Abstract
Exercise pulmonary hypertension is an underappreciated form of physical limitation related to early pulmonary vascular disease. A low diffusing capacity of lungs for carbon monoxide (DLco) can be seen in patients with resting pulmonary hypertension as well as parenchymal lung disease. It remains unclear whether low DLco% identifies early pulmonary vascular disease. We hypothesize that a reduced DLco% differentiates the presence of exercise pulmonary hypertension in patients with parenchymal lung disease. Fifty-six patients referred for unexplained exertional dyspnea with pulmonary function tests within six months of hemodynamic testing underwent exercise right heart catheterization. Exclusion criteria included resting pulmonary arterial or venous hypertension. Receiver operator characteristic curve determined the optimal DLco% cutoffs based on the presence or absence of parenchymal lung disease. Twenty-one (37%) patients had parenchymal lung disease, most common manifesting as chronic obstructive lung disease or interstitial lung disease. In patients with parenchymal lung disease, a DLco of 46% demonstrated 100% sensitivity and 73% specificity for detecting exercise pulmonary hypertension. In patients without parenchymal lung disease, a DLco of 73% demonstrated 58% sensitivity and 94% specificity for detecting exercise pulmonary hypertension. In both cohorts, DLco% below the optimum cutoffs were associated with higher peak mean pulmonary arterial pressure and peak total pulmonary resistance consistent with the hemodynamic definition of exercise pulmonary hypertension. Patients with a DLco < 46% were more often treated with pulmonary vasodilators and had a trend to higher mortality and lung transplant. DLco% is a simple non-invasive screening test for the presence of exercise pulmonary hypertension in our mixed referral population with progressive exertional dyspnea. DLco < 46% with parenchymal lung disease and DLco < 73% without parenchymal lung disease may play a role in differentiating the presence of pulmonary vascular disease prior to invasive hemodynamic testing.
Keywords: chronic obstructive lung disease (COPD), exercise pulmonary hypertension, hemodynamics, interstitial lung disease, pulmonary arterial hypertension
Introduction
Exercise pulmonary hypertension (ePH) is an abnormal pulmonary vascular response to exertion that represents an early form of pulmonary vascular disease.1–4 Confrontational testing, such as stationary bicycling, perturb normal resting hemodynamics in at-risk patients leading to disproportionate increases in pulmonary arterial pressure (PAP) relative to cardiac output (CO).1,2 Although there is no consensus definition for ePH, hemodynamic recommendations describe an abnormal pulmonary vascular response to exercise.5,6 Identification of patients with ePH allows for early diagnosis, identification of functional limitation, and consideration for pharmacologic therapies.4,7–9
Patients with pulmonary hypertension (PH) may have reduced percent predicted diffusing capacity of lungs for carbon monoxide (DLco%), with reported ranges between 60% and 70% of predicted.10–13 DLco measures the transfer of inhaled gas to red blood cells within pulmonary capillaries and reflects the properties of the alveolar–capillary membrane. A reduced DLco in pulmonary arterial hypertension (PAH) may be the consequence of vascular remodeling and is related to proportionate reductions in alveolar–capillary membrane diffusing capacity and total pulmonary capillary blood volume available for gas exchange.10 Decreased DLco% is often seen in patients with underlying structural lung diseases, such as chronic obstructive lung disease (COPD) and interstitial lung disease (ILD).14–16
Patients with parenchymal lung disease are at risk for pulmonary vascular dysfunction.17 Early in the disease state, clinical findings may be subtle while pathologic and radiographic abnormalities are present. Prior to the development of resting PH, tobacco-exposed individuals with COPD have evidence of endothelial dysfunction, intimal hyperplasia, and smooth muscle cell proliferation.18 Imaging of the distal pulmonary vasculature in tobacco-exposed COPD patients can demonstrate pruning.19 Likewise, patients with ILD have early narrowing of the pulmonary vascular bed, though mediated via different mechanisms.20 Both parenchymal lung disease states share features of hypoxic pulmonary vasoconstriction.17 The presence of concomitant PH may not be readily identifiable, unless investigated. COPD patients with early pulmonary vascular disease manifesting as an abnormal pulmonary vascular response to exercise can progress to resting PAH over time.21 Identification of early PH in patients with parenchymal lung disease may provide opportunities to further clinical trials with PAH-specific therapy given the absence of current high-quality data in this population.4
Due to the relatively non-specific symptoms of exertional dyspnea and exercise fatigue, identification of ePH can be difficult. Certain populations such as systemic sclerosis, COPD, ILD, and forms of heart failure may be at increased risk for an abnormal pulmonary vascular response to exercise.2,4,22–24 Without knowing the prevalence of ePH in each group, it is difficult to implement diagnosis-based screening. Although a reduced DLco% may reflect parenchymal lung disease, we suspect that a DLco% below a threshold value may identify patients with occult pulmonary microvasculopathy, revealed by exercise testing. To the best of our knowledge, there are no studies that evaluate the role of DLco% in predicting abnormal cardiopulmonary hemodynamics during exercise by invasive measurements. We hypothesize that DLco% used as a screening tool in a mixed referral population presenting with unexplained exertional dyspnea can identify ePH.
Materials and methods
Study design
We performed a single-center study at the University of Pittsburgh Medical Center, Pittsburgh, PA, USA. Eighty-three consecutive patients referred for unexplained exertional dyspnea underwent supine exercise right heart catheterization (exRHC) between March 2012 and May 2017. From this database query, 56 patients were included for final analysis. Since this study was performed prior to the Sixth World Symposium on Pulmonary Hypertension, the inclusion criteria were based upon the Fifth World Symposium criteria with normal resting hemodynamics defined as a mean pulmonary arterial pressure (mPAP) < 25 mmHg, pulmonary vascular resistance (PVR) < 3.0 WU, and pulmonary arterial wedge pressure (PAWP) < 15 mmHg.25,26 Exclusion criteria included resting PAH (n = 13) and elevated resting or peak PAWP > 25 mmHg (n = 14). Patients were not included if there was documented history of structural heart disease or heart failure with reduced ejection fraction, defined by ejection fraction < 45%. This study was approved by the University of Pittsburgh Institutional Review Board (PRO11070366).
