Abstract
BACKGROUND:
Statins have pleiotropic anti-inflammatory and immunomodulatory effects, yet the effect of statin use on asthma-related emergency department (ED) visits and hospitalizations has remained unclear, especially in Asian populations.
OBJECTIVE:
We sought to examine the effect of statin therapy on asthma-related ED visits and/or hospitalizations.
METHODS:
A cohort study was conducted using data from Taiwan’s National Health Insurance Research Database from 2001 to 2013. A total of 117,595 adult patients with asthma were included. The outcomes were defined as asthma-related ED visits and/or hospitalizations. Multiple Cox proportional hazards models were applied to determine the effect of statin use on asthma-related ED visits and/or hospitalizations.
RESULTS:
There were 3,417 asthma-related ED visits and/or hospitalizations among 117,595 subjects with asthma. Statin users were significantly less likely to experience asthma-related ED visits and/or hospitalizations (adjusted hazard ratio: 0.81; 95% confidence interval: 0.74–0.89) compared with nonstatin users. The risks of asthma-related ED visits and/or hospitalizations were decreased among those with a higher cumulative defined daily dose (DDD), greater average DDD, and longer cumulative-day users than the counterparts.
CONCLUSIONS:
Our study suggests that statin use is associated with the decreased risk of asthma-related ED visits and/or hospitalizations in patients with asthma. A dose-response effect of statin use is also observed in this study. Therefore, future randomized clinical trials would be warranted to further evaluate the association.
Keywords: Statin, Asthma exacerbation, Adulthood asthma
Asthma is a respiratory syndrome defined by variable, reversible airway obstruction and increased hyperreactivity of the airways to various sources of stimuli.1 Approximately 300 million people around the world suffer from asthma, and roughly 1,000 people die from asthma every day.2–4 The estimated prevalence of adult asthma ranges from 0.8% to 13.4% worldwide.5 Previous studies have reported that most asthmatic patients can maintain asthma control with regular treatment.6,7 However, some studies have also documented that suboptimal asthma control is related to more emergency department (ED) visits, consequently leading to increased health care burden.8,9
Hydroxymethylglutaryl-coenzyme A reductase inhibitors, statins, are commonly prescribed for hyperlipidemia and administered for the primary and secondary prevention of cardiovascular disease.10,11 Previous studies have provided evidence that statin therapy reduces high-sensitivity C-reactive protein level and causes pleiotropic anti-inflammatory and immunomodulatory effects.12 Nevertheless, whether statin use can decrease the occurrence of asthma exacerbations has remained inconclusive.13–15 For example, Zeki et al16 suggested that simvastatin inhibits airway hyperreactivity and attenuates airway inflammation. However, Menzies et al17 showed no evidence for therapeutic anti-inflammatory effects of simvastatin on the treatment of asthma.
Therefore, to evaluate effect of statin use on asthma-related outcomes, we conducted a cohort study using a representative sample of an Asian population from Taiwan’s National Health Insurance Research Databases (NHIRD) from 2001 to 2013. The objectives of this study were to investigate the association between statin use and asthma-related outcomes, specifically, asthma-related ED visits and/or hospitalizations. We also examined whether there were dose-response effects of statin use on the aforementioned asthma-related outcomes.
METHODS
Data source
We used registry data derived from 3 different Longitudinal Health Insurance Databases (LHIDs) composed of medical claims data from the NHIRD in Taiwan. In brief, the National Health Insurance (NHI) program has provided mandatory medical care to residents in Taiwan since 1995. The NHIRD contains reimbursement claims data collected by the NHI program, including demographic characteristics, outpatient and inpatient claims data, and prescription records. Previous studies have reported that enrollees represent approximately 98% of the total population in Taiwan.18 Specifically, each LHID was constructed by randomly selecting 1 million enrollees from the NHI program in 2000, 2005, and 2010, separately. As such, a total of roughly 3 million subjects and their medical claims data from January 1, 2002, to December 31, 2013, were included in the present study. The Institutional Review Board of the National Health Research Institutes, Taiwan, approved this study protocol.
Study cohort
In this retrospective cohort study, we included patients aged 20 years and older with asthma. We identified patients as having asthma if they had a diagnosis based on International Classification of Diseases, Ninth Revision, Clinical Modification codes for asthma (493.xx) plus 1 inpatient or 2 outpatient visits for asthma within 1 year during 2002–2013. Of note, the exclusion criteria in this study were as follows: (1) patients with asthma-related ED visits and/or hospitalizations before index date; (2) patients whose gender was missing in the databases; (3) patients whose records were duplicates in at least 2 of 3 LHID datasets; and (4) patients who passed away within 1 year between the diagnosis of asthma and the index date because we were not able to compute the Deyo-Charlson index that was used to control for comorbid conditions. Of note, we found 1 patient with inaccurate records from an LHID set and excluded the patient from the subsequent analyses. Figure 1 (A) illustrates the detailed flow chart regarding inclusion/exclusion criteria for the study patients.
FIGURE 1.
