Abstract
Objectives
To compare responsiveness and longitudinal validity of Disease Activity Score 28 (DAS28), Disease Activity index for PSoriatic Arthritis (DAPSA), Composite Psoriatic Disease Activity Index (CPDAI), Psoriatic ArthritiS Disease Activity Score (PASDAS), GRAppa Composite scorE (GRACE) and Minimal Disease Activity (MDA) in usual care PsA patients, within 1 year after diagnosis.
Methods
Data collected in the Dutch southwest early PsA cohort (DEPAR) were used. Responsiveness was assessed using effect size (ES), standardized response mean (SRM), and discrimination between different general health states. Longitudinal validity was tested using mixed models with outcomes health-related quality of life (HRQOL), productivity and disability.
Results
Responsiveness was highest for PASDAS, with ES 1.00 and SRM 0.95, lowest for DAPSA, with ES 0.73 and SRM 0.71, and in between for DAS28, CPDAI and GRACE. Differences in general health were best discriminated with PASDAS and GRACE. Patients reporting stable or worsening general health could not be distinguished by DAS28 or CPDAI. Discrimination was better using DAPSA, but worse than when using PASDAS and GRACE. Longitudinal evolvement of HRQOL and productivity had the highest association with low disease activity according to GRACE, followed by PASDAS, MDA, DAPSA, DAS28, with the lowest association for CPDAI.
Conclusion
PASDAS and GRACE were superior with respect to responsiveness, and together with MDA best related to longitudinal evolvement of HRQOL, productivity and disability. Responsiveness and longitudinal validity of most outcomes were inferior for DAS28, DAPSA and CPDAI. As alternatives to the continuous measure DAPSA, use of PASDAS or GRACE should be considered.
Keywords: psoriatic arthritis, disease activity, responsiveness, longitudinal validity
Rheumatology key messages
Responsiveness and longitudinal validity of Psoriatic ArthritiS Disease Activity Score, GRAppa Composite scorE and Miminal Disease Activity were superior
Psoriatic ArthritiS Disease Activity Score and GRAppa Composite scorE reflect the spectrum of disease activity better than Disease Activity index for PSoriatic Arthritis.
Introduction
PsA is a heterogeneous disease, with manifestations arthritis, enthesitis, spondylitis, dactylitis, and psoriasis [1, 2]. The goal of treatment of PsA is to optimize function and health-related quality of life (HRQOL), and to prevent structural damage. This can be done by aiming at remission or, if this cannot be achieved, low or minimal disease activity [3, 4]. Disease activity is assessed using composite measures, in which multiple aspects of disease are combined in a total score of level of disease activity. In rheumatoid arthritis, guiding treatment based on measuring disease activity with the Disease Activity Score 28 (DAS28) has improved care and long term outcomes [5]. Though multiple disease activity measures are available and used in research in PsA [6], no consensus has been reached on which measure should be used [7].
The DAS28 has often been used as a disease activity measure [6], although it was not originally developed for use in patients with PsA. As a more PsA-specific measure, the Disease Activity index for PSoriatic Arthritis (DAPSA) was developed using the 66/68 joint count instead of the 28 joint count [8]. Both DAPSA and DAS28 are mainly articular measures. Some have argued that the target for PsA should take into account more than joint involvement alone, for example by using the Composite Psoriatic Disease Activity Index (CPDAI) [9], Psoriatic ArthritiS Disease Activity Score (PASDAS) [10], the GRAppa Composite ScorE (GRACE) [11] or Minimal Disease Activity (MDA) [12]. The latter is a dichotomous measure, while the others are continuous measures similar to the DAPSA and DAS28. For clinical practice, use of either DAPSA or MDA has been advised by an international task force [7]. An overview of the components needed to calculate each measure is given in Table 1.
Table 1.
Components in calculation of disease activity measures
| Component | DAS28 | DAPSA | CPDAI | PASDAS | GRACE | MDA |
|---|---|---|---|---|---|---|
| Clinical assessment | ||||||
| Tender joint count | 28 | 68 | 68 | 68 | 68 | 68 |
| Swollen joint count | 28 | 66 | 66 | 66 | 66 | 66 |
| PASI | × | × | × | |||
| LEI | × | × | × | |||
| Dactylitis count | × | × | ||||
| VAS physician | × | |||||
| Patient questionnaire | ||||||
| VAS global | × | × | × | × | × | |
| VAS skin | × | |||||
| VAS joints | × | |||||
| VAS pain | × | × | ||||
| HAQ | × | × | × | |||
| DLQI | × | |||||
| BASDAI | × | |||||
| ASQoL | × | |||||
| SF-36 PCS | × | |||||
| PsAQoL | × | |||||
| Laboratory assessment | ||||||
| CRP | × | × | × | |||
ASQoL: Ankylosing Spondylitis Quality of Life; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; CPDAI: Composite Psoriatic Disease Activity Index; DAPSA: Disease Activity index for PSoriatic Arthritis; DAS28: Disease Activity Score 28; DLQI: Dermatology Life Quality Index; GRACE: GRAppa Composite ScorE; LEI: Leeds Enthesitis Index; MDA: Minimal Disease Activity; PASDAS: Psoriatic ArthritiS Disease Activity Score; PASI: Psoriasis Area and Severity Index; PsAQoL: Psoriatic Arthritis-specific Quality of Life; SF-36 PCS: Short Form 36 Physical Component Scale; VAS: visual analogue scale.