Patient population
Study patients with unexplained exertional dyspnea and exercise intolerance were referred for the evaluation of pulmonary vascular contribution to their symptoms. Diagnoses of parenchymal lung diseases were confirmed by electronic health record review of outpatient visit diagnosis codes, pulmonary function testing (PFT), and diagnostic chest imaging. Patients with scleroderma met the American College of Rheumatology classification criteria and were followed by board-certified rheumatologists.27 Mortality was obtained by provider documentation in the health system repository and Internet searches for published death notices. No patients were treated with PAH-specific therapies at the time of exRHC.
Pulmonary function testing
PFT was performed within six months of exRHC. Forced expiratory volume in 1 s (FEV1%) and forced vital capacity (FVC%) were obtained via spirometry. DLco% was calculated using the Neas prediction equation and corrected for hemoglobin and carboxyhemoglobin but not for lung volumes.28
Exercise right heart catheterization
We previously published our methods for performing supine exRHC, which were the same for this study.4 Prior to exRHC, a pulmonary artery catheter (Edwards, Irvine, CA, USA) was placed by ultrasound-guidance into the interval jugular vein via modified Seldinger technique. The zero-reference point was mid-thoracic level in the supine position.29 Each study used an individualized incremental increase of 10–25 Watts (W) every 2–3 min (Medical Positioning, Incorporated, Kansas City, MO, USA). Patients maintained 55–65 revolutions per minute on supine bicycle for the duration of the study. Hemodynamic data were recorded every 2–3 min (Xper Cardio Physiomonitoring System, Philips, Melbourne, FL, USA); a minimum of four hemodynamic values from rest (defined as supine legs up) to peak exercise were required. Peak exercise was defined as the maximal perceived effort able to be expended by the patient, consistent with methods from prior studies.1,3–5 CO and cardiac index (CI) were determined by thermodilution technique; the mean of three measurements was used at peak exercise. Supine hemodynamic measurements were averaged over several respiratory cycles to mitigate the breathing effect during exercise.23 We defined ePH by (1) single-point measurement of peak mean pulmonary arterial pressure (mPAPpeak) ≥ 30 mmHg and peak total pulmonary resistance (TPRpeak) ≥ 3.0 WU and (2) slope of the mPAP/CO ratio > 3.0 mmHg/L/min; however, in considering a pooled definition of ePH, we included the (3) change in mPAP/CO from peak and baseline values > 3.0 WU.5 All patients included in the final analysis had a PAWPpeak < 25 mmHg to exclude left-sided heart involvement.
The alpha model
The mechanical descriptor alpha (α) is a measure of pulmonary vascular distensibility and can be used to determine local vascular resistance by assessing vessel diameter. It is calculated from invasive cardiopulmonary hemodynamics through measurement of mPAP, TPR, and PAWP over a range of CO measurements.3 A single value of α was determined using the method of successful iterations. Using the data collected for each individual time point, α was varied to find the best-fit, least square value between measured mPAP and calculated mPAP.30 We previously reported that reduced α, a marker of early pulmonary vascular disease, was partially restored by treatment with pulmonary vasodilators in ePH.4
Statistical analysis
We stratified the initial cohort by presence or absence of parenchymal lung disease as intrinsic pulmonary pathology would manifest as lower baseline DLco%. Continuous variables were indicated as median values with interquartile ranges. We used Kruskal–Wallis test and Fisher’s exact test to evaluate continuous and categorical variables between groups, respectively. We calculated a receiver operating characteristic (ROC) curve of DLco% for the prediction of ePH in these two groups. In each group, we used the Youden index to calculate the optimum cutoff from empirical distribution function of sensitivity and specificity.31 We used area under curve (AUC) comparison to test the statistical significance of adding mPAP to DLco predictive model of ePH. No imputation was performed for missing data. All analyses were performed in Stata 14.2 (StataCorp, College Station, TX, USA). Statistical significance was considered as p < 0.05 and no adjustment was performed for multiplicity.
Results
Baseline subject demographics
Descriptive baseline characteristics of the study population are shown in Table 1. As a group, patients had a median age of 61 years, FEV1 of 82%, FVC of 86%, and DLco of 63%. Twenty-one (37%) patients had underlying parenchymal lung disease, manifesting mostly as COPD (57%) and ILD (29%), in addition to several combined pulmonary fibrosis and emphysema (14%). Those with parenchymal lung disease had lower baseline DLco% (44% vs 70%, p < 0.001). The majority of patients were classified as either World Health Organization Functional Class II or III at the time of exRHC.
Table 1.
Baseline demographics.
| Combined group (n = 56) | No lung disease (n = 35) | Lung disease (n = 21) | p Values | |
|---|---|---|---|---|
| Age (year) | 61 (50–70) | 59 (48–67) | 68 (55–75) | 0.02 |
| Male (n, %) | 24 (43%) | 11 (32%) | 13 (62%) | 0.05 |
| Deceased (n, %) | 6 (11%) | 1 (3%) | 5 (24%) | 0.024 |
| Race (n, %) | ||||
| Caucasian | 48 (86%) | 32 (91%) | 16 (76%) | 0.16 |
| African-American | 5 (9%) | 2 (6%) | 3 (14%) | |
| Asian | 1 (2%) | 1 (3%) | 0 (0%) | |
| Not reported | 2 (4%) | 0 (0%) | 2 (10%) | |
| Parenchymal lung disease (n, %) | 35 (63%) | 35 (100%) | 0 (0%) | <0.001 |
| None | 12 (21%) | 0 (0%) | 12 (57%) | |
| COPD | 6 (11%) | 0 (0%) | 6 (29%) | |
| ILD | 3 (5%) | 0 (0%) | 3 (14%) | |
| CPFE | ||||
| Lung transplant (n, %) | 2 (4%) | 0 (0%) | 2 (10%) | 0.13 |
| Scleroderma (n, %) | 7 (13%) | 4 (12%) | 3 (14%) | >0.9 |
| Smoker (n, %) | 30 (54%) | 13 (37%) | 17 (81%) | 0.002 |
| Total pack yearsa | 13.8 (21.4) | 0 (0–2.5) | 18.0 (9.3–45.0) | <0.001 |
| Pulmonary vasodilator (n, %) | 27 (49%) | 16 (46%) | 11 (55%) | 0.51 |
| Ambrisentan | 5 (19%) | 4 (25%) | 1 (9%) | |
| Bosentan | 1 (4%) | 1 (6%) | 0 (0%) | |
| Riociguat | 5 (19%) | 3 (19%) | 2 (18%) | |
| Selexipag | 1 (4%) | 0 (0%) | 1 (9%) | |
| Sildenafil | 11 (41%) | 10 (63%) | 1 (9%) | |
| Tadalafil | 17 (63%) | 9 (56%) | 8 (73%) | |
| 6MWT (m) | 329 (259–396) | 363 (317–415) | 293 (210–366) | 0.10 |
| WHO Functional Class (n, %)a | 0.20 | |||
| I | 2 (6%) | 0 (0%) | 2 (14%) | |
| II | 21 (62%) | 16 (80%) | 5 (36%) | |
| III | 10 (29%) | 4 (20%) | 6 (43%) | |
| IV | 1 (3%) | 0 (0%) | 1 (7%) | |
| Pulmonary function testing | ||||
| FEV1 (%) | 82 (65–101) | 93 (69–112) | 70 (48–81) | 0.006 |
| FVC (%) | 86 (70–99) | 88 (71–106) | 77 (69–93) | 0.33 |
| DLco (%) | 63 (45–72) | 70 (56–80) | 44 (34–55) | <0.001 |
| Transthoracic echocardiogram | ||||
| TR Jet (m/s) | 2.6 (2.3–3.0) | 2.4 (2.2–2.7) | 3.0 (2.8–3.2) | <0.001 |
| LAVI (mL/m2) | 27 (19–32) | 22.0 (19.0–32.0) | 29.5 (21.0–33.5) | 0.22 |
Values are indicated as median with interquartile range (IQR) unless otherwise specified by “a”.
