Graphical Abstract
There are many aspects involved in the formulation and use of emulsion based drug delivery systems. Apart from the efficiency in delivering drugs at localised sites and good drug release, an important aspect that is often overlooked is the oxidative stability. This is vital because these emulsions are formulated from lipidic ingredients prone to oxidation. Oxidative stability affects emulsion systems and bodily systems upon administration. Preventive measures and peroxidation evaluation are thus crucial.
Keywords: Drug delivery, Emulsions, Lipids, Peroxidation, Oxidative stress
Abstract
Pharmaceutical delivery systems are developed to improve the physicochemical properties of therapeutic compounds. Emulsions are one of these drug delivering systems formulated using water, oils and lipids as main ingredients. Extensive data are usually generated on the physical and chemical characteristics of these oil-in-water and lipid emulsions. However, the oxidative tendency of emulsions is often overlooked. Oxidation impacts the overall quality and safety of these pharmaceutical emulsions. Additionally, introducing oxidatively unstable emulsions into biological systems further promotes oxidation in situ. Products of these reactions then continue to pose serious harm to cells and fuel other physiological oxidation reactions. Consequently, the increase of oxidation products leads to oxidative damage to biological systems. Thus, emulsions with lower lipid peroxidation are more stable and will reduce the negative effects of oxidation in situ. Preventive measures during the formulation of emulsions are important. Many naturally occurring and cost effective substances possess low oxidation tendencies and confer oxidative protection when used in emulsions. Additionally, certain preparatory methods should be employed to reduce or better control lipid peroxidation. Finally, emulsions must be evaluated for their oxidation susceptibility using the various techniques available. Careful attention to the preparation of emulsions and assessment of their oxidative stability will help produce safer emulsions without compromising efficacy.
1. Introduction
Conventional methods of administering certain drugs have resulted in failure of achieving therapeutic concentrations, despite reaching systemically toxic levels [1]. Failure in reaching effective concentrations is mainly due to obstacles in, but not limited to, biological barriers, naturally occurring biochemical reactions and molecular characteristics of the therapeutic compounds themselves. To circumvent these issues, there is a bloom in the development of innovative means to effectively deliver therapeutic compounds, such as using drug delivery systems. These systems safely deliver therapeutic agents at calculated rates to targeted sites and reduce unwanted distribution. These systems have led to improvement in the pharmacokinetics, pharmacodynamics and uptake of various therapeutic molecules. These improvements are due to reduced binding and interaction of the incorporated molecule with endogenous molecules. Furthermore, reduced premature enzymatic degradation is attributed to protection conferred by the carrier system to the encapsulated molecule before it reaches the targeted site [2].
Micro and nano-particulate systems are examples of delivery systems constantly in development. These systems have increased drug loading capacities and controlled release of their contents depending on the release pattern needed for a specific compound [3]. Biodegradable nanoparticles and emulsions made from lipids of physiological origin are specific examples of small-sized systems. Topical formulations and films are preparations that contain lipid nanoparticles and have exhibited excellent tolerability due to their chemical and physical stability. In addition, lipid nanoparticles used for cosmetic dermal application have enhanced properties including controlled occlusion, enhanced penetration, increased hydration and improved bioavailability [4], [5]. Emulsions are heterogeneous systems in which one liquid is dispersed in another liquid in the form of droplets [6]. Lipid based emulsions have evolved as vehicles capable for drug delivery of both hydrophilic and lipophilic drugs [7], apart from having potential uses in chemistry, cosmetics and food technology [8].
Lipidic emulsions are favoured for their biocompatibility, biodegradability, stability, convenient handling and easy and cost effective manufacturing processes [9], [10]. Lipidic emulsions are currently in use as parenteral formulations and investigated for solubilisation of water-insoluble drugs [11]. Lipidic emulsions used in parenteral drug delivery systems have also helped improve analgesic therapy [11]. Furthermore, lipidic emulsions are also being investigated for dermal and transdermal applications [7], apart from possible uses in the care of pre-term and term infants in intensive care units [12]. Nanoemulsions are used for intranasal drugs, pulmonary drugs and vaccine delivery [9]. Oral emulsified formulations are being used for its ability to provide protection against oxidation or hydrolysis [6].
The wide use of emulsions for cosmetic and medical purposes leads to numerous new emulsion based systems being constantly developed in laboratories. Most often, these newly formulated emulsions are well characterised for their drug delivering ability, physical properties and cytotoxicity. Size, for example, is one property that developers try to minimise. Smaller molecules are better preferred for cosmetics in order to generate transparent, easily absorbed products that users perceive as fresh and pure. Nanoemulsions have usage diversity and are easily sterilised through filtration that favour developers [13] and are stable against sedimentation or creaming [14]. The demands for ‘ideal’ preparations have led to thorough optimisations of their preparatory methods including polydispersity and crystallinity [15], light stability, temperature stability and low-energy preparation [16]. However, the lipid's oxidative stability is another important aspect that is often overlooked and thus less frequently evaluated.
There are literatures over the years that report the peroxidation profile of a few types of preparations. The effect of lipid peroxidation was evaluated in parenteral lipidic infusions used in new-born infants [17] and the effects of sugars as pro-oxidants in aqueous emulsion systems [18]. Parenteral lipid emulsions used in infants were found to be prone to lipid peroxidation and that free radicals generated from the process are damaging to human cells (caused haemolysis) [19]. The influence of trace elements on the lipid peroxidation of pure lipid emulsions and lipid-containing all-in-one admixtures used in total parenteral nutrition bags was also investigated [20].
Other studies focused on specific components in certain emulsified preparations. A study reported the influence of emulsion droplet surface charge, metals and other factors on oxidative stability [21]. A 2004 study found that using cactus pear fruit controlled lipid peroxidation in fish oil-in-water emulsion [22]. Another investigation formulated polymeric nanoparticles loaded with melatonin and found that the latter acted as an antioxidant better in nanocapsules and nanospheres rather than when used in nanoemulsions [23].
In this review, the importance of lipid peroxidation in vastly used emulsion systems, its effects towards bodily systems, techniques available to control oxidation and methods to measure oxidative stability are discussed.
2. Emulsion based pharmaceutical delivery systems
Emulsion systems are modern colloidal drug carrier, ternary or pseudo-ternary dispersed systems consisting of specific mixtures of oils (or lipids), water, surfactants and co-surfactants [2], [7]. Emulsions could be manipulated to include many beneficial ingredients during their manufacturing. These ingredients provide the delivery system, besides its simplified preparation, thermodynamic stability, minimal viscosity and ultralow surface tension [24]. Emulsions have polar (hydrophilic) and non-polar (hydrophobic) sites that allow the solubilisation and encapsulation of both hydrophilic and lipophilic drugs at these separate sites. Furthermore, emulsions can be designed to be biodegradable; it reduces toxicity and other undesirable side effects of drugs [25]. Some drugs have also demonstrated radical improvement in their stability when incorporated into emulsion based formulations [26].