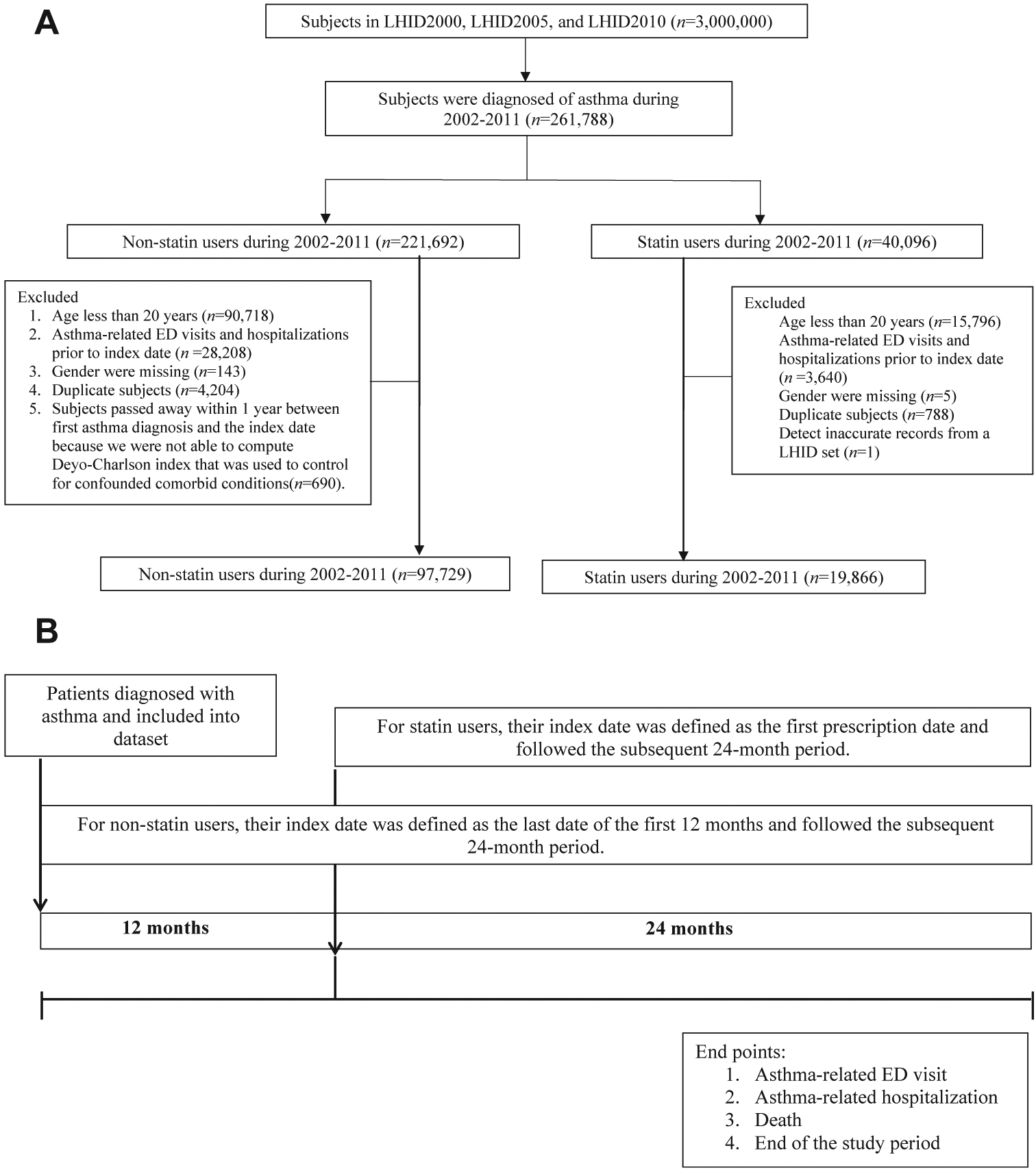
A, Flow diagram of criteria for inclusion and exclusion in the present study, 2002–2011. B, Definition of timeline regarding index date for the study observation period between statin and nonstatin users (see the text for the details). ED, Emergency department; LHID, Longitudinal Health Insurance Database.
Statin exposure
For statin users, we defined the statin index date as the first identified prescription date of statins. Statin users were included in subsequent analyses if were enrolled in the NHI program for the 24-month observation period after the index date (Figure 1, B). We defined the nonstatin users as patients without any statin prescription during the entire 36-month period. The corresponding index date of the nonstatin users was defined as the last date of the first 12 months during the 36-month period (Figure 1, B). We excluded patients with asthma diagnosed in the 12 months before the index date to make the baseline characteristics of the 2 groups comparable. Statins investigated in this study include lovastatin, pravastatin, fluvastatin, simvastatin, atorvastatin, and rosuvastatin.
In this study, we examined 3 different kinds of statin dose effect, including cumulative drug days, cumulative defined daily dose (cDDD), and average DDD. More specifically, the average daily dose of statin use was assessed using DDD, which was defined as “the assumed average maintenance dose per day for a drug used for its main indication in adults.”19 We summed up the total numbers of prescription days as cumulative drug days and classified the cumulative drug days into quartiles: 0 (nonuse; reference group), >0 days and ≤38 days, >38 days and ≤101 days, >101 days and ≤224 days, and >224 cumulative drug days, separately. We also computed the cDDD, and classified the cDDD into quartiles: 0 (nonuse; reference group), > 0 cDDD and ≤28 cDDD, >28 cDDD and ≤74.67 cDDD, >74.67 cDDD and ≤172 cDDD, and >172 cDDD, individually. Of note, cumulative drug days and cDDD for each study patient were calculated based on the 24-month follow-up period. The statin users in this study may or may not continuously fill statin prescriptions over the 24-month period because physicians prescribed statin agents to patients and adjusted dosing based on their condition. The average DDD was computed by dividing the cDDD by the number of statin-use days, and categorized into tertiles: 0 (nonuse; reference group), >0 and ≤0.67, and >0.67 average DDD, respectively.
Data analysis
We compared the distributions of demographic and clinical characteristics between statin users and nonstatin users in our study cohort using the F test for continuous variables and the χ2 test for categorical variables. The primary outcomes in this study were asthma-related ED visits and/or hospitalizations. Multiple Cox proportional hazards models with covariate adjustment were applied to determine the effect of statin use (including statin use [yes/no], cumulative drug days, cDDD, and average DDD of statin use, separately) on asthma-related ED visits and/or hospitalizations. Covariates included age, sex, Deyo-Charlson index (exclude chronic pulmonary disease) in the 12 months before the index date, asthma controller medication in the 12 months before the index date, diagnosis of asthma in the 12 months before the index date, and health care utilization during the 12 months before the index date.20 The Deyo-Charlson index accounted for the following conditions: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, hemiplegia or paraplegia, renal disease, malignancy, and AIDS/HIV. In addition, health care utilization was calculated using the number of ambulatory visits and hospitalizations 1 year before index date. Plots of cumulative hazard function were applied to compare cumulative incidences of asthma-related ED visits and/or hospitalizations between statin users and nonstatin users.21
All of the analyses were performed using SAS version 8.2 (SAS institute, Cary, NC). P values less than .05 were declared to be statistically significant.
