Abstract
Background
Asthma may worsen during adolescence, due to both health risk behaviors and psychosocial stressors commonly encountered during this life stage.
Methods
Cross-sectional study of 24 612 high school students who participated in the 2009 and 2011 National Youth Risk Behavior Survey. Multivariable logistic regression was used to examine the relation between self-reported health risk behaviors or psychosocial stressors and current asthma. Mediation analysis was performed to assess whether depressive symptoms or suicidal behavior contribute to the link between psychosocial stressors and asthma.
Results
Current asthma was reported by 13.1% of the study participants. In a multivariable analysis, female sex, obesity, shorter sleep duration, frequent soda/pop consumption, and marijuana use were each significantly associated with 14–36% increased odds of asthma. Any violent behavior (adjusted odds ratio [OR] = 1.12, 95% confidence interval [CI] = 1.02–1.24), any victimization (OR = 1.43, 95%CI = 1.29–1.58), any suicidal behavior (OR = 1.41, 95%CI = 1.22–1.64) and having felt sad or hopeless in the past year (OR = 1.57, 95%CI = 1.40–1.75) were each associated with current asthma. In a mediation analyses, having felt sad/hopeless and suicidal behaviors accounted for 21% and 14%, respectively, of the victimization-asthma association.
Conclusion
Potentially modifiable risk factors, including obesity, short sleep duration, frequent soda/pop consumption, and psychosocial stressors are associated with asthma in US adolescents. Promoting healthier lifestyles, as well as screening for violence exposure and treating depressive symptoms, could help reduce asthma burden in this population.
Keywords: current asthma, health behavior, psychosocial stressor, YRBS
1 |. INTRODUCTION
Asthma is the most common chronic respiratory disease of childhood. Estimates of the global average prevalence of current wheeze are ~11.5% among 6–7 year-old children and ~14.1% among 13–14 year old adolescents.1 In the United States, estimates of the prevalence of self-reported asthma in children are: ~9.6% in 5–11 year-olds, ~11.2% in 12–14 year-olds, and ~9.9% in 15–17 year-olds.2 In 2013, asthma caused 13.8 million missed school days3 and led to $81.9 billion in total annual costs.4
The determinants of the onset, persistence, and remission of asthma during childhood and adolescence are poorly characterized.5 Although adolescence is often linked to symptom improvement or remission of asthma, adolescents may engage in risky behaviors (ie, tobacco use and substance abuse) that have been associated with worse asthma outcomes, including poor symptom control and medication adherence, and lower health-related quality of life.6–9 Puberty also affects the gender distribution of asthma during adolescence, with rising disease prevalence in girls between ages 10 and 17 years.10–11
We aimed to assess the relation between health risk behaviors (eg, marijuana use, dietary patterns) or psychosocial stressors (eg, exposure to violence and victimization) and current asthma among high school adolescents in the United States. Because current evidence suggests that psychosocial stressors and depression play a role in asthma pathogenesis,12 we examined whether any estimated effects of a psychosocial stressor on asthma are at least partly explained or mediated by depressive symptoms or suicidal behavior.
2 |. METHODS
2.1 |. Subject recruitment and study procedures
The Youth Risk Behavior Surveillance System (YRBSS) was established by the US Centers for Disease Control and Prevention (CDC) to monitor the prevalence of youth behaviors that most influence health. The YRBSS includes national, state, territorial, tribal government, and local school-based surveys of representative samples of 9th through 12th grade students. The National Youth Risk Behavior Survey (YRBS) is conducted every two years and uses a three-stage cluster sampling design to produce a representative sample of 9th through 12th grade students in the US. All regular public, Catholic, and other private school students, in grades 9 through 12, in the 50 States and the District of Columbia are included in the sampling frame. Puerto Rico, the former trust territories, and the Virgin Islands are not included in the sampling frame. A weighting factor is applied to each student record to adjust for nonresponse and the oversampling of black and Hispanic students. For each cycle, the CDC develops a standard questionnaire that sites can use as-is or modify to meet their needs. Trained data collectors follow a common protocol to administer the questionnaires in the schools, coordinate data collection, weight the data, and prepare the data for analysis. The procedure is designed to protect students’ privacy and parental permissions are obtained before participation. This study combined 2009 and 2011 YRBS, during which the average school response rate was 81%. Of a total of 36 245 questionnaires distributed, 31 963 were completed by students. After excluding 128 unusable questionnaires, data from 31 835 questionnaires was included in the YRBS (response rate = 88%). Thus, the overall response rate (school response rate*student response rate) was 71%.13,14 Further details can be found on the YRBSS web site (https://www.cdc.gov/healthyyouth/data/yrbs/data.htm).