All measures have been shown to be related to disease burden. The continuous measures were all able to discriminate between placebo and active treatment groups in trials [13, 14], and were related to treatment change [10, 15] and a patient-acceptable symptom state [16]. Though all measures are able to distinguish two groups with different levels of disease activity, other cross-sectional studies have shown that agreement on a patient level is often moderate [16, 17]. Also, the ReFlap study has shown that agreement between patient opinion and low disease activity according to MDA or DAPSA is limited [18]. For use in clinical practice, we need to know which measure is best at measuring change of disease activity (i.e. responsiveness) and has the strongest relation with patient outcomes (i.e. longitudinal validity), which has not been studied in a usual care population of patients with early disease. Responsiveness has been tested in an analysis of trial data [14], but not in a longitudinal study of usual care patients. Also, little is known on how these measures perform in the early course of disease. We therefore aimed to compare the responsiveness and longitudinal validity of the currently available composite disease activity measure (DAS28, DAPSA, CPDAI, PASDAS, GRACE and MDA) in PsA patients within 1 year after diagnosis. Responsiveness was evaluated using both a distribution-based and an anchor-based approach, and longitudinal validity using associations with HRQOL, productivity and disability as no reference standard for disease activity exists.
Patients and methods
Patients and setting
We used data collected in the Dutch southwest Early Psoriatic Arthritis cohoRt (DEPAR) study, of which details are described elsewhere [19]. In short, DEPAR collects data with the aim of investigating daily clinical practice of PsA patients. Patients with a new diagnosis of PsA are eligible to participate if they had not yet received treatment with disease-modifying antirheumatic drugs (DMARDs) for PsA before the first study visit. Written informed consent was obtained from all participants according to the Declaration of Helsinki. The study was approved by the local medical research ethics committee of Erasmus Medical Centre Rotterdam, the Netherlands (MEC-2012-549). For this analysis, we used data collected between August 2013 and April 2018.
Data collection
In the first year after diagnosis and inclusion in the study, data of patients were collected every 3 months in a study visit. Trained research nurses collected clinical data, including swollen joint count (SJC; 66 joints) and tender joint count (TJC; 68 joints), enthesitis at clinical examination (Leeds Enthesitis Index, LEI [20], dactylitis count, physician global visual analogue scale (VAS), and psoriasis (Psoriasis Area and Severity Index, PASI [21]). Patients filled out questionnaires shortly before or after their visit to the research nurse. In DEPAR, multiple questionnaires are collected to measure patient-reported activity of disease and different outcomes. For this analysis we used the Short Form 36 (SF-36 [22]), HAQ [23], patient global, pain and skin VAS, Productivity Cost Questionnaire (PCQ [24]), Dermatology Life Quality Index (DLQI [25]), Ankylosing Spondylitis Quality of Life Questionnaire (ASQoL [26]), PsA-specific quality of life (PsAQoL [27]), and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI [28]).
Disease activity
At each visit within the first year we calculated scores of DAS28-CRP [29], DAPSA [8], CPDAI [9], PASDAS [10], GRACE [11] and MDA [12]. DAS28-CRP is calculated, using the 28-joint tender and swollen counts, patient global VAS, and CRP level, as follows: DAS28-CRP = 0.56√TJC + 0.28√SJC + 0.36 ln(CRP + 1) + 0.014VAS + 0.96. DAPSA adds the SJC66, TJC68, VAS global (0–10 scale), VAS pain (0–10 scale) and CRP (mg/dl) in a total score. CPDAI assesses grades severity of involvement of five domains (joints, skin, entheses, dactylitis and spine) with a score of 0–3. Spinal disease activity (using BASDAI and ASQoL) was only assessed in patients with axial involvement according to their rheumatologist. Other domains were assessed using SJC, TJC, LEI, PASI, dactylitis count, HAQ, and DLQI. PASDAS is calculated as: PASDAS=(0.18√physician global VAS + 0.159√patient global VAS – 0.253√SF-36 PCS + 0.101 ln(SJC + 1) + 0.48 ln(TJC + 1) + 0.23 ln(LEI + 1) + 0.377 ln(dactylitis count + 1) + 0.102 ln(CRP + 1) + 2) × 1.5. GRACE score was calculated as: GRACE = (1 – AMDF) × 10, in which AMDF is the arithmetic mean of desirability function. In the AMDF, SJC, TJC, HAQ, patient global, pain and skin VAS, PASI and PsAQoL are transformed to a 0–1 score where 0 is completely unacceptable and 1 is normal. The AMDF is a weighted average of these eight scales. MDA is defined as meeting at least 5 out of 7 remission criteria: SJC ⩽ 1, TJC ⩽ 1, LEI ⩽ 1, PASI ⩽ 1, patient global VAS ⩽ 20 mm, patient pain VAS ⩽ 15 mm and HAQ ⩽ 0.5.
