Abstract
The skin comprises a complex coordinated system of epithelial tissue cells and immune cells that ensure adequate immune reactions against trauma, toxins and pathogens, while maintaining tissue homeostasis. Keratinocytes form the outermost barrier of the skin, and sense changes in barrier integrity, intrusion of microbial components and stress molecules. Thus, they act as sentinels that continuously communicate the status of the organ to the cutaneous immune system. Upon damage the keratinocytes initiate a pro-inflammatory signaling cascade that leads to the activation of resident immune cells. Simultaneously, the tissue mediates and supports immune-suppressive functions to contain inflammation locally. After resolution of inflammation, the skin provides a niche for regulatory and effector memory T cells that can quickly respond to reoccurring antigens. In this review we discuss the central role of keratinocyte-derived signals in controlling cutaneous T cell immunity.
1. Introduction
The skin provides the outermost barrier of the body to the environment, and therefore is in constant contact with environmental toxins, pathogens, mechanical stress and commensal bacteria. The skin itself comprises a physical barrier that consists of layers of keratinocytes in different differentiation statuses, and the underlying dermis that mostly consists of extracellular matrix produced by fibroblasts. Due to its exposed location the skin is equipped to sense tissue damage and infection. During an immune response the skin signals to the resident immune cells, thereby initiating clearance of infection and promoting tissue regeneration. Immune responses in the skin have to be strictly balanced to prevent systemic inflammation and maintain tissue integrity, a sensitive process that is supported by the skin tissue cells such as keratinocytes and fibroblasts.
While the role of innate immune cells in skin immunity is of great importance to tissue immunity, this review will primarily highlight the interactions between non-hematopoietic tissue cells, and T cells. Specific sentinel lymphoid structures and epithelial immune functions in other peripheral tissues such as the mucosa-associated lymphoid tissue (MALT) and the bronchus-associated lymphoid tissue (BALT) have been described and reviewed in great detail [1–3], and therefore we aim to highlight the importance of epithelial cells in regulating cutaneous immune responses. We will focus on the unique role of keratinocytes in orchestrating the skin immune system with an emphasis on skin-tropic and skin-resident T cells in skin immune homeostasis and inflammatory responses. We will discuss how keratinocytes sense damage and danger and initiate the signaling cascade leading to specific T cell responses. Additionally, we will introduce mechanisms that establish and maintain immune homeostasis and memory, and discuss how the tissue is able to imprint a specific T cell phenotype.
2. Player introductions – Cellular components of the skin
The skin is a highly organized organ that consists of various layers, all of which have unique functions that contribute to barrier integrity and host-defense. The outer layer of the skin is formed by the epidermis, which is underlaid by the dermis[4]. The epidermis is mainly comprised of keratinocytes (up to 95% of all cells) and can be divided into different layers (see Figure 1) that are formed by sequential differentiation stages of these cells. The life cycle of keratinocytes leads to constant self-renewal of the epidermis. The basal layer (stratum basale) constitutes the inner epidermal layer, which is in contact with the dermis via various structural proteins that form the basement membrane zone. Keratinocytes of the basal layer proliferate and subsequently differentiate and move up (outwards) until they reach the cornified layer (stratum corneum) and are finally shed in a process called desquamation. The stratum corneum consists of dead and flattened keratinocytes that are embedded in a lipid matrix and form the first and outermost physical and chemical barrier against pathogen entry and prevents water loss. Lamellar bodies produced by cells of the underlying stratum granulosum support the chemical barrier function of the stratum corneum by shedding of various lipids, fatty acids and anti-microbial peptides (AMPs). Epidermal barrier integrity and function of the stratum corneum is reviewed in detail elsewhere [5,6]. The importance of an intact skin barrier is exemplified by the increased prevalence of allergic sensitization and development of atopic dermatitis when the epidermal barrier is compromised, for example due to mutations in the filaggrin gene or a perturbed lipid metabolism [7–9]. In addition to these intrinsic genetic factors, epithelial cells are influenced by the environment and the microbiome, which can further determine the outcome of immune responses towards potential allergens and pathogens, which is reviewed in detail in [10]. Although primarily formed by keratinocytes, the stratum basale also contains melanocytes which produce the skin pigment melanin and also contribute to the innate immune function of the skin [11]. The immune functions of the epidermis are greatly supported by a specialized population of antigen-presenting cells, namely CD1a+ Langerhans cells (LCs) [12]. Additionally, innate lymphoid cells [13], γδ T cells (in humans epidermis-associated; primarily Vδ1+) [14] and dendritic-epidermal T cells (DETCs; in mice only [15,16]) as well as adaptive αβ tissue resident T cells (discussed in more detail below) can be found throughout the epidermis [17] (Fig.1).
Figure 1: Keratinocytes are equipped to sense tissue status and maintain T cell niche.
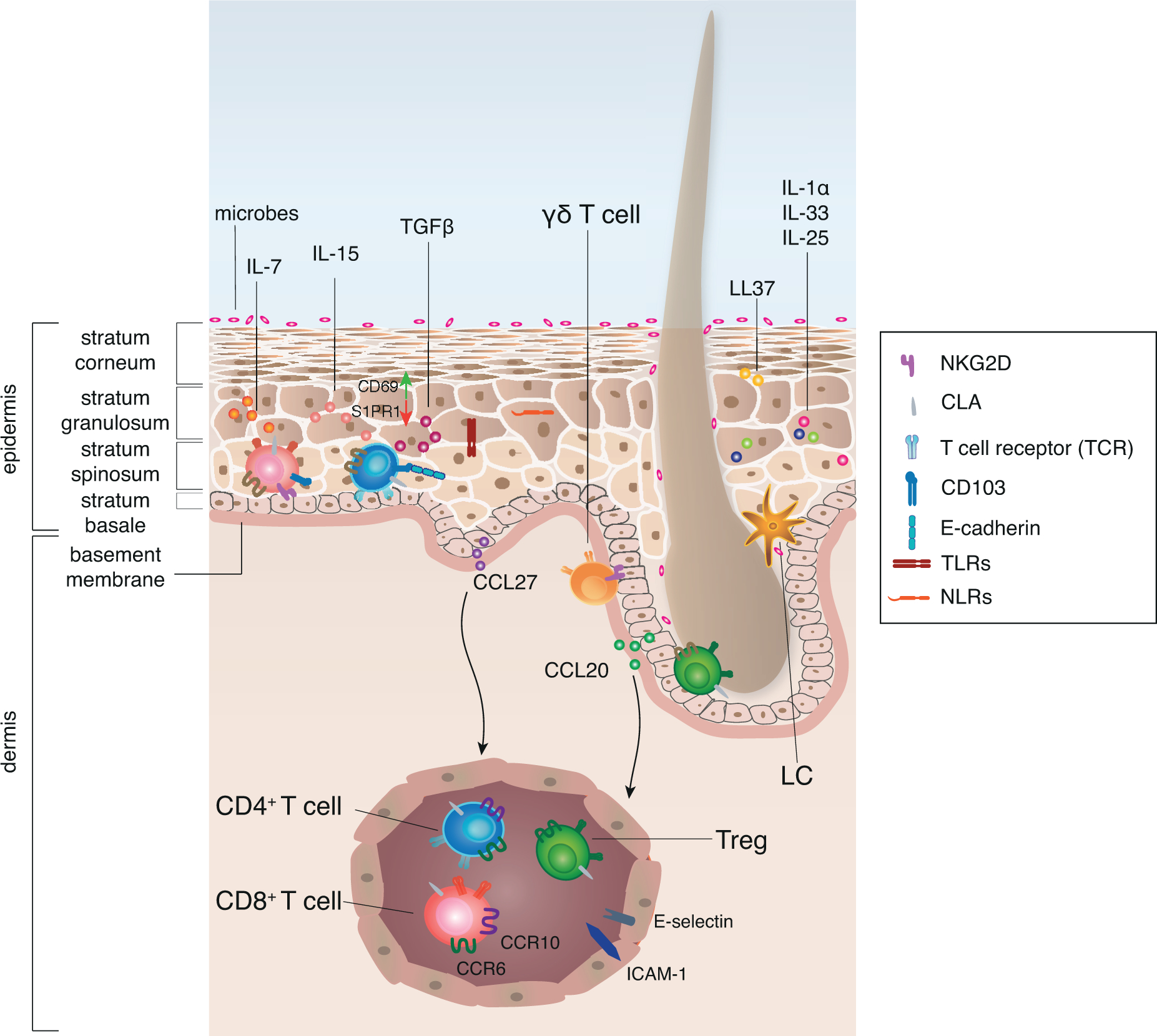
The human epidermis is mainly comprised of keratinocytes and can be divided into different layers. Keratinocytes function as sentinels that are equipped with TLRs and NLRs to sense danger signals, such as microbial components, chemicals or stress signals released upon damage, so called PAMPs and alarmins. Alarmins are produced by keratinocytes themselves and show anti-microbial properties, such as secretion of LL37. Additionally, keratinocytes are equipped with pre-stored cytokines, such as IL-1α, IL-25 and IL-33 that are released upon cell damage. The immune function of the epidermis is supported by specialized populations of antigen-presenting cells, CD1a+ Langerhans cells (LC), as well as innate lymphoid cells, and adaptive tissue-resident T cells. CCL20 produced by keratinocytes of the HF co-localizes LC and Treg and thus HF may serve as a niche for the induction of peripheral tolerance. Similarly, γδ T cells are often associated with adnexal structures.
