Abstract
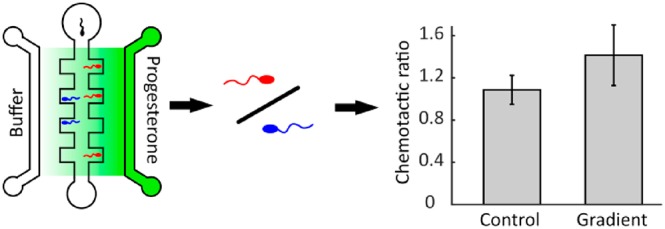
Current male fertility diagnosis tests focus on assessing the quality of semen samples by studying the concentration, total volume, and motility of spermatozoa. However, other characteristics such as the chemotactic ability of a spermatozoon might influence the chance of fertilization. Here we describe a simple, easy to fabricate and handle, flow-free microfluidic chip to test the chemotactic response of spermatozoa made out of a hybrid hydrogel (8% gelatin/1% agarose). A chemotaxis experiment with 1 μM progesterone was performed that significantly demonstrated that boar spermatozoa are attracted by a progesterone gradient.
Current male fertility diagnosis tests focus on assessing the quality of semen samples by studying the concentration of spermatozoa, the total volume of semen, and the motility of the spermatozoa.1 These parameters directly influence the fertility of a man. However, they give a restricted analysis of spermatozoa function in vivo.
The fertilization process of the oocytes in vivo is governed by a set of guidance mechanisms to which spermatozoa respond. Thermotaxis and chemotaxis are postulated to contribute to directing spermatozoa in the fallopian tube toward the oocyte.2 The spermatozoa are initially stored in the isthmic sperm reservoir, where they become capacitated and therefore able to fertilize the oocyte. It is hypothesized that movement from the isthmic reservoir is facilitated by thermotaxis, a process by which a temperature gradient guides the spermatozoa toward the oocyte at the end of the oviduct.2 This first attraction process is long-range, meaning that it is maintained and exists over a relatively long distance along the oviduct from the isthmus reservoir to the oocyte. Chemotaxis is the next guidance process, and it results in the attraction of sperm up a concentration gradient of a particular substance toward the oocyte. This attraction is short-range because peristaltic movements of the oviduct restrict the formation of a long-range concentration gradient. Chemotaxis is therefore the final mechanism that spermatozoa need to actively follow in order to reach the fertilization site.2,3
It has been discovered that human follicular fluid contains several substances that may cause sperm chemotaxis. The substances that can be found in the follicular fluid include progesterone, atrial natriuretic peptide (ANP), heparin, and synthetic N-formylated peptides.4 Progesterone is one of the main ingredients of follicular fluid4 and is present at micromolar concentrations in the vicinity of an oocyte. Given its physiological relevance, progesterone has been suggested as a chemoattractant of spermatozoa. Different concentration ranges of the hormone have been suggested to have different effects on spermatozoa. Picomolar3 and nanomolar concentrations5 were shown to have an attractive effect in chemotaxis. Results show that the concentration causing a reaction is dependent on the species and that progesterone may not be a universal chemoattractant in mammalian species.3,6 Progesterone was also suggested as an agent inducing hyperactivation at concentrations in the micromolar range.5 The highest progesterone concentration is found around the cumulus matrix of the oocyte and is in the same micromolar concentration range that has been reported to cause hyperactivation of spermatozoa.5
Standard techniques used in the lab for the study of cell chemotaxis do not consider random movement of cells. Devices such as those reported by Boyden7 (a transwell-like structure, where the cells migrate through a membrane), Zigmond8 (where cells grow and migrate on a coverslip glass through a bridge between two connected reservoirs), and Dunn and co-workers9 (similar in structure to the Zigmond chamber but with the chemotactic agent containing a reservoir sandwiched between two buffer-containing reservoirs) only allow for unidirectional movement of the cells, namely, toward the potential chemoattractant. This means that, for these devices, one cannot say with certainty that the observed event is chemotactic behavior instead of an increase of the random motility of the cell.
