Highlights
-
•
Thoracic kyphosis and loading of the thoracic spine were compromised by obesity.
-
•
Women classified as obese reported more symptoms of upper torso musculoskeletal pain.
-
•
Women classified as obese reported less time spent in physical activity.
-
•
Strategies to minimize changes to the upper torso in women with obesity are needed.
Keywords: Breast volume, Obesity, Thoracic kyphosis, Upper torso loading
Abstract
Purpose
This study investigated the effects of obesity on breast size, thoracic spine structure and function, upper torso musculoskeletal pain and physical activity participation in women living independently in the community.
Methods
A total of 378 women were divided into 3 groups (Not Overweight: body mass index (BMI) = 22.5 ± 0.2 kg/m2 (mean ± SE); Overweight: BMI = 27.4 ± 0.3 kg/m2; Obese: BMI = 35.4 ± 0.3 kg/m2). Outcome variables of breast volume (mL), thoracic flexion torque (N·m), thoracic kyphosis (degrees), upper torso musculoskeletal pain (score) and time spent in physical activity (min) were calculated and compared among the 3 groups, adjusting for between-group differences in age.
Results
There was a significant main effect of BMI on all outcome variables. Participants classified as Obese displayed significantly larger breasts, had greater thoracic flexion torques and reported less time participating in physical activity relative to the participants who were classified as Not Overweight and Overweight. Participants in the Obese group also displayed significantly more thoracic kyphosis and reported significantly more upper torso musculoskeletal pain compared to their counterparts who were classified as Not Overweight.
Conclusion
This study is the first to demonstrate that increased obesity levels were associated with compromised kyphosis and loading of the thoracic spine, as well as increased symptoms of upper torso musculoskeletal pain and reduced time spent in physical activity in women living in the community. We recommend further research to determine whether evidence-based interventions designed to reduce the flexion torque generated on the thoracic spine can improve these symptoms of upper torso musculoskeletal pain and the ability of women with obesity to participate in physical activity.
1. Introduction
Obesity has been consistently associated with the development of an extensive range of detrimental physical and mental health issues. These issues include, for example, well-documented increases in the relative risk of diabetes mellitus,1 hypertension and dyslipidemia,1, 2 coronary heart disease,3, 4 a range of cancers,5 and most mood, anxiety and personality disorders,6, 7, 8 as well as musculoskeletal problems such as load-induced osteoarthritis.9 Given that obesity has nearly tripled worldwide since 1975,10 with a significant increase particularly in the prevalence of obesity among women,11 it is imperative that individuals, especially women, are encouraged to maintain an active lifestyle. This is because participating in physical activity together with having a healthy diet have been shown to be an effective strategy for reducing obesity10 and the associated higher risk of developing a wide range of negative physical and mental health issues detailed in numerous review articles.12, 13, 14
To be able to maintain an active lifestyle and enjoy the health benefits associated with participating in physical activity, the structural framework of the body should not be unnecessarily compromised. Although the musculoskeletal framework of the body is designed to enable individuals to perform tasks of daily living and recreation with ease, deviations from normal structure or alignment can compromise function, which can in turn cause discomfort, pain and, in some cases, an inability to participate in physical activity.15
Extensive research has shown that obesity can negatively impact upon the structure and function of the musculoskeletal system, particularly the feet and lower limbs. For example, individuals with obesity across the age-spectrum have been found to have broader, thicker, and flatter feet than their counterparts who are not obese.16, 17, 18, 19, 20 Individuals with obesity have also been found to generate significantly higher plantar pressures when standing and walking21, 22, 23, 24, 25, 26, 27, 28 and to alter their feet and lower limb biomechanics during walking relative to individuals who are not overweight.29, 30, 31 The need for individuals who are overweight and obese to continually bear excess body mass has also been associated with the development of musculoskeletal pain and discomfort in the feet.32, 33, 34 In fact, these higher plantar pressures generated during walking by children with obesity have been significantly associated with reduced physical activity and more time spent in sedentary behavior, most likely due to foot pain and discomfort experienced during weight-bearing activities.35, 36 This creates a vicious cycle whereby the reduced participation in physical activity by individuals who are already obese will subsequently perpetuate their obesity.
Clinical data have confirmed that adults with obesity also report a significantly greater prevalence of musculoskeletal pain than individuals who are not obese,37 particularly in lower limb joints, as well as the neck and back.38, 39 McCarthy et al.40 examined the relationship between chronic pain and obesity in a cohort of 840 older individuals (mean age = 80 years, range: 70–101 years) who were systematically sampled from an electoral list. Although pain was most commonly experienced in the legs and feet (44.8%; grouped together), 39.8% of the cohort reported back pain and 31.2% reported neck and shoulder pain. Compared to participants classified as normal weight (body mass index (BMI) = 18.5–24.9 kg/m2), the participants with obesity (BMI = 30.0–34.5 kg/m2) were twice as likely to report chronic pain, whereas participants with severe obesity (BMI > 35 kg/m2) were more than 4 times as likely to report chronic pain. Furthermore, individuals grouped in higher BMI categories reported a significantly higher number of painful body locations and more frequent and severe pain than their counterparts with lower BMI values. The authors speculated the association between chronic pain and obesity was likely due in part to the greater mechanical loading of weight-bearing structures over a long period of time because of excess body mass, together with an increase in inflammatory markers associated with obesity.40
Although comprehensive reviews of the effects of obesity on mechanical loading of the lower extremities and lower back are available,41, 42, 43 only limited research has investigated the effects of obesity on mechanical loading of the upper torso and how this might impact upon upper torso musculoskeletal structure and function. One factor likely to impact upon upper torso loading is breast size and the relative location of the breasts on the trunk.44 Several studies have established that breast size is significantly associated with obesity, whereby women with higher BMI values have larger breasts45, 46, 47 that are more ptotic and splayed further away from the midline of the torso than the breasts of women with lower BMI values.48 Furthermore, large breast size has been associated with an increased prevalence and severity of musculoskeletal pain, particularly pain in the upper torso.44, 49, 50 Because the structure and function of the musculoskeletal system are inter-related, increased upper torso musculoskeletal pain among women with large breasts is thought to reflect compromised function caused by structural changes to the musculoskeletal system, mainly changes to the vertebral column.50, 51, 52, 53 That is, increased loading of the spine caused by the weight of large breasts anterior to the torso will increase the flexion torque acting on the thoracic spine.50 Over a sustained period of time, an increased thoracic flexion torque is likely to increase thoracic kyphosis, which in turn can lead to secondary changes in cervical lordosis, increased tension in the neck extensor muscles, reduced thoracic extensor muscle endurance strength, and an increased experience of musculoskeletal pain.50, 51, 52, 53
Whether increased breast size on the anterior chest wall affects loading of the thoracic spine in women with obesity, and whether this impacts upon their upper torso musculoskeletal pain and ability to participate in physical activity, has not yet been investigated. Therefore, the aim of this study was to investigate the effects of obesity on breast size, thoracic spine structure and function, upper torso musculoskeletal pain, and physical activity participation in a large cohort of women who were living independently in the community. We hypothesized that the larger breasts of women with obesity would generate high flexion torques about the thoracic spine leading to increased thoracic dysfunction, increased upper torso musculoskeletal pain, and decreased time spent participating in physical activity.
