Abstract
Background
The diagnosis of pulmonary arterial hypertension requires right heart catheterization (RHC) which is typically performed via proximal venous access (PVA). Antecubital venous access (AVA) is an alternative approach for RHC that can minimize complications, decrease procedural duration and allow for immediate patient recovery. A direct comparison between the two procedures in patients with pulmonary hypertension (PH) is lacking.
Objectives
To determine the feasibility, safety, and adoption rates of AVA-RHC as compared with ultrasound-guided PVA in a subpopulation of patients with PH.
Methods
All patients who underwent RHC for evaluation of PH between December 2014 and March 2017 at a single large academic medical center were included in this study. Demographic, procedural and outcomes data were retrieved from the medical records.
Results
In total, 159 RHC were included (124 AVA, 35 PVA). The duration of RHC was significantly shorter in the AVA compared with PVA group (53 (IQR 38–70) vs. 80 (IQR 56–95) min, respectively, p < 0.001). 19% of AVA (24/124) procedures were switched to PVA. Failed attempts at AVA were more common in scleroderma (50% failure rate). Success rate of AVA increased from 81.2% to 93.3% from the first to last quartile. Fluoroscopy time was similar in both groups, the difference between the groups in the radiation dose are not statistically significant (54.5 (IQR 25–110) vs. 84.5 (IQR 30–134)).
Conclusion
AVA-RHC is a feasible and safe alternative to PVA in patients with PH who are evaluated for pulmonary arterial hypertension diagnosis. Our experience and rapid adoption rate support the use of AVA as the preferred access site for RHC in uncomplicated PH patients.
Keywords: pulmonary circulation and pulmonary hypertension, collagen vascular diseases
Background
Pulmonary hypertension (PH) is associated with significant morbidity and mortality. It is classified into five subgroups: pulmonary arterial hypertension (PAH) (group 1); PH due to left heart disease (group 2); PH due to lung diseases and/or hypoxia (group 3); chronic thromboembolic pulmonary hypertension (group 4); and PH with unclear multifactorial mechanisms (group 5).1
Current therapies are aimed to improve clinical outcomes and hemodynamic parameters. However, these therapies are only effective for PAH (group 1). The other groups are managed according to their baseline etiologies2 thus, an accurate diagnosis is crucial. The guidelines require a right heart catheterization (RHC) to diagnose PAH, defined by the following hemodynamic measurements: pulmonary capillary wedge pressure (PCWP) ≤15 mmHg, mean pulmonary arterial pressure (PAP) ≥25 mmHg and a pulmonary vascular resistance (PVR) >3 Wood units.3 In addition to its use for diagnosis, RHC provides useful information on the severity of hemodynamic impairment, can guide therapeutic interventions, evaluate response to PAH therapy and establish prognosis.2,3
RHC is typically performed via proximal venous access (PVA), either via the femoral or jugular veins. It is generally considered a safe and well-tolerated procedure.4,5
The traditional approach, however, has several limitations, mainly site access complications such as the accidental puncture of adjacent anatomical structures, patient discomfort, and delayed patient ambulation.4 These concerns deter some patients from undergoing RHC, despite its critical role.6 This is particularly important in patients with PAH who need to repeat RHC for follow-up and therapeutic decisions.
Implementing antecubital vein access (AVA) RHC as the default practice could lessen the barriers to RHC, thereby providing more patients access to this crucial procedure, and improve adherence to guidelines. RHC via an AVA has gained increased popularity over the traditional approach in cardiology literature due to its improved safety profile and comparable success rates.7–9 However, direct comparison between the two procedures in patients with PH is lacking. Additionally, there is insufficient data in the literature comparing procedural duration, fluoroscopy time and radiation dose between the two approaches.7
The aim of the present study was to determine the feasibility, safety, and adoption rates of AVA-RHC as compared with ultrasound-guided PVA in the subpopulation of patients with PH.