All YRBSS questionnaires are self-administered and used to collect information on demographic characteristics, health risk behaviors, psychosocial stressors, and asthma. We excluded from this analysis those who had missing information on asthma or demographic characteristics (n = 4191). Current asthma case was defined by a positive answer to both of the following questions: “Has a doctor or nurse ever told you that you have asthma?” and “Do you still have asthma?”. Participants who had negative answers to these two questions were selected as control subjects. Participants who reported a diagnosis of asthma but no current asthma or vice-versa were excluded from this analysis (n = 3032). Thus, 24 612 participants were included in the current analysis.
Health risk behaviors considered for this analysis were frequency of physical activity ≥60 min/day in the past week (never, 1–3 times, or ≥4 times), average hour of sleep (≥8 or <8 h), weight (normal weight: body mass index (BMI) <85th percentile; overweight: BMI 85th-95th percentile; obese: BMI ≥95th percentile), fruit or vegetable consumption in the past week (≥5 or <5 times/day), regular soda or pop consumption in the past week (never, 1–6 times, or ≥7 times), current smoking (at least 20 cigarettes in the past month), marijuana use in the past month (at least one time), and ever use of illegal drugs (cocaine, heroin, methamphetamines, ecstasy). Psychosocial stressors included six measures of violent behavior (carried any weapon, carried a gun, carried any weapon to school, in a physical fight, injured in a physical fight, and in a physical fight at school) and five measures of victimization (feared to attend school, threatened or injured by a weapon at school, physically abused by dating partner, coerced into sex, and bullied at school). The questionnaires also included three measures of suicidal behavior (considered suicide, planned suicide, and attempted suicide), and inquired about the child having felt sad or hopeless in the past year (a proxy for depressive symptoms). Summary variables were created by collapsing across items, resulting in three variables that measured exposure to any violent behavior, any victimization, and any suicidal behavior.
2.2 |. Statistical analysis
Sampling weights, stratification, and clusters provided in the YRBS data set were incorporated into the analysis, to account for the complex YRBS survey design and to obtain proper estimates and their standard errors. Wald chi-square tests and t tests were used for bivariate analyses of binary and continuous variables, respectively. Logistic regression was used for the multivariable analysis of health risk behaviors, psychosocial stressors, and current asthma. All multivariable models included age, sex, race/ethnicity (white, black or African American, Hispanic/Latino, multiple race/ethnicity, or others), health risk behaviors (except for physical activity which was not significant in the bivariate analysis), and psychosocial stressors. Mediation analysis was performed to assess whether part or all the association between psychosocial stressors (eg, exposure to violence or victimization) and current asthma is explained by depressive symptoms or suicidal behavior, through a mediated or “indirect effect.” This analysis was performed by using the Karlson-Holm-Breen (KHB) decomposition method for binary outcomes,15 which adjusts for the rescaling issues that arise from cross-model comparison of nonlinear models16. Statistical analyses were conducted using the SAS SURVEY procedure by SAS 9.4 software (SAS Institute Inc, Cary, NC), and the mediation analysis was conducted in Stata 14 (StataCorp, College Station, TX).
3 |. RESULTS
The overall prevalence of current asthma in the study population was 13.1%. The main characteristics of participants are presented in Table 1. Compared to adolescents without current asthma (n = 21 390), those with current asthma (n = 3222) were more likely to: be female, African-American, overweight or obese, and current smokers; report fewer hours of sleep; eat fruit or vegetables more frequently; drink soda/pop more frequently; and report use of marijuana or (other) illegal drugs. Adolescents with current asthma were also more likely to report exposure to any violent behavior, any victimization, any suicide behavior, and having felt sad or hopeless in the past year.
TABLE 1.
