Graphical Abstract
Keywords: Duloxetine hydrochloride, Enteric polymers, Preparation, Dissolution profile, Stability
Abstract
The main purpose of the present study was to prepare duloxetine hydrochloride (DXH) enteric-coated pellets using different enteric polymers. Three layers (drug-loaded layer, barrier layer, and enteric-coated layer) were applied to the inert core pellets, successively. The optimal formulation was manufactured by employing suspension layering method in fluidized bed processor (FBP) with varieties of enteric polymers like Aqoat® AS-LF, Eudragit® L30D55 and HPMCP-HP55. The prepared pellets were measured for physical characterization and the in vitro dissolution profile. Scanning electron microscopy (SEM) was conducted to observe the morphology of pellets, and different kinetic models were applied to analyze the release mechanism of Cymbalta® and home-made pellets. The coating weight gain of enteric-coated layer containing Eudragit® L30D55, Aqoat® AS-LF and HP-55 were determined to be 35%, 26% and 24%, respectively. The similarity factors (f2) of self-made capsules with above polymers and commercially available capsules (Cymbalta®) were above 50 in the dissolution medium of pH 6.8 phosphate buffer solution (PBS). SEM figures showed the smooth surfaces of self-prepared pellets using Eudragit® L30D55 and Aqoat® AS-LF, whereas rough surface was found in the HP-55 pellets at day 0, and an impurity was appearing in the condition of 40 °C/75% relative humidity for 1 month. In conclusion, the pellets prepared by utilizing Eudragit® L30D55 and Aqoat® AS-LF were the optimal preparations based on the dissolution profile and stability.
1. Introduction
Duloxetine is a selective serotonin norepinephrine reuptake inhibitor (SNRIs) currently known as a safe and effective antidepressant. It is usually in the form of hydrochloride. Duloxetine hydrochloride (DXH) enteric-coated capsule under the name Cymbalta® has been approved for marketing by the FDA. Cymbalta® is indicated in the United States for the treatment of major depressive disorder, generalized anxiety disorder, diabetic peripheral neuropathic pain, and fibromyalgia [1].
Pellets, multi-part dosage forms, have both pharmaceutical and therapeutic advantages. Their pharmaceutical benefits including the flexibility in development and design enable the administration of incompatible bioactive compounds owing to the low surface-area-to-volume ratio compared with granules and powders. Therapeutic advantages involve enhancement of bioavailability, decrease of irritation and alteration of mechanism of drug release in the gastrointestinal tract (GIT) when administered orally. Consequently, pellets acting as a substrate of drug are able to be coated with large amounts of drugs and excipients.
Owing to the lability of DXH at pH value less than 2.5, enteric polymers should be applied to prevent acid degradation of DXH in the stomach and provide for rapid drug-release in the small intestine. In this study, several enteric polymers have been employed, such as Eudragit® L30D55, hydroxyl propyl methyl cellulose acetate succinate (HPMCAS) and hydroxyl propyl methyl cellulose phthalate (HPMCP). Eudragit® L30D55 is an anionic copolymer of methacrylic acid and ethyl acrylate, and the ratio of carboxyl to ester group is 1:1. The backbone structures of HPMCAS and HPMCP are all water-soluble polymers, HPMC. HPMCP contains a carboxybenzoyl (phthalyl) group, which can define the solubility of HPMCP, while HPMCAS includes acetyl and succinyl groups, and its solubility is determined by their ratio. The polymers have very low solubility in water due to their hydrophobic nature, when their carboxyl groups are in undissociated form. The structure state of polymer shifts to the formation of the ionized form with increasing water solubility as the pH rises. Thus, the pH can be controlled by adjusting the phthalyl content to make HPMCP soluble. HP-55 and HP-50 are dissolved at a pH around 5.5 and 5.0, respectively. In the same way, HPMCAS-LF (Aqoat® AS-LF) with 8% acetyl and 15% succinyl can be soluble at a pH around 5.5. HP-55 and HPMCAS-LF are selected, because Cymbalta® has been proven to release the drug from pH 5.5 PBS. If the described polymers are employed, we should pay attention to their stability with duloxetine. It is the residual free acids group present in HPMCAS or HPMCP that has been found to react with DXH, and the reactions form succinamide or phthalamide impurities accelerated by humidity and heat [2]. Thus, the barrier layer is an indispensable part of pellets to separate the drug from polymers and improve the stability of preparation.
The aim of the present study is to (a) prepare duloxetine hydrochloride enteric-coated pellets using several enteric polymers; (b) evaluate the effect of the type of enteric polymer, coating weight, pH of enteric polymers and curing conditions on gastric stability and in vitro dissolution; and (c) investigate the drug stability by accelerated test.
2. Materials and methods
2.1. Materials
Duloxetine hydrochloride was purchased from Shanghai Wonder Pharmaceutical Co. Ltd. (Shanghai, China). Sucrose-starch nonpareils were from Hangzhou Gaocheng Biotech & Health Co. Ltd. (Hangzhou, China). Hydroxy-propyl methylcellulose E5 (HPMC-E5) was kindly provided by Shanhe Pharmaceutical Co. Ltd. (Anhui, China), and methacrylic acid copolymer (Eudragit® L30D55) was from Evonik (Germany). The polymers of hydroxyl propyl methyl cellulose acetate succinate-LF (Aqoat® AS-LF) and hydroxyl propyl methyl cellulose phthalate (HPMCP) were obtained from Shin-Etsu Chemical Co. Ltd. (Japan) and Samsung Fine Chemicals Co. Ltd. (Korea). No.3 hard gelatin capsule shells were from Suzhou Capsule Co. Ltd. (Suzhou, China). Commercially available duloxetine delayed-release capsules (Cymbalta®, 30 mg/capsule, Eli Lilly and Company, USA) were chosen for comparison. All organic solvents used in HPLC were of high-performance liquid chromatography (HPLC) grade. All other ingredients were of analytical grade.
2.2. Methods
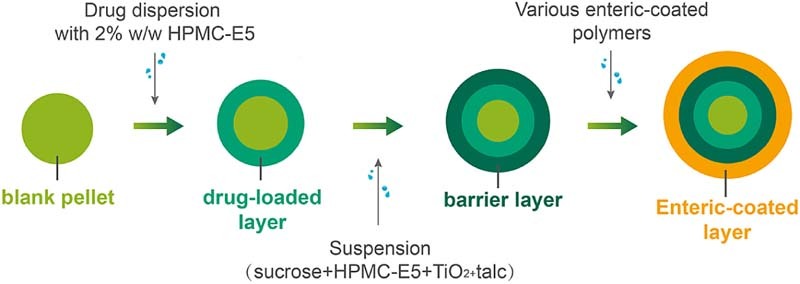
2.2.1. Preparation of pellets
Duloxetine hydrochloride delayed-release pellets were composed of four parts, namely nonpareils (sugar spheres), drug layer, barrier layer and enteric-coated layer successively. All the layers were prepared in a fluidized bed processor (Table 1) by the suspension layer method. Drug layer could also be manufactured by a centrifugal granulator (Table 2).
Table 1.