The study evaluated the total procedure time (defined as the interval between administration of local anesthesia for obtaining venous access and removal of the last catheter). We also compared fluoroscopy time, radiation dose, failure rate over time, and major adverse events between PVA and AVA-RHC approaches.
Methods
The PH service was established in our institute, an 1100-bed tertiary academic medical center, in late 2014. All patient with suspected PAH are referred for evaluation by RHC before commencing definite PH treatment. This study was conducted in accordance with the amended Declaration of Helsinki. Research Ethics Board approved the protocol and waived the need for informed consent to retrieve patient data.
Venous access
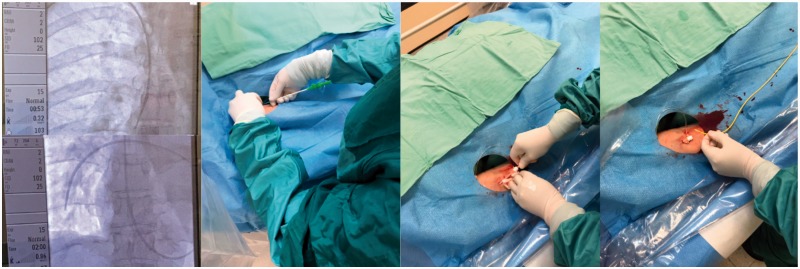
Cannulation of the antecubital vein is accomplished with a 16-18G sheath. The antecubital site is attempted as the initial site of access on all patients. The AVA sheath can then be dilated and used as an access site for RHC (Fig. 1). If AVA cannulation fails after two attempts, then PVA cannulation with ultrasound guidance (V-scan) is attempted with the internal jugular vein as the preferred site.
Fig. 1.
Right heart catheterization via antecubital vein access.
Right heart catheterization
For all of RHC procedure we used a Swan-Ganz catheter (Edwards Lifesciences, USA). The pressure transducer was set to zero level at the mid-thoracic level before the procedure was started. Measurements during RHC include a comprehensive hemodynamic assessment comprising the measurement of cardiac output in two different methods, by thermodilution and by Fick calculation, mixed venous oxygen saturation (SvO2), Pulmonary arterial pressure (PAP), Pulmonary arterial wedge pressure (PAWP), right atrial pressure (RAP) and right ventricular pressure. Most procedures were performed without a guidewire (though when necessary a guidewire was used (ATW cordis catheter, Switzerland) to locate the catheter in the desired section).
Data analysis
We retrospectively analyzed the medical records of all patients who underwent RHC between December 2014 and March 2017. Clinical and demographic data, and procedure and fluoroscopy time were retrieved from the electronic medical records. If a patient underwent multiple RHC then each one was considered a separate encounter. Patients were clustered into three groups according to the initial access site: AVA-RHC, PVA-RHC and cross-over patients who failed. The latter group includes patients who failed AVA-RHC and instead had PVA-RHC. No patients who failed PVA-RHC were transferred to AVA-RHC.
Statistical analysis
Data are presented as case-based. An intention to treat analysis was performed, including all patients referred to RHC, according to the initial vascular approach site. All RHC data during the study period were separated into four quartiles, seven months each. Comparisons of changes over time were done between first and last quartiles. Categorical variables are presented as percentages and compared using the Chi-square. All continuous variables were tested for normal distribution using Shapiro–Wilk test. Normal distributed variables are presented as mean ± standard deviation (SD) or medians and 25%–75% interquartile range (IQR) for non-normally distributed data. Comparison of continuous variables was done either by independent T-test or Mann–Whitney, as appropriate. For statistical analysis, SPSS for Windows, version 21 (IBM, Chicago, IL) was used. A p value ≤0.05 was considered statistically significant.