The various emulsion systems available include dry, micro-, nano-, self-emulsifying, pickering emulsions and multiple emulsions, and among these systems some are more stable than others [8], [27]. Regardless of the type and particle size of these emulsified delivery systems, a successful emulsion should be well evaluated for their physicochemical properties. In addition, emulsions that are formulated for cosmetic use should additionally be able to prevent microbial growth. Another important physical factor that reflects emulsion instability is phase inversion, which is the change from oil-in-water to water-in-oil or vice versa. Other major issues associated with the physical instability of emulsions are flocculation, creaming, coalescence and breaking [28], [29], [30]. Processes recognised as chemical degradation also include hydrolysis, dehydration, isomerisation and racemisation, decarboxylation and elimination, oxidation, photodegradation, drug-excipients and drug–drug interactions [31].
Furthermore, intrinsic contributors to chemical stability include molecular structure of the drug, environmental factors such as temperature, light, pH, buffer species, ionic strength, oxygen, moisture and the inclusion of additives and excipients [31]. However, apart from these physical and chemical properties of delivery systems, oxidative stability is also an essential criterion. This is primarily because direct skin contact and cellular interaction is expected with the usage of these delivery systems.
3. Lipid peroxidation and its effects
3.1. Lipid peroxidation and its effects on emulsion systems
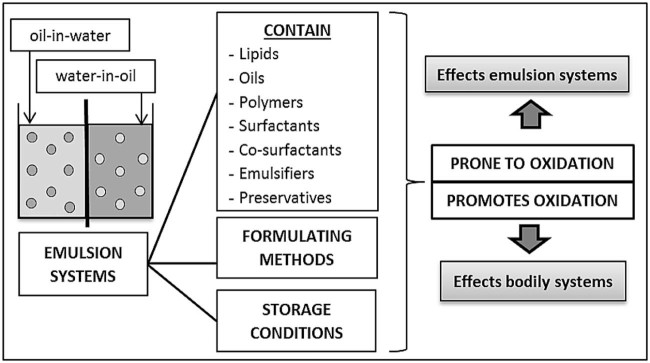
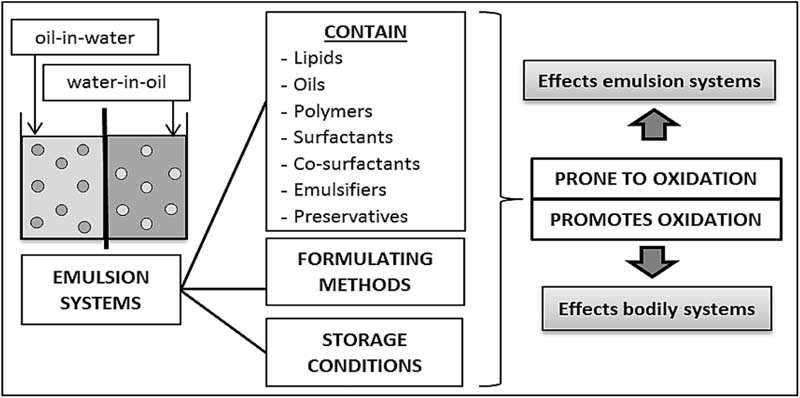
Albeit oxygen being a source of life for many organisms, it is also a primary factor for oxidation. Oxidative damage is produced by oxidation with its specific targets being DNA, protein and lipids [32]. Lipid and oil based formulations are also susceptible to degradation through lipid peroxidation and is a concerning chemical instability [33]. Lipid peroxidation is a common problem with parenteral and oral lipid formulations [34], as they are always formulated using one or more lipophilic and oil based substances. Additionally, the oxidative stability of skincare or cosmetic emulsions helps outline the emulsions' function and optimal storage conditions. Fig. 1 depicts the parameters in emulsion technology that are prone to or cause oxidation.
Fig. 1.
Parameters within emulsion technology that contribute to the oxidative effects of the emulsion systems and in the human physiology.
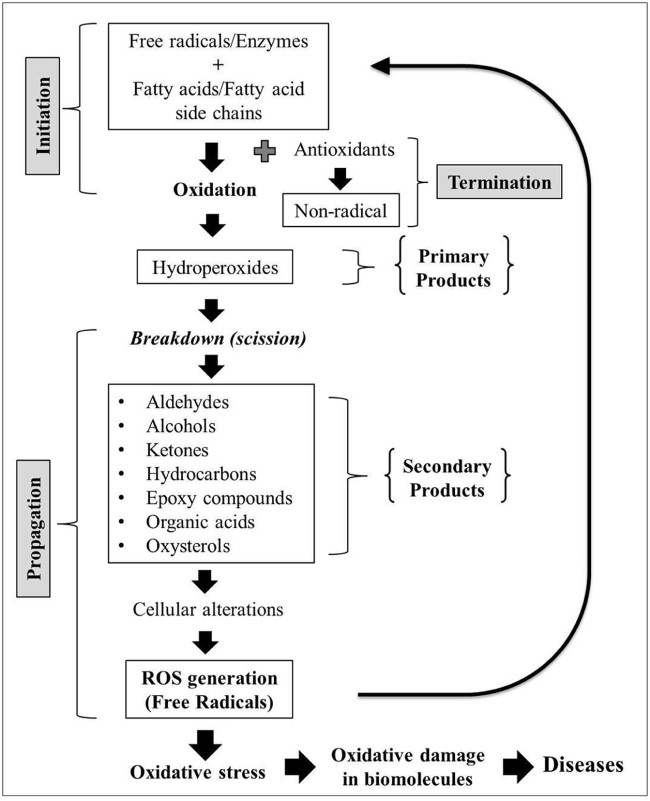
Lipid peroxidation is an auto-oxidation process and consists of initiation, propagation and termination [35]. There are different mechanisms and pathways involved in the lipid peroxidation of emulsified lipids and bulk lipids [33]. Auto-oxidation can occur in bulk fats, fats and oils via a self-sustaining free radical involving mechanism. This occurs through pathways mediated by free radicals and enzymes or without them [35]. Lipid peroxidation is also known to be initiated when a fatty acid or fatty acyl side chain of any chemical entity is attacked by free radicals [36]. These radicals have sufficient reactivity to abstract a hydrogen atom from a methylene carbon belonging to these fatty side chains [37], thus initiating the oxidation reaction. Emulsion systems undergoing lipid peroxidation cause degradation of the encapsulated drug and cause changes to cross linkages, thus retarding dissolution [38]. Lipid peroxidation impacts not only the quality of emulsions by negatively affecting their oil quality but also their safety.
The oxidation process produces many by-products and hydroperoxides are the primary oxidation products when fat and oils undergo oxidation [39]. Hydroperoxides are unstable and are susceptible to decomposition. Secondary or final products consist of a broader number of complex mixtures including volatile, non-volatile and polymeric products, which are produced from the scission of the hydroperoxide products. Examples of secondary oxidation products are aldehydes, ketones, alcohols, hydrocarbons, organic acids and epoxy compounds. These products are also referred to as decay products of the hydroperoxides and their amounts continually increase as the oxidation process continues [40]. Other substances produced by the peroxidation process, such as malonaldehydes and cholesterol oxides, have been reported to have toxic properties as well [41]. Fig. 2 describes the processes involved in lipid peroxidation.
Fig. 2.