Characteristics of study participants by current asthma, 2009–2011 (n = 24 612)
| Characteristics | No asthma (n = 21 390) | Current asthma (n = 3222) |
|---|---|---|
| Age | 16.1 ± 0.02 | 15.9 ± 0.03* |
| Female gender | 11 099 (49.2) | 1756 (53.5)** |
| Race/ethnicity | ||
| White | 9217 (59.2) | 1459 (59.6)** |
| Black or African American | 3604 (13.5) | 681 (16.2) |
| Hispanic/Latino | 3765 (10.4) | 320 (6.6) |
| Multiple race/ethnicity | 3495 (12.1) | 567 (13.5) |
| Others | 1309 (4.7) | 195 (4.1) |
| Body mass index (BMI), percentile | 61.4 ± 0.4 | 65.0 ± 0.7** |
| Normal weight (<85th percentile) | 14 456 (73.4) | 2018 (68.6)** |
| Overweight (85th–<95th percentile) | 3134 (15.0) | 516 (16.9) |
| Obese (≥95th percentile) | 2457 (11.6) | 471 (14.6) |
| Physical activity ≥60 min/day (past week) | ||
| Never | 4331 (18.6) | 624 (17.5) |
| 1–3 times | 6500 (29.3) | 936 (29.4) |
| ≥4 times | 10 459 (52.1) | 1655 (53.1) |
| Average hour of sleep <8 h | 12 840 (67.7) | 2044 (71.1)** |
| Ate fruit or vegetables <5 times/day (past week) | 16 613 (78.6) | 2370 (75.7)** |
| Drank regular soda/pop (past week) | ||
| Never | 4068 (20.4) | 560 (18.3)** |
| 1–6 times | 11 084 (52.4) | 1585 (49.2) |
| ≥7 times | 5918 (27.2) | 1019 (32.5) |
| Smoked ≥20 cigarettes (past month) | 1114 (6.0) | 236 (8.0)** |
| Used marijuana ≥1 time (past month) | 4340 (20.1) | 774 (23.9)** |
| Ever use illegal drugs (cocaine, heroin, methamphetamines, ecstasy) | 1822 (8.4) | 345 (10.5)* |
| Violent behaviors | ||
| Carried any weapon (past month) | 3115 (15.4) | 580 (18.1)** |
| Carried a gun (past month) | 948 (4.5) | 208 (6.4)** |
| Carried any weapon to school (past month) | 935 (4.5) | 239 (7.4)** |
| In a physical fight (past year) | 6493 (29.8) | 1163 (35.0)** |
| Injured in a physical fight (past year) | 634 (2.7) | 186 (5.7)** |
| In a physical fight at school (past year) | 2268 (10.3) | 427 (12.6)** |
| Any violent behavior | 7761 (37.5) | 1344 (42.2)** |
| Victimization | ||
| Feared to attend school (past month) | 1054 (4.3) | 242 (6.8)** |
| Threatened or injured by a weapon at school (past year) | 1323 (6.0) | 307 (9.0)** |
| Physically abused by dating partner (past year) | 1954 (8.3) | 446 (12.0)** |
| Coerced into sex (ever) | 1429 (6.4) | 387 (11.6)** |
| Bullied at school (ever) | 3496 (18.2) | 766 (26.7)** |
| Any victimization | 6579 (31.7) | 1333 (42.2)** |
| Suicide behaviors | ||
| Considered suicide (past year) | 2884 (13.2) | 635 (19.4)** |
| Planned suicide (past year) | 2288 (10.4) | 493 (14.6)** |
| Attempted suicide (past year) | 1236 (5.6) | 324 (9.9)** |
| Any suicide behavior | 3612 (17.7) | 768 (24.7)** |
| Felt sad or hopeless (past year) | 5603 (24.7) | 1119 (34.3)** |
Results shown as mean ± standard error for continuous variables, and as number (%) for binary variables. Numbers might vary because of missingness.
P < 0.05.
P < 0.01 comparison between participants with and without current asthma.
The results of the multivariable analysis of current asthma are shown in models 1–4 in Table 2. In this analysis, being older, being Hispanic or Latino, and eating fruits/vegetables <5 times per week were significantly associated with reduced odds of current asthma. On the other hand, female sex, obesity, and drinking soda/pop >7 times/week were all significantly associated with increased odds of current asthma. Both sleep duration <8 h and report of marijuana use in the past week were significantly associated with increased odds of current asthma in some multivariable models but not in others (Table 2). With regard to psychosocial stressors, depressive symptoms, and suicidal behavior, exposure to any violent behavior (Model 1), any victimization (Model 2), any suicidal behavior (Model 3), and feeling sad or hopeless in the past year (Model 4) were each significantly associated with increased odds of current asthma. When all stressors were included in the same model, victimization and feeling sad/hopeless were significantly and independently associated with current asthma (Supplemental Table S1).
TABLE 2.