Results
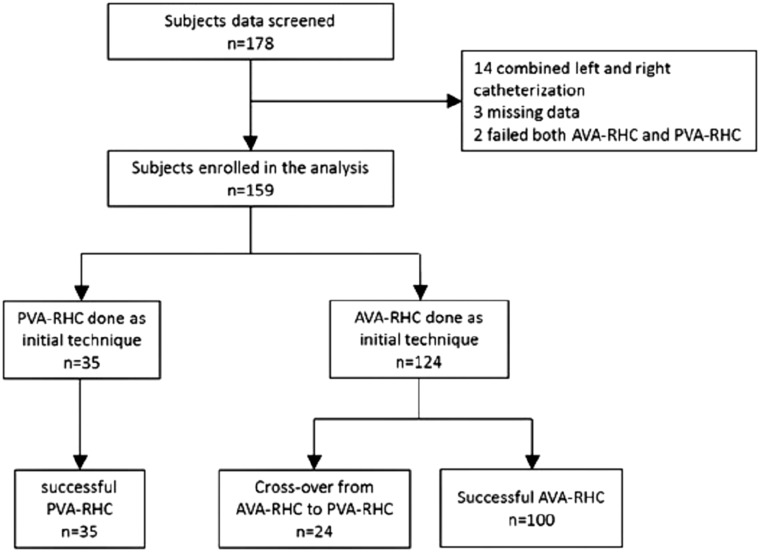
One hundred and seventy-eight consecutive patients who underwent RHCs between December 2014 and March 2017 were screened. We excluded 19 patients from the analysis (14 combined left and right catheterization, 3 missing data, 2 failed both AVA-RHC and PVA-RHC), and hence 159 cases (124 AVA, 35 PVA) were analyzed (Fig. 2). Patient demographics, diagnosis, procedure time, fluoroscopy time and radiation dose are presented in Table 1. There were no significant differences between the two groups except for mean procedure duration which was shorter in the AVA-RHC access vs. PVA-RHC cohorts, (53 (IQR 38–70) vs. 80 (IQR 56–95) min respectively, p < 0.001).
Fig. 2.
Flow chart shows the number of patients who were screened, enrolled and included in the final analysis.
Table 1.
Comparison between PVA and all AVA procedures (including crossover).
| PVA-RHC (n = 35) | All AVA (n = 124) | Statistical significance | |
|---|---|---|---|
| Age | 61.4 ± 17.8 | 64 ± 15.8 | NS |
| Malea | 7 (20) | 37 (29) | NS |
| BMI | 29.8 ± 6.6 | 29 ± 6 | NS |
| Diagnosisa | |||
| Normal PH | 6 (17) | 30 (24) | NS |
| Group 1 | 16 (46) | 46 (37) | NS |
| Group 2 | 7 (20) | 34 (27) | NS |
| Group 3 | 0 | 3 (2.5) | NS |
| Group 4 | 4 (11) | 7 (5.5) | NS |
| Group 5 | 2 (6) | 4 (3) | NS |
| Procedure time (min)b | 80 (56,95) | 53 (38,70) | p < 0.001 |
| Fluoroscopy time (min)b | 2.1 (1.2,4.0) | 2.4 (1.5,4.1) | NS |
| Radiation dose (mGy)b | 84.5 (30.0,134.5) | 54.5 (25.0,110.0) | NS |
Data are presented as absolute number (percentage).
Data are presented as Median (interquartile range).
The success rate among AVA-RHC cases was 81% (100/124 cases). Access for PVA-RHC was almost always successful on the first attempt (34/35 cases, 97%). All failed AVA-RHC were switched to PVA-RHC (the cross-over group, n = 24). Comparison between successful AVA and failed AVA (crossover group) are presented in Table 2. Of note, patients in the cross-over group were significantly older than patients in the successful AVA-RHC group (age 69 ± 11.6 vs. 62.8 ± 16.5 years, p = 0.003). We could not identify other predictors for failure in AVA-RHC except for a history of scleroderma, which had very high failure rate (50% n = 4/8 initial AVA attempts), although the number was insufficient for statistical analysis.
Table 2.