Various process parameters of three layers in fluidized bed processor.
| S. No. | Parameters | Drug layer | Barrier layer | Enteric-coated layer |
|---|---|---|---|---|
| 1 | Inlet temperature (°C) | 55–60 | 50–55 | 45–50/55–60 |
| 2 | Outlet temperature (°C) | 50–55 | 45–50 | 40–45/50–55 |
| 3 | Product temperature (°C) | 40–45 | 38–40 | 35–40/40–45 |
| 4 | Atomization (Bar) | 1.0–1.5 | 1.5–2.0 | 1.5–2.0 |
| 5 | Air flow (m3/h)a | 50–55 | 45–50 | 50–55 |
| 6 | Spray rate (g/min)a | 5–7 | 4–6 | 4–7 |
Adjusted according to the experimental conditions in the process of drug-loading or coating.
Table 2.
Various process parameters of drug layer in centrifugal granulator.
| S. No. | Processing parameter | Value |
|---|---|---|
| 1 | Rotational speed of plate | 200 rpm |
| 2 | Blower rate | 10 × 14 l/min |
| 3 | Air flow rate | 10 l/min |
| 4 | Spray air pressure | 0.5 MPa |
| 5 | Spray rate of solutiona | 4–7 g/min |
| 6 | Rotating rate of powder feederb | 0–15 rpm |
| 7 | Inlet temperature (°C) | 35 |
| 8 | Outlet temperature (°C) | 25 |
The spray rate was 4 g/min for the first 10 min, and gradually increased to 7 g/min until the end.
The rotating rate of feeder was 0 rpm for the first 10 min, and gradually increased to 15 rpm until the end.
HPMC-E5 was applied both as binder and as barrier polymer because of its low molecular weight (MW) and its application of serving as good pelletization aid [3]. When sucrose was added to the barrier layer, the resistance of pellets to acid conditions was remarkably increased [4]. It was suggested that sodium chloride with HPMC provided functional barrier, which avoided the migration of acidic ingredients between layers and thus capacitated stabilization of duloxetine [5]. Eudragit® L30D55, Aqoat® AS-LF and HPMCP-HP55 were the three chosen enteric polymers.
The drug-loaded and barrier-layer-loaded pellets should be dried for 2 h at 40 °C, and then weighted to calculate the weight gain when their temperature reached room temperature. There was difference in the curing condition of three enteric polymers. Eudragit® L30D55 required only 2 hours under 40 °C to be cured, while Aqoat® AS-LF and HPMCP-HP55 were fully cured under 40/60 °C for more time. After the curing, the pellets were collected and weighted to calculate the weight gain. In the following study, we investigated the effect of curing condition on the dissolution.
2.2.1.1. Drug layer
DXH (101 g) was pulverized by dry grinding and passed through 200-mesh sieve. Two hundred grams of blank pellets (600–710 µm) was weighted to FBP or centrifugal granulator. HPMC-E5 (2%, wt/wt) was added to 500 ml distilled water that had been heated to 60–70 °C in advance, stirring constantly until fully dissolved. The suspension of drug layer was built up by incorporating duloxetine hydrochloride intermittently into the HPMC-E5 solution with constant stirring at room temperature. The achieved suspension was coated onto the sugar spheres. In the process, the drug suspension must be stirred constantly. The pellets (24–30 mesh) were ultimately sieved to prepare the barrier layer.
2.2.1.2. Barrier layer
Barrier layer, also called separating layer, physically kept the components in the drug layer and enteric layer from coming into direct contact with each other. Sucrose (32%, wt/wt, based on the total dry weight of barrier layer) was dissolved in the 2% wt/wt solution of the HPMC-E5 in water (400 ml), namely solution A. Micronized talc (50%, wt/wt, based on the total dry weight of barrier layer) was dispersed in distilled water (400 ml), and then an opacifying agent like titanium dioxide (TiO2) (5%, wt/wt, based on the total dry weight of barrier layer) was added to protect the duloxetine from light, namely solution B. Solutions A and B were blended with continuous stirring to be homogeneous. In the process, the barrier-coating dispersion must be stirred continuously. The suspension of separating layer was prepared to gain 15% of drug-loaded pellets. Eventually, the pellets (24 to 30 sieve mesh) were sieved to prepare the enteric-coated layer.
2.2.1.3. Enteric-coated layer
-
a.
Eudragit® L30D55 was added to part of purified water (30%, wt/wt, based on the aqueous dispersion of polymer) under stirring, and then mixed for 5 minutes. Fine talc (7%, wt/wt, based on the aqueous dispersion of polymer) was dispersed with constant stirring in distilled water, which was dropped with triethyl citrate (TEC) (3%, wt/wt, based on the aqueous dispersion of polymer), and then added to the above suspension. The solution was blended for 15 minutes until a homogenous dispersion was formed. The suspension can be neutralized to the wanted pH using 0.1 mol/l sodium hydroxide solution. The prepared pellets were cured for 2 h at 40 °C.
-
b.
HPMCAS-LF (6%, wt/wt, based on the solution) was well-dispersed in the purified water cooled to 15 °C or below. To this suspension, TEC (20%, wt/wt, based on the polymer) and talc (30%, wt/wt, based on the polymer) were added and blended thoroughly until homogeneity was obtained. The enteric suspension should be maintained below 20 °C. A part of or all the enteric polymer was neutralized with ammonium hydroxide, which was able to prevent the spray gun nozzle from blocking at room temperature. The prepared pellets were cured for 3 h at 60 °C.
-
c.
Ethanol and purified water were prepared at the ratio of 8:2 in a clean beaker. HPMCP-HP55 (8%, wt/wt, based on the solution) was slowly added to the obtained solvent and dissolved for 1 hour under constant stirring. TEC (10%, wt/wt, based on the polymer) and talc (30%, wt/wt, based on the polymer) were added to the above solution and a uniform suspension was obtained with continuous stirring. The prepared pellets were cured for 2/4 h at 40 °C.
Eventually, the pellets (20 to 30 sieve mesh) were screened for encapsulation.
2.2.2. Characterization of optimized pellets
Qualified pellets with different kinds of enteric polymers were evaluated by yield of pellets, angle of repose, friability and particle size distribution. The yield of pellets was calculated by the following formula (1)
| (1) |
Fixed funnel method was chosen to determine the angle of repose. The powder of DXH was poured through a funnel to form a cone. The tip of the funnel should be held closely to the growing cone and slowly raised as the pile grows. No pouring the powder when the pile reached a predetermined height or the base reacted to a predetermined width. Rather than attempt to measure the angle of the resulting cone directly, we divided the height by half the width of the base. The inverse tangent of this ratio was the angle of repose.
Friability was tested by a friabilator. Ten grams of home-pellets and 25 glass balls were mixed together to be centrifuged for 10 min at 30 rpm. The pellets were collected and weighted. As a result, the percentage of weight loss of the pellets was calculated [6]. Sieve analysis was applied for particle size and distribution. One hundred grams of pellets was collected and divided into three parts (20–24 mesh, 24–30 mesh and 30–40 mesh) according to the particle size.
A certain amount of weight of pellets was weighed out (equivalent to 30 mg duloxetine), and then were packed in No.3 capsule shell for future use.
2.2.3. Determination of drug content
The drug content was determined by the method of HPLC. Agilent ZORBAX SB-C8 column (3.5 µm, 75 × 4.6 mm) was optimized in a column oven with the temperature maintained 45 °C. The injection volume was 10 µl, the flow rate was 1.5 ml/min, and the UV detector wavelength was set at 230 nm.
For buffer A, 3.4 g/l of monobasic potassium phosphate in water was prepared. Fifteen milliliters of triethylamine was added to 1 l of this solution, and adjusted with phosphoric acid to a pH of 5.5 ± 0.2.