In addition, at each visit patients were classified as having low disease activity according to the five continuous composite measures (DAS28 ⩽ 3.2 [30], DAPSA ⩽ 14 [31], CPDAI ⩽ 4 [11], PASDAS ⩽ 3.2 [11] and GRACE ⩽ 2.3 [11]) and MDA (5/7 remission criteria). In cases where not all disease activity scores could be calculated, the visit was excluded from this analysis. DLQI and ASQoL were not collected in all patients at all visits, resulting in some exclusion by design.
Outcomes
The anchor-question of the SF-36 was used to distinguish categories of change in general health, which was reported by patients as either much improved, somewhat improved, stable, somewhat worsened or much worsened. HRQOL was determined using the SF-36 Physical Component Scale (PCS) and Mental Component Scale (MCS), which were calculated using the Dutch norm scores [32]. Work productivity was assessed using the PCQ every visit, in which patients are asked about work and productivity in the past 4 weeks. We determined employment status, absenteeism, working hours, and productivity loss at work (presenteeism) and productivity loss of unpaid work throughout the first year. Total productivity hours per week was calculated by subtracting hours of absence and productivity loss at work in hours from the total working hours. Productivity loss at work was calculated by multiplying the total hours of productivity loss by the percentage of productivity loss. Disability was assessed using the HAQ.
Statistical analysis
Responsiveness of each measure was compared in a distribution-based approach and an anchor-based approach. The former was done by comparing the effect size (ES, i.e. the difference between baseline and 1 year, divided by the s.d. of the baseline), standardized response mean (SRM, i.e. the difference divided by the s.d. of the difference). Change in disease activity from baseline to 1 year was calculated for all measures. We hypothesized that within the first year after diagnosis, the disease activity would decrease, which justifies the distribution-based approach. In the anchor-based approach we compared change of each measure over 3 months in patients reporting improvement, worsening or stable general health (as determined in the anchor question of the SF-36). The relation between low disease activity according to different disease measures and patient-reported outcomes each 3 months in the first year was assessed using mixed effects models. The variables time and disease activity measure were included in the fixed-effects part, and random intercepts and random slopes were included in the random-effects part. Outcomes were SF-36 PCS, SF-36 MCS, productivity (linear mixed effects models) and HAQ > 0.5 (mixed-effects logistic regression). Relative model fits were compared using the Akaike information criterion, using the fit of disease activity measure DAS28 as reference. Analyses were performed in STATA 15.1 (StataCorp, College Station, TX, USA) and R 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 549 patients included until April 2018, 331 had all disease activity scores available at baseline and were included in this analysis. Excluded patients (n = 218) had missing questionnaires (79%, including 36% by design), or missing clinical data including CRP (21%, Supplementary Fig. 1, available at Rheumatology online). Mean age was 50.7 (s.d. 13) years, median symptom duration was 0.9 (interquartile range (IQR) 0.3–2.8) years and 171 (52%) were male. At baseline, median swollen joint count was 2 (IQR 1–4), median tender joint count was 3 (IQR 1–7), and median PASI score was 2 (IQR 0.5–4.0, Table 2).
Table 2.
Clinical characteristics at baseline (n = 331)
| Characteristic | Value |
|---|---|
| Age, mean (s.d.), years | 50.7 (13) |
| Male, n (%) | 171 (52) |
| Symptom duration, median (IQR), years | 0.9 (0.3–2.8) |
| Swollen joint count (66), median (IQR) | 2 (1–4) |
| Tender joint count (68), median (IQR) | 3 (1–7) |
| LEI > 0, n (%) | 130 (39) |
| LEI if positive, median (IQR) | 2 (1–3) |
| LDI > 0, n (%) | 50 (15) |
| PASI, median (IQR) | 2 (0.5–4.0) |
| VAS Global, mean (s.d.) | 46 (26) |
| VAS Pain, mean (s.d.) | 46 (26) |
| HAQ, median (IQR) | 0.6 (0.4–1.0) |
| DAS28, mean (s.d.) | 3.1 (1.1) |
| DAPSA, mean (s.d.) | 18 (11) |
| CPDAI, mean (s.d.) | 3.9 (1.9) |
| PASDAS, mean (s.d.) | 4.1 (1.2) |
| GRACE, mean (s.d.) | 3.4 (1.5) |
| MDA, mean (s.d.) | 51 (15) |
CPDAI: Composite Psoriatic Disease Activity Index; DAPSA: Disease Activity index for PSoriatic Arthritis; DAS28: Disease Activity Score 28; GRACE: GRAppa Composite ScorE; IQR: interquartile range; LEI: Leeds Enthesitis Index; LDI: Leeds Dactylitis Index; MDA: Minimal Disease Activity; PASDAS: Psoriatic ArthritiS Disease Activity Score; PASI: Psoriasis Area and Severity Index; VAS: visual analogue scale.
