Abstract
Background
Sepsis is a life-threatening situation, and it can be rendered more severe by coagulopathy. We here examine a novel plasma biomarker for sepsis-induced coagulopathy.
Methods
A total of 116 patients diagnosed with sepsis were recruited and divided into two groups by whether they also had coagulopathy. Plasma samples were collected on arrival at the intensive care unit. Fifteen sepsis-alone and 15 sepsis-induced coagulopathy plasma samples were mixed and sent for microRNA sequencing. Differently expressed microRNAs were then validated by quantitative reverse transcriptase polymerase chain reaction in 52 sepsis-alone and 34 sepsis-induced coagulopathy patients; plasma lipocalin-2 was measured as well.
Results
Four microRNAs were selected from microRNA sequencing. Only hsa-mir-92a-3p was differently expressed in the validation set. Its level of expression was significantly lower in sepsis-induced coagulopathy group. Hsa-mir-92a-3p had an area under a receiver operating characteristic curve of 0.660 (95% confidence interval, 0.537, 0.782). The plasma Hsa-mir-92a-3p level was related to activated partial thromboplastin time, prothrombin activity, and plasma lipocalin-2 level. A binary logistic model showed an association between hsa-mir-92a-3p and fibrinogen with SIC.
Conclusions
The utility of hsa-mir-92a-3p as a biomarker for sepsis-induced coagulopathy needs more verification, and the regulatory mechanism of hsa-mir-92a-3p in coagulation disorder and its potency as a therapeutic target must be confirmed.
Keywords: Sepsis, Coagulation disorder, microRNA, Biomarker, Diagnosis
Background
Sepsis is a series of life-threatening organ dysfunction events initiated by dysregulated host response to infection [1]. Disseminated intravascular coagulation (DIC) is a major cause of organ dysfunction in sepsis. It is significantly closely related to mortality and difficult to be recognized before coagulation collapsing [2, 3]. Recently, multiple studies have confirmed that early diagnosis and intervention of DIC is crucial to disease prognosis [4–9], which highlighted the importance of identifying sepsis-induced coagulopathy (SIC) before it is too late.
MicroRNA (miRNA) is a group of endogenous noncoding small RNAs. They are highly conserved and function as posttranscriptional regulators of gene expression [10]. It has been reported that levels of miRNAs are stable across the human circulatory system [11, 12]. MiRNAs have been studied widely as biomarkers for several diseases. MiR-146a, miR-150, miR-4772-5p, miR-15a, miR-223, and miR-499-5p are potential biomarkers for sepsis in human serum/plasma, and down-regulation of miR-574-5p has been shown to be associated with higher risk of mortality [13–18]. However, there has been little study of miRNAs for SIC, according to Wang et al. Some miRNAs have been found to be related to sepsis prognosis. Among these, serum miR-122 was found to be related to coagulation disorder in sepsis patients. Thus, studies on miRNAs in association with SIC are sorely needed.
Lipocalin-2, also called neutrophil gelatinase-associated lipocalin (NGAL), is a 25 kD protein secreted by human neutrophils. Lipocalin-2 is widely used as an index in clinical settings. It can be detected in serum and plasma samples easily and quickly in many inflammatory and metabolic disorders. Its relationship with sepsis has been studied extensively. It has been used as a biomarker for sepsis-associated acute kidney injury and severe sepsis prognosis [19]. One recent study revealed its regulatory role in gut-origin sepsis [20]. Another study indicated that plasma lipocalin-2 levels are elevated in DIC patients and related to disease prognosis [21]. Though the relationship of lipocalin-2 with sepsis and DIC has been studied, no study has yet evaluated its relationship with SIC.
In this study, we sequenced plasma microRNA in sepsis-alone and SIC patients. Four microRNAs were found to be differently expressed including hsa-mir-92a-3p. In the subsequent PCR validation, we observed the hsa-mir-92a-3p expression level was consistent with sequencing results and was associated with plasma lipocalin-2 levels. We then explored the potential of hsa-mir-92a and lipocalin-2 as disease-specific markers.
Methods
Study design and patient characteristics
Patients were diagnosed with sepsis according to “The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)” [1]. Exclusion criteria were as follows: ① younger than 18 years old, ② immunocompromised status, ③ pregnancy, ④ malignancy, ⑤ massive transfusion within 24 h of sample collection.
A total of 116 patients (49 SIC and 67 sepsis-alone) were divided into two groups: SIC group and sepsis-alone (SA) group. SIC was defined as proposed by Iba et al. in 2017 [22]: SIC scores no less than 4 points with total score of prothrombin time and coagulation exceeding 2 points. The SIC score system (Table 1) consisted of three parts: Prothrombin time, which was assessed using PT-INR value; coagulation, assessed by platelet count; and total SOFA, calculated using 4 items (respiratory SOFA, cardiovascular SOFA, hepatic SOFA, and renal SOFA). The SIC score system was proved to be closely correlated with mortality rate. It took the SOFA score into consideration, which was tailored for sepsis-associated coagulopathy [22–24].
Table 1.
Score system for sepsis-induced coagulopathy
| Category | Parameter | 0 | 1 | 2 |
|---|---|---|---|---|
| Prothrombin time | PT-INR | ≤1.2 | > 1.2 | > 1.4 |
| Coagulation | Platelet count (× 109/L) | ≥150 | < 150 | < 100 |
| Total SOFA | SOFA four items | 0 | 1 | ≥2 |
SOFA Sequencing Organ Failure Assessment
Blood sample and plasma MiRNA isolation
Blood samples were collected from all patients within 24 h of admission to the intensive care unit (ICU) and centrifuged at 3000 rpm for 15 min at room temperature. The supernatant was transferred to Eppendorf tubes and stored at − 80 °C until miRNA extraction. Total plasma miRNA was isolated using a serum/plasma miRNA isolation and extraction kit (Tiangen, Beijing, China) according to the manufacturer’s instructions.
