Abstract
Artificial Intelligence (AI) applications in medicine have grown considerably in recent years. AI in the forms of Machine Learning, Natural Language Processing, Expert Systems, Planning and Logistics methods, and Image Processing networks provide great analytical aptitude. While AI methods were first conceptualized for radiology, investigations today are established across all medical specialties. The necessity for proper infrastructure, skilled labor, and access to large, well-organized data sets has kept the majority of medical AI applications in higher-income countries. However, critical technological improvements, such as cloud computing and the near-ubiquity of smartphones, have paved the way for use of medical AI applications in resource-poor areas. Global health initiatives (GHI) have already begun to explore ways to leverage medical AI technologies to detect and mitigate public health inequities. For example, AI tools can help optimize vaccine delivery and community healthcare worker routes, thus enabling limited resources to have a maximal impact. Other promising AI tools have demonstrated an ability to: predict burn healing time from smartphone photos; track regions of socioeconomic disparity combined with environmental trends to predict communicable disease outbreaks; and accurately predict pregnancy complications such as birth asphyxia in low resource settings with limited patient clinical data. In this commentary, we discuss the current state of AI-driven GHI and explore relevant lessons from past technology-centered GHI. Additionally, we propose a conceptual framework to guide the development of sustainable strategies for AI-driven GHI, and we outline areas for future research.
Keywords: Artificial Intelligence, AI Framework, Global Health, Implementation, Sustainability, AI Strategy
1. Introduction
Advancements in medical technology have long inspired global health pioneers, who envision ways that new technologies might bridge deficits in skilled labor, infrastructure, and access to resources in developing nations. In the current era, there have been rapid advancements in medically applied Artificial Intelligence (AI), a versatile, complex, and powerful set of tools capable of performing specific tasks which normally require human intelligence. The applications of AI technologies in medicine have grown considerably and are now developed in nearly every medical specialty.1-5
However, due to the complexity of medical AI systems, and their reliance on access to unique resources, these applications are applied almost exclusively within the developed world. Despite limitations, there is growing interest in leveraging AI to address healthcare issues facing the developing world. Proof of concept AI-based Global Health Initiatives (GHI) exists. I gap in knowledge, however, remains regarding how to develop an effective and sustainable approach to AI-based GHI. In this paper, we provide context to the current state of AI-based GHI, exploring pertinent lessons from past technology-centered global health initiatives. We propose a conceptual model to guide the development of sustainable AI models applied in a global health context and provide clear directions for future research endeavors in order to expand upon this framework.
2. AI and Global Health
A modest number of AI-based global health initiatives have arisen within the last 10 years.6 Several implementation models have been developed for resource-poor settings.7-21 AI model concepts are applicable in: (1) creating intelligent Electronic Health Records (EHR), (2) performing bio-surveillance, (3) diagnosing disease, (4) assisting in clinical decision making, and (5) optimizing planning and scheduling processes.
Design requirements for the creation of large database technologies in low-resource settings have previously been established.22 Adapted for AI initiatives, we pose design requirements to include: (1) a model—access to clean, large, and inclusive datasets for model generation; (2) personnel—skilled programmers and data scientists; (3) processes—an accurate, continued stream of information flow, and availability of knowledgeable physician/subject matter experts to assess end goal achievement; and (4) infrastructure—a dependable network on which models will run, including access to reliable power and network connectivity.22 Achievement of these requirements is the first step toward the realization of a fully formed AI-based GHI. However, to ensure sustainable model deployment, attention must be paid toward durable access to essential resources and sustained capacity for model maintenance. AI is still at baseline a technology that comes with a “principled set of considerations” that will be important for its utilization, standardization, and sustainable deployment in a given GHI.
3. Lessons from Global Health
The literature on technology based GHI provides a number of conceptual frameworks to guide effective and sustainable programs.22 A prominent approach is to break a program down into ‘vertical’ and ‘horizontal’ components. To elaborate, a predominately vertical program is one that relies heavily on outsourced labor, resources, and expertise (from developed nations) with minimal integration into local infrastructure.23,24 In contrast, predominately horizontal programs are those which seek to integrate a particular health care technology into local infrastructures and labor forces. To ensure that technology-driven improvements toward health outcomes are sustainable in resource-poor nations, each initiative should aim to start as, or transition into, an appropriately balanced horizontally initiative. The optimal balance between vertical and horizontal components has been termed a ‘synergistic’ approach.
