Abstract
Efforts to understand biological functions and develop management schemes specific to Bos indicus-influenced cattle raised in tropical and subtropical environments are critical to meet the increasing global demand for protein. In the United States, B. indicus breeds are mostly used to generate B. indicus × B. taurus crosses with increased thermal and parasite tolerance, while retaining some productive characteristics of B. taurus cattle. Although crossbreeding represents a proven strategy to improve cattle adaptation almost immediately, research has also attempted to identify B. taurus genetics that can withstand subtropical and tropical climates. Reduced milk production and delayed reproductive maturation appear to be related with tropical adaptation of B. taurus breeds, as a means to conserve energy under stressful conditions and limited nutrition. Moreover, longevity may be the ultimate adaptation response to unfavorable environments, and retention of bulls and heifers from proven cows is the recommended strategy to improve longevity in B. indicus-influenced herds. Besides selection for longevity, other aspects should be considered when planning reproductive management in tropical and subtropical regions. Bos indicus and B. taurus breeds have multiple differences pertaining to reproductive function, including age at puberty, ovarian dynamics, and pregnancy development. Nutritional strategies such as the stair-step regimen, and use of exogenous progesterone (P4) inserts are options to hasten puberty attainment of late-maturing B. indicus-influenced heifers. Yet, limited pharmacological alternatives are available for reproductive management of B. indicus-influenced females in the United States, which rely on GnRH-based protocols not specifically designed to the reproductive function of B. indicus breeds. In contrast, hormonal protocols based on exogenous P4, estradiol esters, and equine chorionic gonadotropin are available for use in B. indicus females in South America. These include protocols tailored to prepubertal heifers, anestrous cows, and cycling nulliparous or parous females, which often yield pregnancy rates of 50% to fixed-time artificial insemination. The global dairy industry also faces similar challenges in increasing demand and production as the beef industry. Selection of cows capable of sustaining optimal milk yield, reproductive success, and health status in hot and humid conditions is essential for optimal dairy production in subtropical and tropical regions.
Keywords: Bos indicus, genetics, physiology, reproduction, tropical and subtropical environments
Introduction
As discussed extensively in the companion manuscript (Cooke et al., 2020), efforts to understand biological functions and develop management schemes specific to Bos indicus-influenced cattle raised in tropical and subtropical environments are critical to meet the increasing global demand for protein. Vose et al. (2017) projected that annual average temperature of the contiguous United States will increase 1.5 °C by 2050, which will continue to provoke erratic weather patterns and intensify the severity of climate disruptions. Thus, beef production systems that utilize B. indicus cattle are also expected to limit the severity of climate change in the United States and across the globe, given their greater ability in converting low-quality feeds into protein in tropical and subtropical climates compared with B. taurus breeds (Cooke et al., 2020).
Benefits and limitations for using Bos indicus genetics in the U.S. beef industry
Approximately 30% of cattle in the United States contain some Bos indicus genetics, and approximately 40% of beef cows and 50% of the country’s cow–calf producers are located in the southern states where B. indicus cattle and their crosses are located (NASS, 2017). These cattle have greater resistance to internal and external parasites, as well as greater tolerance to elevated ambient temperatures and humidity than most B. taurus beef breeds (Turner, 1980; Hansen, 2004). Because of these traits, B. indicus cattle and many B. indicus × B. taurus crosses experience less severe reductions in feed intake, growth rate, milk yield, and reproduction in the hot, humid climates throughout the southern United States (Hansen, 2004). However, B. indicus cattle often have reduced performance responses in the feedlot, and their carcasses usually grade lower and produce less tender beef than B. taurus counterparts (Turner, 1980). Hence, B. indicus breeds are mostly used in the United States to generate B. indicus × B. taurus crosses with increased thermal and parasite tolerance, while retaining productive characteristics of B. taurus cattle. Alternatively, research efforts have also focused on pursuing B. taurus breeds that can withstand the environmental challenges found in subtropical and tropical regions of the United States.
Genetic basis to cattle adaptation in unfavorable environments
Bos taurus breeds
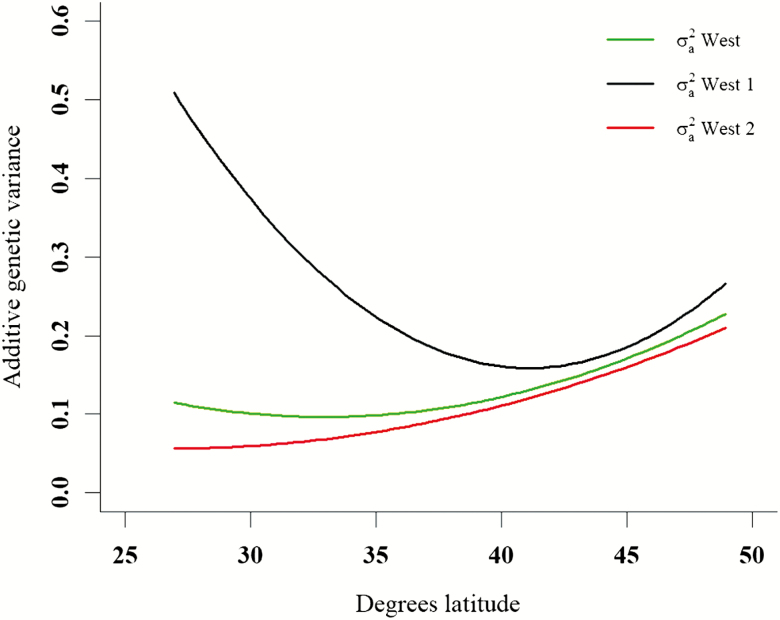
Estimates of additive genetic variance and narrow sense heritability for intramuscular fat in Hereford vary across U.S. geography quantifications evaluated in random regression analyses (Liberona et al., 2020). Percentage intramuscular fat records of Hereford cattle (n = 169,440) were evaluated in random regression models in which the random regression was either linear or quadratic Legendre polynomials across 1) latitude, 2) longitude, 3) latitude within 2 or 4 East to West regions, or 4) longitude within 2 to 4 North to South regions. Additive genetic variance, and consequently, narrow sense heritability for this trait increased for more Northern latitudes or regions. When modeled as a random regression on latitude in 4 regions, the West Coast region had high additive variance in Southern latitudes (Fig. 1). These results appear to uniquely parameterize genotype–environment interactions for these traits and may represent an opportunity to better predict genetic merit contextually.
Figure 1.
Trajectory plots of the additive genetic variance for intramuscular fat across latitude within the overall West region (data divided into West and East regions at 99°W) and subdivided West regions (furthest West subregion with boundary at 104.55°W). Adapted from Liberona et al. (2020).
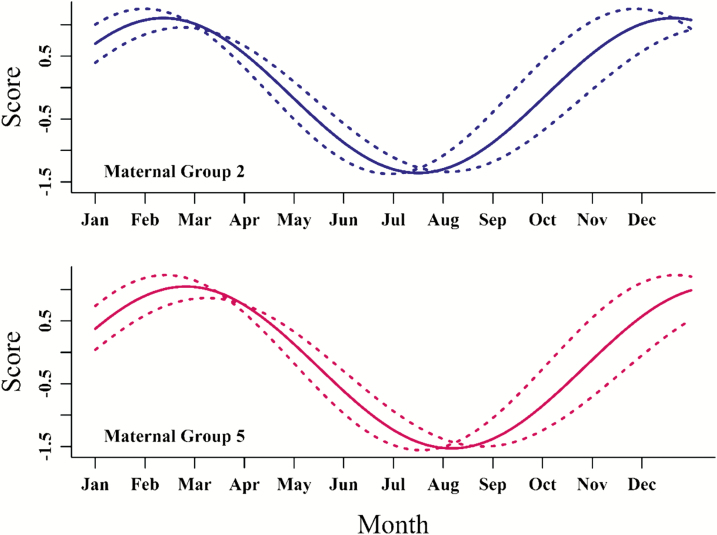
Shedding and regrowth of winter coats of Angus cows in subtropical areas may indicate differential adaptation in cattle that are not otherwise adapted to the subtropics. Angus cattle are not adapted to the subtropical summers of Central Texas. An indication of adaptation that has been associated with productivity is the early-spring shedding of accumulated winter coat (Gray et al., 2011). Angus cows (n = 135) in Central Texas were scored for winter coat shedding and regrowth monthly for 2 yr using subjective scores (Gray et al., 2011) from 1 (completely shed coat) to 5 (complete winter coat; no shedding). Nonlinear regression was used to estimate descriptive parameters of a sine function , in which A represents peak height, B represents the rate of change of scores across a cycle, and C and D represent the horizontal and vertical shifts of the cycle (Riley et al., 2015a). This function was used to estimate the changes in winter coat shedding scores across months for groups of maternal half siblings along with their mother as indicative of genetic groups and for 4 age categories of cows (2, 3, 4 to 8, and 9 yr and older). Different curves (characterized by different parameter estimates) were obtained for maternal lineage groups, which suggests genetic differences in the winter coat shedding and regrowth dynamics. Two groups with distinct curves are shown in Fig. 2. Those differ by high and low values and particularly a shift to the right for one group, indicating later shedding and regrowth of winter coat. A strong age effect was supported by higher and right-shifted curves for 2- and 3-yr-old cows, again indicative of later winter coat shedding. This age effect, however, may be an indication of selection, as those cows that shed winter coat relatively late may not stay in the herd because of associated poor performance.
Figure 2.
Shedding and regrowth of winter hair curve (solid lines) of Angus cows, from 2 different maternal lineages, in subtropical areas modeled as by month (t). A = height of peaks in the oscillatory function above a centralized baseline in the Cartesian plane; B = the angular frequency, that is, the rate of change of scores across a complete fundamental cycle of the process, C = horizontal shift in the cycle and D = represents the vertical offset of the function. Upper panel (blue lines) represents maternal lineage group 2, and lower panel (red lines) represents maternal lineage group 5. In both panels, dotted lines represent 68% confidence bands. Adapted from Riley et al. (2015a).
Angus cattle “native” to Florida, whose ancestors were part of a closed herd since the early 1950s and assumed to have acquired adaptation to those conditions, were compared with Angus from modern U.S. bloodlines in South Florida conditions. Embryos from the 2 groups were transferred into B. indicus × B. taurus females and evaluated from birth through post-weaning (Riley et al., 2011). Calves from the modern Angus lines were significantly larger at birth, weaning, 1 yr of age, and as mature cows than the animals from the Florida line. Body temperature under hot conditions did not differ for the 2 groups. As calves, no differences in quantity of hair coat were observed; however, as mature cows, the females from modern Angus lines had more hair and more difficulty shedding winter coat than the Florida females. Both males and females from the modern Angus lines achieved sexual maturation at younger ages than the Florida Angus. Milk production, measured by weigh–suckle–weigh methodology, was greater in the modern Angus females. Reproductive performance of Florida cows was superior to that of modern Angus females, and cows from the modern lines were removed sooner because of reproductive failure. Results from this study suggest that high milk production is not compatible with adaptation to South Florida conditions, which appears reasonable given the high-energy requirements of milk production. Results also suggest that late reproductive maturation is consistent with tropical adaptation; again, this seems reasonable as an energy conservation or utilization strategy when resources may be limited or offered in stressful conditions.