RESULTS
A total of 117,595 subjects with asthma (19,866 statin users and 97,729 nonstatin users) were included in this study. A detailed flow chart for identifying the study cohort is shown in Figure 1. The distributions of demographic characteristics, medical conditions, and health care utilization are provided in Table I. Statin users were older (mean age and the corresponding standard deviation were 62.99 ± 12.5 years for statin users, and 52.07 ± 18.4 years for nonstatin users) than nonstatin users, and had a higher prevalence of most of the chronic diseases. Compared with nonstatin users, statin users also had a greater number of outpatient and inpatient visits (Table I).
TABLE I.
Demographic and clinical characteristics of study subjects
| Characteristic | Nonstatin users (n = 97,729) | Statin users (n = 19,866) | P value |
|---|---|---|---|
| Age, (y) | 52.07 ± 18.4 | 62.99 ± 12.5 | <.0001 |
| Sex, n (%) | |||
| Female | 52,824 (54) | 11,486 (57.8) | <.0001 |
| Male | 44,905 (46) | 8,380 (42.2) | |
| Deyo-Charlson conditions, n (%) | |||
| Myocardial infarction | 362 (0.4) | 387 (1.9) | <.0001 |
| Congestive heart failure | 3,693 (3.8) | 1,448 (7.3) | <.0001 |
| Peripheral vascular disease | 715 (0.7) | 452 (2.3) | <.0001 |
| Cerebrovascular disease | 5,452 (5.6) | 2,705 (13.6) | <.0001 |
| Dementia | 1,351 (1.4) | 298 (1.5) | .206 |
| Chronic pulmonary disease | 96,415 (98.8) | 10,879 (54.8) | <.0001 |
| Rheumatic disease | 1,593 (1.6) | 411 (2.1) | <.0001 |
| Peptic ulcer disease | 16,069 (16.5) | 4,531 (22.8) | <.0001 |
| Mild liver disease | 7,531 (7.7) | 2,690 (13.5) | <.0001 |
| Diabetes without chronic | 6,107 (6.3) | 6,501 (32.7) | <.0001 |
| Diabetes with chronic | 1,122 (1.2) | 1,262 (6.4) | <.0001 |
| Hemiplegia or paraplegia | 264 (0.3) | 115 (0.6) | <.0001 |
| Renal disease | 2,098 (2.2) | 1,100 (5.5) | <.0001 |
| Any malignancy including leukemia and lymphoma | 3,645 (3.7) | 862 (4.3) | <.0001 |
| Moderate or severe liver disease | 64 (0.07) | 8 (0.04) | .19 |
| Metastatic solid tumor | 198 (0.2) | 22 (0.1) | .006 |
| AIDS/HIV | 33 (0.03) | 8 (0.04) | .66 |
| Cumulative number of outpatient visits, n (%) | |||
| ≤14 | 32,930 (33.7) | 3,155 (15.9) | <.0001 |
| 15–26 | 29,949 (30.6) | 5,880 (29.6) | |
| ≥27 | 34,850 (35.7) | 10,831 (54.5) | |
| Cumulative number of inpatient visits, n (%) | |||
| 0 | 82,011 (83.9) | 15,501 (78) | <.0001 |
| 1 | 10,308 (10.6) | 3,099 (15.6) | |
| ≥2 | 5,410 (5.5) | 1,266 (6.4) | |
We first examined the relationships between statin use and asthma-related ED visits, hospitalizations, and asthma-related ED visits or hospitalizations, separately. The results in Table II indicated that statin use was associated with decreased odds of asthma-related outcomes compared with nonstatin users (adjusted hazards ratio [AHR] = 0.81; 95% confidence interval [CI]: 0.74–0.89 for asthma-related ED visits and/or hospitalizations; AHR = 0.86; 95% CI: 0.76–0.98 for asthma-related ED visits; and AHR = 0.77; 95% CI: 0.67–0.88 for hospitalizations). Similar results were observed in each LHID subset (Table II).
TABLE II.
Cox proportional hazard model for asthma-related ED visits and hospitalizations
| Database | Asthma related ED visits or hospital admissions | Asthma related ED visits | Asthma related hospital admissions | ||||
|---|---|---|---|---|---|---|---|
| Statin | N | Events | Adjusted HR* | Events | Adjusted HR* | Events | Adjusted HR* |
| LHID2000+2005+2010 | |||||||
| No | 97,729 | 2,818 | Ref | 1,614 | Ref | 1,204 | Ref |
| Yes | 19,866 | 599 | 0.81 (0.74, 0.89)† | 322 | 0.86 (0.76, 0.98) | 277 | 0.77 (0.67, 0.88) |
| LHID2000 | |||||||
| No | 35,265 | 1,102 | Ref | 609 | Ref | 493 | Ref |
| Yes | 6,848 | 215 | 0.94 (0.73, 1.19) | 119 | 1.24 (0.87, 1.76) | 96 | 0.75 (0.53, 1.05) |
| LHID2005 | |||||||
| No | 35,156 | 1,015 | Ref | 579 | Ref | 436 | Ref |
| Yes | 7,178 | 222 | 0.73 (0.56, 0.95) | 122 | 0.87 (0.61, 1.25) | 100 | 0.6 (0.4, 0.9) |
| LHID2010 | |||||||
| No | 35,512 | 829 | Ref | 501 | Ref | 328 | Ref |
| Yes | 6,629 | 188 | 0.87 (0.65, 1.18) | 98 | 1.05 (0.68, 1.62) | 90 | 0.76 (0.51, 1.14) |
ED, Emergency department; HR, hazards ratio; LHID, Longitudinal Health Insurance Database.