The human skin provides a variety of survival and maintenance signals such as IL-7, IL-15 and TGF-β to support the maintenance of tissue-resident T cells that ensure quick response to infection. Keratinocyte-derived CCL27 and CCL20 allow for the recruitment of T cells from the blood that express CCR10 and CCR6 respectively to non-inflamed tissue. T cells that sense the chemokine gradient can extravasate through post-capillary venules mediated by E-selectin - CLA interaction and ICAM-1. Upon tissue entry T cells change their surface receptor expression, probably induced by tissue-derived signals. In order to maintain antigen-experienced T cells within the epidermis, downregulation of S1PR1 and upregulation of CD69 are supported by IL-15 and TGF-β. In addition, TGF-β induces upregulation of CD103 on T cells that allows them to interact with E-cadherin on keratinocytes, which supports the retention of resident memory T cells.
The epidermis also gives rise to a number of specialized appendages also called adnexal structures like sebaceous glands and hair follicles (HFs) that extend down into the underlying dermis. HFs fulfill several unique immune functions in the skin and in some ways substitute for organized lymphoid structures that are not present in healthy skin. For instance, HFs have been shown to serve as a reservoir for various commensals [18] and as a preferred site for immune cell location [19–22].
The dermis consists mostly of extracellular matrix (ECM) of elastin fibers and collagens that is secreted by dermal fibroblasts. The ECM gives pliability and stability to the skin and additionally supports infiltrating and resident immune cells, such as mast cells, macrophages, innate lymphoid cells (ILCs), γδ T cells and αβ T cells. These skin-homing immune cells can enter the tissue by extravasation via dermal post-capillary venules that express specific surface molecules and support directed migration (discussed below).
3. Readiness to sense damage
Due to its location, the skin faces constant physical stress, mechanical or chemical damage and encounters environmental toxins and pathogens as well as commensal bacteria. Upon barrier breach and intrusion of microbes the skin is poised to kick-start anti-microbial responses and to recruit and activate effector cells. To do so, keratinocytes, fibroblasts and skin-resident immune cells function as sentinels that are equipped to detect danger signals, such as microbial components, chemicals or certain stress signals released by damaged cells. Below we briefly discuss mechanisms used to sense any disruption of skin homeostasis.
3.1. TLRs and NLRs
Keratinocytes express a wide range of pattern recognition receptors (PRRs). Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are constitutively expressed by keratinocytes and allow sensing of pathogen associated molecular patterns (PAMPs) which are highly conserved microbe-derived motifs [23] (Fig.1). TLRs can be grouped as cell-surface TLRs (TLR 1,2,4,5, and 6) and TLRs that reside in intracellular compartments (TLR 3, 7, 8, and 9) that sense specific motifs derived from bacteria, fungi or viruses. Some TLRs form heterodimers allowing them to sense a broader range of molecules. Keratinocytes constitutively express TLRs 1–6, 9 and 10 (the latter in humans only) [24–26]. Functional TLR2 recognizes ligands expressed by Gram-positive bacteria, and TLR4, TLR5 are specific for Gram-negative lipopolysaccharides and bacterial flagellin, respectively. On the other hand, TLR3 can recognize double stranded RNA originating from viruses, and keratinocytes treated with polyI:C, a TLR3-ligand, produce the inflammatory cytokines TNFα and IL-6 [27], which regulate keratinocyte proliferation as well as T cell differentiation [28]. Interestingly, Th1-derived IFN-γ increases TLR3 expression in keratinocytes, enhancing anti-viral activity against herpes simplex virus type-1, which although a DNA virus, produces dsRNA during its replication [29]. Engagement of TLRs and NLRs activates different transcription factors, such as NF-κB and interferon-regulatory factors (IRFs) that result in the production of pro-inflammatory cytokines and type I interferons, respectively [30].
3.2. Endogenous danger signals: alarmins
In addition to pathogens tissue and cell damage caused by physical insult, excessive heat or cold or chemical insults, as well as UV radiation can cause skin damage and disturb skin function. This damage is signaled via endogenous danger molecules, so called alarmins. Among the produced alarmins are defensins, S100 proteins, heat-shock proteins and cathelicidins (LL37) that show direct anti-microbial action (Fig.1), as well as chemokines and cytokines that activate and recruit innate and adaptive immune cells. Additionally keratinocytes store cytosolic IL-1α that requires no further cleavage and allows for rapid activation of adjacent immune cells in case of cell damage [31]. Similar to IL-1α, IL-25 and IL-33 are stored within the cells and their secretion is released upon contact with proteases or defective barrier (Fig.1 & 2), which results in activation of adaptive and innate immune cells [32–34]. Alarmins can further be secreted by innate and adaptive effector cells when they are activated by PAMPs or other alarmins [23].
Figure 2: Keratinocytes orchestrate damage response and its regulation in human skin.
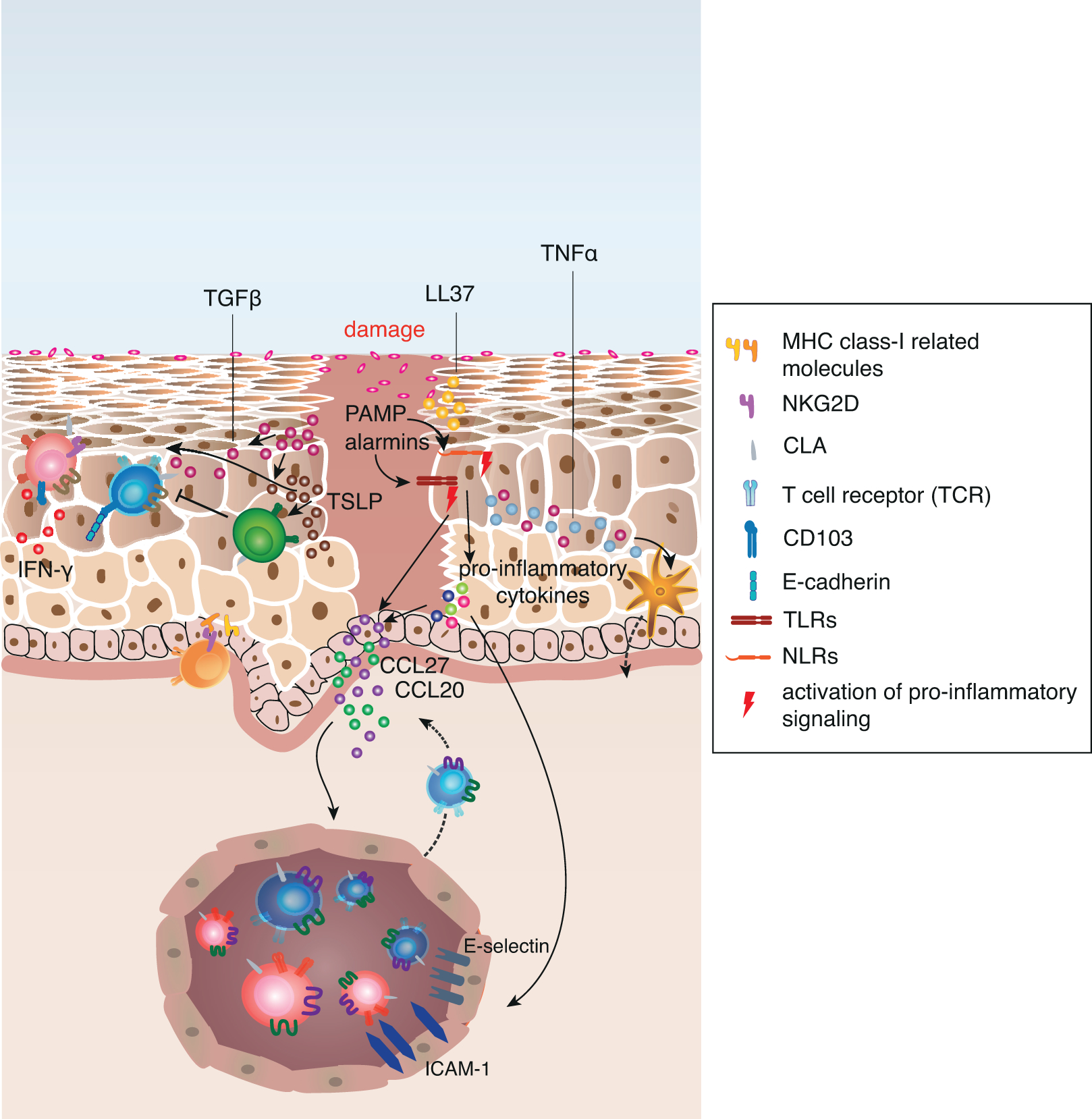
Upon tissue damage pre-stored cytokines are released from damaged cells and initiate immediate effector functions in adjacent cells. Sensing of microbial components and stress molecules (PAMPs and alarmins) by TLRs and NLRs result in the activation of pro-inflammatory signalling pathways such as NF-κB and IRF transcription. This leads to the increased production of pro-inflammatory cytokines, such as IL-1α, IL-1β, IL-18, TNFα and type I interferons. Keratinocyte-derived TNFα activates Langerhans cells to migrate to skin-draining lymph nodes and activate naive or central memory T cells that upregulate CLA expression upon interaction with the LC. Additionally, the pro-inflammatory cytokines mediate the upregulation of chemokines and adhesion molecules on post-capillary venules as well as on adjacent keratinocytes, which promotes recruitment of circulating skin-tropic T cells from the blood.