Bos indicus-influenced breeds
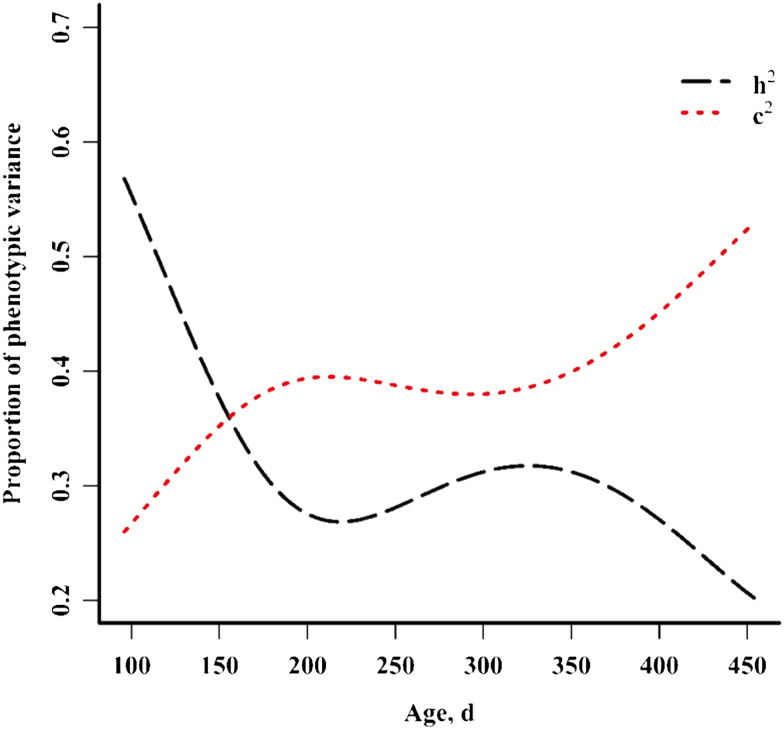
Cattle temperament may be indicative of adaptation and is highly heritable. Over 4,000 B. indicus × B. taurus calves in Western Mississippi were evaluated for 3 measures of temperament at approximately 2-mo intervals from 28 d of age to over 1 yr of age. Random regression methodology (quadratic polynomial) was used to estimate the additive genetic component of temperament corresponding to age at the time of measurement (Littlejohn et al., 2018). This methodology permits estimation of the variance and narrow sense heritability across the parameter space of the covariate (Fig. 3). Results indicated that the heritability of exit velocity, as well as 2 other temperament traits, was highest at young ages but diminished in importance as calves aged. Correspondingly, the proportion of permanent environmental variance increased in importance with calf age. This seems reasonable, and this increase in the permanent environmental variance proportion may represent animal adaptation to external conditions such as handling. These results suggest that selection may be most effective when based on phenotypes recorded early in life, and that improvement of temperament at older ages might be best supported by a strategy of selection based on previous records.
Figure 3.
Plots of heritability (h2) and permanent environmental variance (c2) for exit velocity as a proportion of the phenotypic variance across age of Bos indicus × Bos taurus calves. Adapted from Littlejohn et al. (2018).
Crossbreeding represents a proven strategy to improve adaptation almost immediately. Heterosis influences cattle body temperature maintenance, reproduction, survival, and, to a lesser extent, temperament in subtropical or other stressful environmental conditions such as toxic fescue. Results from Australia, Florida, and Arkansas have documented favorable heterosis for adaptation traits such as rectal temperature and coat score (Prayaga et al., 2009; Riley et al., 2012, 2015b). The almost immediate adaptation to local conditions of B. indicus × B. taurus crossbreds, particularly adapted breeds crossed with nonadapted breeds, and high levels of heterosis probably represent the 2 main reasons that cattlemen in the southern United States use Brahman-crossbred cows.
Female longevity may be the ultimate adaptation trait, as annual compliance to reproductive standards may be an appropriate assessment of a combination of attributes that represent adaptation. Longevity has been documented relative to reproductive success in comparisons of B. indicus × B. taurus cows (Riley et al., 2001; Muntean et al., 2018). In general, maternal performance as indicated by calf quality was not considered when cows were annually evaluated for removal in those investigations. Engle et al. (2018) reported a genomic region on BTA 5 associated with the probability of avoiding Nelore-Angus crossbred cows remaining in the herd until 6 yr of age, also known as stayability. Components of longevity include reproduction success, udder health and structure (Tolleson et al., 2017), and incisor conditions as aged cows (Riley et al., 2001). The additive genetic component of longevity is not well characterized in beef cows, probably because of the time required for assessment of complete phenotypes. The estimation of that component for individuals is difficult for that reason as well as its low heritability. Therefore, selection of bulls and replacement females from aged, proven cows may be the best practical strategy for improvement of longevity in B. indicus-influenced herds in subtropical and tropical environments.
Differences in reproductive function between subspecies
With the expected increases in beef demand over the next decades, reproductive performance of beef females will not only determine the overall efficiency of cow–calf operations and the entire U.S. beef industry, but also have a significant impact on world food supply. When a cow does not become pregnant or fails to give birth to a live offspring, that cow does not provide a productive outcome in the way of protein (calf; the main feedstock for beef production) but still consumes valuable resources. Annual production losses associated with infertility of beef cattle in the United States are estimated at $1 billion (Lamb et al., 2008). According to the USDA, 33% of individual cattle within herds are culled annually because of reproductive failures (USDA, 2010). These failures are known to be greater in B. indicus cattle in tropical and subtropical regions (Reese et al., 2020), although the exact reasons for this outcome have not been identified and addressed.
Physiological differences between B. indicus and B. taurus females
Several research studies were conducted to characterize differences in reproductive physiology between B. indicus and B. taurus breeds (Table 1). The estimated age at puberty (first ovulation or estrus) for B. indicus raised in tropical and subtropical regions ranges from 16 to 40 mo (Nogueira, 2004), which is greater than the average of 15 mo for B. taurus breeds (Randel, 1990). Nonetheless, B. indicus heifers frequently attain the skeletal size required to support a healthy and safe pregnancy well before the establishment of regular estrous cycles. Delayed puberty in B. indicus breeds is attributed to both genetic and environmental factors, whereas crossbreeding is used to attenuate this outcome. As an example, Brangus heifers have an average of 17 to 18 mo of age at puberty (Randel, 1990).
Table 1.
Reproductive physiological differences between Bos indicus and Bos taurus females
| Reproductive characteristic | Bos taurus | Bos indicus | Reference |
|---|---|---|---|
| Age at puberty | ~12 mo | ~24 mo | Fajersson et al. (1991) Abeygunawardena and Dematawewa (2004) Nogueira et al. (2004) |
| Reproductive tract score in heifers at puberty | 52% RTS ≥ 4 | 25% RTS ≥ 4 | Locke et al. (2016) Thomas et al. (2017) |
| Number of small follicles during wave emergence | 15 to 33 | 30 to 60 | Carvalho et al. (2008) Gimenes et al. (2009) Bastos et al. (2010) |
| Follicular diameter at deviation | 8.5 to 9.0 mm | 5.0 to 7.0 mm | Ginther et al. (1996) Sartori et al. (2001) Castilho et al. (2007) |
| Diameter of ovulatory follicle | 13.9 to 17.1 mm | 11.3 to 14.0 mm | Sartori et al. (2004) Sartorelli et al. (2005) Mollo et al. (2007) |
| Diameter of corpus luteum | 20.0 to 30.0 mm | 17.0 to 21.0 mm | Ginther et al. (1989) Figueiredo et al. (1997) Bastos et al. (2010) |
| Preovulatory blood estradiol concentrations | 15 pg/mL | 17 pg/mL | Sartori et al. (2016) |
| Blood progesterone concentrations on day 7 of estrous cycle | 1.6 ng/mL | 3.1 ng/mL | Sartori et al. (2016) |
| Length of estrus behavior | 11 to 21 h | 10 to 15 h | Pinheiro et al. (1998) Bó et al. (2003) |
The ovarian function and circulating hormones of B. indicus and B. taurus have substantial differences even under similar environmental and nutritional conditions. Ovarian dynamics in B. indicus cattle is characterized by the occurrence of 2, 3, or sometimes 4 waves of follicular development vs. the predominantly 2 follicular waves observed in B. taurus breeds (Bó et al., 2003). The follicular dominance period is similar between subspecies, but B. indicus cows have smaller follicular size and greater antral follicle numbers than B. taurus (Bó et al., 2003). A study comparing Holstein and Nelore multiparous cows indicated that at follicular wave emergence, the number of 2 to 5 mm follicles present in the ovaries was 50% greater in Nelore cows compared with Holstein cows (Sartori et al., 2016). In that same study, follicle diameter at deviation and ovulation, as well as corpus luteum volume was less in Nelore cows (Sartori et al., 2016). Another interesting difference is in the oocyte maturation process, where oocytes from B. indicus animals are less sensitive to heat stress (HS) and more likely to develop into an embryo after fertilization compared with oocytes from B. taurus animals in tropical and subtropical conditions (Camargo et al., 2007).
Differences also exist in concentrations of hormones between B. indicus and B. taurus cattle. Brahman cows have greater basal concentrations of insulin-like growth factor I (IGF-I) but reduced concentrations of follicle-stimulating hormone than Angus cows (Alvarez et al., 2000). These latter authors suggested that increased circulating IGF-I may be responsible for the greater follicular number in B. indicus-based breeds. Circulating concentrations of luteinizing hormone (LH) during the preovulatory LH surge is also less in Brahman than B. taurus-based breeds (Randel, 1990; Bó et al., 2003). However, circulating concentrations of insulin, progesterone (P4), and preovulatory estradiol (E2) peak during the estrous cycle were greater in Nelore than Holstein cows (Sartori et al., 2016). It has been proposed that B. indicus have less liver steroid metabolism and increased steroid production by the ovaries than B. taurus, which may be associated with greater circulating insulin and IGF-1 in B. indicus females.
Bos indicus cows often have decreased pregnancy rates compared to B. taurus females due to their reduced ovulation rate (Carvalho et al., 2008). However, if they exhibit estrus, B. indicus fertility tends to be similar to B. taurus females (Randel, 1990). Estrus is the period that the female accepts to be mounted and its behavior is induced by high concentrations of estrogens. It was reported that Brahman and Brahman-based cows have shorter and less intense estrus behavior and they ovulate earlier than Angus cows (Randel, 1990). In addition, B. indicus cows take longer to exhibit estrus after exogenous estradiol injection than Hereford cows. This explains the fact that Brahman cows have a shorter period from the onset of estrus to ovulation than Hereford cows (Randel, 1990). However, recent data using modern estrus detection systems did not observe differences in interval from estrus to ovulation between cow genotypes (Bó et al., 2003). Estrus is also affected by social hierarchy in B. indicus cows (Chenoweth, 1994). Dominant cows tend to delay estrus expression after induction of luteolysis and are less likely to stand to be mounted (Bó et al., 2003).
Pregnancy differences between B. indicus and B. taurus females
Although B. indicus and B. taurus cattle undergo the same reproductive processes to generate a viable offspring, they have different gestation length (291 ± 1 and 282 ± 1 d, respectively; Paschal et al., 1991). Research has also been conducted to elucidate the differences in embryo or fetal development between B. indicus and B. taurus cattle, and contrast pregnancy development associated with the maternal environment vs. the genetics of the embryo. Mercadante et al. (2013) determined that cows containing primarily Angus genetics (> 80% Angus) had larger fetuses than cows with at least 20% Brahman genetics (>20% Brahman) at day 53 of pregnancy. A second study examined fetus size at days 35 and 62 of gestation. Fetus size did not differ in Angus and Brangus cows at day 35, but at day 62, fetuses in Angus cows were larger than fetuses in Brangus cows. In a third study, fetus size did not differ in Angus and Brangus cows on or before day 48 of pregnancy, but fetus size was greater in Angus than Brangus cows at days 54 to 55 of pregnancy. Collectively, these studies indicate B. taurus-influenced fetuses are larger than B. indicus-influenced fetuses around 2 mo of gestation.