For buffer B, 216 mg/l of monobasic ammonium phosphate and 4.5 g/l of dibasic potassium phosphate were prepared in water, and adjusted with ammonia or phosphoric acid to a pH of 7.9–8.3.
Mobile phase was formed from methanol, tetrahydrofuran and buffer A (323:90:587).
The contents of NLT 10 capsules were ground with a mortar and pestle into fine powders. Then powders (equivalent to approximately 90 mg duloxetine) were precisely weighed and transferred into a 100 ml volumetric flask. Half of the mixed diluent (buffer B and acetonitrile with the ratio of 60:40 (v/v)) was added, and the contents in the flask were shaken for 10 minutes after sonication for 3 minutes. The solution was then diluted to the mark with uniform diluent to get a stock solution, which was filtered through a 0.45 µm membrane filter. Five millimeters of the filtrate was diluted to 50 ml with the mixed diluent, and the diluted solution was filtered to get sample solution as before [7].
2.2.4. Drug release measurements and comparisons
Enteric-coated capsules prepared from Eudragit® L30D55, Aqoat® AS-LF and HPMCP-HP55 were tested in 1000 ml of 0.1 mol/l HCL (simulated gastric fluid) followed by the phosphate buffer of pH 6.8 using apparatus 1 (basket apparatus) in USP. One liter of 0.1 mol/l HCL was transferred into each container of the ZRS-8G Test Dissolution Tester (Tianjin University Radio Factory, Tianjin, China), in which medium can be maintained at 37 ± 0.2 °C. DXH capsule equivalent to 30 mg duloxetine was placed in each of the baskets, and the baskets were fixed to shafts and operated dissolution apparatus at 100 rpm for 2 hours. After 2 hours in acid, 0.1 mol/l HCL was carefully drained and the fresh solution of 1000 ml of pH 6.8 phosphate buffer (simulated intestinal fluid), maintained at 37 ± 0.2 °C in advance, was taken into the container. At each specified interval of time, 5.0 ml of the sample was withdrawn from each container and replaced with equal volume of fresh pH 6.8 medium maintained at 37 ± 0.2 °C for 1 h. The collected sample was filtered through 0.45 µm membrane filter and analyzed with HPLC as the assay of drug content [7].
In this study, we evaluated the similarity between home-made capsules and marketed capsule (Cymbalta®) in the pH 6.8 phosphate buffer. As a parameter of similarity evaluation, the similarity factor (f2) plays a significant role in comparing the dissolution profiles. f2 (shown in the following formula (2)) is a logarithmic transformation of the sum-squared error of differences between the reference and the tested preparations over all time points [8].
| (2) |
Log stands for logarithm based on 10. It is recommended that two dissolution profiles can be determined to be similar when f2 value exceeds 50.
2.2.5. Scanning electron microscopy (SEM)
To evaluate the surface morphology of enteric-coated polymer membrane with different polymers, scanning electron microscopy (SEM) (SU8010, HIGUCHI, Japan) images were achieved from Cymbalta® and home-made pellets. The pictures were taken at the magnification of 90× or 80×.
2.2.6. Kinetic models of reference and optimized formulations
Kinetic studies were conducted for reference and optimizing enteric-coated formulations with different polymers. It was inferred that there were three probable ways for drug release from the polymeric membrane to medium: (1) the drug is dispersed into the membrane and permeated through the consistent polymeric network, and then redistributed in the polymeric framework and diffused to the medium; (2) the drug is dispersed to medium via tiny holes or cracks existing in the membrane; and (3) the coalition of the above two [9], [10], [11], [12].
Different mathematical models were applied to study the in vitro dissolution behaviors of the pellets, including zero-order model [13], first-order model [14], Higuchi model [15], Ritger–Peppas model [16], Hixson–Crowell model, Baker–Lonsdale model [17] and Weibull model [18]. Regression analysis was conducted, and then the best fits were calculated based on correlation coefficient as r [12].
Of the models above, Ritger–Peppas model [16] was applied for further analyses of drug release mechanism. Equation of Ritger–Peppas model was shown in the following formula (3). Qt denoted the accumulated release at time (t), kR expressed the constant rate of drug release, while n was an important parameter indicating the release mechanism. If “n” ≤ 0.45, the mechanism was Fickian diffusional. If 0.45 < “n” < 0.89, the release mechanism was non-Fickian dispersion including Fickian-diffusional and relaxation. If the “n” ≥ 0.89, the relaxation played a sole role in drug release.
| (3) |
2.2.7. Stability study
The optimized enteric-coated pellets and Cymbalta® were placed into the condition of 40 °C/75% RH for 1 month. Samples were analyzed for the dissolution profiles, contents and related substances.
3. Results and discussion
3.1. DXH drug-layer pellets prepared by different apparatus
Drug-layer pellets were prepared with two kinds of instruments, centrifugal granulator and fluidized bed. The above equipments were both used to search the optional formulation and preparation technique. It was the same in temperature and relative humidity of environment. The pellets prepared by fluidized bed, had lower friability (0.07%) and higher yield (93.2%) than that prepared by centrifugal granulator (0.18%, 75.3%). Pellets manufactured by centrifugal granulator were coated with barrier suspension by fluidized bed, in the process of which, the powder could desquamate from the drug-layer pellets. Consequently, it was the loss of DXH that led to the rough surface and low yield. In addition, there was a drawback in the preparation process that dust emissions happened due to low density of drug.
3.2. Physical characterization of enteric-coated pellets
The optimal enteric-coated pellets were achieved with 85.3 ± 0.2% of yield, 25.2 ± 0.9° of angle of repose, and 0.07 ± 0.02% of friability. The percentage of pellets obtained from 20–24 mesh, 24–30 mesh, and 30–40 mesh were 88%, 10% and 2%, respectively.
3.3. Effect of particle size of duloxetine hydrochloride on drug release
According to BCS, duloxetine hydrochloride is classified as class-II drug and has limited aqueous solubility. It was proven by the experiment that duloxetine hydrochloride was sparingly soluble in water [19]; thus particle size was decreased to increase the solubility and dissolution rate of duloxetine hydrochloride. Duloxetine hydrochloride was smashed by pulverizer, then, was passed through 80-mesh or 200-mesh sieve. Different particle sizes of duloxetine hydrochloride were sprayed to sugar spheres homogeneously to compare the differences on the drug release. Fig. 1A showed the release profiles of duloxetine from drug-loaded pellets at pH 6.8. It can be concluded that pellets with 200-mesh DXH have higher solubility and dissolution rate than 80-mesh.
Fig. 1.