Responsiveness
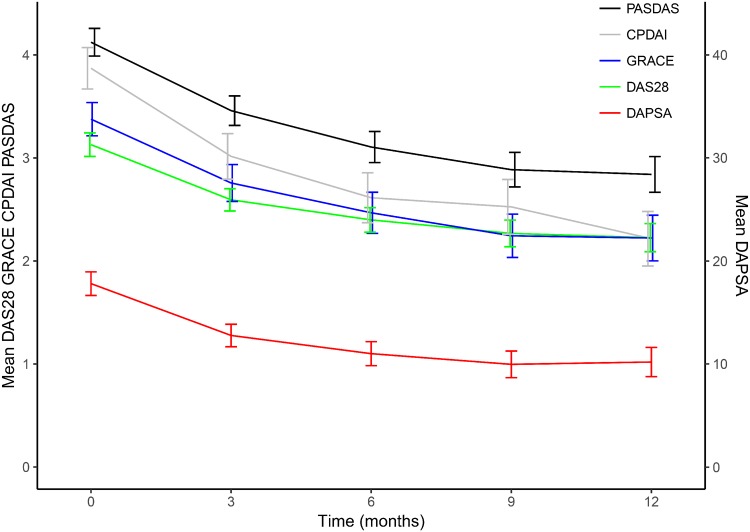
Figure 1 shows average disease activity scores throughout the first year of patients with complete data. Though the average DAS28 (green), CPDAI (grey) and GRACE (blue) scores were similar at twelve months, their initial scores and evolvement differed over the first year. Disease activity was high at baseline and low at 1 year according to DAS28 in 65 (34%), DAPSA in 67 (35%), CPDAI in 51 (27%), PASDAS in 76 (40%), GRACE in 63 (33%) and MDA in 66 (35%).
Fig. 1.
Disease activity scores in the first year
Results shown as mean (95% CI) of patients with complete disease measure data. CPDAI (grey): Composite Psoriatic Disease Activity Index; DAPSA (red): Disease Activity index for PSoriatic Arthritis; DAS28 (green): Disease Activity Score 28; GRACE (blue): GRAppa Composite ScorE; PASDAS (blackPsoriatic ArthritiS Disease Activity Score.
In the distribution-based assessment of responsiveness (i.e. ES and SRM in the first year, Table 3), the PASDAS was the most responsive, as shown with the highest ES (1.00, s.d. 1.05) and highest SRM (0.95, s.d. 1.00). The DAPSA was the least responsive, with an ES of 0.73 (s.d. 1.04) and SRM 0.71 (s.d. 1.00). The responsiveness of DAS28 (ES 0.88, s.d. 1.05; SRM 0.83, s.d. 1.00), CPDAI (ES 0.88, s.d. 1.08; SRM 0.82, s.d. 1.00) and GRACE (ES 0.75, s.d. 0.90; SRM 0.83, s.d. 1.00) were similar and all better than that of DAPSA, but worse than that of PASDAS.
Table 3.
Disease Activity in 1 year (n = 190)
| Baseline disease activity, mean (s.d.) | One-year disease activity, mean (s.d.) | Difference, mean (s.d.) | ES, mean (s.d.) | SRM, mean (s.d.) | Baseline LDA, n (%) | One-year LDA, n (%) | Change to LDA, n (%) | |
|---|---|---|---|---|---|---|---|---|
| DAS28 | 3.11 (1.01) | 2.23 (0.95) | −0.89 (1.07) | 0.88 (1.05) | 0.83 (1.00) | 100 (52) | 155 (82) | 65 (34) |
| DAPSA | 17.6 (10.0) | 10.2 (9.9) | −7.4 (10.4) | 0.73 (1.04) | 0.71 (1.00) | 84 (44) | 139 (73) | 67 (35) |
| CPDAI | 3.89 (1.90) | 2.22 (1.85) | −1.68 (2.06) | 0.88 (1.08) | 0.82 (1.00) | 120 (63) | 164 (86) | 51 (27) |
| PASDAS | 4.05 (1.21) | 2.84 (1.21) | −1.21 (1.27) | 1.00 (1.05) | 0.95 (1.00) | 47 (25) | 118 (62) | 76 (40) |
| GRACE | 3.34 (1.50) | 2.22 (1.55) | −1.12 (1.35) | 0.75 (0.90) | 0.83 (1.00) | 52 (27) | 110 (58) | 63 (33) |
| MDA | 30 (16) | 91 (48) | 66 (35) |
Results shown as mean (s.d.) or n (%). CPDAI: Composite Psoriatic Disease Activity Index; DAPSA: Disease Activity index for PSoriatic Arthritis; DAS28: Disease Activity Score 28; ES: Effect Size; GRACE: GRAppa Composite ScorE; LDA: Low Disease Activity; MDA: Minimal Disease Activity; PASDAS: psoriatic arthritis disease activity score; SRM: Standardized Response Mean.