Mean with standard deviation (SD).
COPD: chronic obstructive pulmonary disease; ILD: interstitial lung disease; CPFE: combined pulmonary fibrosis and emphysema; 6MWT: 6-min walk test; WHO: World Health Organization; TR: tricuspid regurgitant. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; DLco: diffusing capacity of lungs for carbon monoxide; LAVI: left atrial volume index. Patients were not treated with PH-specific therapies at the time of exRHC; medications were later added at the discretion of the treating physician.
Receiver operator characteristic curve
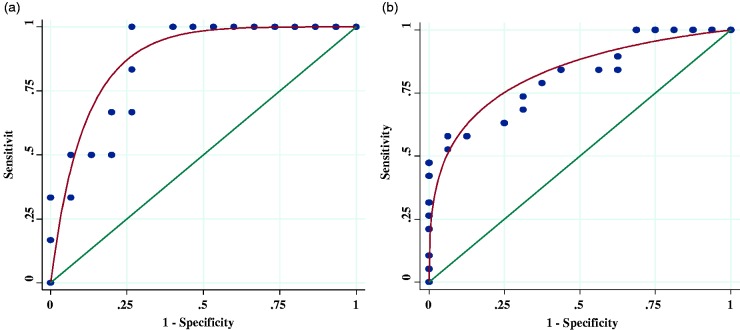
Analysis of optimal DLco% for the detection of ePH is shown in Fig. 1. A DLco of 46% demonstrated 100% sensitivity, 73% specificity, 60% positive predictive value (PPV), and 100% negative predictive value (NPV) with an AUC of 0.87 for detecting ePH in patients with parenchymal lung disease. A DLco of 73% demonstrated 58% sensitivity, 94% specificity, 92% PPV, and 65% NPV with an AUC of 0.81 for detecting ePH in patients without parenchymal lung disease. There was no difference in AUC measurements when adding resting supine legs down mPAP values between 20 and 24 mmHg to the DLco% predictive model (Supplementary Fig. 1).
Fig. 1.
ROC curve analysis. (a) Optimal DLco% (46%) in patients with parenchymal lung disease. (b) Optimal DLco% (73%) in patients without parenchymal lung disease.
AUC: area under curve.
Resting and peak exercise hemodynamics with and without parenchymal lung disease
Resting hemodynamics
Pairwise comparisons of resting hemodynamic parameters between patients with and without parenchymal lung disease are highlighted in Table 2. Patients with parenchymal lung disease had lower baseline DLco%, as indicated in Table 1.
Table 2.
Resting (supine legs up) and peak exercise hemodynamics.
| No lung disease (n = 35) | Lung disease (n = 21) | p Values | |
|---|---|---|---|
| Resting (supine legs up) hemodynamics | |||
| mPAP (mmHg) | 20 (17–23.7) | 20.3 (18–27.7) | 0.36 |
| PAWP (mmHg) | 10.5 (7–13) | 10 (6–13) | 0.59 |
| TPR (WU) | 3.1 (2.5–4.1) | 3.7 (2.7–4.5) | 0.35 |
| PVR (WU) | 1.5 (1.1–1.9) | 1.6 (1.3–2.5) | 0.19 |
| PAC (mL/mmHg) | 3.8 (3.3–5.1) | 2.9 (2.4–3.5) | 0.004 |
| SpO2 (%) | 98 (96.5–100) | 98 (96.5–99) | >0.9 |
| O2 (LPM) | 0 (0–0) | 0 (0–3) | 0.06 |
| Peak hemodynamics | |||
| mPAP (mmHg) | 29.7 (22.7–35) | 34.7 (30.3–41) | 0.03 |
| PAWP (mmHg) | 16 (11–21) | 15 (11–19) | 0.67 |
| TPR (WU) | 2.8 (2.0–3.3) | 3.6 (2.3–4.5) | 0.05 |
| PVR (WU) | 1.3 (0.9–1.7) | 2.3 (1.5–2.6) | 0.01 |
| PAC (mL/mmHg) | 2.4 (2.1–3.3) | 2.2 (1.6–2.6) | 0.04 |
| Alpha (%/mmHg) | 1.1 (0.7–1.6) | 0.7 (0.5–1.1) | 0.05 |
| Slope of mPAP/CO (mmHg/L/min) | 1.9 (0.9–2.6) | 3.0 (1.2–3.7) | 0.06 |
| CO (L/min) | 11.3 (9.1–12.6) | 10.5 (8.6–12.2) | 0.61 |
| CI (L/min/m2) | 6.1 (4.8–6.7) | 5.7 (4.8–6.1) | 0.42 |
| Stroke volume (mL) | 93 (78–108) | 94 (78–118) | 0.52 |
| PA saturation (%) | 71 (65–74) | 69 (66–71) | 0.35 |
| SpO2 (%) | 97 (95–98) | 93 (90.5–96) | 0.02 |
| O2 (LPM) | 0 (0–0) | 0 (0–4) | 0.03 |
Pairwise comparisons between patients with and without parenchymal lung disease. Values are indicated as median with interquartile range (IQR).
mPAP: mean pulmonary artery pressure; PAWP: pulmonary arterial wedge pressure; TPR: total peripheral resistance; PVR: pulmonary vascular resistance; PAC: pulmonary arterial compliance; CO: cardiac output; CI: cardiac index; PA: pulmonary artery.