In addition to these mechanisms, keratinocytes upregulate MHC class I-related gene products on their surface that engage NKG2D on CD8+ T cells and γ5 T cells. Pro-inflammatory cytokines also allow keratinocytes to directly activate antigen-experienced memory CD4+ T cells. Activated T cells in the tissue secrete pro-inflammatory cytokines, (e.g. IFN-γ), that in turn activate chemokine secretion by keratinocytes and increased alarmin and anti-microbial peptide (e.g. LL37) production.
TGF-β and TSLP secretion is increased upon tissue damage, and while both cytokines induce a variety of pro-inflammatory gene products and induce migration of LCs to skin-draining lymph nodes, they are essential to keep the inflammation local and support its resolution by directly signaling to Treg. TGF-β also promotes the local formation of resident memory T cells to provide quick response in case of a secondary antigen encounter.
4. Responding to damage
Homeostatic expression of chemokines by keratinocytes and chemokine receptors by skin-tropic immune cells allows immune cell recruitment and retention within the tissue in absence of inflammation. However, in case of tissue damage or infection rapid changes of the expression of inflammatory chemokines by keratinocytes as well as tissue-resident immune cells allow for the recruitment of effector immune cells and in parallel to rapidly activate the resident immune cells. These changes are triggered by cytokines produced in response to tissue damage or infection.
4.1. Kick-starting the response
Should a barrier breach occur PAMPs and other danger-associated signals (such as alarmins) stimulate TLRs and NLRs (Fig.2). TLR engagement leads to the activation of several response pathways through interferon regulatory factors (IRF), which are transcription factors that regulate gene expression in response to pathogen-derived danger signals. In particular, IRFs specify the transcription and secretion of target proteins, such as type I interferons (e.g. IFN-α, IFN-β) and pro-inflammatory cytokines (e.g. IL-6, TNF-α). Type I interferon secretion by KCs prepares yet unaffected keratinocytes for the coming danger through the regulation of several genes, so called IFN-stimulated genes (ISGs)[30]. ISGs have a wide range of functions including pathogen recognition, inhibition of translation and/or transcription especially directed against viruses and also viral RNA degradation [35]. For example, TLR3-signaling drives IRF6-dependent mRNA-expression for interferon-β (IFN-β), the interleukin-12 (IL-12) family member IL-23p19 by keratinocytes[36]. Similarly, IRF1 and IRF3 expression by keratinocytes regulate anti-viral immunity and MHC class I expression by keratinocytes[37][38]. IRF5 and NF-κB, downstream of TLRs are known to target pro-inflammatory cytokine transcription [39] (Fig.2). Interestingly, IRF6 also regulates keratinocyte migration, which might become relevant in wound repair [40]. On the other hand, NLR signaling leads to the activation of the inflammasome via NLRP3, resulting in the activation of the pro-inflammatory cytokines IL-1β and IL-18. The NLRs NOD1 and NOD2 are constitutively expressed by keratinocytes and sense bacterial products and contribute to inflammation by activating the NF-κB, IRF3 and 5 and MAPK signaling pathways [39,41]. Upon sensing of alarmins or microbial motifs keratinocytes release TNF-α, IL-1α, IL-1β and IL-18. IL-1 acts in an autocrine manner on KC, by inducing the expression of IL-1R and IL-1R antagonist; the latter acts as a competitive inhibitor of IL-1α and is crucial in balancing/resolving inflammatory responses. Additionally IL-1α stimulates the production of other pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α and also stimulates the expression of various adhesion molecules endothelial cells [42] (Fig2).
Importantly, IL-1α secretion by keratinocytes alone is not enough to activate effector T cells in the periphery. Skin macrophages, upon IL-1R signaling secrete CXCL2 that attracts dermal DCs and initiates the formation of dermal DC-T cell clusters in the skin, which promotes antigen-dependent activation of effector T cells [43], emphasizing the importance of the myeloid cell compartment in eliciting cutaneous immune responses. Thus, IL-1α can act as a pro-inflammatory stimulus to a variety of tissue resident immune cells, such as LCs, dermal DCs, macrophages and T cells as well as adjacent keratinocytes and mediates increased leukocyte recruitment to the skin. Similarly, the activation of TLRs leads to the secretion of specific subsets of cytokines that will lead to the activation of epidermal LCs and also dermal dendritic cells.
4.2. Calling in the troops - recruitment of T cells to the skin
In order to recruit antigen-specific adaptive T cells the skin supports the egress of antigen-presenting cells to the draining lymphoid organs (Fig.2). Keratinocyte-derived TNF-α induces the activation and migration of Langerhans cells (LCs) to the skin-draining lymph nodes where they present antigen to T cells [44](Fig.2). The LC-T cell interaction in the skin draining lymph nodes induces expression of cutaneous leukocyte antigen (CLA) on T cells. CLA binds preferentially to its ligand, E-selectin, whose expression on the endothelium is increased in inflamed skin [45].
Effector T cells express certain combination of surface receptors that are used for migration as well as retention in the tissue. The current concept suggests the target tissue is imprinted in the draining lymph nodes by antigen presenting cells [46]. Subsequently, a proportion of T cells that infiltrate the skin will develop into tissue resident memory cells.
Likewise, TNF-α and IL-1β secreted upon damage induce CCL27 expression in adjacent keratinocytes [47] (Fig.2). Thus, via alarmin secretion and TLR/NLR engagement that results in NF-kB pathway activation, keratinocytes can both initiate and amplify the pro-inflammatory signaling cascade, thereby orchestrating infiltration, activation and differentiation of leukocytes (Fig.2). In fact, this intricate process is quite compartmentalized and different keratinocyte populations of the HF have been shown to produce stress signals and chemokines that regulate recruitment of monocyte-derived precursors of LCs to the epidermis [48]. In human and murine HFs, keratinocytes of the infundibulum express CCL20 which signals to the CCR6 receptor to recruit T cells and LCs to the HF [22] (Fig.1). CCL20 and CCL27 attract memory T cells via their CCR6 and CCR10 receptors that are expressed on most skin-tropic lymphocytes, which are characterized by their expression of CLA [49] (Fig.2). The importance of the CCL27-CCR10 axis is underlined by impaired lymphocyte recruitment in the skin if it is lost. Conversely, CCR10 expression is increased in patients that suffer from psoriasis, topic or allergic-contact dermatitis, leading to increased leukocyte recruitment to the skin[50].
Interestingly, the type of injury can result in tailored activation, recruitment and/or local activation of the most effective cell subset. For example, TLR3 signaling (e.g. in response to its synthetic ligand, poly-I:C or its natural ligand, dsRNA) induces IL-1β and IL-18 production, which polarize DC activation promoting T cell differentiation towards the Th1 subtype in vitro [51]. Depending on the specific receptor engaged (e.g. TLR type), keratinocytes secret distinct chemokines that lead to the attraction of different subsets of T cells to the skin. For instance, keratinocytes secrete CXCL9 and CXCL10 upon TLR3 stimulation, both of which are known to recruit Th1 T cells. Similarly, signaling through TLRs 3 and 5 results in increased CCL20 and CCL27 secretion, which promotes memory T cell recruitment specifically to the skin[24]. Thus, fine-tuning of the signals that control the recruitment to the skin is crucial to maintain tissue homeostasis while still being able to respond adequately to damage.
