diastolic filling phase. At the start of diastole, the tricuspid valve opens and the blood starts to flow into the right ventricle. At the end of diastole, the ventricle starts to contract, the tricuspid valve closes, and the ventricle enters the isovolumetric contraction (IVC) phase. The first maximum of the second derivative of RV pressure (d2P/dt2) that precedes max dP/dt marks the transition from diastole to isovolumetric contraction or the EDP.18 Once the pressure in the right ventricle is greater than in pulmonary artery, the pulmonary valve opens and the blood is ejected from the right ventricle into the pulmonary circulation (systole). The end of the isovolumetric contraction is identifiable as the first minimum of d2P/dt2 that follows max dP/dt or the beginning-systolic pressure (BSP). The end-systolic pressure (ESP) is identified as the first minimum of d2P/dt2 that precedes min dP/dt and marks the pulmonary valve closing and the isovolumetric relaxation phase (IVR) beginning. The end of the IVR phase is then marked by the first maximum of d2P/dt2 that follows min dP/dt. A RHC-derived myocardial performance index was then calculated as the ratio of (IVC time + IVR time) and the RV ejection time (Fig. 1).
Fig. 1.
Methods to assess systolic and diastolic RV function using RV pressure waveforms. (a) Segmentation of the (1) RV pressure waveform using (2) pressure velocity (dP/dt) and (3) pressure acceleration (d2P/dt2) to define the isovolumetric contraction (IVC), ejection, isovolumetric relaxation (IVR), and filling regions. An RHC-derived MPI (Tei index) were derived using the ratio of the sum of the isovolumetric contraction (IVCT) and relaxation (IVRT) time to the RV ejection time (RVET) or RV MPI = (IVCT + IVRT) /RVET. (b) Single-beat method to estimate max isovolumetric pressure (Pmax) that was used in the estimation of the RV volume at zero pressure (V0). (c) Assessment of the EDPVR when the RV volume at zero pressure was assumed to be zero (dashed line) or non-zero (V0) (solid line) and derived as the intercept of the Ees with the volume axis.
RV contractility and estimated volumes at zero pressure (V0)
Maximum isovolumic pressure Pmax was determined from a sinusoidal curve fit of the RV pressure waveform during early isovolumetric contraction and late isovolumetric relaxation. Expanding on previous single-beat methods,2,19,20 early isovolumetric contraction was constrained to the region between the first maximum of d2P/dt2 that precedes max dP/dt and max dP/dt (Fig. 1a). Early isovolumetric relaxation was defined as the region from min dP/dt to the first maximum of d2P/dt2 that follows min dP/dt (Fig. 1b). To reduce inter-observer variability in estimates of Pmax, only minor manual modifications of these regions were allowed to optimize the sinusoidal fit. RV ESP–volume relationship was assumed to be linear, ESPVR = Ees(V)+b, and determined from (ESV, mean PAP [mPAP]) and (EDV, Pmax). RV volume at zero pressure (V0) was calculated as V0 = −b/Ees. The Ees/Ea ratio was then calculated as the ratio of (Pmax-RVSP)/RVSP. Using a conversion factor, RV stroke work index (RVSWI) was calculated as 0.0136 ∙ SVI ∙ (mPAP – RAP) in units of g/m/beat/m2.
RV diastolic stiffness from RHC pressure and cMRI RV volumes
The end-diastolic pressure-volume relationship (EDPVR) is described by a non-linear exponential curve P = α (eVβ−1) as previously published.2,3,21 In the single-beat approach, diastolic stiffness is calculated by fitting the non-linear exponential equation to diastolic portion of the pressure-volume curve. The current method uses three pressure-volume points to determine diastolic stiffness coefficient, β, and a curve-fit parameter, α. The three volume points are the RV volume at zero pressure (V0), ESV, and EDV. The corresponding pressure points are zero, min RV pressure, and EDP. Previous studies have shown V0 does not equal zero in PH and increases with right heart failure.20 To determine the effect of RV volume at zero pressure on RV stiffness, calculated values for alpha and beta were compared when V0 was assumed to be 0 mL (βV0=0 and αV0=0) or when V0 is estimated using the single-beat analysis (β V0 and αV0) (Fig. 1c). In both cases, cMRI volumes were used for EDV and ESV, and SV was calculated as EDV–ESV. To determine the effect of SV on RV diastolic stiffness, another set of β and α (βRHC and αRHC), were calculated using RHC-derived SV (SVRHC = cardiac output/heart rate) and CMR-derived EDV so that ESV was EDV – SVRHC. To determine the effect of EDV on RV diastolic stiffness, another set of β and α (β250 and α250) were calculated using RHC-derived SV and normalizing the EDV to 250 mL so that ESV is 250 – SVRHC.
