Abstract
PURPOSE
KEYNOTE-164 (NCT02460198) evaluated the antitumor activity of pembrolizumab in previously treated, metastatic, microsatellite instability–high/mismatch repair–deficient (MSI-H/dMMR) colorectal cancer (CRC).
METHODS
This phase II open-label study involved 128 centers worldwide. Eligible patients were age ≥ 18 years and had metastatic MSI-H/dMMR CRC treated with ≥ 2 prior lines of standard therapy, including fluoropyrimidine, oxaliplatin, and irinotecan with or without anti–vascular endothelial growth factor/epidermal growth factor receptor monoclonal antibody (cohort A) or ≥ 1 prior line of therapy (cohort B). MSI-H/dMMR status was assessed locally. Patients received pembrolizumab 200 mg every 3 weeks for up to 2 years until progression, unacceptable toxicity, or withdrawal. The primary end point was objective response rate by RECIST version 1.1 by independent central review. Secondary end points were duration of response, progression-free survival (PFS), overall survival, safety, and tolerability.
RESULTS
A total of 124 patients with MSI-H/dMMR CRC (61 in cohort A, 63 in cohort B) enrolled. At data cutoff, median follow-up was 31.3 months (range, 0.2-35.6 months) for cohort A and 24.2 months (range, 0.1-27.1 months) for cohort B. Objective response rate was 33% (95% CI, 21% to 46%) and 33% (95% CI, 22% to 46%), respectively, with median duration of response not reached in either cohort. Median PFS was 2.3 months (95% CI, 2.1 to 8.1 months) and 4.1 months (95% CI, 2.1 to 18.9 months). Median overall survival was 31.4 months (95% CI, 21.4 months to not reached) and not reached (95% CI, 19.2 months to not reached). Treatment-related grade 3-4 adverse events occurred in 10 patients (16%) in cohort A and 8 (13%) in cohort B, with the most common occurring in ≥ 2 patients being pancreatitis, fatigue, increased alanine aminotransferase, and increased lipase (2 patients each; 3%) in cohort A.
CONCLUSION
Pembrolizumab is effective with a manageable safety profile in patients with MSI-H/dMMR CRC.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cause of cancer death, with an estimated 881,000 deaths worldwide.1 In the United States, 145,600 new cases and 51,020 deaths are estimated to occur in 2019.2 Approximately 5% of stage IV CRC is microsatellite instability-high (MSI-H) and results from accumulations of high levels of single-base mismatches or short insertions and deletions in repetitive DNA tracts as a result of deficiencies in DNA mismatch repair (dMMR).3 MSI-H/dMMR metastatic CRC often originates on the right side of the colon, is poorly differentiated, and is more closely associated with mutation in the BRAF gene than microsatellite-stable (MSS) CRC, all factors associated with poor outcomes.4,5 Typically, patients with MSI-H/dMMR metastatic CRC are less responsive to conventional chemotherapy and have a poorer prognosis than patients with mismatch repair-proficient or MSS CRC.6,7
Evidence has shown that MSI-H/dMMR tumors achieve durable responses to single-agent programmed death 1 (PD-1) blockade, regardless of tumor type, or in combination with cytotoxic T-lymphocyte–associated antigen-4 inhibitor in MSI-H/dMMR CRC.6-9 In an initial study of 41 patients with heavily pretreated MSI-H/dMMR CRC, MSI-H non-CRC, and MSS cancers, treatment with the PD-1 inhibitor pembrolizumab resulted in objective response rates (ORRs) of 40%, 71%, and 0% respectively.6 In an updated analysis in 78 evaluable patients, pembrolizumab provided an ORR of 52% in patients with MSI-H/dMMR CRC and 54% in patients with MSI-H non-CRC.7 Nivolumab has also demonstrated antitumor activity in MSI-H/dMMR CRC. In a phase II study, nivolumab provided a response rate of 31% and a 12-month overall survival (OS) rate of 73% in heavily pretreated patients with metastatic MSI-H/dMMR CRC, while its combination with the cytotoxic T-lymphocyte–associated antigen-4 inhibitor ipilimumab demonstrated a response rate of 55% and 12-month OS rate of 85%.8,9 Pembrolizumab is approved by the US Food and Drug Administration for the treatment of patients with MSI-H/dMMR solid tumors who experienced progression on prior treatment and had no satisfactory alternative treatment options, regardless of tumor site or histology, and for patients with MSI-H CRC who experienced progression after treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.10 Nivolumab (with or without ipilimumab) is currently approved for the treatment of patients with MSI-H/dMMR CRC tumors who experienced progression after treatment with a fluoropyrimidine, oxaliplatin, and irinotecan.11 Here, we present data from the KEYNOTE-164 study that evaluated the antitumor activity of pembrolizumab after ≥ 2 (cohort A) or ≥ 1 (cohort B) prior lines of therapy in patients with MSI-H/dMMR CRC.
METHODS
Study Design and Patients
KEYNOTE-164 is an international, phase II, open-label, nonrandomized, multicenter study of pembrolizumab in patients with previously treated, unresectable, locally advanced or metastatic MSI-H and/or dMMR CRC. MSI-H and/or dMMR status was verified by local polymerase chain reaction or immunohistochemistry testing. Eligible patients were age ≥ 18 years with previously treated, histologically proven, locally advanced, unresectable or metastatic MSI-H CRC who had undergone ≥ 2 prior lines of standard therapy that included fluoropyrimidine, oxaliplatin, and irinotecan with or without anti–vascular endothelial growth factor or epidermal growth factor receptor monoclonal antibodies (cohort A) and ≥ 1 prior line of systematic therapy (cohort B). Patients who withdrew from standard treatment and were ineligible for retreatment with the same therapies were eligible. In both cohorts, patients with prior adjuvant therapy were counted as having 1 prior line of therapy if that patient’s disease had progressed within 6 months after treatment. In addition, patients had an Eastern Cooperative Oncology Group performance status of 0 or 1, life expectancy > 3 months, ≥ 1 measurable lesion per RECIST version 1.1 (v1.1), and adequate organ function. Patients were excluded if they had prior monoclonal antibody, chemotherapy, targeted small-molecule therapy, or radiation therapy within 2 weeks of study start; prior anti-PD-1, programmed death ligand 1 (PD-L1), or PD-L2 therapy; active autoimmune disease; active malignancy requiring treatment; active infection requiring systemic treatment; known history of HIV, interstitial lung disease, or active noninfectious pneumonitis; and active hepatitis B or C virus infection. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent. The protocol and all amendments were approved by the institutional review board or ethics committee at each participating institution.