Peak exercise hemodynamics
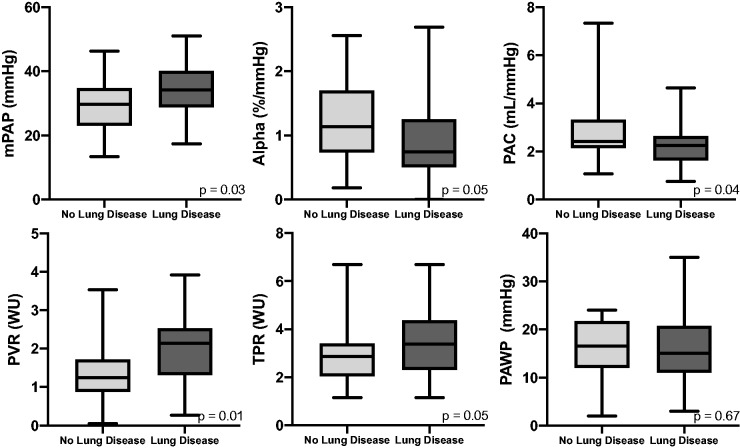
Pairwise comparisons of peak hemodynamic parameters between patients with and without lung disease are highlighted in Table 2 and Fig. 2. Patients with parenchymal lung disease had lower oxygen levels and required more oxygen at peak exercise. Those with parenchymal lung disease also had higher mPAP and PVR, as well as lower pulmonary arterial compliance (PAC). There were no differences in PAWP or TPR.
Fig. 2.
Peak exercise hemodynamics. Box whisker plots comparing cardiopulmonary hemodynamic values between patients with and without parenchymal lung disease. Results indicate median, IQR, minimum, and maximum values.
mPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; TPR: total pulmonary resistance; PAWP: pulmonary arterial wedge pressure; PAC: pulmonary arterial compliance.
Resting and peak exercise hemodynamics stratified by presence of parenchymal lung disease
Resting hemodynamics
Pairwise comparisons of resting hemodynamic parameters categorized by optimal DLco% are shown in Table 3. In patients without parenchymal lung disease, a DLco < 73% demonstrated higher mPAP, but did not display significant differences in TPR or PVR as compared with those with higher DLco. The resting stroke volume was lower in patients with DLco < 73%. In patients with parenchymal lung disease, a DLco < 46% demonstrated higher resting values for mPAP, TPG, TPR, and PVR.
Table 3.
Resting hemodynamics stratified by parenchymal lung disease.
| No lung disease |
p Values | Lung disease |
p Values | |||
|---|---|---|---|---|---|---|
| DLco ≥ 73% (n = 11) | DLco < 73% (n = 21) | DLco ≥ 46% (n = 8) | DLco < 46% (n = 9) | |||
| FVC (%) | 105 (66–116) | 86 (72–96) | 0.20 | 72 (60–91) | 81 (75–107) | 0.15 |
| FEV1 (%) | 111 (67–123) | 89 (71–98) | 0.16 | 69 (48–81) | 73 (37–83) | 0.82 |
| RAP (mmHg) | 7 (3–8) | 6 (3–7) | 0.86 | 4.5 (2.5–7) | 6 (3–6) | 0.81 |
| PASP (mmHg) | 29 (25–38) | 35 (30–41) | 0.10 | 37 (30–39.5) | 48 (42–50) | 0.04 |
| PADP (mmHg) | 11 (9–12) | 15 (11–17) | 0.02 | 9 (6.5–12) | 12 (11–17) | 0.07 |
| mPAP (mmHg) | 18 (14.3–20) | 21.3 (18.3–24.3) | 0.04 | 18.3 (14.3–21.2) | 23.3 (19–30) | 0.03 |
| PAWP (mmHg) | 10 (7–13) | 11 (9–13) | 0.75 | 9.5 (6–14.5) | 10 (6–12) | 0.67 |
| TPR (WU) | 3.1 (2.1–3.3) | 3.2 (2.6–4.3) | 0.14 | 2.9 (2.3–3.5) | 4.5 (3.7–5.6) | 0.02 |
| PVR (WU) | 1.2 (0.6–1.6) | 1.6 (1.3–2.2) | 0.08 | 1.2 (0.9–1.5) | 2.5 (1.9–3.7) | 0.008 |
| PAC (mL/mm Hg) | 5.0 (3.4–5.2) | 3.8 (3.2–4.8) | 0.42 | 3.5 (2.7–4.1) | 2.8 (2.2–3.1) | 0.10 |
| TPG (mm Hg) | 8.7 (8–12) | 11.3 (11–16) | 0.09 | 10.2 (6.8–14) | 17 (15–23) | 0.007 |
| Time constant calculated (s) | 0.37 (0.18–0.42) | 0.36 (0.34–0.48) | 0.26 | 0.32 (0.15–0.32) | 0.31 (0.25–0.68) | 0.29 |
| PA saturation (%) | 72 (67–78) | 69 (64–73) | 0.43 | 69 (67–70) | 69 (66–71) | 0.85 |
| PA pulse pressure (mm Hg) | 21 (16–27) | 22 (19–25) | 0.62 | 27.5 (23.5–28) | 31 (24–37) | 0.44 |
| CO (L/min) | 6.1 (5.3–6.8) | 6.3 (4.9–7.3) | 0.81 | 6.5 (5.7–7.0) | 5.9 (5.2–6.5) | 0.25 |
| CI (L/min/m2) | 3.3 (2.8–3.7) | 3.3 (2.4–3.8) | 0.40 | 3.1 (2.7–3.5) | 3.0 (2.7–3.2) | 0.67 |
| Stroke volume (mL) | 104.8 (85.3–105.4) | 80.7 (70.5–95.6) | 0.05 | 95.3 (71.3–109.7) | 75 (67.5–83.9) | 0.25 |
| SpO2 (%) | 98 (97.5–99.5) | 98.5 (96–100) | 0.78 | 98 (97–99) | 98 (96–99) | 0.68 |
| O2 (LPM) | 0 (0–0) | 0 (0–0) | >0.9 | 0 (0–0) | 2 (0–3) | 0.35 |
FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; RAP: right atrial pressure; PASP: pulmonary artery systolic pressure; PADP: pulmonary artery diastolic pressure; mPAP: mean pulmonary artery pressure; PAWP: pulmonary arterial wedge pressure; TPR: total peripheral resistance; PVR: pulmonary vascular resistance; PAC: pulmonary arterial compliance; TPG: transpulmonary gradient; PA: pulmonary artery; CO: cardiac output; CI: cardiac index.