Microfluidic devices can handle very small sample volumes and are capable of mixing and dispensing fluids and combining reactions and separations. This makes microfluidic devices good platforms for performing various chemical, biochemical, and biological processes.10 Because the flow in a microfluidic device is typically laminar, using microfluidics allows for the formation of a controlled gradient by means of diffusion. This regulation of gradients gives a controlled environment for the assessment of the chemotactic response of bacteria,11−14 somatic cells,15−19 and spermatozoa.6,10,20−24 Microfluidic devices to study chemotaxis can be categorized into flow-based or flow-free devices. As the name suggests, flow-based devices use the laminar flow in a microfluidic device to create a concentration gradient via diffusion between streams, while flow-free devices work in the absence of flow. Flow-based systems provide a large amount of control and stability, which allows for continuously running experiments after setup. The advantage of the flow-free systems, however, is that they can be operated without using pumps.25 The microfluidic chips that are used are mostly made from polydimethylsiloxane (PDMS; an optically clear silicone rubber) or agarose (an optically clear hydrogel).
PDMS is a biocompatible material that is commonly used in the fabrication of microchips because it has several advantages. PDMS is impermeable to water in liquid form, nontoxic to cells, and permeable to gases. Furthermore, PDMS can be easily fabricated and bound to other surfaces.26 Microfluidic chips made from PDMS are used to study the motility and chemotaxis of spermatozoa. For example, Koyama et al.10 used a three-inlet chip to generate a chemical gradient via the two outer channels while inserting the spermatozoa in the middle channel. In their microfluidic device, they combined the ability to generate and control a chemical gradient with transportation of the spermatozoa in order to evaluate chemotaxis of mouse sperm cells toward aqueous extracts from the ovarian tissue. They operated the device under constant flow to obtain a stable gradient and prevent, as they mentioned, trapping events from occurring. The downside of this device is the influence of the flow on the behavior of the spermatozoa and the need for a pumping system.
An example of a flow-free device from PDMS is the one by Xie et al.23 They created a Y-channel to connect three wells together, where cumulus cells were placed in either pool A or B, forming a chemoattractant gradient toward the diffusion chamber, where chemotaxis was recorded. They also noted that a channel of 7 mm would be too long for sperm cells to reach without becoming exhausted.23 In this case, cumulus cells were used to create the gradient, which could be placed inside the chip and did not need an addition of fluid to generate a gradient. If one does not want to employ cells placed in the chip to generate the gradient but wants to add a solution instead, such chips will be difficult to operate without disturbing the gradient or the position of the sample due to direct hydrodynamic coupling of the different wells24 or the need of a set of pumps for their usage.6,10 Additionally small behavior differences might not be visible in these chips with all spermatozoa present in the diffusion area without clear boundaries.10 The flow-free device can be improved by using a hydrogel instead of PDMS. By using a hydrogel, such as the commonly used agarose, one can prevent the hydrodynamic distortion of gradients by providing a wall that allows for diffusion and that strongly reduces convection.
Agarose is a polysaccharide derived from red seaweed.27 It is a biocompatible material that has been successfully used in fabrication of microfluidic chips for the study of chemotaxis of several species.6 The chip used by these authors consists of a sample channel sandwiched in between a sink and a source channel, where the agarose material of the chip separates the channels and only allows diffusion of the chemoattractant. One then obtains a linear gradient over the sample channel. This chip was operated under continuous flow in the source and sink channels and no flow in the sample channel. The movement patterns of the spermatozoa were recorded to determine the average direction. It was used to study chemotaxis of sea urchin and mouse sperm6 and showed a chemotaxis response of sea urchin sperm. However, mouse sperm did not show any chemotactic response to the progesterone gradient presented, also giving interesting insights in the range of chemotaxis due to progesterone. The concentrations of progesterone used in this work were 2.5–250 μM,6 which are above the concentrations of progesterone in the cumulus matrix.5 In this work, we improved the device by operating the entire chip in flow-free conditions, removing the need for a set of pumps for their usage. The chip is made out of a hybrid gelatin/agarose hydrogel, improving the viability of the spermatozoa as compared to agarose. Furthermore, by adding side chambers to the sample channel and counting the sperm cells in these, small behavior differences can become easily quantifiable in these chips. By these modifications, the microfluidic chip is as easy to handle as the commercially available chambers for chemotaxis assays, while allowing for a fast identification of small but reproducible differences in the chemotactic behavior of spermatozoa.