2. Materials and methods
2.1. Participants
A total of 378 Australian women aged 18 years and over who lived in the community volunteered to participate in this study. These participants were recruited by advertising the study widely via the media (i.e., television, radio, newspapers), across all sectors of the University of Wollongong (i.e., students, general staff, academic staff) and through Women's Health Centers. Women were excluded from participating in the study if they were pregnant or breast feeding, because both can affect breast volume. They were also excluded if they had epilepsy that could be induced by the flashing light of a scanner (described below) or were unable to assume the scanning position required to measure breast volume. The women provided written informed consent before participating in the study. All testing was conducted according to the National Health and Medical Research Council Statement on Human Experimentation,54 and all testing procedures were approved by the University of Wollongong Human Research Ethics Committee (HE 13/424).
To determine the effects of obesity on the outcome variables described below, the participants were divided into 3 groups based on the World Health Organization's international BMI classifications (Not Overweight: 18.5–24.9 kg/m², Overweight: 25.0–29.9 kg/m², Obese: ≥ 30.0 kg/m²).55 BMI calculated using the Quetelet index (body mass/height2; kg/m2), provides the most useful population-level measure of overweight and obesity.10 Each participant's height was measured using a portable stadiometer (Model: 214; Seca Corp., Hanover, MD, USA), and body mass was measured using a calibrated Body Composition Analyser (Model: TISC24OMA; Tanita Corp., Arlington Heights, IL, USA). Both measurements were taken while the participants stood barefoot and were wearing minimal clothing.
2.2. Breast size
The size of each participant's breasts was characterized by measuring the volume of both the left and right breasts, following procedures that have been described in detail elsewhere.56 In brief, each participant's breasts were scanned using a hand-held 3-dimensional scanner (ArtecTM Eva 3D Scanner; Artec Group, San Jose, CA, USA) while the participants lay prone across 2 tables, with their breasts freely suspended in a 50-cm space between the 2 tables. Before scanning commenced, small markers (∼ 1 cm in diameter) were adhered directly onto each participant's skin around the outline of each breast in order to highlight the borders of the breasts. The breast scans were then imported into software to create a 3-dimensional model of each breast (Geomagic Studio® software; Version 12.0; 3D Systems, Rock Hill, SC, USA). This was achieved by initially removing the image of each breast from the scan of the participant's torso. A posterior breast wall was then created using a tangential cut plane to mimic the anatomy of the superficial surface of pectoralis major, the soft tissue upon which the breast lies.57 The volume of each left and right breast model was then calculated in mL using the Geomagic Studio® software.56
2.3. Thoracic flexion torque
The flexion torque (N·m) created at the thoracic spine by the weight of each participant's breasts was calculated by multiplying each participant's left and right breast weight (breast volume × breast density (0.94 kg/m³) × acceleration due to gravity (9.81 N))53 by the corresponding moment arm (m). The moment arm for each breast was determined by measuring (using Geomagic Studio® software) the perpendicular distance from the apex of each participant's thoracic spine to the estimated center of gravity of their left and right breasts (calculated automatically by the Geomagic® software based on breast volume distribution). These distances were measured on a 3-dimensional scan of each participant, which was taken while the participants were standing in the anatomical position.
2.4. Thoracic kyphosis
The amount of curvature of each participant's thoracic spine was represented by the thoracic kyphosis angle, which was calculated using a noninvasive method that has been described in detail elsewhere.50, 58 In brief, a flexible strip of plastic-covered metal (Flexicurve, Faber-Castell, Stein, Germany) was molded to the posterior surface of each participant's vertebral column, with its ends aligned with C7 and the L5 to S1 intervertebral space. The molded Flexicurve ruler (Faber-Castell, Stein) was then placed on grid paper where it was traced. From the tracing, a thoracic kyphosis angle was calculated following procedures described by Greendale et al.58 Thoracic kyphosis was measured because increased thoracic kyphosis has been associated with poor mobility in the thoracic spine59 and, in turn, with increased musculoskeletal pain.50, 60
2.5. Upper torso musculoskeletal pain
The upper torso musculoskeletal pain reported to be experienced by each participant was recorded using a color-coded chart, which represented 7 body regions (neck, shoulders, arms, upper back, lower back, breasts, and head). The severity of pain was graded on the chart using a visual analogue scale (visual analog scale: 0 = no pain, 10 = worst pain), and the frequency of experiencing pain was rated from 1 to 3 (1 = rarely (≤1 time per month), 2 = occasionally (≤3 times per month), 3 = frequently (1–3 times per week)).49, 50 The severity grade and frequency score were multiplied for each of the 7 body regions, which were then summed to provide a total upper torso musculoskeletal pain score (maximum score = 210).49, 50
2.6. Time spent in physical activity
To document the amount of physical activity performed in the week preceding the study, each participant completed 8 questions from the Active Australia Survey.61 The questions related to each participant's participation (frequency and duration) in 4 activity types (walking, moderate-intensity activity, vigorous gardening, and vigorous-intensity activity). From the self-reported responses to this survey, the total time each participant estimated that she spent performing physical activity per week (min) was calculated following the analysis guidelines.61 To avoid the effects of over-reporting, data for participants who reported values more than 3 standard deviations greater than the BMI group mean for total time spent in physical activity per week were excluded from analysis (n = 5). Time spent in physical activity was assessed because increased physical activity participation has been associated with reduced musculoskeletal pain in the low back, neck, and shoulder.62
All the variables described above were measured on each participant by a highly trained researcher (CEC) who displayed good intra-rater reliability in performing the measurements. Specifically, the researcher had excellent reliability in scanning and calculating the breast volume variables (Cronbach's α ≥ 0.95). She also had good to excellent reliability when measuring thoracic kyphosis (intraclass correlation coefficient = 0.78; p < 0.05), with the intraclass correlation coefficient value calculated on measurements taken on 3 consecutive days for 6 women who were not associated with the main study.