Adjusted for age, sex, Deyo-Charlson index (exclude chronic pulmonary disease) in the 12 mo before the index date, asthma controller medication in the 12 mo before the index date, diagnosis of asthma in the 12 mo before the index date, and health care utilization in the 12 mo before the index date.
Significant results (P < .05) are in bold.
As noted in Table I, the distributions of demographic characteristics, medical conditions, and health care utilization of the 2 study groups were not comparable at baseline. Thus, we further conducted a propensity score-matched study cohort and evaluated the effect of statin use on asthma-related ED visits, hospitalizations, and asthma-related ED visits or hospitalizations, respectively. In brief, propensity scores, representing the predicted probability of a patient with asthma taking statins, conditional on his/her baseline features, were computed for each patient to make a nonstatin user group matched with a statin user group with a similar demographic and/or clinical profile. We found comparable results between the study cohorts with and without propensity score matching (Table E1, available in this article’s Online Repository at www.jaci-inpractice.org). When further evaluating asthma severity by grouping study patients as mild-to-moderate asthmatic patients if identified from outpatient visits and as severe asthmatic patients if identified from inpatient visits based on their medical claims records, comparable results were observed as those in Table II. Of note, because of the small sample size in the subgroup of severe asthmatic patients, significant results were only observed in the outcome of asthma-related ED visits and/or hospitalizations (Table E2, available in this article’s Online Repository at www.jaci-inpractice.org).
Cumulative incidences of asthma-related outcomes (including asthma-related ED visits, hospitalizations, and asthma-related ED visits and/or hospitalizations) are shown in Figure 2. The risk of asthma-related ED visits, asthma-related hospitalizations, and asthma-related ED visits and/or hospitalizations was significantly lower for statin users compared with their counterparts in the study cohort.
FIGURE 2.
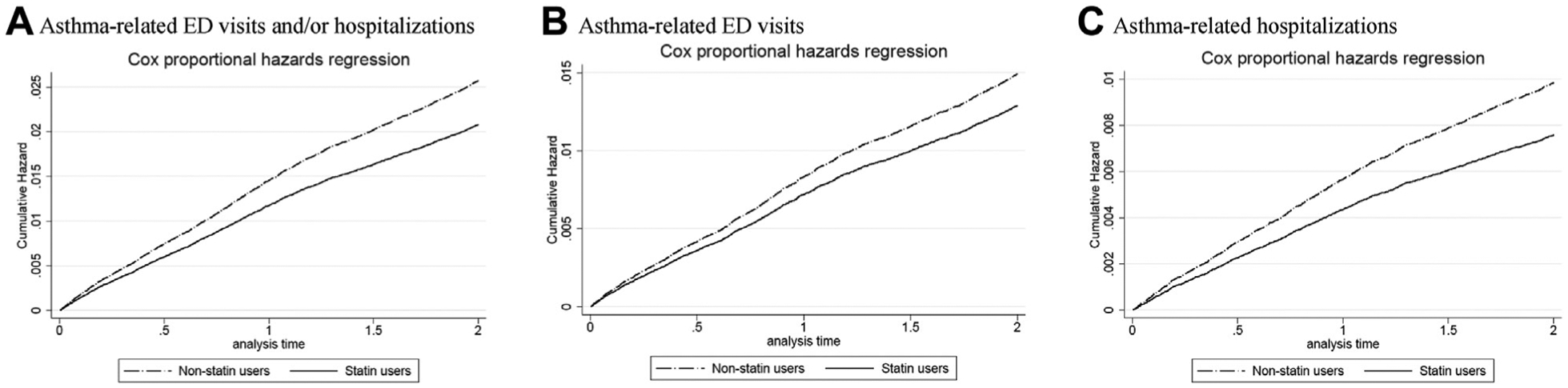
Cumulative incidence rate of asthma-related ED visits and/or hospitalizations between statin users and nonstatin users. ED, Emergency department.
When we investigated dose response effects using cumulative drug days, cDDD, and average DDD, respectively, reduced risks of asthma-related outcomes were observed among statin users (Tables III–V). Specifically, patients who took statins for more cumulative days tended to have a more reduced risk of asthma-related ED visits and/or hospitalizations (AHR = 1.25, 95% CI: 1.09–1.44 for 1–38 days; AHR = 1.00, 95% CI: 0.86–1.17 for 39–101 days; AHR = 0.75, 95% CI: 0.63–0.89 for 102–224 days; AHR = 0.27, 95% CI: 0.21–0.35 for more than 224 days) in all 3 LHID datasets combined along with each of 3 LHID datasets, separately. Similar decreased risks of asthma-related ED visits or hospitalizations are also found in Table III. In addition, results in Tables IV and V suggest that patients with a higher cDDD and average DDD, separately, were associated with decreased odds of asthma-related outcomes compared with nonstatin users.
TABLE III.
