Short abstract
Background
Lesion location is a prognostic factor of disease progression and disability accrual.
Objective
To investigate lesion formation in 11 brain regions, assess correlation between lesion location and physical and cognitive disability measures and investigate treatment effects by region.
Methods
In 2355 relapsing–remitting multiple sclerosis patients from the FREEDOMS and FREEDOMS II studies, we extracted T2-weighted lesion number, volume and density for each brain region; we investigated the (Spearman) correlation in lesion formation between brain regions, studied association between location and disability (at baseline and change over 2 years) using linear/logistic regression and assessed the regional effects of fingolimod versus placebo in negative binomial models.
Results
At baseline, the majority of lesions were found in the supratentorial brain. New and enlarging lesions over 24 months developed mainly in the frontal and sublobar regions and were substantially correlated to pre-existing lesions at baseline in the supratentorial brain (p = 0.37–0.52), less so infratentorially (p = −0.04–0.23). High sublobar lesion density was consistently and significantly associated with most disability measures at baseline and worsening of physical disability over 24 months. The treatment effect of fingolimod 0.5 mg was consistent across the investigated areas and tracts.
Conclusion
These results highlight the role of sublobar lesions for the accrual of disability in relapsing–remitting multiple sclerosis.
Keywords: White matter lesion, multiple sclerosis and neuroinflammation, demyelination, multiple sclerosis: imaging, frontal lobe, fingolimod, disability
Introduction
The presence of lesions in the central nervous system (CNS) is the hallmark of multiple sclerosis (MS)1 and the demonstration of their dissemination through time or space is the basis of MS diagnostic criteria. Although lesions are disseminated throughout the brain, it has long been known there is a predilection for certain brain regions2,3 and lesion location is a prognostic factor of disease progression4 and the type and severity of disability.5–8 Despite the importance of lesion location, lesions are typically only registered as present or absent in the care of MS patients, or summarized by lesion count and volume in clinical trials, irrespective of their location in the brain. This may be one of the reasons why the correlation between radiological summary measures and clinical findings is, at best, modest.9
Studies investigating the relationship between lesion location and disability either focused on one or few specific locations, or applied a voxel-based approach where each voxel was considered an independent entity.4–8,10 We hypothesized that a better understanding could be obtained by taking into account that neighbouring voxels in the same anatomical structure jointly contribute to functional loss. Our aim was to identify the brain regions in which the association with disability measures is particularly strong, that is, stronger than in other areas of the brain. Using a large placebo-controlled, multicenter dataset of relapsing–remitting MS (RRMS) patients, we first aimed to analyze the distribution of white matter (WM) lesions in different brain regions, and to correlate lesion location to physical and cognitive disability scores. Moreover, we aimed to explore the effect of fingolimod on lesion formation in different areas of the brain. Fingolimod is known to inhibit egress of naïve and central memory T cells from lymph nodes,11 but it has also been shown to cross the blood-brain barrier and accumulate in certain areas of the brain, suggesting a more direct effect in the CNS.12 Therefore, we investigated if this property of fingolimod may lead to different effects on the development of new and enlarging (NE) lesions in different regions of the brain.
Materials and methods
Patients and study design
We analyzed the location of WM lesions in RRMS patients pooled from two Phase III, 24-month, placebo-controlled fingolimod trials, FREEDOMS (NCT00289978) and FREEDOMS II (NCT00355134). Overall, 2355 MS patients (fingolimod 0.5 mg (n = 773), fingolimod 1.25 mg (n = 783), placebo (n = 799)) with an RR disease course, and with Expanded Disability Status Scale (EDSS) scores between 0 and 5.5, who had either one relapse in the past year or two relapses in the past 2 years were recruited. Details on the study design and patient eligibility criteria were previously reported.13,14 All patients provided written informed consent and the studies were conducted in accordance with the International Council for Harmonization Guidelines for Good Clinical Practice15 and the Declaration of Helsinki.16
Image processing
All magnetic resonance imaging (MRI) scans were assessed centrally by the Medical Image Analysis Centre in Basel (MIAC AG, Switzerland). MIAC personnel were blinded to treatment allocation and had no access to clinical patient data. Patients were scanned according to a standard MRI protocol, in which 3 mm-thick T1-weighted and dual-echo T2-weighted (T2w) images were obtained at baseline and months (M) 6, 12 and 24 (M6 and M12 scans were not used in the current study). At baseline, all WM lesions on T2w images were segmented according to the standardized procedure of the central reading center (for details, see supplementary materials). At M24, all NE lesions that occurred in the study, namely, between baseline and M24, were segmented following the same procedure in the cohort of patients, who had evaluable T2w images both at baseline and M24. Henceforth, the T2w WM lesions will be referred to as lesions.
Baseline lesions and NE lesions over 24 months were registered to the Montreal Neurological Institute (MNI) space in a multistep procedure detailed in the supplementary methods.
Brain regions
The MNI space defines regions by main brain lobes (as defined by the Talairach atlas), or by WM tracts (as defined by the John Hopkins University white-matter tractography atlas).17 In the former case, six regions were defined in the supratentorial brain: frontal, parietal, occipital, temporal, sublobar (including the corpus callosum, WM around the deep grey matter, dGM, and the lateral ventricles and near the insula) and limbic (including the WM that connects the cingulate gyrus with the hippocampal gyrus and the amygdala). Five regions were defined in the infratentorial brain: the anterior and posterior part of the cerebellum and the midbrain, pons and medulla in the brainstem. The 24 WM tracts are tabulated in Supplementary Table e-1. No distinction was made between the left and right hemispheres.
MRI outcomes
For each patient, the volume and number of lesions at baseline and NE lesions at M24 were computed for every region. Lesion density was calculated as lesion volume (LV) divided by the corresponding volume in a given brain region and can be interpreted as the percentage of tissue affected by MS lesions.