4. A Synergistic Approach to AI based global health initiatives
We propose a conceptual framework to guide sustainable AI-driven GHI. Figure 1 provides a breakdown of four key components for the sustainable development of an AI-driven GHI: (1) Intervention, (2) AI Application, (3) Resource Sustainability Considerations, and (4) Synergistic Program Design. Intervention considerations include the type of healthcare problem one is seeking to solve. After establishing a type of intervention, the appropriateness of individual AI model types may be considered and targeted to optimize model fit. In Figure 1, we chose well-regarded papers per intervention category (intelligent electronic records,10 biosurveillance,8,9,11–14,15,16,25 diagnostics,26–30 clinical decision assistance,7,19,20,31–35 and automated planning and scheduling,36) and investigated each paper to determine which AI application was chosen to meet the intervention’s goal. After an AI model is selected, sustainability considerations must be made. For example, certain models may require more oversight or routine maintenance. Successful synergistic programs are ultimately founded upon a resource-conscious strategy which enables the transition from a predominately vertical program to one with optimal horizontal components.
Figure 1.
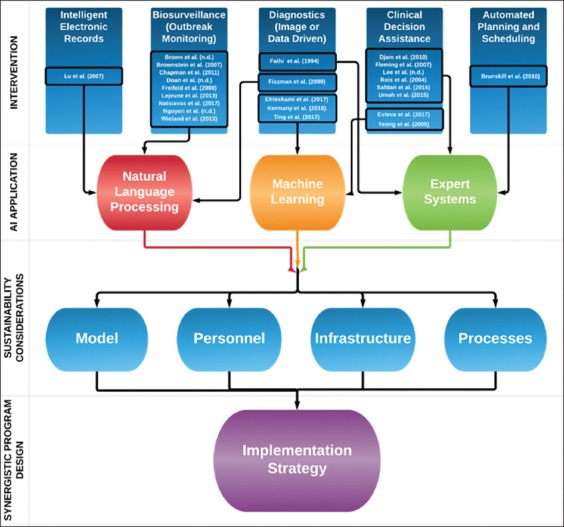
A process for development of an artificial intelligence driven global health initiative.
Our second diagram (Figure 2) serves as a guide for developing a pre-implementation strategy for synergistic AI-driven GHI. Initial determination of which AI application is appropriate for a particular GHI helps to ensure that resource needs specific to that application are accounted for pre-deployment. We break down such resource considerations into the four general categories: (1) Model, (2) Personnel, (3) Infrastructure, and (4) Process.
Figure 2.

A potential strategy for implementation of a synergistic artificial intelligence driven global health initiative.
We recognize that there is not a universal approach to AI implementation in resource poor regions. Our primary aim for this conceptual framework is to facilitate future research on sustainable AI-driven GHI. For example, one might start with a particular healthcare problem (within a resource-poor setting) amenable to an AI-driven solution, select the appropriate AI application, consider that application’s specific resource requirements, and then develop a sustainable strategy for implementation.
5. Synergistic Strategy for Sustainability
AI-based GHI must maintain focus on the end goal of establishing a self-sustaining model in their location of deployment. Therefore, prior to deployment, an ultimate exit strategy for the program must be well developed. At the time of deployment, it is expected that a large portion of the AI system, specifically the model, processes, personnel and infrastructure, may be provided primarily through international support. However, shortly after the program establishes itself in the region of interest, action should begin quickly to strengthen the local correlates of these key resources.
As the use of a particular AI application becomes fully operational, serious consideration should be given towards transitioning most, if not all, personnel and processes over to on-site assets in the region of interest. Appropriate preparations for personnel exchange should be planned at the time of initial deployment, as the transition will require training of local healthcare providers. Infrastructure limitations and technical model features will likely prevent expedient transition toward an optimal utilization of local resources. In some regions, transition to full reliance on a particular local resource could remain a challenge indefinitely. Even in such cases, a locally supported AI system should remain the goal. Achievement of steady-state for a program can be claimed when local infrastructure resources are able to supply the personnel needed to maintain the model and necessary processes with minimal reliance on outside support - the endpoint necessary to enact durable change.