The processes involved in lipid peroxidation that result in negative effects towards pharmaceutical emulsions and biological systems.
3.2. Effects of lipid peroxidation on bodily systems
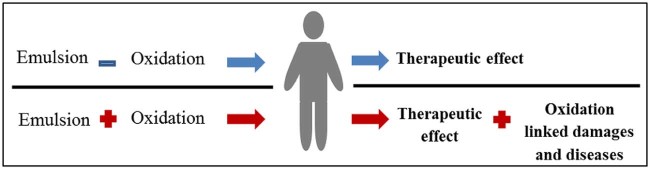
In the body, lipid emulsions are cleared from the blood based on their physicochemical properties and the physiological response of the reticuloendothelial system [42]. Lipid particles within emulsions are removed slower when their droplets are larger and neutrally charged [10]. This occurs with emulsions made from emulsifiers, surfactants and phospholipids with high molecular weights [10]. Thus until emulsions are cleared from the system, biological interaction occur. Emulsions containing oxidatively unstable substances undergo peroxidation that is capable of inducing oxidative stress. Highly reactive ions produced from the oxidation pathways initiate cascades of other oxidation reactions [43]. Fig. 3 depicts the risk of introducing oxidation prone emulsions into the human system.
Fig. 3.
The effects of introducing emulsions with oxidative leniencies into physiological systems.
Oxidative stress related processes are further exacerbated by the state of hydrogen atoms residing within our system. Hydrogen has only a single electron that is prone to removal by any chemical species, leaving behind an unpaired electron on the carbon atom it was originally attached to. The resulting carbon-centred lipid radicals (in aerobic cells) are likely to undergo molecular rearrangement and participate in the oxidation pathways as well. After lipid oxidation reactions are initiated and chain-reacted, lipid peroxides are produced including malonaldehydes and cholesterol oxides (one of the reactive aldehydes and oxysterols respectively) [39], [44]. These compounds interact with each other and affect membrane proteins causing function impairment, increased nonspecific permeability to ions, fluidity changes and inactivation of membrane-bound receptors and enzymes [41].
Cellular membrane alterations triggered by oxidative stress can directly and indirectly contribute to major derangements of cellular metabolism. Altered metabolism gives rise to free intracellular calcium ions that cause the activation of proteases. Proteases then attack the cytoskeleton and nucleases causing further DNA-strand breakage or fragmentation [41], [45]. In addition, peroxide exposure can lead to deformation of the membrane of red blood cells and subsequent leakage of their potassium ions [46].
On another note, prolonged mitochondrial injury may result from reactive oxygen species (ROS) generation, such as the superoxide anions (O2•–) and hydroxyl radicals (•OH). These radicals consist of oxygen free radicals and other chemical entities capable of depleting antioxidant systems, leading to further oxidative stress [46]. Furthermore, oxidative stress leads to production of inflammatory cytokines that also induce the production of free radicals [47]. The overwhelming amount of free radicals will fuel cellular oxidation chain reactions and free radicals like ROS are well recognised as contributors to multiple pathological processes.
Neuronal degeneration occurs with membrane lipids being one of ROS's major targets [48]. The oxidative stress resulting from impairment of antioxidant systems and increased production of ROS has also been associated with hypercholesterolaemia [46]. Other conditions are directly and indirectly associated with lipid peroxidation propagation. These include atherosclerosis (lesions), myocardial infarction, stroke, systemic lupus erythaematosus, erythrocyte dysfunction, ageing and Behcet's, Alzheimer's and Parkinson's diseases. Other related diseases include obsessive compulsive disorder, diabetes mellitus, skin cancer and chronic liver disease [35]. Additionally, the oxidation of triglycerides leads to increase in body temperature and induces toxicity in the central nervous system [26]. It is thus evident that prior to introducing therapeutic oil and lipid based emulsion systems into the body, it is important to ensure that they have very minimal oxidative tendencies. To achieve this, many strategic measures can be undertaken.
4. Preventing lipid peroxidation and maintaining oxidative stability
4.1. Preventive measures during formulation
The ingredients used in formulating emulsions are vital in maintaining oxidative stability. Proteins, sugars, acids, bases, buffers, salts, surfactants and polysaccharides are used in the formulation of oil-in-water emulsions. They may act as either pro-oxidants or antioxidants, depending on their chemical nature, molecular interactions and environmental factors. Total oil content can additionally influence droplet size and viscosity of the emulsion system [2].
A study by Nakaya and co-workers [45] determined the effect of droplet size and emulsifiers on the oxidative stability of oil-in-water emulsions containing polyunsaturated triacylglycerols (TAG), cod liver oil, soybean oil, ester, water and buffer. It was found that oxidative stability was increased when droplet size decreased and that various non-ionic emulsifiers did not influence oxidative stability. Moreover, the study discovered that particle size was influenced by the concentration of surfactants and stabilisers. Increase in water miscible solvent was also found to decrease particle size [45]. However, on the contrary, another study found that there was no correlation between droplet size and oxidative stability of caprylic acid and canola oil containing oil-in-water emulsions [49].
The addition of oil and lipids is unavoidable in the formulation of emulsions. Thus there are specific complementary ingredients that can help provide formulations with additional oxidative stability. For instance, the only chain-breaking antioxidant in human lipids is alpha(α)-tocopherol or vitamin E. Evidence shows that when l-ascorbic acid is formulated with vitamin E, they protect each other from breakdown and thus replenish each other's activity [50], [51]. Ascorbic acid is capable of reducing α-tocopheroxyl radical formed when α-tocopherol reacts with a radical to regenerate α-tocopherol [52]. In oil-in-water emulsions, oxides located at droplet surfaces and pro-oxidants located in the aqueous phase tend to interact. Tocopherol as a non-polar antioxidant is more efficiently retained in oil droplets and was found to accumulate at interfaces where oxidation is more likely to occur [53]. This similar effect was seen when preservatives and antioxidants (natural or synthetic) were used in various cosmetic preparations, subsequently maximising their stability [54], [55], [56], [57].
Propyl gallate is also a phenol based radical terminator suggested to improve oxidative stability. Ascorbyl palmitate is another oxygen scavenger like ascorbic acid that works well with phenol-based antioxidants. However, care must be taken when using them as these compounds can also promote lipid peroxidation by acting as reducers when metal ions are present [33]. Other antioxidants reported to protect encapsulated drugs and unsaturated fatty acid chains from oxidation include β-carotene, butylated hydroxy toluenes (BHT) and butylated hydroxyanisole (BHA) [58], [59]. Furthermore, antioxidants depend strongly on pH and electrochemical properties of emulsions to be effective in retarding oxidation [60], hence monitoring pH levels during formulation and storage is crucial.