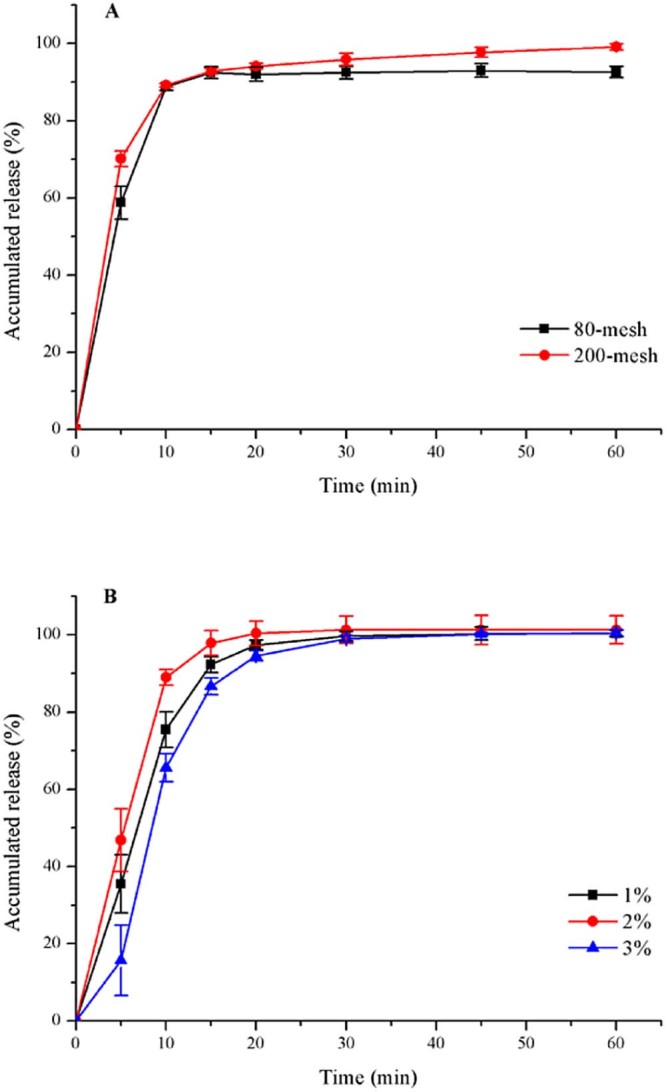
Release profiles (mean ± SD, n ≥ 3) of duloxetine from drug-loaded pellets with (A) different particle sizes of drug; (B) different concentrations of HPMC-E5 solution, at pH 6.8.
3.4. Inspection of the concentration of HPMC-E5 in DXH solution
In the process of drug-loading, HPMC-E5 played a role of adhesion, so the drug could adhere to the sugar-spheres. Different concentrations of HPMC-E5 solution (1.0%, 2.0% and 3.0%, wt/wt) were prepared to estimate their impact on the preparation procedure and drug release. 1.0% HPMC solution was sprayed on the blank pellets, which could result in high friability because of the low viscosity. The viscosity of 3.0% HPMC-E5 solution was high enough to make the pellets agglomerate on the side of fluidized bed so that the drug-spraying process could not run continuously. However, 2.0% HPMC-E5 solution could prevent the above problems from occurring and enabled the industrial manufacture to reality. Fig. 1B expressed the dissolution profiles of duloxetine drug-loaded pellets coated with different concentrations of adhesion. It was concluded that 2.0% HPMC-E5 solution was the optimized adhesion because of its higher dissolution and smoother preparation process.
3.5. The impact of environmental aspect (temperature and relative humidity) on drug-laying and coating
Drug-laying and barrier-laying temperature could be controlled in the range of 40–50 °C. Too low temperature could lead to long working hours. Too high temperature could reduce thestability of duloxetine. Besides, the temperature should be regulated along with relative humidity.
Temperature not only had impact on the enteric-coating process, but also determined the physico-chemical property of membrane. Too high or low temperature could consequently make an effect on the intactness of the enteric-membrane and influence the drug-release behavior. Different temperatures were demanded over the membrane formation of the three polymers. In the process of coating with Eudragit® L30D55, it was required that inlet temperature was regulated at 40–45 °C to make the temperature of pellets controlled at 28–30 °C. Inlet temperature could be adjusted to 55–60 °C during the coating with Aqoat® AS-LF and HPMCP-HP55. Moreover, to prevent nozzle clogging, it was recommended to chill the coating suspension with ice bath when using Aqoat® AS-LF, if necessary, to maintain the temperature under 25 °C [4].
Furthermore, relative humidity was another principal element affecting coating procedure. Electrostatic phenomenon would generate between pellets and inner wall of fluidized bed when the relative humidity was extremely low, resulting in inhomogeneous coating. Especially, the phenomenon was remarkable when using organic solvent as enteric-coating solvent. Nevertheless, pellets would agglomerate in case of the high relative humidity that led to the adverse fluidization condition of pellets. According to the experience, the coating process could be accomplished successfully in the environment at a controlled relative humidity of 30%–50% [12].
3.6. Release of duloxetine from pellets coated with Eudragit® L30D55
3.6.1. Effect of the coating weight on dissolution profile in pH 6.8 PBS
First of all, the enteric-coated solution was prepared to make 18% coating weight gain. Then, some enteric-coated pellets were taken out from discharge hole of FBP respectively when the coating weight gain reached 10%, 12% and 15%. As seen in Fig. 2A, percentage of duloxetine released in the acid stage medium after 2 h decreased with the increase of coating amount. The release of duloxetine was restrained completely in the acid medium if the enteric-coated weight of pellets was added to 18%. It was because the diffusion path length increased. The accumulative drug-release of pellets at pH 6.8 was instead increased when making the coating amount gained. It was the reaction of 0.1 mol/l HCL with polymer or duloxetine probably that interfered in the dissolution of drug in the following pH 6.8 PBS.
Fig. 2.
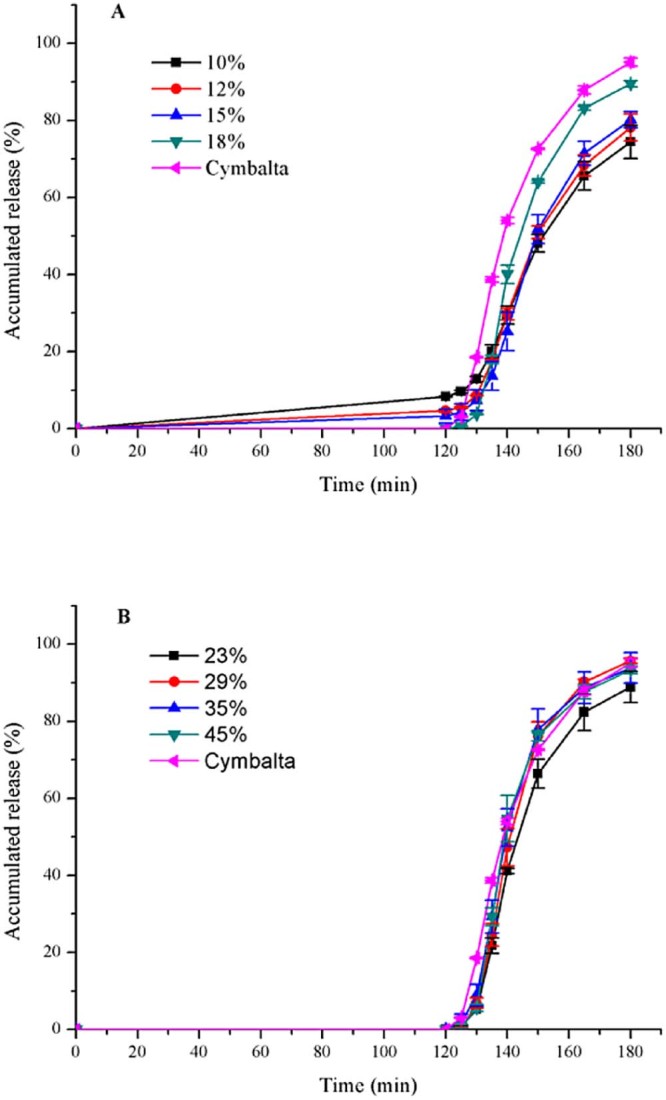
Release profiles (mean ± SD, n ≥ 3) of duloxetine pellets coated with Eudragit® L30D55 (A) affected by weight gain (10–18%); (B) affected by weight gain (23–45%), and Cymbalta® after 2 h 0.1 mol/l HCL and subsequently at pH 6.8.