6. Discussion
Overall, the available studies on AI-driven GHI are still extremely limited. Current literature is largely conceptual in nature, with a majority of studies outlining potential use cases for AI within particular resource-poor settings. However, as the application of medical AI reaches scale in these developed countries, it is critical for research on the sustainable implementation of this technology in resource-poor settings to grow in parallel.
Our proposed synergistic model for AI-driven GHI used the four components distilled from numerous GHI—model, personnel, infrastructure, and processes—as inputs in a vertical-to-horizontal transition framework. The ultimate aim of this model is to enable any AI-driven GHI to seamlessly transition from a non-sustainable, international-dependent intervention into a sustainable, locally-driven intervention capable of affecting permanent change over a large period of time.
We recognize the limitations inherent in the current form of this model. Further research and iterative refinements are necessary for this model to reach operational utility. A significant amount of data is still needed in order to conduct a proper analysis of the optimal time-to-transition from a vertical heavy program to a supportive horizontal program for each AI-driven GHI. We assert that these limitations may be addressed through the work of future studies who use our conceptual framework to further refine our model and define optimal transition points.
Future research in this area should begin by cataloging current GHI capable of being augmented by AI. To better understand the vertical to horizontal transition, we recommend a comprehensive analysis of the long-term resource and infrastructure demands for each AI application being considered for use in a GHI. Finally, we propose research to develop healthcare focused AI education programs to train local healthcare providers who would be involved in an AI-driven GHI.
6. Conclusion and Global Health Implications
Our model for the implementation of sustainable AI-driven GHI aims to provide a clear path toward improved health outcomes in resource poor settings through the support of these developing countries as a whole. The potential for AI to transform the practice of medicine is now abundantly clear. Yet, amongst this excitement, we must not lose sight of our ultimate goal of empowering these developing nations to fully support themselves. In this new frontier of medicine, we stand to learn as much from the countries we help as they might from us.
Key Messages.
Strategies for sustainable implementation artificial intelligence driven global health initiatives should be developed prior to its wide scale utilization.
Future research on artificial intelligence driven global health initiatives should seek to characterize long-term resource and infrastructure needs.
Acknowledgements
None.
Footnotes
Conflicts of Interest: No Conflicts of Interest.
Financial Disclosure: No disclosures.
Funding/Support: No Funding.
Ethics Approval: Not Applicable.
References
- 1.Topol EJ. High-performance medicine:the convergence of human and artificial intelligence. Nature Medicine. 2019;25(1):44–56. doi: 10.1038/s41591-018-0300-7. doi:10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- 2.Chan HP, Doi K, Vyborny CJ, Lam KL, Schmidt RA. Computer-aided detection of microcalcifications in mammograms Methodology and preliminary clinical study. Investigational Radiology. 1988;23(9):664–671. [PubMed] [Google Scholar]
- 3.Wang X, Peng Y, Lu L, Lu Z, Bagheri M, Summers RM. ChestX-Ray8:Hospital-Scale Chest X-Ray Database and Benchmarks on Weakly-Supervised Classification and Localization of Common Thorax Diseases In 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR) Vol 2017-Janua. IEEE. 2017:3462–3471. doi:10.1109/CVPR.2017.369. [Google Scholar]
- 4.Nam JG, Park S, Hwang EJ, et al. Development and Validation of Deep Learning–based Automatic Detection Algorithm for Malignant Pulmonary Nodules on Chest Radiographs. Radiology. 2019;290(1):218–228. doi: 10.1148/radiol.2018180237. doi:10.1148/radiol.2018180237. [DOI] [PubMed] [Google Scholar]
- 5.Strodthoff N, Strodthoff C. Detecting and interpreting myocardial infarction using fully convolutional neural networks. Physiological Measurements. 2019;40(1):015001. doi: 10.1088/1361-6579/aaf34d. doi:10.1088/1361-6579/aaf34d. [DOI] [PubMed] [Google Scholar]