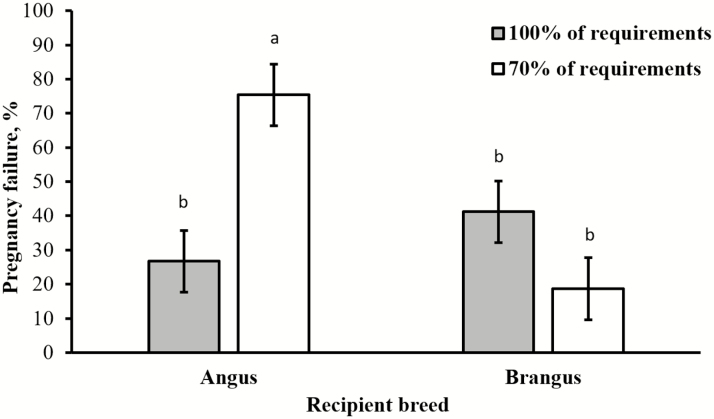
Fontes et al. (2019) further investigated how inclusion of B. indicus genetics in both maternal and fetal systems alters fetal plane of development during early gestation, particularly when nutrient availability is limited as in subtropical and tropical regions. The most significant findings of this study were the differences in pregnancy failure between the embryo and embryo recipient breeds under the nutritional restriction (Fig. 4). Angus recipients submitted to the nutritional restriction had increased pregnancy failure compared with Angus recipients receiving a maintenance diet, and also compared with Brangus recipients in both restricted and maintenance diets. Regardless of the recipient cow breed, those in the restricted diet that received an Angus embryo experienced greater pregnancy failure than counterparts in the restricted diet receiving Brangus embryos. Collectively, these results indicate that the magnitude of the detrimental effect of nutrient restriction differs between B. taurus and B. indicus cattle. Bos taurus genotype in both maternal and fetal systems decreased the probability of pregnancy when nutrient restriction was superimposed, corroborating that B. indicus-influenced cows were less susceptible to experience embryonic loss when exposed to a negative nutritional plane.
Figure 4.
Effects of recipient breed (Angus or Brangus) on pregnancy failure at day 28 of gestation. Cows received diets to provide 100% or 70% of their daily nutrient requirements. Dietary treatments were designed based on the specific requirements assumed for Angus and Brangus breeds, according to the National Academies of Sciences, Engineering, and Medicine (2016). A recipient breed × diet interaction was detected (P < 0.01), and values with different superscripts (a,b) differ (P < 0.05). Adapted from Fontes et al. (2019).
Subspecies genotype also affects plasma pregnancy-associated glycoprotein (PAG) concentrations during pregnancy. These proteins are part of a large family of aspartic proteases detected in the maternal circulation throughout most of pregnancy (Xie et al., 1997; Telugu et al., 2009; Pohler et al., 2016). Recent work determined that PAG concentrations are greater in B. indicus-influenced cows early gestation compared with B. taurus females (Mercadante, et al., 2013; Pohler et al., 2016). There also were positive correlations between fetal size and PAG concentrations within breed. Although differences in both fetal size and PAG concentrations exist between breeds, there still is a trend for pregnancies containing larger fetuses to produce more PAGs. Nonetheless, B. indicus-based genetics produce smaller fetuses but greater plasma PAG concentrations. Elevated plasma PAG concentrations exist in physiological scenarios with placental insufficiencies and pregnancy loss (Perry et al., 2005; Thompson et al., 2010; Pohler et al., 2016). Too little mechanistic information exists to provide insight into whether a placental insufficiency phenomenon exists in B. indicus cattle or fetuses. A more reasonable presumption is that differences in PAG profiles reflect yet another genotype-dependent alteration in the normal progression of pregnancy between B. taurus and B. indicus cattle. Recent work suggests that the embryo genotype and more specifically the parental genotype are the critical factor in determining PAG production and secretion (Franco et al., 2018; Reese et al., 2019). Because PAGs are placental products and studies in rodents have reported major influence from the paternal genome on placental development (Kaufman et al., 1977; Surani et al., 1984; Barton et al., 1985), one could reason the major influence that sire genotype and subspecies might have on circulating PAG concentrations during pregnancy.
Current concepts in female reproductive management
Management of Bos indicus-influenced replacement heifers in the United States
Nutrition is one of the primary environmental factors that regulate the puberty process in cattle. Nutritional management involving continuous, high rates of gain is one option for promoting the timely onset of puberty using the concept of “targeted” BW. This approach sets a target of 60% to 65% of mature BW as a practical rule of thumb for individual heifers to have reached puberty. The physiological precept of this principle is that heifers are genetically programmed to reach puberty at a predetermined size (Lamond, 1970; Taylor and Fitzhugh, 1971). Recent studies indicate that such targets also represent a minimum level of adiposity and a threshold circulating level of leptin (Williams et al., 2002). Therefore, it is clear that genetic composition will have a major impact on what 65% of mature BW represents. Heifers that ultimately will have a large frame size at maturity (eg, frame score 9 and mature BW > 650 kg) will need to reach a much heavier BW to reach puberty compared with those with an expected mature frame size of 1 and mature BW of <450 Kg (Fox et al., 1988). However, puberty can be accelerated or delayed by nutrition both before and after weaning. Wiltbank et al. (1966) indicated that BW gain during the preweaning period may have a greater influence on age at puberty than postweaning BW gain. This falls in line with the concept of precocious puberty (puberty reached at 10 mo of age or less) resultant from early-age (4 to 7 mo of age) exposure to high-density diets in B. taurus heifers (Gasser et al. 2006).
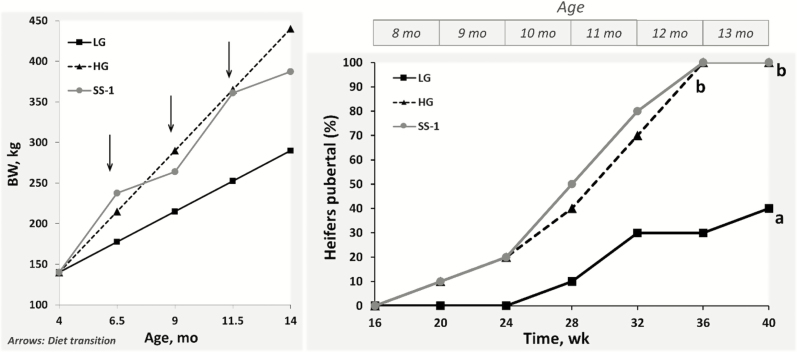
Although there is opportunity for manipulating early calfhood nutrition to accelerate puberty, this can result in excessive fattening, impairment of mammary development, and risk of unwanted, ill-timed pregnancies. Strategies to nutritionally program heifers in a manner that promotes timely onset of puberty while avoiding excessive fattening are needed. This is particularly relevant to tropical and subtropical regions, where later-maturing breeds predominate. In this regard, recent studies in B. indicus-influenced beef heifers demonstrate that metabolic programming of processes underlying puberty can be shifted temporally by using a stair-step compensatory growth model (Cardoso et al., 2014; Fig. 5). These studies suggest that feeding heifers a high-concentrate diet during critical windows of development (4 to 9 mo of age) results in changes in the metabolic endocrine status, characterized by elevated circulating concentrations of leptin, insulin, and IGF-I. These hormones are known to stimulate puberty onset, allowing optimal timing of sexual maturation in replacement beef heifers (Jones et al., 1991; Garcia et al., 2002). The use of nutritional strategies such as the stair-step regimen can also substantially reduce the feeding costs associated with the development of replacement heifers.
Figure 5.
Stair-step, compensatory growth model for development of Bos indicus-influenced beef heifers. Left panel depicts different rates of BW gain of heifers weaned at approximately 4 mo of age and subjected to 1 of 3 nutritional treatments: high BW gain (HG), low BW gain (LG), or stair-step BW gain (SS-1). Right panel depicts the cumulative percentage of heifers that attained puberty throughout the study. Adapted from Cardoso et al. (2014).
Exogenous P4 can induce cyclicity in prepubertal heifers, and estrus synchronization protocols that include supplemental P4 can be strategically utilized by cattle producers to hasten reproductive development and pregnancy attainment in B. indicus-influenced heifers. The effectiveness of these strategies, however, is highly dependent on heifer nutritional and metabolic status. Acceptable pregnancy rates to fixed-time artificial insemination (AI) were observed when B. indicus and B. indicus × B. taurus heifers were fed to reach approximately 300 kg prior to breeding at 12 to 15 mo of age, and exposed to a P4 and estradiol-based puberty induction protocol (Day and Nogueira, 2013). The use of estradiol in estrus synchronization, however, is not legal in the United States but is approved in South America, which will be discussed later in this manuscript. Nonetheless, there are currently no definite GnRH-based protocols for estrus synchronization and fixed-time AI in B. indicus-influenced heifers. The use of a short-term GnRH-based protocol has been evaluated in B. indicus × B. taurus replacements (Oosthuizen et al., 2018). This protocol consists in 100 µg of GnRH + 25 mg prostaglandin F2α (PGF) analogue injection + controlled internal P4 releasing insert (CIDR) on day 0, followed by 50 mg of PGF and CIDR removal on day 5, and 100 µg of GnRH and fixed-time AI 66 ± 2 h later. Oosthuizen et al. (2018) reported that this strategy resulted in a greater proportion of heifers becoming pregnant within the first 21 d of the breeding season, whereas others have reported inconsistent results when utilizing a similar protocol for Brahman heifers (Yelich and Bridges, 2012). Hence, there is a need for the development of pharmacological strategies tailored specifically for B. indicus-influenced heifers in the United States that do not rely on estradiol-based products.
Management of Bos indicus-influenced cows in the United States
Similar to replacement heifers, there are limited pharmacological alternatives specifically developed for cows with substantial B. indicus influence in the United States. A protocol similar to that described by Oosthuizen et al. (2018) is the current recommendation for mature B. indicus-influenced cows. A PGF injection is given at the beginning of the protocol in conjunction with the first GnRH, and a second injection of PGF is administered 8 h after CIDR removal. Fixed-time AI is also performed 66 h after CIDR removal. The rationale behind the PGF injection at CIDR insertion is based on data indicating that B. indicus females appear to be more sensitive to the effects of P4 on gonadotropin release (Randel, 1984; Carvalho et al., 2008). Hence, decreasing concentrations of P4 may facilitate follicular development and consequently improve pregnancy rates in these females. More recently, Scarpa et al. (2019) recommended that the initial GnRH injection should not be administered, which allows for the elimination of the second PGF injection 8 h after CIDR removal.