All single-beat pressure-volume loop analyses of RV systolic and diastolic function were performed in Matlab (Matlab 2016b, The MathWorks, Natick, MA, USA).
Statistics
Data were presented as mean ± standard deviation. Study data were collected and securely managed using REDCap (Research Electronic Data Capture) tools hosted at University of Arizona.22 Bland–Altman analysis was used to compare methods. Paired Wilcoxon Signed-Rank tests were used to test the effect of therapy and Wilcoxon Signed-Rank tests were used to test the differences between mild and severe PAH patients. A P value ≤ 0.05 was considered significant.
Results
The 32 incident PAH patients (26 men, 5 women; mean age = 55 ± 12 years) had a same-day RHC and CMR at baseline when they were initially treatment-naïve and three months after the initiation of parenteral prostacyclin therapy. The baseline hemodynamics and therapeutic response have been previously published.17 Briefly, three months of therapy significantly decreased mPAP, pulmonary vascular resistance (PVR), and RAP (Table 1). This cohort was used to test the methods for determining RV diastolic stiffness because of the temporally related RHC and CMR measurements at the three-month time point.
Table 1.
Demographic and hemodynamic characteristics of the incident and prevalent PAH cohorts.
| Incident PAH before therapy (n = 32) | Incident PAH after 3 months of therapy | Prevalent mild PAH (n = 11) | Prevalent severe PAH (n = 10) | |
|---|---|---|---|---|
| Age (years) | 57 ± 13 | 67 ± 15 | 56 ± 10* | |
| Sex (F/M) | 26/6 | 8/3 | 4/6 | |
| Heart rate (bpm) | 82 ± 11 | 86 ± 12 | 70 ± 7 | 88 ± 14* |
| mPAP (mmHg) | 58 ± 11 | 49 ± 9† | 30 ± 3 | 61 ± 7* |
| PCWP (mmHg) | 10 ± 4 | 8 ± 4† | 9 ± 4 | 14 ± 6 |
| Cardiac output (L/min) | 4.2 ± 1.1 | 5.4 ± 1.7† | 5.9 ± 1.3 | 6.2 ± 1.2 |
| PVR (mmHg/L/min) | 12.6 ± 4.3 | 8.5 ± 3.6† | 3.8 ± 1.4 | 8.2 ± 2.0* |
| RAP (mmHg) | 13 ± 6 | 7 ± 4† | 5 ± 3 | 15 ± 8* |
| PA saturation (%) | 60 ± 9 | 65 ± 6† | 70 ± 6 | 64 ± 7 |
P < 0.05 vs. prevalent mild PAH.
P < 0.05 vs. incident PAH before therapy.
mPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure.
RV volume at zero pressure has a minimal effect on RV diastolic stiffness coefficient (β)
In incident patients on three months of treprostinil therapy, there was a non-significant difference in β when V0 was assumed to be zero (0.049 ± 0.018 mL–1) or a single-beat estimate of V0 (0.050 ± 0.018 mL–1) (Fig. 2a). In both estimates of β, CMR-derived ESV and EDV were used in the calculations. The average Pmax was 141 ± 33 mmHg with an average V0 of 42 mL (interquartile range [IQR] = −6–108 mL) when systolic RV pressure is used for the ESP. In a Bland–Altman comparison of the calculated β, there was minimal bias and small confidence intervals (CI) (bias = −0.0003 mL–1, CI = −0.0015–0.0008 mL–1; Fig. 2b). Similarly, V0 had no effect on the average β using RHC-derived SV (data not shown).
Fig. 2.
RV volume at zero pressure (V0) has a minimal effect on the RV diastolic stiffness coefficient (β). (a) Box plot of the RV diastolic stiffness coefficient, β, when V0 was assumed to equal zero (V0 = 0) or assumed to be a non-zero number (V0). (b) In a Bland–Altman analysis, there was minimal bias and small limits of agreement. Note: The Bland–Altman plot has been scaled to match Figs. 3d and 4b for comparison.
RV stroke volume has a significant effect on the precision of RV diastolic stiffness coefficient (β)
Estimates of RV diastolic stiffness coefficient (β) are sensitive to the methods used to determine the SV. Despite no significant differences in the average CMR-derived SV and RHC-derived SV, there was a decrease in the precision of SV (Fig. 3a and b). The bias between RHC- and CMR-derived SV was –5 mL with limits of agreement of −42–33 mL. The average β had a slight but non-significant decrease when derived using RHC-derived SV (βRHC, SVRHC) compared to CMR-derived SV (βCMR, SVCMR), 0.047 ± 0.019 mL–1 vs. 0.049 ± 0.018 mL–1, respectively (Fig. 3c). There was minimal bias between the derived β (bias = 0.002) but there was decreased precision in the measurements (CI = −0.03–0.03) (Fig. 3d). The median time difference between when the RHC and CMR were performed was one day (IQR = −1.5–7 days), which is a potential contributing factor to decreased precision in the measures of SV. There were seven individuals with at least 14 days between their RHC and CMR.