2.7. Statistical analysis
Descriptive statistics for all outcome variables were calculated for the participants, grouped according to the 3 BMI groups (Not Overweight, Overweight, and Obese). Those variables that were not normally distributed were either log transformed (breast volume and thoracic flexion torque) or square root transformed (the time spent in physical activity and upper torso musculoskeletal pain) to meet the normality and homogeneity of variance assumptions underlying parametric statistics. Because the participant groups were not matched for age, a one-way analysis of variance was used to determine whether there was any significant difference in age among the 3 BMI groups. Then, a one-way analysis of covariance, adjusting for age, was used to compare the effect of BMI on the outcome variables. Bonferroni post hoc tests were used to determine where any significant differences existed among the 3 BMI groups. The α level was set at p < 0.05, and all calculations were performed using the SPSS (Version 21.0; IBM Corp., Armonk, NY, USA).
3. Results
3.1. Participant age, BMI and breast volume
Descriptive statistics pertaining to the age, BMI, and breast volume of the participants, grouped by BMI category, is presented in Table 1. On average, data for the study participants were representative of Australian women aged 18 years and over.63, 64 There was a significant main effect of BMI on age F(2,374) = 29.180, p < 0.001), whereby participants clustered in the Overweight and Obese categories were significantly older than participants who were classified as Not Overweight, and participants classified as Obese, on average, were significantly older than participants classified as Overweight (Table 1). When adjusting for this difference in age, there was a significant main effect of BMI on breast volume (left: F(2,374) = 119.715, p < 0.001; right: F(2,374) = 124.063, p < 0.001). Post hoc analysis confirmed that the breast volume of all 3 groups differed significantly to each other. Participants classified as Obese, on average, displayed the largest breast volumes; and women classified as Not Overweight displayed the smallest breast volumes, irrespective of whether it was their left or right breast (Table 1).
Table 1.
Values for the participants' age, BMI, and breast volume, grouped by BMI category.
| Variables | Not Overweight (n = 163) | Overweight (n = 103) | Obese (n = 112) |
|---|---|---|---|
| Age (year)* | 37.2 ± 1.4 | 45.2 ± 1.9 | 54.4 ± 1.7 |
| (34.3–40.0) | (41.7–48.8) | (50.9–57.8) | |
| BMI (kg/m2)⁎⁎ | 22.5 ± 0.2 | 27.4 ± 0.3 | 35.4 ± 0.3 |
| (21.9–22.8) | (26.9–28.0) | (34.9–35.9) | |
| Breast volume (mL) | |||
| Right breast⁎⁎ | 367 ± 32 | 683 ± 38 | 1077 ± 38 |
| (305–430) | (608–759) | (1002–1153) | |
| Left breast⁎⁎ | 378 ± 30 | 705 ± 37 | 1052 ± 37 |
| (319–438) | (632–778) | (980–1125) | |
Notes: Data are presented as mean ± SE (and 95% confidence intervals). Untransformed values are shown in the table for ease of interpretation, although statistical comparisons were conducted on the transformed data. The BMI and breast volume data were adjusted for the effects of age.
Abbreviation: BMI= body mass index.
p < 0.001 significant differences among all 3 groups.
p < 0.001 significant differences among all 3 groups when the transformed data were controlled for the effects of age.
3.2. Thoracic flexion torque
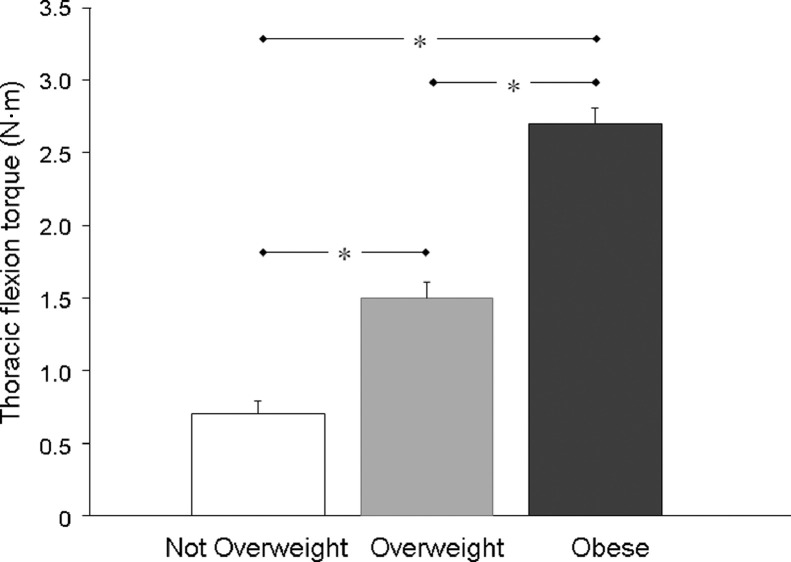
The mean (± SE) flexion torques created on the thoracic spine by the left and right breasts of the participants, grouped by BMI category, are displayed in Fig. 1. Adjusting for age, there was a significant main effect of BMI on the thoracic flexion torque data (left: F(2,374) = 115.484, p < 0.001; right: F(2,374) = 161.461, p < 0.001). Post hoc analysis revealed that there were significant differences between all 3 participant groups, whereby the participants classified as Obese displayed the largest thoracic flexion torques and the participants classified as Not Overweight displayed the smallest values for this variable (Fig. 1).
Fig. 1.
Mean (± SE) values for the thoracic flexion torque (N·m) displayed by the participants, grouped by body mass index category (Not Overweight: n = 163; Overweight: n = 103; Obese: n = 112). Untransformed values of the right thoracic flexion torque only are shown because the left side values were very similar. Statistical comparisons were conducted on the transformed data. * p < 0.05 among all 3 groups.