Disability scores
The EDSS and its scores of functional subsystems (EDSS bowel and bladder, EDSS-BB; EDSS brainstem, EDSS-BS; EDSS pyramidal, EDSS-PY; EDSS sensory, EDSS-SE; EDSS cerebellar, EDSS-CB; EDSS cerebral, EDSS-CE; EDSS visual, EDSS-VI),18 as well as the MS functional composite subscores (i.e. Timed 25-Foot Walk Test, T25FWT; 9-Hole Peg Test, NHPT; and Paced Auditory Serial Addition Test, PASAT)19 were used as disability measures.
Statistical analysis
All evaluable data were analyzed according to the intent-to-treat principle. Pre-existing lesions at baseline and NE lesions over 24 months were analyzed separately, the percentage of patients with lesions (pre-existing and NE) in each region was calculated. To study the natural process of lesion formation in MS, the M24 analysis was performed on untreated (placebo) patients only. The distribution of lesions by brain region and for the whole brain (WB) was described by mean and standard deviations of LV, number and density.
To investigate the association of MS lesions in specific brain regions with disability or disability worsening, we fitted two models separately to the data for each time point with the following model formulations to derive the maximum likelihood estimations:
| (1) |
| (2) |
| (3) |
| (4) |
where: represents the dependent variable in the regressions and is the disability score (i.e. either EDSS total score, EDSS functional system scores, PASAT, T25FWT, or NHPT) at baseline; β0 is the intercept of the model; is the variable representing the trial (i.e. FREEDOMS or FREEDOMS II) and the corresponding estimate; is the patient’s age and the corresponding estimate; is the patient’s gender and the corresponding estimate; is the patient’s disease duration and the corresponding estimate; is the lesion density in the WB at baseline and the corresponding estimate; is the lesion density in region computed at baseline and the corresponding estimate; represents the dependent variable and is the disability worsening at M24; is the density of the NE lesions in the WB at M24 and the corresponding estimate; and is the density of the NE lesions in region computed at M24 and the corresponding estimate.
In summary, model (1) investigates the association between the WB lesion density and each disability score at baseline, ignoring lesion location, and model (3) quantifies the association between the WB NE lesion density and the disability worsening at M24. Models (2) and (4) distinguish among different brain regions but they are otherwise similar to the WB models. We used models (1) and (3) to quantify the association of ‘an average WB lesion’ with disability measures in terms of the beta-estimate, which we then used as a benchmark to identify the locations in which the association with the disability or disability worsening is stronger than the average lesion. Mathematically:
| (5) |
| (6) |
To assess the reproducibility of our association results (point estimates with 95% confidence limits based on all data), we resampled 1000 bootstrap samples from the data. To each bootstrap sample we refitted the models to verify whether we could re-identify a statistically significant and stronger association between lesions in the specific brain region and the disability measure than between ‘an average WB lesion’ and the same disability measure. We were interested in those association results which had a high reproducibility (arbitrarily defined as being reproducible in >70% of the bootstrap samples).
In the baseline analysis, for continuous or continuous-like disability scores (PASAT, T25FWT, NHPT and EDSS), we used linear regression models. To normalize residuals, we used a logit transformation of PASAT (i.e. ) and the inverse transformation for T25FWT (i.e.). The EDSS subscores were transformed in binary variables: 0 if the subscore was equal to 0 (no symptoms), and 1 if the subscore was greater than 0 (symptoms present). Consequently, a logistic regression model was selected.
In the M24 analysis, disability worsening was a binary variable (where 0 = not worsened, 1 = worsened) and its definition was dependent on the score considered (Supplementary Table e-2). Logistic regression was then used; odds ratios and confidence intervals were derived from fitting the models using the whole dataset (i.e. not bootstrapped).
In addition to this analysis, at M24 we computed the correlation between pre-existing lesions at baseline and the formation of NE lesions in a pair-wise combination of brain regions (Spearman rank correlations). We also investigated the probability of each region being affected by the highest amount of NE lesions as a function of the location of the majority of pre-existing baseline lesions.
Analysis of the effect of MS treatment on lesion formation
We analyzed whether MS treatment (fingolimod) had a similar effect on lesion formation throughout the brain, or whether some brain regions were more responsive to the treatment than others. The following model was fit to all data (i.e. fingolimod and placebo patients) for each brain region separately using a negative binomial:
| (7) |
where: is the dependent variable and represents the number of NE lesions at M24; is the intercept of the model; is the trial and the corresponding estimate; is the treatment option (i.e. placebo, fingolimod 0.5 mg, fingolimod 1.25 mg) and the corresponding estimate; and is the number of lesions at baseline and the corresponding estimate.
In this analysis, we used lesion number instead of volume or density for consistency with the original analysis.14 From the obtained from the model, a lesion rate ratio (LRR) between fingolimod and placebo was obtained. The treatment effect of fingolimod vs placebo (% change) was calculated as , negative numbers favour fingolimod.
All statistical analyses were conducted using R (www.r-project.org).
Data availability
This is a post hoc analysis of data from patients who had participated in two fingolimod Phase III clinical trials. Anonymized data not published within this article will be made available on request from any qualified investigator.
Results
Baseline characteristics
Out of a total of 2355 randomized RRMS patients in the pooled FREEDOMS and FREEDOMS II trials, 1907 (81%) had evaluable lesion location information at baseline, and 1351 (72% of the 1871 patients, who completed both studies) at both baseline and M24. The baseline characteristics of both subsets of patients were consistent with the baseline characteristics of all patients in the original trials (Table 1). The majority of MS patients were women, with a mean age of ∼38 years, mean disease duration of ∼9 years and mean EDSS 2.4.
Table 1.