Subsequently, the coating weight was increased to 23%, 29%, 35% and 45%. The release amount of above pellets was 0% in the 0.1 mol/l HCL solution. As we can see in Fig. 2B, the accumulative release of home-made pellets at 60 min corresponded to Cymbalta® if the enteric-coated weight of home-made pellets increased to 29%. According to f2 (Table 3), duloxetine pellets coated with 35% is the optimal formation for Eudragit® L30D55.
Table 3.
The f2 of dissolution profiles of home-made and marketed pellets in pH 6.8 PBS.
| Enteric polymers | Enteric-coated weight gain (%) | f2 |
|---|---|---|
| Eudragit® L30D55 | 23 | 48 |
| 29 | 54 | |
| 35 | 61a | |
| 45 | 51 | |
| Aqoat® AS-LF without NH3 | 17 | 36 |
| 20 | 68a | |
| 24 | 60 | |
| Aqoat® AS-LF with NH3 | 25 | 49 |
| 30 | 53 | |
| 35 | 47 | |
| HPMCP-HP55 | 17 | 59 |
| 26 | 63 | |
| 34 | 57 |
The maximums of similarity factor of preparations used by different polymers were emphasized.
However, in the first 20 minutes, the pellets coated with 35% Eudragit® L30D55 were released more slowly than the marketed. There was a probability that the structure of polymers was a key for the dissolution rate. As a result, we utilized other enteric-coated polymers to make the experiment.
3.7. Release of duloxetine from pellets coated with Aqoat® AS-LF
3.7.1. Effect of the coating weight on dissolution profile in pH 6.8 PBS
As seen in Fig. 3A, percentage of duloxetine released in the acidic medium after 2 h decreased with the increase of coating amount. The release of drug in the acid medium was reduced to less than 10%, when enteric-coated weight of pellets added to 20%. According to the resistance to acid and f2 (Table 3), duloxetine pellets coated with 24% was the best formulation for Aqoat® AS-LF. When using Aqoat® AS-LF, the dissolution rate was more rapid compared to Eudragit® L30D55.
Fig. 3.
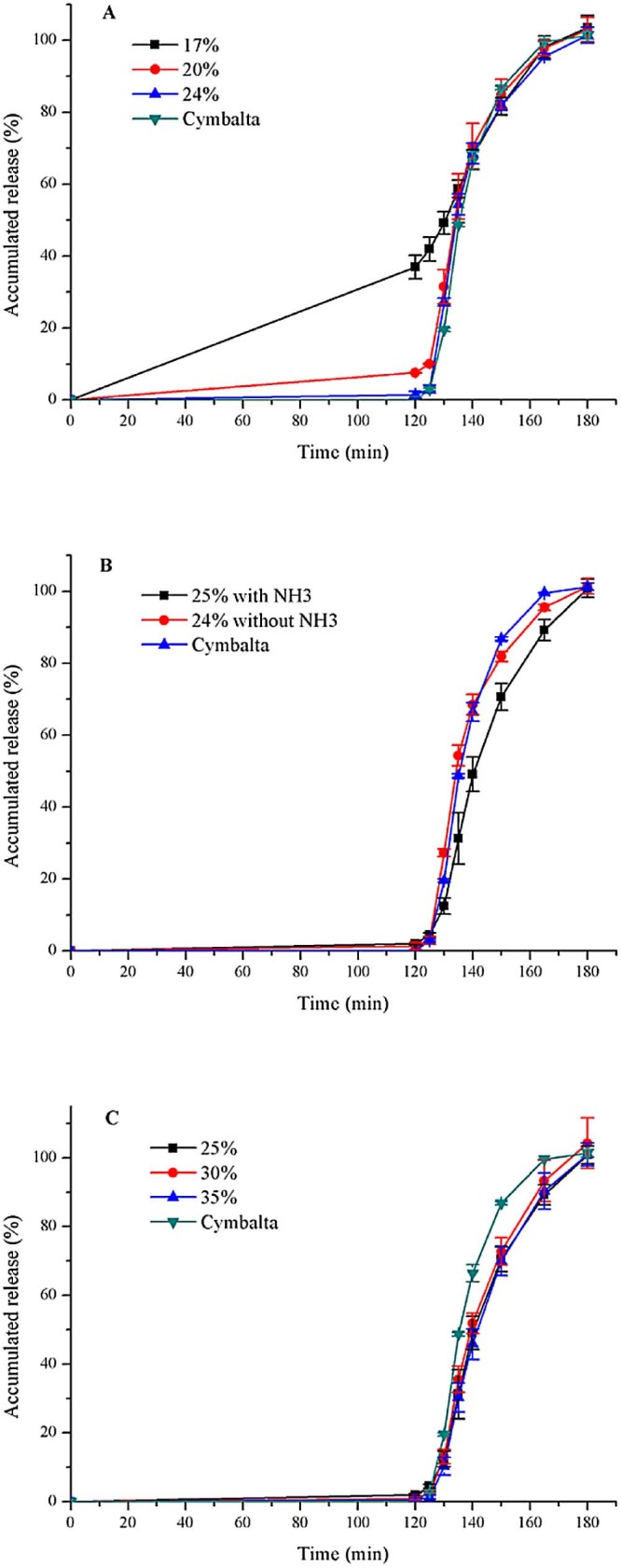
Release profiles (mean ± SD, n ≥ 3) of duloxetine pellets coated with Aqoat® AS-LF (A) affected by weight gain without NH3; (B) affected by the enteric suspension with or without NH3; (C) affected by weight gain with NH3, and Cymbalta® after 2 h 0.1 mol/l HCL and subsequently at pH 6.8.
3.7.2. Effect of pH of enteric polymer on dissolution profile in pH 6.8 PBS
We preferred to apply the enteric polymer as an aqueous solution. Aqoat® AS-LF was insoluble in water, but it could be easily dissolved by neutralizing the polymer preferably with ammonium [4]. As seen in Fig. 3B, with weight gain of enteric polymers unchanged, enteric-coated pellets with NH3 had lower dissolution rate. If enteric weight increased to 30%, the dissolution rate was higher than 25% (Fig. 3C). Moreover, f2 of pellets (30% weight-gain) was enhanced to over 50 (Table 3).
3.8. Release of duloxetine from pellets coated with HPMCP-HP55
3.8.1. Effect of curing condition on dissolution profile in pH 6.8 PBS
It was proposed that the curing temperature or time was increased to improve film formation and hence coating performance [20]. Pellets with HPMCP were found to form phthalamide impurity when accelerated by heat and humidity [2]. Therefore, only the curing time was modified from 2 to 4 hours, maintaining the curing temperature at 40 °C. Fig. 4A expressed that increasing curing time to 4 h slightly decreased release after 2 h in 0.1 mol/l HCL (1.08% → 0%), while it significantly increased release at pH 6.8.
Fig. 4.