Hazard ratios of asthma-related ED visits and hospitalizations associated with cumulative drug days of statin use
| Database | Asthma-related ED visits or hospitalizations | Asthma-related ED visits | Asthma-related hospitalizations | ||||
|---|---|---|---|---|---|---|---|
| Statin | N | Events | AHR* (95% CI) | Events | AHR* (95% CI) | Events | AHR* (95% CI) |
| LHID2000+2005+2010 | |||||||
| 0 (no use) | 97,729 | 2.818 | Ref | 1,614 | Ref | 1,204 | Ref |
| 1 (drug day ≤ 38) | 4,842 | 217 | 1.25 (1.09, 1.44)† | 115 | 1.3 (1.07, 1.58) | 102 | 1.23 (1, 1.51) |
| 2 (38 < drug day ≤ 101) | 4,953 | 185 | 1 (0.86, 1.17) | 102 | 1.1 (0.89, 1.34) | 83 | 0.93 (0.74, 1.16) |
| 3 (101 < drug day ≤ 224) | 5,154 | 144 | 0.75 (0.63, 0.89) | 74 | 0.77 (0.61, 0.97) | 70 | 0.75 (0.59, 0.96) |
| 4 (drug day > 224) | 4,917 | 53 | 0.27 (0.21, 0.35) | 31 | 0.32 (0.22, 0.45) | 22 | 0.23 (0.15, 0.35) |
| LHID2000 | |||||||
| 0 (no use) | 35,265 | 1,102 | Ref | 609 | Ref | 493 | Ref |
| 1 (drug day ≤ 42) | 1,734 | 76 | 1.63 (1.14, 2.32) | 43 | 1.96 (1.16, 3.32) | 33 | 1.42 (0.87, 2.31) |
| 2 (42 < drug day ≤ 101) | 1,667 | 67 | 1.09 (0.71, 1.68) | 39 | 1.53 (0.84, 2.79) | 28 | 0.81 (0.43, 1.53) |
| 3 (101 < drug day ≤ 224) | 1,722 | 53 | 0.96 (0.61, 1.5) | 26 | 1.31 (0.7, 2.43) | 27 | 0.74 (0.39, 1.4) |
| 4 (drug day > 224) | 1,725 | 19 | 0.31 (0.16, 0.6) | 11 | 0.45 (0.18, 1.1) | 8 | 0.23 (0.08, 0.61) |
| LHID2005 | |||||||
| 0 (no use) | 34,721 | 1,015 | Ref | 579 | Ref | 436 | Ref |
| 1 (drug day ≤ 42) | 1,767 | 85 | 1.47 (0.99, 2.17) | 43 | 1.79 (1.08, 2.97) | 42 | 1.17 (0.63, 2.16) |
| 2 (42 < drug day ≤ 102) | 1,692 | 63 | 0.7 (0.41, 1.2) | 37 | 0.73 (0.34, 1.58) | 26 | 0.67 (0.31, 1.44) |
| 3 (102 < drug day ≤ 217) | 1,749 | 55 | 0.74 (0.46, 1.2) | 30 | 0.87 (0.46, 1.66) | 25 | 0.63 (0.31, 1.3) |
| 4 (drug day > 217) | 1,869 | 19 | 0.28 (0.14, 0.55) | 12 | 0.38 (0.17,0.87) | 7 | 0.18 (0.06, 0.58) |
| LHID2010 | |||||||
| 0 (no use) | 31,515 | 829 | Ref | 501 | Ref | 328 | Ref |
| 1 (drug day ≤ 30) | 1,661 | 74 | 1.99 (1.31, 3.01) | 42 | 3.04 (1.77, 5.25) | 32 | 1.28 (0.66, 2.47) |
| 2 (30 < drug day ≤ 98) | 1,657 | 59 | 0.72 (0.41, 1.28) | 27 | 0.57 (0.21, 1.57) | 32 | 0.82 (0.41, 1.64) |
| 3 (98 < drug day ≤ 217) | 1,695 | 39 | 0.78 (0.45, 1.36) | 20 | 0.69 (0.28, 1.72) | 19 | 0.85 (0.43, 1.69) |
| 4 (drug day > 217) | 1,616 | 16 | 0.42 (0.22, 0.8) | 9 | 0.53 (0.21, 1.32) | 7 | 0.35 (0.14, 0.88) |
AHR, Adjusted hazards ratio; CI, confidence interval; ED, emergency department; LHID, Longitudinal Health Insurance Database.
Adjusted for age, sex, Deyo-Charlson index (exclude chronic pulmonary disease) in the 12 mo before the index date, asthma controller medication in the 12 mo before the index date, diagnosis of asthma in the 12 mo before the index date, and health care utilization in the 12 mo before the index date.
Significant results (P < .05) are in bold.
TABLE V.
Hazard ratios of asthma-related ED visits and hospitalizations associated with average DDD of statin use
| Database | Asthma-related ED visits or hospitalizations | Asthma-related ED visits | Asthma-related hospitalizations | ||||
|---|---|---|---|---|---|---|---|
| Statin | N | Events | AHR* (95% CI) | Events | AHR* (95% CI) | Events | AHR* (95% CI) |
| LHID2000+2005+2010 | |||||||
| 0 (no use) | 97,730 | 2,818 | Ref | 1,614 | Ref | 1,204 | Ref |
| 1 (ave_DDD ≤ 0.67) | 11,499 | 388 | 0.88 (0.79, 0.98)† | 203 | 0.92 (0.79, 1.07) | 185 | 0.85 (0.73, 1) |
| 2 (ave_DDD > 0.67) | 8,366 | 211 | 0.71 (0.61, 0.82) | 119 | 0.79 (0.65, 0.95) | 92 | 0.64 (0.52, 0.79) |
| LHID2000 | |||||||
| 0 (no use) | 35,265 | 1,102 | Ref | 609 | Ref | 493 | Ref |
| 1 (ave_DDD ≤ 0.67) | 3,985 | 132 | 0.96 (0.72, 1.3) | 72 | 1.21 (0.78, 1.87) | 60 | 0.82 (0.54, 1.23) |
| 2 (ave_DDD > 0.67) | 2,863 | 83 | 0.9 (0.63, 1.28) | 47 | 1.28 (0.79, 2.08) | 36 | 0.65 (0.38, 1.1) |
| LHID2005 | |||||||
| 0 (no use) | 35,158 | 1,015 | Ref | 579 | Ref | 436 | Ref |
| 1 (ave_DDD ≤ 0.67) | 4,185 | 146 | 0.69 (0.49, 0.97) | 73 | 0.75 (0.47, 1.2) | 73 | 0.64 (0.39, 1.05) |
| 2 (ave_DDD > 0.67) | 2,991 | 76 | 0.78 (0.54, 1.13) | 49 | 1.04 (0.65, 1.68) | 27 | 0.54 (0.29, 1) |
| LHID2010 | |||||||
| 0 (no use) | 31,512 | 829 | Ref | 501 | Ref | 328 | Ref |
| 1 (ave_DDD ≤ 0.67) | 3,814 | 126 | 1 (0.7, 1.42) | 70 | 1.2 (0.71, 2.02) | 56 | 0.88 (0.55, 1.41) |
| 2 (ave_DDD > 0.67) | 2,815 | 62 | 0.71 (0.46, 1.11) | 28 | 0.87 (0.46, 1.64) | 34 | 0.61 (0.33, 1.14) |
AHR, Adjusted hazards ratio; ave_DDD, average DDD; CI, confidence interval; DDD, defined daily dose; ED, emergency department; LHID, Longitudinal Health Insurance Database.