4.3. Cellular communication is a two-way street
By the above-described mechanisms keratinocytes trigger pro-inflammatory signaling cascades that orchestrate infiltration, activation and differentiation of leukocytes via alarmin secretion and TLR/NLR engagement. However, in addition to these pathways, damaged and stressed keratinocytes may also communicate with their surroundings by altering their surface phenotype. An example is the induction of certain MHC class-I related gene products such as MICA, MICB and ULBP in humans and Rae-1, Mult and H60 in mouse (Fig.2). All of these are ligands of NKG2D, an activating receptor that is expressed on a variety of resident or infiltrating immune cells in the skin [52,53]. Expression of these molecules is generally considered to mark “altered self’ and activate the immune system in order to eradicate dysfunctional/stressed cells. NKG2D is an activating receptor first described on NK cells, but additionally, it is expressed by γδ T cells, CD8+ T cells and NKT cells [54–56]. Furthermore, freshly isolated γδ T cells from human skin all express NKG2D and even maintain the expression in subsequent cell culture. Thus the skin is able to directly signal damage to effector T cells via upregulation of these various makers of cellular stress [57]. In accordance with that idea, under pro-inflammatory conditions keratinocytes can express MHC II and ICAM-1 on their surface and lead to direct activation of antigen-experienced memory CD4+ T cells [58]. However, cellular communication between keratinocytes and immune cells of the skin is reciprocal. While keratinocytes can either directly or indirectly activate skin T cells, T cell-derived cytokines IL-17 and IL-22 in turn have been shown to promote the production of anti-microbial peptides and proteins as well as alarmins by keratinocytes, thereby actively supporting the anti-microbial properties of the skin and amplifying the inflammatory response [59]. Additionally, IL-17 and IFN-γ are known to trigger abnormal keratinocyte proliferation in psoriasis. Interestingly, the antimicrobial peptide LL37 that is overexpressed by keratinocytes in psoriatic skin may serve as a self-antigen for T cells in psoriatic patients [60].
Taken together, by sensing changes in the barrier integrity, intrusion of microbial components and stress molecules, keratinocytes act as sentinels that continuously communicate the status of the organ to the resident immune system. NF-κB that is activated by TLR/NLR signaling in response to danger represents one important link between innate and adaptive immune system of the skin as it increases expression of adhesion molecules, chemokines and cytokines all of which support the recruitment of additional leukocytes to the site of inflammation [61]. Fast effector mechanisms such as release of pre-stored effector cytokines and direct activation of resident cells ensure the rapid and effective response that includes protection against infection and initiation of tissue-repair.
5. Immune surveillance
The ability to respond to danger signals (infection, damage) greatly relies on the interplay between keratinocytes and immune cells. While keratinocytes sense the initial signals, they quickly communicate changes or loss of tissue homeostasis to resident immune cells whose response then supports to re-establish tissue integrity and homeostasis. T cells are important players in skin immunity, which we will discuss in more detail in the following section.
5.1. A home for T cells - skin forms a niche
The presence of memory T cells in the skin is crucial to ensure quick and adequate microbial response to infection in case of barrier breach. Hence to support tissue homeostasis it is advantageous for the skin to provide a niche for resident T cells that can respond quickly and promote cutaneous immune responses. The importance of T cells in skin immunity is also underlined by the impressive numbers of T cells in the skin. In humans it harbors twice the number of T cells than the whole blood circulation, and 98% of all skin-tropic T cells (characterized by CLA+CCR4+) are located in the skin while only 2% are circulating cells [62]. For decades the classical view of T cell immunity in the skin suggested that effector cells were continually circulating between the tissue, the draining lymph nodes and the blood. However, using an elegant xeno-transplantation model Boyman et al. found that skin resident T cells are sufficient to mediate immune responses, and that, at least in Psoriasis, the initiation of cutaneous immune responses can occur independent of circulating T cells [63]. Not surprisingly and in line with their diverse functions, memory T cell populations of human skin are heterogeneous in terms of surface receptor expression and function [64]. There is emerging evidence that tissue-derived signals themselves can imprint these tissue signatures [65,66]. In accordance with that idea, epidermal keratinocytes can induce CCR8 and CLA expression on T cells via yet unknown soluble factors, thereby controlling the localization of skin-selective T cells[67]. To support these T cells within the tissue, keratinocytes under steady state (i.e. in absence of inflammation) provide a variety of survival and maintenance signals such as IL-7, IL-15 and TGF-β [20,68,69] (Fig.1).
5.2. Counter-regulation of inflammatory responses - keeping the balance
While initiating and instructing effector T cell responses is important to ensure clearance of infections, it is also crucial to keep inflammation local. By contrast, uncontrolled immune responses against innocuous antigens, like allergens or self-antigens within the skin can lead to chronic inflammatory skin diseases such as psoriasis and atopic dermatitis. Many regulatory mechanisms are mediated by keratinocytes, either directly, for example via secretion of TGF-β, TSLP or IL-1 antagonists (as discussed before) or indirectly by supporting the generation and function of suppressive regulatory T cells (Fig.2).
5.2.1. Modulation of inflammatory responses by pro-inflammatory molecules
Supporting the generation and maintenance of tissue T cells in the skin is just one part of keeping the balance between inflammation and homeostasis. In the course of an ongoing immune response it is vital to directly activate suppressive Treg to modulate inflammation and prevent development of systemic inflammatory conditions. One mechanism to directly support regulatory T cell (Treg) function during inflammation is the secretion of TSLP by keratinocytes. TSLP (thymic stromal lymphopoietin), an important player of skin immunity, is rapidly expressed by stressed keratinocytes and induces a variety of innate and adaptive immune cells [70]. Expression of TSLP can induce the expression of many pro-inflammatory factors like Tslp, Tfgb, Bmp7 as well as genes for pro-inflammatory cytokines, l17d, Il1f9, Il24 and Kitl and the chemokine genes Ccl22, Cxcl1, Cxcl9 and Cxcl. However, it has also been shown to signal specifically to skin-associated Treg that express the TSLP-receptor and prevent rapid progression of a local skin response to a lethal systemic condition [71]. Thus, in addition to their well-characterized role in pro-inflammatory responses, keratinocytes also directly support immunosuppressive responses that are critical for re-establishing homeostasis of the organism.
Similarly, TGF-β, whose release and maturation is increased in damaged or inflamed skin and plays an important role in tissue remodeling and immune homeostasis of the skin [72], shows pro-inflammatory as well as immunosuppressive functions that can balance the outcome of an immune response. On the one hand, TGF-β can activate DCs to migrate to the skin-draining lymph nodes and induce T cell homing to the skin in contact hypersensitivity [73]. On the other hand, it can mediate the reduction of IFN-γ production and cytotoxic functions of CD8+ T cells [74], induce the formation of resident memory T cells in the epidermis of the skin [65,69]. TGF-β also supported the in vitro generation of skin allograft-protective Treg in presence of TGF-β, IL-2 and retinoic acid [75].
5.2.2. Skin supports residence and generation of regulatory T cells
In addition to fine-tuning pro-inflammatory and suppressive responses, the skin harbors a great number of Treg that stably reside within the tissue [21]. Recently it was confirmed that more than 95% of Treg that reside in healthy human skin are antigen-experienced memory Treg (mTreg). Interestingly these mTreg preferentially reside near HFs in both human and murine skin [20,21], (Fig.1). It is likely that HFs serve as a niche for increased presentation of microbial and other HF-associated antigens that are not expressed in the thymus, and might thus play an important role as a niche for the induction of peripheral tolerance towards commensals. HFs might also be central in the initial seeding of the skin with Treg during development. Treg are among the first ab T cells to enter the skin and neonatal murine skin recruits Treg within a certain window of time via CCL20 signaling to induce commensal-specific tolerance [22]. Interestingly the peak of Treg infiltration in neonatal skin coincides with postnatal HF morphogenesis [76]. Thereby the HF recruits Treg to the entry point of commensals potentially supporting development of peripheral Treg. These tissue-Treg then directly support regenerative processes of HFs by expression of the Notch ligand family member Jagged 1 (Jag1) [77]. Additionally, Treg support the regeneration of the tissue during wound healing by attenuating IFNg production via EGFR-dependent mechanisms [78]. These data indicate that Treg fulfill various functions to support tissue homeostasis and indeed, Treg in the skin are heterogeneous in their ontogeny and function [79]. The importance of antigen-specific tissue resident mTreg in resolving autoimmune inflammation could be shown with an inducible mouse model for skin autoimmunity. Using tetracycline-induced ovalbumin expression by keratinocytes, Rosenblum et al. demonstrated that the persistent expression of self-antigen favors the induction of suppressive Treg that resolved organ-specific autoimmunity in mice [80,81]. Interestingly, exposure to persistently expressed self-antigen led to the preferential maintenance of mTreg in this model, which was dependent on skin-derived IL-7 [20]. This demonstrates that keratinocyte-derived signals such as IL-7 [82,83], as well as antigen persistence in the skin can switch the balance from pro-inflammatory to suppressive immune responses.