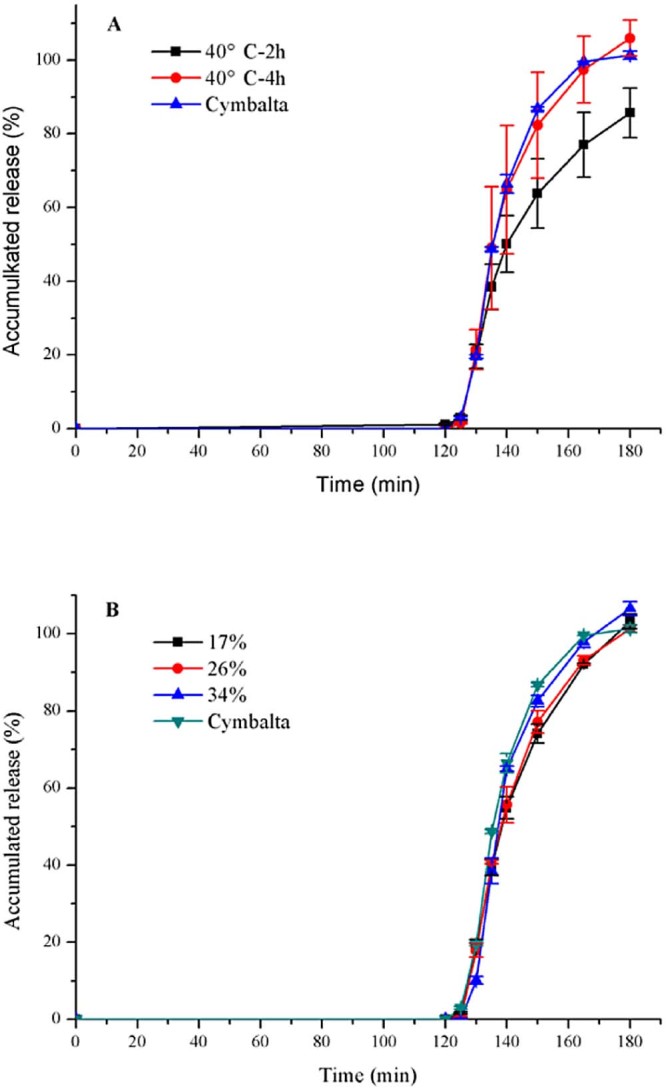
Release profiles (mean ± SD, n ≥ 3) of duloxetine pellets coated (A) with 17% HPMCP-HP55 affected by curing time under 40 °C; (B) affected by weight gain with HP-55, and Cymbalta® after 2 h 0.1 mol/l HCL and subsequently at pH 6.8.
3.8.2. Effect of the coating weight on dissolution profile in pH 6.8 PBS
As seen in Fig. 4B, percentage of duloxetine released in 0.1 mol/l HCL after 2 h had remained at 0% with the increase of coating weight gain. According to f2 (Table 3), with HPMCP-HP55, duloxetine enteric-pellets coated with 26% was the best formulation. When HP-55 was used, the dissolution rate was the same with Cymbalta®.
3.9. Effect of the type of enteric polymer on dissolution profile in pH 6.8 PBS
The dissolution profile of preparations with the above three polymers was summarized in Fig. 5. We selected the three enteric-coated pellets with the same weight gain, and then the figure could suggest that the different enteric polymers have varied resistance to acids and release rate in intestinal juice.
Fig. 5.
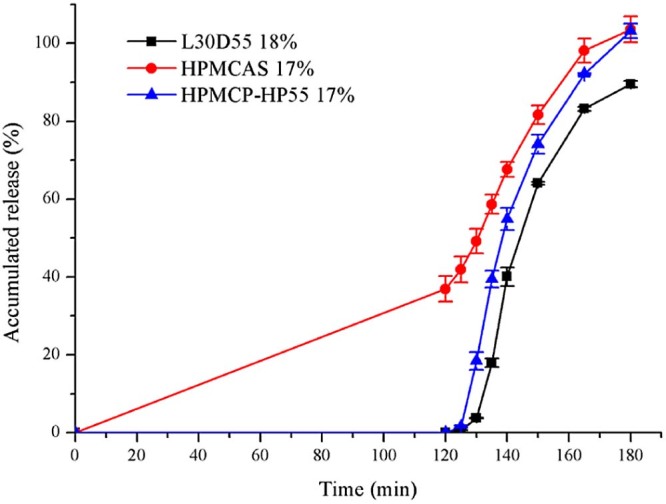
Release profiles (mean ± SD, n ≥ 3) of duloxetine pellets coated with different enteric polymers after 2 h 0.1 mol/l HCL and subsequently at pH 6.8.
When aqueous coatings are employed, it is necessary to apply more coating polymers to achieve the desired effect such as resistance to gastric fluid or delay in release, than with comparable organic coatings [21]. In our research, Aqoat® AS-LF was well-dispersed in the distilled water, while HPMCP-HP55 was dissolved in the 80% ethanol solution. Consequently, the pellets could better achieve gastro-resistance with 17% HPMCP-HP55 than with 17% Aqoat® AS-LF.
It becomes clear that a factor governing the rate of solution and solubility of HPMCAS is the electrostatic free energy of dissociation of carboxyl groups [22]. That could be true for HPMCP-HP55 and Eudragit® L30D55. When the polymers are in the higher pH condition, they could combine with hydroxyl ions to form salt. Due to the repulsive interaction of ionized carboxyl, the empty space between the molecules would become larger leading to the loose structure, and thus drug was released from the film. The backbone structure of HPMCAS and HPMCP are the same, HPMC, which is different from Eudragit® L30D55. Eudragit® L30D55 is an anionic copolymer of methacrylic acid and ethyl acrylate. The monomers of HPMCAS and HPMCP have two carboxyl groups, while that of Eudragit® L30D55 has only one. It was probably less carboxyl group that resulted in the lower dissolution rate for Eudragit® L30D55. Further research is needed to prove this.
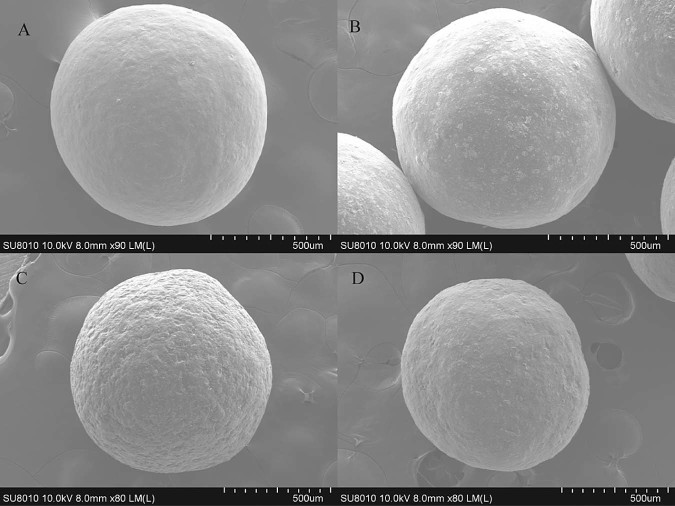
3.10. SEM experiments
Enteric-coated pellets were examined under scanning electron microscopy at ×90 or ×80 magnifications. The electron photomicrographs were shown in Fig. 6. The surfaces of the coated pellets were all round. Pellets coated by Eudragit® L30D55 and Aqoat® AS-LF were smooth as Cymbalta®, while the one coated with HPMCP-HP55 was rough extraordinarily.
Fig. 6.