Looking into ingredients acting as surfactants, phosphatidylcholine (also known as lecithin) is a complex mixture of phosphatides consisting of phosphatidylcholines, phosphatidylserine, phosphatidylinositol, triglycerides and fatty acids [61]. Lecithins are components of cell membranes and are used extensively in pharmaceutical applications as an emulsifying, dispersing and stabilising agent. It is listed as generally regarded as safe (GRAS) and accepted in the Food and Drug Association (FDA) Inactive Ingredients Guide for Parenterals [61]. The antioxidative properties of phospholipids like lecithin were revealed by their addition to processed vegetable oils and animal fats [62]. Lecithin is a high density lipoprotein that is included in formulations as a phospholipid [63]. In emulsions, lecithin acts as an emulsifying and stabilising agent by reducing interfacial tension [64]. Lecithin may have the ability to form relatively thick and viscoelastic membranes around lipid droplets in emulsion systems. This ability is partly responsible for lecithin's ability to retard lipid oxidation and has efficient synergy with other natural antioxidants [53], [65]. In biological systems, different auto-oxidation mechanisms are expected to be shown by lecithin. Being a cholesterol acyltransferase, it is able to directly hydrolyse oxidized polar phospholipids and transfer oxidation products like lipid hydroperoxide from low-density lipoprotein (LDL) to high-density lipoprotein (HDL). These proteins are then subsequently degraded by HDL enzymes or the liver, preventing the initiation of free radical chain reactions through their oxidation [63].
Oleic acid is a naturally resourced monounsaturated fatty acid found in large quantities in olive oil. Intake of olive oil was shown to reduce serum cholesterol by diminishing oxidative stress and inflammatory mediators while promoting antioxidant defences [66]. A study by Waraho et al. [67] discovered that the addition of oleic acid into emulsions increased lipid hydroperoxides (by up to 5%) but did not cause the increase of oxidation rates. It was thus suggested that the hydroperoxides are not strong pro-oxidants of free fatty acids in oil-in-water emulsions. The pro-oxidant activity of oleic acid is linked to its ability to attract pro-oxidant metals to the emulsion droplet surface. However, the weak pro-oxidant ability of oleic acid was decreased when the emulsion's pH was lowered [67].
Cells treated with olive oil or oleic acid containing emulsion have showed a lesser production of ROS compared to poly-unsaturated fatty acid or soybean oil-based lipid emulsion. This finding was supported by a preclinical rodent study and a study involving children requiring parenteral nutrition [46]. A similar outcome was seen when olive oil based lipid emulsions were tested alongside soybean-based lipid emulsions in critically ill neonates [68]. Other components in olive oil have also shown potential for usage in emulsions. For example, squalene demonstrated antioxidant activity by protecting lipids of oil-in-water emulsions from undergoing peroxidation and squalane increased oxidative stability better than squalene [69].
It is also important to understand the chemical nature of oils used for formulating emulsions. The melting point of oils increases with the increase of fatty acid chain lengths and decreases with increasing degree of unsaturation and a lowered melting point improves the oil's resistance to oxidation [58]. Saturated medium chain triglycerides (MCTs) have greater solubilisation and are resistant to peroxidation [70], [71]. In addition, mono-unsaturated fatty acids are less prone to peroxidation compared to di- or poly-unsaturated fatty acids [70], [71]. Another kind of lipid, known as sphingolipid, is used in the formulation of sphingosomes (a more stable form of liposomes). Sphingolipid is able to avoid aggregation and leakage caused by the oxidation and hydrolysis of phospholipids [27]. The antioxidant profile of parenteral emulsions formulated from fish oil was improved when levels of α-tocopherol was increased in the formula, thus reducing the risk of oxidative stress affecting children [72].
There are other ingredients that may help control oxidation in emulsions. The incorporation of an oil soluble thioglycolic acid into various oils inhibited the oxidative degradation of an anti-HIV drug [73]. Eskandar et al. found that the presence of silica particles in emulsions initially stabilised with lecithin and improved their long term physical stability [74]. Chitosan is a biodegradable hydrophilic polymer [3] with good biocompatibility, high charge density, muco-adhesion properties and is non-toxic [75]. In another study, regardless of storage conditions, poloxamer–chitosan emulgator film was able to stabilise the castor oil-based nano-sized emulsion in terms of mean droplet diameter [25]. Another beneficial stabilising ingredient is hydrophilic polymers [3], including pluronic, a well-known poloxamer. Preservatives added to emulsions to prevent the growth of harmful bacteria, yeast and mould [76], [77], [78], [79] may provide some protection against oxidation but this needs further investigation. In another research, the metal chelators deferoxamine (DFO) and ethylenediaminetetraacetic acid (EDTA) were capable of reducing lipid peroxidation in oil-in-water emulsions containing sodium chloride (an oxidation promoter) [80].
However, in addition to good choices of ingredients, their ‘location’ within the formulated emulsion is as important in order to improve their roles as surfactants, emulsifier and antioxidant. Lipid oxidation occurs more rapidly for oils situated in the air interface than in the bulk oil. As a result, antioxidants should preferably be located at the air or water interfaces to be more affective at retarding oxidation than when evenly distributed throughout the oil [53]. Furthermore, the orientation of lipid molecules affects their access to pro-oxidants or antioxidants residing within the water phase. The free radicals that form at droplet surfaces in the water phase may interact with lipids located in the vicinity or deep within the droplet. This creates an association between the rate of lipid oxidation, diffusion and contact incidences between oxidants, free radicals, hydroperoxides and lipids in each regional phase [53]. Nevertheless, these naturally occurring reactions may be circumvented using novel strategies. For example, the addition of surfactants (which coat and stabilise droplets) also possessing antioxidant properties would be an advantage. These surfactants may help prevent oxidation interactions or collectively reduce surfaces available for interactions.
On the contrary, the presence of certain substances in emulsions can initiate or propagate peroxidation processes. It is therefore necessary that these substances are reduced or eliminated during formulation and storage. Trace metals are naturally occurring impurities in emulsions; however, they are largely responsible for promoting lipid oxidation and increasing the concentration of peroxyl radicals [81]. Heavy metals are strong oxidation catalysts including iron (Fe), copper (Cu), lead (Pb), tin (Sn) and residual hydrogenation catalysts such as platinum (Pt) and palladium (Pd) [82]. In emulsions that contain marine polyunsaturated lipids, the presence of low molecular weight iron was shown to affect stability [83].
In emulsions formulated for cosmetic use, many of the most effective topical based ingredients are inherently unstable and prone to oxidation. Examples include retinoids (a class of chemical compounds related to vitamin A), l-ascorbic acid and agents used in sunscreens [84]. These ingredients may cause the product to oxidise before it is able to interact and work on the skin. Additionally, the ingredients used must be highly pure and refined in order to be free from pigments, decomposition products, sterols, free hydrocarbons and peroxides. These complexes are able to initiate oxidation and destabilise compounds susceptible to oxidation [10], [82].
Other additional precautions should also be taken during the formulation process. It had been suggested that the entire emulsion preparation process should be conducted under nitrogen atmosphere when possible. This becomes crucial more so when excipients and drugs used in the formulations are sensitive to oxidation [26]. Other preparatory based preventive measures include protecting the formulation from light and packaging them in an inert atmosphere [85]. These environments help prevent the presence of harmful contaminants in the emulsions (Table 1).
Table 1.