Adjusted for age, sex, Deyo-Charlson index (exclude chronic pulmonary disease) in the 12 mo before the index date, asthma controller medication in the 12 mo before the index date, diagnosis of asthma in the 12 mo before the index date, and health care utilization in the 12 mo before the index date.
Significant results (P < .05) are in bold.
TABLE IV.
Hazard ratios of asthma-related ED visits and hospitalizations associated with cumulative DDD of statin use
| Database | Asthma-related ED visits or hospitalizations | Asthma-related ED visits | Asthma-related hospitalizations | ||||
|---|---|---|---|---|---|---|---|
| Statin | N | Events | AHR* (95% CI) | Events | AHR* (95% CI) | Events | AHR* (95% CI) |
| LHID2000+2005+2010 | |||||||
| 0 (no use) | 97,729 | 2,818 | Ref | 1,614 | Ref | 1,204 | Ref |
| 1 (cDDD ≤ 28) | 5,185 | 239 | 1.25 (1.09, 1.43)† | 116 | 1.2 (0.99, 1.45) | 123 | 1.33 (1.1, 1.61) |
| 2 (28 < cDDD ≤ 74.67) | 4,913 | 174 | 0.95 (0.81, 1.11) | 101 | 1.09 (0.89, 1.34) | 73 | 0.82 (0.65, 1.04) |
| 3 (74.67 < cDDD ≤ 172) | 4,825 | 121 | 0.67 (0.56, 0.81) | 65 | 0.72 (0.56, 0.93) | 56 | 0.64 (0.49, 0.84) |
| 4 (cDDD > 172) | 4,943 | 65 | 0.34 (0.27, 0.44) | 40 | 0.42 (0.31, 0.58) | 25 | 0.27 (0.18, 0.4) |
| LHID2000 | |||||||
| 0 (no use) | 35,265 | 1,102 | Ref | 609 | Ref | 493 | Ref |
| 1 (cDDD ≤ 28) | 1,776 | 86 | 1.62 (1.14, 2.28) | 41 | 1.57 (0.89, 2.74) | 45 | 1.65 (1.07, 2.56) |
| 2 (28 < cDDD ≤ 74.67) | 1,729 | 58 | 1.1 (0.71, 1.7) | 39 | 1.82 (1.04, 3.17) | 19 | 0.65 (0.32, 1.32) |
| 3 (74.67 < cDDD ≤ 172) | 1,650 | 44 | 0.78 (0.47, 1.28) | 24 | 1.25 (0.66, 2.4) | 20 | 0.47 (0.21, 1.07) |
| 4 (cDDD > 172) | 1,693 | 27 | 0.39 (0.21, 0.71) | 15 | 0.55 (0.24, 1.25) | 12 | 0.29 (0.12, 0.7) |
| LHID2005 | |||||||
| 0 (no use) | 34,721 | 1,015 | Ref | 579 | Ref | 436 | Ref |
| 1 (cDDD ≤ 28) | 1,858 | 82 | 1.07 (0.7, 1.64) | 38 | 1.23 (0.69, 2.18) | 44 | 0.93 (0.49, 1.78) |
| 2 (28 < cDDD ≤ 75) | 1,699 | 75 | 1.07 (0.68, 1.67) | 43 | 1.08 (0.57, 2.05) | 32 | 1.07 (0.58, 1.98) |
| 3 (75 < cDDD ≤ 172) | 1,753 | 43 | 0.59 (0.34, 1.01) | 26 | 0.77 (0.39, 1.52) | 17 | 0.41 (0.17, 1.01) |
| 4 (cDDD > 172) | 1,767 | 22 | 0.36 (0.2, 0.67) | 15 | 0.55 (0.27, 1.13) | 7 | 0.19 (0.06, 0.61) |
| LHID2010 | |||||||
| 0 (no use) | 31,512 | 829 | Ref | 501 | Ref | 328 | Ref |
| 1 (cDDD ≤ 28) | 1,742 | 83 | 1.9 (1.27, 2.83) | 45 | 2.48 (1.42, 4.36) | 38 | 1.53 (0.86, 2.69) |
| 2 (28 < cDDD ≤ 71) | 1,666 | 50 | 1.03 (0.61, 1.73) | 26 | 1 (0.43, 2.3) | 24 | 1.06 (0.55, 2.04) |
| 3 (71 < cDDD ≤ 168.33) | 1,606 | 38 | 0.65 (0.36, 1.18) | 16 | 0.55 (0.2, 1.5) | 22 | 0.73 (0.35, 1.51) |
| 4 (cDDD > 168.33) | 1,615 | 17 | 0.26 (0.12, 0.59) | 11 | 0.54 (0.22, 1.34) | 6 | 0.07 (0.01, 0.53) |
AHR, Adjusted hazards ratio; cDDD, cumulative DDD; CI, confidence interval; DDD, defined daily dose; ED, emergency department; LHID, Longitudinal Health Insurance Database.
Adjusted for age, sex, Deyo-Charlson index (exclude chronic pulmonary disease) in the 12 mo before the index date, asthma controller medication in the 12 mo before the index date, diagnosis of asthma in the 12 mo before the index date, and health care utilization in the 12 mo before the index date.
Significant results (P < .05) are in bold.