Procedures
All enrolled patients received intravenous pembrolizumab 200 mg every 3 weeks for up to 35 cycles (approximately 2 years) or until disease progression, unacceptable toxicity, or study withdrawal. Patients who discontinued treatment for reasons other than progression were followed until progression, initiation of a new anticancer therapy, withdrawal of consent, or loss to follow-up.
Tumor response was assessed every 9 weeks per RECIST v1.1 by independent central review. During follow-up, survival was assessed every 9 weeks. Patients who achieved a confirmed complete response (CR) and had received at least 8 cycles of pembrolizumab with ≥ 2 cycles beyond the date of CR had the option of discontinuing pembrolizumab or continuing study therapy. Eligible patients who stopped pembrolizumab after 35 cycles or who stopped after attaining a CR could resume pembrolizumab, at the discretion of the investigator, for an additional 17 cycles (approximately 1 year) after they had experienced radiographic progression. Adverse events (AEs), including serious and predefined AEs of clinical interest, were monitored throughout the study and for 30 days (90 days for serious AEs) after pembrolizumab discontinuation and were graded by investigators according to the National Cancer Institute CTCAE (version 4).
Outcomes
The primary end point was ORR (the proportion of patients with CR or partial response [PR]) as assessed by independent central radiology review per RECIST v1.1. Secondary end points were duration of response (DOR; time from first documented CR or PR until disease progression [PD] or death as a result of any cause, whichever occurred first), disease control rate (DCR; proportion of patients with CR + PR plus stable disease [SD] for ≥ 24 weeks before PD), progression-free survival (PFS; time from first study treatment to first documented PD or death, whichever occurred first), OS (time from first study treatment to death as a result of any cause), safety, and tolerability. Patients without documented death at data cutoff were censored at the date of last follow-up.
Statistical Analyses
Cohorts A and B were evaluated independently. In cohort A, for ORR (RECIST v1.1 by central review), with a sample size of 60 patients and assuming a true response rate of 35% with pembrolizumab, the study had a 93% power to reject the null hypothesis of a response rate of 15% with pembrolizumab, with a one-sided α of 2.5% at final analysis. The boundary for statistical success corresponded to an observed response rate of at least 26.7% with a one-sided α of 2.5%. In cohort B, with a sample size of 60 patients and at least 19 responders observed, the lower bound of the 95% CI would be > 20%. Point estimates and exact Clopper-Pearson CIs were provided for response rate and DCR (per RECIST v1.1 by central review) in both cohorts. Kaplan-Meier estimates were provided for DOR and PFS (per RECIST v1.1 by central review) and OS. Safety was assessed by descriptive analyses. The efficacy and safety analysis populations included all patients who received ≥ 1 dose of pembrolizumab. Statistical analyses were done using SAS 9.4 software (SAS Institute, Cary, NC).
Data Availability
Merck Sharp & Dohme’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone Web site or through e-mail to dataaccess@merck.com.
RESULTS
Patients
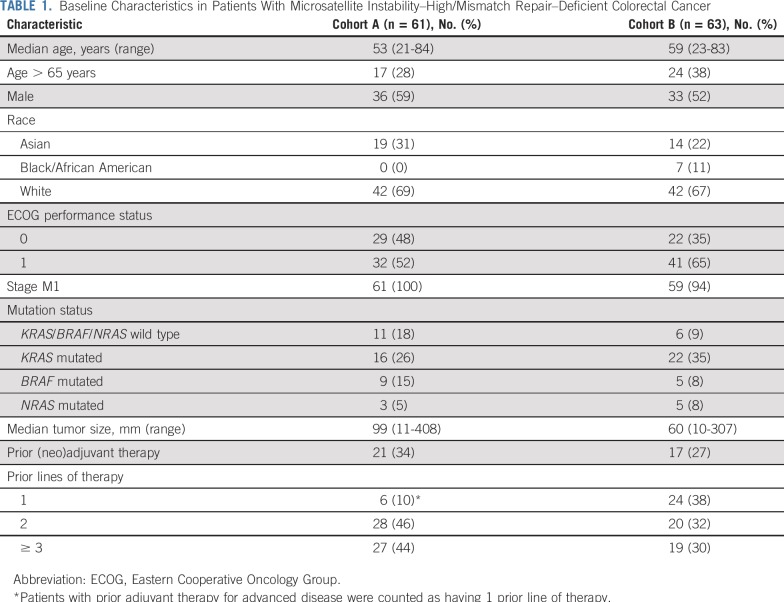
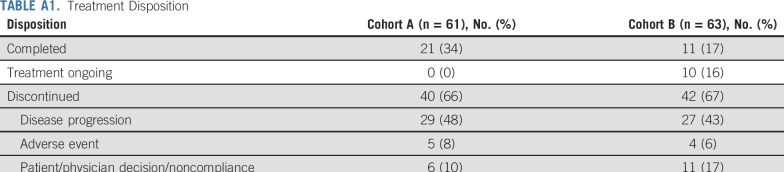
Between September 14, 2015, and September 12, 2017, 61 patients with advanced, metastatic MSI-H/dMMR CRC who received ≥ 2 prior therapies (cohort A) and 63 who received ≥ 1 prior therapy (cohort B) from 128 sites in 7 countries were enrolled. The median duration of follow-up was 31.3 months (range, 0.2-35.6 months) for patients in cohort A and 24.2 months (range, 0.1-27.1 months) for patients in cohort B. The patient characteristics in each cohort were consistent with those of a stage IV MSI-H/dMMR CRC population (Table 1). Of the 61 patients in cohort A and 63 patients in cohort B, all patients in cohort A and 59 (94%) in cohort B had stage M1 disease, and 27 (44%) patients in cohort A and 19 (30%) in cohort B had ≥ 3 prior therapies. As of the data cutoff date of September 4, 2018, all patients in cohort A had completed treatment, and treatment was ongoing in 10 patients (16%) in cohort B. A total of 40 patients (66%) in cohort A and 42 (67%) in cohort B discontinued treatment largely because of PD (Appendix Table A1, online only).