Methods to prevent lipid peroxidation and maintain or improve oxidative stability in pharmaceutical emulsions.
| Approach | Method/Include | Action/Property | Effect |
|---|---|---|---|
| Content based | Vitamin C (ascorbic acid), Vitamin E (α-tocopherol), propyl gallate, ascorbyl palmitate | Antioxidant at air or water interfaces | Maximise/improve oxidative stability, retards oxidation |
| Oleic acid | Antioxidant | Reduce oxidation | |
| β-carotene, butylated hydroxy toluenes (BHT) and butylated hydroxyanisole (BHA) | Encapsulate drug and unsaturated fatty acid chains | Provide protection against oxidation | |
| Lecithin | Emulsifier, stabiliser, dispersing agent | Reduce interfacial tension, form thick and viscoelastic membranes around lipid droplets, synergistic with other natural antioxidants | |
| Saturated medium chain triglycerides (MCT) | Good solubility | Resistant to peroxidation | |
| Mono-unsaturated fatty acids | Less prone to peroxidation compared to di- or poly-unsaturated fatty acids | Reduce oxidation | |
| Sphingolipid | Avoid aggregation and content leakage caused by oxidation and hydrolysis of phospholipids | Maintain formulation stability preventing further oxidative damage | |
| Thioglycolic acid (oil soluble) | Oxidative inhibitor | Inhibit oxidative degradation of certain drugs – maintaining stability | |
| Silica particles | Stabiliser | Improve long term physical stability | |
| Poloxamer–chitosan | As emulgating film | Maintain droplet diameter – stabilising castor oil-based nano-sized emulsion | |
| Hydrophilic polymers (poloxamer, pluronic) | Stabiliser | Provide stability for emulsions | |
| Metal chelators deferoxamine (DFO) and ethylenediaminetetraacetic acid (EDTA) | Metal binding | Reduce lipid peroxidation | |
| Preservatives | Prevent growth of harmful bacteria, yeast and mould | May provide certain protection against oxidation | |
| Surfactants with antioxidant property | Coat droplets | May reduce surfaces available for oxidation thus preventing oxidation interactions | |
| Preparation based | Reduce or remove trace metals | Initiate and propagate peroxidation | Reduce oxidation reactions |
| Alteration to surfactants' and stabilisers' concentrations (in general) | Decrease droplet/particle size | Increase oxidative stability | |
| Use highly pure and refined ingredients | Free from pigments, decomposition products, sterols, free hydrocarbons and peroxides | Reduce contaminating agents | |
| Nitrogen atmosphere | Create controlled environment | Protect oxidation prone components | |
| Protection from light | Create controlled environment | Protect emulsions | |
| Packaging in inert atmosphere | Create controlled environment | Eliminate oxidation prone contaminants | |
| Monitoring pH levels during formulation and storage | Antioxidant effects | Control factors (electrochemical, pH) that effect the actions of antioxidants |
Furthermore, in view of the many types of emulsions available, it is possible to compare their oxidative stability in response to different choices of ingredients. For example, dry emulsions show improved stability and sustained better release for oral delivery of lipophilic, sparingly soluble, easily oxidised and light sensitive drugs. Cochleates were shown to be more stable systems due to their lower lipid oxidation tendencies [27]. It has also been reported that nanoemulsions are more stable than liposomes and vesicles [9].
4.2. Evaluating lipid peroxidation
The oxidative stability of emulsions can be evaluated using various methods. Notably, the effects of oxidative instability may not affect the organoleptic characteristics of emulsions such as colour, liquefaction and phase separation. As a result, the occurring peroxidation may not be directly observable, thus monitoring involves test-based assessments. There are various assays to evaluate the oxidative stability of lipidic emulsions. These assays help in identifying individual ingredients potentially contributing to or reducing oxidative stability. The presence of secondarily produced aldehydes for instance can be detected using the anisidine value (q-AV) determination technique. This method was described by the American Oil Chemists' Society (AOCS) and estimates the amount of alpha and beta unsaturated aldehydes, 2-alkenals and 2,4-alkadienals. These products continually increase and are detected by the generation of hydroperoxides. Hydroperoxides are formed by the reaction of p-methoxyaniline (anisidine) with unsaturated aldehydes, under acidic conditions. The chromogen generated is then quantified spectrophotometrically and converted to anisidine values (p-AnV or AV) using a formula [40].
Other methods include ultra-violet (UV) absorption, iodometric, ferric-xylenol orange (monitors lipid hydroperoxide intermediate formation), thiobarbituric acid reactive substances (TBARS; lipid peroxidation is indicated by malonaldehyde concentration) and Rancimat assay (measures the change of electrical conductivity caused by the formation of volatile dicarboxylic acids) [86], [87], [88]. Another method measures the rate of dissolved oxygen by fatty acids in emulsions and liposomes [83]. High performance liquid chromatography (HPLC) was also used to evaluate the lipid peroxidation of corn oil-in-water emulsions stabilised by the non-ionic surfactant Tween 20. In the study, hexanol was specifically monitored as the oxidation product [80].
5. Conclusion
Emulsions are used as drug delivery systems and have shown good potential in the pharmaceutical and cosmetic industries. However, lipids and oils contained in these pharmaceutical emulsions undergo lipid peroxidation. Products produced from the oxidation processes negatively influence emulsions and bodily systems in many ways. Hence, it is important to practice better formulating techniques. During formulation, choices of ingredients and the amounts in which they are used are vital in ensuring oxidative stability. The effects of certain ingredients on the oxidative stability of emulsion can be investigated to guide choices. These include studying how polar and non-polar ingredients are positioned in the different phases of emulsification and their possible molecular roles and interactions.
The oxidative stability of emulsions should always be considered as one important aspect of safety; hence evaluations should be done strategically. One recommendation is the use of more than one method to evaluate oxidative stability and these assessments should be conducted repeatedly. For example, the usage of conjugated diene hydroperoxides, weight gain, peroxide value and thiobarbituric acid reactive substance assays were employed to analyse the effects of just one extract towards an oil-in-water emulsion [22].
After the preparation of the emulsions, the influence of different storage environments on oxidative stability should also be assessed. This is primarily due to the complexity of the progression of lipid oxidation. Some methods measure the loss of initial reactants such as hydroperoxides, lipids, antioxidant and oxygen, while others detect the intermediary products generated, like conjugated dienes and hydroperoxides. Other known methods measure alcohols, aldehydes, ketones and hydrocarbons, which are secondary products formed by lipid oxidation [40].
In addition, there are emerging methods that are able to extract specific oils and further isolate and quantify individual peroxidation products through chromatography. The future may also see emerging antibody-based methods for measuring lipid peroxidation in biological systems. There are possibilities that in vivo studies can be applied to assess the extent of oxidative damage caused by the peroxidation products. Bodily fluids (such as plasma) may be assessed for biomarkers produced in response to post-exposure of highly oxidising emulsions. More studies should be conducted on the effects of lipid peroxidation in emulsions used for parenteral nutrition in certain populations. These include home-treated adults [89], patients with sepsis [90] and a broad range of cancer patients [91], [92].
Oxidative stress and death caused by the stress is often associated with the ageing and obese population [93], [94]. Hence, introducing oxidatively stable emulsions becomes an important safety criterion and prevention measure in these patients. It is thus vital that lipid and oil based emulsions intended for their use are well characterised for their oxidative stability. With emerging novel methods, it is also possible to conduct periodic monitoring of the effects of oxidation post-administration.