Scanning electron microscopy figures of optimal pellets (A: Cymbalta®; B: Aqoat® AS-LF; C: HPMCP-HP55; D: Eudragit® L30D55).
The aqueous dispersion of HP-55 could be micronized and film formation was improved by minimizing its particle size [23]. In addition to the loss of plasticizer (TEC) and lessened protection against moisture, it was possible for dispersion coatings to show a more porous membrane or cracks along with remaining particle borders, particularly [24]. The drug release characteristics of the preparation could be altered and sticking would occur with a decline in the functional property of the films. Sticking could be refrained by means of applying a plus overcoat of water-soluble cellulose material such as HPC or HPMC [25]. Consequently, we will attempt to add a finishing layer over the enteric layer to improve the elegance of the pellets and its storage. The same impact can be exerted on the HP-55 if decreasing the polymer size.
3.11. The mechanism of drug release
The different kinetic models were applied to marketed reference and the different enteric-coated formulations, and the results were shown in Table 4. It was observed that First-order model, Hixson–Crowell model and Weibull model were all fitted for Cymbalta®, and Weibull model was the best. Home-made pellets applied with polymers of Eudragit® L30D55, Aqoat® AS-LF and HPMCP HP-55 were consistent with the marketed. The “n” of Ritger–Peppas model was 0.7386 of Cymbalta®, while the “n” were 0.8150, 0.6225 and 0.7642 of Eudragit® L30D55, Aqoat® AS-LF and HPMCP-HP55, respectively. As a consequence, we can draw the conclusion that both free diffusion and relaxation play a key role in the drug release of Cymbalta® and home-made products.
Table 4.
Kinetic models of drug release and correlation coefficient.
| Kinetic model | Equation | r | ||||
|---|---|---|---|---|---|---|
| Cymbalta® | Home-made | |||||
| Eudragit® L30D55 | Aqoat® AS-LF | HPMCP (HP-55) | ||||
| Zero-order model | 0.8873 | 0.9273 | 0.8429 | 0.9337 | ||
| First-order model | 0.9750 | 0.9561 | 0.9741 | 0.9753 | ||
| Higuchi diffusion model | 0.9234 | 0.8810 | 0.9293 | 0.9118 | ||
| Ritger–Peppas model | 0.9502 | 0.9414 | 0.9442 | 0.9599 | ||
| Baker–Lonsdale model | 0.8746 | 0.8347 | 0.8843 | 0.8597 | ||
| Hixson–Crowell model | 0.9762 | 0.9550 | 0.9761 | 0.9718 | ||
| Weibull model | 0.9972 | 0.9909 | 0.9923 | 0.9957 | ||
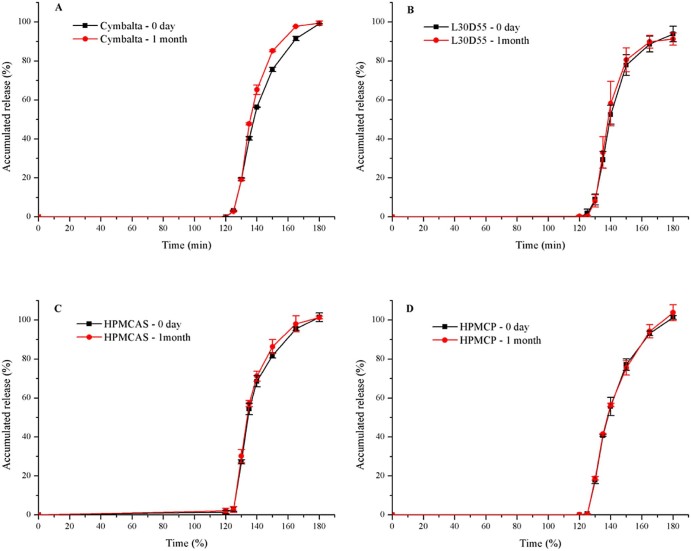
3.12. Results of stability study
The optimized enteric-coated pellets were selected on the basis of abovementioned results. Fig. 6 showed that the dissolution profiles of marketed and home-made capsules had no noticeable difference after storage under the condition of 40 °C/75% relative humidity for 1 month.
As shown in Table 5, the home-made pellets manufactured using Eudragit® L30D55 and Aqoat® AS-LF were relatively stable. The pellets could be unable under the accelerated test for longer than one month, and hence home-made pellets and Cymbalta® need more evaluation. However, there was an unknown impurity in the HP-55 pellets after storage at 40 °C/75% relative humidity for 1 month (Fig. 8). It was demonstrated that the residual free acids existing in the enteric polymers HPMCP and HPMCAS in pellets form phthalamide and succinamide impurities respectively, under the accelerated condition of heat and humidity. It was postulated that phthalic and succinic substituents could be cleaved from the polymers, leading to the formation of either anhydrides or free acids. It was proposed that the reaction was motivated by the migration of either (1) the anhydride or free acid or (2) the drug substance through the formulations. Formation of the impurities was minimized by way of increasing the thickness of the barrier-layer [2]. The structure of the unknown impurity can be analyzed by nuclear magnetic resonance (NMR).
Fig. 7.
Release profiles (mean ± SD, n ≥ 3) of (A) Cymbalta® and home-made pellets by (B) Eudragit® L30D55; (C) Aqoat® AS-LF; (D) HPMCP-HP55, in day 0 and after accelerated test of 1 month after 2 h 0.1 mol/l HCL and subsequently at pH 6.8.
Table 5.
Stability of marketed and home-made pellets exposed to the accelerated condition.
| Product | Time | Content property | Related substance(%) | Content(%) | Dissolution profile | |
|---|---|---|---|---|---|---|
| Cymbalta® | 0-day | White-pellets | – | 99.90 | Fig. 7A | |
| 1-month | White-pellets | – | 99.32 | |||
| Homemade | Eudragit® L30D55 | 0-day | White-pellets | – | 98.98 | Fig. 7B |
| 1-month | White-pellets | – | 98.52 | |||
| Aqoat® AS-LF | 0-day | White-pellets | – | 99.95 | Fig. 7C | |
| 1-month | White-pellets | – | 100.16 | |||
| HPMCP (HP-55) | 0-day | White-pellets | – | 100.00 | Fig. 7D | |
| 1-month | White-pellets | 0.2 | 98.56 | |||
Fig. 8.
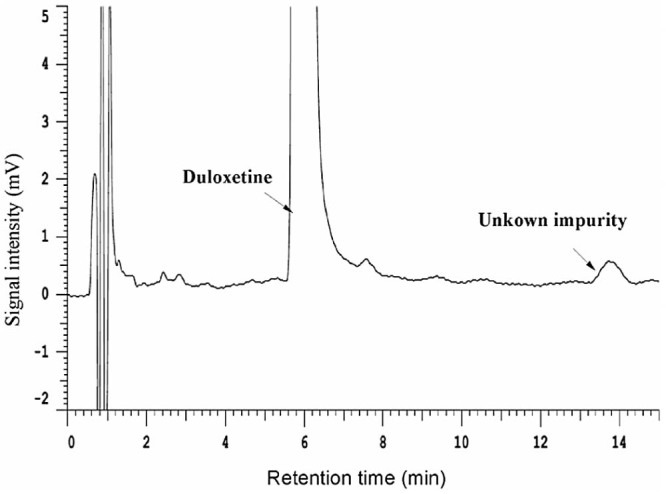
HPLC chromatogram of duloxetine hydrochloride pellets prepared by HPMCP-HP55 stressed at 40 °C/75% relative humidity for 1 month.