Fig. 3.
RV SV has a significant effect on the precision of RV diastolic stiffness coefficient (β). (a) There were no significant differences between the CMR-derived SV and the RHC-derived SV. (b) In a Bland–Altman analysis, there were large limits of agreement between estimates of SV. (c) Similarly, there were no significant differences in the derived diastolic stiffness coefficient (β) when CMR-derived and RHC-derived SVs were used. (d) There was a decrease in the precision of the diastolic stiffness coefficient (β) due to the different SVs.
The precision of the calculated metrics of systolic RV function are affected by RV SV. Similar to RV diastolic stiffness, there were no significant differences in average end-systolic elastance (Ees; Supplemental Fig. 1a) and Ea (Supplemental Fig. 1c) when calculated using RHC-derived or CMR-derived SV. There was minimal bias in the derived Ees (bias = 0.09 mmHg/mL) but there was decreased precision in the measurements (CI = −0.85–1.04 mmHg/mL, Supplemental Fig. 1b). Similar results were found for Ea with a bias of 0.04 (CI = −0.68–0.76 mmHg/ml, Supplemental Fig. 1d).
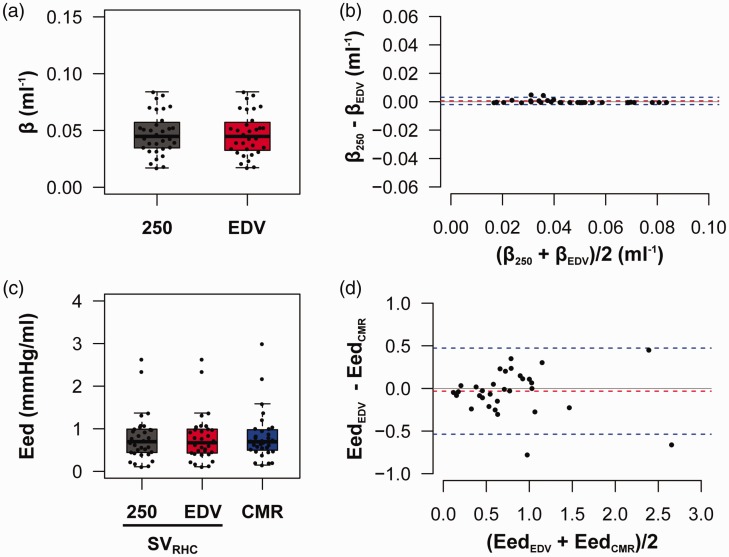
End-diastolic elastance is independent of absolute RV volumes
There was a non-significant difference in β when EDV was normalized to 250 mL or measured by CMR (Fig. 4a). In both estimates of β, V0 was assumed to be equal to zero and the RHC-derived SVs were used in the calculations. In a Bland–Altman comparison, there was minimal bias and small CIs in the calculated β (bias = 0.0006 mL–1, CI = −0.002–0.003 mL–1) (Fig. 4b). Similar to β, the measured EDV had a minimal effect on the calculated end-diastolic elastance (Eed; Fig. 4c) but the SV (SVRHC or SVCMR) has a larger influence on the calculated Eed with a bias of −0.03 mmHg/mL and CI of −0.51–0.46 between EedCMR and EedEDV (Fig. 4d).
Fig. 4.
RV diastolic stiffness coefficient (β) and Eed are independent of absolute RV EDV. Normalizing EDV to 250 mL had no significant effect on the stiffness coefficient (β) in terms of the (a) median or (b) the bias or limits of agreement. There were no significant differences in Eed, when EDV was normalized to a constant volume. (d) Similar to the stiffness coefficient (β), SV did have a significant effect on the limits of agreement on the calculated Eed.
Myocardial Performance Index from RV pressure waveforms
The MPI is a global measure of systolic and diastolic RV function that is normally derived using echocardiographic images. From the segmentation of the RV pressure waveform, it is feasible to determine the isovolumetric contraction and relaxation time as well as the ejection time to determine a RHC-derived MPI. At three months in the incident PAH patients, the isovolumetric contraction time was 49 ± 18 ms compared to the isovolumetric relaxation time of 68 ± 33 ms. The time to max RV pressure was 221 ± 31 ms from the point of end-diastole. The ejection time was 38.4 ± 6% and the filling time was 44.9 ± 6% of the cardiac cycle. Based on the changes in pressure acceleration (d2P/dt2), the ejection time was 271 ± 47 ms. In the initial RHC at baseline, the isovolumetric contraction time was similar to three months at 46 ± 15 ms. The isovolumetric relaxation time at baseline (88 ± 40 ms) was significantly longer than at three months (P = 0.02). From baseline to three months, there was a significant decrease in the isovolumetric relaxation time. At baseline, the ejection time was 35.5 ± 5% and the filling time was 46.4 ± 6% of the cardiac cycle. There were no significant differences in the RHC-derived MPI from baseline to three months in these patients (Table 2).