In conclusion, it is a challenge to formulate drug delivery systems that have good efficacy for their specific intended purpose but are also physically and chemically stable. However, there are increasing numbers of morbidities caused by oxidation processes induced by exposure to oxidation prone entities. This creates an urgent and unavoidable need for monitoring the oxidative profile of emulsions using better techniques and strategic approaches.
Funding
No financial cost was incurred in the preparation of this manuscript. The authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors for the preparation of this manuscript.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Acknowledgements
The authors wish to acknowledge Dr Tamilvanan Shunmugaperumal for highlighting the importance of lipid peroxidation in oil-in-water based drug delivery emulsion systems to the corresponding author and Professor Michael J. Rathbone and Dr Chitneni Mallikarjun for the valuable intellectual guidance. Further acknowledgements are to Drs Kavitha Mohandas and Mohd Javed Qureshi for the useful discussions.
Footnotes
Peer review under responsibility of Shenyang Pharmaceutical University.
References
- 1.Gong C.Y., Shi S., Dong P.W. In vitro drug release behavior from a novel thermosensitive composite hydrogel based on Pluronic f127 and poly (ethylene glycol)-poly (ε-caprolactone)-poly (ethylene glycol) copolymer. BMC Biotechnol. 2009;9:8. doi: 10.1186/1472-6750-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta S. Biocompatible microemulsion systems for drug encapsulation and delivery. Curr Sci. 2011;101:174–188. [Google Scholar]
- 3.Mohanraj V.J., Chen Y. Nanoparticles – a review. Trop J Pharm Res. 2006;5:561–573. [Google Scholar]
- 4.Müller R.H., Petersen R.D., Hommoss A. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev. 2007;59:522–530. doi: 10.1016/j.addr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Pardeike J., Hommoss A., Müller R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Asadujjaman M.D., Mishuk A.U. Novel approaches in lipid based drug delivery systems. J Drug Deliv Ther. 2013;3:124–130. [Google Scholar]
- 7.Heuschkel S., Goebel A., Neubert R.H. Microemulsions – modern colloidal carrier for dermal and transdermal drug delivery. J Pharm Sci. 2008;97:603–631. doi: 10.1002/jps.20995. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R., Kumar M.S., Mahadevan N.A. Multiple emulsions: a review. Int J of Adv in Pharmac Ana. 2012;2:9–19. [Google Scholar]
- 9.Lovelyn C., Attama A.A. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;2:626–639. [Google Scholar]
- 10.Angare D., Giri T., Tripathi D.K. Unexplored areas and new findings in lipid emulsion serving as potential drug carrier for lipophilic drugs: a review. Trends in Medical Research. 2012;7:1–24. [Google Scholar]
- 11.Kalepu S., Manthina M., Padavala V. Oral lipid-based drug delivery systems – an overview. Acta Pharmaceutica Sinica B. 2013;3:361–372. [Google Scholar]
- 12.Driscoll D.F., Bistrian B.R., Demmelmair H. Pharmaceutical and clinical aspects of parenteral lipid emulsions in neonatology. Clin Nutr. 2008;27:497–503. doi: 10.1016/j.clnu.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Sonneville-Aubrun O., Simonnet J.T., L'alloret F. Nanoemulsions: a new vehicle for skincare products. Adv Colloid Interface Sci. 2004;108:145–149. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Solans C., Izquierdo P., Nolla J. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10(3):102–110. [Google Scholar]
- 15.Gutiérrez J.M., González C., Maestro A. Nano-emulsions: new applications and optimization of their preparation. Curr Opin Colloid Interface Sci. 2008;13(4):245–251. [Google Scholar]
- 16.Davidov-Pardo G., McClements D.J. Nutraceutical delivery systems: resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem. 2015;167:205–212. doi: 10.1016/j.foodchem.2014.06.082. [DOI] [PubMed] [Google Scholar]
- 17.Wispe J.R., Bell E.F., Roberts R.J. Assessment of lipid peroxidation in newborn infants and rabbits by measurements of expired ethane and pentane: influence of parenteral lipid infusion. Pediatr Res. 1985;19(4):374–379. doi: 10.1203/00006450-198519040-00012. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi R., Tatsumi Y., Asano M. Effect of metal salts and fructose on the autoxidation of methyl linoleate in emulsions. Agric Biol Chem. 1988;52(3):849–850. [Google Scholar]
- 19.Pitkanen O., Hallman M., Andersson S. Generation of free radicals in lipid emulsion used in parenteral nutrition. Pediatr Res. 1991;29(1):56–59. doi: 10.1203/00006450-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Steger P.J., Mühlebach S.F. Lipid peroxidation of intravenous lipid emulsions and all-in-one admixtures in total parenteral nutrition bags: the influence of trace elements. J Parenter Enteral Nutr. 2000;24(1):37–41. doi: 10.1177/014860710002400137. [DOI] [PubMed] [Google Scholar]
- 21.Mei L., McClements D.J., Wu J. Iron-catalyzed lipid oxidation in emulsion as affected by surfactant, pH and NaCl. Food Chem. 1998;61(3):307–312. [Google Scholar]
- 22.Siriwardhana N., Jeon Y.J. Antioxidative effect of cactus pear fruit (Opuntia ficus-indica) extract on lipid peroxidation inhibition in oils and emulsion model systems. Eur Food Res Technol. 2004;219(4):369–376. [Google Scholar]
- 23.Schaffazick S.R., Pohlmann A.R., de Cordova C.A. Protective properties of melatonin-loaded nanoparticles against lipid peroxidation. Int J Pharm. 2005;289(1):209–213. doi: 10.1016/j.ijpharm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Madhav S., Gupta D. A review on microemulsion based system. Int J Pharm Sci Res. 2011;2:1888–1899. [Google Scholar]
- 25.Tamilvanan S., Kumar B.A., Senthilkumar S.R. Stability assessment of injectable castor oil-based nano-sized emulsion containing cationic droplets stabilized by poloxamer–chitosan emulsifier films. AAPS PharmSciTech. 2010;11:904–909. doi: 10.1208/s12249-010-9455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hippalgaonkar K., Majumdar S., Kansara V. Injectable lipid emulsions – advancements, opportunities and challenges. AAPS PharmSciTech. 2010;11:1526–1540. doi: 10.1208/s12249-010-9526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chime S.A., Onyishi I.V. Lipid-based drug delivery systems (LDDS): recent advances and applications of lipids in drug delivery. Afr J Pharm Pharmacol. 2013;7:3034–3059. [Google Scholar]
- 28.Garti N., Aserin A. Pharmaceutical emulsions: double emulsions and microemulsions. In: Benita S., editor. Microencapsulation methods and industrial applications. Marcel Dekker; New York: 1996. pp. 411–534. [Google Scholar]