Baseline characteristics of the datasets used in this study compared to the original FREEDOMS and FREEDOMS II trials.
| Characteristics | FREEDOMS and FREEDOMS II (n = 2355) |
Baseline dataset (n = 1907) |
M24 dataset (n = 1351) |
|---|---|---|---|
| Women, n | 1733 | 1362 | 980 |
| Placebo/ | |||
| Fingolimod 0.5 mg/ | 773/783/799 | 631/635/641 | 444/467/440 |
| Fingolimod 1.25 mg | |||
| Age, years | |||
| Mean ± SD | 38.6 ± 8.8 | 38.5 ± 8.8 | 38.3 ± 8.8 |
| Median (range) | 39 (17–57) | 39 (17–57) | 39 (17–57) |
| DD, years | |||
| Mean ± SD | 9.3 ± 7.4 | 9.1 ± 7.3 | 8.9 ± 7.2 |
| Median (range) | 8 (0–50) | 7 (0–50) | 7 (0–50) |
| EDSS score | |||
| Mean ± SD | 2.4 ± 1.3 | 2.4 ± 1.3 | 2.4 ± 1.3 |
| Median (range) | 2 (0–6.5) | 2 (0–6) | 2 (0–6) |
| T2w lesion volume, mL | |||
| Mean ± SD | 5.9 ± 7.8 | 5.7 ± 7.6 | 5.6 ± 7.2 |
| Median (range) | 3 (0–69) | 3 (0–69) | 3 (0–55) |
| Normalized brain volume, mL | |||
| Mean ± SD | 1518 ± 84 | 1520 ± 84 | 1523 ± 80 |
| Median (range) | 1522 (1144–1764) | 1524 (1144–1756) | 1528 (1217–1756) |
DD: disease duration; EDSS: Expanded Disability Status Scale; M: month; SD: standard deviation; T2w: T2 weighted.
Lesion distribution and relation to disability at baseline by location
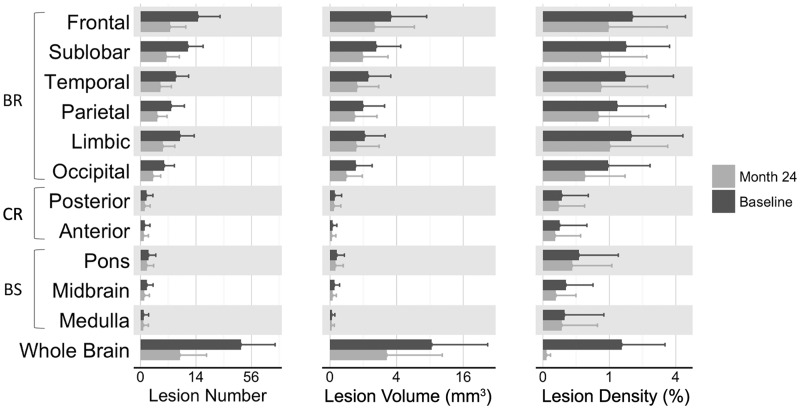
At baseline, most patients had lesions in the frontal lobe and the sublobar region, whereas fewer patients had lesions in the cerebellum or the brainstem (Table 2). The highest LV and number were also located in the frontal lobe, followed by the sublobar and limbic areas (Figure 1). When adjusting for the size of the different lobes (i.e. considering lesion density), the density of MS lesions was similar throughout the supratentorial brain with relatively fewer lesions only in the occipital lobe (Figure 1).
Table 2.
Percentage of all patients with preexisting lesions at baseline and placebo-treated patients with NE lesions (at M24) by location.
| Brain regions | Baseline (%) | Month 24 (%) |
|---|---|---|
| BR | ||
| Frontal | 96.2 | 68.2 |
| Sublobar | 95.8 | 67.1 |
| Temporal | 89.4 | 58.8 |
| Parietal | 83.9 | 51.8 |
| Limbic | 91.1 | 61.5 |
| Occipital | 76.7 | 39.6 |
| CR | ||
| Posterior | 11.8 | 8.1 |
| Anterior | 6.7 | 4.3 |
| BS | ||
| Pons | 19.2 | 12.4 |
| Midbrain | 15.1 | 6.5 |
| Medulla | 3.9 | 3.1 |
BR: supratentorial brain; BS: brainstem; CR: cerebellum; M: month; NE: new/enlarging.
Figure 1.
Distribution of all T2 lesions at baseline (Baseline), and of new or enlarging lesions between baseline and month 24 (M24). Mean and standard deviation of lesion number, volume and density in each brain region and in the whole brain at baseline (i.e. pre-existing lesions at the study entry) and at M24 (i.e. new or enlarging lesions between baseline and M24). At baseline, mean and standard deviation were derived from the whole cohort, whereas at M24 only from placebo-treated patients to report the lesion formation in untreated patients. BR: supratentorial brain; BS: brainstem; CR: cerebellum.
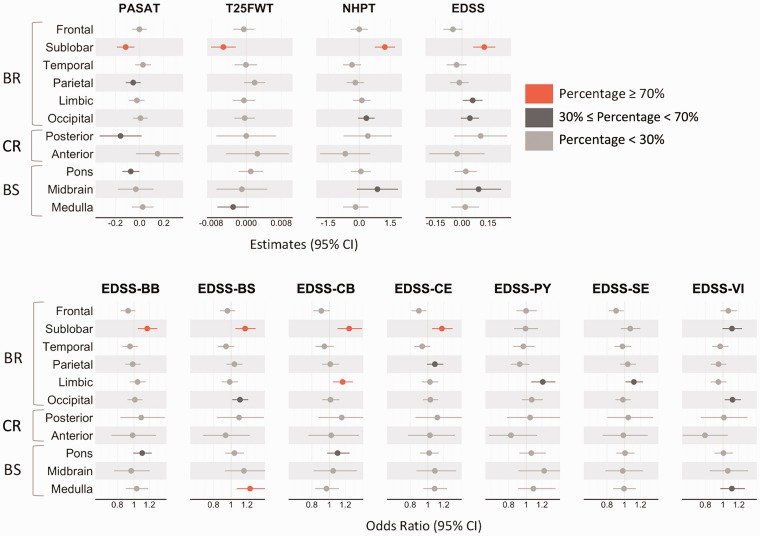
The association of sublobar lesions with most disability measures (i.e. PASAT, T25FWT, NHPT, EDSS, EDSS-BB, EDSS-BS, EDSS-CB and EDSS-CE) was stronger than the association of ‘the average WB lesion’ with the same disability scales (Figure 2). Other consistently significant correlations between lesion location and disability scores included associations between the medulla and the EDSS-BS, and the limbic lobe and EDSS-CB.