Hiseq sequencing and target MiRNA selection
The plasma samples of 15 SIC patients and 15 SA patients were pooled in two separate groups. Total miRNA were isolated using miRcute miRNA extraction and isolation kit (Tiangen, Beijing, China); gene sequencing was completed by the Beijing Genomics Institute (BGI, Beijing, China).
MiRNAs selected for further validating all met the following two criteria (1) over 20 read numbers; (2) log2Ratio ≥ 2 [25, 26].
Target MiRNA validation via qRT-PCR
CDNA was synthesized using a miRcute Enhanced miRNA cDNA First-line Synthesis Kit (kr211) and real-time qPCR was performed with a SYBR Green miRNA assay (Tiangen, Beijing, China). Briefly, 8 μl of isolated miRNA was added to a 20 μl system, 42 °C for 60 min followed by 95 °C 3 min for reverse transcription. Two μl of cDNA was added to a 20-μl system tested with ABI 7500 Real-time PCR System in duplication. The program was set according to the manufacture’s instruction.
Plasma Lipocalin-2 assessment
Lipocalin-2 levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Life Technologies, CA, US) with a range of 7.81 to 500 pg/ml. Concentration was determined from standard curves. ELISA was performed in duplicate according to the manufacturer’s instructions.
Statistical analysis
Expression levels of selected miRNAs detected by qRT-PCR are here presented with raw Ct values. Results for normally distributed continuous variables were given as means±standard errors and compared between groups using Student’s t tests. Results for non-normally distributed continuous variables are summarized as medians (interquartile ranges) and compared using the Mann-Whitney U tests. Pearson’s analysis was used to evaluate the relationships between pairs of variables. Statistical significance was set at P < 0.05. SPSS 20.0 software was used for all statistical analyses.
A binary logistic regression model was generated after the following hypothesis was tested: ① There is a linear relationship between the continuous independent variable and the logit conversion value of the dependent variable; ② There is no multicollinearity between independent variables; ③ There are no obvious outliers, leverage points, or strong influence points. The variables that showed statistically significant differences in univariate analysis were entered into the binary logistic regression model. Variables were excluded for existence of multicollinearity between independent variables. Finally, six variables were taken into the final analysis as shown in the results.
Results
Patients
A total of 116 sepsis patients were included between Nov 2014 and Aug 2017 from emergency intensive care units, respiratory intensive care units, and surgery intensive care units of Beijing Chao-Yang Hospital. Eighty-six patients were included for the validation set and were divided into SIC group (n = 34) and SA group (n = 52). Patients’ clinical characteristics are presented in Table 2. Patients from the two groups were matched by sex (P = 0.983), age (P = 0.316) and disease severity measured by Acute Physiology and Chronic Health Evaluation (APACHE) II scores (P = 0.635). Sources of infection causing sepsis included pulmonary infection, biliary and pancreatic infection, peritonitis, urinary infection, and cerebral infection. There were significantly more cases of pulmonary infection (P < 0.001) and peritonitis (P < 0.001) in the SIC group than in the SA group. Patients were matched for other causes: biliary system and pancreatic infection (P = 0.276), urinary infection (P = 0.380), and cerebral infection (P = 1.000). Most global coagulation tests differed significantly between the two groups except D-dimer (P = 0.097) and thrombin time (TT) (P = 0.101). There was no difference between the two groups with respect to creatinine (P = 0.575) or blood urea nitrogen (BUN) (P = 0.299) levels.
Table 2.
Patients’ clinical characteristics for validation set (n = 86)
| Variables | Sepsis-induced coagulopathy (n = 34) | Sepsis alone (n = 52) | P-value |
|---|---|---|---|
| Sex, F/M | 61.90% | 62.50% | P = 0.983a |
| Age, years | 59.56 ± 17.131 | 63.48 ± 17.927 | P = 0.316b |
| Source of infection% | |||
| Pulmonary | 58.00% (29) | 42.00% (21) | P < 0.001a |
| Biliary system and pancreatic | 45.50% (5) | 54.50% (6) | P = 0.276a |
| Peritonitis | 78.90% (15) | 21.10% (4) | P < 0.001a |
| Urinary system | 60.00% (3) | 40.00% (2) | P = 0.380a |
| Cerebral | 0% (0) | 100% (1) | P = 1.000a |
| SOFA scores | 6.68 ± 3.67 | 6.04 ± 3.16 | P = 0.393b |
| APACHE II scores | 17.35 ± 7.88 | 16.60 ± 6.73 | P = 0.635b |
| 28-day mortality% | 67.7% (21) | 32.3% (10) | P < 0.001a |
| PLT (×10^9/L) | 108.03 ± 91.99 | 211.10 ± 96.93 | P < 0.001b |
| D-dimer (mg/dL) | 7.12 (3.00, 18.24) | 4.60 (2.38, 8.64) | P < 0.001c |
| APTT (s) | 44.80 (38.10, 61.10) | 35.75 (31.70, 43.23) | P < .001c |
| PT (s) | 14.90 (13.48, 17.33) | 12.75 (11.53, 13.88) | P < 0.001c |
| PA% | 66.53 ± 17.89 | 81.10 ± 11.98 | P < 0.001c |
| INR | 1.26 (1.16, 1.47) | 1.11 (1.03, 1.18) | P < 0.001c |
| Fbg (mg/dL) | 275.13 ± 136.45 | 421.50 ± 173.30 | P < 0.001b |
| TT (s) | 20.00 (18.28, 24.88) | 19.10 (17.98, 20.70) | P = 0.055c |
| Cr (mmol/L) | 75.65 (52.90, 137.20) | 74.25 (53.80, 127.65) | P = 0.575c |
| BUN (mmol/L) | 10.12 (5.78, 14.07) | 8.54 (5.06, 12.78) | P = 0.299c |
aχ2 test, bStudent’s t test, cMann-Whitney U test, SOFA Sequential organ failure assessment, APACHE II Acute physiology and chronic health evaluation, PLT Platelet, APTT activated partial thromboplastin time, PT Prothrombin time, PA Prothrombin activity, INR International normalized ratio, Fbg Fibrinogen, TT Thrombin Time, Cr Creatine, BUN Blood urea nitrogen
Another 30 patients (15 SIC patients and 15 SA patients) were included for gene sequencing, demographic variables were presented in Table 3.