Estrus expression near the time of fixed-time AI is correlated with pregnancy success in both B. taurus and B. indicus females due to positive impacts on ovarian function and uterine environment, which affects embryo development and pregnancy maintenance (Sá Filho et al., 2010; Davoodi et al., 2016; Pohler et al., 2016). Preovulatory estradiol associated with estrus expression directly affects pregnancy establishment and maintenance through several physiological events including gamete transport and preparation of uterine environment (Hawk and Cooper, 1975; Buhi, 2002; Pohler et al., 2012). A recent study from Franco et al. (2018) tested whether pregnancy rates differed among sires when B. indicus cows expressed estrus before fixed-time AI, and if breed or sire affected the fertility. Not surprisingly, there was a major influence of sire on pregnancy rates and subsequent pregnancy loss; however, what was surprising is the effect subspecies of the sire played. Cows inseminated with an Angus sire had a 1.73-fold increase in pregnancy rates when they expressed estrus before or at the time of fixed-time AI (61.0% vs. 35.2%, respectively). Within cows inseminated with a Nelore sire, a 1.22-fold increase noted (72.6% vs. 59.2%, respectively). Perhaps B. indicus embryos are capable of withstanding HS and other environment challenges during early gestation compared with B. taurus counterparts. Barros et al. (2006) reported increased survival of B. indicus embryos in elevated temperatures during early gestation and embryonic development compared to B. taurus genotypes. However, Block et al. (2002) reported no effect of the genetic background of the spermatozoa in relation to embryo survival. There is not a clear reason for variation in pregnancy success and interaction with estrus expression among sire subspecies. Nonetheless, differences may exist in sperm characteristics (genetic or molecular) that explain why some sires seem to be more resilient to changes in the uterine environment compared with others, which may be related with changes in sperm longevity (Franco et al., 2018).
Management of Bos indicus-influenced females in South America
Bos indicus breeds also predominate in South America countries such as Brazil, which contains the largest commercial beef herd of the world (USDA, 2019). Research conducted in this region during the past 20 yr yielded the development of reproductive tools specifically tailored to B. indicus-influenced females reared in tropical and subtropical environments, particularly hormonal treatments to control ovarian dynamics and allow the use of fixed-time AI (Vasconcelos et al., 2014).
As in many other tropical and subtropical regions of the globe, the prevalence of anestrous females at the beginning of the breeding season is a major reproductive challenge to Brazilian cow–calf producers. This outcome is a combination of environmental stressors and the physiological features of B. indicus breeds. Strategies to induce cyclicity are paramount to reproductive management of these females, including use of exogenous P4 via CIDR insertion and temporary calf removal in parous cows (Vasconcelos et al., 2014). These strategies, in addition to PGF and other exogenous hormones approved for use outside the United States [estradiol benzoate, estradiol cypionate, and equine chorionic gonadotropin (eCG)] constitute the latest reproductive technologies used by the Brazilian cow–calf industry. The use of estradiol benzoate and cypionate are not only less expensive, but also more efficient in initiating a new follicular wave in B. indicus females compared with GnRH-based protocols (Baruselli et al. 2004). Accordingly, Meneghetti et al. (2009) developed a synchronization + fixed-time AI protocol for B. indicus cows that consisted in CIDR insertion + 2 mg of estradiol benzoate on day 0, CIDR withdrawal + 12.5 mg of PGF + 0.5 mg of estradiol cypionate + temporary calf removal on day 9, followed by fixed-time AI + calf return on day 11. This protocol resulted in synchronization and pregnancy rates of 90% and 50%, respectively, whereas the CIDR can be used as many as 4 times with no detrimental effects on pregnancy success (Table 2). Subsequently, Sá Filho et al. (2009) evaluated additional strategies to improve the protocol developed by Meneghetti et al. (2009) and noted that replacing temporary calf removal with eCG administration (400 IU) yielded similar pregnancy rates (Table 2). Nonetheless, combining eCG administration and calf removal did not yield any additional benefits to reproductive success, given that calf removal stimulated LH secretion in a manner that additional gonadotropin support from eCG was not required for final follicular development (Sá Filho et al. 2009). Therefore, the hormonal protocol proposed by Meneghetti et al. (2009) should include either calf removal or eCG administration, but not both, to maximize reproductive performance of B. indicus cows.
Table 2.
Ovulation and pregnancy rates to fixed-time AI of Bos indicus cows submitted to ovulation synchronization protocols with different hormonal strategies
| Hormonal strategy | Ovulation rate, % | Pregnancy rate, % |
|---|---|---|
| Ovulatory stimulus1 | ||
| Estradiol cypionate (n = 315) | 89.5 | 50.8 |
| Estradiol benzoate (n = 318) | 90.9 | 51.9 |
| GnRH (n = 312) | 91.0 | 54.2 |
| CIDR uses2 | ||
| New (n = 69) | 79.7 | 43.5 |
| One use (n = 180) | 80.0 | 48.9 |
| Two uses (n = 224) | 83.9 | 50.4 |
| Three uses (n = 105) | 85.7 | 49.5 |
| eCG to replace calf removal3 | ||
| Negative control (n = 221) | 81.9 | 41.6b |
| 300 IU of eCG (n = 208) | 80.8 | 46.6ab |
| 400 IU of eCG (n = 223) | 83.9 | 54.3a |
| Calf removal (n = 239) | 86.6 | 51.5a |
| eCG associated with calf removal4 | ||
| Calf removal (n = 297) | 88.9 | 53.2 |
| Calf removal + 200 IU of eCG (n = 299) | 93.0 | 51.8 |
| Calf removal + 400 IU of eCG (n = 291) | 90.0 | 52.2 |
| Time of PGF treatment and eCG5 | ||
| PGF on day 7 + 0 IU eCG (n = 178) | 80.3b | 47.7b |
| PGF on day 7 + 300 IU eCG (n = 172) | 90.7a | 56.4a |
| PGF on day 9 + 0 IU eCG (n = 174) | 64.4c | 27.0c |
| PGF on day 9 + 300 IU eCG (n = 178) | 89.3a | 45.5b |
Adapted from Meneghetti et al. (2009), Peres et al. (2009), and Sá Filho et al. (2009).
1Cows received estradiol cypionate (0.5 mg) on day 9, estradiol benzoate (1.0 mg) on day 10, or GnRH (100 µg) on day 11 of the protocol. No treatment effects were noted (P > 0.10).
2Cows received a CIDR (originally containing 1.9 g of progesterone; Zoetis, São Paulo, SP, Brazil) from days 0 to 9 of the protocol. No treatment effects were noted (P > 0.10).
3Cows received no treatment (negative control), 300 IU of equine chorionic gonadotropin (eCG), 400 IU of eCG, or calf removal (48 h) on day 9 of the protocol. Means with different superscripts differ (a, b) differ (P < 0.05).
4Cows received calf removal (48 h) or calf removal eCG at 200 or 400 IU on day 9 of the protocol. No treatment differences were noted (P > 0.10).
5Cows received 12.5 mg of prostaglandin F2α (PGF) analogue on day 7 or 9, and 0 IU or 300 IU of eCG on day 9 of the protocol. Means with different superscripts differ (a, b, c) differ (P < 0.05).
Hormonal protocols to induce puberty attainment in nulliparous B. indicus heifers in Brazil have also been investigated, and include the use of exogenous P4 via CIDR insertion as in the United States. Claro Junior et al. (2010) reported that using a CIDR for 12-d hastened puberty and improved estrus detection and pregnancy rates at the beginning of the breeding season in prepubertal B. indicus heifers (Table 3). Moreover, a previously used CIDR is preferable to a new CIDR to induce puberty and improve reproductive performance during the breeding season (Table 3). Administering eCG and estradiol cypionate at the time of CIDR removal can also hasten puberty and pregnancy attainment in prepubertal B. indicus heifers (Rodrigues et al., 2013). Accordingly, Rodrigues et al. (2014) investigated the use of a puberty induction protocol based on a used CIDR for 12 d and administration of 200 IU of eCG + 0.5 mg of estradiol cypionate at CIDR removal. Heifers were also assigned to a fixed-time AI protocol (Meneghetti et al., 2009) 12 d after CIDR removal, and authors reported that 45.5% of heifers became pregnant to fixed-time AI. Subsequently, Rodrigues et al. (2016) applied the aforementioned puberty induction + fixed-time AI protocol to pubertal and prepubertal heifers, and reported similar conception rates between cyclicity groups (as described by Vasconcelos et al., 2017). Hence, authors concluded that combining this puberty induction protocol with fixed-time AI resulted in successful pregnancy in heifers inseminated with their first ovulation, diverging from the concept that fertility of the pubertal ovulation is not adequate (Byerley et al. 1987).
Table 3.
Reproductive responses of prepubertal Bos indicus heifers receiving a new CIDR (CIDR1, originally containing 1.9 g of progesterone; Zoetis, São Paulo, SP, Brazil), a CIDR previously used 3 times (CIDR4), or no CIDR insert1
| Reproductive response | No CIDR (n = 113) | CIDR1 (n = 237) | CIDR4 (n = 239) |
|---|---|---|---|
| Progesterone on day 0, ng/mL | 0.37a | 2.31b | 1.20c |
| Follicle diameter on day 0, mm | 9.45a | 9.72a | 11.42b |
| Estrus detection in 7 d, % | 19.5a | 42.6b | 39.3b |
| Pregnancy rate in 7 d, % | 5.3a | 14.3b | 18.4b |
| Estrus detection in 45 d, % | 52.2a | 72.1b | 75.3b |
| Pregnancy rate in 45 d, % | 27.4a | 39.2b | 47.7c |
| Pregnancy rate in 90 d, % | 72.6a | 83.5b | 83.7b |
Treatments were applied from days −12 to 0 relative to the breeding season, which consisted in artificial insemination on estrus detection for 45 d followed by 45 d of natural service. Adapted from Claro Junior et al. (2010).
1Within a row, means without a common superscript (a, b, c) differ (P < 0.05).
Contrary to its benefits in stimulating cyclicity in prepubertal and anestrous females, providing exogenous P4 to cycling cattle during estrus synchronization elevates circulating concentrations of this hormone beyond optimal levels, reducing development of the dominant follicle and subsequent fertility (Dias et al. 2009; Sá Filho et al. 2009). Hence, Peres et al. (2009) modified the estrus synchronization protocol developed by Meneghetti et al. (2009) to advance PGF injection to day 7, and administer 300 IU of eCG on day 9 of the protocol to nonlactating B. indicus cows. Cows receiving this new protocol had the greatest ovulation and pregnancy rates compared with cohorts receiving the other combinations of PGF and eCG administration (Table 2). Hence, administration of PGF earlier in the protocol in addition to eCG is recommended to improve fertility of cycling cattle submitted to fixed-time AI protocols using P4 associated with estradiol.
Dairy production in tropical and subtropical climates
Global dairy production must also increase 63% by 2050 to feed world’s growing population, and support a projected 20% increase in milk consumption per capita (Food and Agricultural Organization [FAO], 2009). Similar to beef production, this increase in dairy supply is expected from tropical and subtropical regions of the planet, where dairy production needs to cope with the environmental challenges associated with hot and humid climates.
Heat stress and reproduction in dairy cows
The majority of studies examining HS today are conducted in tropical or subtropical areas (eg, southern United States, Mexico, Brazil, Israel) where the effects of HS on production animals are evident. However, animals housed in northern latitudes (Central Europe, northern United States, Canada) can also experience HS, where the summer season is relatively short but warm and there is a constant presence of radiant solar energy and high humidity (Schüller et al., 2014). Hence, several research efforts have attempted to characterize and mitigate the effects of HS on dairy production, with special attention to cattle reproduction and individual tolerance to HS.