3.3. Thoracic kyphosis
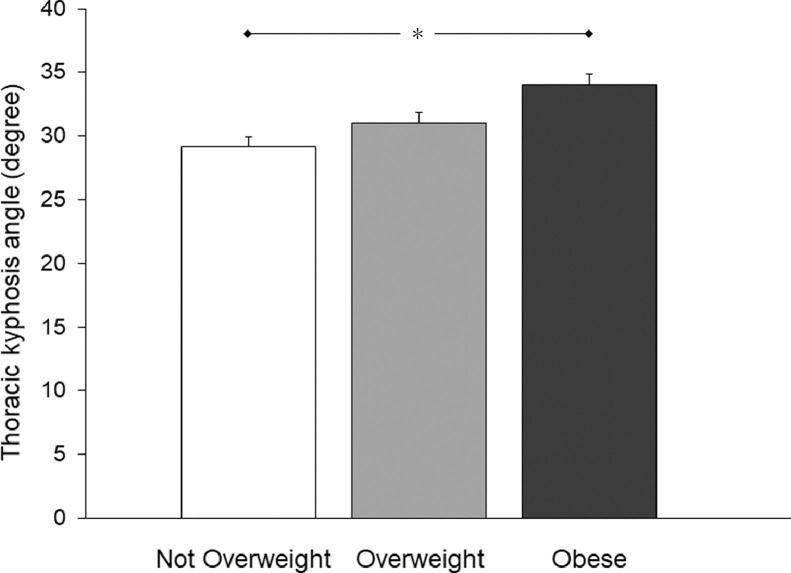
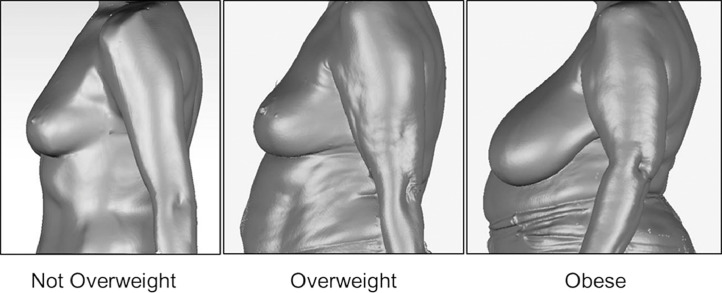
Fig. 2 shows thoracic kyphosis angles measured for the participants, grouped by BMI category. There was a significant main effect of BMI on thoracic kyphosis (F(2,374) = 6.886, p = 0.001). When the data were adjusted for age, the participants classified as Obese displayed significantly greater thoracic kyphosis compared to their counterparts who were classified as Not Overweight (Fig. 2). Typical examples of the breast characteristics and thoracic kyphosis displayed by participants classified as Not Overweight, Overweight, and Obese are represented in Fig. 3.
Fig. 2.
Mean (± SE) values for the thoracic kyphosis angles (degree) displayed by the participants, grouped by body mass index category (Not Overweight: n = 163; Overweight: n = 103; Obese: n = 112). The data were normally distributed. * p < 0.05 between Not Overweight and Obese groups.
Fig. 3.
A typical example of the breast characteristics and thoracic kyphosis displayed by 1 participant from each of the 3 body mass index categories as indicated.
3.4. Upper torso musculoskeletal pain
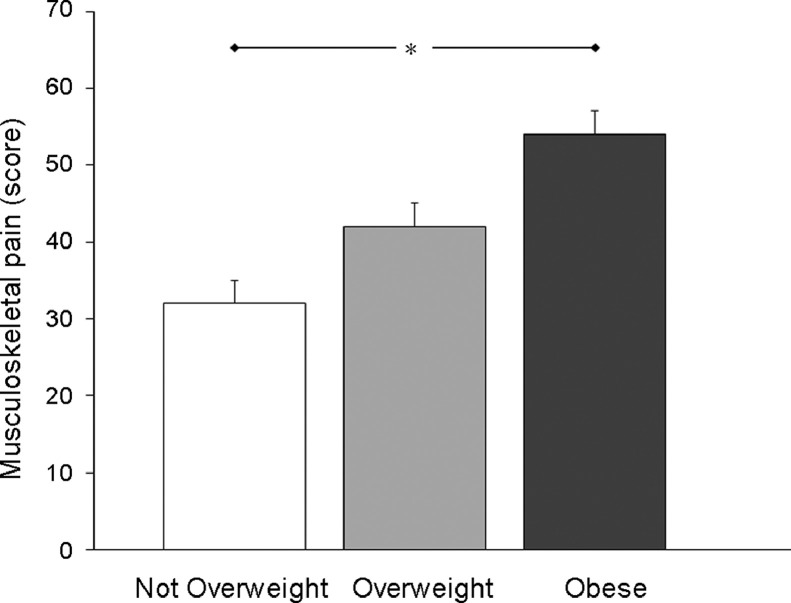
Descriptive data for the upper torso musculoskeletal pain scores reported by the participants, grouped by BMI category, are presented in Fig. 4. When adjusting for age, there was a significant main effect of BMI on the total pain scores (F(2,374) = 12.479, p < 0.001). Post hoc analysis of the data revealed that the participants classified as Obese reported experiencing significantly more upper torso musculoskeletal pain than participants classified as Not Overweight (Fig. 4).
Fig. 4.
Mean (± SE) scores for the upper torso musculoskeletal pain reported by the participants, grouped by body mass index category (Not Overweight: n = 163; Overweight: n = 103; Obese: n = 112). Untransformed values are shown for ease of interpretation. Statistical comparisons were conducted on the transformed data. * p < 0.05 between Not Overweight and Obese groups.
3.5. Time spent in physical activity
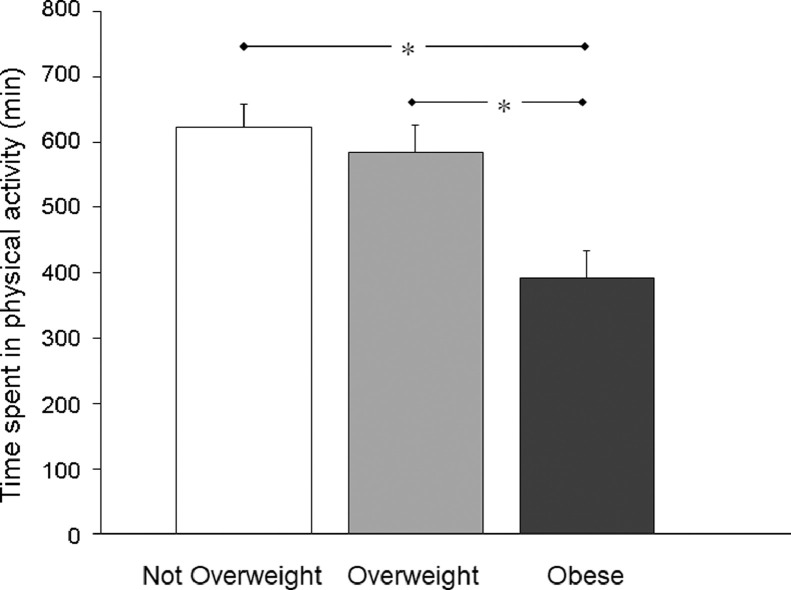
The total time (min) the participants reported they spent participating in physical activity, on average, grouped by BMI category, is displayed in Fig. 5. When controlling for age, analysis of the transformed data revealed a significant main effect of BMI on time spent in physical activity (F(2,369) = 9.692, p < 0.001). Post hoc analysis indicated that the participants classified as Obese spent significantly less time being physically active than the participants classified as either Not Overweight or Overweight (Fig. 5). There was no significant difference between the participants classified as Not Overweight and Overweight for this variable.
Fig. 5.
Mean (± SE) values for the time the participants reported they spent participating in physical activity (min), grouped by body mass index category (Not Overweight: n = 160; Overweight: n = 102; Obese: n = 111). Untransformed values are shown in the figure for ease of interpretation, although statistical comparisons were conducted on the transformed data. * p < 0.05 between Not Overweight and Obese groups; and between Overweight and Obese groups.