Table 3.
Patients’ clinical characteristics for gene sequencing (n = 30)
| Variables | Sepsis-induced coagulopathy (n = 15) | Sepsis alone (n = 15) | P-value |
|---|---|---|---|
| Sex, F/M | 4/11 | 8/7 | P = 0.136a |
| Age, years | 62.60 ± 13.91 | 65.13 ± 20.40 | P = 0.694b |
| Cause of sepsis% | |||
| Pulmonary infection | 75% (9) | 25% (3) | P = 0.025a |
| Biliary system and pancreatic | 20% (1) | 80% (4) | P = 0.330a |
| Peritonitis | 40% (4) | 60% (6) | P = 0.439a |
| Urinary system | 50% (1) | 50% (1) | P = 1.000a |
| Cerebral | 0% (0) | 100% (1) | P = 1.000a |
| SOFA scores | 9.00 ± 4.33 | 6.21 ± 3.39 | P = 0.060b |
| APACHE II scores | 20.20 ± 8.19 | 15.59 ± 7.77 | P = 0.125b |
| PLT (×10^9/L) | 131.20 ± 87.78 | 156.87 ± 76.27 | P = 0.400b |
| D-dimer (mg/dL) | 13.94 ± 12.41 | 5.72 ± 5.49 | P = 0.026b |
| APTT (s) | 50.80 (39.70, 60.70) | 34.90 (33.40, 53.20) | P = 0.062c |
| PT (s) | 17.00 (14.20, 20.10) | 12.90 (12.50, 18.00) | P = 0.022c |
| PA% | 62.15 ± 19.45 | 70.51 ± 14.57 | P = 0.193b |
| INR | 1.51 ± 0.54 | 1.30 ± 0.29 | P = 0.197b |
| Fbg (mg/dL) | 294.60 (140.60, 340.80) | 310.30 (254.50, 533.40) | P = 0.262c |
| TT (s) | 21.50 (19.20, 114.45) | 23.00 (18.70, 282.90) | P = 0.787c |
| Cr (mmol/L) | 137.40 (74.05, 232.75) | 129.56 (77.80, 301.15) | P = 0.967c |
| BUN (mmol/L) | 11.32 ± 6.49 | 11.45 ± 6.38 | P = 0.959b |
aχ2 test, bStudent’s t test, cMann-Whitney U test, SOFA Sequential organ failure assessment, APACHE II Acute physiology and chronic health evaluation, PLT Platelet, APTT Activated partial thromboplastin time, PT Prothrombin time, PA Prothrombin activity, INR International normalized ratio, Fbg Fibrinogen, TT Thrombin Time, Cr Creatine, BUN Blood urea nitrogen
Hsa-mir-92a-3p expressed differently between SIC group and SA group
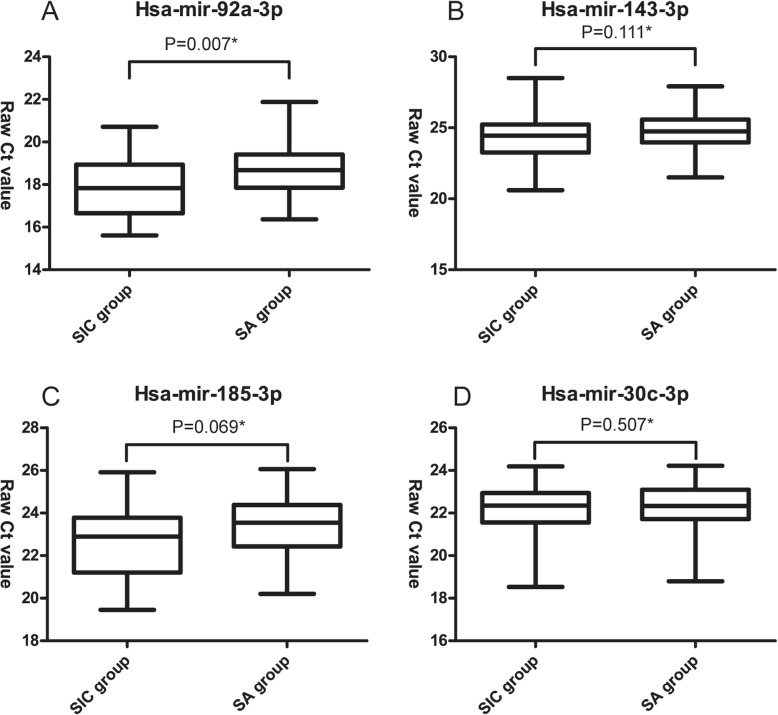
According to the results of gene sequencing, we chose hsa-miR-143-3p, hsa-miR-185-3p, hsa-miR-92a-3p, and hsa-miR-30c-3p for further measurement; mean reads and log2 ratio of four miRNAs are shown in Table 4. The Student’s t test revealed significance only for level of hsa-mir-92a-3p: SIC group vs. SA group (17.86 ± 1.44 vs. 18.66 ± 1.21, P = 0.007) (Fig. 1).