Multivariable analysis of health risk behaviors, psychosocial stressors, and current asthma
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|
|---|---|---|---|---|
| Risk factors | Odds ratio, 95% confidence interval | |||
| Age | 0.95 (0.91, 0.99)* | 0.96 (0.92, 1.00) | 0.96 (0.92, 1.01) | 0.95 (0.91, 0.99)* |
| Female gender | 1.34 (1.18, 1.51)** | 1.29 (1.15, 1.44)** | 1.28 (1.14, 1.44)** | 1.22 (1.09, 1.38)** |
| Race/ethnicity | ||||
| White | 1.0 | 1.0 | 1.0 | 1.0 |
| Black/African American | 1.05 (0.89, 1.24) | 1.10 (0.94, 1.29) | 1.10 (0.91, 1.32) | 1.09 (0.93, 1.29) |
| Hispanic/Latino | 0.64 (0.53, 0.78)** | 0.67 (0.55, 0.81)** | 0.67 (0.55, 0.83)** | 0.64 (0.53, 0.79)** |
| Multiple race/ethnicity | 1.02 (0.85, 1.24) | 1.02 (0.85, 1.23) | 1.03 (0.86, 1.25) | 1.01 (0.84, 1.21) |
| Others | 0.81 (0.65, 1.01) | 0.82 (0.65, 1.02) | 0.81 (0.66, 1.00) | 0.79 (0.63, 1.21) |
| Body mass index (BMI) | ||||
| Normal weight | 1.0 | 1.0 | 1.0 | 1.0 |
| Overweight | 1.20 (1.00, 1.55)* | 1.18 (0.98, 1.41) | 1.18 (0.98, 1.42) | 1.17 (0.98, 1.41) |
| Obese | 1.34 (1.16, 1.55)** | 1.36 (1.17, 1.57)** | 1.34 (1.16, 1.56)** | 1.33 (1.15, 1.54)** |
| Average hour of sleep <8 h | 1.15 (1.01, 1.29)* | 1.14 (1.01, 1.28)* | 1.13 (0.99, 1.28) | 1.12 (0.99, 1.27) |
| Ate fruit or vegetables <5 times/day (past week) | 0.83 (0.73, 0.95)* | 0.84 (0.73, 0.96)* | 0.85 (0.74, 0.99)* | 0.83 (0.73, 0.95)* |
| Drank regular soda/pop (past week) | ||||
| Never | 1.0 | 1.0 | 1.0 | 1.0 |
| 1–6 times | 1.11 (0.96, 1.27) | 1.11 (0.97, 1.27) | 1.12 (0.98, 1.29) | 1.09 (0.96, 1.25) |
| ≥7 times | 1.27 (1.11, 1.47)** | 1.27 (1.11, 1.46)** | 1.31 (1.14, 1.52)** | 1.25 (1.09, 1.44)** |
| Smoked ≥20 cigarettes (past week) | 1.14 (0.93, 1.40) | 1.09 (0.89, 1.35) | 1.12 (0.90, 1.39) | 1.12 (0.91, 1.38) |
| Used marijuana ≥1 time (past week) | 1.14 (1.01, 1.28)* | 1.12 (0.99, 1.27) | 1.13 (1.00, 1.28)* | 1.13 (0.99, 1.27) |
| Use illegal drugs (ever)a | 0.97 (0.78, 1.21) | 0.96 (0.77, 1.19) | 0.95 (0.76, 1.17) | 0.94 (0.75, 1.18) |
| Psychosocial stressors | ||||
| Any violent behavior | 1.12 (1.02, 1.24)* | - | - | - |
| Any victimization | - | 1.43 (1.29, 1.58)** | - | - |
| Any suicide behavior | - | - | 1.41 (1.22, 1.64)** | - |
| Felt sad or hopeless (past year) | - | - | - | 1.57 (1.40, 1.75)** |
Including cocaine, heroin, methamphetamines, and ecstasy.
P < 0.05.
P < 0.01.
To examine whether the severity of victimization is associated with current asthma, we repeated the multivariable analysis of asthma after stratification by total number of experienced victimization types (Table 3). Compared to participants who reported no victimization, those who reported two or more than three different types of victimization had 1.68–2.44 times significantly higher odds of asthma, with similar results in male and female adolescents.
TABLE 3.
Multivariable analysis of severity of victimization and current asthma, by gender
| All participants |
Male |
Female |
|
|---|---|---|---|
| Severity of victimizationa | Odds ratio, 95% confidence interval | ||
| Any victimization | 1.43 (1.29, 1.58)** | 1.40 (1.23, 1.60)** | 1.44 (1.24, 1.68)** |
| None | 1.0 | 1.0 | 1.0 |
| One type | 1.27 (1.12, 1.44)** | 1.27 (1.08, 1.48)** | 1.26 (1.07, 1.51)** |
| Two types | 1.68 (1.40, 2.02)** | 1.63 (1.28, 2.08)** | 1.70 (1.26, 2.31)** |
| More than three types | 2.44 (1.94, 3.07)** | 2.20 (1.60, 3.03)** | 2.85 (1.89, 4.29)** |
Models included age, sex (all participants), race/ethnicity, BMI, average hour of sleep, consumption of fruit or vegetable, and soda/pop, smoking, and used marijuana or illegal drugs.