Materials and Methods
Spermatozoa Sample
Fresh boar semen was obtained from a local artificial insemination center (Varkens KI Twenthe, Fleringen, The Netherlands) at a concentration of 20 × 106 cells/mL. Before their use in experiments, the spermatozoa are placed in a 37 °C water bath for 20 min to preheat and become activated.
Viability Testing of Different Hydrogels
Three different hydrogel solutions were prepared for viability testing:
(1) Agarose (agarose for routine use, A9539, Sigma-Aldrich) was dissolved in phosphate-buffered saline (PBS) at 85 °C under stirring conditions to prepare a 1% (w/v) solution.
(2) A gelatin solution (8% w/v) (gelatin from porcine skin, G1890, Sigma-Aldrich) was prepared in the same manner as the agarose mixture.
(3) A gelatin/agarose mixture (8:1 (w/w)) was prepared by dissolving gelatin (16% w/v) and agarose (2% w/v) in PBS separately and mixing with 1:1 (v/v) ratio under stirring and heating (at 85 °C) for approximately 20 min, until the mixture was clear.
The influence of hydrogel materials on the viability of the spermatozoa was assessed with SYBR 14/propidium iodide (PI) live/dead staining. The spermatozoa were incubated in a 1000× dilution of SYBR 14 (stock 1 mM, ex/em 488/518 nm, Life Technologies, Eugene, OR, U.S.A.) for 20 min and a 100× dilution of PI (stock 2.4 mM, ex/em 535/617 nm, Life Technologies) for 5 min at room temperature. The cells were then pipetted onto the hydrogel solution. The ratio of live/dead spermatozoa at the different time points was divided by the ratio of live/dead spermatozoa of the initial time point. The data of three experiments were plotted, and a linear trend line was drawn (intercept at 0, 1) to obtain the number of cells that would lose their viability per minute.
Chip Fabrication
A positive mold for the chip was designed in SolidWorks (schematic of the design can be found in Figure 1 and printed on a Formlabs Form 2 3D printer (Figure 2, left). The design contains a 2 cm by 3 cm chip, which has channels with features in the order of millimeters. The height of the channels is 350 μm. A schematic of the design with the details of the channel measurements can be seen in Figure 1. PDMS (10:1 v/v, Sylgard 184, Dow Corning, Midland, MI, U.S.A.) was poured onto the 3D-printed mold, degassed, and cured at 60 °C overnight (Figure 2, middle), creating a negative mold.
Figure 1.
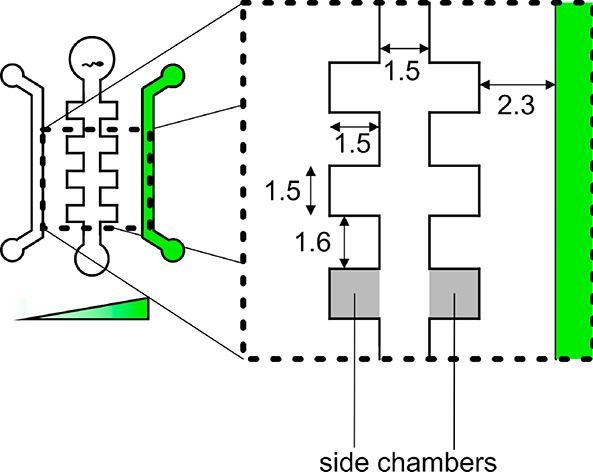
Schematic of the chip design. Left and right channels are the sink and source, respectively. The middle channel is prefilled with buffer and loaded with spermatozoa. Side chambers are used as boundaries for the visualization of spermatozoa. The dimensions are given in mm.