Table 4.
Differently expressed miRNAs in miRNAs sequencing
| miR_name | SA Reads | SIC Reads | log2Ratio | P-value | q-value |
|---|---|---|---|---|---|
| hsa-miR-92a-3p | 9184 | 9651 | −4.96 | 0 | 0 |
| hsa-miR-143-3p | 5959 | 9641 | −4.33 | 0 | 0 |
| hsa-miR-30c-5p | 645 | 335 | −5.97 | 0 | 0 |
| hsa-miR-185-5p | 663 | 2718 | −2.99 | 0 | 0 |
miR microRNA, SA Sepsis Along, SIC Sepsis Induced Coagulopathy
Fig. 1.
Comparisons of the levels of four miRNAs between SIC group (n = 34) and SA group (n = 52). a. Comparison of plasma hsa-mir-92a-3p between SIC and SA group, b. Comparison of plasma hsa-mir-143-3p between SIC and SA group, c. Comparison of plasma hsa-mir-185-3p between SIC and SA group, d. Comparison of plasma hsa-mir-30c-3p between SIC and SA group, * by Student’s t test, SIC = Sepsis-Induced Coagulopathy, SA = Sepsis-Along
Plasma Hsa-mir-92a level related to global coagulation index
We recorded patients’ global coagulation tests results including D-dimer, activated partial thromboplastin time (APTT), prothrombin activity (PA), fibrinogen (FBG), and thrombin time (TT). Statistical analysis revealed a strong relationship between hsa-mir-92a and PA (Pearson’s correlation coefficients = 0.266, P = 0.013) and hsa-mir-92a and APTT (Pearson’s correlation coefficients = − 0.325, P = 0.002).
Plasma Hsa-miR-92a level related to plasma Lipocalin-2 level
We measured plasma lipocalin-2 levels and found plasma lipocalin-2 expression to differ between the SIC and SA groups (246.05 ± 169.95 pg/ml vs. 168.84 ± 105.30 pg/ml, P = 0.011) (Fig. 2) and to be related to plasma hsa-miR-92a level (Pearson’s correlation coefficients = − 0.282, P = 0.009).
Fig. 2.
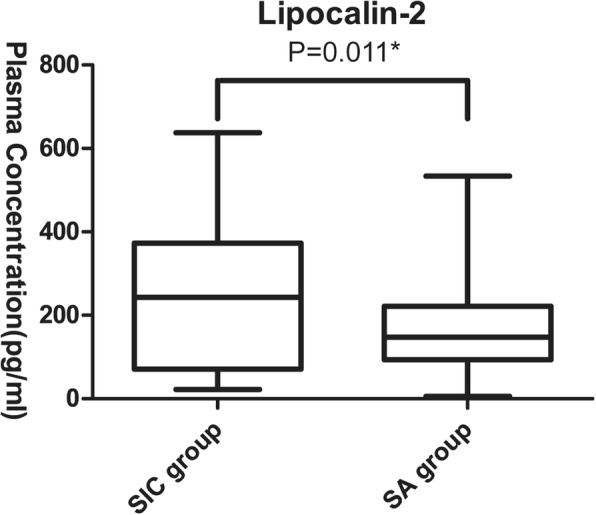
Comparisons of the levels of lipocalin-2 between SIC group (n = 34) and SA group (n = 52). * Student’s t test, SIC = Sepsis-Induced Coagulopathy, SA = Sepsis-Along
Diagnostic values of Hsa-miR-92a and Lipocalin-2
Results showed hsa-miR-92a and lipocalin-2 could be used to distinguish sepsis-induced coagulopathy from sepsis-alone patients. To compare the diagnostic values of these two biomarkers, receiver operating characteristic (ROC) curves were generated and area under the curve (AUC) was calculated. Hsa-miR-92a had an AUC of 0.660 (P = 0.011, 95% CI, 0.537, 0.782) in predicting the absence of SIC whereas lipocalin-2 had an AUC of 0.619 (P = 0.084, 95% CI, 0.485, 0.752) in predicting SIC (Fig. 3, Fig. 4). When the cutoff point was set at 261.01 pg/mL, lipocalin-2 had the highest specificity of 84.6% (Table 5).
Fig. 3.