6. Skin remembers - T cell memory
Inflammatory responses and the control of the same are important mechanisms in healthy human skin tissue. Based on the exposed position of the skin recurring encounters with a specific antigen are likely to occur over the life of an individual, such as bacterial antigen upon barrier breach. In order to quickly respond to reoccurring antigens, formation and maintenance of T cell memory allowing for a quick secondary response is crucial.
6.1. T cell memory formation is mediated by keratinocyte-derived factors
As we will discuss later in more detail, entry into the skin is crucial for progenitors of tissue-resident memory T cells (TRM) to turn on the TRM transcription profile [69]. Although the exact mechanisms of TRM development are not fully understood yet, the modulation of the sphingosine-1-phosphate receptor (S1PR1) signaling by upregulation of CD69 plays a central role in memory formation (Fig.1). The transcriptional downregulation of S1RP1 seems to be required for the establishment of CD8+ memory T cells, since forced expression of S1R1P leads to failure of CD8+ TRM induction in the skin of mice [84]. In addition to CD69, keratinocyte-derived factors such as TGF-β, TNF-α and IL-33 [65,69,84] lead to transcriptional downregulation of S1RP1 independent of TCR stimulation. The presence of CD69 alone may not be sufficient to induce tissue-residency and it is likely that other receptors or ligands are involved in memory T cell maintenance in the skin. E-cadherin that is expressed by keratinocytes, acts as an interaction partner for CD103, which is part of the αEβ7 integrin. CD103 is selectively upregulated upon tissue entry of T cells even in absence of local antigen stimulation [85] which is essential to localize to epithelial tissues and retain TRM in the skin [69]. Expression of CD103 on human CD4+ and CD8+ T cells in vitro is induced by keratinocyte-derived TGF-β [86]. Additionally, Watanabe et al. showed that keratinocytes alone are able to induce CD103 expression in T cells [64] (Fig.1).
Thus, keratinocytes directly support the establishment of resident memory T cells within the tissue, which is beneficial when it comes to secondary infections and allows for an even faster and more effective anti-microbial response. Given their heterogeneity [64] it is apparent that memory T cells in the skin show different effector functions. While Treg have already been shown to promote wound healing in mice [78] it is likely that there are other memory T cell subsets that support tissue regeneration similar to skin-resident innate cells [87,88].
6.2. Skin imprints transcriptional profile of resident memory T cells
The skin tissue imprints not only memory formation and trafficking-receptor expression, but also a tissue specific transcriptional profile that is shared among resident memory T cells across various tissues [89,90]. While TRM are retained in the tissues via surface molecules such as CD69 and/or CD103 and typically express these markers, the heterogeneity of tissue-resident T cells is not covered by these two markers alone [91]. Specific gene expression patterns that support residency and function of TRM in the skin rely on entry of the cells into the epidermis driven by keratinocyte-derived chemokines (CXCL9, CXCL10 upon viral infection) as well as keratinocyte-derived cytokines such as IL-15 and TGFβ [69](Fig.1). Moreover, human CD69+CD4+ and CD8+ memory T cells in human lung and spleen share a core transcriptional profile that distinguishes them from CD69-CD4+ effector memory T cells found in tissue and circulation [92]. Among the shared characteristics are adhesion and inhibitory molecules such as CD49a, CD103 and CD101, PD-1, as well as the production of pro-inflammatory cytokines such as IFNg, IL-2 and IL-17 to rapidly respond upon activation in the tissue, as well as regulatory cytokines (IL-10) to limit excessive tissue damage [89]. Although this shared transcriptional signature was observed across several human organs, they did not include skin tissue and it will be interesting to see if the described transcriptional profile can be extended to cutaneous resident memory T cells. Even though tissue resident T cells share a common transcriptional profile, a recent study by Wong et al. [66] using mass cytometry (CyTOF), revealed the significant influence of the tissue sites on the differentiation of memory T cells. The group analyzed eight different human tissues, including skin, liver, cord blood, PBMC, mucosal tissues and lymphoid tissues for their resident populations of memory T cell. They discovered that CD69+ T cells are diverse and comprise various subsets of tissue-resident cells that gain their tissue specificity by distinct combinations of trafficking and functional markers. Using a barcoding system combined with mass cytometry they analyzed trafficking, function, differentiation and activation of different T helper subsets and could reveal that it is not the expression of a single trafficking marker alone, but the combination of different receptors, that determines the tissue specificity. Additionally, TRM populations are highly diverse across human tissues and show clear tissue signatures that are independent of their T helper subtype. For example, CCR2 is highly expressed on Th1 cells in the blood but almost absent on Th1 cells in the lung [93]. Similarly, Duhen et al. identified a Th22 subpopulation in human PBMC that identifies by expression of various skin homing receptors (CCR4, CCR10, CLA) and produces IL-22 but no IL-17 [94]. However, CCR10 was only lowly expressed on IL-22 secreting cells across different tissues, indicating that there are important differences between blood-derived T cells and tissue-derived T cells in activation, cytokine secretion and probably function [66]. In addition to tissue-specific differences between TRM, species-specific differences have recently been described. Homolog-of BLIMP in T cells (Hobit) transcription factor was found to have a crucial role in CD8+ TRM differentiation in vivo in mice [90]. However, when looking at the differentially expressed genes in human TRM cells, Kumar et al could not find increased expression of Hobit in the human tissues, emphasizing potential differences in the molecular control of human and mouse TRM differentiation [89].
Interestingly, while the majority of skin-resident T cells will remain resident for a long period of time [95], the view that all TRM cells remain sessile has recently been challenged [96]. Also the notion that CD69 expression was sufficient to impart tissue residency was challenged by the finding of re-circulating CD69+ CD8+ T cells in murine studies. This study found that a proportion of non-lymphoid cells resident in the skin may exit the tissue upon re-challenge with the same antigen to become resident in draining lymphoid organs where they may boost the magnitude of recall responses [96] [97]. It is tempting to hypothesize that the migration of resident T cells from non-lymphoid tissues to the draining lymph nodes is promoted by the tissue itself, via modulation of the maintenance signals (such as IL-15 or TGF-β), possibly upon re-encounter of an antigen or danger signals that drive the spread of memory T cells generated on one skin site, in order to protect distant skin sites.
Despite significant advances in understanding the regulation of cutaneous immunity, mechanistic studies on the specific contributions of individual cell types and signals in the genesis and severity of human inflammatory skin diseases remain difficult. Particularly the reliance on animal models is problematic due to fundamental structural differences in the skin in humans versus mice, and a lack of direct correspondence between cutaneous T cell populations in these species. Currently, skin-tropic human T cells are often studied from the peripheral blood where they can be readily identified based on their expression of the cutaneous lymphocyte antigen (CLA) and skin-homing chemokine receptors such as CCR4, CCR8 and CCR10. However, the developmental and functional relationship of these cells in the blood with the different populations of T cells in the skin is still poorly understood. Thus, mechanistic studies require the further development and refinement of humanized mouse models such as xeno-grafting models that are designed to study cutaneous TRM biology [64].
7. Conclusions
The skin is one of the largest immunological organs that due to its exposed location as a barrier organ has to ensure adequate immune monitoring. For fast and effective clearance of infections the skin provides a niche for various immune cells of the innate and adaptive immune system. Memory, effector and regulatory T cell populations are induced and maintained by skin-derived signals and in turn support tissue protective and regenerative functions such as alarmin secretion or wound healing [59] [78].
Due to its size the skin is vulnerable to various insults that can occur simultaneously at distinct sites. Thus, the tissue has developed mechanisms to support fast clearance of infections via locally restricted inflammatory responses, while at the same time preventing the development of organ-wide or even systemic inflammation. To this end the skin employs signaling molecules that are locally restricted and often induce pro-inflammatory responses while initiating suppressive functions [71,98].
In summary, in this review we highlighted the central role of the epidermal barrier as the main orchestrator of cutaneous immune responses. There are many more interesting aspects of the skin immune system that we did not discuss here, such as the role of the extracellular matrix in modulating cell trafficking during an immune response or the crosstalk between the commensal microbiota and the tissue, which are reviewed elsewhere[99][100]. However, the existing data underline the importance of tissue-derived signals that should be considered when studying immunological processes in tissues. We believe that elucidating the immune-regulatory functions of the skin will be key in the development of treatment options for diseases such as chronic wounds of the skin, autoimmune skin diseases or skin cancer.