Figure 2.

(Left) 3D-printed mold; (Middle) PDMS mold; (Right) hydrogel chip. Scale bar is 2 cm.
To make the chips, the prepared hydrogel solution was poured onto the PDMS mold and left to cure until solid (Figure 2, right). Afterward, inlets and outlets were punched from the hydrogel with a 3 mm punch (Harris Uni-Core) to access the channels with a micropipette for filling with buffer and progesterone solutions and introducing the spermatozoa.
The hydrogel chips were then bonded to the glass slides to prevent leakage. For the bonding process, glass slides were first cleaned with a plasma cleaner. For better adhesion between the glass and the hydrogel, the surface of the glass slides was silanized with (3-aminopropyl)triethoxysilane (APTES) and treated with glutaraldehyde. The chips were first submerged in 10% w/w APTES (Sigma-Aldrich) in deionized (DI) water for 30 min. The glass slides were then rinsed with DI water before being submerged in 10% w/w glutaraldehyde (Sigma-Aldrich) in phosphate-buffered saline (PBS, Sigma-Aldrich) for another 30 min and again rinsed with DI water and blow-dried. The hydrogel chips were then bonded to the glass slides. This was only performed for the gelatin and mixed-gel chips, as the agarose had been ruled out in the viability studies.
Fifty μL of 100 μg/mL poly(l-lysine)-grafted-poly(ethylene glycol) solution (PLL-g-PEG, SuSoS, Dübendorf, Switzerland) was pipetted into the chamber and left to incubate for 20 min to reduce adhesion of the spermatozoa on the glass slide. Afterward, sperm diluent (Beltsville thawing solution (BTS), Solusem, Aim Worldwide, Vught, The Netherlands) was added into the center channel. Subsequently, 1 μM progesterone (Sigma-Aldrich) in 1× PBS was added in one well at one side of the diffusion chamber, while the other well contained only 1× PBS. Finally, 0.5 μL of sperm solution (20 × 106 spermatozoa/mL) was pipetted into the sperm inlet. To prevent evaporation, the chip was covered with a glass slide and put on a hot plate at 37 °C.
Gradient Formation
To optimize the time needed to form a gradient, experiments were performed with fluorescein sodium salt solution (Sigma-Aldrich; diluted to 0.005%, diffusion coefficient 4.25 × 10–6 cm2 s–1).28 The fluorescein solution was added to one of the side-channels, and the fluorescein distribution was observed with fluorescence microscopy 4× objective for 140 min with 5 min intervals.
Progesterone Experiments
Progesterone experiments were performed with 1 μM progesterone solution that was prepared from a stock solution (8 μg/mL). Progesterone solution was injected into one of the side-channels, and the gradient was settled in approximately 120 min. Afterward, the chips were put onto the hot plate (37 °C). Spermatozoa solution (0.5 μL; 2 × 106 cell/mL) was then injected into the middle channel, and the side chambers were observed with light microscopy (Nikon Eclipse TE2000-U).
Our chip contains several side chambers (Figure 1) into which the spermatozoa can swim. Only the spermatozoa in the side chambers are counted as being attracted. We define the chemotactic ratio as the number of cells directed to the chemoattractant divided by the number of cells that have swum in the opposite direction. A value of >1 is seen as chemoattractive, while a value of <1 is chemorepulsive. A value of 1 is no reaction.