Heat abatement strategies employed by dairy cows include increased respiration rate, drinking, sweating, and standing time (West, 2003). Cows under HS have reduced milk production, DMI, and physical activity (West, 2003). Their reproductive capabilities will decrease as HS disrupts follicular development, steroidogenesis, oocyte quality, and embryo development (Collier et al., 1982; De Rensis and Scaramuzzi, 2003). López-Gatius et al. (2004) reported that pregnancy loss for cows inseminated during the warm period in Spain was 3.7 times greater compared with animals inseminated during the cool season. Ultimately, the conception rates in the summer months can decrease 20% to 30% when compared to winter months, and even exceed a 76% decrease (De Rensis et al., 2002). Although the reduction in conception rate is associated with warmer months, fertility continues to remain low in autumn months despite the fact that ambient temperatures decrease and cows are no longer exposed to immediate HS (Wolfenson et al., 2000). This phenomenon accounts for more than 30% of “low summer fertility syndrome” (Wolfenson et al., 2000) and speculated to be driven by the delayed effects of HS on the ovaries and follicles (Roth et al., 2001).
Individual variation and the basis for thermal-tolerance measurement
The information presented in this section is resulted from a large field trial conducted within a controlled environment that allowed for precise temperature humidity index (THI) measurements, and enrollment of homogeneous groups of cows being exposed to an identical environment down to the level of pen position in the barn (Polsky et al., 2017). During the analyses of this data set, special attention was given to control outcomes known to affect vaginal temperature. For that reason, the statistical models used for the analyses included parity, body condition score (BCS), milk production, and THI.
Research by Polsky et al. (2017) was conducted for a full year on a commercial dairy farm located in Brazil. The herd (1,700 lactating Holstein cows) had a 305-d average yield of 11,438 kg per cow. Lactating cows (n = 641; 209 primiparous; 432 multiparous) with more than 50 d in milk were enrolled onto the study on a weekly basis. Cows were housed in a cross-ventilated free stall barn with grooved concrete floors. Cows were blocked in groups of 300 animals in pens with 2 rows of deep-bedded sand stalls (1 cow per stall). Milking was performed thrice daily (at approximately 0500, 1300, and 2100 h). Cows were fed a diet balanced to meet or exceed the nutritional requirements of lactating dairy cows producing 45 kg/d of 3.5% fat corrected milk (NRC, 2001). Feed was delivered twice daily at approximately 0700 h and 1600 h and pushed up 3 times/d. A humidity and temperature-measuring instrument (HOBO U23 Pro v2 Relative Temperature/Humidity Data Logger, Onset HOBO Data Loggers, Cape Cod, MA) was placed inside the barn. Temperature and humidity data were recorded every 10 min for 3 consecutive days. A temperature recording data logger (Thermochron iButton, Lawrenceburg, KY) was coupled to a CIDR (with no P4), allowing the sensors to be in direct contact with the vaginal wall, and recorded vaginal temperature continuously every 10 min for 9 d prior to fixed-time AI.
Vaginal temperature outcomes
One of the objectives of study was to determine the best way to quantify the animal’s experience of HS, and how heat load is related to productive and reproductive responses. Vaginal thermometers are a noninvasive method that allows body temperature to be continuously recorded. Different metrics of temperature variation at varying thresholds were examined, including temperature bouts (number of episodes when the cow’s body temperature exceeded 39.1 °C), peaks of highest temperature, temperature amplitude, and total heat load (area under the curve for the total time and highest temperature episode greater than 39.1 °C). Ultimately, percentage of time (in minutes) 9 to 11 d prior to breeding when the cow’s vaginal temperature was ≥39.1 °C (PCT39) was chosen. This metric was the most functional to represent HS because of the significantly negative relationship with estrus expression, pregnancy success, and lack of collinearity with THI. This measurement also agrees with the findings observed by Vasconcelos et al. (2011) during an embryo transfer experiment. Future research should continue to examine new ways to explore continuous temperature measurements that quantify HS, which incorporate the animal’s individual daily variation and basal body temperatures.
The mean vaginal temperature during 3 d of recording period was 38.9 ± 0.24 °C, with mean maximum and minimum temperatures of 39.7 ± 0.46 °C and 38.0 ± 0.75 °C, respectively. The average vaginal temperature amplitude was 1.70 ± 0.87 °C. The mean PCT39 was 27.6 ± 23.1%, with a Q1 and Q3 distribution of 8.5% and 40.8%, respectively. The mean calculated THI during the hot season was 68.9 ± 2.9, with maximum and minimum values of 73.8 and 61.5, respectively. The mean calculated THI during the dry season was 64.3 ± 2.0, with maximum and minimum values of 68.3 and 61.0, respectively. The Pearson correlation coefficient between PCT39 and THI was 0.30 (P < 0.001). The maximum vaginal temperature and amplitude were also weakly correlated (P < 0.01) to THI (r = 0.28 and 0.17, respectively).
Associations with parity, BCS, milk production, and THI.
Body condition score and milk production were recorded at the time of thermometer insertion. Risk factors associated with PCT39 were analyzed in relation to parameters such as parity, BCS, milk production, and THI. The results showed that there were effects of BCS, milk production, and THI on PCT39. Cows with low BCS (≤2.75) remained longer with vaginal temperature above 39.1 °C (higher PCT39) relative to cows with moderate BCS (P = 0.01). Primiparous cows with milk production above the median (38.2 kg/d) presented higher PCT39 than primiparous cows below the median (P = 0.01), and the same relationship was observed for multiparous (median, 46.2 kg/d). Generally, the higher environmental temperature indeed increased on average time spent with vaginal temperature above 39.1 °C; however, the overall correlation between PCT39 and THI was surprisingly weak (r = 0.30). Among PCT39 and milk production, a weak correlation (r = 0.04) was also found.
The overall take from the production, animal parameters, and THI correlations with PCT39 was that the effect of individual variation was much larger than initially expected. The apparent uncoupling of THI, PCT39, and milk production, for example, shows that a sizable population of cows can endure a THI > 68 or 70, produce >40 kg/d of milk and still avoid long periods of time with hyperthermia.
Association with pregnancy rates.
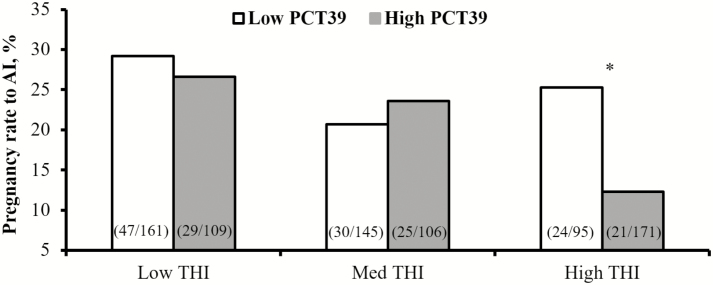
Results produced an interesting interaction between PCT39 and THI. A decrease in pregnancy rates was only observed in a subpopulation of cows with high PCT39 under high THI conditions (Fig. 6). The negative effect of ambient temperature on pregnancy rate has been well documented. Morton et al. (2007) estimated that a daily maximum THI of 72 or more from 35 d before to 6 d after breeding decreases conception rates of lactating dairy cows by 30%. Even exposure to THI ≥ 73 for 1 h on the day of breeding is sufficient to decrease the conception rate by 5%, whereas pregnancy rates of Holsteins significantly decline from 28.5% at low THI (<70) to 14.8% at high THI (>80) conditions (Schüller et al., 2014). Data from Demetrio et al. (2007) and Vasconcelos et al. (2011) also demonstrated that elevated body temperature measured on the day of embryo transfer had a negative effect on conception rates and embryonic retention. Despite the literature consensus, results from Polsky et al. (2017) demonstrate that even under elevated THI prior to breeding, there is a subgroup of cows who can thrive under these conditions and show similar pregnancy success to AI compared with cows who are under low heat load in low THI conditions. Pereira et al. (2013) found no difference for pregnancy success to AI in cows exposed to 1 bout of HS, or estrus cycle synchronization for animals exposed to 2 or more events of HS. The physiological mechanism behind maintaining high pregnancy rates under high THI conditions by sustaining moderate vaginal temperatures during the day is unclear. Future research should aim to elucidate this relationship, and promote dairy cattle welfare and production in tropical and subtropical regions of the globe.
Figure 6.
Pregnancy rates (%) to AI according to the interaction between THI (low: ≤65, med: 65 to 70, high: ≥70) and percentage of time (min) 9 to 11 d before breeding when PCT39 was classified as high (≥22.90%) or low (<22.90%). Values within parenthesis report pregnancy cows divided by total cows receiving AI. Within THI group, *P < 0.03. Adapted from Polsky et al. (2017).
Summary and conclusions
Bos indicus breeds are mostly used in the U.S. beef industry to generate B. indicus × B. taurus crosses with increased thermal and parasite tolerance, while retaining the productive features of B. taurus cattle. Research has also attempted to identify B. taurus genetics that can withstand subtropical and tropical climates. Reduced heritability for marbling, hastened shedding of accumulated winter hair coat, reduced milk production, and delayed reproductive maturation appear to be related with tropical adaptation of B. taurus breeds; the latter two as means to conserve energy under stressful conditions and limited nutrition. Longevity is considered the ultimate adaptation response to unfavorable environments, and retention of offspring from proven cows is the recommended strategy to improve this trait in B. indicus-influenced herds. Other aspects should also be considered when planning breeding and reproductive management in tropical and subtropical regions, given that B. indicus and B. taurus breeds have multiple differences in reproductive functioning. Limited pharmacological alternatives are available for reproductive management of B. indicus-influenced females in the United States, which rely on GnRH-based protocols mostly designed for B. taurus breeds. In South America, estradiol-based protocols were specifically tailored for B. indicus females, consistently yielding pregnancy rates ≥ 50% to fixed-time AI across parities. The dairy industry is also challenged with increasing global demand for milk and its products. Selection of dairy cows capable of sustaining optimal milk yield and reproductive success in hot and humid conditions is essential for worldwide dairy production. Collectively, efforts to understand the biological challenges and optimize beef and dairy production in subtropical and tropical climates are still warranted, and will contribute toward global agricultural sustainability and food security.
Glossary
Abbreviations
- AI
artificial insemination
- BCS
body condition score
- CIDR
controlled internal progesterone releasing insert
- CIDR1
CIDR used for the first time
- CIDR4
CIDR previously used 3 times
- DMI
dry matter intake
- E2
estradiol
- eCG
equine chorionic gonadotropin
- HS
heat stress
- IGF-I
insulin-like growth factor I
- LH
luteinizing hormone
- P4
progesterone
- PAG
pregnancy-associated glycoprotein
- PCT39
vaginal temperature ≥ 39.1°C
- PGF
prostaglandin F2α
- SS-1
stair-step BW gain
- THI
temperature humidity index
Acknowledgments
This manuscript is based on presentations at the Beef Species Symposium: Cattle adapted to Tropical and Subtropical Environments, during the 2019 Annual Meeting of the American Society of Animal Science, 8 to 11 July, 2019, Austin, TX. All authors had equal contribution to this manuscript, and order of authorship (besides the corresponding author) is based on alphabetical order. Authors from Texas A&M—Department of Animal Science are part of the Area of Excellence in Cattle Adapted to Tropical and Subtropical Environments.
Conflict of interest statement
No conflict of interest to disclose.