Comparison between Successful and failed (crossover) AVA procedures.
| Successful AVA (n = 100) | Crossover (n = 24) | Statistical significance | |
|---|---|---|---|
| Age | 62.8 ± 16.4 | 69.3 ± 11.6 | 0.03 |
| Malea | 30 (30) | 7 (29) | NS |
| BMI | 29.5 ± 6.3 | 28 ± 4.6 | NS |
| Diagnosisa | |||
| Normal PH | 26 (26) | 4 (17) | NS |
| Group 1 | 33 (33) | 13 (54) | 0.06 |
| Group 2 | 27 (27) | 7 (29) | NS |
| Group 3 | 3 (3) | 0 | NS |
| Group 4 | 7 (7) | 0 | NS |
| Group 5 | 4 (4) | 0 | NS |
| Procedure timeb | 47 (34,65) | 74 (62,85) | <0.001 |
| Fluoroscopy Timeb | 2.1 (1.5,4.0) | 3.1 (1.6,8.1) | NS |
| Radiation dose (mGy)b | 52 (24,101) | 101 (40,124) | NS |
Data are presented as absolute number (percentage).
Data are presented as Median (interquartile range).
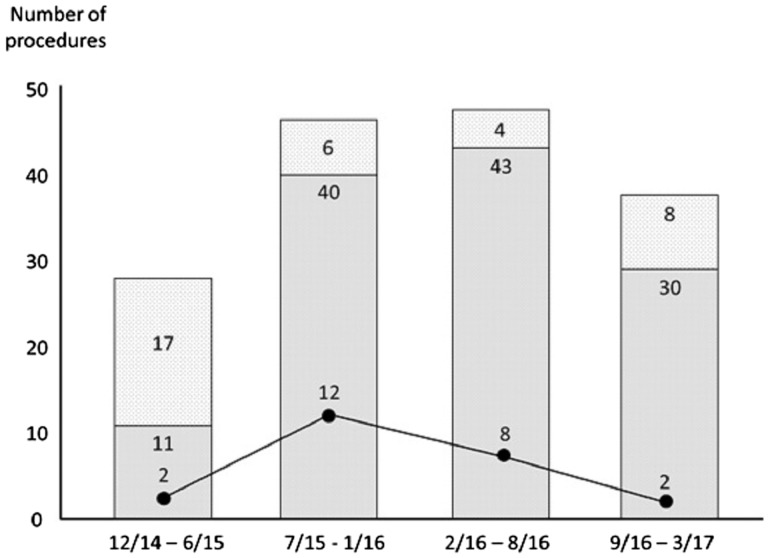
Success rate of AVA cannulation increased from 72.5% in the first 14 months to 85% in the second half (p = NS) and went as high as 93.3% in the last quartile (Fig. 3).
Fig. 3.
Number of procedures plotted against time. Light grey: PVA; Medium grey: AVA; Failed AVA (crossover group) are represented by the solid line. Number of failures decreases with time.
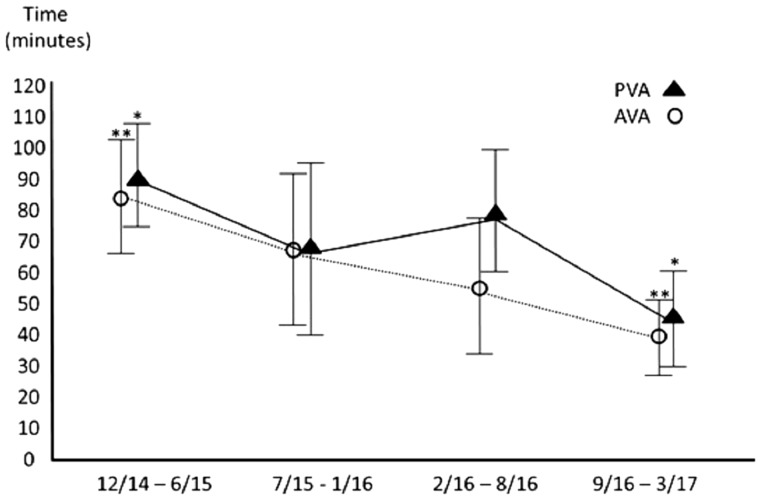
As time progressed and experience increased, the time needed for both AVA-RHC and PVA-RHC decreased (Fig. 4). The average time needed for AVA-RHC was consistently shorter than the time needed for PVA-RHC (53 (IQR 38–70) min vs. 80 (IQR 56–95) min p < 0.001 across all quartiles). Interestingly, even in the cross-over group the procedure time was shorter, compared to the original PVA-RHC group (74 (IQR 6–85) min vs. 80 (IQR 56–95) min, respectively, p = NS). Also, fluoroscopy time was similar in both group, while the absorbed radiation dose (presented by mGy units) was smaller in the AVA group compared to PVA group (54.5 vs. 84.5, respectively), although the difference was not statistically significant.