Highlights:
Keratinocytes sense changes in the skin barrier, damage, cell stress, and intrusion of microbes
Keratinocytes act as sentinels that communicate the status of the skin
Keratinocyte-derived signals orchestrate and shape cutaneous immune responses
Keratinocytes help to maintain tissue homeostasis
Skin tissue supports long-lived memory T cells to promote immune protection
Acknowledgements
The authors’ work has been supported by a joint grant from the NIH to IKG and DJC (R01AI127726). IKG is also supported by a grant from the Dystrophic Epidermolysis Bullosa Research Association (DEBRA) International and DEBRA Austria. MMK is recipient of a DOC fellowship awarded by the Austrian Academy of Sciences.
Abbreviations:
- CLA
Cutaneous leukocyte antigen
- ECM
extracellular matrix
- HFs
hair follicles
- ILCs
innate lymphoid cells
- IRFs
interferon-regulatory factors
- LCs
Langerhans cells
- mTreg
memory Treg
- NLRs
nucleotide-binding oligomerization domain (NOD)-like receptors
- PRRs
pattern recognition receptors
- PAMPs
pathogen associated molecular patterns
- TLRs
Toll-like receptors
- Treg
regulatory T cells
- TRM
tissue resident memory
- TSLP
thymic stromal lymphopoietin
Footnotes
Declarations of interest: none
References
- [1].Coskun M, Intestinal epithelium in inflammatory bowel disease, Front. Med 1 (2014) 24 10.3389/fmed.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gandhi VD, Vliagoftis H, Airway epithelium interactions with aeroallergens: role of secreted cytokines and chemokines in innate immunity, Front. Immunol 6 (2015) 147 10.3389/fimmu.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peterson LW, Artis D, Intestinal epithelial cells: regulators of barrier function and immune homeostasis, Nat. Rev. Immunol 14 (2014) 141–153. 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- [4].Kolarsick PAJ, Kolarsick MA, Goodwin C, Anatomy and Physiology of the Skin, J. Dermatol. Nurses Assoc 3 (2011) 203 10.1097/JDN.0b013e3182274a98. [DOI] [Google Scholar]
- [5].Baroni A, Buommino E, De Gregorio V, Ruocco E, Ruocco V, Wolf R, Structure and function of the epidermis related to barrier properties, Clin. Dermatol 30 (2012) 257–262. 10.1016/j.clindermatol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- [6].Menon GK, Cleary GW, Lane ME, The structure and function of the stratum corneum, Int. J. Pharm 435 (2012) 3–9. 10.1016/j.ijpharm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- [7].Kubo A, Nagao K, Amagai M, Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases, J. Clin. Invest 122 (2012) 440–447. 10.1172/JCI57416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].S. J and Strobel S, Skin Barrier Dysfunction and Systemic Sensitization to Allergens Through the Skin, Curr. Drug Targets - Inflamm. Allergy (2005). http://www.eurekaselect.com/90764/article (accessed May 7, 2018). [DOI] [PubMed]
- [9].Hammad H, Lambrecht BN, Barrier Epithelial Cells and the Control of Type 2 Immunity, Immunity. 43 (2015) 29–40. 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- [10].Lambrecht BN, Hammad H, The immunology of the allergy epidemic and the hygiene hypothesis, Nat. Immunol 18 (2017) 1076–1083. 10.1038/ni.3829. [DOI] [PubMed] [Google Scholar]
- [11].Tapia CV, Falconer M, Tempio F, Falcón F, López M, Fuentes M, Alburquenque C, Amaro J, Bucarey SA, Di Nardo A, Melanocytes and melanin represent a first line of innate immunity against Candida albicans, Med. Mycol 52 (2014) 445–454. 10.1093/mmy/myu026. [DOI] [PubMed] [Google Scholar]
- [12].Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P, Epidermal Langerhans cells—Changing views on their function in vivo, Immunol. Lett 106 (2006) 119–125. 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- [13].Bonefeld CM, Geisler C, The role of innate lymphoid cells in healthy and inflamed skin, Immunol. Lett 179 (2016) 25–28. 10.1016/j.imlet.2016.01.005. [DOI] [PubMed] [Google Scholar]
- [14].Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL, A role for human skin-resident T cells in wound healing, J. Exp. Med 206 (2009) 743–750. 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hayday AC, [gamma][delta] cells: a right time and a right place for a conserved third way of protection, Annu. Rev. Immunol 18 (2000) 975–1026. 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- [16].Havran WL, Chien YH, Allison JP, Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors, Science. 252 (1991) 1430–1432. [DOI] [PubMed] [Google Scholar]
- [17].Pasparakis M, Haase I, Nestle FO, Mechanisms regulating skin immunity and inflammation, Nat. Rev. Immunol 14 (2014) 289–301. 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- [18].Montes LF, Wilborn WH, Location of bacterial skin flora, Br. J. Dermatol 81 (1969) Suppl 1:23+. [DOI] [PubMed] [Google Scholar]
- [19].Gilliam AC, Kremer IB, Yoshida Y, Stevens SR, Tootell E, Teunissen MB, Hammerberg C, Cooper KD, The human hair follicle: a reservoir of CD40+ B7-deficient Langerhans cells that repopulate epidermis after UVB exposure, J. Invest. Dermatol 110 (1998) 422–427. 10.1046/j.1523-1747.1998.00162.x. [DOI] [PubMed] [Google Scholar]
- [20].Gratz IK, Truong H-A, Yang SH-Y, Maurano MM, Lee K, Abbas AK, Rosenblum MD, Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues, J. Immunol. Baltim. Md 1950 190 (2013) 4483–4487. 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, Yang SH-Y, Anthony BA, Sverdrup FM, Krow-Lucal E, MacKenzie TC, Johnson DS, Meyer EH, Löhr A, Hsu A, Koo J, Liao W, Gupta R, Debbaneh MG, Butler D, Huynh M, Levin EC, Leon A, Hoffman WY, McGrath MH, Alvarado MD, Ludwig CH, Truong H-A, Maurano MM, Gratz IK, Abbas AK, Rosenblum MD, Memory regulatory T cells reside in human skin, J. Clin. Invest 124 (2014) 1027–1036. 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scharschmidt TC, Vasquez KS, Pauli ML, Leitner EG, Chu K, Truong H-A, Lowe MM, Sanchez Rodriguez R, Ali N, Laszik ZG, Sonnenburg JL, Millar SE, Rosenblum MD, Commensal Microbes and Hair Follicle Morphogenesis Coordinately Drive Treg Migration into Neonatal Skin, Cell Host Microbe. 21 (2017) 467–477.e5. 10.1016/j.chom.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bianchi ME, DAMPs, PAMPs and alarmins: all we need to know about danger, J. Leukoc. Biol 81 (2007) 1–5. 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- [24].Lebre MC, van der Aar AMG, van Baarsen L, van Capel TMM, Schuitemaker JHN, Kapsenberg ML, de Jong EC, Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9, J. Invest. Dermatol 127 (2007) 331–341. 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- [25].Terhorst D, Kalali BN, Ollert M, Ring J, Mempel M, The role of toll-like receptors in host defenses and their relevance to dermatologic diseases, Am. J. Clin. Dermatol 11 (2010) 1–10. 10.2165/11311110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [26].Baker BS, Ovigne J-M, Powles AV, Corcoran S, Fry L, Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis, Br. J. Dermatol 148 (2003) 670–679. [DOI] [PubMed] [Google Scholar]
- [27].Borkowski AW, Park K, Uchida Y, Gallo RL, Activation of TLR3 in keratinocytes increases expression of genes involved in formation of the epidermis, lipid accumulation and epidermal organelles, J. Invest. Dermatol 133 (2013) 2031–2040. 10.1038/jid.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tanaka T, Narazaki M, Kishimoto T, IL-6 in Inflammation, Immunity, and Disease, Cold Spring Harb. Perspect. Biol 6 (2014) a016295 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kajita AI, Morizane S, Takiguchi T, Yamamoto T, Yamada M, Iwatsuki K, Interferon-Gamma Enhances TLR3 Expression and Anti-Viral Activity in Keratinocytes, J. Invest. Dermatol 135 (2015) 2005–2011. 10.1038/jid.2015.125. [DOI] [PubMed] [Google Scholar]
- [30].Kawamura T, Ogawa Y, Aoki R, Shimada S, Innate and intrinsic antiviral immunity in skin, J. Dermatol. Sci 75 (2014) 159–166. 10.1016/j.jdermsci.2014.05.004. [DOI] [PubMed] [Google Scholar]
- [31].Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR, Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice., J. Clin. Invest 90 (1992) 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dickel H, Gambichler T, Kamphowe J, Altmeyer P, Skrygan M, Standardized tape stripping prior to patch testing induces upregulation of Hsp90, Hsp70, IL-33, TNF-α and IL-8/CXCL8 mRNA: new insights into the involvement of “alarmins”, Contact Dermatitis. 63 (2010) 215–222. 10.1111/j.1600-0536.2010.01769.x. [DOI] [PubMed] [Google Scholar]