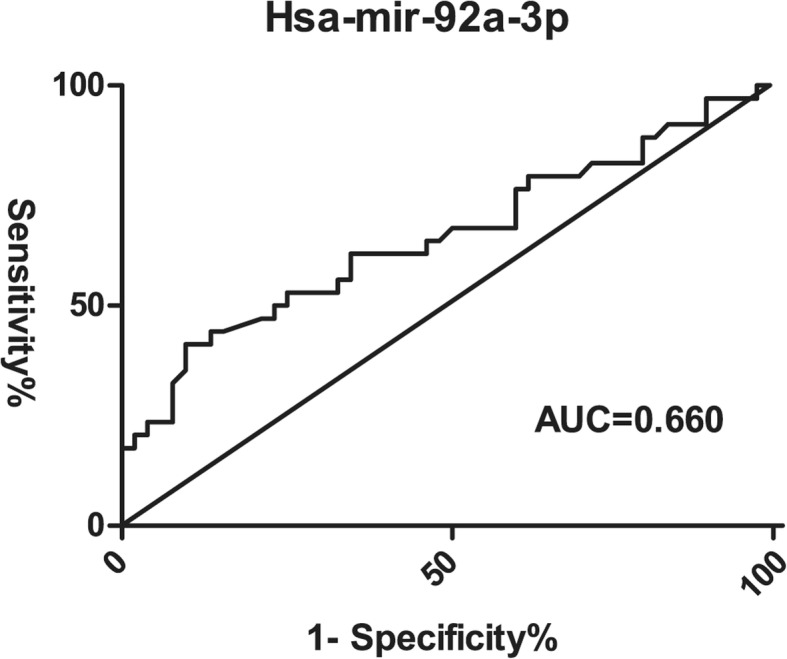
Receiver operating characteristic curves of hsa-mir-92a-3p for SIC group (n = 34) and SA group (n = 52). AUC = Area Under Curve
Fig. 4.
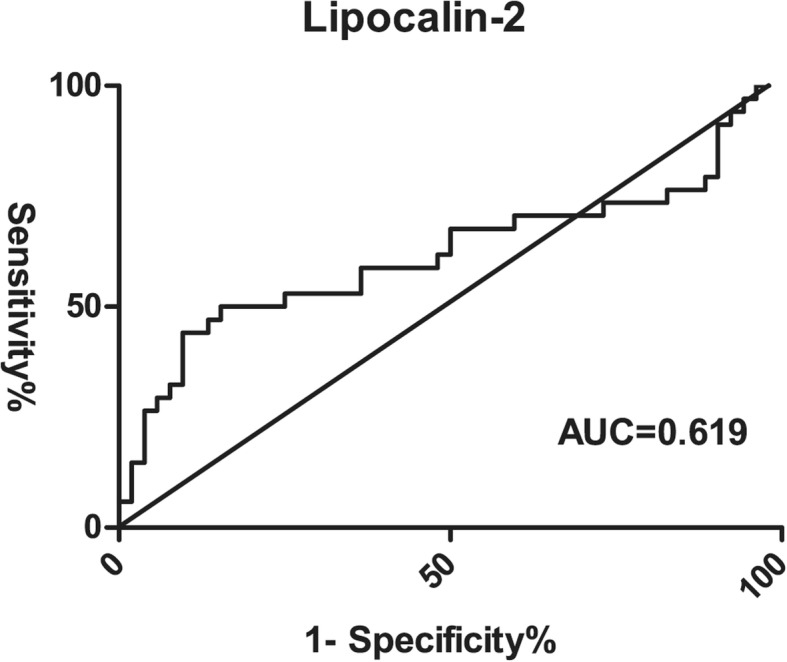
Receiver operating characteristic curves of lipocalin-2 for SIC group (n = 34) and SA group (n = 52). AUC = Area Under Curve
Table 5.
Diagnostic values of has-miR-92a and lipocalin-2
| Biomarker | AUC (95% CI) | Cutoff point | Sensitivity% | Specificity% |
|---|---|---|---|---|
| Hsa-miR-92a | 0.660 (0.537, 0.782) | 17.18 | 41.20% | 58.80% |
| lipocalin-2 | 0.619 (0.485, 0.752) | 261.01 pg/mL | 50% | 84.60% |
| Hsa-miR-92a + lipocalin-2 | 0.687 (0.578, 0.782) | 0.46139 | 52.94% | 84.62% |
AUC Area under curve
We then generated the ROC for a combination of hsa-mir-92a and lipocalin-2 (Fig. 5). The AUC for hsa-mir-92a + lipocalin-2 was 0.687 (P = 0.003, 95% CI, 0.578, 0.782).
Fig. 5.
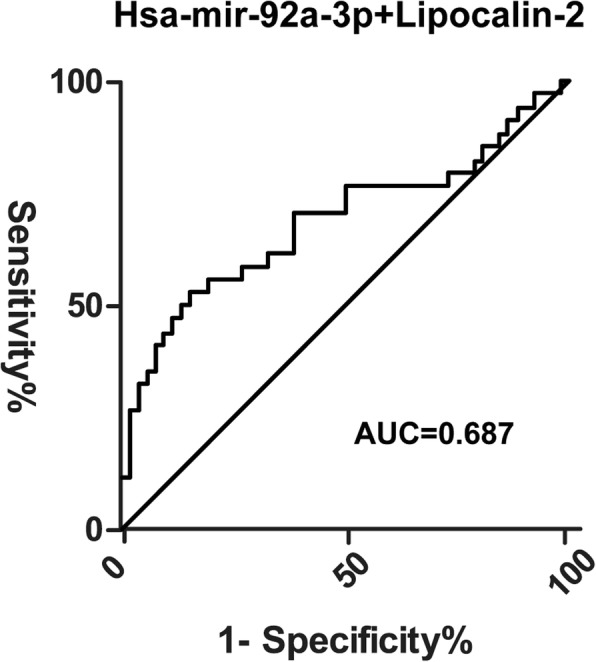
Receiver operating characteristic curves of hsa-mir-92a-3p and lipocalin-2 for SIC group (n = 34) and SA group (n = 52). AUC = Area Under Curve
Risk factors for Sepsis-induced coagulopathy
As shown, hsa-mir-92a was differently expressed between SIC and SA groups. Because we hypothesized sepsis accompanied by coagulation disorder indicated more disease severity, APACHEII score was also taken into analysis. The analysis of basic information indicated a difference in the number of patients with each specific source of pulmonary infection and peritonitis; these variables were taken into a binary logistic regression model.