4. Discussion
This study highlights the effects of obesity on breast size and loading and structure of the thoracic spine, as well as upper torso musculoskeletal pain and time spent in physical activity reported by a cohort of community-dwelling women. Of primary concern was the finding that the women classified as Obese reported significantly more upper torso musculoskeletal pain and less time participating in physical activity relative to their counterparts who were classified as Not Overweight. The implications of these unique findings are discussed below.
The women in the present study reflected Australian and worldwide data whereby 57% of this cohort of 378 women living independently in the community were classified as Overweight or Obese.10, 65 The older age of the women classified as Obese also reflects a worldwide trend of the likelihood of being overweight or obese increasing with advancing age.11, 65 Given that the world's population is aging, the increasing proportion of older adults with obesity is likely to place a substantial future burden on health-care systems resulting from obesity-related disability and institutionalisation.12 It is therefore imperative that evidence-based intervention strategies are developed to minimize the detrimental health issues associated with obesity, including the increased thoracic kyphosis and higher levels of upper torso musculoskeletal pain reported by women classified as Obese in the present study.
Consistent with previous research,45, 46, 47 participants classified as Obese in the present study displayed breast volumes, on average, that were 3 times that of the breast volumes displayed by women who were classified as Not Overweight. Coltman et al.49 reported that breast volume was the strongest predictor of upper torso musculoskeletal pain in a large cohort of women, whereby women with the greatest upper torso musculoskeletal pain had hypertrophic breasts (breast volumes > 1200 mL46). The participants classified as Obese in the present study had breast volumes, on average, that were just below this hypertrophic threshold, although individual participants classified as Obese had breast volumes as large as 3100 mL per breast. It is therefore not surprising that the participants classified as Obese reported experiencing 1.7 times more upper torso musculoskeletal pain than their counterparts who were classified as Not Overweight.
Several strategies have been proposed to alleviate the upper torso musculoskeletal pain symptoms experienced by women with large breasts49, 50 and are therefore likely to be applicable to women with obesity. These strategies include decreasing the flexion torque on the thoracic spine by decreasing breast mass through weight loss50 or, in extreme cases, breast reduction surgery.66 Increasing the level of breast support provided to women with large breasts via a high support and well-fitted bra50, 53, 66 could also counteract the higher thoracic flexion torque found in the women classified as Obese in the current study. Previous research has found that the bra-breast forces generated in women with large breasts are lower in high-support bras compared to low-support bras.53 Correct bra fit, however, is essential if a bra is to function in the way it was designed.44, 67 Therefore, education on how to correctly fit a bra should also be included when treating women with obesity who present with upper torso musculoskeletal pain. Increasing the trunk's ability to counteract the flexion torque generated by the breasts by increasing the strength of the posterior muscles of the thoracic spine in order to resist the flexion torque is also recommended as a treatment strategy.50 McGhee et al.50 noted that scapular-retractor strength exercises for women with large breasts should be endurance based, and involve low load-high repetitions because endurance rather than maximal strength is compromised in these women. These strategies recommended for women with large breasts appear to be applicable to the women classified as Obese in the present study given that these women displayed significantly greater thoracic flexion torque than their counterparts classified either as Overweight or Not Overweight. In fact, the mean flexion torque generated on the thoracic spine by the participants classified as Obese was nearly 4 times greater than that generated by the participants classified as Not Overweight and nearly twice that of the participants classified as Overweight.
Because the structure and function of the musculoskeletal system are inter-related, it is thought that sustained loading of the thoracic spine caused by the weight of large breasts anterior to the torso is likely to increase thoracic kyphosis.50 This was evident in the present study whereby the participants classified as Obese displayed significantly increased thoracic kyphosis relative to their counterparts classified as Not Overweight, although the thoracic kyphosis angles were all within the normal range.68 Increased thoracic kyphosis is of concern because it can lead to secondary changes in the upper torso musculoskeletal system, including cervical lordosis, increased tension in the neck extensor muscles, reduced thoracic extensor muscle endurance strength, reduced range of motion of the shoulder complex and increased experience of musculoskeletal pain.50, 51, 52, 53,59 It is therefore imperative that medical and allied health professional who are treating women with obesity monitor changes to the thoracic spine of their patients and consider implementing strategies to decrease the flexion torque acting on the thoracic spine of the women as discussed above. Manual therapy and exercises aimed at decreasing thoracic kyphosis are also likely to be effective treatment strategies for women with large breasts who present with upper torso musculoskeletal pain.50 Decreasing thoracic kyphosis might also improve the posture of the lumbar and cervical spine and associated symptoms.52
Consistent with the current study, previous research has also shown significantly greater thoracic kyphosis angles in women with large breasts.50 This finding, however, was in contrast to the findings of other researchers who reported finding no difference in the thoracic kyphosis angle displayed by young women with large breasts and young women with small breasts.68, 69 The increase in thoracic kyphosis secondary to the flexion torque caused by large breasts is likely to be a long-term consequence caused by sustained loading of the thoracic spine and is therefore not yet evident in younger individuals. In contrast, the need of women with obesity to continually bear this additional load on the thoracic spine across their lifetime is likely to have caused this result. However, given that the current study was cross-sectional in nature and therefore cannot confirm cause and effect, further research using a longitudinal study design is required to verify this notion.
Although greater breast mass associated with obesity is likely to be a strong contributor to the increased upper torso musculoskeletal pain reported by the participants in the present study classified as Obese, it was not the only contributing factor. That is, some women classified as Not Overweight also reported experiencing upper torso musculoskeletal pain. Furthermore, women with obesity are more likely to somatize and to suffer more mood, anxiety and personality disorders, including depression,6, 7, 8 than women who are not overweight, which can impact on pain and pain perception.38 Pain is also multifactorial in origin.49 For example, the association between chronic pain and obesity has been speculated to be, in part, due to an increase in inflammatory markers associated with obesity, as well the greater mechanical loading due to excess body mass.40 Further research is therefore recommended to investigate the multitude of factors likely to contribute to the upper torso pain experienced by women with obesity.
It is imperative that individuals are encouraged to maintain an active lifestyle because participating in physical activity has been shown to be an effective strategy for reducing obesity and the associated higher risk of developing a wide range of negative physical and mental health issues.10 In the present study, it is of concern that the participants classified as Obese spent significantly less time each week participating in physical activity compared to the other study participants. In fact, the participants classified as Obese reported, on average, participating in 36% and 30% less physical activity than the participants classified as Not Overweight and Overweight, respectively. Due to the cross-sectional nature of our study, we cannot claim that the variables quantified (breast volume, thoracic flexion torque, thoracic kyphosis, and upper torso musculoskeletal pain) prevented the women from being physically active. However, previous research has revealed that higher loading of the plantar surfaces of the feet during walking by children with obesity is significantly associated with reduced physical activity and more time spent in sedentary behavior, most likely due to foot pain and discomfort experienced during weight-bearing activities.35, 36 Further research is therefore warranted to determine whether implementing strategies to reduce loading of the thoracic spine can decrease the symptoms associated with upper torso musculoskeletal pain and, in turn, encourage women to participate in more physical activity.