Figure 2.
Association between lesions at baseline in each region and the different disability scores. The estimates, odds ratios and the CIs plotted here were derived from the model defined as (2) using the whole dataset. The color associated with each estimate and CI derived from the bootstrap analysis indicates the percentage of times in which the association between lesions in specific locations and disability is stronger than the one between ‘average whole brain lesions’ and disability: values are 0–100%, higher values indicate better reproducibility. For the bootstrap analysis results, see Supplementary Table e-3. BR: supratentorial brain; BS: brainstem; CI: confidence interval; CR: cerebellum; EDSS: Expanded Disability Status Scale; EDSS-BB: EDSS bowel and bladder; EDSS-BS: EDSS brainstem; EDSS-CB: EDSS cerebellar; EDSS-CE: EDSS cerebral; EDSS-PY: EDSS pyramidal; EDSS-SE: EDSS sensory; EDSS-VI: EDSS visual; NHPT: 9-Hole Peg Test; PASAT: Paced Auditory Serial Addition Test; T25FWT: Timed 25-Foot Walk Test.
New lesion formation in untreated MS patients over 24 months by location
Similar to baseline, a high percentage of untreated patients developed NE lesions in the supratentorial brain, whereas fewer (less than 13%) developed NE lesions in the cerebellum and in the brainstem (Table 2). The average number of NE lesions per patient that developed in different regions over 24 months in untreated patients were as follows (Figure 1): frontal, 4.04±5.39; sublobar, 3.05±3.85; temporal, 1.80±2.57; parietal, 1.32±1.88; limbic, 2.32±3.09; and occipital, 0.72±1.16. In contrast, only 0.09±0.33 and 0.05±0.24 NE lesions occurred in the posterior and the anterior part of the cerebellum, respectively. In the brainstem, 0.19±0.62 NE lesions appeared in the pons, 0.07±0.29 in the midbrain and 0.07±0.25 in the medulla.
We found substantial positive correlations (p (spearman correlation) ranged from 0.37 to 0.52) between pre-existing lesion locations and NE lesion locations in the supratentorial brain. Patients who had lesions in the supratentorial region of the brain at baseline were also likely to develop NE lesions in the supratentorial region itself. Lesions in the infratentorial region were less correlated to other regions (weaker correlations ranged from −0.04 to 0.23).
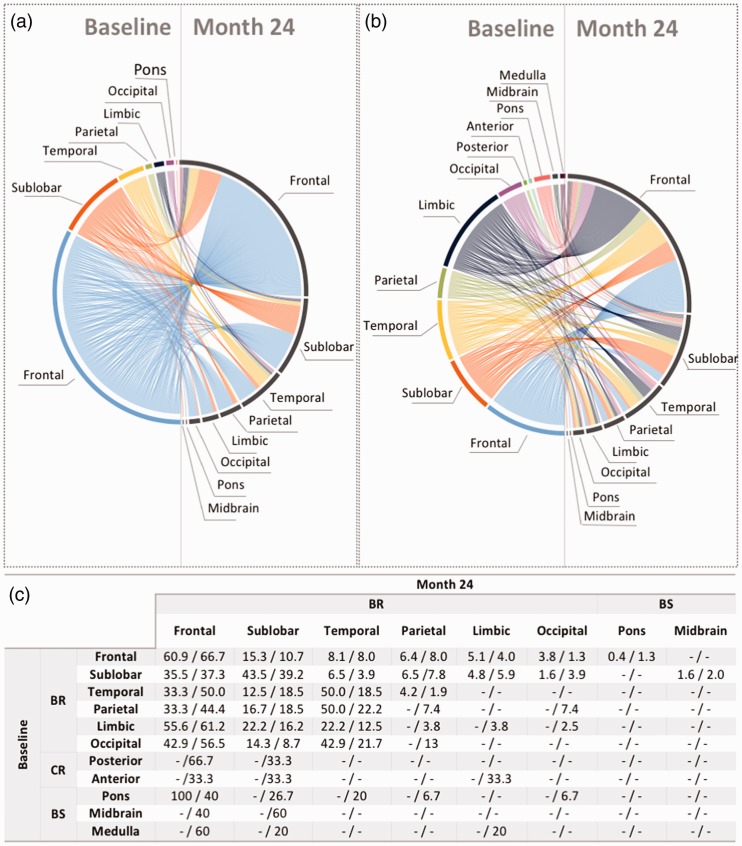
The relationship between the regions characterized by the highest volume (or density) of lesions at baseline and the ones with the highest volume (or density) of NE lesions at M24 are plotted in Figure 3(a) and 3(b) and the associated probabilities are reported in Figure 3(c). Irrespective of where the highest LV was present at baseline, the highest volume of NE lesions developed in the frontal lobe and in the sublobar region (Figure 3(a)). Patients who had the highest LV at baseline in the brainstem or cerebellum were also the most likely to develop NE lesions in the frontal lobe or in the sublobar region. The predilection of MS lesions for these regions was even more pronounced when the analysis was based on lesion density (Figure 3(b)).