DISCUSSION
In this large cohort study consisting of patients with asthma insured by Taiwan’s National Health Plan during the period of 2001 to 2013, we found that statin users had reduced risk of asthma-related ED visits and/or hospitalizations compared with nonstatin users. In addition, the risk of asthma-related ED visits and hospitalizations was decreased among those with higher cDDD, greater average DDD, and longer cumulative-day statin use than their counterparts. These findings suggest that statins may have a beneficial effect on preventing asthma exacerbations and are associated with decreased risks of asthma-related ED visits and/or hospitalization in an Asian population.
Our study findings are supported by previous studies. Previously, using the Population-based Effectiveness in Asthma and Lung Diseases (PEAL) Network population in the USA, Tse et al13 found that statin use in patients with asthma is associated with decreased asthma-related ED visits and oral corticosteroid use. In another study using a different population in the USA, Tse et al22 reported that statin use in individuals with asthma taking inhaled corticosteroids is associated with decreased asthma-related ED visits. Furthermore, using data derived from another subset of NHIRD (2000) in Taiwan, Huang et al23 have also found that statin use is associated with a lower incidence of hospitalization in patients with asthma. Whereas Huang et al studied 1 subset of NHIRD, our study includes 3 subsets, demonstrating that these findings are generalizable to multiple years. Moreover, we were able to identify a dose-dependent relationship with higher daily and cumulative dose of statin use being associated with fewer exacerbations from asthma.
Understanding the pathophysiology behind the effects of statins on asthma-related outcomes is beyond the scope of this study; however, the pleiotropic anti-inflammatory and immunomodulatory effects of statins are well documented in the literature,12 and in animal models of experimental asthma.24 Nevertheless, the actions of statin on the respiratory tract are still unclear, and several small randomized controlled trials on statin in asthma have demonstrated limited benefits across various laboratory and clinical endpoints.15,17,25 Bhattacharjee et al26 summarized the effects of statin to mitigate against asthma as having 2 parts. The first effect is to reduce airway inflammation by reducing airway inflammatory cells influx,27 decreasing inflammatory cytokine,28 and nitric oxide production,29 and the second effect is to counter airway remodeling by mitigating airway epithelial dam-age,30 inhibiting subepithelial fibrosis,31,32 and inhibiting contractile regulatory proteins in the airway smooth muscle.33,34 Taken together, in view of the multifaceted nature of statin action, further investigations are needed to validate the potential beneficial effect of statin therapy observed in this study.
The strength of our study is that it is a large, nationwide, population-based study. Because Taiwan has a NHI plan, all Taiwanese have equal access to medications and health care; thus, heterogeneity in access to statins and asthma medications is not likely to play a role in asthma-related outcomes investigated in the present study. In addition, because all Taiwanese are insured by the same NHI plan, we are able to confidently capture all health care utilization. In addition, because prescriptions are covered by the NHI program in Taiwan, prescriptions are generally filled for residents in Taiwan including patients with asthma examined in this study. In general, pharmacy departments are available inside all teaching hospitals and almost all regional hospitals in Taiwan. Moreover, we are able to assess a dose-response relationship between statin use and asthma-related outcomes, which to the best of our knowledge has not been explored in other studies. Despite the strengths of our study, a few caveats should be noted in the present study. First, asthma is a heterogeneous disease consisting of several asthma phenotypes and/or endotypes, and our medical claims database is unable to differentiate which patients with asthma will benefit most from statins. In addition to phenotypic heterogeneity, it would also be of interest to explore whether statin treatment is associated with asthma severity. Second, our study is an observational study and we cannot determine whether statins cause decreased asthma-related ED visits and hospitalizations. Third, information on potential confounding factors such as smoking, alcohol, obesity, and quality of education at office visits is not available in the NHIRD. Therefore, it is likely that residual confounding effects could exist because of unmeasured variables. Fourth, we examined only the beneficial effects of statin use in Taiwanese study participants, limiting the generalizability of our results.
CONCLUSIONS
In this nationwide population-based cohort of patients with asthma, statin use is associated with decreased asthma-related ED visits and hospitalizations. Our study also suggests that there is a dose-response effect of statin use on asthma-related ED visits and hospitalizations in an Asian population. As such, future randomized clinical trials would be of importance to examine the effect of statin therapy on asthma exacerbations among adult populations and to confirm the findings from this study.
Supplementary Material
What is already known about this topic?
Statins have pleiotropic anti-inflammatory and immunomodulatory effects, yet the effect of statin use on asthma-related emergency department (ED) visits and hospitalization has remained unclear.
What does this article add to our knowledge?
In line with some previous studies, this study suggests that statins may have a beneficial effect on preventing asthma exacerbations and are associated with a decreased risk of asthma-related ED visits and/or hospitalization in an Asian population.
How does this study impact current management guidelines?
This study indicates that statin use is associated with decreased risk of asthma-related ED visits and/or hospitalization. Future randomized clinical trials would be of importance to examine the effect of statin therapy on asthma exacerbations.