Results and Discussion
Hydrogel Chip Fabrication via Double Casting
The microfluidic chip is made from a hydrogel via a double casting method. A positive mold of the chip is 3D-printed, resulting in a negative PDMS mold of the chip. PDMS is known for its biocompatibility and contains no compounds that can leach into the gel as compared to the 3D-printed material. More importantly, the use of PDMS makes the mold flexible, which allows the chip to be easily removed from the mold because the hydrogel chips are easy to break. The molds contain simple semi-3D structures that are large enough to be 3D-printed and could be micromilled as well, making fabrication of new designs fast and easy. The double casting method also means that many molds can be created from a single master mold, making the process even cheaper and massively parallelizable if needed.
Optimization of Chip Composition
Three different gel compositions were tested for spermatozoa viability. These three gels were chosen for their mechanical properties and optical transparency and for allowing the diffusion of chemicals. Gels with higher concentrations are structurally stable but are less optically transparent. Lower-concentration gels are too fragile to handle and will rupture even with careful handling.
Agarose chips are generally used for diffusion studies in microfluidic chips, but it had been observed in preliminary experiments that it causes early exhaustion or death of the spermatozoa. Therefore, we performed a viability assay on the three different gels (1% agarose, 8% gelatin, and a hybrid 1% agarose/8% gelatin) for the spermatozoa with a duration of about 20 min. From these experiments we found that the spermatozoa that were located on the agarose showed a larger decline in viability (2.4%/min) as compared to the spermatozoa on the pure gelatin (0.5%/min) and the mixture of agarose/gelatin (0.7%/min). The spermatozoa on the control slide showed a decline in viability of 0.1%/min. The pure gelatin would be preferred as a material because its influence on the viability was the least when compared to the control. However, the gelatin structures melt at 37 °C, which is the optimal temperature to mimic physiological conditions. Therefore, the mixture of agarose and gelatin was chosen because this hybrid form can ensure the structural integrity of the chips, while cell death is suppressed as compared to the agarose chips.
Time Window for Diffusion Gradient
The formation of the chemical gradient in our chips was investigated. For this, a solution of fluorescein sodium salt (MW 330.3) was used as a model for the progesterone (MW 314.5) because of its similarity in molecular weight and, hence, diffusivity. We found that the gradient takes 2 h to develop (Figure 3), after which a stable gradient is present during our experiments. After 2 h, one can see the linear slope of the gradient, which lies in the sperm channel and stays within 2.5% of its initial slope for at least 20 min.
Figure 3.
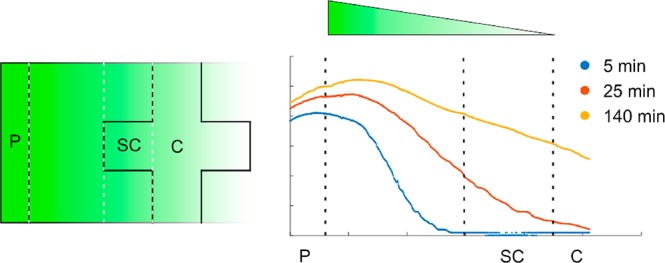
(Left) Gradient of the fluorescein over the chip. P = progesterone loading channel, SC = side chamber, and C = main channel. (Right) Gradients in the chip at t = 5, 20, and 135 min in au. Because of the imperfect illumination of the chip, the left edge is less illuminated, which causes the maximum to lie outside of the source channel.
To ascertain whether the spermatozoa would be able to be directly inserted while keeping the gradient undisturbed, we tested the interruption of the formed gradient after adding 0.5 μL of sperm solution to the center channel. This has been validated by fluorescence images during addition of 0.5 μL of DI water after the gradient formation by fluorescein sodium salt, where no difference of the gradient could be observed (data not shown). This fits the calculations, as the height of the channel is 0.35 mm, while the width is 1.5–3 mm. The displacement of the liquid in the channel by the addition of 0.5 μL would be less than 1 mm (0.95 mm), which is less than the entrance length of the channel, causing no disruption in the gradient in the channel further onward.