TABLE 1.
Baseline Characteristics in Patients With Microsatellite Instability–High/Mismatch Repair–Deficient Colorectal Cancer
Efficacy
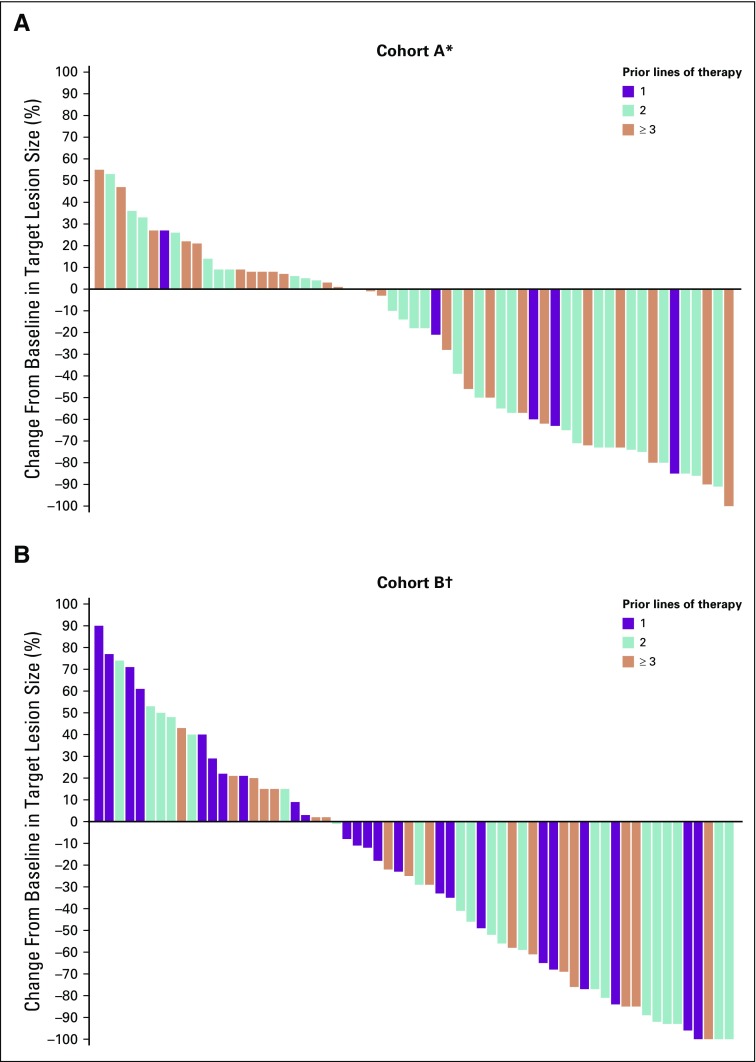
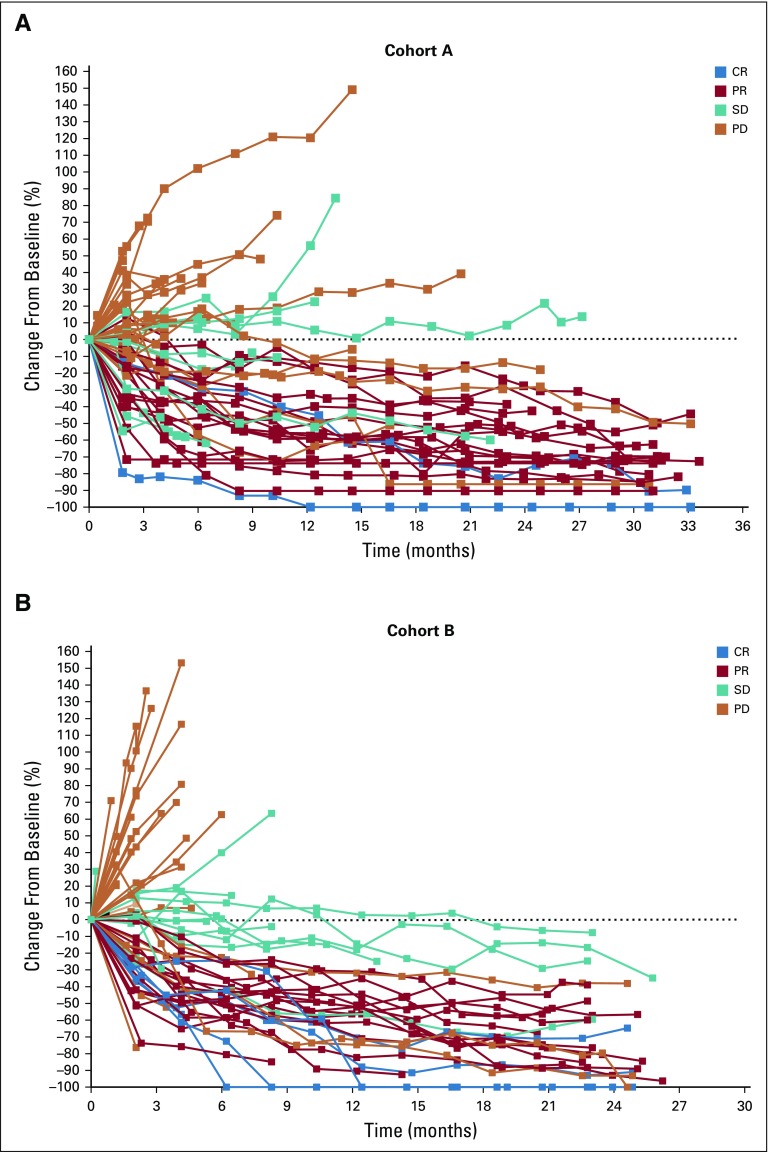
In cohort A, 20 (33%; 95% CI, 21% to 46%) of the 61 patients had a confirmed objective response (2 CRs and 18 PRs) per RECIST v1.1 by central review (Table 2). Eleven patients (18%; 95% CI, 9% to 30%) had SD with a DCR of 51% (95% CI, 38% to 64%). The median time to response was 4.3 months (range, 1.8-24.9 months), with the median DOR not reached (range, 6.2 to 31.3+ months). Eighty-five percent of responses were ongoing at analysis, and an estimated 95% of patients had a DOR of ≥ 12 months. Similarly, in cohort B, 21 (33%; 95% CI, 22% to 46%) of the 63 patients had a confirmed response (5 CRs and 16 PRs) per RECIST v1.1 by central review (Table 2), and 15 (24%; 95% CI, 14% to 36%) had prolonged SD (Appendix Fig A1, online only) with a DCR of 57% (95% CI, 44% to 70%). The median time to response was 3.9 months (range, 1.8-12.5 months), with the median DOR not reached (range, 4.4 to 23.6+ months). Seventy-six percent of responses were ongoing at time of analysis, and an estimated 95% of patients had a DOR of ≥ 12 months. Thirty-four (56%) of the 61 patients in cohort A and 39 (62%) of the 63 in cohort B had a reduction from baseline in target lesion size (Fig 1). Changes from baseline in tumor size showed a general reduction in tumor burden over time, including in some patients with SD (Appendix Fig A1).
TABLE 2.
Best Response (RECIST Version 1.1 by Central Review) in Patients With Microsatellite Instability–High/Mismatch Repair–Deficient Colorectal Cancer
FIG 1.
Best percent change from baseline in target lesion size (RECIST version 1.1 by central review) by prior lines of therapy in patients with microsatellite instability–high colorectal cancer in (A) cohort A and (B) cohort B. (*) Two patients in cohort A did not have postbaseline assessments and are not included. (†) One patient in cohort B did not have postbaseline assessments and is not included.
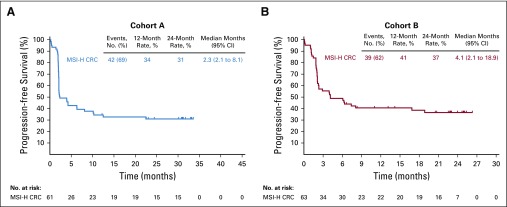