Table 2.
Differences in RHC-derived measures of RV function in incident treatment-naïve PAH patients after three months of therapy.
| Incident PAH before therapy (n = 32) | Incident PAH after 3 months of therapy | |
|---|---|---|
| RV systolic function | ||
| Max dP/dt (mmHg/s) | 662 ± 245 | 598 ± 180 |
| Contractile Index (L/s) | 7.34 ± 2.51 | 7.97 ± 2.26 |
| Ees (mmHg/mL) | 1.47 ± 0.71 | 1.12 ± 0.63* |
| RV afterload/work | ||
| Ea (mmHg/mL) | 1.98 ± 0.78 | 1.37 ± 0.62* |
| Stroke work (g/m/beat/m2) | 16.45 ± 6.31 | 19.26 ± 5.5* |
| RV-PA coupling | ||
| Ees/Ea | 0.82 ± 0.39 | 0.89 ± 0.44 |
| RV diastolic function | ||
| Min dP/dt (mmHg/s) | −714 ± 182 | −655 ± 169* |
| RHC-derived MPI | 0.53 ± 0.25 | 0.46 ± 0.23 |
| RV diastolic stiffness coefficient (mL–1) | 0.063 ± 0.022 | 0.047 ± 0.018* |
| Eed (mmHg/mL) | 1.26 ± 0.69 | 0.79 ± 0.56* |
P < 0.05 vs. incident PAH before therapy.
Ees, end-systolic elastance; Ea, arterial elastance; MPI, Myocardial Performance Index; Eed, end-diastolic elastance.
RHC-based analysis of RV function in incident and prevalent cases of PAH
Using only the RHC measured RV pressure waveform and cardiac output, it is feasible to detect improvement in RV function in incident patients on three months of therapy (Table 2) and discriminate differences in RV function between mild PAH patients and severe PAH patients (Table 3). In the incident patients, there was a non-significant decrease in max dP/dt and increase in contractile index. In comparison, there was a significant decrease in the Ees. The significant decrease in Ees is likely a result of decreased arterial elastance and PVR due to the therapy. There were no significant changes in Ees/Ea, min dP/dt, or MPI. RV diastolic function did improve with decreased RV diastolic stiffness coefficient (β) and Eed. These findings are in keeping with the previously published results in this cohort on RV function using CMR-derived RV volumes in the calculation of RV function.17
Table 3.
Differences in RHC-derived measures of RV function in prevalent PAH patients with mild and severe disease.
| Prevalent mild PAH (n = 11) | Prevalent severe PAH (n = 10) | |
|---|---|---|
| RV systolic function | ||
| Max dP/dt (mmHg/s) | 383 ± 150 | 750 ± 293* |
| Contractile Index (L/s) | 8.41 ± 2.76 | 8.07 ± 2.24 |
| Ees (mmHg/mL) | 0.73 ± 0.47 | 1.37 ± 0.76* |
| RV afterload/work | ||
| Ea (mmHg/mL) | 0.57 ± 0.17 | 1.35 ± 0.40* |
| Stroke work (g/m/beat/m2) | 15.59 ± 3.38 | 20.58 ± 7.16 |
| RV-PA coupling | ||
| Ees/Ea | 1.36 ± 0.82 | 0.98 ± 0.40 |
| RV diastolic function | ||
| Min dP/dt (mmHg/s) | −425 ± 134 | −756 ± 225* |
| RHC-derived MPI | 0.34 ± 0.12 | 0.49 ± 0.21 |
| RV diastolic stiffness coefficient (mL–1) | 0.029 ± 0.011 | 0.043 ± 0.012* |
| Eed (mmHg/mL) | 0.33 ± 0.31 | 0.83 ± 0.37* |
P < 0.05 vs. prevalent mild PAH.
Ees, end-systolic elastance; Ea, arterial elastance; MPI, Myocardial Performance Index; Eed, end-diastolic elastance.