- [33].Kim BE, Bin L, Ye Y-M, Ramamoorthy P, Leung DYM, IL-25 enhances HSV-1 replication by inhibiting filaggrin expression, and acts synergistically with Th2 cytokines to enhance HSV-1 replication, J. Invest. Dermatol 133 (2013) 2678–2685. 10.1038/jid.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kouzaki H, Tojima I, Kita H, Shimizu T, Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells, Am. J. Respir. Cell Mol. Biol 49 (2013) 741–750. 10.1165/rcmb.2012-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bowie AG, Unterholzner L, Viral evasion and subversion of pattern-recognition receptor signalling, Nat. Rev. Immunol 8 (2008) 911–922. 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ramnath D, Tunny K, Hohenhaus DM, Pitts CM, Bergot A-S, Hogarth PM, Hamilton JA, Kapetanovic R, Sturm RA, Scholz GM, Sweet MJ, TLR3 drives IRF6-dependent IL-23p19 expression and p19/EBI3 heterodimer formation in keratinocytes, Immunol. Cell Biol. 93 (2015) 771–779. 10.1038/icb.2015.77. [DOI] [PubMed] [Google Scholar]
- [37].Ye W, Xu Y, Wang Y, Dong Y, Xi Q, Cao M, Yu L, Zhang L, Cheng L, Wu X, Xu Z, Lei Y, Zhang F, Hantaan virus can infect human keratinocytes and activate an interferon response through the nuclear translocation of IRF-3, Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis 29 (2015) 146–155. 10.1016/j.meegid.2014.11.009. [DOI] [PubMed] [Google Scholar]
- [38].Zhou F, Chen J, Zhao K-N, Human papillomavirus 16-encoded E7 protein inhibits IFN-γ-mediated MHC class I antigen presentation and CTL-induced lysis by blocking IRF-1 expression in mouse keratinocytes, J. Gen. Virol 94 (2013) 2504–2514. 10.1099/vir.0.054486-0. [DOI] [PubMed] [Google Scholar]
- [39].Chen Y-J, Li J, Lu N, Shen X-Z, Interferon regulatory factors: A key to tumour immunity, Int. Immunopharmacol 49 (2017) 1–5. 10.1016/j.intimp.2017.05.010. [DOI] [PubMed] [Google Scholar]
- [40].Biggs LC, Naridze RL, DeMali KA, Lusche DF, Kuhl S, Soll DR, Schutte BC, Dunnwald M, Interferon regulatory factor 6 regulates keratinocyte migration, J. Cell Sci 127 (2014) 2840–2848. 10.1242/jcs.139246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Strober W, Murray PJ, Kitani A, Watanabe T, Signalling pathways and molecular interactions of NOD1 and NOD2, Nat. Rev. Immunol 6 (2006) 9–20. 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- [42].Juráňová J, Franková J, Ulrichová J, The role of keratinocytes in inflammation, J. Appl. Biomed 15 (2017) 169–179. 10.1016/j.jab.2017.05.003. [DOI] [Google Scholar]
- [43].Natsuaki Y, Egawa G, Nakamizo S, Ono S, Hanakawa S, Okada T, Kusuba N, Otsuka A, Kitoh A, Honda T, Nakajima S, Tsuchiya S, Sugimoto Y, Ishii KJ, Tsutsui H, Yagita H, Iwakura Y, Kubo M, guan Ng L, Hashimoto T, Fuentes J, Guttman-Yassky E, Miyachi Y, Kabashima K, Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin, Nat. Immunol 15 (2014) 1064–1069. 10.1038/ni.2992. [DOI] [PubMed] [Google Scholar]
- [44].Kimber I, Cumberbatch M, Stimulation of Langerhans Cell Migration by Tumor Necrosis Factor α (TNF-α), J. Invest. Dermatol 99 (1992) S48–S50. 10.1111/1523-1747.ep12668986. [DOI] [PubMed] [Google Scholar]
- [45].Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS, Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells, Nature. 389 (1997) 978–981. 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- [46].Baaten BJG, Cooper AM, Swain SL, Bradley LM, Location, Location, Location: The Impact of Migratory Heterogeneity on T Cell Function, Front. Immunol 4 (2013). 10.3389/fimmu.2013.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, Zlotnik A, CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells, Proc. Natl. Acad. Sci. U. S. A 96 (1999) 14470–14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, Ueha S, Horiuchi K, Tanizaki H, Kabashima K, Kubo A, Cho Y, Clausen BE, Matsushima K, Suematsu M, Furtado GC, Lira SA, Farber JM, Udey MC, Amagai M, Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin, Nat. Immunol 13 (2012) 744–752. 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM, CC-Chemokine Receptor 6 Is Expressed on Diverse Memory Subsets of T Cells and Determines Responsiveness to Macrophage Inflammatory Protein 3α, J. Immunol 162 (1999) 186–194. [PubMed] [Google Scholar]
- [50].Homey B, Alenius H, Müller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bünemann E, Lehto M, Wolff H, Yen D, Marxhausen H, To W, Sedgwick J, Ruzicka T, Lehmann P, Zlotnik A, CCL27-CCR10 interactions regulate T cell-mediated skin inflammation, Nat. Med 8 (2002) 157–165. 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- [51].Cristina Lebre M, Antons JC, Kalinski P, Schuitemaker JHN, van Capel TMM, Kapsenberg ML, de Jong EC, Double-Stranded RNA-Exposed Human Keratinocytes Promote Th1 Responses by Inducing a Type-1 Polarized Phenotype in Dendritic Cells: Role of Keratinocyte-Derived Tumor Necrosis Factor α, Type I Interferons, and Interleukin-18, J. Invest. Dermatol 120 (2003) 990–997. 10.1046/j.1523-1747.2003.12245.x. [DOI] [PubMed] [Google Scholar]
- [52].Cerwenka A, Lanier LL, NKG2D ligands: unconventional MHC class I like molecules exploited by viruses and cancer, Tissue Antigens. 61 (2003) 335–343. 10.1034/j.1399-0039.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- [53].Raulet DH, Roles of the NKG2D immunoreceptor and its ligands, Nat. Rev. Immunol 3 (2003) 781–790. 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- [54].Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T, Activation of NK Cells and T Cells by NKG2D, a Receptor for Stress-Inducible MICA, Science. 285 (1999) 727–729. 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- [55].Houchins JP, Yabe T, McSherry C, Bach FH, DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells, J. Exp. Med 173 (1991) 1017–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH, The Role of the NKG2D Immunoreceptor in Immune Cell Activation and Natural Killing, Immunity. 17 (2002) 19–29. 10.1016/S1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- [57].Ebert LM, Meuter S, Moser B, Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance, J. Immunol. Baltim. Md 1950 176 (2006) 4331–4336. [DOI] [PubMed] [Google Scholar]
- [58].Black APB, Ardern-Jones MR, Kasprowicz V, Bowness P, Jones L, Bailey AS, Ogg GS, Human keratinocyte induction of rapid effector function in antigen-specific memory CD4+ and CD8+ T cells, Eur. J. Immunol 37 (2007) 1485–1493. 10.1002/eji.200636915. [DOI] [PubMed] [Google Scholar]
- [59].MacLeod AS, Mansbridge JN, The Innate Immune System in Acute and Chronic Wounds, Adv. Wound Care 5 (2016) 65–78. 10.1089/wound.2014.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, Chamilos G, Feldmeyer L, Marinari B, Chon S, Vence L, Riccieri V, Guillaume P, Navarini AA, Romero P, Costanzo A, Piccolella E, Gilliet M, Frasca L, The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis, Nat. Commun 5 (2014) 5621 10.1038/ncomms6621. [DOI] [PubMed] [Google Scholar]
- [61].Barnes PJ, Nuclear factor-kappa B, Int. J. Biochem. Cell Biol 29 (1997) 867–870. [DOI] [PubMed] [Google Scholar]
- [62].Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K-I, Dowgiert RK, Kupper TS, The vast majority of CLA+ T cells are resident in normal skin, J. Immunol. Baltim. Md 1950 176 (2006) 4431–4439. [DOI] [PubMed] [Google Scholar]
- [63].Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO, Spontaneous Development of Psoriasis in a New Animal Model Shows an Essential Role for Resident T Cells and Tumor Necrosis Factor-α, J. Exp. Med 199 (2004) 731–736. 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Watanabe R, Gehad A, Yang C, Campbell L, Teague JE, Schlapbach C, Elco C, Huang V, Matos TR, Kupper TS, Clark RA, Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells, Sci. Transl. Med 7 (2015) 279ra39 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D, Antigen independent differentiation and maintenance of effector-like resident memory T cells in tissues, J. Immunol. Baltim. Md 1950 188 (2012) 4866–4875. 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wong MT, Ong DEH, Lim FSH, Teng KWW, McGovern N, Narayanan S, Ho WQ, Cerny D, Tan HKK, Anicete R, Tan BK, Lim TKH, Chan CY, Cheow PC, Lee SY, Takano A, Tan E-H, Tam JKC, Tan EY, Chan JKY, Fink K, Bertoletti A, Ginhoux F, Curotto de Lafaille MA, Newell EW, A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures, Immunity. 45 (2016) 442–456. 10.1016/j.immuni.2016.07.007. [DOI] [PubMed] [Google Scholar]