Five types of victimization included in the analysis are: feared to attend school in the past month, threatened or injured by a weapon at school in the past year, physically abused by dating partner in the past year, ever coerced into sex, and ever bullied at school.
P < 0.05.
P < 0.01.
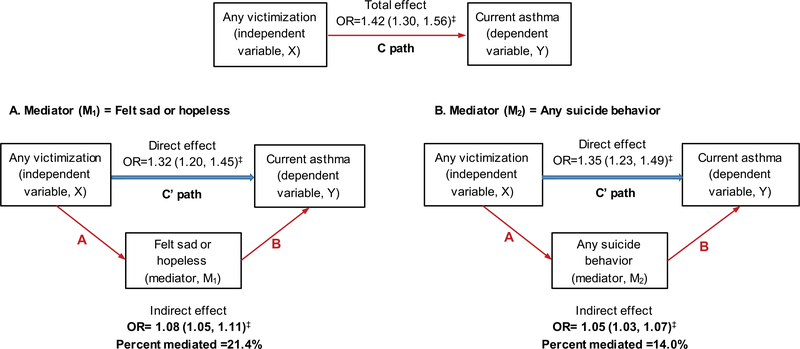
Next, we performed a mediation analysis to assess whether the association between victimization and asthma is mediated through feeling of sadness/hopelessness or suicidal behavior. For ease of interpretation, feeling sad/hopeless in the past year (Figure 1, panel A) and any suicidal behavior (Figure 1, panel B) were analyzed in two separate models. In this analysis, both feeling sad/hopeless in the past year and any suicidal behavior were significant mediators of the victimization-asthma association, explaining 21% and 14%, respectively, of the estimated effects. Similarly, feeling sad/hopeless in the past year and any suicidal behavior significantly mediated 20–29% of the association between exposure to any violent behavior and asthma (Supplemental Figure S1).
FIGURE 1.
Analytic diagram of the relationship between any victimization (X), felt sad or hopeless (mediator, M1, panel A), any suicide behavior (mediator, M2, panel B), and current asthma (Y). In mediation/decomposition analysis, the original association (X-Y or C path) is significant. When a mediator M is introduced, the direct effect (C′) is markedly reduced. The difference between C and C′ is explained by B (eg, the indirect effect via M explains a significant proportion or all the effect of X on Y). Models adjusting for age, gender, race/ethnicity, BMI, average hour of sleep, consumption of fruit or vegetable, and soda/pop, smoking, and used marijuana or illegal drugs. ‡P < 0.01
4 |. DISCUSSION
In this population-based study, exposure to any violent behavior, any victimization, any suicidal behavior, and feeling sad/hopeless in the past year were each associated with current asthma among US adolescents. We estimated that suicidal behavior and depressive symptoms in the past year explain 14–21% of the observed association between victimization and asthma, and 20–29% of the observed association between exposure to any violent behavior and asthma. We also found that female sex, obesity, and frequent soda/pop consumption are associated with asthma, with similar but less consistently significant results for an association between shorter sleep duration or report of marijuana use and current asthma.
Temporal changes in dietary patterns and obesity may partly explain the asthma epidemic in industrialized countries.17,18 Whereas a Western diet (rich in proinflammatory foods and poor in antioxidant foods) has been linked to asthma incidence and prevalence,19 an “anti-inflammatory diet” has been associated with decreased asthma symptoms.20 Meanwhile, obesity is associated with asthma, morbidity from asthma, impaired lung function, and metabolic dysregulation in children and adults.18 Conversely, weight loss results in improvement of asthma-related outcomes.21
In our study, frequent consumption of regular soda or pop and obesity were independently associated with current asthma. Consumption of sugary beverages (high-calorie, low-nutrient) can cause obesity22 and has been linked to asthma.23 Sugar-containing beverages have been associated with nearly twofold increased odds of asthma in children,24 and higher consumption of soft drinks has been linked to asthma25 and hospitalizations for asthma in adults26. Although the mechanisms underlying these observations are unclear, high fructose corn syrup may cause intestinal formation of proinflammatory advanced glycation end products (AGEs).27,28 We found less frequent consumption of fruits or vegetables in control subjects, a paradoxical finding that may be partly explained by changes in dietary patterns after an asthma diagnosis (“reverse causation”).