Peak exercise hemodynamics
Pairwise comparisons of peak hemodynamic parameters categorized by optimal DLco% are shown in Table 4. In patients without parenchymal lung disease, DLco < 73% demonstrated higher mPAP, TPR, mPAP/CO slope, and change in mPAP/CO, as well as lower CI. There were no differences in PVR or α. In patients with parenchymal lung disease, DLco < 46% demonstrated higher mPAP, TPR, PVR, and time constant, as well as lower CO, CI, PAC, and α. There were no differences in slope of the mPAP/CO ratio or change in mPAP/CO. Patients with ILD and COPD were stratified into less severe and more severe disease using FVC and FEV1 cutoffs of 60%, respectively; resting and peak exercise cardiopulmonary hemodynamics were not dependent on the severity of underlying parenchymal lung disease (data not shown).
Table 4.
Peak exercise hemodynamics stratified by parenchymal lung disease.
| No lung disease |
p Values | Lung disease |
p Values | |||
|---|---|---|---|---|---|---|
| DLco ≥ 73% (n = 12) | DLco < 73% (n = 23) | DLco ≥ 46% (n = 10) | DLco < 46% (n = 11) | |||
| Power (W) | 100 (100–150) | 75 (60–100) | 0.006 | 75 (60–125) | 50 (40–60) | 0.02 |
| Duration (min) | 18.0 (14.4–20.3) | 12.4 (9.0–18.5) | 0.022 | 14.0 (12.6–18.3) | 9.4 (6–12) | 0.014 |
| mPAP (mmHg) | 24.1 (20.3–30.2) | 32.7 (25.7–37) | 0.02 | 30.5 (22.3–34.7) | 39.7 (33.7–42.3) | 0.04 |
| PAWP (mmHg) | 11.5 (9–15.5) | 18 (13–22) | 0.013 | 13 (11–19) | 15 (11–22) | 0.67 |
| TPR (WU) | 2.1 (1.5–2.9) | 3.0 (2.1–3.8) | 0.014 | 2.3 (1.8–3.3) | 4.5 (3.8–5.2) | <0.001 |
| PVR (WU) | 1.1 (0.8–1.6) | 1.4 (1.0–1.8) | 0.19 | 1.1 (0.6–1.7) | 2.5 (2.3–3.8) | 0.001 |
| Slope of mPAP/CO (mmHg/L/min) | 1.2 (0.9–1.8) | 2.1 (1.4–3.1) | 0.04 | 1.2 (0.5–3.8) | 3.1 (2.9–3.7) | 0.06 |
| R2 for slope of mPAP/CO | 0.67 (0.49–0.78) | 0.66 (0.36–0.94) | 0.81 | 0.70 (0.38–0.80) | 0.61 (0.42–0.72) | >0.9 |
| Δ mPAP/CO (WU) | 1.2 (1.0–2.0) | 2.7 (1.7–4.3) | 0.003 | 2.6 (1.4–4.1) | 3.3 (2.8–9.8) | 0.09 |
| Alpha (%/mmHg) | 1.3 (1.0–1.6) | 0.9 (0.7–1.5) | 0.29 | 1.1 (1.0–1.6) | 0.6 (0.3–0.7) | 0.005 |
| PAC (mL/mmHg) | 3.1 (2.1–3.9) | 2.3 (2.1–2.9) | 0.12 | 2.7 (2.3–2.8) | 1.6 (1.3–1.9) | 0.001 |
| TPG (mmHg) | 18 (14–22) | 17.3 (13–22) | 0.77 | 19.5 (15–23.7) | 26 (22–32) | 0.04 |
| PA saturation (%) | 40.0 (34.1–47.6) | 42.8 (32.1–50.4) | 0.53 | 38.6 (34.4–44.7) | 44.1 (40.3–48.4) | 0.13 |
| PA pulse pressure (mmHg) | 34.5 (26.5–38.5) | 37 (29–44) | 0.22 | 46.5 (40–51) | 50 (41–54) | 0.55 |
| CO (L/min) | 12.2 (11.2–13.6) | 10.7 (8.6–12.3) | 0.06 | 12.7 (11.4–14.9) | 8.6 (7.6–10.5) | 0.001 |
| CI (L/min/m2) | 6.6 (5.8–8.1) | 5.6 (4.7––6.4) | 0.03 | 6.3 (5.4–6.9) | 4.8 (4.2–5.8) | 0.005 |
| Stroke volume (mL) | 101.3 (87.3–116.2) | 85.9 (72.4–100) | 0.11 | 120.1 (100.9–133.4) | 80.6 (63.2–87.6) | 0.002 |
mPAP: mean pulmonary artery pressure; PAWP: pulmonary arterial wedge pressure; TPR: total peripheral resistance; PVR: pulmonary vascular resistance; PAC: pulmonary arterial compliance; TPG: transpulmonary gradient; PA: pulmonary artery; CO: cardiac output; CI: cardiac index.
Cardiopulmonary hemodynamics and DLco% correlations
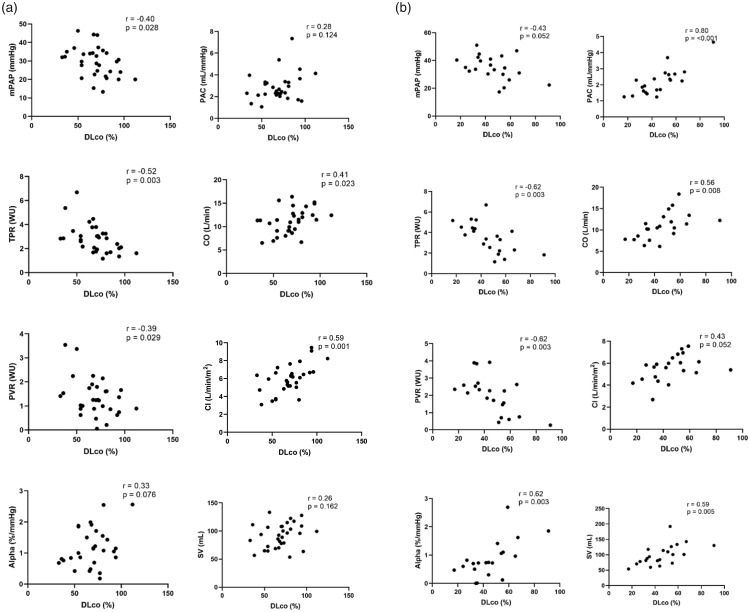
Correlations between DLco% and peak hemodynamics are shown in Fig. 3. In patients without parenchymal lung disease, DLco% negatively correlated with mPAP, TPR, and PVR. In patients with parenchymal lung disease, DLco% positively correlated with α and negatively correlated with TPR and PVR. There were no differences in PAWP in either group (data not shown).