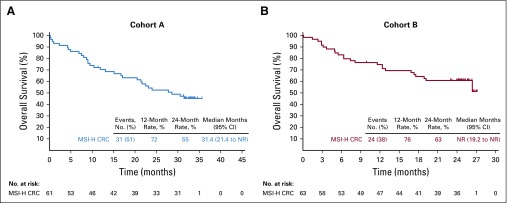
In the 61 patients of cohort A, there were 42 (69%) PFS events (RECIST v1.1 by central review) at data cutoff. The median PFS was 2.3 months (95% CI, 2.1 to 8.1 months) with estimated 12- and 24-month PFS rates of 34% and 31%, respectively (Fig 2A). In the 63 patients in cohort B, there were 39 (62%) PFS events at data cutoff. The median PFS was 4.1 months (95% CI, 2.1 to 18.9 months) with estimated 12- and 24-month PFS rates of 41% and 37%, respectively (Fig 2B). In cohort A, the median OS was 31.4 months (95% CI, 21.4 months to not reached) with estimated 12- and 24-month OS rates of 72% and 55%, respectively (Fig 3A). In cohort B, the median OS was not reached (95% CI, 19.2 months to not reached) with 12- and 24-month OS rates of 76% and 63%, respectively (Fig 3B).
FIG 2.
Kaplan-Meier estimates of progression-free survival (RECIST version 1.1 by central review) in patients with microsatellite instability–high colorectal cancer (MSI-H CRC) in (A) cohort A and (B) cohort B.
FIG 3.
Kaplan-Meier estimates of overall survival in patients with microsatellite instability–high colorectal cancer (MSI-H CRC) in (A) cohort A and (B) cohort B. NR, not reached.
Response Rate by Prior Lines of Therapy
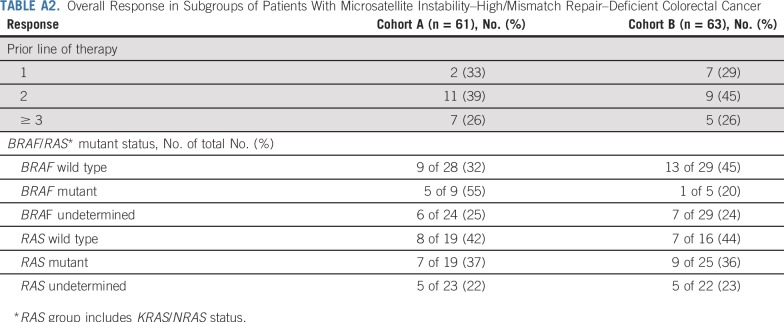
In cohort A, 6 patients (10%) had 1 prior therapy, 28 (46%) had 2 prior therapies, and 27 (44%) had ≥ 3 prior therapies (Table 1). Patients experienced a reduction from baseline in target lesion size regardless of number of prior therapies (Fig 1). In cohort A, responses were observed in 2 (33%) of 6 patients with 1 prior therapy, 11 (39%) of 28 with 2 prior therapies, and 7 (26%) of 27 with ≥ 3 prior therapies (Appendix Table A2, online only). In cohort B, 24 (38%), 20 (32%), and 19 (30%) patients had 1, 2, or ≥ 3 prior therapies, respectively, and patients experienced a reduction from baseline in target lesion size regardless of number of prior therapies. In cohort B, responses were observed in 7 (29%) of 24 patients with 1 therapy, 9 (45%) of 20 with 2 prior therapies, and 5 (26%) of 19 with ≥ 3 prior therapies (Appendix Table A2).