Sleep deficits have been associated with asthma, perhaps through altering innate immune responses.29,30 Shortened sleep duration has been associated with asthma31 and decreased lung function32 in adolescents. Conversely, asthma has been associated with obstructive sleep apnea33 and poor quality of sleep.34 Short sleep duration is common among school-aged children and adolescents,35,36 and thus healthy sleep habits (ie, 8–10 h of sleep per night) could improve their general health.
Marijuana use has been associated with asthma in some but not all previous studies.37 Potential respiratory effects of marijuana smoking include cytologic changes in the respiratory tract, respiratory symptoms (such as cough, wheeze, shortness of breath, and sputum production), and impaired lung function.38 However, occasional use or a low cumulative dose of marijuana was not significantly associated with lung function in a 20-year longitudinal study that accounted for cigarette smoking.39 Although we lacked information on the dose or duration of marijuana use, our results for marijuana use and asthma merit further investigation.
Emerging evidence supports that psychosocial stressors at the individual-, family-, and community-levels have a negative impact on asthma.40,41 Plausible causal mechanisms for stress-related asthma includes alterations in the hypothalamic-pituitary-adrenocortical (HPA) axis and the autonomic nervous system (ANS), as well as expression changes for genes that regulate behavioral, autonomic, neuroendocrine, and immunologic responses.12 Anxiety and depression are more common in adults42 and adolescents43 with asthma than in those without asthma, and this could be explained by persistent secretion of cortisol and catecholamines, down-regulation of glucocorticoid and β2-adrenergic receptors, and increased oxidative stress and airway inflammation.44 Moreover, suicidal behavior (a manifestation of severe depression) has been associated with severe or poorly controlled asthma.45 We previously reported that depressive symptoms46 and a lifetime history of suicide attempts (independently of depressive symptom)47 are associated with current asthma in adults. In the current study, feeling sad or hopeless and any suicidal behavior (considered, planed, or attempted suicide) were significantly associated with 41–57% increased odds of current asthma. However, feeling sad or hopeless is a proxy measure of depressive symptoms, and a validated screening instrument for depression would offer a more precise assessment of depression in adolescents.
Consistent with a prior report from another YRBS cycle,48 we found that any victimization and any violent behavior were significantly associated with current asthma in adolescents. Our study expands on prior findings by showing that the strength of this association increased as the number of victimization types increased, suggesting a dose-response relationship.
In children and adults, physical or sexual abuse49 and lifetime exposure to violent events in the neighborhood50 have been linked to asthma and morbidity from asthma.51,52 Exposure to violence and any victimization could influence asthma through depressive symptoms, and we indeed found that the association between victimization and asthma was partly explained by feelings of sadness or hopelessness and by any suicidal behavior, with similar findings for the association between any violent behavior and asthma. Consistent with our results, a previous study showed that depressive symptoms and anhedonia partly explained an association between perceived neighborhood stress or exposure to violence and asthma symptoms in children aged 10–17 years.53 While mediation does not imply causation, our results are consistent with a significant contribution of depressive symptoms to the victimization-asthma and violence-asthma links, and further suggest that early detection of victimization or violence and appropriate treatment of depressive symptoms could help adolescents with asthma.
We recognize several study limitations. First, we cannot determine temporal relationships between health risk behaviors or psychosocial stressors and asthma in this cross-sectional study. Thus, we cannot exclude “reverse causation” as an alternative explanation for some of our findings (eg, asthma could lead to depressive symptoms). Second, both misclassification of asthma and recall bias on health risk behaviors are possible. However, misdiagnosis of asthma is less likely in adolescents than in young children. Third, we lack data on potential confounders, including family history of asthma, allergic sensitization, access to health care, and air pollution. Fourth, we lack data on specific Hispanic subgroups. Thus, our finding of reduced odds of asthma in Hispanics may be due to under-representation of Puerto Ricans (who are more heavily affected by asthma than Mexican Americans or Central Americans) in the YRBS, which excluded the island of Puerto Rico. Finally, only selective food items and short-term dietary consumption were evaluated in the YBRS.
In summary, asthma remains a significant public health burden among adolescents in the United States. This study identifies potentially modifiable risk factors for asthma morbidity in adolescents, including poor dietary and sleep habits, obesity, and violence-related depressive symptoms. Promoting a healthy weight and diet, encouraging sufficient sleep, and early detection and treatment of violence and depressive symptoms could help reduce the asthma burden in US adolescents.
Supplementary Material
ACKNOWLEDGMENTS
Dr. Forno’s contribution was supported by grant HL125666 from the U.S. National Institutes of Health (NIH). Dr. Celedón’s contribution was supported by grants HL117191, HL119952, and MD011764 from the US NIH. Dr. Celedón has received research materials from Merck and GSK (inhaled steroids), and Pharmavite (vitamin D and placebo capsules), to provide medications at no cost to participants in NIH-funded studies, unrelated to the current work.