The γ-scintigraphy studies, in vivo, had revealed that there was a substantial time delayed (up to 2 h) for such pellets to disintegrate in the small intestine after the emptying of the stomach, with different enteric-polymers owning various disintegration times [26], [27]. Therefore, dissolution research is recommended to be evaluated in the dissolution medium of lower pH like pH 5.5 PBS and pH 6.0 PBS, instead of pH 6.8 PBS [28].
4. Conclusions
Duloxetine hydrochloride enteric-coated capsules were successfully prepared in a laboratory-scale, with three polymers including Eudragit® L30D55, Aqoat® AS-LF and HPMCP-HP55. The in vitro release behaviors of above three preparations were similar to that of Cymbalta®. However, the pellets prepared with HPMCP-HP55 had poorer stability than that with two other polymers. We can draw a conclusion that drug release can be affected if the coating weight, pH of enteric polymers and curing conditions are changed. In conclusion, the pellets prepared by utilizing Eudragit® L30D55 and Aqoat® AS-LF were the optimal preparations based on the drug release and stability. The self-made pellets with HP-55 will be manufactured through thicker barrier-layer or by adding a finishing layer to enhance the stability.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Eli Lilly and Company Duloxetine (marketed as Cymbalta®) prescribing and labeling information. 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021427s046lbl.pdf accessed 27.03.15.
- 2.Jansen P.J., Oren P.L., Kemp C.A. Characterization of impurities formed by interaction of duloxetine HCl with enteric polymers hydroxypropyl methylcellulose acetate succinate and hydroxypropyl methylcellulose phthalate. J Pharm Sci. 1998;87(1):81–85. doi: 10.1021/js970133r. [DOI] [PubMed] [Google Scholar]
- 3.Chatlapalli R., Rohera B.D. Physical characterization of HPMC and HEC and investigation of their use as pelletization aids. Int J Pharm. 1998;161:179–193. [Google Scholar]
- 4.Anderson N.R., Oren P.L., Ogura T. 1996. Duloxetine enteric pellets. US patent; US 5508276; 16 Apr. [Google Scholar]
- 5.Jaklič M.T., Jurečič R. Development of intermediate layer with improved diffusional barrier. Sci Pharm. 2010;78:717. [Google Scholar]
- 6.Shravani D., Lakshmi P.K., Balasubramaniam J. Preparation and optimization of various parameters of enteric coated pellets using the Taguchi L9 orthogonal array design and their characterization. Acta Pharm Sin B. 2011;1(1):56–63. [Google Scholar]
- 7.United States Pharmacopeia and National Formulary (USP 35-NF 30) United States Pharmacopeial Convention; Rockville: 2012. Duloxetine delayed release capsules. [Google Scholar]
- 8.Moore J.W., Flanner H.H. Mathematical comparison of dissolution profiles. Pharm Technol. 1996;20:64–74. [Google Scholar]
- 9.Siepmann F., Wahle C., Leclercq B. pH-sensitive film coatings: towards a better understanding and facilitated optimization. Eur J Pharm Biopharm. 2008;68:2–10. doi: 10.1016/j.ejpb.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K., Nara E., Akiyama Y. Development of an oral sustained release drug delivery system utilizing pH-dependent swelling of carboxyvinyl polymer. J Control Release. 2006;111:309–315. doi: 10.1016/j.jconrel.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q.W., Flament M.P., Siepmann F. Curing of aqueous polymeric film coatings: importance of the coating level and type of plasticizer. Eur J Pharm Biopharm. 2010;74:362–370. doi: 10.1016/j.ejpb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Wang J., Sun Y., Li B. Preparation and evaluation of tamsulosin hydrochloride sustained-release pellets modified by two-layered membrane techniques. Asian J Pharm Sci. 2015;10(1):31–39. [Google Scholar]
- 13.Samuelov Y., Donbrow M., Friedman M. Sustained release of drugs from ethylcellulose–polyethylene glycol films and kinetics of drug release. J Pharm Sci. 1979;68:325–329. doi: 10.1002/jps.2600680318. [DOI] [PubMed] [Google Scholar]
- 14.Lapidus H., Lordi N.G. Some factors affecting the release of a water-soluble drug from a compressed hydrophilic matrix. J Pharm Sci. 1966;55:840–843. doi: 10.1002/jps.2600550818. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50:874–875. doi: 10.1002/jps.2600501018. [DOI] [PubMed] [Google Scholar]
- 16.Ritger P.L., Peppas N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5:37–42. [PubMed] [Google Scholar]
- 17.Baker R.W., Lonsdale H.K. Controlled release: mechanisms and release. In: Tanquary A.C., Lacey R.E., editors. Controlled release of biologically active agents. Plenum; New York: 1974. pp. 15–71. [Google Scholar]
- 18.Costa P., Lobo J.M.S. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 19.Vyas V.U., Nagesh N., Mittapalli P.K. 2009. Pharmaceutical formulations comprising duloxetine. US patent; US 20090226517A1; 10 Sep. [Google Scholar]
- 20.Huyghebaert N., Vermeire A., Remon J.P. In vitro evaluation of coating polymers for enteric coating and human ileal targeting. Int J Pharm. 2005;298:26–37. doi: 10.1016/j.ijpharm.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Osterwald H., Bauer K.H. Gegenüberstellung von dünndarmlöslichen Filmüberzügen einiger synthetischer Polymere auf festen Arzneiformen aus wäßrigen und aus organischen Umhüllungszubereitungen. Act Pharm Technol. 1980;26:201–209. [Google Scholar]
- 22.Takahashi A., Kato T., Kamiya F. Dissolution mechanism for hydroxypropyl methylcellulose acetate succinate used in the enteric coating of tablets. Kobunshi Ronbunshu. 1985;42(11):803–808. [Google Scholar]
- 23.Thoma K., Bechtold K. Influence of aqueous coatings on the stability of enteric coated pellets and tablets. Eur J Pharm Biopharm. 1999;47:39–50. doi: 10.1016/s0939-6411(98)00086-1. [DOI] [PubMed] [Google Scholar]
- 24.Murthy K.S., Enders N.A., Mahjour M. A comparative evaluation of aqueous enteric polymers in capsule coatings. Pharm Technol. 1986;10:36–46. [Google Scholar]
- 25.Oshlack B., Chasin M., Pedi F. 1994. Controlled-release formulations coated with aqueous dispersions of ethylcellulose. EP patent; EP 0630646(A1); 28 Dec. [Google Scholar]
- 26.Bogentoft C., Alpsten M., Ekenved G. Absorption of acetylsalicylic acid from enteric-coated tablets in relation to gastric emptying and in-vivo disintegration. J Pharm Pharmacol. 1984;36:350–351. doi: 10.1111/j.2042-7158.1984.tb04394.x. [DOI] [PubMed] [Google Scholar]
- 27.Cole E.T., Scott R.A., Connor A.L. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int J Pharm. 2002;231:83–95. doi: 10.1016/s0378-5173(01)00871-7. [DOI] [PubMed] [Google Scholar]
- 28.Ebel J.P., Jay M., Beihn R.M. An in vitro/in vivo correlation for the disintegration and onset of drug release from enteric-coated pellets. Pharm Res. 1993;10:233–238. doi: 10.1023/a:1018986827350. [DOI] [PubMed] [Google Scholar]