In a retrospective analysis of prevalent PAH patients with mild PAH (n = 11; mPAP = 30 ± 3 mmHg) and severe PAH (n = 10; mPAP = 61 ± 7 mmHg), there is a measurable decline in RV function. Despite a lack of CMR-derived RV volumes, we are able to detect decreased RV systolic function, increased afterload, and increased RV diastolic stiffness (Table 2). Prevalent patients with severe PAH had increased max dP/dt and Ees but a non-significant change in the contractile index (max dP/dt / RVSP). There was significant increase in arterial elastance and RV stroke work. There was a non-significant decrease in Ees/Ea but there was a significant decrease in RV diastolic function. There was a significant decrease in min dP/dt and increase in RV diastolic stiffness and Eed (Table 2). The contractile index, Ees/Ea, and RHC-derived MPI had minimal power at distinguishing between these groups.
There are significant correlations between systolic and diastolic function in the incident and prevalent PAH patients. Systolic function (Ees) correlates with RV diastolic stiffness coefficient (β) in the current cohort of PAH patients (Fig. 5a, c, and e). In treatment-naïve PAH patients, there was no correlation between Ees and Eed (P = 0.2, Fig. 5b) but there is a correlation between Ees and Eed after three months of prostacyclin therapy (r2 = 0.31, P < 0.001, Fig. 5d) and in the prevalent patients with PAH (Fig. 5e).
Fig. 5.
Correlations between right ventricular systolic and diastolic function in incident and prevalent patients with PAH. Systolic function (Ees) correlates with RV diastolic stiffness coefficient (β) in incident (a, c) and prevalent (e) PAH patients. Correlation between Ees and Eed is more variable with no significant correlation in treatment-naïve PAH patients (b) and significant correlations after three months of therapy (d) and in a prevalent cohort of PAH patients (f).
Discussion
The current study suggests the feasibility of identifying specific stiffness and elastic properties of the RV from the standard RHC using RHC pressure and thermodilution cardiac output. These methods allow for additional information on RV function to be determined when RV-specific imaging measurements are: (1) not available from MRI or echocardiograph; (2) there is a large delay between a RHC and CMR or echo; and/or (3) the initial MRI or echocardiography exams were not read for RV-specific parameters. The precision of the calculated RV diastolic stiffness is largely a function of the measured SV. EDV and RV volume at zero pressure (V0) have little influence on the calculated RV diastolic stiffness and Eed. In the absence of CMR-derived RV volumes, it is feasible to detect changes in RV function in incident and prevalent PH patients (Table 3). The Ees and Eed can be utilized to assess whether the change is mainly due to systolic or diastolic changes in function.
Assessment of RV function in retrospective cohorts
Assessment of RV diastolic function has been limited to patients that have had a RHC and cMRI because the diastolic stiffness coefficient and Eed are derived from RHC–CMR pressure-volume loops. The EDP–volume relationship utilizes three RV volumes including: RV volume at zero pressure (V0); ESV; and EDV. A previous study showed that as there is RV remodeling and dilation with PH, RV volume at zero pressure (V0) increases and cannot be assumed to be equal to zero.20 Using the single-beat method and the estimation of Pmax, we found a median V0 of 42 mL (IQR = −6–108 mL). The inclusion of V0 in the calculation of diastolic stiffness had minimal effects on the estimates. These results suggest that the single-beat method and estimates of Pmax are not necessary when early and late diastolic pressure measurements and SV are available. Our results demonstrate that SV has the largest influence on the calculated β and Eed with absolute volumes exerting minimal influence. Eliminating the need for absolute volumes in the calculation of RV diastolic function allows the assessment of RHC-derived RV diastolic stiffness in more retrospective studies and further assessment of the prognostic importance of RV diastolic stiffness. Ees and Ea have a similar dependence on SV where there were no significant differences in the average values for the cohorts but there was a decrease in precision of the measurements (Supplemental Fig. 1). Despite the decrease in precision, the small biases would suggest this method could be used to assess differences in cohort studies. The use of CMR is recommended in >50% of the guidelines but the application of CMR is limited by access, missing technical skills for performing and analyzing exams, poor vender standardization, reader expertise, and reimbursement issues.23
It is feasible to assess changes in ventricular contractility and relaxation by max dP/dt and min dP/dt.10–13 Our data show that as severity of PAH increases, there is an increase in max dP/dt and decrease in min dP/dt (Tables 2 and 3). In incident PAH patients, there were no significant changes in max or min dP/dt after three months of therapy. As markers of RV function, max and min dP/dt are largely load-dependent and as such are likely less sensitive to therapy-induced changes in RV function as Ees and Eed in incident patients with PAH that have higher PVR. Select clinical hemodynamic monitoring systems have the capability of calculating max and min dP/dt but have not included them in the standard clinical catheterization reports.