Chemotaxis Tests
After establishing the chemical gradient in the hydrogel chips, where the gradient in the main channel is linear, several experiments on the chemotaxis of spermatozoa were performed with a progesterone solution of 1 μM (the concentration found around the cumulus cells). For the control, we used 6 chips, and for the chemotaxis conditions (alternating left and right to prevent influence from any chirality of the spermatozoa), 21 chips were used. The average amount of cells observed was 249 per condition (minimum 78). Afterward, the means of the two conditions (progesterone or no progesterone) were compared using a two-tailed t test with independent variances and showed a significant difference (p < 0.01, Figure 4). The chemotactic ratio that was found was 1.41 for the cells that had been exposed to a gradient of progesterone versus 1.09 for the control. The average for left and right cells were 1.39 and 1.44, respectively; a two-tailed t test showed that the values for left-oriented chemotaxis were not significantly different from those of the right-oriented chemotaxis (p = 0.71). In a single chip, there is the option to take the average of up to four chambers, which allows for comparison of single-chip experiments. In one case, however, the results would be not significant with a p-value of 0.067; therefore, we would advise use of at least three chips to ensure that the results can be trusted. Compared to other studies, our chemotactic ratio seems to be a bit higher (1.4 versus 1.2,23 and various ratios from different ovary extracts from 1 to 3 with an average of 1.210), but this might be caused by the different species used, the difference in the chemoattractant, the geometry, or the presence of flow, where the first study23 has a longer Y-shaped channel and used cumulus cells instead of a progesterone solution and the second study10 has ovary extracts at different concentrations in the presence of flow.
Figure 4.
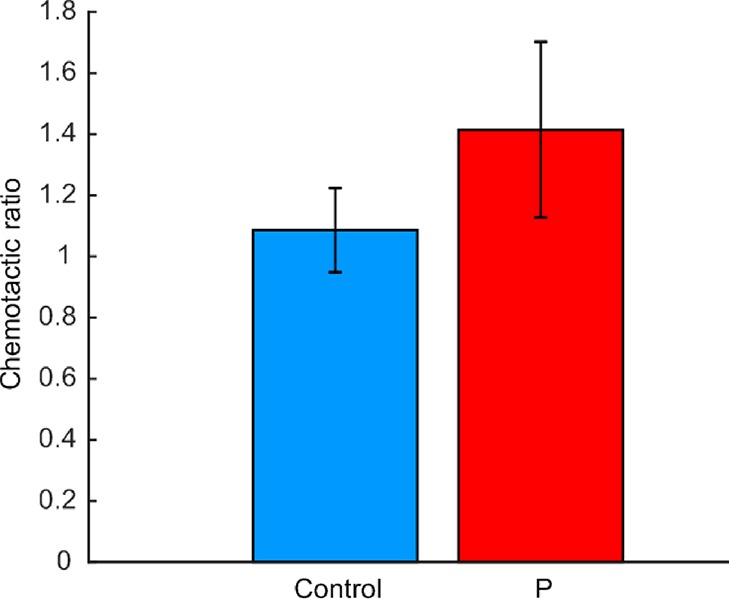
Chemotactic ratio of the spermatozoa. N = 6 for the control, N = 21 for progesterone (P). Error bars are one standard deviation.
Conclusion
Here we showed the development of a flow-free microfluidic chip to test the chemotactic response of spermatozoa. Compared to other designs, our chip is easy to handle while allowing for a fast identification of small but reproducible differences in the chemotactic behavior of spermatozoa. For our design, a hybrid hydrogel (8% gelatin/1% agarose) was shown to be optimal because of its biocompatibility and availability to work at biologically relevant temperatures. Additionally, the 3D-printed design allows for fast production of the hydrogel chips without cleanroom fabrication. Multiple PDMS molds can be made from one 3D-printed mold for easy upscaling. Another advantage is that the PDMS molds are flexible and allow for easy removal of low w/v hydrogel chips without damaging them.