- 6.Wahl B, Cossy-Gantner A, Germann S, Schwalbe NR. Artificial intelligence (AI) and global health:how can AI contribute to health in resource-poor settings? BMJ Global Health. 2018;3(4):e000798. doi: 10.1136/bmjgh-2018-000798. doi:10.1136/bmjgh-2018-000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming G, Merwe M, van der McFerren G. Fuzzy expert systems and GIS for cholera health risk prediction in southern Africa. Environmental Model Software. 2007;22(4):442–448. doi:10.1016/J.ENVSOFT.2005.12.008. [Google Scholar]
- 8.Natsiavas P, Maglaveras N, Koutkias V. A Public Health Surveillance Platform Exploiting Free-Text Sources via Natural Language Processing and Linked Data:Application in Adverse Drug Reaction Signal Detection Using PubMed and Twitter. In: Riaño D, Lenz R, Reichert M, editors. Knowledge Representation for Health Care ProHealth 2016, KR4HC 2016 Lecture Notes in Computer Science vvol 10096. Springer: Cham; 2017. [Google Scholar]
- 9.Wieland ML, Wu ST, Kaggal VC, Yawn BP. Tracking health disparities through natural-language processing. American Journal of Public Health. 2013;103(3):448–449. doi: 10.2105/AJPH.2012.300943. doi:10.2105/AJPH.2012.300943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu HM, King CC, Wu TS, et al. Intelligence and Security Informatics:Biosurveillance. Berlin, Heidelberg: Springer Berlin Heidelberg; 2007. Chinese Chief Complaint Classification for Syndromic Surveillance; pp. 11–22. doi:10.1007/978-3-540-72608-1_2. [Google Scholar]
- 11.Lejeune G, Brixtel R, Lecluze C, Doucet A, Lucas N. Added-Value of Automatic Multilingual Text Analysis for Epidemic Surveillance. In: Peek N, Marín Morales R, Peleg M, editors. Artificial Intelligence in Medicine. AIME 2013. Lecture Notes in Computer Science. Vol. 7885. Berlin, Heidelberg: Springer; 2013. [Google Scholar]
- 12.Doan S, Ngo QH, Kawazoe A, Collier N. Global Health Monitor-A Web-Based System for Detecting and Mapping Infectious Diseases. [Accessed April 20 2019];Proceedings of the Third International Joint Conference on Natural Language Processing. II https://www.aclweb.org/anthology/I08-2140 . [Google Scholar]
- 13.Chapman W.W, Gundlapalli A.V, South B.R, Dowling J.N. Natural Language Processing for Biosurveillance. In: Castillo-Chavez C, Chen H, Lober W, Thurmond M, Zeng D, editors. Infectious Disease Informatics and Biosurveillance. Integrated Series in Information Systems. Vol. 27. Boston, MA: Springer; 2011. [Google Scholar]
- 14.Brownstein J S, Freifeld C.C. HealthMap:the development of automated real-time internet surveillance for epidemic intelligence. EuroSurveillance. 2007;12(48):pii=3322. doi: 10.2807/esw.12.48.03322-en. https://doi.org/10.2807/ esw.12.48.03322-en . [DOI] [PubMed] [Google Scholar]
- 15.Freifeld CC, Mandl KD, Reis BY, Brownstein JS. HealthMap:Global Infectious Disease Monitoring through Automated Classification and Visualization of Internet Media Reports. J ournal of American Medical Informatics Association. 2008;15(2):150–157. doi: 10.1197/jamia.M2544. doi:10.1197/jamia.M2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen MT, Nguyen TT. Extraction of Disease Events for a Real-time Monitoring System. International Journal of Computational Vision and Robotics. 2015;5(3) doi:10.1145/2542050.2542084. [Google Scholar]
- 17.Kiranantawat K, Sitpahul N, Taeprasartsit P, et al. The First Smartphone Application for Microsurgery Monitoring. Plastic Reconstruction Surgery. 2014;134(1):130–139. doi: 10.1097/PRS.0000000000000276. doi:10.1097/PRS.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 18.Gulshan V, Peng L, Coram M, et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. J ournal of the American Medical Association. 2016;316(22):2402. doi: 10.1001/jama.2016.17216. doi:10.1001/jama.201617216. [DOI] [PubMed] [Google Scholar]
- 19.Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115–118. doi: 10.1038/nature21056. doi:10.1038/ natur 21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeong EK, Hsiao TC, Chiang HK, Lin C-W. Prediction of burn healing time using artificial neural networks and reflectance spectrometer. Burns. 2005;31(4):415–420. doi: 10.1016/j.burns.2004.12.003. doi:10.1016/J.BURNS.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Onu CC, Udeogu I, Ndiomu E, et al. Ubenwa:Cry-based Diagnosis of Birth Asphyxia November 2017. [Accessed May 29 2019]. http://arxiv.org/abs/1711.06405 .