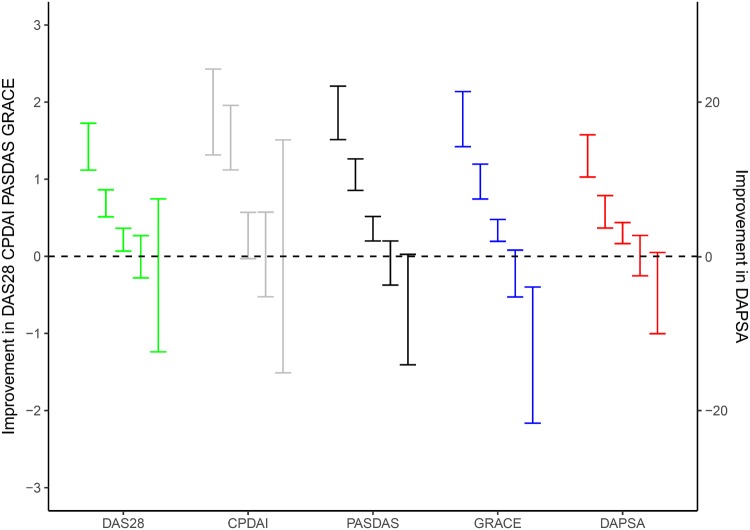
In the anchor-based assessment of responsiveness, improvement in disease activity score was related to change in general health as assessed with the anchor-question of the SF-36 of the first 3 months. Of the 265 patients with complete baseline and 3 months’ assessments, general health as compared with 3 months ago was much improved in 39 patients (15%), somewhat improved in 78 (29%), stable in 100 (38%), somewhat worsened in 41 (15%) and much worsened in 7 patients (3%, Table 4). The improvement in disease activity scores in these five categories of change in general health are shown in Table 4 and Fig. 2 (much improved on the left to much worsened on the right). DAS28 and DAPSA differentiated much improved from other states [mean change 1.42 (95% CI: 1.12, 1.73) for DAS28 and 13 (95% CI: 10.3, 15.8) for DAPSA], but did not differentiate between somewhat improved, stable, somewhat worsened and much worsened. CDPAI did not differentiate between any of the general health states. Both PASDAS and GRACE differentiated between much improved [mean change 1.86 (95% CI: 1.51, 2.21) for PASDAS and 1.78 (95% CI: 1.42, 2.14) for GRACE], somewhat improved [mean change 1.06 (95% CI: 0.86, 1.26) for PASDAS and 0.97 (95% CI: 0.74, 1.20) for GRACE], and stable [mean change 0.36 (95% CI: 0.20, 0.52) for PASDAS and 0.34 (95% CI: 0.19, 0.48) for GRACE], but not between somewhat worsened and much worsened.
Table 4.
Difference in disease activity scores and general health in the first 3 months (n = 265)
| DAS28 | CPDAI | PASDAS | GRACE | DAPSA | |
|---|---|---|---|---|---|
| Much improved (n = 39) | 1.42 (1.12, 1.73) | 1.87 (1.31, 2.43) | 1.86 (1.51, 2.21) | 1.78 (1.42, 2.14) | 13 (10.3, 15.8) |
| Somewhat improved (n = 78) | 0.69 (0.51, 0.86) | 1.54 (1.12, 1.96) | 1.06 (0.86, 1.26) | 0.97 (0.74, 1.2) | 5.8 (3.7, 7.9) |
| Stable (n = 100) | 0.22 (0.07, 0.36) | 0.27 (−0.03, 0.57) | 0.36 (0.2, 0.52) | 0.34 (0.19, 0.48) | 3 (1.6, 4.4) |
| Somewhat worsened (n = 41) | −0.01 (−0.28, 0.27) | 0.02 (−0.52, 0.57) | −0.09 (−0.37, 0.2) | −0.22 (−0.53, 0.08) | 0.1 (−2.5, 2.7) |
| Much worsened (n = 7) | −0.25 (−1.24, 0.75) | 0 (−1.51, 1.51) | −0.69 (−1.41, 0.03) | −1.28 (−2.16, −0.4) | −4.8 (−10, 0.5) |
Results shown as mean (95% CI). CPDAI: Composite Psoriatic Disease Activity Index; DAPSA: Disease Activity index for PSoriatic Arthritis; DAS28: Disease Activity Score 28; GRACE: GRAppa Composite ScorE; PASDAS: Psoriatic ArthritiS Disease Activity Score.