Response Rate by Mutation Status
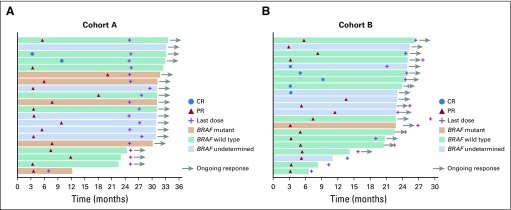
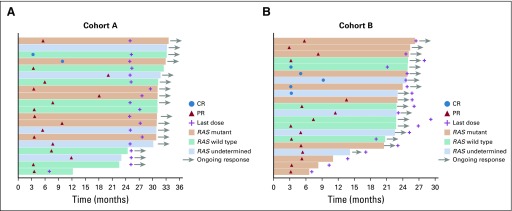
Eleven patients (18%) in cohort A and 7 (11%) in cohort B had KRAS/BRAF/NRAS wild-type tumors. Nine patients (15%) in cohort A and 5 (8%) in cohort B had BRAF-mutant tumors, and 16 (26%) in cohort A and 22 (35%) in cohort B had KRAS-mutant tumors (Table 1). In cohort A, responses were observed in 5 (55%) of 9 patients with BRAF-mutant tumors and in 7 (37%) of 19 with KRAS/NRAS-mutant tumors (Appendix Table A2; Appendix Fig A2 and A3 online only). In cohort B, responses were observed in 1 (20%) of 5 patients with a BRAF-mutant tumor and in 9 (36%) of 25 with KRAS/NRAS-mutant tumors (Appendix Table A2; Appendix Fig A2 and A3).
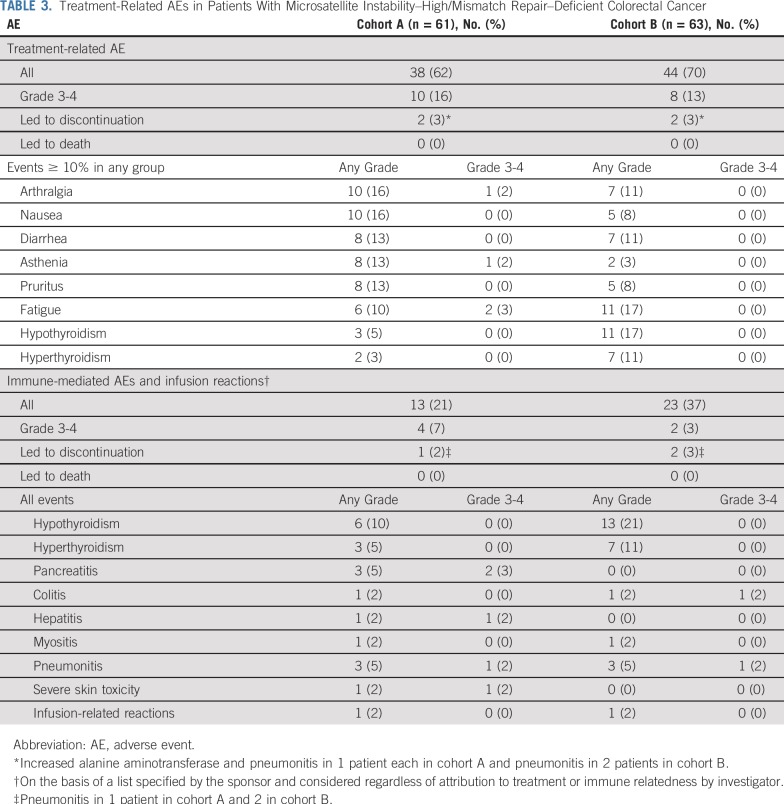
Safety
In cohort A, 38 (62%) of 61 patients had any-grade treatment-related AEs, with 10 (16%) having a grade 3-4 treatment-related AE (Table 3). Two patients (3%) discontinued because of treatment-related AEs of increased alanine aminotransferase and pneumonitis in 1 patient each. Treatment-related AEs with incidence of ≥ 10% were arthralgia and nausea in 10 patients (16%) each; diarrhea, asthenia, and pruritus in 8 patients (13%) each; and fatigue in 6 patients (10%). The most common grade 3-4 treatment-related AEs among these patients were fatigue in 2 (3%) and asthenia in 1 (2%). In cohort B, 44 (70%) of 63 patients had any-grade treatment-related AEs, with 8 (13%) having a grade 3-4 treatment-related AE. Two patients (3%) discontinued because of a treatment-related AE of pneumonitis. Treatment-related AEs with incidence of ≥ 10% were fatigue and hypothyroidism in 11 patients (17%) each and hyperthyroidism, arthralgia, and diarrhea in 7 patients (11%) each. There were no grade 3-4 treatment-related AEs among these patients. No grade 5 treatment-related AEs occurred in either cohort (Table 3).
TABLE 3.
Treatment-Related AEs in Patients With Microsatellite Instability–High/Mismatch Repair–Deficient Colorectal Cancer
Immune-mediated AEs or infusion reactions occurred in 13 (21%) of the 61 patients in cohort A (Table 3). Most events were grade 1-2 in severity, with 4 patients (7%) having a grade 3-4 immune-mediated AE. Grade 3-4 immune-mediated AEs were pancreatitis in 2 patients (3%) and hepatitis, pneumonitis, and severe skin toxicity in 1 patient (2%) each. Twenty-three (37%) of the 63 patients in cohort B had immune-mediated AEs or infusion reactions, with 2 patients (3%) having a grade 3-4 immune-mediated AE. Grade 3-4 immune-mediated AEs were colitis and pneumonitis in 1 patient (2%) each (Table 3). One patient (2%) in each cohort discontinued because of an immune-mediated AE of pneumonitis. No grade 5 immune-mediated AEs occurred in either cohort.