Fig. 3.
Peak hemodynamic and DLco% correlations. (a) Plots in patients without parenchymal lung disease. (b) Plots in patients with underlying parenchymal lung disease.
mPAP: mean pulmonary arterial pressure; PAC: pulmonary arterial compliance; TPR: total pulmonary resistance; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance; DLco%: diffusing capacity of lungs for carbon monoxide; SV: stroke volume.
Classification of ePH
Pairwise comparisons of frequency of peak hemodynamics in patients with and without parenchymal lung disease satisfying ePH criteria are shown in Table 5. Patients with both DLco < 73% without parenchymal lung disease and DLco < 46% with parenchymal lung disease demonstrated higher frequencies of mPAPpeak > 30 mmHg and TPRpeak > 3.0 WU, satisfying criteria for the pooled definition of ePH, described as mPAP > 30 mmHg and TPR > 3 WU, slope of mPAP/CO ratio > 3 mmHg/L/min, and change in mPAP/CO > 3 WU.
Table 5.
ePH classification and clinical outcomes.
| No lung disease |
p Values | Lung disease |
p Values | |||
|---|---|---|---|---|---|---|
| DLco ≥ 73% (n = 12) | DLco < 73% (n = 23) | DLco ≥ 46% (n = 10) | DLco < 46% (n = 11) | |||
| ePH classification | ||||||
| mPAP > 30 and TPR > 3 (n, %) | 1 (8%) | 11 (48%) | 0.027 | 2 (20%) | 10 (91%) | 0.002 |
| Slope of mPAP/CO ratio > 3 (n, %) | 0 (0%) | 6 (27%) | 0.069 | 3 (30%) | 7 (64%) | 0.20 |
| Δ mPAP/CO > 3 (n, %) | 1 (8%) | 10 (44%) | 0.055 | 3 (33%) | 8 (73%) | 0.18 |
| ePH by all three hemodynamic methodsa (n, %) | 1 (8%) | 15 (65%) | 0.002 | 4 (40%) | 11 (100%) | 0.004 |
| Clinical outcomes | ||||||
| Vasodilator therapy | 5 (42%) | 11 (48%) | 0.73 | 3 (30%) | 8 (80%) | 0.025 |
| Lung transplant or mortality (n, %) | 0 (0%) | 1 (4%) | >0.9 | 1 (10%) | 6 (55%) | 0.063 |
Patients classified according to three proposed ePH definitions. Vasodilator therapy was initiated after exercise testing at the discretion of the patient’s physician.
ePH by all three hemodynamic methods defined as mPAP > 30 mmHg with TPR > 3 WU and slope of the mPAP/CO ratio > 3 mmHg/L/min and change in mPAP/CO > 3 WU.
ePH: exercise pulmonary hypertension; mPAP: mean pulmonary arterial pressure; TPR: total peripheral resistance; CO: cardiac output.
Clinical outcomes in ePH
Pairwise comparisons of lung transplantation or mortality in patients with and without parenchymal lung disease are shown in Table 5. Patients with parenchymal lung disease and DLco < 46% had higher frequency of either lung transplant or mortality (55% vs 10%), although not statistically significant. This, however, was associated with a Hazard Ratio (HR) of 11.6 (p = 0.027) for mortality in those with parenchymal lung disease (data not shown).
Discussion
We identified the optimal DLco% to screen for ePH in a mixed referral population enriched with parenchymal lung disease presenting with exertional dyspnea. In patients with normal resting hemodynamics and parenchymal lung disease, DLco < 46% demonstrated higher peak mPAP, PVR, TPR, time constant, and α, in addition to lower PAC and CO, consistent with early pulmonary vascular disease. In patients without parenchymal lung disease, DLco < 73% also demonstrated higher peak cardiopulmonary hemodynamics. To our knowledge, this is the first study to evaluate the predictive value of DLco% as a non-invasive screening tool for the identification of ePH in a referral population.
In clinical practice, it can be difficult to determine whether an exRHC is needed to identify patients with ePH. The purpose of this study was to evaluate the utility of DLco% as a screening tool for ePH in a mixed population prior to undergoing exRHC. We demonstrated that DLco% is a simple, non-invasive diagnostic test to screen patients with and without parenchymal lung disease who may be at risk for pulmonary vascular disease. We suggest that patients with parenchymal lung disease and DLco < 46% with normal resting hemodynamics undergo invasive exercise testing to evaluate for ePH.
To date, limited studies have assessed ePH in patients with parenchymal lung disease. Hilde et al. performed invasive incremental exercise testing in 72 patients with COPD and mean DLco of 57%, normal resting pulmonary pressures, and mPAP/CO slope of 4.6 mmHg/L/min; Degani-Costa et al. performed incremental exercise testing in 27 patients with ILD and mean DLco of 40% and mPAP/CO slope of 4.0 mmHg/L/min.24,32 However, neither study evaluated the potential role of DLco% as a screening test in identifying abnormal exercise hemodynamics. We hypothesize that our DLco% cutoff may be generalizable to patients with COPD and ILD, although there may be limitations based upon gender and race.28
Based on our ROC curve analyses, we report that DLco < 46% demonstrated 100% sensitivity, 73% specificity, 60% PPV, and 100% NPV for detecting ePH in our population of patients with parenchymal lung disease. We also report that DLco < 73% demonstrated 58% sensitivity, 94% specificity, PPV 92%, and NPV 65% for detecting ePH in our population of patients without parenchymal lung disease. The application of DLco% as a screening test for ePH is strongest for patients with parenchymal lung disease who likely have pulmonary vascular involvement associated with lung destruction. In patients without parenchymal lung disease, a DLco of > 73% identifies patients likely to not have ePH. It is possible that our cohort without parenchymal lung disease were composed of a heterogenous population, with less severe pulmonary vascular involvement, as suggested by lower peak exercise hemodynamics.