Funding information
National Heart, Lung, and Blood Institute, Grant numbers: HL117191, HL119952, MD011764
Footnotes
CONFLICTS OF INTEREST
The other authors report no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Lai CK, Beasley R, Crane J, et al. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2009;64: 476–483. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2016 National Health Interview Survey (NHIS) Data-Most Recent Asthma Data. 2018.
- 3.Centers for Disease Control and Prevention. AsthmaStats: Asthma-related Missed School Days among Children aged 5–17 Years. 2015.
- 4.Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thorac Soc. 2018; 15:348–356. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. Lancet Respir Med. 2017;5:224–234. [DOI] [PubMed] [Google Scholar]
- 6.Desai M, Oppenheimer JJ. Medication adherence in the asthmatic child and adolescent. Curr Allergy Asthma Rep. 2011;11:454–464. [DOI] [PubMed] [Google Scholar]
- 7.Bitsko MJ, Everhart RS, Rubin BK. The adolescent with asthma. Paediatr Respir Rev. 2014;15:146–153. [DOI] [PubMed] [Google Scholar]
- 8.de Benedictis D, Bush A. Asthma in adolescence: is there any news? Pediatr Pulmonol. 2017;52:129–138. [DOI] [PubMed] [Google Scholar]
- 9.Bender BG. Risk taking, depression, adherence, and symptom control in adolescents and young adults with asthma. Am J Respir Crit Care Med. 2006;173:953–957. [DOI] [PubMed] [Google Scholar]
- 10.Fu L, Freishtat RJ, Gordish-Dressman H, et al. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann Am Thorac Soc. 2014;11:939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vink NM, Postma DS, Schouten JP, Rosmalen JG, Boezen HM. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126:498–504 e491–496. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg SL, Miller GE, Brehm JM, Celedon JC. Stress and asthma: novel insights on genetic, epigenetic, and immunologic mechanisms. J Allergy Clin Immunol. 2014;134:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Division of Adolescent and School Health. 2009. National Youth Risk Behavior Survey Data Users Manual.
- 14.Centers for Disease Control and Prevention Division of Adolescent and School Health. 2011. National Youth Risk Behavior Survey Data Users Manual.
- 15.Karlson A, Holm A. Decomposing primary and secondary effects: a new decomposition method. Res Soc Stratif Mobil. 2011;29:221–237. [Google Scholar]
- 16.Kohler U, Karlson K, Holm A. Comparing coefficients of nested nonlinear probability models. Stata J. 2011;11:420–438. [Google Scholar]
- 17.Han YY, Forno E, Holguin F, Celedon JC. Diet and asthma: an update. Curr Opin Allergy Clin Immunol. 2015;15:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141:1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brigham EP, Kolahdooz F, Hansel N, et al. Association between Western diet pattern and adult asthma: a focused review. Ann Allergy Asthma Immunol. 2015;114:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han YY, Forno E, Shivappa N, Wirth MD, Hebert JR, Celedon JC. The dietary inflammatory index and current wheeze among children and adults in the United States. J Allergy Clin Immunol Pract. 2018;6: 834–841 e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forno E, Celedon JC. The effect of obesity, weight gain, and weight loss on asthma inception and control. Curr Opin Allergy Clin Immunol. 2017;17:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care. 2015;4:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S, Blanck HM, Sherry B, Jones SE, Pan L. Regular-soda intake independent of weight status is associated with asthma among US high school students. J Acad Nutr Diet. 2013;113:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berentzen NE, van Stokkom VL, Gehring U, et al. Associations of sugar-containing beverages with asthma prevalence in 11-year-old children: the PIAMA birth cohort. Eur J Clin Nutr. 2015;69:303–308. [DOI] [PubMed] [Google Scholar]
- 25.Shi Z, Dal Grande E, Taylor AW, Gill TK, Adams R, Wittert GA. Association between soft drink consumption and asthma and chronic obstructive pulmonary disease among adults in Australia. Respirology. 2012;17:363–369. [DOI] [PubMed] [Google Scholar]
- 26.Cisneros R, Gonzalez M, Brown P, Schweizer D. Soda consumption and hospital admissions among Californian adults with asthma. J Asthma. 2017;54:371–375. [DOI] [PubMed] [Google Scholar]