- 22.Jawhari B, Ludwick D, Keenan L, Zakus D, Hayward R. Benefits and challenges of EMR implementations in low resource settings:a state-of-the-art review. BMC Medical Informatics Decision Making. 2016;16(1):116. doi: 10.1186/s12911-016-0354-8. doi:10.1186/s12911-016-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Msuya J. Horizontal and Vertical Delivery of Health Services. What Are The Trade Offs? 2694 http://documents.worldbank.org/curated/en/914491468761944686/Horizontal-and-vertical-delivery-of-health-services-what-are-the-trade-offs. [Google Scholar]
- 24.Atun RA, Bennett S, Duran A. When Do Vertical (Stand-Alone) Programmes Have a Place in Health Systems? 2008. [Accessed June 9 2019]. http://www.euro.who.int/pubrequest .
- 25.Brown P, Oktay C, Cevik A, et al. Sensitivity and Specificity of an Ngram Method for Classifying Emergency Department Visits into the Respiratory Syndrome in the Turkish Language. Academic Emergency Medicine. 2007 10.1197/j.aem.2007.03.1230. [Google Scholar]
- 26.Fathi-Torbaghan M, Meyer D. MEDUSA:A Fuzzy Expert System for Medical Diagnosis of Acute Abdominal Pain. Methods of Information in Medicine. 1994;33(5):522–529. doi:10.1055/s-0038-1635055. [PubMed] [Google Scholar]
- 27.Fiszman M, Chapman WW, Aronsky D, Evans RS, Haug PJ. Automatic Detection of Acute Bacterial Pneumonia from Chest X-ray Reports. J ournal of American Medical Informatics Association. 2000;7(6):593–604. doi: 10.1136/jamia.2000.0070593. doi:10.1136/jamia.2000.0070593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehteshami Bejnordi B, Veta M, Johannes van Diest P, et al. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women With Breast Cancer. J ournal of the American Medical Association. 2017;318(22):2199. doi: 10.1001/jama.2017.14585. doi:10.1001/jama.2017.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kermany DS, Goldbaum M, Cai W, et al. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell. 2018;172(5):1122–1131. e9. doi: 10.1016/j.cell.2018.02.010. doi:10.1016/J.CELL.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Ting DSW, Cheung CY-L, Lim G, et al. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images From Multiethnic Populations With Diabetes. J ournal of the American Medical Association. 2017;318(22):2211. doi: 10.1001/jama.2017.18152. doi:10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djam X, Wajiga G, Kimbi Y, Blamah N. A Fuzzy Expert System for the Management of Malaria. International Journal of Pure and Applied Sciences and Technology. 2011;5(2):84–108. [Google Scholar]
- 32.Lee CS. Wang MHA Fuzzy Expert System for Diabetes Decision Support Application IEEE Transactions on Systems Man and Cybernetics Part B (Cybernetics) 41(1):139–153. doi: 10.1109/TSMCB.2010.2048899. [DOI] [PubMed] [Google Scholar]
- 33.Reis MAM, Ortega NRS, Silveira PSP. Fuzzy expert system in the prediction of neonatal resuscitation. Brazilian Journal of Medical and Biological Research =Rev Bras Pesqui medicas e Biol. 2004;37(5):755–764. doi: 10.1590/s0100-879x2004000500018. [DOI] [PubMed] [Google Scholar]
- 34.Safdari R, Kadivar M, Langarizadeh M, Nejad AF, Kermani F. Developing a Fuzzy Expert System to Predict the Risk of Neonatal Death. Acta Inform Med. 2016;24(1):34–37. doi: 10.5455/aim.2016.24.34-37. doi:10.5455/aim.2016.24.34-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umoh UA, Umoh U, Nyoho E. A Fuzzy Intelligent Framework for Healthcare Diagnosis and Monitoring of Pregnancy Risk Factor in Women. J ournal of Health, Medicine and Nursing. 2015;18:2015. [Google Scholar]
- 36.Brunskill E, Lesh N. Routing for Rural Health:Optimizing Community Health Worker Visit Schedules 2010 AAAI Spring Symposium - Technical Report. [Accessed June 3 2019]. https://www.aaai.org/ocs/index.php/SSS/SSS10/paper/viewPaper/1139 .