Finally, we used a binary logistic regression model to assess the influence of the plasma level of hsa-mir-92a, APACHEII score, pulmonary infection and peritonitis, D-dimer level, and fibrinogen for SIC. There were linear relationships between dependent variables and covariates and one case with a ZResid of 3.386 times the standard deviation. These were analyzed further. The model was significant, 0.531 for Hosmer and Lemeshow goodness of fit test. Among the six independent variables included in the model, mir-92a and fibrinogen had statistical significance. For each unit of increase in plasma mir-92a level, the risk of SIC decreased by 32.4%, and for each unit of increase in plasma fibrinogen level, the risk of SIC decreased by 0.7%. The result and odds ratio with its 95% CI are given in Table 6.
Table 6.
Risk factors for sepsis-induced coagulopathy
| 95% CI | |||||
|---|---|---|---|---|---|
| Variables | DF | P value | OR | Lower | Upper |
| Hsa-mir-92a | 1 | 0.035 | 0.658 | 0.446 | 0.970 |
| Fibrinogen | 1 | 0.001 | 0.993 | 0.989 | 0.997 |
| D-dimer | 1 | 0.093 | 1.050 | 0.992 | 1.111 |
| APACHEII | 1 | 0.937 | 1.003 | 0.932 | 1.080 |
| Pulmonary Infection | 1 | 0.402 | 0.600 | 0.182 | 1.979 |
| Peritonitis | 1 | 0.857 | 0.853 | 0.150 | 4.856 |
| Constant | 1 | 0.019 | 10,504.227 | ||
The binary logistic model included six variables: plasma level of hsa-mir-92a, Fibrinogen, D-dimer, APACHE II score, pulmonary infection and Peritonitis. APACHEII Acute physiology and chronic health evaluation, DF Degree of freedom, OR Odds ratio, CI Confident interval
Discussion
Sepsis is a life-threatening situation. It is often accompanied by coagulation disorder, and it can leave clinicians stymied in the attempt to identify the optimum treatment. It would be useful to find new biomarkers and therapeutic targets. In this study, we found novel biomarkers for SIC. After Hiseq sequencing, we located four differently expressed miRNAs in human plasma and validated our findings in a larger sample. Plasma lipocalin-2 was measured as well. We found significant differences in both hsa-mir-92a and lipocalin-2 levels between SIC and SA groups. There were higher plasma levels of hsa-mir-92a in the SA group than in the SIC group, but the higher plasma lipocalin-2 level indicated a greater possibility of SIC. We also found a strong relationship between hsa-mir-92a and prothrombin activity, activated partial thromboplastin time, and lipocalin-2.
MiR-92a has been widely studied in tumorigenesis and metastasis of several different kinds of tumors. MiR-92a is significantly up-regulated in colon rectal cancer tissues and correlated with metastases and poor prognosis [27]. It also participates in gene regulation processes in other kinds of tumors including non-Hodgkin’s lymphoma, acute lymphoid leukemia, multiple myeloma, breast cancer, lung cancers, hepatocellular carcinoma, and esophageal squamous cell carcinoma, acting as both tumor suppressor and oncogene [28–34].
Mir-92a was found to be involved in angiogenesis and endothelial cell function [35]. Bonauer et al. found that human endothelial cells (ECs) express the miR-17–92 cluster, particularly its member miR-92a. Forced overexpression of miR-92a in human ECs impairs EC function, and inhibition of miR-92a can enhance vessel growth. One in vivo study with a mouse hind limb ischemia model revealed that the number of capillaries and smooth muscle actin-positive arterioles increases after antagomir-92a treatment. In searching for possible pathways and putative target genes for mir-92a, the mRNAs for integrin subunits ɑ5 (ITGA5) and ɑv, sphingosine-1-phosphate receptor 1 (S1PR1), and mitogen-activated kinase 4 (MKK4) were found to show less expression in response to miR-92a overexpression. Further study on downstream pathways by silencing ITGA5 expression, resembling the effect of miR-92a on mRNA expression, revealed the expression levels of a second group of genes might be secondarily regulated as a consequence of ITGA5 downregulation. These genes include CD31, whose product is a platelet/endothelial cell adhesion molecule, and VWF, whose product is a Von Willebrand factor. The mir-92a-ITGA5-VWF axis could lead to low expression of Von Willebrand factor and cause instability of FVIII, finally resulting in prolonged APTT.
Lipocalin-2 has been studied as a clinical biomarker for DIC in its different forms: plasma free and total lipocalin-2 levels were significantly higher in patients with overt-DIC than in those without overt-DIC. The free lipocalin-2 level also showed a significant prognostic value in DIC [36]. In the search for a possible regulatory mechanism for mir-92a and lipocalin-2, studies have focused on apoptotic cell-derived sphingosine-1-phosphate (S1P), which induces the production and release of lipocalin-2 by human macrophages through the S1P-S1PR1-signal transducer and activator of transcription 3 (STAT3)-lipocalin-2 axis in lymphangiogenesis and tumor metastasis [37, 38]. Mir-92a has been confirmed to play a role in the regulation of S1PR1 [35]. Increased mir-92a could lead to downregulation of S1PR1 and upregulation of lipocalin-2. However, this model still needs confirmation.