- 29.Im-Emsap W., Paeratakul O., Siepmann J. Disperse systems. In: Banker G.S., Rhodes C.T., editors. Modern pharmaceutics. Marcel Dekker; New York: 2002. pp. 250–257. [Google Scholar]
- 30.Sinko P.J. Chemical kinetics and stability. In: Sinko P.J., editor. Martin's physical pharmacy and pharmaceutical sciences. Lippincott Williams & Wilkins; Baltimore: 2006. pp. 402–432. [Google Scholar]
- 31.Yoshioka S., Stella V.J. Kluwer Academic/Plenum Publishers; New York: 2000. Stability of drugs and dosage forms; pp. 4–34. [Google Scholar]
- 32.Kohen R., Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods of their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 33.Cannon J.B. Chemical and physical stability considerations for lipid-based drug formulations. Amer Pharm Rev. 2007;10:132–138. [Google Scholar]
- 34.Shrestha H., Bala R., Arora S. Lipid-based drug delivery systems. J Pharm. 2014;2014 doi: 10.1155/2014/801820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gahalain N., Chaudhary J., Kumar A. Lipid peroxidation: an overview. Int J Pharm Sci Res. 2011;2:2757–2766. [Google Scholar]
- 36.Kumar M.S., Singh P., Rath S.K. Protective effect of quercetin on chloroquine-induced oxidative stress and hepatotoxicity in mice. Malar Res Treat. 2013;2013 doi: 10.1155/2013/141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shilpa H.D., Anita R.B. Malondialdehyde as a marker of lipid peroxidation in acute myocardial infarction patients. MRIMS J Health Sci. 2013;1:20–22. [Google Scholar]
- 38.Zara S., Nabila M. Optimizing oral drug delivery using lipid based formulations. Int Res J Pharm. 2014;5:514–522. [Google Scholar]
- 39.Pereira I.R., Bertolami M.C., Faludi A.A. Lipid peroxidation and nitric oxide inactivation in postmenopausal women. Arq Bras Cardiol. 2003;80(4):415–423. doi: 10.1590/s0066-782x2003000400005. [DOI] [PubMed] [Google Scholar]
- 40.Shahidi F., Zhong Y. Lipid oxidation: measurement methods. In: Shahidi F., editor. Bailey's industrial oil and fat products. John Wiley & Sons; New Jersey: 2005. pp. 357–385. [Google Scholar]
- 41.Halliwell B., Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715S–724S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y., Wu X.T., Li N. Structured triglyceride for parenteral nutrition: meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. 2006;15:406–411. [PubMed] [Google Scholar]
- 43.Uttara B., Singh A.V., Zamboni P. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakaya K., Ushio H., Matsukawa S. Effects of droplet size on the oxidative stability of oil-in-water emulsions. Lipids. 2005;40:501–507. doi: 10.1007/s11745-005-1410-4. [DOI] [PubMed] [Google Scholar]
- 46.Roche L.D. Oxidative stress: the dark side of soybean-oil-based emulsions used in parenteral nutrition. Oxid Antioxid Med Sci. 2012;1:11–14. [Google Scholar]
- 47.Lander H.M. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11(2):118–124. [PubMed] [Google Scholar]
- 48.Lee J., Kosaras B., Del Signore S.J. Modulation of lipid peroxidation and mitochondrial function improves neuropathology in Huntington's disease mice. Acta Neuropathol. 2011;121:487–498. doi: 10.1007/s00401-010-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osborn H.T., Akoh C.C. Effect of emulsifier type, droplet size, and oil concentration on lipid oxidation in structured lipid-based oil-in-water emulsions. Food Chem. 2004;84:451–456. [Google Scholar]
- 50.Lin J.Y., Selim M.A., Shea C.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol. 2003;48:866–874. doi: 10.1067/mjd.2003.425. [DOI] [PubMed] [Google Scholar]
- 51.Sheraz M., Ahmed S., Ahmad I. Formulation and stability of ascorbic acid in topical preparations. Syst Rev Pharm. 2011;2:86. [Google Scholar]
- 52.Niki E., Noguchi N., Tsuchihashi H. Interaction among vitamin C, vitamin E, and beta-carotene. Am J Clin Nutr Title. 1995;62(6):1322S–1326S. doi: 10.1093/ajcn/62.6.1322S. [DOI] [PubMed] [Google Scholar]
- 53.McClements D.J., Decker E.A. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J Food Sci. 2000;65:1270–1282. [Google Scholar]
- 54.Malinowska P., Gliszczyńska-Świgło A., Szymusiak H. Protective effect of commercial acerola, willow, and rose extracts against oxidation of cosmetic emulsions containing wheat germ oil. Eur J Lipid Sci Technol. 2014;116:1553–1562. [Google Scholar]
- 55.Idha K., Gunawan I. Natural antioxidants in cosmetics. In: Rahman A., editor. Studies in natural products chemistry. Elsevier; United Kingdom: 2013. p. 491. [Google Scholar]
- 56.Papageorgiou S., Varvaresou A., Tsirivas E. New alternatives to cosmetics preservation. J Cosm Sci. 2010;61:107. [PubMed] [Google Scholar]
- 57.Jee J.P., Lim S.J., Park J.S. Stabilization of all-trans retinol by loading lipophilic antioxidants in solid lipid nanoparticles. Eur J Pharm Biopharm. 2006;63:134–139. doi: 10.1016/j.ejpb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Vishal J., Sawaranlata S., Vyas A. Oral submicron emulsion – a batter approach for oral delivery of BCS class-II drugs. Pharmacia. 2012;1:73–83. [Google Scholar]
- 59.Mukhopadhyay A.K. Commercial implications of antioxidants. In: Mukhopadhyay A.K., editor. Antioxidants – natural and synthetic. Amani Int'l Publishers; Germany: 2007. p. 118. [Google Scholar]
- 60.de Lima A.A., Sussuchi E.M., De Giovani W.F. Electrochemical and antioxidant properties of anthocyanins and anthocyanidins. Croatica Chemica Acta. 2007;80:29–34. [Google Scholar]
- 61.Yanasarn N., Sloat B.R., Cui Z. Nanoparticles engineered from lecithin-in-water emulsions as a potential delivery system for docetaxel. Int J Pharm. 2009;379:174–180. doi: 10.1016/j.ijpharm.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramadan M.F. Quercetin increases antioxidant activity of soy lecithin in a triolein model system. Food Sci Technol. 2008;41:581–587. [Google Scholar]
- 63.Podrez E.A. Anti-oxidant properties of high-density lipoprotein and atherosclerosis. Clin Exp Pharmacol Physiol. 2010;37:719–725. doi: 10.1111/j.1440-1681.2010.05380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pichot R., Spyropoulos F., Norton I.T. Mixed-emulsifier stabilised emulsions: investigation of the effect of monoolein and hydrophilic silica particle mixtures on the stability against coalescence. J Colloid Interface Sci. 2009;329:284–291. doi: 10.1016/j.jcis.2008.09.083. [DOI] [PubMed] [Google Scholar]
- 65.Azizkhani M., Zandi P. Effects of some natural antioxidant mixtures on margarine stability. Pak J Agri Sci. 2010;47:251–257. [Google Scholar]