Fig. 2.
Anchor-based analysis: difference in disease activity scores and general health in the first 3 months (n = 265)
Difference in disease activity score in the first 3 months in categories of change in general health, left to right: much improved, somewhat improved, stable, somewhat worsened, much worsened. Results shown as mean (95% CI). CPDAI (grey): Composite Psoriatic Disease Activity Index; DAPSA (red): Disease Activity index for PSoriatic Arthritis; DAS28 (green): Disease Activity Score 28; GRACE (blue): GRAppa Composite ScorE; PASDAS (black): Psoriatic ArthritiS Disease Activity Score.
Longitudinal associations with outcomes
The associations between the longitudinal evolvement of disease activity measure and longitudinal evolvement of outcomes of SF-36 PCS, SF-36 MCS, productivity and HAQ were assessed using mixed effects models. To compare longitudinal validity of measures relative to each other, model fits of the mixed models were compared with the Akaike information criterion relative to DAS28 (a lower Akaike information criterion corresponds to a better fit, Supplementary Table 1, available at Rheumatology online). For SF-36 PCS, the longitudinal evolvement had the highest association with low disease activity according to the GRACE, followed by PASDAS, MDA, DAPSA, DAS28, with the lowest association for CPDAI. Regarding productivity, the association was highest for GRACE and PASDAS as well, followed by DAPSA, MDA, CPDAI and DAS28. The evolvement of SF-36 MCS was poorly related to any disease measure and model fit was comparable. Regarding the outcome of disability (HAQ > 0.5), the order of association (high to low) was MDA, CPDAI, GRACE, DAPSA, PASDAS and DAS28. A summary of the relative performance for each aspect of responsiveness and longitudinal validity as discussed before is shown in Supplementary Table 2, available at Rheumatology online.
Discussion
In this study, we compared responsiveness and longitudinal validity of DAS28, DAPSA, CPDAI, PASDAS, GRACE and MDA in current usual care of newly diagnosed PsA patients. Using the change of disease activity over the first year as expressed in the ES and SRM, responsiveness of PASDAS was highest and that of DAPSA was lowest. Using change in general health status as anchor, all measures except CPDAI were able to discriminate patients that reported improved or stable general health. Stable and worsened general health, however, could only be discriminated by GRACE and PASDAS. Longitudinal evolvement of HRQOL and productivity was best captured with GRACE, PASDAS and MDA and less by DAPSA, DAS28 and CPDAI. A similar relation was seen with the outcome of disability using the HAQ, but with the difference that CDPAI had the second-best relation with HAQ.
This study is the first to assess responsiveness and longitudinal validity of all composite measures in usual care of early disease. Responsiveness has been studied using data from a trial by Helliwell and Kavanaugh; they reported that PASDAS, AMDF (i.e. the GRACE score in a different form) and DAS28 had the highest and similar responsiveness, while a modified CDPAI and DAPSA showed lower responsiveness [14]. This confirms part of our findings. They, however, analysed data from a trial including patients with active disease and a predominantly polyarticular phenotype. This patient selection probably explains why the articular measure DAS28 had a better responsiveness with higher ES and SRM than we observed. Regarding validity, the relation of a disease measure and HRQOL has been studied in cross-sectional studies for MDA: patients in MDA report better HRQOL than patients not in MDA [33, 34]. This study is the first to test the validity longitudinally, by assessing the evolvement of outcomes in relation to disease activity over time.
The superiority of PASDAS, GRACE and MDA in terms of longitudinal validity could be attributed to their multidimensionality, but also to their use of more extensive questionnaires than for VAS scores alone. These questionnaires of HRQOL and disability used in PASDAS, GRACE and MDA are already closely related to the outcomes used in our analysis, resulting in a better performance. Analysis of relative contribution of each component was outside the scope of this work, but we will discuss the relation between use of questionnaires in disease measures here. On the one hand, the composites measures using questionnaires of HRQOL are a better representation of impact of disease to guide treatment decisions. HRQOL also belongs to the core set of domains for the assessment of patients with PsA according to the OMERACT [35]. On the other hand, generic questionnaires of HRQOL are more likely to be affected by factors other than disease activity and its burden that they aim to measure. Comorbidities for example are known to influence both disease activity measures and outcomes [36–38]. Also, some have argued that the HAQ is influenced by structural damage as well, which would make it impossible for some patients to be in remission despite absence of active inflammation [7]. In our analysis of early disease, however, we suspect disability is mostly determined by inflammation. Moreover, not only are questionnaires influenced by other factors than active disease: an increase in acute phase reactants—considered to be an objective measure of disease activity—can have other causes than an increase in PsA activity. Regardless, a composite measure needs the interpretation of a physician, who can choose not to change treatment if a higher disease activity score has other causes than PsA activity. A higher specificity for low disease activity is in that case of greater importance than a higher sensitivity.