There are limitations to this study. First, without a basal level of miRNA expression in healthy controls, we could not determine whether the differently expressed miRNAs also differed between the SIC group and healthy controls in the same way. Second, because no optimum normalizer exists for such critical conditions as sepsis and severe infection [39], raw Ct value was taken into further analysis, which can cause bias due to individual difference and different sample degeneration states.
Conclusions
In this work, we evaluated differently expressed miRNAs between SA and SIC patients. After validation, plasma hsa-mir-92a was confirmed as a diagnostic biomarker for sepsis-induced coagulopathy and related to global coagulation index and plasma lipocalin-2 level. Further study is needed to confirm the regulatory role of hsa-mir-92a-3p in coagulation disorders and assess therapeutic potency.
Acknowledgements
Not applicable.
Abbreviations
- APACHEII
Acute Physiology and Chronic Health Evaluation
- APTT
Activated Partial Thromboplastin Time
- AUC
Area Under Curve
- BGI
Beijing Genomics Institute
- BUN
Blood Urea Nitrogen
- CI
Confidence Interval
- DIC
Disseminated Intravascular Coagulation
- ECs
Endothelial Cells
- ELISA
Enzyme-Linked Immunosorbent Assay
- FBG
Fibrinogen
- ICU
Intensive Care Unit
- INR
International Normalized Ratio
- ITGA5
Integrin Subunits ɑ5
- miRNAs
MicroRNAs
- MKK4
Mitogen-activated Kinase Kinase 4
- NGAL
Neutrophil Gelatinase-Associated Lipocalin
- PA
Prothrombin Activity
- PT
Prothrombin Time
- qRT-PCR
Quantitative Reverse Transcription-Polymerase Chain Reaction
- ROC
Receiver Operating Characteristic
- S1P
Sphingosine-1-Phosphate
- S1PR1
Sphingosine-1-Phosphate Receptor 1
- SA
Sepsis-Along
- SIC
Sepsis-Induced Coagulopathy
- SOFA
Sequential Organ Failure Assessment
- STAT3
Signal Transducer and Activator of Transcription 3
- TT
Thrombin Time
- VWF
Von Willebrand Factor
Authors’ contributions
YW was in charge of study design, data analysis, and was a major contributor in writing the manuscript and experiment operation. HW helped with literature retrieval and revised the manuscript. CZ participated in design of the study and literature search. RG performed the literature search, data analysis and revised the manuscript. CFZ participated in conducting of Enzyme-linked immunosorbent assay. HY helped to revise the manuscript. ZT helped with study design and revised the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (NO. 81500003), Beijing Natural Science Foundation of China (NO. 7172084), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ID: ZYLX201312) and the Beijing Municipal Administration of Hospitals’ Ascent Plan (ID: DFL20150302). The funders mentioned above financially sponsored this research in reagents, equipments, literature searching and also covered article processing fee.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by Ethics Committee of the Beijing Chaoyang Hospital. All of the patients gave their written informed consents.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yishan Wang and Huijuan Wang contributed equally to this work.
References
- 1.Singer M, Deutschman CS, Seymour CW. The third international consensus definitions for Sepsis and septic shock (Sepsis-3) Jama. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 3.Dhainaut JF, Yan SB, Joyce DE. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004;2(11):1924–1933. doi: 10.1111/j.1538-7836.2004.00955.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamakawa K, Umemura Y, Hayakawa M. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20(1):229. doi: 10.1186/s13054-016-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iba T, Thachil J. Present and future of anticoagulant therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation: a perspective from Japan. Int J Hematol. 2016;103(3):253–261. doi: 10.1007/s12185-015-1904-z. [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa M, Yamakawa K, Saito S. Recombinant human soluble thrombomodulin and mortality in sepsis-induced disseminated intravascular coagulation. A multicentre retrospective study. Thromb Haemost. 2016;115(6):1157–1166. doi: 10.1160/TH15-12-0987. [DOI] [PubMed] [Google Scholar]
- 7.Tagami T, Matsui H, Horiguchi H. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2014;12(9):1470–1479. doi: 10.1111/jth.12643. [DOI] [PubMed] [Google Scholar]
- 8.Iba T, Gando S, Thachil J. Anticoagulant therapy for sepsis-associated disseminated intravascular coagulation: the view from Japan. J Thromb Haemost. 2014;12(7):1010–1019. doi: 10.1111/jth.12596. [DOI] [PubMed] [Google Scholar]
- 9.Nishida O, Ogura H, Egi M. The Japanese clinical practice guidelines for Management of Sepsis and Septic Shock 2016. Acute Med Surg. 2018;5(1):3–89. doi: 10.1002/ams2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagos-Quintana M, Rauhut R, Lendeckel W. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell PS, Parkin RK, Kroh EM. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Ba Y, Ma L. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 13.Ma Y, Vilanova D, Atalar K. Genome-wide sequencing of cellular microRNAs identifies a combinatorial expression signature diagnostic of sepsis. PLoS One. 2013;8(10):e75918. doi: 10.1371/journal.pone.0075918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HJ, Zhang PJ, Chen WJ. Evidence for serum miR-15a and miR-16 levels as biomarkers that distinguish sepsis from systemic inflammatory response syndrome in human subjects. Clin Chem Lab Med. 2012;50(8):1423–1428. doi: 10.1515/cclm-2011-0826. [DOI] [PubMed] [Google Scholar]