Literature Cited
- Abeygunawardena H., and Dematawewa C. M.. . 2004. Pre-pubertal and postpartum anestrus in tropical Zebu cattle. Anim. Reprod. Sci. 82-83:373–387. doi: 10.1016/j.anireprosci.2004.05.006 [DOI] [PubMed] [Google Scholar]
- Alvarez P., Spicer L. J., Chase C. C. Jr., Payton M. E., Hamilton T. D., Stewart R. E., Hammond A. C., Olson T. A., and Wettemann R. P.. . 2000. Ovarian and endocrine characteristics during an estrous cycle in Angus, Brahman, and Senepol cows in a subtropical environment. J. Anim. Sci. 78:1291–1302. doi: 10.2527/2000.7851291x [DOI] [PubMed] [Google Scholar]
- Barros C. M., Pegorer M. F., Vasconcelos J. L., Eberhardt B. G., and Monteiro F. M.. . 2006. Importance of sperm genotype (indicus versus taurus) for fertility and embryonic development at elevated temperatures. Theriogenology 65:210–218. doi: 10.1016/j.theriogenology.2005.09.024 [DOI] [PubMed] [Google Scholar]
- Barton S. C., Adams C. A., Norris M. L., and Surani M. A.. . 1985. Development of gynogenetic and parthenogenetic inner cell mass and trophectoderm tissues in reconstituted blastocysts in the mouse. J. Embryol. Exp. Morphol. 90:267–285. [PubMed] [Google Scholar]
- Baruselli P. S., Reis E. L., Marques M. O., Nasser L. F., and Bó G. A.. . 2004. The use of hormonal treatments to improve reproductive performance of anestrous beef cattle in tropical climates. Anim. Reprod. Sci. 82–83:479–486. doi: 10.1016/j.anireprosci.2004.04.025 [DOI] [PubMed] [Google Scholar]
- Bastos M. R., Mattos M. C. C., Meschiatti M. A. P., Surjus R. S., Guardieiro M. M., Mourão G. B., Pires A. V., Pedroso A. M., Santos F. A. P., and Sartori R.. . 2010. Ovarian function and circulating hormones in nonlactating Nelore versus Holstein cows. Acta Sci. Vet. 38:776. [Google Scholar]
- Block J., Chase C. C. Jr., and Hansen P. J.. . 2002. Inheritance of resistance of bovine preimplantation embryos to heat shock: Relative importance of the maternal versus paternal contribution. Mol. Reprod. Dev. 63:32–37. doi: 10.1002/mrd.10160 [DOI] [PubMed] [Google Scholar]
- Bó G. A., Baruselli P. S., and Martinez M. F.. . 2003. Pattern and manipulation of follicular development in Bos indicus cattle. Anim. Reprod. Sci. 78:307–326. doi: 10.1016/S0378-4320(03)00097-6 [DOI] [PubMed] [Google Scholar]
- Buhi W. C. 2002. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction 123:355–362. doi: 10.1530/rep.0.1230355 [DOI] [PubMed] [Google Scholar]
- Byerley D. J., Staigmiller R. B., Berardinelli J. G., and Short R. E.. . 1987. Pregnancy rates of beef heifers bred either on puberal or third estrus. J. Anim. Sci. 65:645–650. doi: 10.2527/jas1987.653645x [DOI] [PubMed] [Google Scholar]
- Camargo, L. S., J. H. Viana, A. D. Ramos, R. V. Serapião, W. F. de Sa, A. D. Ferreira, M. F. Guimarães, and V. R. do Vale Filho. 2007. Developmental competence and expression of the Hsp 70.1 gene in oocytes obtained from Bos indicus and Bos taurus dairy cows in a tropical environment. Theriogenology 68:626–632. doi:10.1016/j.theriogenology.2007.03.029. doi: 10.1016/j.theriogenology.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Cardoso R. C., Alves B. R., Prezotto L. D., Thorson J. F., Tedeschi L. O., Keisler D. H., Park C. S., Amstalden M., and Williams G. L.. . 2014. Use of a stair-step compensatory gain nutritional regimen to program the onset of puberty in beef heifers. J. Anim. Sci. 92:2942–2949. doi: 10.2527/jas.2014-7713 [DOI] [PubMed] [Google Scholar]
- Carvalho J. B. P., Carvalho N. A. T., Reis E. L., Nichi M., Souza A. H., and Baruselli P. S.. . 2008. Effect of early luteolysis in progesterone-based timed AI protocols in Bos indicus, Bos indicus × Bos taurus, and Bos taurus heifers. Theriogenology 69:167–175. doi: 10.1016/j.theriogenology.2007.08.035 [DOI] [PubMed] [Google Scholar]
- Castilho C., Garcia J. M., Renesto A., Nogueira G. P., and Brito L. F.. . 2007. Follicular dynamics and plasma FSH and progesterone concentrations during follicular deviation in the first post-ovulatory wave in Nelore (Bos indicus) heifers. Anim. Reprod. Sci. 98:189–196. doi: 10.1016/j.anireprosci.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Chenoweth P. J. 1994. Aspects of reproduction in female Bos indicus cattle: A review. Aust. Vet. J. 71:422–426. doi: 10.1111/j.1751-0813.1994.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Claro Junior I., Sá Filho O. G., Peres R. F., Aono F. H., Day M. L., and Vasconcelos J. L.. . 2010. Reproductive performance of prepubertal Bos indicus heifers after progesterone-based treatments. Theriogenology 74:903–911. doi: 10.1016/j.theriogenology.2010.04.015 [DOI] [PubMed] [Google Scholar]
- Collier R. J., Doelger S. G., Head H. H., Thatcher W. W., and Wilcox C. J.. . 1982. Effects of heat stress during pregnancy on maternal hormone concentrations, calf birth weight and postpartum milk yield of Holstein cows. J. Anim. Sci. 54:309–319. doi: 10.2527/jas1982.542309x [DOI] [PubMed] [Google Scholar]
- Cooke R. F., Daigle C. L., Moriel P., Smith S. B., Tedeschi L. O., and Vendramini J. M. B.. . 2020. Cattle adapted to tropical and subtropical environments: social, nutritional, and carcass quality considerations. J. Anim. Sci. doi: 10.1093/jas/skaa014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi S., Cooke R. F., Fernandes A. C., Cappellozza B. I., Vasconcelos J. L., and Cerri R. L.. . 2016. Expression of estrus modifies the gene expression profile in reproductive tissues on Day 19 of gestation in beef cows. Theriogenology 85:645–655. doi: 10.1016/j.theriogenology.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Day M. L., and Nogueira G. P.. . 2013. Management of age at puberty in beef heifers to optimize efficiency of beef production. Anim. Front. 3:6–11. doi: 10.2527/af.2013-0027 [DOI] [Google Scholar]
- Demetrio D. G., Santos R. M., Demetrio C. G., and Vasconcelos J. L.. . 2007. Factors affecting conception rates following artificial insemination or embryo transfer in lactating Holstein cows. J. Dairy Sci. 90:5073–5082. doi: 10.3168/jds.2007-0223 [DOI] [PubMed] [Google Scholar]
- De Rensis F., Marconi P., Capelli T., Gatti F., Facciolongo F., Franzini S., and Scaramuzzi R. J.. . 2002. Fertility in postpartum dairy cows in winter or summer following estrus synchronization and fixed time AI after the induction of an LH surge with GnRH or hCG. Theriogenology 58:1675–1687. doi: 10.1016/s0093-691x(02)01075-0 [DOI] [PubMed] [Google Scholar]
- De Rensis F., and Scaramuzzi R. J.. . 2003. Heat stress and seasonal effects on reproduction in the dairy cow – A review. Theriogenology 60:1139–1151. doi: 10.1016/s0093-691x(03)00126-2 [DOI] [PubMed] [Google Scholar]
- Dias C. C., Wechsler F. S., Day M. L., and Vasconcelos J. L.. . 2009. Progesterone concentrations, exogenous equine chorionic gonadotropin, and timing of prostaglandin F (2alpha) treatment affect fertility in postpuberal Nelore heifers. Theriogenology 72:378–385. doi: 10.1016/j.theriogenology.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Engle B. N., Herring A. D., Sawyer J. E., Riley D. G., Sanders J. O., and Gill C. A.. . 2018. Genome-wide association study for stayability measures in Nellore-Angus crossbred cows. J. Anim. Sci. 96:1205–1214. doi: 10.1093/jas/sky067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajersson P., Barradas H. V., Roman-Ponce H., and Cook R. M.. . 1991. The effects of dietary protein on age and weight at the onset of puberty in Brown Swiss and Zebu heifers in the tropics. Theriogenology 35:845–855. doi: 10.1016/0093-691x(91)90425-d [DOI] [PubMed] [Google Scholar]
- Figueiredo R. A., Barros C. M., Pinheiro O. L., and Soler J. M.. . 1997. Ovarian follicular dynamics in Nelore breed (Bos indicus) cattle. Theriogenology 47:1489–1505. doi: 10.1016/s0093-691x(97)00156-8 [DOI] [PubMed] [Google Scholar]
- Fontes P. L. P., Oosthuizen N., Ciriaco F. M., Sanford C. D., Canal L. B., Pohler K. G., Henry D. D., Mercadante V. R. G., Timlin C. L., Ealy A. D., . et al. 2019. Impact of fetal vs. maternal contributions of Bos indicus and Bos taurus genetics on embryonic and fetal development1. J. Anim. Sci. 97:1645–1655. doi: 10.1093/jas/skz044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agricultural Organization (FAO). 2009. How to feed the world in 2050. In: Proc. Expert Meeting on How to Feed the World in 2050 FAO Headquarters, Rome, Italy. [Google Scholar]
- Fox D. G., Sniffen C. J., and O’Connor J. D.. . 1988. Adjusting nutrient requirements of beef cattle for animal and environmental variations. J. Anim. Sci. 66:1475–1495. doi: 10.2527/jas1988.6661475x [DOI] [Google Scholar]
- Franco G. A., Peres R. F. G., Martins C. F. G., Reese S. T., Vasconcelos J. L. M., and Pohler K. G.. . 2018. Sire contribution to pregnancy loss and pregnancy-associated glycoprotein production in Nelore cows. J. Anim. Sci. 96:632–640. doi: 10.1093/jas/sky015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. R., Amstalden M., Williams S. W., Stanko R. L., Morrison C. D., Keisler D. H., Nizielski S. E., and Williams G. L.. . 2002. Serum leptin and its adipose gene expression during pubertal development, the estrous cycle, and different seasons in cattle. J. Anim. Sci. 80:2158–2167. doi: 10.2527/2002.8082158x [DOI] [PubMed] [Google Scholar]
- Gasser C. L., Behlke E. J., Grum D. E., and Day M. L.. . 2006. Effect of timing of feeding a high-concentrate diet on growth and attainment of puberty in early-weaned heifers. J. Anim. Sci. 84:3118–3122. doi: 10.2527/jas.2005-676 [DOI] [PubMed] [Google Scholar]
- Gimenes L. U., Fantinato Neto P., Arango J. S. P., Ayres H., and Baruselli P. S.. . 2009. Follicular dynamics of Bos indicus, Bos taurus and Bubalus bubalis heifers treated with norgestomet ear implant associated or not to injectable progesterone. Anim. Reprod. 6:256. [Google Scholar]
- Ginther O. J., Knopf L., and Kastelic J. P.. . 1989. Temporal associations among ovarian events in cattle during oestrous cycles with two and three follicular waves. Reproduction 87:223–230. doi: 10.1530/jrf.0.0870223 [DOI] [PubMed] [Google Scholar]
- Ginther O. J., Wiltbank M. C., Fricke P. M., Gibbons J. R., and Kot K.. . 1996. Selection of the dominant follicle in cattle. Biol. Reprod. 55:1187–1194. doi: 10.1095/biolreprod55.6.1187 [DOI] [PubMed] [Google Scholar]
- Gray K. A., Smith T., Maltecca C., Overton P., Parish J. A., and Cassady J. P.. . 2011. Differences in hair coat shedding, and effects on calf weaning weight and BCS among Angus dams. Livest. Sci. 140:68–71. doi: 10.1016/j.livsci.2011.02.009 [DOI] [Google Scholar]
- Hansen P. J. 2004. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 82–83:349–360. doi: 10.1016/j.anireprosci.2004.04.011 [DOI] [PubMed] [Google Scholar]
- Hawk H. W., and Cooper B. S.. . 1975. Improvement of sperm transport by the administration of estradiol to estrous ewes. J. Anim. Sci. 41:1400–1406. doi: 10.2527/jas1975.4151400x [DOI] [PubMed] [Google Scholar]
- Jones E. J., Armstrong J. D., and Harvey R. W.. . 1991. Changes in metabolites, metabolic hormones, and luteinizing hormone before puberty in Angus, Braford, Charolais, and Simmental heifers. J. Anim. Sci. 69:1607–1615. doi: 10.2527/1991.6941607x [DOI] [PubMed] [Google Scholar]
- Kaufman M. H., Barton S. C., and Surani M. A. H.. . 1977. Normal postimplantation development of mouse parthenogenetic embryos to the forelimb bud stage. Nature 265:53–55. doi: 10.1038/265053a0 [DOI] [PubMed] [Google Scholar]
- Lamb G. C., Dahlen C., and Maddox M.. . 2008. What is the economic impact of infertility in beef cattle?http://edis.ifas.ufl.edu/an208 (Accessed 20 September 2019).