Fig. 4.
Time (min) needed for completion of the procedure. */**Statistical significance.
There were no significant adverse events in the AVA-RHC, and only one in the PVA group (carotid artery puncture resulting in a large hematoma).
Given the safety and convenience of AVA-RHC, it is not surprising that the practice of PVA-RHC decreased over time in our facility from 61% in the first quarter to 21% during the last quarter (p < 0.001), reflecting a high adoption rate.
Discussion
RHC has a vital role in the diagnosis, management and follow-up of patients with PH. Despite the recently increased use of AVA-RHC,8–10 the majority continue to be performed via the more “traditional” proximal locations. This study demonstrates that RHC by AVA can be performed safely, efficiently and accurately by a pulmonologist in the subpopulation of patients with PH.
Procedure duration for AVA-RHC was less than PVR-RHC, likely due to the extra time needed for cannulation of a proximal venous access point as compared to the peripheral antecubital vein. Of note, our analysis was based on the time taken from the initial attempt at cannulation. The crossover group was also included, and time spent on the initial, failed attempts was added to the overall time.
Like any other procedure, proficiency with technical aspects of AVA-RHC requires training and experience. In our cohort, there was a clear improvement in competence in a relatively short period, demonstrated by a reduced procedure duration, thereby decreasing radiation exposure (in both AVA-RHC and PVA-RHC). More importantly, improved procedural skill led to lower failure rates over time.
We found that both PVA-RHC and AVA-RHC procedures are safe, with only one adverse event in the PVA-RHC cohort when the carotid artery was accidentally punctured, causing a large hematoma which required hospitalization for overnight observation.
Fluoroscopy time and radiation dose were numerically smaller in the AVA group compared with PVA group. Williams et al.10 showed decreased procedure and fluoroscopy time compared with a historical cohort in an unselected population undergoing right and left heart catheterization via an antecubital fossa vein and the radial artery, compared to femoral access. Similarly, Shan et al.8 demonstrated lower fluoroscopy time using the aforementioned approach. Our study failed to demonstrate a similar reduction in fluoroscopy time, owing either to the small numbers in each cohort, or due to the fact that our PVA cannulation was performed exclusively at the internal jugular vein using ultrasound guidance, as opposed to blind femoral access which can be more cumbersome PVA.11
Despite its advantages, RHC-AVA is not suitable for all patients, particularly those (elderly patients, obese patients, drug users) in whom AVA cannulation is challenging.12 Similarly, contralateral AVA access should be attempted in patients with unilateral anatomical distortions (after mastectomy or shunts). In our cohort consisting only of patients from the PH clinic, higher rates of failure were noted in elderly patients and those with scleroderma, due to the thickening of their skin and the damage to peripheral vessels.
Like any other procedure, mastering the technical aspects of RHC-AVA requires training and experience. In our study, there was an obvious improvement in competence over time, demonstrated by reduction in the time needed for completion of AVA-RHC and the decrease in failure rate. This improvement was achieved in relatively short period of time.