In this analysis we assessed responsiveness and longitudinal association with outcomes of different measures, but for adaptation in clinical practice feasibility needs to be considered as well. A less feasible measure will only be accepted for use in clinical practice if it has a sufficiently better performance. All measures have a joint assessment and some form of general assessment using a VAS score. The measures differ in terms of joint count (i.e. 28 or 66/68), use of acute phase reactants, assessment of other PsA manifestations and use of questionnaires other than VAS. In clinical practice, assessment of musculoskeletal disease activity should include all joints, presence of enthesitis and presence of dactylitis. Psoriasis could be assessed with a body surface area instead of a PASI score. Acute phase reactants are often already measured, along with toxicity screening for DMARDs. The biggest feasibility problem will most likely be the questionnaires: CPDAI needs four questionnaires, GRACE needs two questionnaires and three VAS scores, and PASDAS needs one questionnaire and one VAS score. With increasing use and possibilities of electronic health records, and focus on value based healthcare, collecting patient-reported outcomes in clinical care will become more feasible. Regarding feasibility in our cohort, complete data of all measures were available in 65% of visits in our cohort, but it will probably be different in clinical practice. More data on feasibility of use of these measures in clinical practice are needed.
A strength of this study is that it tested the validity of composite measures in a usual care population, including patients with monoarthritis, oligoarthritis and other phenotypes besides polyarthritis alone, which is the population of interest when composite measures are to be used in clinical practice. Also, it is the first study testing these composite measures early in the course of disease, within the first year after diagnosis. We showed that the relative performance differed from responsiveness in a clinical trial, probably owing to a difference in disease history, disease activity and phenotype. The patients eligible to participate in clinical trials often have high disease activity, so the responsiveness is expected to be higher. Further, we tested all disease measures using clinical assessments and questionnaires as instructed by developers of the disease measure.
Our study has some limitations as well. With our choice of excluding patients with incomplete baseline data and follow-up visits with incomplete data (some by design), our sample size and power was reduced. Also, the estimate of the longitudinal relation between disease activity and outcomes itself might be biased, but not the performance relative to each other. Last, as discussed before, with the lack of a gold standard for disease activity we chose anchors and outcomes of general health as reference, while the SF-36 has not been established as a disease activity anchor. We hypothesized that within the first year of disease in which treatment for PsA is initiated, the majority of change in general health is the result of change in disease activity. We cannot rule out that in some patients the associations between general health and some disease measures is increased owing to factors other than disease activity.
In conclusion, the disease activity measures PASDAS and GRACE had the highest responsiveness, were best able to discriminate between different health states, and together with MDA were best related to longitudinal evolvement of HRQOL, productivity and disability. Responsiveness and longitudinal validity was inferior for the disease activity measures DAS28, DAPSA and CPDAI. Though MDA and DAPSA are recommended for use in clinical practice by an international task force, as an alternative to DAPSA we suggest use of the continuous measures PASDAS or GRACE.
Supplementary Material
Acknowledgements
We gratefully thank all participating patients and participating rheumatologists and research nurses. In addition, we would like to thank Esther Röder and the research team for their support.
Funding: Research support for this analysis was funded by Pfizer (ASPIRE grant WI171979). The company had no role in the study design, collection of data, analysis or interpretation of data, nor on the preparation or approval of the manuscript and the decision to submit the manuscript for publication.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Mease PJ. Psoriatic arthritis: update on pathophysiology, assessment and management. Ann Rheum Dis 2011;70(Suppl 1):i77–84. [DOI] [PubMed] [Google Scholar]
- 2. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P.. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64:ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gossec L, Smolen JS, Ramiro S. et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 4. Coates LC, Kavanaugh A, Mease PJ. et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 5. Stoffer MA, Schoels MM, Smolen JS. et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis 2016;75:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalyoncu U, Ogdie A, Campbell W. et al. Systematic literature review of domains assessed in psoriatic arthritis to inform the update of the psoriatic arthritis core domain set. RMD Open 2016;2:e000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smolen JS, Schols M, Braun J. et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018;77:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schoels M, Aletaha D, Funovits J. et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69:1441–7. [DOI] [PubMed] [Google Scholar]