DISCUSSION
The data from the KEYNOTE-164 study confirm that pembrolizumab provides durable responses with a manageable safety profile in patients with previously treated MSI-H/dMMR advanced or metastatic CRC. Pembrolizumab is approved for patients with previously treated MSI-H/dMMR CRC after fluoropyrimidine, oxaliplatin, and irinotecan, and for patients with MSI-H/dMMR non-CRC solid tumors after ≥ 1 prior therapy, regardless of tumor type or origin. This first US Food and Drug Administration approval of a tumor-agnostic anticancer therapy was based on data that showed an ORR of 39.6% and evidence of durable clinical benefit in 149 patients with MSI-H/dMMR cancers across 5 clinical studies, including 61 from cohort A of the phase II KEYNOTE-164 study and 19 from the KEYNOTE-158 study of pembrolizumab in patients with MSI-H/dMMR CRC and non-CRC, respectively.10,12 The current updated analysis provides data from both cohort A (MSI-H/dMMR CRC after ≥ 2 prior therapies) and cohort B (MSI-H/dMMR CRC after ≥ 1 prior therapy) of KEYNOTE-164 with longer follow-up. A companion article by Marabelle et al13 presents data from KEYNOTE-158 (ClinicalTrials.gov identifier: NCT02628067), which studied pembrolizumab in patients with previously treated metastatic MSI-H/dMMR non-CRC solid tumors.
With a median duration of follow-up of 31 months in cohort A, pembrolizumab provided an ORR of 33%, including 2 CRs, in patients with MSI-H/dMMR CRC and prior treatment with fluoropyrimidine, oxaliplatin, and irinotecan. Similarly, in cohort B, with a median duration of follow-up of 24 months, the ORR was 33%, including 5 CRs. The median DOR was not reached in either cohort, and the median OS was 31 months in cohort A and not reached in cohort B, which supports the durability of the clinical benefit of pembrolizumab in some patients with MSI-H/dMMR CRC. While the lines of therapy for cohorts A and B are largely overlapping, cohort B was composed of 38% of patients receiving pembrolizumab after 1 line of therapy and, therefore, represented tumors earlier in the disease course. Our observations are in line with data from the neoadjuvant setting suggesting that treatment with PD-1 blockade earlier or even treatment of naïve tumors can be more effective than treatment of more advanced and refractory cases.14 The phase III KEYNOTE-177 study (ClinicalTrials.gov identifier: NCT02563002) is evaluating the antitumor activity of first-line pembrolizumab compared with standard chemotherapy for patients with MSI-H/dMMR metastatic CRC and should help to further refine this observation.
In the current study, 46% of patients in cohort A and 49% in cohort B had BRAF- or RAS-mutant tumors. Although the numbers of patients with MSI-H/dMMR CRC BRAF- or RAS-mutant tumors were small, responses in 19 of these patients were still ongoing at the time of analysis. These data compare favorably with response rates historically observed with EGFR inhibitors (approximately 7-8%) and chemotherapy in patients with KRAS-, RAS-, or BRAF-mutant metastatic CRC.15,16 The demonstration of durability of responses with pembrolizumab in MSI-H/dMMR CRC in various disease subsets supports the use of pembrolizumab in patients with MSI-H/dMMR CRC regardless of mutation status and 1, 2, or ≥ 3 prior lines of treatment. Furthermore, the safety profile is consistent with that observed with pembrolizumab across multiple tumor types.12 No new safety signals were identified.
Limitations of the current study include the small subgroup size, which impairs interpretation of subgroup analyses, and lack of a comparator. In addition, MSI-H or dMMR status was assessed locally because availability and collection of tissue samples limited central confirmation. These tissue limitations precluded identification of potential biomarkers, such as baseline immune cell infiltrate, PD-L1 expression, and tumor mutation burden, that may correlate with efficacy. Nonetheless, an understanding of the response to PD-1 blockade in MSI/dMMR tumors is of high interest and may highlight novel mechanisms of response that may affect the field of immunotherapy. Tumor intrinsic (ie, mutation spectra, heterogeneity) and extrinsic (ie, tumor microenvironment, HLA restriction) properties will be important factors to evaluate in future cohorts.
In summary, data from KEYNOTE-164 confirm the durable clinical benefit of pembrolizumab in patients with previously treated MSI-H/dMMR metastatic CRC. Pembrolizumab is an important addition to the treatment options for these patients.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers and all investigators and their site personnel, Ruixue Wang and Jiao Nan Li (MSD China, Beijing, China) for statistical analysis support, Luana Atherly-Henderson (Merck Sharp & Dohme, a subsidiary of Merck & Co Inc., Kenilworth, NJ, USA (MSD)) for medical writing assistance, Jonathan Cheng (MSD) for critical review of the manuscript, and Conrad Messam (MSD) for critical clinical study support.
APPENDIX
FIG A1.
Percent change from baseline in target lesion size (RECIST v1.1) with time in patients with microsatellite instability–high/mismatch repair–deficient colorectal cancer in (A) Cohort A and (B) Cohort B. CR, complete response; PD, disease progression; PR, partial response; SD, stable disease.
FIG A2.
Treatment exposure and duration of response by BRAF status in patients with microsatellite instability–high/mismatch repair–deficient colorectal cancer in (A) Cohort A and (B) Cohort B. CR, complete response; PR, partial response.
FIG A3.
Treatment exposure and duration of response by RAS status in patients with microsatellite instability–high/mismatch repair–deficient colorectal cancer in (A) Cohort A and (B) Cohort B. CR, complete response; PR, partial response.
TABLE A1.
Treatment Disposition
TABLE A2.