- [67].McCully ML, Ladell K, Hakobyan S, Mansel RE, Price DA, Moser B, Epidermis instructs skin homing receptor expression in human T cells, Blood. 120 (2012) 4591–4598. 10.1182/blood-2012-05-433037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, Manton JH, Heath WR, Mueller SN, Persistence of skin-resident memory T cells within an epidermal niche, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 5307–5312. 10.1073/pnas.1322292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T, The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin, Nat. Immunol 14 (2013) 1294–1301. 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- [70].Ziegler SF, Artis D, Sensing the outside world: TSLP regulates barrier immunity, Nat. Immunol 11 (2010) 289–293. 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kashiwagi M, Hosoi J, Lai J-F, Brissette J, Ziegler SF, Morgan BA, Georgopoulos K, Direct control of regulatory T cells by keratinocytes, Nat. Immunol 18 (2017) 334–343. 10.1038/ni.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro., Proc. Natl. Acad. Sci. U. S. A 83 (1986) 4167–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mohammed J, Gunderson AJ, Khong H-H, Koubek RD, Udey MC, Glick AB, TGFβ1 Overexpression by Keratinocytes Alters Skin Dendritic Cell Homeostasis and Enhances Contact Hypersensitivity, J. Invest. Dermatol 133 (2013) 135–143. 10.1038/jid.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Thomas DA, Massagué J, TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance, Cancer Cell. 8 (2005) 369–380. 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- [75].Moore C, Tejon G, Fuentes C, Hidalgo Y, Bono MR, Maldonado P, Fernandez R, Wood KJ, Fierro JA, Rosemblatt M, Sauma D, Bushell A, Alloreactive regulatory T cells generated with retinoic acid prevent skin allograft rejection, Eur. J. Immunol 45 (2015) 452–463. 10.1002/eji.201444743. [DOI] [PubMed] [Google Scholar]
- [76].Paus R, Müller-Röver S, Van Der Veen C, Maurer M, Eichmüller S, Ling G, Hofmann U, Foitzik K, Mecklenburg L, Handjiski B, A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis, J. Invest. Dermatol 113 (1999) 523–532. 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- [77].Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong H-A, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, Taravati K, Tan MR, Ricardo-Gonzalez RR, Nosbaum A, Bertolini M, Liao W, Nestle FO, Paus R, Cotsarelis G, Abbas AK, Rosenblum MD, Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation, Cell. 169 (2017) 1119–1129.e11. 10.1016/j.cell.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nosbaum A, Prevel N, Truong H-A, Mehta P, Ettinger M, Scharschmidt TC, Ali NH, Pauli ML, Abbas AK, Rosenblum MD, Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing, J. Immunol. Baltim. Md 1950 196 (2016) 2010–2014. 10.4049/jimmunol.1502139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gratz IK, Campbell DJ, Organ-specific and memory treg cells: specificity, development, function, and maintenance, Front. Immunol 5 (2014) 333 10.3389/fimmu.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gratz IK, Rosenblum MD, Maurano MM, Paw JS, Truong H-A, Marshak-Rothstein A, Abbas AK, Cutting edge: Self-antigen controls the balance between effector and regulatory T cells in peripheral tissues, J. Immunol. Baltim. Md 1950 192 (2014) 1351–1355. 10.4049/jimmunol.1301777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK, Response to self antigen imprints regulatory memory in tissues, Nature. 480 (2011) 538–542. 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Al-Rawi M. a. A., Rmali K, Watkins G, Mansel RE, Jiang WG, Aberrant expression of interleukin-7 (IL-7) and its signalling complex in human breast cancer, Eur. J. Cancer Oxf. Engl. 1990 40 (2004) 494–502. 10.1016/j.ejca.2003.10.016. [DOI] [PubMed] [Google Scholar]
- [83].Interleukin 7 is produced by murine and human keratinocytes, J. Exp. Med 178 (1993) 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Skon CN, Lee J-Y, Anderson KG, Masopust D, Hogquist KA, Jameson SC, Transcriptional downregulation of S1pr1 is required for establishment of resident memory CD8+ T cells, Nat. Immunol 14 (2013) 1285–1293. 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T, Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 7037–7042. 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pauls K, Schön M, Kubitza RC, Homey B, Wiesenborn A, Lehmann P, Ruzicka T, Parker CM, Schön MP, Role of Integrin αE(CD103)β7 for Tissue-Specific Epidermal Localization of CD8+ T Lymphocytes, J. Invest. Dermatol 117 (2001) 569–575. 10.1046/j.0022-202x.2001.01481.x. [DOI] [PubMed] [Google Scholar]
- [87].Rak GD, Osborne LC, Siracusa MC, Kim BS, Wang K, Bayat A, Artis D, Volk SW, IL-33-dependent group 2 innate lymphoid cells promote cutaneous wound healing, J. Invest. Dermatol 136 (2016) 487–496. 10.1038/JID.2015.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A, Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling, J. Clin. Invest 119 (2009) 3573–3585. 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho S-H, Lerner H, Friedman AL, Shen Y, Farber DL, Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites, Cell Rep. 20 (2017) 2921–2934. 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mackay LK, Minnich M, Kragten NAM, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, Braun A, Wynne-Jones E, Behr FM, Stark R, Pellicci DG, Godfrey DI, Belz GT, Pellegrini M, Gebhardt T, Busslinger M, Shi W, Carbone FR, van Lier RAW, Kallies A, van Gisbergen KPJM, Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes, Science. 352 (2016) 459–463. 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- [91].Zielinski CE, Human T cell immune surveillance: Phenotypic, functional and migratory heterogeneity for tailored immune responses, Immunol. Lett 190 (2017) 125–129. 10.1016/j.imlet.2017.08.001. [DOI] [PubMed] [Google Scholar]
- [92].Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJC, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL, Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets, Immunity. 38 (2013) 187–197. 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC, Rules of chemokine receptor association with T cell polarization in vivo, J. Clin. Invest 108 (2001) 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F, Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells, Nat. Immunol 10 (2009) 857–863. 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- [95].Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS, Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity, Nature. 483 (2012) 227–231. 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Beura LK, Wijeyesinghe S, Thompson EA, Macchietto MG, Rosato PC, Pierson MJ, Schenkel JM, Mitchell JS, Vezys V, Fife BT, Shen S, Masopust D, T Cells in Nonlymphoid Tissues Give Rise to Lymph-Node-Resident Memory T Cells, Immunity. 48 (2018) 327–338.e5. 10.1016/j.immuni.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Klicznik MM, Morawski PA, Höllbacher B, Varkhande S, Motley S, Rosenblum MD, Long SA, Brachtl G, Duhen T, Campbell D, Gratz IK, Continual exit of human cutaneous resident memory CD4 T cells that seed distant skin sites, BioRxiv. (2018). 10.1101/361758. [DOI] [Google Scholar]
- [98].Chen W, Ten Dijke P, Immunoregulation by members of the TGFβ superfamily, Nat. Rev. Immunol 16 (2016) 723–740. 10.1038/nri.2016.112. [DOI] [PubMed] [Google Scholar]
- [99].Scharschmidt TC, Establishing Tolerance to Commensal Skin Bacteria: Timing Is Everything, Dermatol. Clin 35 (2017) 1–9. 10.1016/j.det.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Belkaid Y, Bouladoux N, Hand TW, Effector and memory T cell responses to commensal bacteria, Trends Immunol. 34 (2013) 299–306. 10.1016/j.it.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