Recent studies report that patients, particularly those with scleroderma, with resting mPAP between 21 and 24 mmHg are at increased risk for developing resting PH and ePH.7,33,34 In fact, the updated definition of precapillary PH was recently lowered to mPAP > 20 mmHg.26 We evaluated the addition of resting mPAP 21–24 mmHg to our DLco% model and found no added benefit in the prediction of ePH. While it was unanticipated that the inclusion of an additional hemodynamic risk factor did not have a positive effect, we attribute this to the heterogeneous composition of our study population.
In our study population, DLco% identified a higher frequency of ePH in patients with and without parenchymal lung disease. From the currently accepted methods for determining ePH, low DLco% best identified ePH in both the presence and absence of parenchymal lung disease using the single-point measurement definition of mPAPpeak ≥ 30 mmHg and TPRpeak ≥ 3.0 WU. It is important to note that the highest inclusion of patients with ePH was using the pooled definition of abnormal exercise hemodynamic definitions (mPAP > 30 mmHg and TPR > 3 WU, slope of the mPAP/CO ratio > 3 mmHg/L/min, and change in mPAP/CO > 3 WU); using this method, 100% of patients with parenchymal lung disease and DLco < 46% met criteria for ePH.
In parenchymal lung disease, destruction of the alveolar–capillary bed, ventilation–perfusion mismatch, and chronic hypoxic pulmonary vasoconstriction result in elevated pulmonary pressures and decreased membrane diffusion.35–37 Unfortunately, there are no descriptive histologic studies or imaging studies of the pulmonary vasculature in the ePH population. There are, however, surrogate hemodynamic markers, including α and PAC, used to assess the relative health of resistive vessels.38,39 A recent study of healthy volunteers in hypoxic environments revealed exercise-induced decreases in α, consistent with the concept of hypoxia-induced vascular changes.39 We report a reasonable positive correlation between DLco% and α (R = 0.63) at peak exercise in patients with parenchymal lung disease, suggesting a lack of pulmonary vascular recruitment and reduced pulmonary vascular distensibility in this population. We previously reported that DLco% correlated with PVR and PAC in a population of ePH patients enriched with lung disease.4 In the same population, we showed that a reduction in α is modifiable with pulmonary vasodilator therapy.21 Therefore, patients with parenchymal lung disease and DLco < 46% who meet criteria for ePH should undergo further clinical trials with PAH-specific therapy.
We found that resting and exercise cardiopulmonary hemodynamics were independent of the severity of parenchymal lung disease, further supporting our claim that the optimal DLco% cutoff as demonstrated by our ROC curve analyses best predicted ePH, not the severity of the underlying parenchymal lung disease.
We report that patients with ePH, parenchymal lung disease, and DLco < 46% had a higher frequency of pulmonary vasodilator treatment. While a substantial portion (49%) of patients were prescribed PAH-specific therapies, none were actively on these medications at the time of their exRHC. In some cases, vasodilator therapy was initiated after exercise testing by our team; in other cases, patients presented to our specialty referral center already on these medications. Although our study was not powered to assess the effect of pulmonary vasodilators on mortality or transplant, we note a trend toward higher rates of combined clinical outcome of lung transplantation or mortality; the HR of 11.6 for mortality also illustrated the severity of illness in this population.
There are several limitations to our study. First, this was a single-center U.S. cohort study performed at a specialty referral center affiliated with a tertiary academic center, which may restrict its applicability to the general population. Second, the study cohorts were not equally represented by gender, which may affect DLco% results.28 Third, it is unclear if DLco% may be generalizable to ethnic groups, due to the differences in DLco% technique and lack of many non-Caucasian comparators.28,40 Fourth, our small sample size of patients who fit the inclusion criteria may be underpowered to detect comparisons and correlations. Patients did not perform cardiopulmonary exercise testing, so there were no available data for respiratory exchange ratio or maximal oxygen uptake. Finally, gas exchange measurements were not readily available during exercise.
Future studies will aim to perform a prospective assessment utilizing DLco% cutoffs of 46% and 73% as screening metrics in the invasive diagnostic testing of ePH in patients with and without parenchymal lung disease referred for unexplained exertional dyspnea. Ultimately, larger, multi-center studies are needed to assess this applicability.
Supplemental Material
Supplemental material, PUL891912 Supplemental material for Lower DLco% identifies exercise pulmonary hypertension in patients with parenchymal lung disease referred for dyspnea by Richard H. Zou, William D. Wallace, S. Mehdi Nouraie, Stephen Y. Chan and Michael G. Risbano in Pulmonary Circulation
Author contributions
M.G.R. conceived the study design. R.H.Z. and W.D.W. performed manual chart review for verification of bioinformatics data. M.N. performed computational extraction and statistical analysis of the data. R.H.Z., W.D.W., S.Y.C., and M.G.R. wrote the manuscript. All authors participated in interpretation of results and revising the manuscript.
Conflict of interest
S.Y.C. has served as a consultant for Zogenix, Vivus, Aerpio, and United Therapeutics. S.Y.C. is a director, officer, and shareholder in Numa Therapeutics. S.Y.C. holds research grants from Actelion and Pfizer. Patent applications (S.Y.C.) have been filed regarding targeting metabolism in pulmonary hypertension. M.G.R holds research grants from Actelion, Gilead, and United Therapeutics. The authors declare no other conflicts of interest.
Funding
This work was supported by NIH grants R01 HL124021, HL 122596, HL 138437, and UH2 TR002073 as well as the American Heart Association Established Investigator Award 18EIA33900027 (S.Y.C.).