With our flow-free device, we showed that the spermatozoa are attracted by a progesterone gradient in the physiological range. Therefore, our device is capable of investigating the chemotactic behavior of spermatozoa, paving the road to investigate this effect for other chemicals to get a better fundamental understanding of the guiding mechanism.
Acknowledgments
We acknowledge financial support from the SRO Biomedical Microdevices, Technical Medical Centre, University of Twente, The Netherlands, and the Dutch Technology Foundation STW (VENI grant). We also thank KI Twenthe for the kind supply of the boar semen samples.
The authors declare no competing financial interest.
References
- WHO . WHO Laboratory Manual for the Examination and Processing of Human Semen; World Health Organization: 2010. [Google Scholar]
- Bahat A.; Eisenbach M. Sperm thermotaxis. Mol. Cell. Endocrinol. 2006, 252 (1), 115–119. 10.1016/j.mce.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B.; Kashikar N. D.; Weyand I. Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. 2008, 70, 93–117. 10.1146/annurev.physiol.70.113006.100654. [DOI] [PubMed] [Google Scholar]
- Morisawa M.; Yoshida M. Activation of motility and chemotaxis in the spermatozoa: From invertebrates to humans. Reprod. Med. Biol. 2005, 4 (2), 101–114. 10.1111/j.1447-0578.2005.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E.; Luconi M.; Muratori M.; Marchiani S.; Tamburrino L.; Forti G. Nongenomic activation of spermatozoa by steroid hormones: Facts and fictions. Mol. Cell. Endocrinol. 2009, 308 (1), 39–46. 10.1016/j.mce.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Chang H.; Kim B. J.; Kim Y. S.; Suarez S. S.; Wu M. Different Migration Patterns of Sea Urchin and Mouse Sperm Revealed by a Microfluidic Chemotaxis Device. PLoS One 2013, 8 (4), e60587 10.1371/journal.pone.0060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962, 115 (3), 453–466. 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J. Cell Biol. 1977, 75 (2), 606–616. 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha D.; Dunn G. A.; Brown A. F. A new direct-viewing chemotaxis chamber. J. Cell. Sci. 1991, 99 (4), 769–775. [DOI] [PubMed] [Google Scholar]
- Koyama S.; Amarie D.; Soini H. A.; Novotny M. V.; Jacobson S. C. Chemotaxis assays of mouse sperm on microfluidic devices. Anal. Chem. 2006, 78 (10), 3354–3359. 10.1021/ac052087i. [DOI] [PubMed] [Google Scholar]
- Salek M. M.; Carrara F.; Fernandez V. I.; Guasto J. S.; Stocker R. Bacterial chemotaxis in a microfluidic T-maze reveals strong phenotypic heterogeneity in chemotactic sensitivity. Nat. Commun. 2019, 10 (1), 1877. 10.1038/s41467-019-09521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T.; Shimizu T. S.; Stocker R. Bacterial chemotaxis in linear and nonlinear steady microfluidic gradients. Nano Lett. 2010, 10 (9), 3379–3385. 10.1021/nl101204e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H.; Cremer P. S.; Manson M. D. A sensitive, versatile microfluidic assay for bacterial chemotaxis. Proc. Natl. Acad. Sci. U. S. A. 2003, 100 (9), 5449–5454. 10.1073/pnas.0931258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J.; Young L.; Kim S.; Fogarty E. A.; Heilman S. M.; Zhou P.; Shuler M. L.; Wu M.; DeLisa M. P. A three-channel microfluidic device for generating static linear gradients and its application to the quantitative analysis of bacterial chemotaxis. Lab Chip 2006, 6 (3), 381–388. 10.1039/B511958H. [DOI] [PubMed] [Google Scholar]