Assessment of RV stroke volume
Our results suggest that the calculated values of the RV diastolic stiffness coefficient β are largely dependent on the measurement of SV and less so on the absolute ventricular volumes. Comparison of RV diastolic function between patients or over time should be limited to β or Eed that have been calculated using the same methods due to differences between CMR-derived and RHC-derived RV SVs. Previous studies have found a poor correlation between the CMR-derived and direct Fick-derived SVs.24 When analyzing RV diastolic function in large retrospective patient populations, RHC-derived measures of SV should be sufficient to detect severity of PAH and therapeutic changes (Table 2). Cardiac output and SV measurements can be different between methods and it is recommended to use thermodilution derived cardiac outputs over estimated Fick measurements in large retrospective studies.25 It is suggested that 3D echocardiography could be used in experienced centers to assess RV volumes and SV;26 however, there are limitations in PAH patients due to increased RV dimensions and an underestimation of RV volumes compared to CMR.27
RHC-derived Myocardial Performance Index
Non-invasive measures of RV function from echocardiography and CMR such as RV strain, RVEF, TAPSE, and other measure provide valuable information and are important variables for following RV function.7,28–30 The echocardiography-derived MPI (or Tei index) allows for a global assessment of systolic and diastolic ventricular function in one parameter. The MPI is the ratio of the isovolumetric phases (contraction and relaxation) to ejection time and has been shown to be of prognostic importance.15 In the current study, we utilized the rates of RV pressure change to identify the isovolumetric contraction and relaxation regions. Using those regions and the corresponding ejection time, we calculated a RHC-derived MPI. The values of RHC-derived MPI (0.34 ± 0.12 to 0.53 ± 0.25) are similar to those derived from non-invasive echo measurements. One study found the MPI index ranged from 0.33 ± 0.33 in controls to 0.63 ± 0.15 in patients with chronic thromboembolic pulmonary hypertension (CTEPH).31 They found that patients with PAH had a MPI of 0.56 ± 0.13 that is similar to the RHC-derived MPI in our incident PAH patients (0.53 ± 0.25). Another study evaluating the utility of the MPI index for assessing disease severity, found a similar increase in MPI due to PAH (controls = 0.26 ± 0.12, PAH = 0.54 ± 0.34) and that treatment decreased MPI from 0.55 ± 0.3 to 0.42 ± 0.17 at follow-up.32 We found a similar decrease in RHC-derived MPI from 0.53 ± 0.25 to 0.46 ± 0.23 in incident PAH patients after three months of treprostinil therapy. While a high MPI is associated with worse ventricular performance,16 we were unable to detect significant changes possibly because the MPI is highly sensitive to changes in loading conditions and more a global measure of RV diastolic and systolic RV function.
Correlation between systolic and diastolic function in PAH patients
Questions remain as to the relationship between Ees and Eed in patients with PAH. A recent study found there to be considerable reserve in Ees/Ea in patients with PAH where Ees/Ea has to decrease to < 0.89 before failure due to large increases in RV contractility and the homoemetric response in the RV.33 At the same time, there is an increase in Eed that associates with CMR indicators of RV fibrosis. Our current results in patients with PAH and a previous results in a mixed PH cohort show there are significant correlations between RV diastolic stiffness coefficient (β) and systolic function (Ees) (Fig. 5a, c, and e).2 The diastolic stiffness coefficient is a term that describes the curvature of the EDP–volume relationship where Eed and another measure of diastolic function is the slope of the EDPVR at a specific volume or, in this case, EDV.
A previous study found only weak correlations between Eed and Ees (r2 = 0.25, P < 0.001) in baseline patients before therapy and no correlation between Eed and Ees (r2 = 0.03, P = 0.264) after the patients were treated.3 The loss of the significant correlation after patients were treated suggests that the RV diastolic stiffening and the systolic adaptations are largely independent processes. Contrary to these finding, we found that in treatment-naïve PAH patients there was no correlation between Ees and Eed (P = 0.2, Fig. 5b) but there is a correlation between Ees and Eed after three months of prostacyclin therapy (r2 = 0.31, P < 0.001; Fig. 5d). Severity of disease is a potential contributing factor to the different findings the two studies. In the Trip et al. paper, the baseline Eed was 0.56 mmHg/mL3 (range = 0.42–0.94 mmHg/mL3) where the incident patients in our study had baseline Eed of 1.06 mmHg/mL (range = 0.68–1.80 mmHg/mL). We can see that over a wider range of diastolic function (0.68–1.80 mmHg/mL) before treatment, there is no significant correlation between Ees and Eed. Due to the non-linearity of the EDPVR it is possible for patients to have the same diastolic stiffness coefficient (β) and different Eed if their right ventricles are operating at different pressure and volumes. Additional studies are needed using follow-up RHC or imaging studies to determine whether RV diastolic stiffness uncouples from Ees during RV remodeling to PH.