Overall Response in Subgroups of Patients With Microsatellite Instability–High/Mismatch Repair–Deficient Colorectal Cancer
Footnotes
Presented at the American Society of Clinical Oncology 2017 Annual Meeting, Chicago, IL, June 2-6, 2017; the European Society of Medical Oncology (ESMO) Congress 2017, Madrid, Spain, September 8-12, 2017; the ESMO World Congress on Gastrointestinal Cancer 2018, Barcelona, Spain, June 20-23, 2018; and ESMO Congress 2019, Barcelona, Spain, September 27-October 1, 2018.
Supported by Merck Sharp & Dohme, a subsidiary of Merck & Co Inc., Kenilworth, NJ, USA. Academic advisors and representatives of the sponsor participated in designing the study. Data collected by investigators and their site personnel were analyzed and interpreted by senior academic authors and representatives of the sponsor.
Clinical trial information: NCT02460198.
See accompanying article on page 1
AUTHOR CONTRIBUTIONS
Conception and design: Dung T. Le, Takayuki Yoshino, Petr Kavan, Todd Crocenzi, Luis A. Diaz Jr
Provision of study material or patients: Dung T. Le, Tae-Won Kim, Eric Van Cutsem, Ravit Geva, Matthew Burge, Bert O’Neil, Petr Kavan, Takayuki Yoshino, Rosine Guimbaud, Hiroya Taniguichi, Elena Elez, Patrick M. Boland, Todd Crocenzi, Chloe E. Atreya, Luis A. Diaz Jr, Thierry André
Collection and assembly of data: Dung T. Le, Eric Van Cutsem, Dirk Jäger, Petr Kavan, Takayuki Yoshino, Salah-Eddin Al-Batran, Todd Crocenzi, Chloe E. Atreya, Luis A. Diaz Jr
Data analysis and interpretation: Dung T. Le, Tae Won Kim, Eric Van Cutsem, Dirk Jäger, Hiroki Hara, Matthew Burge, Petr Kavan, Takayuki Yoshino, Chloe E. Atreya, Patrick M. Boland, Todd Crocenzi, Yi Cui, Tong Dai, Patricia Marinello, Luis A. Diaz Jr, Thierry André
Manuscript writing: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability–High/Mismatch Repair–Deficient Metastatic Colorectal Cancer: KEYNOTE-164
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Dung T. Le
Honoraria: Merck
Consulting or Advisory Role: Merck, Bristol-Myers Squibb
Research Funding: Merck, Bristol-Myers Squibb, Aduro Biotech, Curegenix, Medivir
Patents, Royalties, Other Intellectual Property: Inventor of technology, “Microsatellite Instability as a Pharmacogenomic Marker of Therapeutic Response to Immune Checkpoint Inhibition”
Tae Won Kim
Employment: Asan Medical Center
Research Funding: Sanofi (Inst)
Eric Van Cutsem
Consulting or Advisory Role: Bayer AG, Eli Lilly, Roche, Servier, Bristol-Myers Squibb, Celgene, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Halozyme, Array BioPharma
Research Funding: Amgen (Inst), Bayer AG (Inst), Boehringer Ingelheim (Inst), Eli Lilly (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Ipsen (Inst), Merck (Inst), Merck KGaA (Inst), Servier (Inst), Bristol-Myers Squibb (Inst)
Ravit Geva
Leadership: Pyxis
Honoraria: Bristol-Myers Squibb, MSD, Medison, Roche, Novartis, Janssen Pharmaceuticals, Pfizer, Takeda Pharmaceuticals
Consulting or Advisory Role: Bayer AG, Novartis, MSD, BOL Pharma
Patents, Royalties, Other Intellectual Property: Options, BOL Pharma
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Serono, Bayer AG, Medison
Dirk Jäger
Consulting or Advisory Role: Roche (Inst), Genentech (Inst), Bristol-Myers Squibb (Inst), BioNTech AG (Inst), Amgen (Inst)
Hiroki Hara
Honoraria: Chugai Pharma, Taiho Pharmaceutical, Merck Serono, Yakult Honsha, Eli Lilly, Ono Pharmaceutical, Takeda Pharmaceuticals, Bristol-Myers Squibb, Sanofi, MSD, Daiichi Sankyo, Kyowa Hakko Kirin
Consulting or Advisory Role: Ono Pharmaceutical, MSD
Research Funding: AstraZeneca (Inst), Chugai Pharma (Inst), Merck Serono (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Boehringer Ingelheim (Inst), Dainippon Sumitomo Pharma (Inst), Daiichi Sankyo (Inst), Pfizer (Inst), LSK BioPharma (Inst), Eisai (Inst), Incyte (Inst), BeiGene (Inst)
Matthew Burge
Stock and Other Ownership Interests: Bristol-Myers Squibb, Pfizer, Gilead Sciences, CSL Limited, Coclear
Honoraria: Roche, Amgen, Merck KGaA, Servier, Merck Sharp & Dohme
Consulting or Advisory Role: Amgen, Roche, Ipsen
Patents, Royalties, Other Intellectual Property: Funding for an investigator-initiated prospective trial (Inst)
Travel, Accommodations, Expenses: Amgen, Roche
Bert O’Neil
Employment: Eli Lilly
Consulting or Advisory Role: Bristol-Myers Squibb, Merck
Petr Kavan
Honoraria: Amgen, Ipsen, Novartis, Bayer AG
Consulting or Advisory Role: Novartis, Pfizer, Amgen, Celgene, Taiho Pharmaceutical, LEO Pharma
Travel, Accommodations, Expenses: Pfizer, Ipsen, Taiho Pharmaceutical, Novartis
Takayuki Yoshino
Research Funding: Chugai Pharma (Inst), Sanofi (Inst), Sumitomo Dainippon (Inst), GlaxoSmithKline (Inst), Novartis (Inst), MSD (Inst), Daiichi Sankyo (Inst), PAREXEL International (Inst), Ono Pharmaceutical (Inst)
Rosine Guimbaud
Consulting or Advisory Role: Servier, MSD Oncology, Roche, Pierre Fabre, AstraZeneca
Travel, Accommodations, Expenses: Roche, Bristol-Myers Squibb
Hiroya Taniguchi