- 66.Matalon S., Ji H.L. Oleic acid damages ion transport and promotes alveolar edema: the dark side of healthy living. Am J Respir Crit Care Med. 2005;171:424–425. doi: 10.1164/rccm.2411005. [DOI] [PubMed] [Google Scholar]
- 67.Waraho T., Cardenia V., Rodriguez-Estrada M.T. Prooxidant mechanisms of free fatty acids in stripped soybean oil-in-water emulsions. J Agric Food Chem. 2009;57:7112–7117. doi: 10.1021/jf901270m. [DOI] [PubMed] [Google Scholar]
- 68.Webb A.N., Hardy P., Peterkin M. Tolerability and safety of olive oil–based lipid emulsion in critically ill neonates: a blinded randomized trial. Nutrition. 2008;24:1057–1064. doi: 10.1016/j.nut.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 69.Fox C.B. Squalene emulsions for parenteral vaccine and drug delivery. Molecules. 2009;4:3286–3312. doi: 10.3390/molecules14093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manuel-y-Keenoy B., Nonneman L., De Bosscher H. Effects of intravenous supplementation with alpha-tocopherol in patients receiving total parenteral nutrition containing medium-and long-chain triglycerides. Eur J Clin Nutr. 2002;56:121–128. doi: 10.1038/sj.ejcn.1601294. [DOI] [PubMed] [Google Scholar]
- 71.Kris-Etherton P.M. Monounsaturated fatty acids and risk of cardiovascular disease. Circulation. 1999;100:1253–1258. doi: 10.1161/01.cir.100.11.1253. [DOI] [PubMed] [Google Scholar]
- 72.Baena-Gómez M.A., Aguilar M.J., Mesa M.D. Changes in antioxidant defense system using different lipid emulsions in parenteral nutrition in children after hematopoietic stem cell transplantation. Nutrients. 2015;7:7242–7255. doi: 10.3390/nu7095335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strickley R.G., Anderson B.D. Solubilization and stabilization of an anti-HIV thiocarbamate, NSC 629243, for parenteral delivery, using extemporaneous emulsions. Pharm Res. 1993;10:1076–1082. doi: 10.1023/a:1018935311304. [DOI] [PubMed] [Google Scholar]
- 74.Eskandar N.G., Simovic S., Saupe A. 2006 International conference on nanoscience and nanotechnology. IEEE; 2006. The influence of silica nanoparticles on the preparation and stability of O/W emulsions. July 3. [Google Scholar]
- 75.Sinha V.R., Singla A.K., Wadhawan S. Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 2004;274:1–33. doi: 10.1016/j.ijpharm.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 76.Darr D., Dunston S., Faust H. Effectiveness of antioxidants (Vitamin C and E) with and without sunscreens as topical photoprotectants. Acta Derm Vcnereol (Stockh) 1996;76:264–268. doi: 10.2340/0001555576264268. [DOI] [PubMed] [Google Scholar]
- 77.Lin F.H., Lin J.Y., Gupta R.D. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol. 2005;125:826–832. doi: 10.1111/j.0022-202X.2005.23768.x. [DOI] [PubMed] [Google Scholar]
- 78.Maia A.M., Baby A.R., Pinto C.A. Influence of sodium metabisulfite and glutathione on the stability of vitamin C in O/W emulsion and extemporaneous aqueous gel. Int J Pharm. 2006;322:130–135. doi: 10.1016/j.ijpharm.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 79.Tournas J.A., Lin F.H., Burch J.A. Ubiquinone, idebenone, and kinetin provide ineffective photoprotection to skin when compared to a topical antioxidant combination of vitamins C and E with ferulic acid. J Invest Dermatol. 2006;126:1185–1187. doi: 10.1038/sj.jid.5700232. [DOI] [PubMed] [Google Scholar]
- 80.Cui L., Cho H.T., McClements D.J. Effects of salts on oxidative stability of lipids in Tween-20 stabilized oil-in-water emulsions. Food Chem. 2016;197:1130–1135. doi: 10.1016/j.foodchem.2015.11.099. [DOI] [PubMed] [Google Scholar]
- 81.Hogg N., Kalyanaraman B. Nitric oxide and lipid peroxidation. Biochim Biophys Acta (BBA)-Bioenergetics. 1999;1411:378–384. doi: 10.1016/s0005-2728(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 82.Stuchlík M., Zák S. Lipid-based vehicle for oral drug delivery. Biomed Pap Med Fac Univ Palacky Olomouc. 2001;145:17–26. [PubMed] [Google Scholar]
- 83.Kristinova V., Aaneby J., Mozuraityte R. The effect of dietary antioxidants on iron-mediated lipid peroxidation in marine emulsions studied by measurement of dissolved oxygen consumption. Eur J Lipid Sci Technol. 2014;116:857–871. [Google Scholar]
- 84.Ivana V. The science behind skincare. Tropical treatments [proceedings on the internet] 2014. https://www.prime-journal.com/the-science-behind-skincare/ Available from: [Accessed 14 November 2014]
- 85.Bhalekar M.R., Harinarayana D., Madgulkar A.R. Improvement of photostability in formulation: a review. Asian J Chem. 2008;20:5095–5108. [Google Scholar]
- 86.Souza L.C., Campa A. Pharmacological parameters of intravenously administered amphotericin B in rats: comparison of the conventional formulation with amphotericin B associated with a triglyceride-rich emulsion. J Antimicrob Chemother. 1999;44:77–84. doi: 10.1093/jac/44.1.77. [DOI] [PubMed] [Google Scholar]
- 87.Devasagayam T.P., Boloor K.K., Ramasarma T. Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J Biochem Biophys. 2003;40:300–308. [PubMed] [Google Scholar]
- 88.Park H.Y., Rho H.S., Kim D.H. Modified rancimat method for evaluation of antioxidative effect against skin lipids. Bull Korean Chem Soc. 2010;31:1751–1752. [Google Scholar]
- 89.Jones C.J., Calder P.C. Influence of different intravenous lipid emulsions on fatty acid status and laboratory and clinical outcomes in adult patients receiving home parenteral nutrition: a systematic review. Clin Nutr. 2016 doi: 10.1016/j.clnu.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 90.Gultekin G., Sahin H., Inanc N. Impact of Omega-3 and Omega-9 fatty acids enriched total parenteral nutrition on blood chemistry and inflammatory markers in septic patients. Pak J Med Sci. 2014;30(2):299–304. [PMC free article] [PubMed] [Google Scholar]
- 91.Feng Y.L., Lee C.S., Chiu C.C. Appropriateness of parenteral nutrition usage in cancer patients. Nutr Cancer. 2015;67(6):1014–1017. doi: 10.1080/01635581.2015.1053501. [DOI] [PubMed] [Google Scholar]
- 92.Zhang B., Wei G., Li R. n-3 fatty acid-based parenteral nutrition improves postoperative recovery for cirrhotic patients with liver cancer: a randomized controlled clinical trial. Clin Nutr. 2016 doi: 10.1016/j.clnu.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 93.Tucsek Z., Toth P., Sosnowska D. Obesity in aging exacerbates blood–brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2014;69(10):1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schöttker B., Saum K.U., Jansen E.H. Oxidative stress markers and all-cause mortality at older age: a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2015;70(4):518–524. doi: 10.1093/gerona/glu111. [DOI] [PubMed] [Google Scholar]