Figure 3.
The relationship between the regions with the highest number of lesions at baseline and the regions with the highest amount of NE lesions. To study the natural process of lesion formation in MS, the M24 analysis was performed on untreated (placebo) patients only. The left side of the circular plots represents the brain regions in which the patients had the highest number of lesions at baseline, whereas the right side represents the regions in which the patients developed the highest number of NE lesions during the 24 months of the trials. Each line represents a patient and connects the region where they had the highest number of lesions at baseline with the region in which they developed the highest number of new/enlarging lesions at M24. The circular plot in (a) used the LV to determine the highest amount, whereas the one in (b) used the lesion density. The table in (c) expresses the probabilities associated with the circular plots in (a) and (b) as LV/lesion density. Irrespective of the location of the highest number of lesions at baseline, the highest number of new lesions developed mostly in the frontal lobe, sublobar region and the temporal lobe. BR: supratentorial brain; BS: brainstem; CR: cerebellum; LV: lesion volume; M: month; NE: new/enlarging; MS: multiple sclerosis.
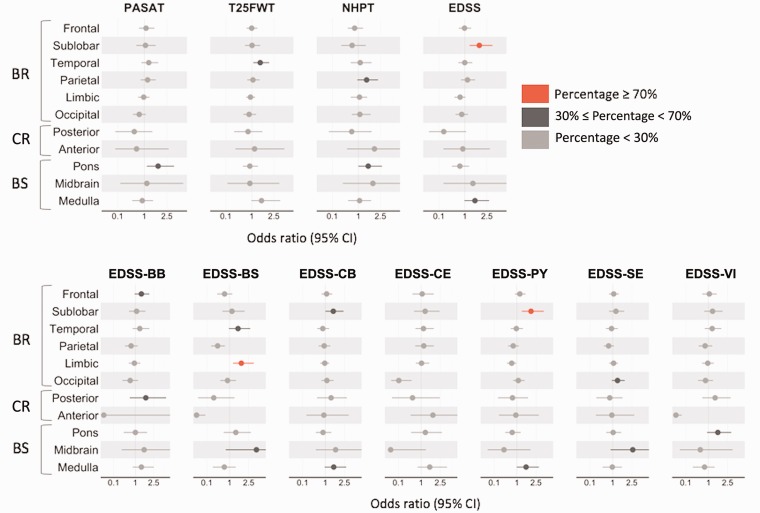
New lesion formation and disability worsening
Development of NE lesions in the sublobar region was associated with worsening of physical disability as measured by the EDSS total score and EDSS-PY. The association between new sublobar lesions and EDSS worsening was significant and stronger than the association between ‘the average WB lesion’ and disability worsening (Figure 4). A stronger relationship was also found between lesions in the limbic area and worsening on the EDSS-BS score. The detailed results from the bootstrap analysis, that is, how many times the bootstrap samples showed a stronger association with disability than the WB, are reported in Supplementary Tables e-3, e-4 and e-5.
Figure 4.
Association between NE lesions in each region and the different disability worsening. The odds ratios and CIs plotted here were derived from the model defined as (4) using only the placebo-treated patients of the M24 dataset. The colour associated with each odds ratio and CI derived from the bootstrap analysis indicates the percentage of times in which the association between NE lesions in specific locations and disability worsening is stronger than the one between ‘average whole brain lesions’ and disability worsening: values are 0–100%, higher values indicate better reproducibility. A high NE lesion density in the sublobar region showed a stronger association with EDSS and EDSS-PY worsening than the association obtained using the NE lesion density defined in the whole brain. A stronger relationship was also found between NE lesion density in the limbic area and the EDSS-BS worsening. For the bootstrap analysis results, see Supplementary Table e-4. BR: supratentorial brain; BS: brainstem; CI: confidence interval; CR: cerebellum; EDSS: Expanded Disability Status Scale; EDSS-BB: EDSS bowel and bladder; EDSS-BS: EDSS brainstem; EDSS-CB: EDSS cerebellar; EDSS-CE: EDSS cerebral; EDSS-PY: EDSS pyramidal; EDSS-SE: EDSS sensory; EDSS-VI: EDSS visual; NE: new/enlarging; NHPT: 9-Hole Peg Test; PASAT: Paced Auditory Serial Addition Test; T25FWT: Timed 25-Foot Walk Test.
The effect of MS treatment on lesion formation by location
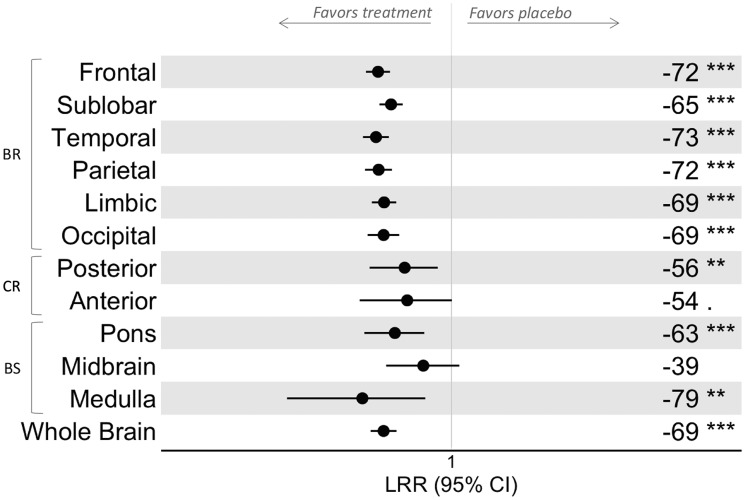
Fingolimod 0.5 mg significantly and consistently reduced NE lesions compared with placebo in the supratentorial brain (p<0.001, in all regions) (Figure 5). The percentage reduction with fingolimod 0.5 mg treatment was relatively homogeneous in the supratentorial brain, whereas it was more variable in the infratentorial area, with the highest relative reduction in the medulla (79%, p<0.01) and the lowest in the midbrain (39%, not significant). The treatment effect in each region was broadly consistent with the overall effect for the WB. The effect of treatment with fingolimod 1.25 mg was similar to that of fingolimod 0.5 mg.