- 9. Mumtaz A, Gallagher P, Kirby B. et al. Development of a preliminary composite disease activity index in psoriatic arthritis. Ann Rheum Dis 2011;70:272–7. [DOI] [PubMed] [Google Scholar]
- 10. Helliwell PS, FitzGerald O, Fransen J. et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 2013;72:986–91. [DOI] [PubMed] [Google Scholar]
- 11. Helliwell PS, FitzGerald O, Fransen J.. Composite disease activity and responder indices for psoriatic arthritis: a report from the GRAPPA 2013 meeting on development of cutoffs for both disease activity states and response. J Rheumatol 2014;41:1212–7. [DOI] [PubMed] [Google Scholar]
- 12. Coates LC, Fransen J, Helliwell PS.. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 13. FitzGerald O, Helliwell P, Mease P. et al. Application of composite disease activity scores in psoriatic arthritis to the PRESTA data set. Ann Rheum Dis 2012;71:358–62. [DOI] [PubMed] [Google Scholar]
- 14. Helliwell PS, Kavanaugh A.. Comparison of composite measures of disease activity in psoriatic arthritis using data from an interventional study with golimumab. Arthritis Care Res (Hoboken) 2014;66:749–56. [DOI] [PubMed] [Google Scholar]
- 15. Acosta Felquer ML, Ferreyra Garrott L, Marin J. et al. Remission criteria and activity indices in psoriatic arthritis. Clin Rheumatol 2014;33:1323–30. [DOI] [PubMed] [Google Scholar]
- 16. Michelsen B, Diamantopoulos AP, Hoiberg HK. et al. Need for improvement in current treatment of psoriatic arthritis: study of an outpatient clinic population. J Rheumatol 2017;44:431–6. [DOI] [PubMed] [Google Scholar]
- 17. Chimenti MS, Triggianese P, Conigliaro P. et al. A 2-year observational study on treatment targets in psoriatic arthritis patients treated with TNF inhibitors. Clin Rheumatol 2017;36:2253–60. [DOI] [PubMed] [Google Scholar]
- 18. Gorlier C, Orbai AM, Puyraimond-Zemmour D. et al. Comparing patient-perceived and physician-perceived remission and low disease activity in psoriatic arthritis: an analysis of 410 patients from 14 countries. 2019;78:201–8. [DOI] [PubMed] [Google Scholar]
- 19. Wervers K, Vis M, Tchetveriko I. et al. Burden of psoriatic arthritis in different definitions of disease activity: comparing minimal disease activity and disease activity index for psoriatic arthritis. Arthritis Care Res (Hoboken) 2018;70:1764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Healy PJ, Helliwell PS.. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 21. Fredriksson T, Pettersson U.. Severe psoriasis—oral therapy with a new retinoid. Dermatologica 1978;157:238–44. [DOI] [PubMed] [Google Scholar]
- 22. Ware JE Jr, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 23. Fries JF, Spitz P, Kraines RG, Holman HR.. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 24. Bouwmans C, Krol M, Severens H. et al. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value Health 2015;18:753–8. [DOI] [PubMed] [Google Scholar]
- 25. Finlay AY, Khan GK.. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–6. [DOI] [PubMed] [Google Scholar]
- 26. Doward LC, Spoorenberg A, Cook SA. et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis 2003;62:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKenna SP, Doward LC, Whalley D. et al. Development of the PsAQoL: a quality of life instrument specific to psoriatic arthritis. Ann Rheum Dis 2004;63:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garrett S, Jenkinson T, Kennedy LG. et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 29. Fransen J, Welsing PM, de Keijzer RM, Van Riel P.. Disease activity scores using C-reactive protein: cRP may replace ESR in the assessment of RA disease activity. Ann Rheum Dis 2004;62:151. [Google Scholar]
- 30. van Riel PL, van Gestel AM.. Clinical outcome measures in rheumatoid arthritis. Ann Rheum Dis 2000;59(Suppl 1):i28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schoels MM, Aletaha D, Alasti F, Smolen JS.. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016;75:811–8. [DOI] [PubMed] [Google Scholar]
- 32. Aaronson NK, Muller M, Cohen PD. et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 1998;51:1055–68. [DOI] [PubMed] [Google Scholar]
- 33. van Mens LJJ, Turina MC, van de Sande MGH. et al. Residual disease activity in psoriatic arthritis: discordance between the rheumatologist's opinion and minimal disease activity measurement. Rheumatology (Oxford) 2018;57:283–90. [DOI] [PubMed] [Google Scholar]
- 34. Queiro R, Canete JD, Montilla C. et al. Minimal disease activity and impact of disease in psoriatic arthritis: a Spanish cross-sectional multicenter study. Arthritis Res Ther 2017;19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gladman DD, Mease PJ, Strand V. et al. Consensus on a core set of domains for psoriatic arthritis. J Rheumatol 2007;34:1167–70. [PubMed] [Google Scholar]
- 36. Husted JA, Thavaneswaran A, Chandran V, Gladman DD.. Incremental effects of comorbidity on quality of life in patients with psoriatic arthritis. J Rheumatol 2013;40:1349–56. [DOI] [PubMed] [Google Scholar]
- 37. Salaffi F, Carotti M, Gasparini S, Intorcia M, Grassi W.. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual Life Outcomes 2009;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brikman S, Furer V, Wollman J. et al. The effect of the presence of fibromyalgia on common clinical disease activity indices in patients with psoriatic arthritis: a cross-sectional study. J Rheumatol 2016;43:1749–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.