Clinical challenges associated with RV waveform analysis
The segmentation of the RV waveform to determine min RV pressure and the EDP are major contributors to variability in the assessment of RV diastolic function. Contributing factors include the method used to filter/smooth the signal (Butterworth filter or smoothing) and the filter settings are potential contributors to the variability. The algorithm standardizes the determination of the max and min of the pressure waveform, dP/dt, and d2P/dt2.
Advanced hemodynamic analysis on clinically obtained RV pressure waveforms from standard fluid-filled catheters can be challenging due to the quality of the recorded waveforms and can increase the variability in the values obtained for RV function (Supplemental Fig. 2). Steady waveforms (Supplemental Fig. 2A) are easy to analyze and will have little beat to beat variability compared to waveforms that exhibit large swings in pressure due to respiratory variability (Supplemental Fig. 2B). Additional factors that will increase the beat-to-beat variability and determination of a single representative beat include excessive high frequency noise (Supplemental Fig. 2C) and arrhythmias (Supplemental Fig. 2D).
A major clinical challenge in using the single-beat method to assess RV systolic function is the estimation of Pmax. In a multi-center analysis,18 three observers at each center each estimated Pmax in patient with PAH using the first derivative and a novel second derivative method. There was good inter-reader agreement between readers with an intraclass correlation coefficient (ICC) of 0.95 for the first derivative method and 0.98 for the second derivative method but there were still large 95% CIs when comparing the methods. The second derivative method reduced inter-observer variability but over constrained the fits and under-estimated Pmax. More work is needed to develop standardized methods that are physiologically accurate with less inter- and intra-observer variability. It is possible to apply the proposed methods to assess RV systolic and diastolic function to real-time catheterization data with a dedicated analysis program that considers these challenges.
Conclusion
In the absence of measured RV volumes, it is feasible to estimate RV diastolic stiffness and Eed from clinical signal-beat pressure volume curves by normalizing RV EDV to a constant volume and using RHC-derived SVs. Eed is more sensitive to the measured SV and the RAP than the absolute ESV and EDV.
Supplemental Material
Supplemental material, PUL850993 Supplemental material for Surfing the right ventricular pressure waveform: methods to assess global, systolic and diastolic RV function from a clinical right heart catheterization by Rebecca R. Vanderpool, Reena Puri, Alexandra Osorio, Kelly Wickstrom, Ankit A. Desai, Stephen M. Black, Joe G.N. Garcia, Jason X.-J. Yuan and Franz P. Rischard in Pulmonary Circulation
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
RRV is funded by the Arizona Biomedical Research Centre (ABRCNIA-ADHS18-19887); FPR is funded by the National Heart Lung and Blood Institute (NHLBI U01 HL 125208). JXJY is funded by the National Heart Lung and Blood Institute (NHLBI R35 HL 135807).
ORCID iD
Rebecca R. Vanderpool https://orcid.org/0000-0001-6038-0568
References
- 1.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The Relationship Between the Right Ventricle and its Load in Pulmonary Hypertension. J Am Coll Cardiol 2017; 69: 236–243. [DOI] [PubMed] [Google Scholar]
- 2.Vanderpool RR, Pinsky MR, Naeije R, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 2015; 101: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trip P, Rain S, Handoko ML, et al. Clinical relevance of right ventricular diastolic stiffness in pulmonary hypertension. Eur Respir J 2015; 45: 1603–12. [DOI] [PubMed] [Google Scholar]
- 4.Benza RL, Gomberg-maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: The REVEAL Risk Score Calculator 2.0 and Comparison with ESC/ERS-Based Risk Assessment Strategies. Chest 2019. DOI: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. [DOI] [PubMed] [Google Scholar]
- 6.Lahm T, Douglas IS, Archer SL, et al. Assessment of right ventricular function in the research setting: Knowledge gaps and pathways forward an official American thoracic society research statement. Am J Respir Crit Care Med 2018; 198: e15–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vonk Noordegraaf A, Chin KM, Haddad F, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 2019; 53: 1801900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLure LER, Brown A, Lee WN, et al. Non-invasive stroke volume measurement by cardiac magnetic resonance imaging and inert gas rebreathing in pulmonary hypertension. Clin Physiol Funct Imaging 2011; 31: 221–226. [DOI] [PubMed] [Google Scholar]
- 9.Wetterslev M, Møller-Sørensen H, Johansen RR, et al. Systematic review of cardiac output measurements by echocardiography vs. thermodilution: the techniques are not interchangeable. Intensive Care Med 2016; 42: 1223–1233. [DOI] [PubMed] [Google Scholar]