Honoraria: Takeda Pharmaceuticals, Chugai Pharmaceutical, Merck Serono, Taiho Pharmaceutical, Bayer AG, Eli Lilly Japan, Yakult Honsha, Sanofi
Elena Elez
Consulting or Advisory Role: Amgen, Roche, Merck Serono, Sanofi, Servier, Bayer AG, Pierre Fabre
Research Funding: Merck Serono, Sanofi (Inst)
Travel, Accommodations, Expenses: Roche, Merck Serono, Sanofi, Amgen
Salah-Eddin Al-Batran
Stock and Other Ownership Interests: IKF Klinische Krebsforschung GmbH
Consulting or Advisory Role: Roche, Celgene, Eli Lilly, Bristol-Myers Squibb, Merck, Nordic Bioscience, Merck Sharp & Dohme
Speakers’ Bureau: Eli Lilly, Roche, Celgene, AIO GmbH, Forum für Medizinische Fortbildung, MCI Group, Nordic Bioscience, Promedior
Research Funding: Celgene, Eli Lilly, Medac, Hospira, Sanofi, German Cancer Aid, German Research Foundation, Federal Ministry of Education and Research of Germany, Merck, Roche, Vifor Pharma
Patrick M. Boland
Honoraria: Boston Biomedical
Consulting or Advisory Role: Sirtex Medical, Merrimack
Research Funding: Boehringer Ingelheim (Inst), Merck (Inst), Theradiag (Inst), Advaxis (Inst), Bayer AG (Inst), Genentech (Inst), Roche (Inst), Boston Biomedical (Inst), Ipsen (Inst), Merrimack (Inst), Athenex (Inst),
Todd Crocenzi
Research Funding: Bristol-Myers Squibb (Inst), AstraZeneca (Inst), MedImmune (Inst)
Chloe E. Atreya
Consulting or Advisory Role: Array BioPharma, Pionyr Immunotherapeutics
Research Funding: Novartis, Merck, Bristol-Myers Squibb, Guardant Health, Genentech (Inst)
Travel, Accommodations, Expenses: Roche
Tong Dai Stock and Other Ownership Interests: Merck
Yi Cui
Employment: MSD China
Stock and Other Ownership Interest: Merck Sharp & Dohme
Patricia Marinello
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Luis A. Diaz Jr
Leadership: Personal Genome Diagnostics, Jounce Therapeutics
Stock and Other Ownership Interests: PapGene, Personal Genome Diagnostics, Jounce Therapeutics, Zydecom Corporation, Thrive Detect, Neophore, Amgen (I)
Consulting or Advisory Role: Merck, Personal Genome Diagnostics, Cell Design Labs, Lyndra, Caris Life Sciences, Genocea Biosciences, Zydecom, Neophore
Research Funding: Merck (Inst)
Patents, Royalties, Other Intellectual Property: US-2010041048-A1: Circulating Mutant DNA to Assess Tumor Dynamics, US-2015344970-A1: Personalized Tumor Biomarkers, WO-2010118016-A2: Digital Quantification of DNA Methylation, US-2005202465-A1: Thymidylate Synthase Gene and Metastasis, US-2014227271-A1: Somatic Mutations in Atrx in Brain Cancer, WO-2012094401-A2: Genes Frequently Altered in Pancreatic Neuroendocrine Tumors, US-2013323167-A1: Detecting and Treating Solid Tumors Through Selective Disruption of Tumor Vasculature, EP-2912468-B1: Papanicolaou Test for Ovarian and Endometrial Cancers, US-9976184-B2: Mutations in Pancreatic Neoplasms, US-2017267760-A1: Checkpoint Blockade and Microsatellite Instability, US-2018171413-A1: Head and Neck Squamous Cell Carcinoma Assays, US-2018171413-A1: Head and Neck Squamous Cell Carcinoma Assays, US-2018171413-A1: Head and Neck Squamous Cell Carcinoma Assays, US-2018086832-A1: HLA-Restricted Epitopes Encoded by Somatically Mutated Genes, US-2018258490-A1: Assaying Ovarian Cyst Fluid, US-2016208340-A1: TERT Promoter Mutations in Urothelial Neoplasia, US-2015252415-A1: Arid1b and Neuroblastoma, WO-2018071796-A2: Compositions and Methods for Identifying Functional Anti-Tumor T-Cell Responses, EP-3322824-A1: Detection of Tumor-Derived DNA in Cerebrospinal Fluid, US-2016273049-A1: Systems and Methods for Analyzing Nucleic Acid (Inst), US-2018135044-A1: Non-Unique Barcodes in a Genotyping Assay (Inst), US-2017016075-A1: Neoantigen Analysis (Inst)
Travel, Accommodations, Expenses: Merck
Thierry André
Honoraria: Roche, Genentech, Bristol-Myers Squibb, Servier, Xbiotech, Bayer AG, Sanofi, Amgen, PRMA Consulting, Pierre Fabre, Vantana
Consulting or Advisory Role: Roche, Genentech, Amgen, Bristol-Myers Squibb, Mundipharma, HalioDX, MSD Oncology, Servier, Guardant Health, Bayer AG, AstraZeneca, MedImmune, Tesaro, Clovis Oncology
Travel, Accommodations, Expenses: Roche, Genentech, Amgen, Bristol-Myers Squibb, MSD Oncology, Vantana
No other potential conflicts of interest were reported.
REFERENCES
- 1. International Agency for Research on Cancer: Cancer Fact Sheet, March 2019. https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009;100:266–273. doi: 10.1038/sj.bjc.6604867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H) Ann Oncol. 2014;25:1032–1038. doi: 10.1093/annonc/mdu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 10.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med. 2017;377:1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 11. Bristol-Myers Squibb, Princeton, NJ, USA: OPDIVO (nivolumab): US prescribing information, 2018.
- 12. Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA: Keytruda (pembrolizumab): US prescribing information, 2019.
- 13.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 16.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone Web site or through e-mail to dataaccess@merck.com.