Acknowledgments
Y-TT and T-CY are supported in part by grants from the Ministry of Science and Technology and National Health Research Institutes (PI: Tsai, NSC 101-2314-B-400-009-MY2, MOST 103-2314-B-400-004-MY3, PH-104-PP-14, PH-104-SP-05, PH-104-SP-16, PH-105-SP-05, and PH-105-SP-04; PI: Yao, NSC 101-2314-B-182A-044, NSC 102-2314-B-182A-048, MOST 103-2314-B-182-030, MOST 104-2314-B-182-046-MY2, and MOST 106-2314-B-182-051-MY3). This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes (Registered numbers: 99081, 99136, 99287, 101014, NHRID-101-548). The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
Abbreviations used
- AHR
Adjusted hazards ratio
- cDDD
Cumulative DDD
- CI
Confidence interval
- DDD
Defined daily dose
- ED
Emergency department
- LHID
Longitudinal Health Insurance Database
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research Database
Footnotes
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Gong HJ. Wheezing and Asthma. 3rd ed Boston: Butterworth-Heinemann; 1990. [Google Scholar]
- 2.Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 19902013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 19902013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet2015;385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellwood P, Asher MI, Billo NE, Bissell K, Chiang CY, Ellwood EM, et al. The Global Asthma Network rationale and methods for Phase I global surveillance: prevalence, severity, management and risk factors. Eur Respir J 2017;49: 1601605 10.1183/13993003.01605-2016. [DOI] [PubMed] [Google Scholar]
- 5.Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy 2010; 65:152–67. [DOI] [PubMed] [Google Scholar]
- 6.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control study. Am J Respir Crit Care Med 2004;170:836–44. [DOI] [PubMed] [Google Scholar]
- 7.Bateman ED, Bousquet J, Busse WW, Clark TJ, Gul N, Gibbs M, et al. Stability of asthma control with regular treatment: an analysis of the Gaining Optimal Asthma controL (GOAL) study. Allergy 2008;63:932–8. [DOI] [PubMed] [Google Scholar]
- 8.Hammer SC, Robroeks CM, van Rij C, Heynens J, Droog R, J£bsis Q, et al. Actual asthma control in a paediatric outpatient clinic population: do patients perceive their actual level of control? Pediatr Allergy Immunol 2008;19:626–33. [DOI] [PubMed] [Google Scholar]
- 9.Carroll WD, Wildhaber J, Brand PL. Parentmisperception of control in childhood/adolescent asthma: the Room to Breathe survey. Eur Respir J 2012;39:90–6. [DOI] [PubMed] [Google Scholar]
- 10.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA 1999;282:2340–6. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995;333:1301–7. [DOI] [PubMed] [Google Scholar]
- 12.Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, et al. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation 2015;132:1224–33. [DOI] [PubMed] [Google Scholar]
- 13.Tse SM, Li L, Butler MG, Fung V, Kharbanda EO, Larkin EK, et al. Statin exposure is associated with decreased asthma-related emergency department visits and oral corticosteroid use. Am J Respir Crit Care Med 2013;188: 1076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostroukhova M, Kouides RW, Friedman E. The effect of statin therapy on allergic patients with asthma. Ann Allergy Asthma Immunol 2009;103:463–8. [DOI] [PubMed] [Google Scholar]
- 15.Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax 2008;63: 1070–5. [DOI] [PubMed] [Google Scholar]
- 16.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med 2009;180:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol 2007;119:328–35. [DOI] [PubMed] [Google Scholar]
- 18.Wu CS, Chang CM, Tsai YT, Huang YW, Tsai HJ. Antipsychotic treatment and the risk of hip fracture in subjects with schizophrenia: a 10-year population-based case-control study. J Clin Psychiatry 2015;76:1216–23. [DOI] [PubMed] [Google Scholar]
- 19.WHO Collaborating Centre for Drug Statistic Methodology. Guidelines for ATC Classification and DDD Assignment. Oslo: WHO; 2009. [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn 1958;53:457–81. [Google Scholar]
- 22.Tse SM, Charland SL, Stanek E, Herrera V, Goldfarb S, Litonjua AA, et al. Statin use in asthmatics on inhaled corticosteroids is associated with decreased risk of emergency department visits. Curr Med Res Opin 2014;30:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang CC, Chan WL, Chen YC, Chen TJ, Chou KT, Lin SJ, et al. Statin use in patients with asthma: a nationwide population-based study. Eur J Clin Invest 2011;41:507–12. [DOI] [PubMed] [Google Scholar]
- 24.Huang CF, Peng HJ, Wu CC, Lo WT, Shih YL, Wu TC. Effect of oral administration with pravastatin and atorvastatin on airway hyperresponsiveness and allergic reactions in asthmatic mice. Ann Allergy Asthma Immunol 2013; 110:11–7. [DOI] [PubMed] [Google Scholar]
- 25.Braganza G, Chaudhuri R, McSharry C, Weir CJ, Donnelly I, Jolly L, et al. Effects of short-term treatment with atorvastatin in smokers with asthma—a randomized controlled trial. BMC Pulm Med 2011;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharjee D, Chogtu B, Magazine R. Statins in asthma: potential beneficial effects and limitations. Pulm Med 2015;2015:835204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Le W, Ahuja R, Cho DY, Hwang PH, Upadhyay D. Inhibition of inflammatory mediators: role of statins in airway inflammation. Otolaryngol Head Neck Surg 2011;144:982–7. [DOI] [PubMed] [Google Scholar]
- 28.Madonna R, Di Napoli P, Massaro M, Grilli A, Felaco M, De Caterina A, et al. Simvastatin attenuates expression of cytokine-inducible nitric-oxide synthase in embryonic cardiac myoblasts. J Biol Chem 2005;280:13503–11. [DOI] [PubMed] [Google Scholar]
- 29.Zeki AA, Bratt JM, Rabowsky M, Last JA, Kenyon NJ. Simvastatin inhibits goblet cell hyperplasia and lung arginase in a mouse model of allergic asthma: a novel treatment for airway remodeling? Transl Res 2010;156: 335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner NA, O’Regan DJ, Ball SG, Porter KE. Simvastatin inhibits MMP-9 secretion from human saphenous vein smooth muscle cells by inhibiting the RhoA/ROCK pathway and reducing MMP-9 mRNA levels. FASEB J 2005;19: 804–6. [DOI] [PubMed] [Google Scholar]
- 31.Schaafsma D, Dueck G, Ghavami S, Kroeker A, Mutawe MM, Hauff K, et al. The mevalonate cascade as a target to suppress extracellular matrix synthesis by human airway smooth muscle. Am J Respir Cell Mol Biol 2011;44: 394–403. [DOI] [PubMed] [Google Scholar]
- 32.Capra V, Rovati GE. Rosuvastatin inhibits human airway smooth muscle cells mitogenic response to eicosanoid contractile agents. Pulm Pharmacol Ther 2014;27:10–6. [DOI] [PubMed] [Google Scholar]
- 33.Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H. Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. Am J Respir Cell Mol Biol 2006;35:722–9. [DOI] [PubMed] [Google Scholar]
- 34.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010;181:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