This study has several limitations. First, this is a single center experience, representing our own data and experience. Second, in this study, ultrasound was not used to access AVA. The use of ultrasound for a complicated peripheral vein may have even increased success rates with AVA, shortening procedure time and lowering failure rates and third we did not evaluate neither patient nor clinician satisfaction. Nevertheless, previous studies of coronary catheterization13 demonstrated improved preference to peripheral access rather “traditional” femoral access, and there is no reason to believe different preferences with RHC. Given the safety and efficiency of the procedure, even in the crossover group, we believe that AVA-RHC should be the preferred method in most patients having RHC for the evaluation of PH.
In conclusion, we have demonstrated that AVA-RHC is a safe and feasible procedure. There were no major complications, procedural duration was shorter and radiation exposure was similar. With time and experience, and improved patient selection, failure rate is expected to decrease, thus making AVA-RHC even more appealing. Further work is necessary to refine patient selection especially among those evaluated for PH, and to compare patient satisfaction evaluate how AVA-RHC is perceived by patients and caregivers.
Authors’ contribution
All authors have participated in the work and have reviewed and agree with the content of the article. All authors have contributed to the article’s conception and design, drafting and critically revising the article for important intellectual content, and final approval of the version to be published. All authors listed have contributed sufficiently to the project to be included as authors.
Conflict of interest
The author(s) declare the following conflicts of interest: Avital Avriel: Industry Advisory Board: Actelion Pharmaceuticals Israel; Jonathan Wiesen: consulting for Our Crowd, Pulm One, MySpecialistMD; Michael Kassirer, Avi Shimony, Gal Tzangen, Amir Bar-Shai, Miri Merkin, Gabriel Rosenstein, Doron Zahger and Carlos Cafri report no conflicts of interest in this work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Avital Avriel https://orcid.org/0000-0002-6196-4044
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–D50. [DOI] [PubMed] [Google Scholar]
- 4.Hoeper MM, Lee SH, Voswinckel R, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol 2006; 48: 2546–2552. [DOI] [PubMed] [Google Scholar]
- 5.Zuckerman WA, Turner ME, Kerstein J, et al. Safety of cardiac catheterization at a center specializing in the care of patients with pulmonary arterial hypertension. Pulm Circ 2013; 3: 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med 2013; 173: 887–889. [DOI] [PubMed] [Google Scholar]
- 7.Lo TS, Ratib K, Chong AY, et al. Impact of access site selection and operator expertise on radiation exposure; a controlled prospective study. Am Heart J 2012; 164: 455–461. [DOI] [PubMed] [Google Scholar]
- 8.Shah S, Boyd G, Pyne CT, et al. Right heart catheterization using antecubital venous access: feasibility, safety and adoption rate in a tertiary center. Catheter Cardiovasc Interv 2014; 84: 70–74. [DOI] [PubMed] [Google Scholar]
- 9.Roule V, Ailem S, Legallois D, et al. Antecubital vs femoral venous access for right heart catheterization: benefits of a flashback. Can J Cardiol 2015; 31: 1497–1496. [DOI] [PubMed] [Google Scholar]
- 10.Williams PD, Palmer S, Judkins C, et al. Right and left heart catheterization via an antecubital fossa vein and the radial artery – a prospective study. J Invasive Cardiol 2014; 26: 669–673. [PubMed] [Google Scholar]
- 11.Rogers T, Lederman JR. Right heart catheterization from the arm: back to the first principles. Catheter Cardiovasc Interv 2014; 84: 75–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregg SC, Murthi SB, Sisley AC, et al. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care 2010; 25: 514–519. [DOI] [PubMed] [Google Scholar]
- 13.Kiemeneij F, Laarman GJ, Odekerken D, et al. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial, and femoral approaches: the access study. J Am Coll Cardiol 1997; 29: 1269–1275. [DOI] [PubMed] [Google Scholar]