- Torisawa Y. S.; Mosadegh B.; Bersano-Begey T.; Steele J. M.; Luker K. E.; Luker G. D.; Takayama S. Microfluidic platform for chemotaxis in gradients formed by CXCL12 source-sink cells. Integr. Biol. 2010, 2 (11–12), 680–686. 10.1039/c0ib00041h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann E. K.; Berthier E.; Young E. W.; Shelef M. A.; Wernimont S. A.; Huttenlocher A.; Beebe D. J. Microfluidic kit-on-a-lid: a versatile platform for neutrophil chemotaxis assays. Blood 2012, 120 (14), e45–e53. 10.1182/blood-2012-03-416453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia D.; Liu S. Y.; Tharp W. G.; Samadani A.; Toner M.; Poznansky M. C. Microfluidic system for measuring neutrophil migratory responses to fast switches of chemical gradients. Lab Chip 2006, 6 (2), 191–198. 10.1039/B511877H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C.; Allen S. G.; Ingram P. N.; Buckanovich R.; Merajver S. D.; Yoon E. Single-cell migration chip for chemotaxis-based microfluidic selection of heterogeneous cell populations. Sci. Rep. 2015, 5, 9980. 10.1038/srep09980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.; Heilshorn S. C. Microfluidic Investigation of BDNF-Enhanced Neural Stem Cell Chemotaxis in CXCL12 Gradients. Small 2013, 9 (4), 585–595. 10.1002/smll.201202208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y.-J.; Maeng J.-H.; Hwang S. Y.; Ahn Y. Design, Fabrication, and Testing of a Microfluidic Device for Thermotaxis and Chemotaxis Assays of Sperm. SLAS Technol. 2018, 23 (6), 507–515. 10.1177/2472630318783948. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Xiao R. R.; Yin T.; Zou W.; Tang Y.; Ding J.; Yang J. Generation of Gradients on a Microfluidic Device: Toward a High-Throughput Investigation of Spermatozoa Chemotaxis. PLoS One 2015, 10 (11), e0142555 10.1371/journal.pone.0142555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.; Wang S.; Sun Z.; Niu R.; Wang J. In vivo influence of sodium fluoride on sperm chemotaxis in male mice. Arch. Toxicol. 2014, 88 (2), 533–539. 10.1007/s00204-013-1099-0. [DOI] [PubMed] [Google Scholar]
- Xie L.; Ma R.; Han C.; Su K.; Zhang Q.; Qiu T.; Wang L.; Huang G.; Qiao J.; Wang J.; Cheng J. Integration of Sperm Motility and Chemotaxis Screening with a Microchannel-Based Device. Clin. Chem. 2010, 56 (8), 1270–1278. 10.1373/clinchem.2010.146902. [DOI] [PubMed] [Google Scholar]
- Ko Y. J.; Maeng J. H.; Lee B. C.; Lee S.; Hwang S. Y.; Ahn Y. Separation of Progressive Motile Sperm from Mouse Semen Using On-chip Chemotaxis. Anal. Sci. 2012, 28 (1), 27–27. 10.2116/analsci.28.27. [DOI] [PubMed] [Google Scholar]
- Li J.; Lin F. Microfluidic devices for studying chemotaxis and electrotaxis. Trends Cell Biol. 2011, 21 (8), 489–497. 10.1016/j.tcb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Sia S. K.; Whitesides G. M. Microfluidic devices fabricated in Poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24 (21), 3563–3576. 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- Neves N. M.; Reis R. L.. Biomaterials from nature for advanced devices and therapies; Wiley Online Library: 2015; 10.1002/9781119126218. [DOI] [Google Scholar]
- Culbertson C. T.; Jacobson S. C.; Michael Ramsey J. Diffusion coefficient measurements in microfluidic devices. Talanta 2002, 56 (2), 365–373. 10.1016/S0039-9140(01)00602-6. [DOI] [PubMed] [Google Scholar]