- 15.Wang HJ, Zhang PJ, Chen WJ. Four serum microRNAs identified as diagnostic biomarkers of sepsis. J Trauma Acute Care Surg. 2012;73(4):850–854. doi: 10.1097/TA.0b013e31825a7560. [DOI] [PubMed] [Google Scholar]
- 16.Wang JF, Yu ML, Yu G. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun. 2010;394(1):184–188. doi: 10.1016/j.bbrc.2010.02.145. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Wang HC, Chen C. Differential expression of plasma miR-146a in sepsis patients compared with non-sepsis-SIRS patients. Exp Ther Med. 2013;5(4):1101–1104. doi: 10.3892/etm.2013.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Meng K, Chen W. Serum miR-574-5p: a prognostic predictor of sepsis patients. Shock. 2012;37(3):263–267. doi: 10.1097/SHK.0b013e318241baf8. [DOI] [PubMed] [Google Scholar]
- 19.Kjeldsen L, Johnsen AH, Sengelov H. Isolation and primary structure of lipocalin-2, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268(14):10425–10432. [PubMed] [Google Scholar]
- 20.Lu F, Inoue K, Kato J. Functions and regulation of lipocalin-2 in gut-origin sepsis: a narrative review. Crit Care. 2019;23(1):269. doi: 10.1186/s13054-019-2550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang W, Zhu S, Pan C. Predictive utilities of neutrophil gelatinase-associated lipocalin (NGAL) in severe sepsis. Clin Chim Acta. 2018;481:200–206. doi: 10.1016/j.cca.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Iba T, Nisio M, Levy J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9):e017046. doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umemura Y, Yamakawa K, Ogura H. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2016;14(3):518–530. doi: 10.1111/jth.13230. [DOI] [PubMed] [Google Scholar]
- 24.Ding R, Wang Z, Lin Y. Comparison of a new criteria for sepsis-induced coagulopathy and international society on thrombosis and Haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 3.0: a retrospective study. Blood Coagul Fibrinolysis. 2018;29(6):551–558. doi: 10.1097/MBC.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu R, Zhang C, Hu Z. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47(5):784–791. doi: 10.1016/j.ejca.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Zhu E, Zhao F, Xu G. MirTools: microRNA profiling and discovery based on high-throughput sequencing. Nucleic Acids Res. 2010;38:W392–W397. doi: 10.1093/nar/gkq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang G, Zhou H, Xiao H. MicroRNA-92a functions as an oncogene in colorectal cancer by targeting PTEN. Dig Dis Sci. 2014;59(1):98–107. doi: 10.1007/s10620-013-2858-8. [DOI] [PubMed] [Google Scholar]
- 28.Ohyashiki K, Umezu T, Yoshizawa S. Clinical impact of down-regulated plasma miR-92a levels in non-Hodgkin's lymphoma. PLoS One. 2011;6(2):e16408. doi: 10.1371/journal.pone.0016408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshizawa S, Ohyashiki JH, Ohyashiki M. Downregulated plasma miR-92a levels have clinical impact on multiple myeloma and related disorders. Blood cancer. 2012;2(1):e53. doi: 10.1038/bcj.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson S, Moller C, Jirstrom K. Downregulation of miR-92a is associated with aggressive breast cancer features and increased tumour macrophage infiltration. PLoS One. 2012;7(4):e36051. doi: 10.1371/journal.pone.0036051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin HY, Chiang CH, Hung WC. STAT3 upregulates miR-92a to inhibit RECK expression and to promote invasiveness of lung cancer cells. Br J Cancer. 2013;109(3):731–738. doi: 10.1038/bjc.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashita Y, Osada H, Tatematsu Y. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65(21):9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 33.Shigoka M, Tsuchida A, Matsudo T. Deregulation of miR-92a expression is implicated in hepatocellular carcinoma development. Pathol Int. 2010;60(5):351–357. doi: 10.1111/j.1440-1827.2010.02526.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen ZL, Zhao XH, Wang JW. MicroRNA-92a promotes lymph node metastasis of human esophageal squamous cell carcinoma via E-cadherin. J Biol Chem. 2011;286(12):10725–10734. doi: 10.1074/jbc.M110.165654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonauer A, Carmona G, Iwasaki M. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 36.In JW, Kim JE, Jeong JS. Diagnostic and prognostic significance of neutrophil gelatinase-associated lipocalin-2 in disseminated intravascular coagulation. Clin Chim Acta. 2014;430:145–149. doi: 10.1016/j.cca.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Syed SN, Jung M, Weigert A. S1P provokes tumor Lymphangiogenesis via macrophage-derived mediators such as IL-1beta or Lipocalin-2. Mediat Inflamm. 2017;2017:7510496. doi: 10.1155/2017/7510496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung M, Oren B, Mora J. Lipocalin-2 from macrophages stimulated by tumor cell-derived sphingosine 1-phosphate promotes lymphangiogenesis and tumor metastasis. Sci Signal. 2016;9(434):ra64. doi: 10.1126/scisignal.aaf3241. [DOI] [PubMed] [Google Scholar]
- 39.Benz F, Roderburg C, Vargas CD. U6 is unsuitable for normalization of serum miRNA levels in patients with sepsis or liver fibrosis. Exp Mol Med. 2013;45:e42. doi: 10.1038/emm.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.