- Lamond D. R. 1970. The influence of undernutrition on reproduction in the cow. Anim. Breed. Abstr. 38:359–372. [Google Scholar]
- Liberona J. D., Langdon J., Herring A., Blackburn H., Speidel S., Sanders S., and Riley D. G.. . 2020. Random regression of Hereford percentage intramuscular fat on geographical coordinates. J. Anim. Sci. doi: 10.1093/jas/skz359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohn B. P., Riley D. G., Welsh T. H. Jr., Randel R. D., Willard S. T., and Vann R. C.. . 2018. Use of random regression to estimate genetic parameters of temperament across an age continuum in a crossbred cattle population. J. Anim. Sci. 96:2607–2621. doi: 10.1093/jas/sky180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke J. W. C., Thomas J. M., Bishop B. E., Abel J. M., Poock S. E., Brown D. S., Decker J. E., and Patterson D. J.. . 2016. The show-me-select replacement heifer program: adding value to beef herds in Missouri. J. Anim. Sci. 94(E-Suppl. 5):271. doi: 10.2527/jam2016-0583 [DOI] [Google Scholar]
- López-Gatius F., Santolaria P., Yániz J. L., Garbayo J. M., and Hunter R. H. F.. . 2004. Timing of early foetal loss for single and twin pregnancies in dairy cattle. Reprod. Domest. Anim. 39:429–433. doi: 10.1111/j.1439-0531.2004.00533.x [DOI] [PubMed] [Google Scholar]
- Meneghetti M., Sá Filho O. G., Peres R. F. G., Lamb G. C., and Vasconcelos J. L. M.. . 2009. Fixed-time artificial insemination with estradiol and progesterone for Bos indicus cows I: basis for development of protocols. Theriogenology 72:179–189. doi: 10.1016/j.theriogenology.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Mercadante P. M., Waters K. M., Mercadante V. R., Lamb G. C., Elzo M. A., Johnson S. E., Rae D. O., Yelich J. V., and Ealy A. D.. . 2013. Subspecies differences in early fetal development and plasma pregnancy-associated glycoprotein concentrations in cattle. J. Anim. Sci. 91:3693–3701. doi: 10.2527/jas.2012-6130 [DOI] [PubMed] [Google Scholar]
- Mollo M. R., Rumpf R., Martins A. C., Mattos M. C. C., Lopes G. Jr., Carrijo L. H. D., and Sartori R.. . 2007. Ovarian function in Nelore heifers under low or high feed intake. Acta Sci. Vet. 35:958. [Google Scholar]
- Morton J. M., Tranter W. P., Mayer D. G., and Jonsson N. N.. . 2007. Effects of environmental heat on conception rates in lactating dairy cows: Critical periods of exposure. J. Dairy Sci. 90:2271–2278. doi: 10.3168/jds.2006-574 [DOI] [PubMed] [Google Scholar]
- Muntean C. T., Herring A. D., Riley D. G., Gill C. A., Sawyer J. E., and Sanders J. O.. . 2018. Evaluation of F1 cows sired by Brahman, Boran, and Tuli bulls for reproductive, maternal, and cow longevity traits. J. Anim. Sci. 96:2545–2552. doi: 10.1093/jas/sky169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASS. 2017. National Agricultural Statistics Service, agricultural statistics board. USDA, Washington, DC. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2016. Nutrient requirements of beef cattle. 8th ed Animal Nutrition Series. National Academy Press, Washington, DC. doi: 10.17226/19014 [DOI] [Google Scholar]
- Nogueira G. P. 2004. Puberty in South American Bos indicus (Zebu) cattle. Anim. Reprod. Sci. 82–83:361–372. doi: 10.1016/j.anireprosci.2004.04.007 [DOI] [PubMed] [Google Scholar]
- NRC. 2001. Nutrient requirements of dairy cattle. 7th rev. ed.National Research Council, National Academy Press, Washington, DC. [Google Scholar]
- Oosthuizen N., Fontes P. L. P., Sanford C. D., Ciriaco F. M., Henry D. D., Canal L. B., DiLorenzo N., and Lamb G. C.. . 2018. Estrus synchronization and fixed-time artificial insemination alter calving distribution in Bos indicus influenced beef heifers. Theriogenology 106:210–213. doi: 10.1016/j.theriogenology.2017.10.028 [DOI] [PubMed] [Google Scholar]
- Paschal J. C., Sanders J. O., and Kerr J. L.. . 1991. Calving and weaning characteristics of Angus-, Gray Brahman-, Gir-, Indu-Brazil-, Nellore-, and Red Brahman-sired F1 calves. J. Anim. Sci. 69:2395–2402. doi: 10.2527/1991.6962395x [DOI] [PubMed] [Google Scholar]
- Pereira M. H. C., Rodrigues A. D. P., Martins T., Oliveira W. V. C., Silveira P. S. A., Wiltbank M. C., and Vasconcelos J. L. M.. . 2013. Timed artificial insemination programs during the summer in lactating dairy cows: comparison of the 5-d Cosynch protocol with an estrogen/progesterone-based protocol. J. Dairy Sci. 96:6904–6914. doi: 10.3168/jds.2012-6260 [DOI] [PubMed] [Google Scholar]
- Peres R. F. G., Claro Júnior I., Sá Filho O. G., Nogueira G. P., and Vasconcelos J. L. M.. . 2009. Strategies to improve fertility in Bos indicus postpubertal heifers and nonlactating cows submitted to fixed-time artificial insemination. Theriogenology 72:681–689. doi: 10.1016/j.theriogenology.2009.04.026 [DOI] [PubMed] [Google Scholar]
- Perry G. A., Smith M. F., Lucy M. C., Green J. A., Parks T. E., MacNeil M. D., Roberts A. J., and Geary T. W.. . 2005. Relationship between follicle size at insemination and pregnancy success. Proc. Natl. Acad. Sci. USA 102:5268–5273. doi: 10.1073/pnas.0501700102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro O. L., Barros C. M., Figueiredo R. A., do Valle E. R., Encarnação R. O., and Padovani C. R.. . 1998. Estrous behavior and the estrus-to-ovulation interval in Nelore cattle (Bos indicus) with natural estrus or estrus induced with prostaglandin F2 alpha or norgestomet and estradiol valerate. Theriogenology 49:667–681. doi: 10.1016/s0093-691x(98)00017-x [DOI] [PubMed] [Google Scholar]
- Pohler K. G., Geary T. W., Atkins J. A., Perry G. A., Jinks E. M., and Smith M. F.. . 2012. Follicular determinants of pregnancy establishment and maintenance. Cell Tissue Res. 349:649–664. doi: 10.1007/s00441-012-1386-8 [DOI] [PubMed] [Google Scholar]
- Pohler K. G., Peres R. F. G., Green J. A., Graff H., Martins T., Vasconcelos J. L. M., and Smith M. F.. . 2016. Use of bovine pregnancy-associated glycoproteins to predict late embryonic mortality in postpartum Nellore beef cows. Theriogenology 85:1652–1659. doi: 10.1016/j.theriogenology.2016.01.026 [DOI] [PubMed] [Google Scholar]
- Polsky L. B., Madureira A. M. L., Filho E. L. D., Soriano S., Sica A. F., Vasconcelos J. L. M., and Cerri R. L. A.. . 2017. Association between ambient temperature and humidity, vaginal temperature, and automatic activity monitoring on induced estrus in lactating cows. J. Dairy Sci. 100:8590–8601. doi: 10.3168/jds.2017-12656 [DOI] [PubMed] [Google Scholar]
- Prayaga K. C., Corbet N. J., Johnston D. J., Wolcott M. L., Fordyce G., and Burrow H. M.. . 2009. Genetics of adaptive traits in heifers and their relationship to growth, pubertal and carcass traits in two tropical beef cattle genotypes. Anim. Prod. Sci. 49:413–425. doi: 10.1071/EA08247 [DOI] [Google Scholar]
- Randel R. D. 1984. Seasonal effects on female reproductive functions in the bovine (Indian breeds). Theriogenology 21:170–185. doi: 10.1016/0093-691X(84)90315-7 [DOI] [Google Scholar]
- Randel R. D. 1990. Nutrition and postpartum rebreeding in cattle. J. Anim. Sci. 68:853–862. doi: 10.2527/1990.683853x [DOI] [PubMed] [Google Scholar]
- Reese S. T., Franco G. A., Poole R. K., Cooke R. F., and Pohler K. G.. . 2020. Pregnancy loss in beef cattle: a meta-analysis. Anim. Reprod. Sci. doi: 10.1016/j.anireprosci.2019.106251 [DOI] [PubMed] [Google Scholar]
- Reese S. T., Geary T. W., Araujo G. F., Moraes J. G. N., Spencer T. E., and Pohler K. G.. . 2019. Pregnancy associated glycoproteins (PAGs) and pregnancy loss in high vs subfertility classified heifers. Theriogenology 135:7–12. doi: 10.1016/j.theriogenology.2019.05.026 [DOI] [PubMed] [Google Scholar]
- Riley D. G., Arthington J. D., Chase C. C. Jr., Coleman S. W., Griffin J. L., Rae D. O., Mader T. L., and Olson T. A.. . 2011. Evaluation of 2 sources of Angus cattle under South Florida subtropical conditions. J. Anim. Sci. 89:2265–2272. doi: 10.2527/jas.2010-3579 [DOI] [PubMed] [Google Scholar]
- Riley D. G., Burke J. M., Chase C. C. Jr., and Coleman S. W.. . 2015b. Genetic effects for reproductive performance of straightbred and crossbred Romosinuano and Angus cows in a temperate zone. Livest. Sci. 180:22–26. doi: 10.1016/j.livsci.2015.06.024 [DOI] [Google Scholar]
- Riley D. G., Chase C. C. Jr., Coleman S. W., and Olson T. A.. . 2012. Genetic assessment of rectal temperature and coat score in Brahman, Angus, and Romosinuano crossbred and straightbred cows and calves under subtropical summer conditions. Livest. Sci. 148:109–118. doi: 10.1016/j.livsci.2012.05.017 [DOI] [Google Scholar]
- Riley D. G., Sanders J. O., Knutson R. E., and Lunt D. K.. . 2001. Comparison of F1 Bos indicus x Hereford cows in central Texas: II. Udder, mouth, longevity, and lifetime productivity. J. Anim. Sci. 79:1439–1449. doi: 10.2527/2001.7961439x [DOI] [PubMed] [Google Scholar]
- Riley D. G., Sawyer J. E., and Sanders J. O.. . 2015a. Trigonometric functions representing annual winter coat shedding and regrowth in Angus cows. Livest. Sci. 180:41–46. doi: 10.1016/j.livsci.2015.07.004 [DOI] [Google Scholar]