ORCID iD
Michael G. Risbano https://orcid.org/0000-0003-3334-7046.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Herve P, Lau EM, Sitbon O, et al. Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J 2015; 46: 728–737. [DOI] [PubMed] [Google Scholar]
- 2.Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013; 128: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 3.Lau EM, Chemla D, Godinas L, et al. Loss of vascular distensibility during exercise is an early hemodynamic marker of pulmonary vascular disease. Chest 2016; 149: 353–361. [DOI] [PubMed] [Google Scholar]
- 4.Wallace WD, Nouraie M, Chan SY, et al. Treatment of exercise pulmonary hypertension improves pulmonary vascular distensibility. Pulm Circ 2018; 8: 2045894018787381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godinas L, Lau EM, Chemla D, et al. Diagnostic concordance of different criteria for exercise pulmonary hypertension in subjects with normal resting pulmonary artery pressure. Eur Respir J 2016; 48: 254–257. [DOI] [PubMed] [Google Scholar]
- 6.Kovacs G, Herve P, Barbera JA, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J 2017; 50: 1–18. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira RKF, Faria-Urbina M, Maron BA, et al. Functional impact of exercise pulmonary hypertension in patients with borderline resting pulmonary arterial pressure. Pulm Circ 2017; 7: 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saggar R, Khanna D, Shapiro S, et al. Brief report: effect of ambrisentan treatment on exercise-induced pulmonary hypertension in systemic sclerosis: a prospective single-center, open-label pilot study. Arthritis Rheum 2012; 64: 4072–4077. [DOI] [PubMed] [Google Scholar]
- 9.Segrera SA, Lawler L, Opotowsky AR, et al. Open label study of ambrisentan in patients with exercise pulmonary hypertension. Pulm Circ 2017; 7: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farha S, Asosingh K, Xu W, et al. Hypoxia-inducible factors in human pulmonary arterial hypertension: a link to the intrinsic myeloid abnormalities. Blood 2011; 117: 3485–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low AT, Medford AR, Millar AB, et al. Lung function in pulmonary hypertension. Respir Med 2015; 109: 1244–1249. [DOI] [PubMed] [Google Scholar]
- 12.Sun XG, Hansen JE, Oudiz RJ, et al. Pulmonary function in primary pulmonary hypertension. J Am Coll Cardiol 2003; 41: 1028–1035. [DOI] [PubMed] [Google Scholar]
- 13.Jing ZC, Xu XQ, Badesch DB, et al. Pulmonary function testing in patients with pulmonary arterial hypertension. Respir Med 2009; 103: 1136–1142. [DOI] [PubMed] [Google Scholar]
- 14.Arunthari V, Burger CD, Lee AS. Correlation of pulmonary function variables with hemodynamic measurements in patients with pulmonary arterial hypertension. Clin Respir J 2011; 5: 35–43. [DOI] [PubMed] [Google Scholar]
- 15.Bailey KL. The importance of the assessment of pulmonary function in COPD. Med Clin North Am 2012; 96: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez FJ, Flaherty K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006; 3: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbera JA. Mechanisms of development of chronic obstructive pulmonary disease-associated pulmonary hypertension. Pulm Circ 2013; 3: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson G, Turner AF, Balchum OJ, et al. Vascular changes in pulmonary emphysema. The radiologic evaluation by selective and peripheral pulmonary wedge angiography. Am J Roentgenol Radium Ther Nucl Med 1967; 100: 374–396. [PubMed] [Google Scholar]
- 20.Panagiotou M, Church AC, Johnson MK, et al. Pulmonary vascular and cardiac impairment in interstitial lung disease. Eur Respir Rev 2017; 26: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med 2001; 164: 219–224. [DOI] [PubMed] [Google Scholar]
- 22.Saggar R, Khanna D, Furst DE, et al. Exercise-induced pulmonary hypertension associated with systemic sclerosis: four distinct entities. Arthritis Rheum 2010; 62: 3741–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boerrigter BG, Waxman AB, Westerhof N, et al. Measuring central pulmonary pressures during exercise in COPD: how to cope with respiratory effects. Eur Respir J 2014; 43: 1316–1325. [DOI] [PubMed] [Google Scholar]
- 24.Degani-Costa LH, Levarge B, Digumarthy SR, et al. Pulmonary vascular response patterns during exercise in interstitial lung disease. Eur Respir J 2015; 46: 738–749. [DOI] [PubMed] [Google Scholar]
- 25.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 26.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980; 23: 581–590. [DOI] [PubMed] [Google Scholar]
- 28.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of U.S. adults. Am J Respir Crit Care Med 1996; 153: 656–664. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs G, Avian A, Olschewski A, et al. Zero reference level for right heart catheterisation. Eur Respir J 2013; 42: 1586–1594. [DOI] [PubMed] [Google Scholar]
- 30.Lalande S, Yerly P, Faoro V, et al. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol 2012; 590: 4279–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J 2005; 47: 458–472. [DOI] [PubMed] [Google Scholar]
- 32.Hilde JM, Skjorten I, Hansteen V, et al. Haemodynamic responses to exercise in patients with COPD. Eur Respir J 2013; 41: 1031–1041. [DOI] [PubMed] [Google Scholar]
- 33.Lau EM, Godinas L, Sitbon O, et al. Resting pulmonary artery pressure of 21-24 mmHg predicts abnormal exercise haemodynamics. Eur Respir J 2016; 47: 1436–1444. [DOI] [PubMed] [Google Scholar]
- 34.Valerio CJ, Schreiber BE, Handler CE, et al. Borderline mean pulmonary artery pressure in patients with systemic sclerosis: transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheum 2013; 65: 1074–1084. [DOI] [PubMed] [Google Scholar]
- 35.Potter WA, Olafsson S, Hyatt RE. Ventilatory mechanics and expiratory flow limitation during exercise in patients with obstructive lung disease. J Clin Invest 1971; 50: 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behnia M, Wheatley CM, Avolio A, et al. Alveolar-capillary reserve during exercise in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2017; 12: 3115–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farha S, Laskowski D, George D, et al. Loss of alveolar membrane diffusing capacity and pulmonary capillary blood volume in pulmonary arterial hypertension. Respir Res 2013; 14: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palatini P, Casiglia E, Gasowski J, et al. Arterial stiffness, central hemodynamics, and cardiovascular risk in hypertension. Vasc Health Risk Manag 2011; 7: 725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol 2005; 288: L419–L425. [DOI] [PubMed] [Google Scholar]
- 40.Chhabra SK, Kumar R, Gupta UA. Prediction equations for diffusing capacity (transfer factor) of lung for North Indians. Lung India 2016; 33: 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PUL891912 Supplemental material for Lower DLco% identifies exercise pulmonary hypertension in patients with parenchymal lung disease referred for dyspnea by Richard H. Zou, William D. Wallace, S. Mehdi Nouraie, Stephen Y. Chan and Michael G. Risbano in Pulmonary Circulation