- 10.Tabima DM, Hacker TA, Chesler NC. Measuring right ventricular function in the normal and hypertensive mouse hearts using admittance-derived pressure-volume loops. Am J Physiol Heart Circ Physiol 2010; 299: H2069–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachman TN, Bursic JJ, Simon MA, et al. A novel acquisition technique to utilize swan-ganz catheter data as a surrogate for high-fidelity micromanometry within the right ventricle and pulmonary circuit. Cardiovasc Eng Technol 2013; 4: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tedford RJ, Mudd JO, Girgis RE, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail 2013; 6: 953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCabe C, White PA, Hoole SP, et al. Right ventricular dysfunction in chronic thromboembolic obstruction of the pulmonary artery. J Appl Physiol 2014; 116: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason DT. Usefulness and limitations of the rate of rise of intraventricular pressure (dp/dt) in the evaluation of myocardial contractility in man*. Am J Cardiol 1969; 23: 516–527. [DOI] [PubMed] [Google Scholar]
- 15.Tei C, Dujardin KS, Hodge DO, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr 1996; 9: 838–847. [DOI] [PubMed] [Google Scholar]
- 16.Mertens LL, Friedberg MK. Imaging the right ventricle—current state of the art. Nat Rev Cardiol 2010; 7: 551–563. [DOI] [PubMed] [Google Scholar]
- 17.Vanderpool RR, Desai AA, Knapp SM, et al. How prostacyclin therapy improves right ventricular function in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellofiore A, Vanderpool R, Brewis MJ, et al. A novel single-beat approach to assess right ventricular systolic function. J Appl Physiol 2018; 124: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brimioulle S, Wauthy P, Ewalenko P, et al. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol 2003; 284: H1625–630. [DOI] [PubMed] [Google Scholar]
- 20.Trip P, Kind T, van de Veerdonk MC, et al. Accurate assessment of load-independent right ventricular systolic function in patients with pulmonary hypertension. J Heart Lung Transplant 2013; 32: 50–55. [DOI] [PubMed] [Google Scholar]
- 21.Rain S, Handoko ML, Trip P, et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 2013; 128: 2016–2025. [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Knobelsdorff-Brenkenhoff F, Schulz-Menger J. Role of cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology. J Cardiovasc Magn Reson 2015; 18: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauritz G-J, Marcus JT, Boonstra A, et al. Non-invasive stroke volume assessment in patients with pulmonary arterial hypertension: left-sided data mandatory. J Cardiovasc Magn Reson 2008; 10: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opotowsky AR, Hess E, Maron BA, et al. Thermodilution vs estimated Fick cardiac output measurement in clinical practice. JAMA Cardiol 2017; 2: 1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–271. [DOI] [PubMed] [Google Scholar]
- 27.Shimada YJ, Shiota M, Siegel RJ, et al. Accuracy of right ventricular volumes and function determined by three-dimensional echocardiography in comparison with magnetic resonance imaging: a meta-analysis study. J Am Soc Echocardiogr 2010; 23: 943–953. [DOI] [PubMed] [Google Scholar]
- 28.Aloia E, Cameli M, D’Ascenzi F, et al. TAPSE: An old but useful tool in different diseases. Int J Cardiol 2016; 225: 177–183. [DOI] [PubMed] [Google Scholar]
- 29.Badano LP, Ginghina C, Easaw J, et al. Right ventricle in pulmonary arterial hypertension: haemodynamics, structural changes, imaging, and proposal of a study protocol aimed to assess remodelling and treatment effects. Eur J Echocardiogr 2010; 11: 27–37. [DOI] [PubMed] [Google Scholar]
- 30.Huang SJ, Nalos M, Smith L, et al. The use of echocardiographic indices in defining and assessing right ventricular systolic function in critical care research. Intensive Care Med 2018; 44: 868–883. [DOI] [PubMed] [Google Scholar]
- 31.Giusca S, Popa E, Amzulescu MS, et al. Is right ventricular remodeling in pulmonary hypertension dependent on etiology? An echocardiographic study. Echocardiography 2016; 33: 546–554. [DOI] [PubMed] [Google Scholar]
- 32.Ogihara Y, Yamada N, Dohi K, et al. Utility of right ventricular Tei-index for assessing disease severity and determining response to treatment in patients with pulmonary arterial hypertension. J Cardiol 2014; 63: 149–153. [DOI] [PubMed] [Google Scholar]
- 33.Tello K, Dalmer A, Axmann J, et al. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail 2019; 12: e005512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL850993 Supplemental material for Surfing the right ventricular pressure waveform: methods to assess global, systolic and diastolic RV function from a clinical right heart catheterization by Rebecca R. Vanderpool, Reena Puri, Alexandra Osorio, Kelly Wickstrom, Ankit A. Desai, Stephen M. Black, Joe G.N. Garcia, Jason X.-J. Yuan and Franz P. Rischard in Pulmonary Circulation