As with any research, the limitations of this study need to be acknowledged. Although BMI provides a useful population-level measure of overweight and obesity, it is acknowledged that BMI might not correspond to the same degree of fatness in different individuals.10 Furthermore, although validated and reliable methods were used to record upper torso musculoskeletal pain scores and time spent in physical activity, the data were self-reported. Finally, because the study was cross-sectional in design, any cause-and-effect relationships should be interpreted with caution. Because the proportion of the participants who were obese throughout most of their lifespan is unknown, we cannot determine whether the structural and functional changes to the thoracic spine were acute or developed over an extended period of time. Further longitudinal research is therefore recommended to assess the effects of obesity on the upper torso musculoskeletal system and pain reported by women and how these effects impact their ability to participate in physical activity. Longitudinal research is also recommended to evaluate the effectiveness and timing of treatment aimed at modifying upper torso musculoskeletal symptoms in women with obesity across the lifespan.
5. Conclusion
Our study demonstrates that kyphosis and loading of the thoracic spine were compromised by obesity in women living in the community. Increased obesity levels were also associated with increased symptoms of upper torso musculoskeletal pain and reduced time spent in physical activity. Further research is recommended to determine whether interventions designed to reduce the flexion torque generated on the thoracic spine are effective in relieving loading of the thoracic spine structures and, in turn, improving symptoms of upper torso musculoskeletal pain and the ability of women with obesity to enjoy the health benefits associated with participating in physical activity.
Acknowledgments
Acknowledgments
We wish to thank the women who volunteered their time to participate in this study. We would also like to acknowledge the Illawarra, Penrith, Hunter and Central Coast Women's Health Centres across New South Wales for the use of their facilities, as well as their enthusiasm and support. This research has been conducted with the support of the Australian Government Research Training Program Scholarship and the Sports Medicine Australia Research Foundation, which provided partial funding towards this study. The authors have no professional relationship with a for-profit company that would benefit from this research.
Authors’ contributions
JRS participated in the conception and design of the study, interpreting the data, preparing the first draft of the manuscript and editing subsequent versions of the manuscript; CEC participated in the study design, collected all the data and participated in data analysis, statistical analysis, interpretation of the data and manuscript editing; DEM participated in the conception and design of the study, interpreting the data and editing the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Bays H.E., Chapman R.H., Grandy S. The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract. 2007;61:737–747. doi: 10.1111/j.1742-1241.2007.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown C.D., Higgins M., Donato K.A., Rohde F.C., Garrison R., Obarzanek E. Body mass index and the prevalence of hypertension and dyslipidemia. Obes Res. 2000;8:605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- 3.Todd Miller M., Lavie C.J., White C.J. Impact of obesity on the pathogenesis and prognosis of coronary heart disease. J Cardiometab Syndr. 2008;3:162–167. doi: 10.1111/j.1559-4572.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S., Hawken S., Ôunpuu S., Dans T., Avezum A., Lanas F. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 5.Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.DeJesus R.S., Breitkopf C.R., Ebbert J.O., Rutten L.J., Jacobson R.M., Jacobson D.J. Associations between anxiety disorder diagnoses and body mass index differ by age, sex and race: a population based study. Clin Pract Epidemiol Ment Health. 2016;12:67–74. doi: 10.2174/1745017901612010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mather A.A., Cox B.J., Enns M.W., Sareen J. Associations of obesity with psychiatric disorders and suicidal behaviors in a nationally representative sample. J Psychosom Res. 2009;66:277–285. doi: 10.1016/j.jpsychores.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Petry N.M., Barry D., Pietrzak R.H., Wagner J.A. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70:288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 9.Guss J.D., Ziemian S.N., Luna M., Sandoval T.N., Holyoak D.T., Guisado G.G. The effects of metabolic syndrome, obesity, and the gut microbiome on load-induced osteoarthritis. Osteoarthritis Cartilage. 2019;27:129–139. doi: 10.1016/j.joca.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Obesity and Overweight Factsheet, 2018. Available at: www.who.int/news-room/fact-sheets/detail/obesity-and-overweight; 2019. [accessed 07.01.2019].
- 11.Hales C.M., Fryar C.D., Carroll M.D., Freedman D.S., Ogden C.L. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319:1723–1725. doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decaria J.E., Sharp C., Petrella R.J. Scoping review report: obesity in older adults. Int J Obes(Lond) 2012;36:1141–1150. doi: 10.1038/ijo.2012.29. [DOI] [PubMed] [Google Scholar]
- 13.Kulie T., Slattengren A., Redmer J., Counts H., Eglash A., Schrager S. Obesity and women's health: an evidence-based review. J Am Board Fam Med. 2011;24:75–85. doi: 10.3122/jabfm.2011.01.100076. [DOI] [PubMed] [Google Scholar]
- 14.Reilly J.J., Methven E., McDowell Z.C., Hacking B., Alexander D., Stewart L. Health consequences of obesity. Arch Dis Child. 2003;88:748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steele J.R., Riddiford-Harland D.L., Mickle K.J. Excessive weight bearing compromises foot structure and function across the lifespan. In: Geffin A., Benayahu D., editors. Springer-Verlag; Berlin, Heidelberg: 2014. pp. 149–179. (The mechanobiology of obesity and related diseases. Studies in mechanobiology, tissue engineering and biomaterials). [Google Scholar]