- Rodrigues A. D. P., Peres R. F. G., Day M. L., and Vasconcelos J. L. M.. . 2016. Effect of eCG and P4 level in timed AI programs in Bos indicus and Bos indicus × Bos taurus heifers. J Anim. Sci. 99(E-Suppl. 5):502. doi: 10.2527/jam2016-1067 [DOI] [Google Scholar]
- Rodrigues A. D. P., Peres R. F. G., Lemes A. P., Martins T., Pereira M. H. C., Carvalho E. R., Day M. L., Vasconcelos J. L. M.. . 2014. Effect of interval from induction of puberty to initiation of a timed AI protocol on pregnancy rate in Nellore heifers. Theriogenology 82:760–766. doi: 10.1016/j.theriogenology.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Rodrigues A. D., Peres R. F., Lemes A. P., Martins T., Pereira M. H., Day M. L., and Vasconcelos J. L.. . 2013. Progesterone-based strategies to induce ovulation in prepubertal Nellore heifers. Theriogenology 79:135–141. doi: 10.1016/j.theriogenology.2012.09.018 [DOI] [PubMed] [Google Scholar]
- Roth Z., Arav A., Bor A., Zeron Y., Braw-Tal R., and Wolfenson D.. . 2001. Improvement of quality of oocytes collected in the autumn by enhanced removal of impaired follicles from previously heat-stressed cows. Reproduction 122:737–744. [PubMed] [Google Scholar]
- Sá Filho O. G., Dias C. C., Lamb G. C., and Vasconcelos J. L.. . 2010. Progesterone-based estrous synchronization protocols in non-suckled and suckled primiparous Bos indicus beef cows. Anim. Reprod. Sci. 119:9–16. doi: 10.1016/j.anireprosci.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Sá Filho O. G., Meneghetti M., Peres R. F., Lamb G. C., and Vasconcelos J. L.. . 2009. Fixed-time artificial insemination with estradiol and progesterone for Bos indicus cows II: strategies and factors affecting fertility. Theriogenology 72:210–218. doi: 10.1016/j.theriogenology.2009.02.008 [DOI] [PubMed] [Google Scholar]
- Sartorelli E. S., Carvalho L. M., Bergfelt D. R., Ginther O. J., and Barros C. M.. . 2005. Morphological characterization of follicle deviation in Nelore (Bos indicus) heifers and cows. Theriogenology 63:2382–2394. doi: 10.1016/j.theriogenology.2004.08.017 [DOI] [PubMed] [Google Scholar]
- Sartori R., Fricke P. M., Ferreira J. C., Ginther O. J., and Wiltbank M. C.. . 2001. Follicular deviation and acquisition of ovulatory capacity in bovine follicles. Biol. Reprod. 65:1403–1409. doi: 10.1095/biolreprod65.5.1403 [DOI] [PubMed] [Google Scholar]
- Sartori R., Gimenes L. U., Monteiro P. L. Jr., Melo L. F., Baruselli P. S., and Bastos M. R.. . 2016. Metabolic and endocrine differences between Bos taurus and Bos indicus females that impact the interaction of nutrition with reproduction. Theriogenology 86:32–40. doi: 10.1016/j.theriogenology.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Sartori R., Haughian J. M., Shaver R. D., Rosa G. J., and Wiltbank M. C.. . 2004. Comparison of ovarian function and circulating steroids in estrous cycles of Holstein heifers and lactating cows. J. Dairy Sci. 87:905–920. doi: 10.3168/jds.S0022-0302(04)73235-X [DOI] [PubMed] [Google Scholar]
- Scarpa J. O., O’Neil M. M., Cardoso R. C., Stanko R. L., and Williams G. L.. . 2019. Follicle dynamics and fertility at fixed-time AI of Bos indicus-influenced beef cows synchronized with the 5-Day Bee Synch + CIDR protocol with or without GnRH on day 0. J. Anim. Sci. 95(E-Supp 4):234. doi: 10.2527/asasann.2017.479 [DOI] [Google Scholar]
- Schüller L. K., Burfeind O., and Heuwieser W.. . 2014. Impact of heat stress on conception rate of dairy cows in the moderate climate considering different temperature-humidity index thresholds, periods relative to breeding, and heat load indices. Theriogenology 81:1050–1057. doi: 10.1016/j.theriogenology.2014.01.029 [DOI] [PubMed] [Google Scholar]
- Surani M. A., Barton S. C., and Norris M. L.. . 1984. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 308:548–550. doi: 10.1038/308548a0 [DOI] [PubMed] [Google Scholar]
- Taylor C. S., and Fitzhugh H. A. Jr. 1971. Genetic relationships between mature weight and time taken to mature within a breed. J. Anim. Sci. 33:726–731. doi: 10.2527/jas1971.334726x [DOI] [PubMed] [Google Scholar]
- Telugu B. P., Spate L., Prather R. S., and Green J. A.. . 2009. Acid peptidase activity released from in vitro produced porcine embryos: a candidate marker to predict developmental competence. Mol. Reprod. Dev. 76:417–428. doi: 10.1002/mrd.20965 [DOI] [PubMed] [Google Scholar]
- Thomas J. M., Locke J. W. C., Bishop B. E., Abel J. M., Ellersieck M. R., Yelich J. V., Poock S. E., Smith M. F., and Patterson D. J.. . 2017. Evaluation of the 14-d CIDR-PG and 9-d CIDR-PG protocols for synchronization of estrus in Bos indicus-influenced and Bos taurus beef heifers. Theriogenology 92:190–196. doi: 10.1016/j.theriogenology.2017.01.020 [DOI] [PubMed] [Google Scholar]
- Thompson I. M., Cerri R. L., Kim I. H., Green J. A., Santos J. E., and Thatcher W. W.. . 2010. Effects of resynchronization programs on pregnancy per artificial insemination, progesterone, and pregnancy-associated glycoproteins in plasma of lactating dairy cows. J. Dairy Sci. 93:4006–4018. doi: 10.3168/jds.2009-2941 [DOI] [PubMed] [Google Scholar]
- Tolleson M. W., Gill C. A., Herring A. D., Riggs P. K., Sawyer J. E., Sanders J. O., and Riley D. G.. . 2017. Association of udder traits with single nucleotide polymorphisms in crossbred Bos indicus–Bos taurus cows. J. Anim. Sci. 95:2399–2407. doi: 10.2527/jas.2017.1475 [DOI] [PubMed] [Google Scholar]
- Turner J. W. 1980. Genetic and biological aspects of Zebu adaptability. J. Anim. Sci. 50:1201–1205. doi: 10.2527/jas1980.5061201x [DOI] [PubMed] [Google Scholar]
- USDA. 2010. Beef 2007–08, Part IV: Reference of beef cow-calf management practices in the United States, 2007–08. #523.0210. USDA:APHIS:VS, CEAH, Fort Collins, CO. [Google Scholar]
- USDA. 2019. Livestock and poultry: World markets and trade – Foreign agricultural service.https://apps.fas.usda.gov/psdonline/circulars/livestock_poultry.pdf. Accessed on 20 September 2019.
- Vasconcelos J. L. M., Carvalho R., Peres R. F. G., Rodrigues A. D. P., Meneghetti M., Claro Junior I., Aono F. H., Costa W., Lopes C. N., Cooke R. F., . et al. 2017. Reproductive programs for beef cattle: Incorporating management and reproductive techniques for better fertility. Anim. Reprod. 14:547–557. doi: 10.21451/1984-3143-AR998 [DOI] [Google Scholar]
- Vasconcelos J. L., Cooke R. F., Jardina D. T., Aragon F. L., Veras M. B., Soriano S., Sobreira N., and Scarpa A. B.. . 2011. Associations among milk production and rectal temperature on pregnancy maintenance in lactating recipient dairy cows. Anim. Reprod. Sci. 127:140–147. doi: 10.1016/j.anireprosci.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Vasconcelos J. L. M., de Sá Filho O. G., and Cooke R. F.. . 2014. Impacts of reproductive technologies on beef production in South America. Adv. Exp. Med. Biol. 752:161–180. doi: 10.1007/978-1-4614-8887-3_8 [DOI] [PubMed] [Google Scholar]
- Vose R. S., Easterling D. R., Kunkel K. E., LeGrande A. N., and Wehner M. F.. . 2017. Temperature changes in the United States. In: Wuebbles D. J., Fahey D. W., Hibbard K. A., Dokken D. J., Stewart B. C., and Maycock T. K., editors, Climate science special report: Fourth national climate assessment, volume I. U.S. Global Change Research Program, Washington, DC: p. 185–206. doi: 10.7930/J0N29V45 [DOI] [Google Scholar]
- West J. W. 2003. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 86:2131–2144. doi: 10.3168/jds.S0022-0302(03)73803-X [DOI] [PubMed] [Google Scholar]
- Williams G. L., Amstalden M., Garcia M. R., Stanko R. L., Nizielski S. E., Morrison C. D., and Keisler D. H.. . 2002. Leptin and its role in the central regulation of reproduction in cattle. Domest. Anim. Endocrinol. 23:339–349. doi: 10.1016/s0739-7240(02)00169-8 [DOI] [PubMed] [Google Scholar]
- Wiltbank J. N., Gregory K. E., Swiger L. A., Ingalls J. E., Rothlisberger J. A., and Koch R. M.. . 1966. Effects of heterosis on age and weight at puberty in beef heifers. J. Anim. Sci. 25:744–751. doi: 10.2527/jas1966.253744x [DOI] [Google Scholar]
- Wolfenson D., Roth Z., and Meidan R.. . 2000. Impaired reproduction in heat-stressed cattle: basic and applied aspects. Anim. Reprod. Sci. 60–61:535–547. doi: 10.1016/S0378-4320(00)00102-0 [DOI] [PubMed] [Google Scholar]
- Xie S., Green J., Bixby J. B., Szafranska B., DeMartini J. C., Hecht S., and Roberts R. M.. . 1997. The diversity and evolutionary relationships of the pregnancy associated glycoproteins, an aspartic proteinase subfamily consisting of many trophoblast-expressed genes. Proc. Natl. Acad. Sci. USA 94:12809–12816. doi: 10.1073/pnas.94.24.12809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelich J. V., and Bridges G. A.. . 2012. Synchronization response: Bos taurus vs. Bos indicus cattle. In: Proceedings of the 2012 Beef Improvement Federation Research Symposium and Annual Meeting Houston, TX p. 77–98. [Google Scholar]