Figure 5.
Treatment effect of fingolimod 0.5 mg on the occurrence of NE lesions by location. In each brain region and in the whole brain, the effect of fingolimod on lesion formation was investigated using a negative binomial in which the new/enlarging lesion number was the dependent variable and treatment, clinical trial and baseline lesion number were the independent variables. The LRR between the fingolimod-treated and placebo patients was calculated, as were the CIs. The treatment effect of fingolimod versus placebo (% change) on the number of new or enlarging lesions was calculated as (LRR-1)*100 and is reported on the right side of the plot (a negative number favours fingolimod), together with the p value level (i.e. p > 0.1; . 0.1 ≤ p < 0.05; *0.05 ≤ p < 0.01; **0.01 ≤ p < 0.001; ***p ≤ 0.001). BR: supratentorial brain; BS: brainstem; CI: confidence interval; CR: cerebellum; LRR: lesion rate ratio; NE: new/enlarging.
Lesion distribution and its relation to disability and treatment effect by WM tracts
The main results obtained using the WM tracts are reported in Supplementary Figures e-1, e-2, e-3 and e-4 and are consistent with those obtained for the lobes.
Discussion
We analyzed the distribution of T2w WM lesions in the brains of MS patients and their association with disability. We chose to analyze T2w lesions because they allowed us to investigate the impact of lesion burden accumulated over a lifetime (baseline lesions), while enabling us to study new lesion formation over 24 months. Our large dataset of 2355 patients provided a unique opportunity to investigate the spatial distribution of lesion formation in both untreated (placebo) and treated (fingolimod) patients.
MS lesions developed preferentially in the supratentorial brain, particularly the frontal lobe and the sublobar region. A higher occurrence of lesions in the upper part of the brain is consistent with voxel-wise lesion distribution maps previously derived from smaller cohorts.6,8 Even in patients who had majority of lesions in another brain region at study entry, NE lesions developed preferentially in the frontal lobe and the sublobar region. Since the 19th century, MS lesions have been found to be centered around small veins where inflammation occurs.20–22 We hypothesize that a high degree of vascularization may be the biological reason for the predilection of MS lesions for those regions. This hypothesis is supported by evidence that WM lesions occur more frequently in regions characterized by higher perfusion, such as the sublobar region.23 In agreement with this hypothesis, a lower amount of lesions was found in areas with lower perfusion, such as the external capsule, fornix-stria terminalis and cingulum-hippocampus.24
Analyzing the relationship between WM lesions and disability at baseline, we identified sublobar lesions as key contributors to various aspects of disability including motor impairment and cognitive loss. In fact, lesions in the sublobar region showed significant and highly reproducible associations with most of the tested physical and cognitive disability measures and these associations were stronger than the correlations between ‘the average WB lesion’ (without making a distinction of lesion location) and the same disability measures. A possible explanation for the prominent role of the sublobar region could be the relay function of the thalamus. The sublobar region has also been identified previously as a region of special interest for worsening in MS.5–8,10 The sublobar region was reported as the brain area with most rapid shrinkage and an atrophic dGM was prognostic of disability worsening.25 Charil et al.5 evaluated the correlation between lesion occurrence and EDSS functional subscores. For the EDSS-BS, one cluster of significant voxels was found in the insula, a structure included in the Talairach definition of the sublobar region. Moreover, the association we found between the sublobar region and PASAT was also detected by Vellinga et al.8 Other notable associations were found for brainstem lesions and brainstem function, and for lesions in the limbic lobe and cerebellar functions.
When analyzing the association of NE lesion formation and disability worsening, very few regions (the sublobar region for EDSS total score and EDSS-PY, and the limbic area for EDSS-BS) showed a stronger association with disability scores than ‘the average WB lesion’. Overall, this relationship was less clear than the association between lesion location at baseline and disability. The paucity of the areas that showed an association between NE lesions and disability suggests that a substantial number of lesions have no immediate clinical translation and sometimes multiple lesions are needed before clinical relapse and/or progression occur.26,27 Although individual NE lesions may not always lead to clinical symptoms, the cumulative lesion burden (i.e. the T2 LV) has been identified as a main contributor to brain atrophy, which is associated with clinical worsening of MS.28–30 In our study, we used a definition of new or enlarging lesions, which was also predefined as an endpoint in these Phase III trials.13,14 We did not distinguish between NE lesions, which may potentially follow different underlying pathological mechanisms. This could be a contributing factor in not observing a stronger association between new lesions and new disability symptoms over the duration of the trials.
In our study, the effect of fingolimod was remarkably homogeneous throughout the brain regions and WM tracts analyzed. We are speculating that the higher treatment effect variability in the lower brain (as indicated by the wide confidence intervals) was due to the lower lesion numbers and low statistical power compared with the supratentorial brain.
Of the initially randomized patients, 19% of baseline and 25% of M24 scans could not be included in this analysis mainly due to technical reasons (quality of the MRI scans and suitability of the images to be warped to MNI space) or because of a lacking 24 months MRI dataset. The similarity of baseline characteristics between patients with evaluable data in our study and the overall trial population as well as the blinded assessment of MRIs, argues against a relevant selection bias. The analyzed patients represent a typical cohort of RRMS patients.