- 27.DeChristopher LR, Uribarri J, Tucker KL. The link between soda intake and asthma: science points to the high-fructose corn syrup, not the preservatives: a commentary. Nutr Diabetes. 2016;6:e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gugliucci A Formation of fructose-mediated advanced glycation end products and their roles in metabolic and inflammatory diseases. Adv Nutr. 2017;8:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koinis-Mitchell D, Craig T, Esteban CA. Klein RB. Sleep and allergic disease: a summary of the literature and future directions for research. J Allergy Clin Immunol. 2012;130:1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116:1188–1198. [DOI] [PubMed] [Google Scholar]
- 31.Bakour C, O’Rourke K, Schwartz S, Wang W, Sappenfield W, Couluris M. Sleep duration, obesity, and asthma, in Florida adolescents: analysis of data from the Florida Youth Risk Behavior Survey (2009–2013). Sleep Breath. 2017;21:1039–1045. [DOI] [PubMed] [Google Scholar]
- 32.Meltzer LJ, Faino A, Szefler SJ, Strand M, Gelfand EW, Beebe DW. Experimentally manipulated sleep duration in adolescents with asthma: feasibility and preliminary findings. Pediatr Pulmonol. 2015;50:1360–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. JAMA. 2015;313:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mastronarde JG, Wise RA, Shade DM, Olopade CO, Scharf SM. American Lung Association Asthma Clinical Research C. Sleep quality in asthma: results of a large prospective clinical trial. J Asthma. 2008;45:183–189. [DOI] [PubMed] [Google Scholar]
- 35.Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American academy of sleep medicine. J Clin Sleep Med. 2016;12:785–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matricciani L, Olds T, Petkov J. In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev. 2012;16:203–211. [DOI] [PubMed] [Google Scholar]
- 37.Chatkin JM, Zani-Silva L, Ferreira I, Zamel N. Cannabis-associated asthma and allergies. Clin Rev Allergy Immunol. 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol. 2014;46:65–81. [DOI] [PubMed] [Google Scholar]
- 39.Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012;307:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trueba AF, Ritz T. Stress, asthma, and respiratory infections: pathways involving airway immunology and microbial endocrinology. Brain Behav Immun. 2013;29:11–27. [DOI] [PubMed] [Google Scholar]
- 41.Yonas MA, Lange NE, Celedon JC. Psychosocial stress and asthma morbidity. Curr Opin Allergy Clin Immunol. 2012;12:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott KM, Von Korff M, Ormel J, et al. Mental disorders among adults with asthma: results from the World Mental Health Survey. Gen Hosp Psychiatry. 2007;29:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinberg L, Aldea I, Messias E. Asthma, depression, and suicidality: results from the2007, 2009, and 2011 youth risk behavior surveys. J Nerv Ment Dis. 2015;203:664–669. [DOI] [PubMed] [Google Scholar]
- 44.Van Lieshout RJ, Bienenstock J, MacQueen GM. A review of candidate pathways underlying the association between asthma and major depressive disorder. Psychosom Med. 2009;71:187–195. [DOI] [PubMed] [Google Scholar]
- 45.Goodwin RD. Asthma and suicide: current knowledge and future directions. Curr Psychiatry Rep. 2012;14:30–35. [DOI] [PubMed] [Google Scholar]
- 46.Han YY, Forno E, Marsland AL, Miller GE, Celedon JC. Depression, asthma, and bronchodilator response in a nationwide study of US adults. J Allergy Clin Immunol Pract. 2016;4:68–73 e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han YY, Forno E, Canino G, Celedon JC. Psychosocial risk factors and asthma among adults in Puerto Rico. J Asthma. 2018;1–9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swahn MH, Bossarte RM. The associations between victimization, feeling unsafe, and asthma episodes among US high-school students. Am J Public Health. 2006;96:802–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen RT, Canino GJ, Bird HR, Celedon JC. Violence, abuse, and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2008; 178:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sternthal MJ, Jun HJ, Earls F, Wright RJ. Community violence and urban childhood asthma: a multilevel analysis. Eur Respir J. 2010; 36:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coogan PF, Wise LA, O’Connor GT, Brown TA, Palmer JR, Rosenberg L. Abuse during childhood and adolescence and risk of adult-onset asthma in African American women. J Allergy Clin Immunol. 2013;131:1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramratnam SK, Han YY, Rosas-Salazar C, et al. Exposure to gun violence and asthma among children in Puerto Rico. Respir Med. 2015;109:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tobin ET, Zilioli S, Imami L, Saleh DJ, Kane HS, Slatcher RB. Neighborhood stress, depressive symptoms, and asthma morbidity in youth. J Pediatr Psychol. 2016;41:952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.