- 16.Chen J.P., Chung M.J., Wang M.J. Flatfoot prevalence and foot dimensions of 5-to 13-year-old children in Taiwan. Foot Ankle Int. 2009;30:326–332. doi: 10.3113/FAI.2009.0326. [DOI] [PubMed] [Google Scholar]
- 17.Mauch M., Grau S., Krauss I., Maiwald C., Horstmann T. Foot morphology of normal, underweight and overweight children. Int J Obes (Lond) 2008;32:1068–1075. doi: 10.1038/ijo.2008.52. [DOI] [PubMed] [Google Scholar]
- 18.Mickle K.J., Steele J.R., Munro B.J. The feet of overweight and obese young children: are they flat or fat? Obesity (Silver Spring) 2006;14:1949–1953. doi: 10.1038/oby.2006.227. [DOI] [PubMed] [Google Scholar]
- 19.Riddiford-Harland D.L., Steele J.R., Storlien L.H. Does obesity influence foot structure in prepubescent children? Int J Obes Relat Metab Disord. 2000;24:541–544. doi: 10.1038/sj.ijo.0801192. [DOI] [PubMed] [Google Scholar]
- 20.Wearing S.C., Grigg N.L., Lau H.C., Smeathers J.E. Footprint-based estimates of arch structure are confounded by body composition in adults. J Orthop Res. 2012;30:1351–1354. doi: 10.1002/jor.22058. [DOI] [PubMed] [Google Scholar]
- 21.Dowling A.M., Steele J.R., Baur L.A. Does obesity influence foot structure and plantar pressure patterns in prepubescent children? Int J Obes Relat Metab Disord. 2001;25:845–852. doi: 10.1038/sj.ijo.0801598. [DOI] [PubMed] [Google Scholar]
- 22.Dowling A.M., Steele J.R., Baur L.A. What are the effects of obesity in children on plantar pressure distributions? Int J Obes Relat Metab Disord. 2004;28:1514–1519. doi: 10.1038/sj.ijo.0802729. [DOI] [PubMed] [Google Scholar]
- 23.Gravante G., Russo G., Pomara F., Ridola C. Comparison of ground reaction forces between obese and control young adults during quiet standing on a baropodometric platform. Clin Biomech (Bristol, Avon) 2003;18:780–782. doi: 10.1016/s0268-0033(03)00123-2. [DOI] [PubMed] [Google Scholar]
- 24.Hills A.P., Hennig E.M., McDonald M., Bar-Or O. Plantar pressure differences between obese and non-obese adults: a biomechanical analysis. Int J Obes Relat Metab Disord. 2001;25:1674–1679. doi: 10.1038/sj.ijo.0801785. [DOI] [PubMed] [Google Scholar]
- 25.Mickle K.J., Steele J.R., Munro B.J. Does excess mass affect plantar pressure in young children? Int J Pediatr Obes. 2006;1:183–188. doi: 10.1080/17477160600881734. [DOI] [PubMed] [Google Scholar]
- 26.Monteiro M., Gabriel R., Aranha J., Neves E., Castro M., Sousa M. Influence of obesity and sarcopenic obesity on plantar pressure of postmenopausal women. Clin Biomech (Bristol, Avon) 2010;25:461–467. doi: 10.1016/j.clinbiomech.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Riddiford-Harland D.L., Steele J.R., Baur L.A. Are the feet of obese children fat or flat? Revisiting the debate. Int J Obes(Lond) 2011;35:115–120. doi: 10.1038/ijo.2010.119. [DOI] [PubMed] [Google Scholar]
- 28.Yan S.H., Zhang K., Tan G.Q., Yang J., Liu Z.C. Effects of obesity on dynamic plantar pressure distribution in Chinese prepubescent children during walking. Gait Posture. 2013;37:37–42. doi: 10.1016/j.gaitpost.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Dufek J.S., Currie R.L., Gouws P.L., Candela L., Gutierrez A.P., Mercer J.A. Effects of overweight and obesity on walking characteristics in adolescents. Hum Mov Sci. 2012;31:897–906. doi: 10.1016/j.humov.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Mahaffrey R., Morrison S.C., Bassett P., Drechsler W.I., Cramp M.C. Biomechanical characteristics of lower limb gait waveforms: associations with body fat in children. Gait Posture. 2018;61:220–225. doi: 10.1016/j.gaitpost.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Nantel J., Brochu M., Prince F. Locomotor strategies in obese and non-obese children. Obesity (Silver Spring) 2006;14:1789–1794. doi: 10.1038/oby.2006.206. [DOI] [PubMed] [Google Scholar]
- 32.Mickle K.J., Steele J.R. Obese older adults suffer foot pain and foot-related functional limitation. Gait Posture. 2015;42:442–447. doi: 10.1016/j.gaitpost.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Stovitz S.D., Pardee P.E., Vazquez G., Duval S., Schwimmer J.B. Musculoskeletal pain in obese children and adolescents. Acta Paediatr. 2008;97:489–493. doi: 10.1111/j.1651-2227.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- 34.Tanamas S.K., Wluka A.E., Berry P., Menz H.B., Strauss B.J., Davies-Tuck M. Relationship between obesity and foot pain and its association with fat mass, fat distribution, and muscle mass. Arthritis Care Res. 2012;64:262–268. doi: 10.1002/acr.20663. [DOI] [PubMed] [Google Scholar]
- 35.Mickle K.J., Cliff D.P., Munro B.J., Okely A.D., Steele J.R. Relationship between plantar pressures, physical activity and sedentariness among preschool children. J Sci Med Sport. 2011;14:36–41. doi: 10.1016/j.jsams.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Riddiford-Harland D.L., Steele J.R., Cliff D.P., Okely A.D., Morgan P.J., Jones R.A. Lower activity levels are related to higher plantar pressures in overweight children. Med Sci Sports Exerc. 2015;47:357–362. doi: 10.1249/MSS.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 37.Blümel J.E., Arteaga E., Mezones-Holguín E., Zúñiga M.C., Witis S., Vallejo M.S. Obesity is associated with a higher prevalence of musculoskeletal pain in middle-aged women. Gynecol Endocrinol. 2017;33:378–382. doi: 10.1080/09513590.2016.1269741. [DOI] [PubMed] [Google Scholar]
- 38.Hooper M.M., Stellato T.A., Hallowell P.T., Seitz B.A., Moskowitz R.W. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes. 2007;31:114–120. doi: 10.1038/sj.ijo.0803349. [DOI] [PubMed] [Google Scholar]
- 39.Peltonen M., Lindroos A.K., Torgerson J.S. Musculoskeletal pain in the obese: a comparison with a general population and long-term changes after conventional and surgical obesity treatment. Pain. 2003;104:549–557. doi: 10.1016/S0304-3959(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy L.H., Bigal M.E., Katz M., Derby C., Lipton R.B. Chronic pain and obesity in elderly people: results from the Einstein aging study. J Am Geriatr Soc. 2009;57:115–119. doi: 10.1111/j.1532-5415.2008.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wearing S.C., Hennig E.M., Byrne N.M., Steele J.R., Hills A.P. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obes Rev. 2006;7:239–250. doi: 10.1111/j.1467-789X.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 42.Wearing S.C., Hennig E.M., Byrne N.M., Steele J.R., Hills A.P. The impact of childhood obesity on musculoskeletal form. Obes Rev. 2006;7:209–218. doi: 10.1111/j.1467-789X.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 43.Wearing S.C., Hennig E.M., Byrne N.M., Steele J.R., Hills A.P. The biomechanics of restricted movement in adult obesity. Obes Rev. 2006;7:13–24. doi: 10.1111/j.1467-789X.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 44.Spencer L., Briffa K. Breast size, thoracic kyphosis & thoracic spine pain - association & relevance of bra fitting in post-menopausal women: a correlational study. Chiropr Man Ther. 2013;21:20. doi: 10.1186/2045-709X-21-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown N., White J., Milligan A., Risius D., Ayres B., Hedger W. The relationship between breast size and anthropometric characteristics. Am J Hum Biol. 2012;24:158–164. doi: 10.1002/ajhb.22212. [DOI] [PubMed] [Google Scholar]