Working in MNI space and using existing atlases provided an advantage in ensuring a common space and standardized terminology for the comparison of individual patient level data. This approach acknowledges that lesions in the same anatomical structure have a higher probability of acting synergistically on disability outcomes, thus improving interpretability and power to detect clinical correlations as compared to a naïve voxel-based analysis. Nevertheless, this assumption is not trivial because lesions in close proximity to one another might affect different pathways, or in contrast lesions with considerable spatial distance between them might impact the same WM tract. Thus, we added the analysis by tracts; however, no closer correlations to clinical outcomes were found, probably because such an analysis would need more granular clinical and functional-anatomic resolution.
Overall, our results emphasize the prominent role of sublobar lesions in the accrual of disability in patients with RRMS.
Supplemental Material
Supplemental material, MSO906844 Supplemental Material for White matter lesion location correlates with disability in relapsing multiple sclerosis by Laura Gaetano, Baldur Magnusson, Petya Kindalova, Davorka Tomic, Diego Silva, Anna Altermatt, Stefano Magon, Nicole Müller-Lenke, Ernst-Wilhelm Radue, David Leppert, Ludwig Kappos, Jens Wuerfel, Dieter A Häring and Till Sprenger in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Acknowledgments
We thank Richa Chhabra (Novartis Healthcare, Hyderabad, India) for providing medical writing support, which encompassed formatting the manuscript, referencing, preparing tables and figures as per journal guidelines and incorporating the authors’ revisions and finalizing the draft for submission, all under the direction of the authors. The study sponsor (Novartis Pharma) participated in the design and conduct of the study, data collection, data management, data analysis, data interpretation and preparation, review and approval of the manuscript as well as in the writing of the report; and in the decision to submit the paper for publication. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
Laura Gaetano, Medical Image Analysis Center, Switzerland; Department of Neurology, University Hospital Basel, Switzerland.
Baldur Magnusson, Novartis Pharma, Switzerland.
Petya Kindalova, Department of Statistics, University of Oxford, UK.
Diego Silva, Novartis Pharma, Switzerland.
Anna Altermatt, Medical Image Analysis Center, Switzerland; Department of Biomedical Engineering, University of Basel, Switzerland.
Stefano Magon, Medical Image Analysis Center, Switzerland; Department of Neurology, University Hospital Basel, Switzerland.
Nicole Müller-Lenke, Medical Image Analysis Center, Switzerland.
Ernst-Wilhelm Radue, Biomedical Research and Training, Biomedical Research, Switzerland.
Ludwig Kappos, Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research, Biomedicine and Biomedical Engineering, University Hospital and University of Basel, Switzerland.
Jens Wuerfel, Medical Image Analysis Center, Switzerland.
Dieter A Häring, Novartis Pharma, Switzerland.
Till Sprenger, Medical Image Analysis Center, Switzerland; Department of Neurology, DKD Helios Klinik Wiesbaden, Germany.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Laura Gaetano was a temporary employee of Novartis and was employed at MIAC, where the imaging data were analyzed. She is currently employed by F. Hoffman-La Roche, but her current institution was not involved in this study. Baldur Magnusson, Davorka Tomic and Dieter A Häring are employees of Novartis Pharma. Petya Kindalova was a temporary employee of Novartis while working on any analyses related to the current study. She is currently a PhD student at the University of Oxford and the current institution was not involved in the analyses. Diego Silva was an employee of Novartis during the conduct of the study. Anna Altermatt has nothing to disclose. Stefano Magon has received research support from the Swiss MS Society, Swiss National Science Foundation, University of Basel and Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung sowie der medizinischen Bildauswertung Universitätsspital Basel. Nicole Müller-Lenke has nothing to disclose. Ernst-Wilhelm Radue has received research support from Actelion, Bayer Schering, Biogen Idec, Genzyme, Merck Serono, Novartis and Sanofi. David Leppert has received personal compensation for consulting and speaking, and travel reimbursement from Quanterix, Orion and Sanofi. Ludwig Kappos’s institution (University Hospital Basel) has received the following exclusively for research support: steering committee, advisory board and consultancy fees (Actelion, Addex, Bayer HealthCare, Biogen Idec, Biotica, Genzyme, Lilly, Merck, Mitsubishi, Novartis, Ono Pharma, Pfizer, Receptos, Sanofi, Santhera, Siemens, Teva, UCB and Xenoport); speaker fees (Bayer HealthCare, Biogen Idec, Merck, Novartis, Sanofi and Teva); support of educational activities (Bayer HealthCare, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi and Teva); royalties (Neurostatus products); licence fees for Neurostatus products; and grants (Bayer HealthCare, Biogen Idec, European Union, Merck, Novartis, Roche Research Foundation, Swiss MS Society and Swiss National Research Foundation). Jens Wuerfel is CEO of MIAC. He is funded by the European Union (Horizon2020). Till Sprenger has received no personal compensation. The current or previous employers of Till Sprenger have received compensation for consulting or speaking from Biogen Idec, Eli Lilly, Allergan, Actelion, ATI, Desitin, Electrocore, Merck Serono, Mitsubishi Pharma, Novartis, Roche, Teva and Sanofi Genzyme. He has received research support from Novartis Pharma, the Swiss MS Society and the Swiss National Science Foundation.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Novartis Pharma, Basel, Switzerland.
ORCID iDs
Laura Gaetano https://orcid.org/0000-0002-5214-2858
Ludwig Kappos https://orcid.org/0000-0003-4175-5509
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Polman CH, Reingold SC, Banwell Bet al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald Criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownell B, Hughes JT. The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry 1962; 25: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikuta F, Zimmerman HM. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurol 1976; 26: 26–28. [DOI] [PubMed] [Google Scholar]
- 4.Dalton CM, Bodini B, Samson RSet al. Brain lesion location and clinical status 20 years after a diagnosis of clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler J 2012; 18: 322–328. [DOI] [PubMed] [Google Scholar]
- 5.Charil A, Zijdenbos AP, Taylor Jet al. Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: Application to 452 patient data sets. Neuroimage 2003; 19: 532–544. [DOI] [PubMed] [Google Scholar]
- 6.Kincses ZT, Ropele S, Jenkinson Met al. Lesion probability mapping to explain clinical deficits and cognitive performance in multiple sclerosis. Mult Scler J 2011; 17: 681–689. [DOI] [PubMed] [Google Scholar]
- 7.Rossi F, Giorgio A, Battaglini Met al. Relevance of brain lesion location to cognition in relapsing multiple sclerosis. Plos One 2012; 7: e44826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vellinga MM, Geurts JJG, Rostrup Eet al. Clinical correlations of brain lesion distribution in multiple sclerosis. J Magn Reson Imaging 2009; 29: 768–773. [DOI] [PubMed] [Google Scholar]
- 9.Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 2002; 15: 239–245. [DOI] [PubMed] [Google Scholar]
- 10.Altermatt A, Gaetano L, Magon Set al. Clinical correlations of brain lesion location in multiple sclerosis: Voxel-based analysis of a large clinical trial dataset. Brain Topogr 2018; 31: 886–894. [DOI] [PubMed] [Google Scholar]
- 11.Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: Therapeutic effects in the immune and the central nervous system. Brit J Pharmacol 2009; 158: 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter SF, Bowen JD, Reder AT. The direct effects of fingolimod in the central nervous system: Implications for relapsing multiple sclerosis. CNS Drugs 2016; 30: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabresi PA, Radue EW, Goodin D. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, Phase 3 trial. Lancet Neurol 2014; 13: 545–556. [DOI] [PubMed] [Google Scholar]
- 14.Kappos L, Radue EW, O’Connor Pet al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. New Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 15.ICH Harmonised Tripartite Guidelines, Guideline For Good Clinical Practice E6(R1). Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (2018, accessed 28 September 2018).
- 16.World Medical Association. World Medical Association Declaration of Helsinki Ethical Principles for medical research involving human subjects. Jama 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 17.Hua K, Zhang JY, Wakana Set al. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008; 39: 336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtzke JF. Rating neurologic impairment in multiple-sclerosis: an Expanded Disability Status Scale (EDSS). Neurol 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 19.Fischer JS, Rudick RA, Cutter GRet al. The Multiple Sclerosis Functional Composite measure (MSFC): An integrated approach to MS clinical outcome assessment. Mult Scler 1999; 5: 244–250. [DOI] [PubMed] [Google Scholar]
- 20.Charcot JM. Leçons sur les maladies du système nerveux faites à la salpêtrière. Recueillies et publ par Bourneville Hachette Livre BNF; 1880.
- 21.Rindfleisch E. Histologisches Detail zu der grauen Degeneration von Gehirn und Rückenmark (Zugleich ein Beitrag zu der Lehre von der Entstehung und Verwandlung der Zelle). Arch für Pathol Anat und Physiol und für Klin Med [online serial] 1863; 26: 474–483. [Google Scholar]
- 22.Tallantyre EC, Brookes MJ, Dixon JEet al. Demonstrating the perivascular distribution of MS lesions in vivo with 7-Tesla MRI. Neurol 2008; 70: 2076–2078. [DOI] [PubMed] [Google Scholar]
- 23.Holland CM, Charil A, Csapo Iet al. The relationship between normal cerebral perfusion patterns and white matter lesion distribution in 1,249 patients with multiple sclerosis. J Neuroimaging 2012; 22: 129–136. [DOI] [PubMed] [Google Scholar]
- 24.Giezendanner S, Fisler MS, Soravia LMet al. Microstructure and cerebral blood flow within white matter of the human brain: A TBSS analysis. Plos One. 2016; 11: e0150657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaetano L, Haring DA, Radue EWet al. Fingolimod effect on gray matter, thalamus, and white matter in patients with multiple sclerosis. Neurol 2018; 90: e1324–e32. [DOI] [PubMed] [Google Scholar]
- 26.Barkhof F, Scheltens P, Frequin STet al. Relapsing-remitting multiple sclerosis: Sequential enhanced MR imaging vs clinical findings in determining disease activity. AJR 1992; 159: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 27.Frank JA, Stone LA, Smith MEet al. Serial contrast-enhanced magnetic resonance imaging in patients with early relapsing-remitting multiple sclerosis: Implications for treatment trials. Ann Neurol. 1994; 36 Suppl: S86–90. [DOI] [PubMed] [Google Scholar]
- 28.Miller DH, Lublin FD, Sormani MPet al. Brain atrophy and disability worsening in primary progressive multiple sclerosis: Insights from the INFORMS study. Ann Clin Transl Neurol 2018; 5: 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radue EW, Barkhof F, Kappos Let al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurol 2015; 84: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sormani MP, Kappos L, Radue EWet al. Defining brain volume cutoffs to identify clinically relevant atrophy in RRMS. Mult Scler J 2017; 23: 656–664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSO906844 Supplemental Material for White matter lesion location correlates with disability in relapsing multiple sclerosis by Laura Gaetano, Baldur Magnusson, Petya Kindalova, Davorka Tomic, Diego Silva, Anna Altermatt, Stefano Magon, Nicole Müller-Lenke, Ernst-Wilhelm Radue, David Leppert, Ludwig Kappos, Jens Wuerfel, Dieter A Häring and Till Sprenger in Multiple Sclerosis Journal—Experimental, Translational and Clinical
Data Availability Statement
This is a post hoc analysis of data from patients who had participated in two fingolimod Phase III clinical trials. Anonymized data not published within this article will be made available on request from any qualified investigator.