- 46.Coltman C.E., Steele J.R., McGhee D.E. Breast volume is affected by body mass index but not age. Ergonomics. 2017;60:1576–1585. doi: 10.1080/00140139.2017.1330968. [DOI] [PubMed] [Google Scholar]
- 47.Janiszewski P.M., Saunders T.J., Ross R. Breast volume is an independent predictor of visceral and ectopic fat in premenopausal women. Obesity (Silver Spring) 2010;18:1183–1187. doi: 10.1038/oby.2009.336. [DOI] [PubMed] [Google Scholar]
- 48.Coltman C.E., Steele J.R., McGhee D.E. Effects of age and body mass index on breast characteristics: a cluster analysis. Ergonomics. 2018;61:1232–1245. doi: 10.1080/00140139.2018.1481229. [DOI] [PubMed] [Google Scholar]
- 49.Coltman C.E., Steele J.R., McGhee D.E. Can breast characteristics predict upper torso musculoskeletal pain? Clin Biomech (Bristol, Avon) 2018;53:46–53. doi: 10.1016/j.clinbiomech.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 50.McGhee D.E., Coltman K.A., Riddiford-Harland D.L., Steele J.R. Upper torso pain and musculoskeletal structure and function in women with and without large breasts: a cross sectional study. Clin Biomech (Bristol, Avon) 2018;51:99–104. doi: 10.1016/j.clinbiomech.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Findikcioglu K., Findikcioglu F., Ozmen S., Bulam H., Sezgin B. The impact of breast size on the vertebral column: a radiologic study. Aesthetic Plast Surg. 2007;31:23–27. doi: 10.1007/s00266-006-0178-5. [DOI] [PubMed] [Google Scholar]
- 52.Findikcioglu K., Findikcioglu F., Bulam H., Sezgin B., Ozmen S. The impact of breast reduction surgery on the vertebral column. Ann Plast Surg. 2013;70:639–642. doi: 10.1097/SAP.0b013e31823fac41. [DOI] [PubMed] [Google Scholar]
- 53.McGhee D.E., Steele J.R., Zealey W.J., Takacs G.J. Bra-breast forces generated in women with large breasts while standing and during treadmill running: implications for sports bra design. Appl Ergon. 2013;44:112–118. doi: 10.1016/j.apergo.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 54.The National Health and Medical Research Council, the Australian Research Council and Universities Australia. Common wealth of Australia, Canberra. National Health & Medical Research Council Statement on Human Experimentation 2007 (updated 2018). Available at: www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2007-updated-2018. [accessed 11.04.2019].
- 55.World Health Organization . WHO.; Geneva: 2006. BMI classification.www.assessmentpsychology.com/icbmi.htm Available at: [accessed 11.04.2019] [Google Scholar]
- 56.Coltman C.E., McGhee D.E., Steele J.R. Three-dimensional scanning in women with large, ptotic breasts: implications for bra cup sizing and design. Ergonomics. 2017;60:439–445. doi: 10.1080/00140139.2016.1176258. [DOI] [PubMed] [Google Scholar]
- 57.Yip J.M., Mouratova N., Jeffery R.M., Veitch D.E., Woodman R.J., Dean N.R. Accurate assessment of breast volume: a study comparing the volumetric gold standard (direct water displacement measurement of mastectomy specimen) with a 3D laser scanning technique. Ann Plast Surg. 2012;68:135–141. doi: 10.1097/SAP.0b013e31820ebdd0. [DOI] [PubMed] [Google Scholar]
- 58.Greendale G.A., Nili N.S., Huang M.H., Seeger L., Karlamangla A.S. The reliability and validity of three non-radiological measures of thoracic kyphosis and their relations to the standing radiological Cobb angle. Osteoporos Int. 2011;22:1897–1905. doi: 10.1007/s00198-010-1422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crawford H.J., Jull G.A. The influence of thoracic posture and movement on range of arm elevation. Physiother Theor Pr. 1993;9:143–148. [Google Scholar]
- 60.Perriman D.M., Scarvell J.M., Hughes A.R., Lueck C.J., Dear K.B., Smith P.N. Thoracic hyperkyphosis: a survey of Australian physiotherapists. Physiother Res Int. 2012;17:167–178. doi: 10.1002/pri.529. [DOI] [PubMed] [Google Scholar]
- 61.Australian Institute of Health and Welfare . Australian Government; Canberra, ACT: 2003. The Active Australia Survey: a guide and manual for implementation, analysis and reporting. [Google Scholar]
- 62.Vuori L. Exercise and physical health: musculoskeletal health and functional capabilities. Res Q Exerc Sport. 1995;66:276–285. doi: 10.1080/02701367.1995.10607912. [DOI] [PubMed] [Google Scholar]
- 63.Australian Bureau of Statistics . Australian Government; Canberra, ACT: 2012. Profiles of health, Australia, 2011–13. [Google Scholar]
- 64.Australian Bureau of Statistics . Australian Government; Canberra, ACT: 2015. Population by age and sex, Regions of Australia, 2015. [Google Scholar]
- 65.Australian Bureau of Statistics . Australian Government; Canberra, ACT: 2018. 4364.0.55.001 - National Health Survey: first results, 2017–18. [Google Scholar]
- 66.Greenbaum A.R., Heslop T., Morris J., Dunn K.W. An investigation of the suitability of bra fit in women referred for reduction mammaplasty. Brit J Plast Surg. 2003;56:230–236. doi: 10.1016/s0007-1226(03)00122-x. [DOI] [PubMed] [Google Scholar]
- 67.McGhee D.E., Steele J.R. Breast elevation and compression decrease exercise-induced breast discomfort. Med Sci Sports Exerc. 2010;42:1333–1338. doi: 10.1249/MSS.0b013e3181ca7fd8. [DOI] [PubMed] [Google Scholar]
- 68.Oatis C.A. 3rd ed. Wolters Kluwer; Philadelphia, PA: 2016. Kinesiology: the mechanics and pathomechanics of human movement; pp. 932–933. [Google Scholar]
- 69.Wood K., Cameron M., Fitzgerald K. Breast size, bra fit and thoracic pain in young women: a correlational study. Chiropr Osteopat. 2008;16:1. doi: 10.1186/1746-1340-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]